Abstract
In this research article, we report the synthesis and structural characterization of a family of first-row metal complexes bearing redox-active ligands with tunable H-bonding donors. We observed that these coordination complexes can adopt three different geometries and that they are stabilized by intramolecular multicenter H-bonding interactions, which are systematically modified by changing the metal ion (Co, Ni, Cu, Zn), the ligand scaffold (variations in the diamine and ureanyl substituents used) and the solvent of crystallization.
Keywords: H-Bonding, first-row transition metals, intramolecular multicenter H-bonding interactions
Graphical Abstract

1. Introduction
Metalloenzymes promote an array of essential biological functions including biosynthesis, electron transfer and oxygen transport [1, 2]. In the active center of these natural catalysts, a metal ion is bound by natural ligands, which sometimes provide or accept electrons during catalysis. The secondary coordination sphere of the active center is molded by non-covalent interactions, usually H-bonds, that tune the reactivity of these metal complexes by enhancing substrate selectivity, enforcing rigidity for fast electron transfer or favoring certain reaction pathways (e.g. homolytic vs. heterolytic O–O bond cleavage). A paradigmatic example is cytochrome P450, a heme metalloenzyme involved in various challenging oxidations such as hydroxylation of unactivated hydrocarbons and epoxidation of olefins [3, 4]. It is proposed that during catalytic turnover, a hemecysteine moiety reduces O2 in the presence of the substrate to form an iron(III)-hydroperoxide intermediate (Cmpd 0) before undergoing heterolytic O–O bond cleavage to generate a very reactive iron(IV)-oxo π-cation radical (Figure 1) [5, 6]. It is suggested that the secondary coordination sphere plays a critical role in delivering H+ at the right time and at the right place to facilitate this reductive O–O cleavage.
Figure 1.
Role of H-bonding in the heterolytic O–O bond cleavage in cytochrome P450.
Metal-dependent enzymes have inspired inorganic chemists to develop model systems capable of reproducing the structure, spectroscopy and reactivity of their active centers [7–9]. Over the last decades, understanding of how changes in the primary coordination sphere affect the reactivity of 3d metal complexes has advanced immensely [10, 11]. Rational and systematic design of the secondary coordination sphere has been more challenging and less investigated [12, 13]. The Borovik lab has pioneered the development of metal complexes that feature intramolecular H-bonding interactions [14]. The trianionic form of the H6buea ligand scaffold (tris[(N′-tert-butylureayl)-N-ethylene]amine) has been used to synthesize mononuclear iron and manganese metal-(hydro)oxo complexes (Figure 2, left) [15–17]. The isolation of these intermediates at ambient temperature is rare due to the tendency of high-valent metal-oxo(hydroxo) complexes to be reduced and produce dimers, which is believed to be prevented by the strong H-bonding interactions between the ligand scaffold and the M-oxo(hydroxo) moiety. Interestingly, the bidentate version of this ligand sys-tem (H4buea) was utilized to stabilize a dicobalt(III) bis-μ-oxo complex at room temperature [18, 19].
Figure 2.
High-valent metal-(hydro)oxo complexes stabilized by intramolecular H-bonding interactions.
We recently reported a family of copper complexes bearing bidentate redox-active ligands with tunable H-bonding donors (Figure 3) [20]. The crystal structures of the complexes revealed intramolecular multicenter H-bonding interactions between the ureanyl H-donors and the other ligand and/or metal ion. The copper complexes were found to stabilize five distinct oxidation states via reduction of the CuII center and by oxidation of the dianionic redox-active ligand scaffolds (L2−) to their imino-radical (L−) and bisimino forms (L). Some of the copper complexes were used as catalysts for the aerobic dehydrogenation of alcohols at room temperature. Our detailed mechanistic analysis suggested that oxidation of the substrate occurred via an unusual reaction pathway for galactose–oxidase model systems in which both dioxygen and ligand scaffold accept electrons and protons from the substrate simultaneously (note: in galactose oxidase, the reduction of O2 and the oxidation of the substrate are two separate processes in a so-called “ping-pong” mechanism) [21]. In this research article, we analyze the coordination chemistry of a family of metal complexes, including other first-row transition metals than Cu (Co, Ni, Zn), in which the intramolecular multicenter H-bonding interactions can be tuned by ligand modification (i.e. changes in diamine backbone and ureanyl substituents) and crystallization conditions.
Figure 3.
Copper complexes bearing redox-active ligands with tunable H-bonding groups.
