Structured abstract
Purpose of review:
There is increasing interest in the long-term health and comorbid conditions associated with endometriosis for both women and neonates. The purpose of this review was to synthesize and discuss the current state of the literature investigating endometriosis and risk of adverse pregnancy outcomes.
Recent findings:
Methodologic considerations for studying endometriosis and adverse pregnancy outcomes include complexities regarding the comparison population, endometriosis definition, sample size, residual confounding, and interactions. The current research on endometriosis and adverse pregnancy outcomes should be interpreted cautiously. To date, evidence suggests that endometriosis may be associated with higher risk of ectopic pregnancy, placenta previa, preterm birth, and cesarean section. While an association with miscarriage and stillbirth has been consistently observed, the relative risk was small.
Summary:
Pregnant women with endometriosis may be at higher risk for certain adverse pregnancy outcomes and may therefore benefit from additional monitoring. However, additional research is needed to confirm these associations and should focus on ensuring studies have internal and external validity, as well as, investigate the potential for differences in endometriosis phenotypes. Moreover, future research should focus on understanding potential mechanisms of association and better understanding how early interventions, through increased monitoring or screening during pregnancy, may improve outcomes.
Keywords: endometriosis, pregnancy, miscarriage, pre-term birth, fetal growth
Introduction
Endometriosis is a gynecologic disease that affects approximately 10% of women and occurs when endometrial-like tissue thrives outside of the uterus. Endometriosis is heterogeneous in its phenotype and symptoms, with women most often experiencing dysmenorrhea, dyspareunia, dyschezia, or infertility [1, 2]. The relationship between endometriosis and risk of subsequent adverse pregnancy outcomes is complex. Much of the current research to date comes from low-quality or small studies, and therefore generalizing from these findings may lead to inaccurate or false conclusions. We are tasked with disentangling the “myth” from “reality” for understanding the relationship between endometriosis and adverse pregnancy outcomes. In this commentary, we will discuss the study-design and methodologic complexities to consider when interpreting results in this field and then summarize the current state of the research. We will conclude with an outline of gaps in knowledge and critical next steps for moving this field forward.
Methodologic Complexities of Studying Endometriosis and Pregnancy
Endometriosis is a challenging condition to rigorously study in epidemiologic research [3]. There are many complexities related to the disease definition and appropriate comparison population. Most of the research on endometriosis and adverse pregnancy outcomes has been conducted among women recruited from an infertility clinic, which may not generalize to the two-thirds of women with endometriosis who do not present with infertility [4, 5], and also may conflate the effect of infertility on pregnancy outcomes independent of endometriosis [6-8].
Many adverse pregnancy outcomes are rare, i.e. occur in less than ten percent of pregnancies [9], while endometriosis is estimated to occur in approximately ten percent of women of reproductive age [3]. Thus, it is often challenging to find a sufficiently large sample population that has adequate statistical power and that additionally has detailed, valid data on both endometriosis and pregnancy outcomes. A meta-analyses can combine information across small studies, but if studies with biases are included in the meta-analysis, then the results of the meta-analysis cannot improve the validity of the independent, biased studies themselves [10]. In addition, study differences in the population sampled, endometriosis and outcomes definitions, statistical modeling, etc. may render the meta-analytic summary statistic uninformative.
The clinical gold-standard of endometriosis diagnosis is surgical visualization [11]. However, the path to receive a diagnosis of endometriosis is complex for many women, with an average diagnostic delay between symptom onset and disease diagnosis of seven years [4]. Endometriosis diagnoses has also been shown to vary by race/ethnicity and socio-economic status [12, 13], with affluent white women traditionally having greater access to care than other marginalized groups. In order to receive an endometriosis diagnosis, both the patient and her healthcare provider must recognize her symptoms as possibly caused by endometriosis, and the provider must refer the patient to appropriate, specialized care. The different types of providers through which endometriosis is ultimately diagnosed influence population sampling, validity, misclassification, and generalizability. This influences the group diagnosed with endometriosis but also, in any study population, there may be women with undiagnosed endometriosis misclassified into the unexposed group. The proportion of misclassification of endometriosis is correlated with financial, cultural, and systemic barriers to accessing care that are also associated with adverse pregnancy outcomes.
How women are defined as having been diagnosed with endometriosis is another complexity of endometriosis epidemiology. Some studies have restricted their “definition” of endometriosis to surgically treated and/or confirmed endometriosis, while other studies have defined endometriosis more broadly (e.g. self-report, clinician report but no surgical confirmation, radiologic imaging). These differences in endometriosis definition could lead to misclassification, which could impact the estimate of the associations.