2. Results and discussion
2.1. Synthesis of ligands
The bidentate ligands were synthesized by mixing the corresponding diamine with two equivalents of isocyanate (Figure 4) [19, 20]. In most cases, the ligands were isolated as white powders in good yields (see Supplementary Material for further details). 1H-NMR analysis of ligands indicated that changes in the diamine backbone or the ureanyl substituent perturbed the electronic environment of ureanyl protons (Hα and Hα′). For tBuPhLH2, the 1H-NMR peaks corresponding to Hα and Hα′ are at 7.5 and 6. 3 ppm, respectively, while for PhPhLH2 (same backbone as tBuPhLH2 but with a different ureanyl substituent), the Hα and Hα′ NMR resonances shifted to 8.0 and 9.0 ppm, respectively, indicative of the electron-withdrawing nature of the Ph group in comparison with tBu. For tBuEtLH2 (different backbone but same ureanyl substituent), the signals for Hα and Hα′ shifted to 5.7 and 5.6 ppm, respectively, which is also in agreement with the stronger donating ability of the 1,2-ethyldiamine compared to the 1,2-phenyldiamine backbone.
Figure 4.
Synthesis of ligands. Note: the synthesis and characterization of tBuPhLH2, PhPhLH2, (S)-MeBzLH2, tBu-4,5-Me2-PhLH2, Ph-4,5-Me2-PhLH2 and PhEtLH2 was previously described by our research group [20]. The synthesis of tBuEtLH2 was reported by Borovik and coworkers [19].
2.2. Syntheses of metal complexes
The metal complexes were synthesized under anaerobic conditions in DMA (dimethylacetamide) by reacting 1 equiv of ligand with 2 equiv of KH followed by addition of 0.5 equiv of the metal source (Figure 5). The complexes were purified and isolated as crystals by layering diethyl ether into DMA or DMF (dimethylformamide) solutions of the metal complexes. These metal compounds were characterized by different means, including elemental analysis, single crystal X-ray crystallography, NMR (i.e. 1H-NMR for diamagnetic complexes), UV-vis, and FT-IR (see complete characterizations in the Supplementary Material).
Figure 5.
Synthesis of metal complexes. Note: the synthesis and characterization of tBuPhCuII, PhPhCuII, (S)-MeBzCuII, tBu-4,5-Me2-PhCuII, Ph-4,5-Me2-PhCuII, tBuEtCuII, and PhEtCuII was previously described by our research group [20]; the synthesis and characterization of tBuEtFeII, tBuEtNiII, and tBuEtZnII was reported by Borovik and coworkers [19].
2.3. Single crystal X-ray crystallography analysis
Crystalline samples of the metal compounds were analyzed by single crystal X-ray crystallography (see Table 1 and Supplementary Material). We found that these complexes can adopt three different geometries, square-planar (D4h), tetrahedral (Td) or twisted pseudo-tetrahedral (D2d) geometries depending on the metal, ligand and the solvent used in the crystallization (Figure 6) [1]. We observed H-bonding interactions between the ureanyl Hα′ and the Nα atoms of the other ligand and some weak interactions between the ureanyl Hα′ and the metal center in an intramolecular multicenter fashion [12, 22, 23].
Table 1.
Selected distances, twist angle, solvent of crystallization and geometry for the metal complexes described in this article.
| Complexa,b | d(Nα…Hα′) (Å) | d(M…Hα′) (Å) | αTW (°) | Solv. | Geometry |
|---|---|---|---|---|---|
| tBuPhCuIIDMF | 2.154(19)–2.204(19) | 2.72(2)–2.80(2) | 0 | DMF | D4h |
| tBuPhCuIIDMA | 2.19(3)–2.25(3) | 2.60(2)–2.67(2) | 45.9 | DMA | D2d |
| AdPhCuII | 2.166(17)–2.303(18) | 2.777(19)–2.814(19) | 0 | DMA | D4h |
| PhPhCuIIDMF | 2.137(16)–2.165(16) | 2.677(17)–2.728(17) | 0 | DMF | D4h |
| PhPhCuIIDMA | 2.140(18)–2.501(18) | 2.56(2)–2.734(19) | 56.4 | DMA | D2d |
| 4-MeO-PhPhCuII | 2.121(19)–2.206(19) | 2.64(2)–2.86(2) | 0 | DMF | D4h |
| 4-Cl-PhPhCuII | 2.132(19)–2.17(2) | 2.71(2)–2.74(2) | 0 | DMF | D4h |
| 2,6-Me2-PhPhCuII | 2.16(2) | 2.73(2)–2.82(3) | 0 | DMF | D4h |
| 2,6-F2-PhPhCuII | 2.128(17)–2.338(17) | 2.556(18)–2.639(18) | 52.7–57.6c | DMF | D2d |
| 2,6-Cl2-PhPhCuIIDMF | 2.249(17)–2.324(17) | 2.586(18)–2.598(18) | 58.0 | DMF | D2d |
| 2,6-Cl2-PhPhCuIIDMA | 2.252(18)–2.33(2) | 2.61(2)–2.68(2) | 54.4 | DMA | D2d |
| (S)-MeBzPhCuII | 2.21(3)–2.33(3) | 2.54(3)–2.63(3) | 51.3 | DMA | D2d |
| (R)-MeBzPhCuII | 2.22(3)–2.34(3) | 2.58(3)–2.67(3) | 51.4 | DMA | D2d |
| tBu-4,5-Me2-PhCuII | 2.236(16)–2.279(16) | 2.786(17)–2.835(17) | 0 | DMF | D4h |
| Ad-4,5-Me2-PhCuII | 2.14(2)–2.19(2) | 2.77(3) | 0 | DMA | D4h |
| Ph-4,5-Me2-PhCuIIDMF | 2.13(2)–2.35(3) | 2.46(4)–2.58(4) | 51.5–57.6c | DMF | D2d |
| Ph-4,5-Me2-PhCuIIDMA | 2.13(2)–2.35(3) | 2.46(4) – 2.58(4) | 57.8 | DMA | D2d |
| tBuEtCuII | 2.28(2)–2.63(2) | 2.67(2)–2.78(2) | 48.0 | DMF | D2d |
| PhEtCuII | 2.10(2)–2.32(2) | 2.48(2)–2.70(2) | 46.3–48.4c | DMF | D2d |
| PhPhCoII | 2.477(18)–2.571(18) | 2.500(19)–2.55(2) | 88.2 | DMA | Td |
| PhPhNiII | 2.062(14)–2.103(14) | 2.637(16)–2.693(16) | 0 | DMF | D4h |
| PhPhZnII | 2.519(18)–2.585(18) | 2.529(19)–2.55(2) | 88.4 | DMA | Td |
Tables with all the crystallographic data can be found in the Supplementary Material.
The crystal structure of tBuPhCuII, PhPhCuII, (S)-MeBzCuII, tBu-4,5-Me2-PhCuII, Ph-4,5-Me2-PhCuII, tBuEtCuII, and PhEtCuII was previously described by our research group [20].
Complex with two conformations.
Figure 6.
Intramolecular multicenter H bonding.
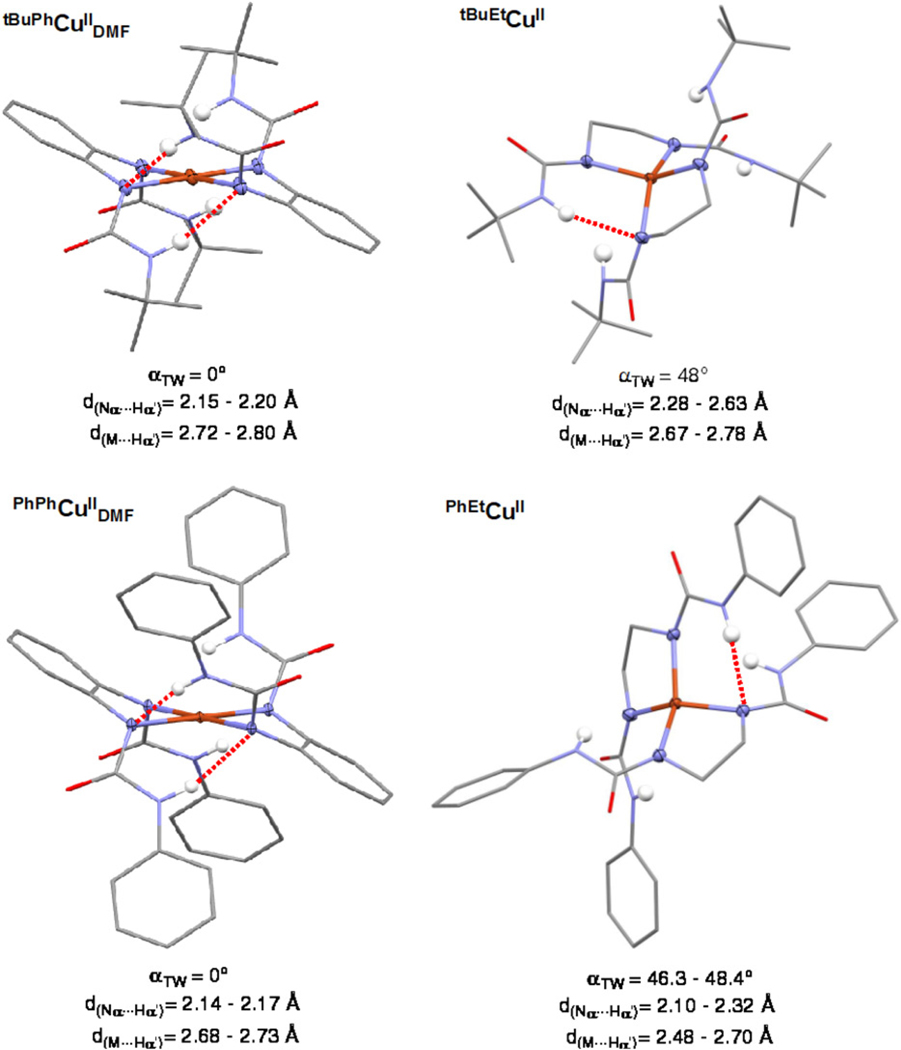
We found that these H-bonding interactions are dependent on the geometry of the complex: for square-planar complexes (twist-angle, αTW = 0) short Nα…Hα′ (~2.1–2.3 Å) and long M…Hα′ distances (~2.3–2.9 Å) are found, which systematically varied to longer Nα…Hα′ (~2.3–2.8 Å) and shorter M…Hα′ (~2.6–2.9 Å) distances upon increase of the twist angle αTW (Figure 6).