When studying endometriosis and pregnancy outcomes, the pathway to diagnosis is further complicated by whether the woman also experiences infertility symptoms. It is a myth that all women with endometriosis experience infertility. Approximately one-third of women with endometriosis present with infertility [4]. While this is twice the prevalence of the general population [14], our research group reported that among the Nurses’ Health Study II (NHSII) cohort (n=116,429), >80% of women with endometriosis achieved a successful pregnancy [5]. Research restricted to infertility clinics have inherent selection bias, as only half of couples who experience infertility consult a healthcare provider about their infertility and fewer go on to receive treatment [15, 16]. Among those couples who are able to access infertility care, some women diagnosed with endometriosis during an infertility evaluation would not have been diagnosed otherwise had they not tried to conceive, recognized difficulty conceiving, and presented for an infertility evaluation.
Much of the research to date has come from clinical studies of women undergoing infertility treatment that oversample endometriosis cases who were diagnosed due to their infertility. These studies cannot tease apart the influence of endometriosis from the influence of infertility or fertility treatment. Indeed, fertility treatment, independent of endometriosis, has been associated with greater risk of many adverse pregnancy outcomes [6, 7]. Lastly, these comparisons may underestimate true endometriosis-specific risk by comparing women with endometriosis to women with other infertility diagnoses that may independently be at higher risk for adverse pregnancy outcomes [17, 8, 18].
Lastly, not all of the prior research has been able to account for potential confounding variables. Confounding variables in this context are variables that are associated with both endometriosis and the adverse pregnancy outcome that are not on the causal pathway between endometriosis and the outcome. Potential confounding variables are specific to each adverse pregnancy outcome, but may include maternal age, maternal body mass index, smoking history, parity, infertility history, dietary intake, and alcohol intake. Some study designs or data structures (e.g. registry data, electronic health records, claims data) may not have collected this information. Inability to take these potential confounding variables into account may lead to incorrect effect estimates and ultimately incorrect conclusions impacted by residual confounding [19].
Hypothesized Mechanisms
There is increasing literature suggesting that women with endometriosis may have higher risk of long-term health outcomes [20, 3], including cardiovascular disease and cancer, compared to women without endometriosis. The hypothesized mechanisms behind these associations vary by disease. There are several hypothesized pathways that are suggested for why women with endometriosis may have higher risk of adverse pregnancy outcomes (Figure 1) [21, 22]. Women with endometriosis have greater levels of inflammation both locally and systemically [23, 24]. Inflammation may contribute to the etiology of gestational diabetes, hypertensive disorders of pregnancy (gestational hypertension and preeclampsia), and preterm birth [25-27]. Additionally women with endometriosis may have inadequate uterine contractility [28] and deficient placentation that may influence implantation, fetal growth, placental abnormalities, and gestation length [29]. Moreover, progesterone resistance of the endometrium in women with endometriosis may impact oocyte quality, pregnancy maintenance, and gestational length, which has downstream contributions on implantation and embryo development [21, 30, 29].
Figure 1.
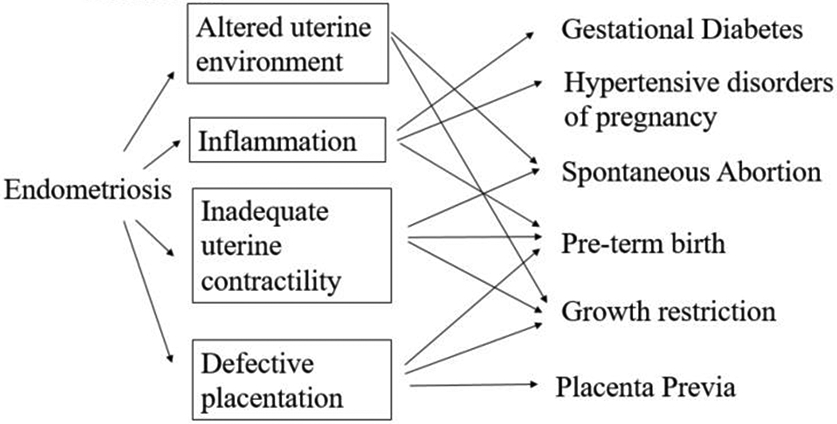
Hypothesized mechanisms between endometriosis and adverse pregnancy outcomes
Current Summary of the Research on Endometriosis and Adverse Pregnancy Outcomes
As of September 2019, there have been numerous studies, and to our knowledge, six meta-analyses of the relation between endometriosis and at least one pregnancy outcome (Table 1).
Table 1.