2.3.1. Effect of different metals on the geometry of the complexes
X-ray diffraction analysis of the Co, Ni, Cu, and Zn complexes bearing the same ligand scaffold PhPhL2− revealed that the identity of the metal ion has a deep impact on the geometry of the complex (Figure 7). We found that PhPhCuIIDMF and PhPhNiII are both square-planar with short Nα…Hα′ distances (~2.1 Å) and long M…Hα′ distances (~2.7 Å). On the other hand, the analogous PhPhCoII and PhPhZnII systems are tetrahedral (αTW=88°) with longer Nα…Hα′ distances (~2.6 Å) and slightly shorter M…Hα′ distances (~2.5 Å).
Figure 7.
Effect of the metal identity on the geometry of the metal complexes.
2.3.2. Effect of solvent of crystallization
X-ray diffraction analysis of the Cu complexes also revealed that these complexes can adopt two different geometries D4h and D2d (Figure 8). Strikingly, the geometry of tBuPhCuII and PhPhCuII seemed to be strongly dependent on the solvent of crystallization. For example, tBuPhCuIIDMF and PhPhCuIIDMF are orange crystals with D4h geometry in DMF but crystallization in DMA led to purple crystals with D2d geometry (tBuPhCuIIDMA and PhPhCuIIDMA).
Figure 8.
Effect of the solvent of crystallization on the geometry of the copper complexes.
In general, we observed that DMF tends to stabilize D4h geometries while DMA produces D2d complexes, but some exceptions can be found. For example, the Cu complexes bearing ligand scaffolds with adamantyl groups in the ureanyl substituent (AdPhCuII and Ad-4,5-Me2-PhCuII) were crystallized in DMA and in both cases, a D4h geometry was observed. Other complexes such as Ph-4,5-Me2-PhCuII and 2,6-Cl2-PhPhCuII adopted the D2d geometry in both solvents with comparable M-Nα, Nα…Hα′, M…Hα′ distances and similar twist angles. We analyzed the crystal structures and found that the potassium counterion played a critical role in stabilizing the different geometries (Figure 9). We observed that for the Cu complexes adopting D4h geometry, the potassium ions were coordinated to the two O atoms of the ureanyl substituents (i.e. each K+ coordinated to two O atoms of the same ligand). On the other hand, the Cu complexes that adopted D2d geometry were coordinated only by one potassium ion per ligand scaffold. We found that in both situations, either DMF or DMA are also coordinated to K+ but that DMF seems to favor the D4h arrangement and DMA the D2d.
Figure 9.
Effect of the solvent of crystallization on the geometry of the copper complexes.
2.3.3. Effect of the dimane backbone
We also observed that the geometry of the copper complexes depended on the diamine backbone utilized (Figure 10). The CuII complexes with the more flexible ethylenediamine backbone (tBuEtL2− and PhEtL2−) stabilize D2d geometries. On the other hand, the more rigid o-phenylenediamine backbones (tBuPhL2− and PhPhL2− in Figure 10) favor the formation of D4h structures.
Figure 10.
Effect of the dimaine backbone on the geometry of the copper complexes.
2.3.4. Effect of the ureanyl substituent
Small changes in the structure of the ligands also led to a change in the geometry from D4h to D2d: 2,6-Cl2-PhPhCuII, 2,6-F2-PhPhCuII, and 2,6-Me2-PhPhCuII were all crystallized in DMF but only the latter had a D4h geometry (Figure 11). Like in the other examples described above, the 2,6-Me2-PhPhCuII D4h structure showed shorter Nα…Hα′ distances (~2.2 Å) and longer M…Hα′ distances (~2.8 Å) when compared to the 2,6-Cl2-PhPhCuII and 2,6-F2-PhPhCuII D2d structures (Nα…Hα′ distances ~2.3 Å; M…Hα′ distances ~2.6 Å).
Figure 11.
Effect of the ureanyl substituents on the geometry of the copper complexes.
2.3.5. Intramolecular multicenter hydrogen bonds, general trends
With all the structural information in hand, we plotted the M–Hα′ distances against the Nα…Hα′ distances (Figure 12) [22] and found that the metal complexes adopting square-planar geometry (D4h, αTW=0°) were clustered at the top left of the chart, which illustrates their short Nα…Hα′ and long M…Hα′ distances (area marked in orange). A second group of complexes comprising the Cu compounds with twisted pseudo-tetrahedral geometry (D2d, 40° < αTW < 60°) can also be identified, which show slightly shorter M…Hα′ distances and longer Nα…Hα′ (marked in red). A third group of compounds includes the CoII and ZnII complexes with geometries close to tetrahedral (αTW~90°) in which the Nα…Hα′ and M…Hα′ distances are very similar (area marked in grey in Figure 12). We also noticed that within the D4h and D2d groups of metal complexes (marked in orange and red), most of the data points form linear correlations, in which an increase of the N…Hα′ leads to a proportional increase of the M…Hα′ (slope ~ 1).
Figure 12.
Scattegram in which average Nα…Hα′ distances are plotted against average M…Hα′ distances. Note: the X-ray diffraction analysis of tBuPhCuII, PhPhCuII, (S)-MeBzCuII, tBu-4,5-Me2-PhCuII, Ph-4,5-Me2-PhCuII, tBuEtCuII, and PhEtCuII was previously described by our research group [20].
From the structural analysis of this family of metal complexes (22 crystal structures included in this work and the tBuEtFeII, tBuEtNiII, tBuEtCoII, and tBuEtZnII structures previously reported by Borovik [19]) we can extract some general trends. First, CuII and NiII favor square-planar (D4h) and distorted tetrahedral geometries (D2d) and the rest of the metals favor tetrahedral (Td) geometry [19]. Second, for the Cu complexes, the D2d geometry is generally favored with DMA as the crystallization solvent and the D4h geometry is favored when DMF is used as solvent of crystallization, although some exceptions can be found (e.g. D4h Ad-4-5-Me2-PhCuII was obtained in DMA). Third, for the CuII complexes, the more flexible ethylenediamine backbone (e.g. tBuEtL2− and PhEtL2−) favors D2d geometries while the more rigid o-phenylenediamine (e.g. tBuPhL2−and PhPhL2−) favors stabilization of D4h structures.
3. Conclusions and perspectives
In this work, we described the synthesis and structural characterization of metal complexes bound by bidentate redox-active ligands with tunable H-bonds. We found that these complexes were stabilized via intramolecular multicenter H-bonding interactions between the ligands and the metal center, and that the directionality of these interactions depended on the geometry of the complexes. The ability of the Cu complexes to adopt at least two coordination environments suggests that these intramolecular H-bonding interactions are flexible and able to rearrange and stabilize different geometries, which we believe plays a critical role in the catalytic oxidation of alcohols (i.e. we proposed that H-bonding interactions are able to stabilize a reactive Cu/O2 species and to direct the coordination of substrate prior to C–H oxidation).