Summary of previous meta-analyses summarizing endometriosis and adverse pregnancy outcomes
| Strength of Association |
Outcome | Meta-analysis Odds Ratio |
Number of studies |
I2 | Among Spontaneous Conceptions |
Among ART Conceptions |
Citation | Potential Study Limitations |
|---|---|---|---|---|---|---|---|---|
| Consistent, Strong Association | Placenta Previa | 3.31 (2.37-4.63) | 18 | 77% | 6.83 (2.10-2.24) | 3.33 (1.52-7.30) | [30] | Rare outcome, may be prone to recall/selection bias |
| 3.03 (1.50-6.13) | 10 cohort studies | 75% | [31] | |||||
| Cesarean section | 1.86 (1.51-2.29) | 20 | 90% | 1.76 (1.51-2.06) | 1.24 (0.89-1.71) | [30] | Many potential indications, including over medicalization | |
| 1.57 (1.39-1.78) | 13 cohort studies | 74% | [31] | |||||
| Preterm birth | 1.70 (1.40-2.06) | 23 | 92% | 1.70 (1.38-2.10) | 1.27 (1.04-1.55) | [30] | Strongly correlated with other adverse outcomes including fetal growth | |
| 1.63 (1.32-2.01) | 14 cohort studies | 74% | [31] | |||||
| 1.49 (1.30-1.70) | 12 | 51% | 1.59 (1.32-1.90) | 1.43 (1.14-1.79) | [44] | |||
| Consistent, Modest Association | Miscarriage | 1.75 (1.29-2.37) | 9 cohort studies | 93% | [31] | Challenging to capture early miscarriage, may over sample planned conceptions, may not be captured in registry data | ||
| 1.31 (1.07-1.59) | 18 | 0% | [32] | |||||
| Stillbirth | 1.29 (1.10-1.52) | 7 | 5% | 7.16 (0.74-69.57) | [30] | Rare outcome | ||
| Inconsistent, Modest/Small Association | Gestational Diabetes | 1.26 (1.03-1.55) | 12 | 31% | 1.30 (0.85-1.98) | 1.08 (0.73-1.60) | [30] | Prevalence and screening practices changing over time |
| 1.14 (0.86-1.51) | 12 | 56% | [49] | |||||
| Preeclampsia | 1.18 (1.01-1.39) | 13 | 63% | 1.21 (0.94-1.56) | 0.89 (0.48-1.67) | [30] | Rare outcome | |
| 1.08 (0.91-1.30) | 15 | 73% | 1.21 (0.94-1.56) | 0.74 (0.41-1.35) | [38] | |||
| 1.04 (0.83-1.29) | 12 cohort studies | 44% | [31] | |||||
| Gestational Hypertension/Preeclampsia | 1.21 (1.05-1.39) | 24 | 77% | 1.12 (0.90-1.39) | 0.79 (0.56-1.11) | [30] | May combine two diseases with separate etiology | |
| 0.90 (0.59-1.37) | 12 cohort studies | 94% | [31] | |||||
| Inconsistent, Small Association | Small for Gestational Age | 1.28 (1.11-1.49) | 19 | 64% | 1.13 (0.92-1.40) | 1.04(0.83-1.30) | [30] | |
| 1.27 (1.03-1.57) | 10 cohort studies | 35% | [31] | |||||
| 1.16 (1.05-1.28) | 5 | 0% | [44] | |||||
| Low birth weight | 1.13 (1.00-1.27) | 12 | 7% | 1.52 (1.13-2.05) | 0.87 (0.59-1.27) | [30] | May not take into account gestational age | |
| 0.96 (0.73-1.27) | 3 | 0% | [44] |
Miscarriage
Several systematic reviews have shown that women with endometriosis are at greater risk of subsequent miscarriage [31, 29, 32]. In the most recent meta-analysis of nine cohort studies, women with endometriosis were found to have a 1.75-fold higher risk of miscarriage compared to their counterparts with no history of endometriosis (95% Confidence Interval (CI)(1.29-2.37)) [31]. This finding was supported by subsequent results from two of the largest studies to date. A national record linkage study in Scotland, with 5,735 women with endometriosis, found a 76% greater risk of miscarriage among women with endometriosis (OR=1.76 (1.44-2.15)) compared to women without endometriosis [33]. Following the publication of the meta-analysis, another population-based cohort reported that an absolute risk of miscarriage of 19.3% in women with laparoscopically confirmed endometriosis compared to 12.3% among women without endometriosis (OR=1.40 (1.31-1.49)) [22]. However, the risk of miscarriage for women with endometriosis was stronger for pregnancies in women at younger ages (aged < 35 years) and in first pregnancies [22].
Although, the majority of studies have reported a greater risk of miscarriage in women with endometriosis, the most recent meta-analysis calculated high variability among studies (I2=93%) [31]. Variation in findings may be due to inherent differences and assumptions associated with sampled populations, miscarriage definitions, and study designs. Self-reported miscarriage assumes women can accurately recognize and recall their miscarriage, therefore individuals who have had a longer duration of trying to conceive or who are experiencing subfertility may be more likely to recall early miscarriages compared to fertile women. Registry-based studies may also fail to capture miscarriages early in pregnancy or miscarriages for women who do not inform their healthcare provider.
Ectopic Pregnancy
The existing literature on ectopic pregnancy is sparse but relatively consistent, suggesting that those with endometriosis have a greater risk of ectopic pregnancy. A registry-based Danish cohort study of 123,335 women followed for 15 years, found that women with endometriosis had a nearly two-fold greater risk of ectopic pregnancy compared to women without endometriosis (RR=1.9 (1.8-2.1)) [34]. These findings have been supported by a national-record linkage study in Scotland, which found that the risk of ectopic pregnancy was nearly three-fold greater for women with endometriosis (OR=2.70 (1.09-6.72)) [33]. Research from the NHSII cohort also supports this overall trend but found a more modest effect size in models adjusted for potential confounders; women with laparoscopically confirmed endometriosis had a 2.3 fold greater risk of ectopic pregnancy in age-adjusted models (95% CI= 1.90-2.86), which attenuated to 1.5 fold after adjustment for body mass index and history of infertility (95% CI= 1.19-1.80)[22].
Stillbirth
Like ectopic pregnancy, stillbirth is a rare pregnancy outcome (3.3 stillbirths per 1,000 births) [35]. A recent meta-analysis of seven prior studies calculated an overall summary odds ratio of 1.29 (1.10-1.52) [30]. Population-based cohort data, published after the meta-analysis, reported a nearly identical modest higher risk of stillbirth for women with endometriosis (OR= 1.27 (1.01-1.60)) [22].
Gestational Diabetes Mellitus
Research has been mixed regarding an association between endometriosis and risk of gestational diabetes mellitus (GDM). GDM affects between 2 to 5% of all pregnancies and is associated with risk of other downstream health consequences, such as stillbirth, macrosomia, preeclampsia, need for cesarean delivery, and maternal development of type 2 diabetes mellitus after pregnancy.
A recent meta-analysis of 12 studies, which included over 3,200 women with endometriosis, found that women with endometriosis had a small, 26% greater risk of gestational diabetes (95% CI= 1.03-1.55) compared to women without endometriosis [30]. Two recent prospective cohort studies support the overall meta-analysis finding, observing a 35% greater risk of GDM for women with endometriosis overall [22, 36].
Some studies have shown that women with any diagnosis of infertility are at greater risk of GDM [37], thus a possible association between endometriosis and GDM may be erroneously attenuated when comparing women with endometriosis to other women with infertility or undergoing ART. Indeed, the overall association in the meta-analysis was attenuated when the analysis was restricted to studies conducted only among women utilizing ART (n=5; OR=1.08) [30]. , Additionally, recent research found that the relationship with GDM was attenuated among women with a history of infertility (P-value, test for heterogeneity = 0.03) [22].
Hypertensive Disorders of Pregnancy
A recent meta-analysis, which combined 24 studies, suggested that women with endometriosis had a modest risk of gestational hypertension and/or preeclampsia (OR=1.21 (1.05-1.39))[30]. However, among meta-analyses that have restricted their samples to cohort studies, the association was attenuated (OR=0.90 [31]; OR=1.08 [38]). However, there was great variation among all studies, with I2=77% and among twelve cohort studies, with I2=94% (Table 1). Data from the NHSII, published after the meta-analyses, found that endometriosis was associated with a modest increased risk of preeclampsia and hypertensive disorders of pregnancy (RR= 1.30 (1.16-1.45))[22]. Conversely, a recent Japanese cohort study and the national-registry study from Scotland, support the findings of the meta-analysis among cohort studies, suggesting that endometriosis was not associated with preeclampsia or other hypertensive disorders of pregnancy [36, 33].
There have been several hypothesized mechanisms between endometriosis and hypertensive disorders of pregnancy. Endometriosis or the eutopic endometrium of women with endometriosis may influence uterine peristaltic activity causing micro-trauma and impaired implantation due to endometrial defects [39]. Inflammation and abnormal implantation coupled with abnormal placentation may increase risk for hypertensive disorders among women with endometriosis.