Our current efforts are focused on expanding this family of complexes in terms of metals used (more examples of Fe, Co, Ni, Zn), ligands (diamine and ureanyl variations) and crystallization conditions (solvent, counterions, etc.). We believe that all of this structural information will allow us to predict the geometry and H-bonding interactions upon modification of the metal complex systems. To the best of our knowledge, this is one of the first research articles in which intramolecular H-bonding interactions are systematically varied and analyzed. Ongoing studies are focused on analyzing the impact of these changes on the reactivity and spectroscopy of these complexes. We are also interested in quantifying, experimentally and/or theoretically, the strength of these intramolecular multicenter H-bonding interactions [24].
Supplementary Material
Acknowledgments
Funding
We thank the Robert A. Welch Foundation (grant N-1900 to I.G.B.) and the National Institutes of Health (NIH award number R15GM128078 to I.G.B.) for financial support. Prof. Swart thanks MINECO (CTQ2014-59212-P and CTQ2015-70851-ERC to M.S.), Gen-Cat (2014SGR1202 to M.S.), FEDER (UNGI10-4E-801 to M.S.).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, L. Tian. Chem. Rev, 114, 3659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kopp DA, Lippard SJ. Curr. Opin. Chem. Biol, 6, 568 (2002). [DOI] [PubMed] [Google Scholar]
- [3].Rittle J, Green MT. Science, 330, 933 (2010). [DOI] [PubMed] [Google Scholar]
- [4].Green MT. Curr. Opin. Chem. Biol, 13, 84 (2009). [DOI] [PubMed] [Google Scholar]
- [5].Meunier B, de Visser SP, S. Shaik. Chem. Rev, 104, 3947 (2004). [DOI] [PubMed] [Google Scholar]
- [6].Shaik S, Cohen S, Wang Y, Chen H, Kumar D, W. Thiel. Chem. Rev, 110, 949 (2010). [DOI] [PubMed] [Google Scholar]
- [7].Costas M, Mehn MP, Jensen MP, L. Que. Chem. Rev, 104, 939 (2004). [DOI] [PubMed] [Google Scholar]
- [8].Elwell CE, Gagnon NL, Neisen BD, Dhar D, Spaeth AD, Yee GM, Tolman WB. Chem. Rev, 117, 2059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Trammell R, Rajabimoghadam K, Garcia-Bosch I. Chem. Rev, 119, 2954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oloo WN, Que L. Acc. Chem. Res, 48, 2612 (2015). [DOI] [PubMed] [Google Scholar]
- [11].Cho J, Sarangi R, Nam W. Acc. Chem. Res, 45, 1321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Natale D, Mareque-Rivas JC. Chem. Commun, 425 (2008). [DOI] [PubMed] [Google Scholar]
- [13].Shook RL, Borovik AS. Inorg. Chem, 49, 3646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cook SA, Borovik AS. Acc. Chem. Res, 48, 2407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Macbeth CE, Golombek AP, Young VG, Yang C, Kuczera K, Hendrich MP, Borovik AS. Science, 289, 938 (2000). [DOI] [PubMed] [Google Scholar]
- [16].Shirin Z, Hammes BS, Young VG, Borovik AS. J. Am. Chem. Soc, 122, 1836 (2000). [Google Scholar]
- [17].Lacy DC, Gupta R, Stone KL, Greaves J, Ziller JW, Hendrich MP, Borovik AS. J. Am. Chem. Soc, 132, 12188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Larsen PL, Parolin TJ, Powell DR, Hendrich MP, Borovik AS. Angew. Chem. Int. Ed, 42, 85 (2003). [DOI] [PubMed] [Google Scholar]
- [19].MacBeth CE, Larsen PL, Sorrell TN, Powell D, Borovik AS. Inorg. Chim. Acta, 341, 77 (2002). [Google Scholar]
- [20].Rajabimoghadam K, Darwish Y, Bashir U, Pitman D, Eichelberger S, Siegler MA, Swart M, Garcia-Bosch I. J. Am. Chem. Soc, 140, 16625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Whittaker JW. Chem. Rev, 103, 2347 (2003). [DOI] [PubMed] [Google Scholar]
- [22].Braga D, Grepioni F, Tedesco E, Biradha K, Desiraju GR. Organometallics, 16, 1846 (1997). [Google Scholar]
- [23].Jeffrey GA, Maluszynska H, Mitra J. Int. J. Biol. Macromol, 7, 336 (1985). [Google Scholar]
- [24].Dahl EW, Kiernicki JJ, Zeller M, Szymczak NK. J. Am. Chem. Soc, 140, 10075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.