Placental anomalies
Prior research has consistently suggested a substantial association between endometriosis and placenta previa [29]. Meta-analyses on the topic have consistently reported a 3-fold [31] to 3 and half fold [30] greater risk. While the I2 has been >70%, this appears to be driven by the magnitude of the relative risk (e.g. 2-fold versus 7-fold), not differences in whether there is or is not a greater risk observed (Table 1). The recent registry-based data from Scotland also found that women with endometriosis had a 2-fold greater risk of placenta previa [33]. While there has been concern that the greater risk associated with endometriosis may be driven by utilization of fertility treatment, the risk of placenta previa for women with endometriosis has been shown to be elevated in both spontaneously-conceived and ART-conceived pregnancies. Indeed, the risk appears to be greater among women with endometriosis who spontaneously conceived, however the number of studies among this population is small (OR=6.83; 3 studies) [30]. As prior dilation and curettage from miscarriage and prior cesarean section increase risk of placenta previa [40], there may be interactions that yield even greater risk for women with endometriosis who are at higher risk of each compared to women without endometriosis.
Cesarean Section
There are many potential indications for utilization of cesarean section including concurrent risk from adverse pregnancy and perinatal outcomes and a high rate of cesarean section among ART deliveries [41]. In 2017, the CDC reported that 32.0% of U.S. deliveries were by cesarean section [42]. Researchers have consistently found an association between endometriosis and risk of cesarean section in meta-analyses, including a summary of 20 studies that quantified a 90% greater risk (OR=1.86 (1.51-2.29))[30], and a meta-analysis restricted to cohort studies that reported a 57% greater risk (OR=1.57 (1.39-1.78); n=13)[31]. The relationship with endometriosis was reported to be modestly stronger among women who spontaneously conceived (OR=1.76; n=6 studies) compared with ART conceptions (OR=1.24; n=7 studies)[30]. In addition to other adverse outcomes influencing cesarean section, this may be driven by the heightened access to care and the over medicalization of care among women with endometriosis [43].
Preterm Birth
Research has been consistent in showing an association between endometriosis and preterm birth [30, 31]. Prior meta-analyses have reported elevated risk of preterm birth ranging from 49% [44] to 70% [30], which again yielded relatively high I2 values but are clinically consistent (Table 1). A recent meta-analysis of cohort studies (n=9) found that pregnancies to women with endometriosis had a greater risk of preterm birth in both women who conceived spontaneously (OR=1.59, 95% CI: 1.32-1.90) and women who utilized ART (OR= 1.43, 95% CI: 1.14-1.79) [44]. Lalani and colleagues reported a similar pattern in their meta-analysis: the association was stronger among spontaneous pregnancies (OR=1.70; n=7 studies), but in ART pregnancies, endometriosis still conferred statistically and clinically significant risk (OR=1.27) (n=10 studies) [30]. Some studies have also found greater risk of severe preterm birth (<34 weeks) (OR=1.58) [31], and also when divided between very preterm births (<32 weeks, OR=1.91 (1.16-3.15)) and moderate preterm birth (32-36 weeks, OR= 1.64 (1.33-2.03)) [39]. Very few studies have taken into account fetal growth, which may mediate the relationship between endometriosis and preterm birth.
There are several proposed mechanisms of association for endometriosis and preterm birth. Pro-inflammatory mediators (PGE2, COX-2, interleukin-8) are higher in the peritoneal fluid of women with endometriosis, which may cause uterine muscle contractions and cervical ripening, resulting in preterm birth [36]. Additionally, women with endometriosis have been shown to have progesterone resistance of the endometrium and pelvic endometriosis may interfere with placentation, thus increasing risk of preterm birth [36].
Measures of fetal growth
Research investigating the relationship between endometriosis and measurements of fetal growth (birth weight, size for gestational age) have reported mixed findings. Prior meta-analyses have consistently shown a small association (OR=1.28 [30]; OR=1.27 [31]; OR=1.16 [44]) between endometriosis and small for gestational age (<10th percentile birth weight by age). However, studies have been inconsistent regarding an association between endometriosis and low birth weight (LBW) (<2500 grams) [45, 46, 44, 33, 17]. A meta-analysis of 12 studies reported a small 13%, increased risk of LBW for women with endometriosis [30]. More recent research reported an absolute risk of LBW of 5.6% in women with endometriosis compared to 3.6% in women without endometriosis [22]. They also reported that the relationship was stronger among women without a history of infertility versus those who ever experienced infertility (OR=1.26 vs. 1.09), however this difference was not statistically significant. The limited prior data comparing spontaneous conceptions to ART conceptions support a similar overall pattern—that is, the association between endometriosis and fetal growth, SGA and LBW, tended to be stronger among spontaneous pregnancies [45, 30]. This comparison may be affected by the suggested higher risk of LBW and SGA for ART pregnancies [47, 18].
Summary of the state of the literature and critical next steps
While there has been a growing interest in understanding the relationship between endometriosis and adverse pregnancy outcomes, there is a paucity of large studies with the depth and breadth of data to explore potential confounding and sub-groups at higher risk. Many of the current studies investigating these associations have been restricted to clinical infertility settings, which limit generalizability of findings. Registry and health-record based studies also have inherent limitations such as under reporting certain adverse outcomes (e.g. miscarriage, gestational diabetes), misclassification of exposures and outcomes, and lack data on potential confounding factors.
While many studies and meta-analyses on endometriosis and adverse pregnancy outcomes exist, it is important to remember that meta-analyses are not able to overcome inherent confounding or biases in the studies they combine. The prior meta-analyses indicated high heterogeneity (I2>75%) for most adverse pregnancy outcomes (Table 1). Variation across studies may be influenced by differences across study designs and populations in endometriosis definitions, population selection, residual confounding, and misclassification of endometriosis and the adverse pregnancy outcomes [48]. Some meta-analyses have attempted to include higher quality studies by restricting their sample to cohort studies [31], although a well-designed case-control study may be more robust to bias than a poorly designed cohort study [3].
Given the greater risk of adverse pregnancy outcomes among women with infertility and among ART pregnancies, some prior research and meta-analyses have explored and observed effect modification by infertility history and ART utilization. Moreover, as the quality of evidence to support these associations continues to improve, research should focus on mechanism and translation – considering biologic targets, screening protocols, and early-interventions among women with endometriosis with and without infertility and from spontaneous and ART conceptions that may reduce excess risk of adverse pregnancy outcomes.
In the current literature, endometriosis has consistently and strongly (Summary ORs: 1.5-3.3) been associated with placenta previa, cesarean section, ectopic pregnancy, and preterm birth (Table 1). While associations with miscarriage and stillbirth have been consistently observed, the magnitude of the associations reported have been more modest (Summary ORs: 1.3-1.7). There have also been modest but less consistent associations reported for the relationship between endometriosis and risk of GDM, preeclampsia, and gestational hypertension. The relationship between endometriosis and fetal growth has been wholly inconsistent.
A consistent pattern of heterogeneity has emerged when looking separately among spontaneous conceptions and among ART/infertile conceptions. The associations between endometriosis and several adverse pregnancy outcomes (placenta previa, cesarean section, preterm birth) have been stronger among spontaneous conceptions (Table 1). The mechanism behind this pattern may be two-fold. 1) It is well established that ART, as well as certain infertility diagnoses, are themselves associated with greater risk for many adverse pregnancy outcomes. Thus, when analyses are conducted among ART conceptions, the independent risk conferred by endometriosis may not be greater than the baseline elevated risk conferred by ART or by other infertility diagnoses necessitating the use of ART. Thus, the quantified relative risk of endometriosis on adverse pregnancy outcomes among ART pregnancies or among infertile women is attenuated compared to the effect of endometriosis among spontaneous pregnancies. 2) Women with endometriosis who experience infertility and ultimately utilize ART may be different from women with endometriosis who do not experience infertility and thus do not utilize ART. Women with endometriosis who do not experience infertility may represent a phenotype of endometriosis that has severe pain symptoms, as these women are more likely to be diagnosed because of their pain presentation. Therefore, the stronger relationship among spontaneous conceptions for endometriosis on adverse pregnancy outcomes may represent a pain-related endometriosis phenotype. Most likely, both mechanisms contribute to the greater risk of adverse pregnancy outcomes for women with endometriosis among spontaneous conceptions compared to ART/infertile conceptions, perhaps through shared and unique pathophysiology.
Conclusion
It is a myth that all women with endometriosis will experience infertility; many women with endometriosis are able to conceive successfully and most conceive spontaneously. It may also be a myth that patients with endometriosis have the same risk of adverse pregnancy outcomes as other women, as they may have higher risk for some adverse pregnancy outcomes and therefore may benefit from additional monitoring. Future well-designed and well-characterized studies are needed to improve our understanding of the health of pregnancies to women with endometriosis, to elucidate mechanisms of association, and to better understand the possible role of additional monitoring and screening during pregnancy among this population.
Footnotes
Human and Animal Rights and Informed Consent:
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99. doi:S0140673604174035 [pii] 10.1016/S0140-6736(04)17403-5 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nature reviews Disease primers. 2018;4(1):9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 3.Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best practice & research Clinical obstetrics & gynaecology. 2018. doi: 10.1016/j.bpobgyn.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–73 e8. doi:S0015-0282(11)00876-4 [pii] 10.1016/j.fertnstert.2011.05.090 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016. doi:dew085 [pii] 10.1093/humrep/dew085 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SS, Dukhovny D, Gopal D, Cabral H, Missmer S, Diop H et al. Health of Infants After ART-Treated, Subfertile, and Fertile Deliveries. Pediatrics. 2018;142(2). doi: 10.1542/peds.2017-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern JE, Liu CL, Cabral HJ, Richards EG, Coddington CC, Hwang S et al. Birth outcomes of singleton vaginal deliveries to ART-treated, subfertile, and fertile primiparous women. Journal of assisted reproduction and genetics. 2018;35(9):1585–93. doi: 10.1007/s10815-018-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luke B, Stern JE, Hornstein MD, Kotelchuck M, Diop H, Cabral H et al. Is the wrong question being asked in infertility research? Journal of assisted reproduction and genetics. 2016;33(1):3–8. doi: 10.1007/s10815-015-0610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3):495–502. doi: 10.1097/AOG.0b013e3181d0c328. [DOI] [PubMed] [Google Scholar]
- 10.Leucht S, Kissling W, Davis JM. How to read and understand and use systematic reviews and meta-analyses. Acta psychiatrica Scandinavica. 2009;119(6):443–50. doi: 10.1111/j.1600-0447.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362(25):2389–98. doi:362/25/2389 [pii] 10.1056/NEJMcp1000274 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farland LV, Horne AW. Disparity in endometriosis diagnoses between racial/ethnic groups. BJOG : an international journal of obstetrics and gynaecology. 2019;126(9):1115–6. doi: 10.1111/1471-0528.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bougie O, Yap MI, Sikora L, Flaxman T, Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology. 2019;126(9):1104–15. doi: 10.1111/1471-0528.15692. [DOI] [PubMed] [Google Scholar]
- 14.Stephen EH, Chandra A. Updated projections of infertility in the United States: 1995-2025. Fertil Steril. 1998;70(1):30–4. doi:S0015-0282(98)00103-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 15.Farland LV, Collier AR, Correia KF, Grodstein F, Chavarro JE, Rich-Edwards J et al. Who receives a medical evaluation for infertility in the United States? Fertil Steril. 2016;105(5):1274–80. doi: 10.1016/j.fertnstert.2015.12.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra A, Stephen EH. Infertility service use among U.S. women: 1995 and 2002. Fertil Steril. 2010;93(3):725–36. doi:S0015-0282(08)04409-9 [pii] 10.1016/j.fertnstert.2008.10.049 [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015;103(6):1438–45. doi: 10.1016/j.fertnstert.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missmer SA. Why so null? Methodologic necessities to advance endometriosis discovery. Paediatr Perinat Epidemiol. 2019;33(1):26–7. doi: 10.1111/ppe.12540. [DOI] [PubMed] [Google Scholar]
- 19.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of internal medicine. 2017;167(4):268–74. doi: 10.7326/m16-2607. [DOI] [PubMed] [Google Scholar]
- 20.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015. doi:dmv013 [pii] 10.1093/humupd/dmv013 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22(1):104–15. doi: 10.1093/humupd/dmv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet Gynecol. 2019. doi: 10.1097/aog.0000000000003410.* This study is a recent large, prospective cohort study.
- 23.Mu F, Harris HR, Rich-Edwards JW, Hankinson SE, Rimm EB, Spiegelman D et al. A Prospective Study of Inflammatory Markers and Risk of Endometriosis. Am J Epidemiol. 2017. doi: 10.1093/aje/kwx272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17(2):426–31. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/s0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf M, Sauk J, Shah A, Vossen Smirnakis K, Jimenez-Kimble R, Ecker JL et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27(1):21–7. [DOI] [PubMed] [Google Scholar]
- 27.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162(12):1198–206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16(6):725–44. doi: 10.1093/humupd/dmq016. [DOI] [PubMed] [Google Scholar]
- 29.Leone Roberti Maggiore U, Ferrero S, Mangili G, Bergamini A, Inversetti A, Giorgione V et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update. 2016;22(1):70–103. doi: 10.1093/humupd/dmv045. [DOI] [PubMed] [Google Scholar]
- 30.Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, Wen SW et al. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum Reprod. 2018. doi: 10.1093/humrep/dey269.** This study is a recent, comprehensive meta-analysis on fetal growth, preeclampsia, gestational diabetes, placenta previa, cesarean section, preterm birth, stillbirth.
- 31.Zullo F, Spagnolo E, Saccone G, Acunzo M, Xodo S, Ceccaroni M et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil Steril. 2017;108(4):667–72.e5. doi: 10.1016/j.fertnstert.2017.07.019.** This study is a recent meta-analysis on placenta previa, cesarean section, preterm birth, miscarriage, preeclampsia, and fetal growth.
- 32.Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;44(3):261–78. doi: 10.1002/uog.13366. [DOI] [PubMed] [Google Scholar]
- 33.Saraswat L, Ayansina D, Cooper K, Bhattacharya S, Miligkos D, Horne AW. Pregnancy outcomes in women withendometriosis: a national record linkage study. BJOG: An International Journal of Obstetrics and Gynaecology. 2016. doi: 10.1111/1471-0528.1392.* This study is a recent large registry-based study on pregnancy outcomes in Scotland.
- 34.Hjordt Hansen MV, Dalsgaard T, Hartwell D, Skovlund CW, Lidegaard O. Reproductive prognosis in endometriosis. A national cohort study. Acta Obstet Gynecol Scand. 2014;93(5):483–9. doi: 10.1111/aogs.12373. [DOI] [PubMed] [Google Scholar]
- 35.Stephansson O, Kieler H, Granath F, Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod. 2009;24(9):2341–7. doi: 10.1093/humrep/dep186. [DOI] [PubMed] [Google Scholar]
- 36.Harada T, Taniguchi F, Onishi K, Kurozawa Y, Hayashi K, Harada T et al. Obstetrical Complications in Women with Endometriosis: A Cohort Study in Japan. PLoS One. 2016;11(12):e0168476. doi: 10.1371/journal.pone.0168476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobias DK, Chavarro JE, Williams MA, Buck Louis GM, Hu FB, Rich-Edwards J et al. History of infertility and risk of gestational diabetes mellitus: a prospective analysis of 40,773 pregnancies. Am J Epidemiol. 2013;178(8):1219–25. doi:kwt110 [pii] 10.1093/aje/kwt110 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Lopez FR, Calvo-Latorre J, Alonso-Ventura V, Bueno-Notivol J, Martinez-Dominguez SJ, Chedraui P. Systematic review and meta-analysis regarding the association of endometriosis and preeclampsia in women conceiving spontaneously or through assisted reproductive technology. Pregnancy hypertension. 2018. doi: 10.1016/j.preghy.2018.01.003.** This study is a recent, large meta-analysis on preeclampsia.
- 39.Glavind MT, Forman A, Arendt LH, Nielsen K, Henriksen TB. Endometriosis and pregnancy complications: a Danish cohort study. Fertil Steril. 2017;107(1):160–6. doi: 10.1016/j.fertnstert.2016.09.020.* Recent, large registry-based study
- 40.Faiz AS, Ananth CV. Etiology and risk factors for placenta previa: an overview and meta-analyses of observational studies. The Journal of Maternal-Fetal & Neonatal Medicine. 2003;13(3):175–90. doi: 10.1080/jmf.13.3.175.190. [DOI] [PubMed] [Google Scholar]
- 41.Ochsenkuhn R, Strowitzki T, Gurtner M, Strauss A, Schulze A, Hepp H et al. Pregnancy complications, obstetric risks, and neonatal outcome in singleton and twin pregnancies after GIFT and IVF. Arch Gynecol Obstet. 2003;268(4):256–61. doi: 10.1007/s00404-003-0518-5 [doi]. [DOI] [PubMed] [Google Scholar]
- 42.Martin J, Hamilton B, Osterman M, Driscoll A, Drake P. National Vital Statistics Reports: U.S. Departmenr of Health and Human Services, Statistics DoV;2018.
- 43.Stern JE, Liu CL, Cabral HJ, Richards EG, Coddington CC, Missmer SA et al. Factors associated with increased odds of cesarean delivery in ART pregnancies. Fertil Steril. 2018;110(3):429–36. doi: 10.1016/j.fertnstert.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez-Lopez FR, Villagrasa-Boli P, Munoz-Olarte M, Morera-Grau A, Cruz-Andres P, Hernandez AV et al. Association Between Endometriosis and Preterm Birth in Women With Spontaneous Conception or Using Assisted Reproductive Technology: A Systematic Review and Meta-Analysis of Cohort Studies. Reprod Sci. 2018;25(3):311–9. doi: 10.1177/1933719117749760. [DOI] [PubMed] [Google Scholar]
- 45.Luke B, Stern JE, Kotelchuck M, Declercq ER, Cohen B, Diop H. Birth Outcomes by Infertility Diagnosis Analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). J Reprod Med. 2015;60(11-12):480–90. [PMC free article] [PubMed] [Google Scholar]
- 46.Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet Gynecol. 2019;134(3):527–36. doi: 10.1097/AOG.0000000000003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tough SC, Greene CA, Svenson LW, Belik J. Effects of in vitro fertilization on low birth weight, preterm delivery, and multiple birth. The Journal of pediatrics. 2000;136(5):618–22. doi: 10.1067/mpd.2000.105132. [DOI] [PubMed] [Google Scholar]
- 48.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Lopez FR, Martinez-Dominguez SJ, Vinas A, Perez-Tambo R, Lafita A, Lajusticia H et al. Endometriosis and gestational diabetes mellitus risk: a systematic review and meta-analysis. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2018;34(5):363–9. doi: 10.1080/09513590.2017.1397115. [DOI] [PubMed] [Google Scholar]


