Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common and progressive disease characterised by chronic cough, airflow limitation and recurrent exacerbations. Since COPD exacerbations are linked to rising mortality and reduced quality of life, the condition poses a substantial burden on individuals, society and the healthcare system. Effective management of COPD exacerbations that includes treatment of related conditions in people with COPD is thus recognised as a relevant clinical question and an important research topic. Gastroesophageal reflux disease (GERD) is a known comorbidity of COPD, and pulmonary microaspiration of gastric acid is thought to be a possible cause of COPD exacerbations. Therefore, reducing gastric acid secretion may lead to a reduction in COPD exacerbations. Proton pump inhibitors (PPIs) are one of the most commonly prescribed medications and are recommended as first‐line therapy for people with GERD because of their inhibitory effects on gastric acid secretion. Treatment with PPIs may present a viable treatment option for people with COPD.
Objectives
To evaluate the efficacy and safety of PPI administration for people with COPD, focusing on COPD‐specific outcomes.
Search methods
We searched the Cochrane Airways Register of Trials and conventional clinical trial registers from inception to 22 May 2020. We also screened bibliographies of relevant studies.
Selection criteria
Parallel‐group and cluster‐randomised controlled trials (RCTs) that compared oral PPIs versus placebo, usual care or low‐dose PPIs in adults with COPD were eligible for inclusion. We excluded cross‐over RCTs, as well as studies with a duration of less than two months.
Data collection and analysis
Two independent review authors screened search results, selected studies for inclusion, extracted study characteristics and outcome data, and assessed risk of bias according to standard Cochrane methodology. We resolved discrepancies by involving a third review author. Primary outcomes of interest were COPD exacerbations, pneumonia and other serious adverse events. Secondary outcomes were quality of life, lung function test indices, acute respiratory infections and disease‐specific adverse events. We extracted data on these outcome measures and entered into them into Review Manager software for analysis.
Main results
The search identified 99 records, and we included one multicentre RCT that randomised 103 adults with COPD. The 12‐month RCT compared an oral PPI (lansoprazole) and usual care versus usual care alone. It was conducted at one tertiary care hospital and three secondary care hospitals in Japan. This study recruited participants with a mean age of 75 years, and excluded people with symptoms or history of GERD. No placebo was used in the usual care arm.
Among the primary and secondary outcomes of this review, the study only reported data on COPD exacerbations and acute respiratory infections (the common cold). As we only included one study, we could not conduct a meta‐analysis.
The included study reported that 12 of the 50 people on lansoprazole had at least one exacerbation over a year, compared to 26 out of 50 on usual care (risk ratio 0.46, 95% CI 0.26 to 0.81). The frequency of COPD exacerbations per person in a year was also lower in the PPI plus usual care group than in the usual care alone group(0.34 ± 0.72 vs 1.18 ± 1.40; P < 0.001). The number of people with at least one cold over the year was similar in both groups: 26 people on lansoprazole and 27 people in the usual care group. We judged the evidence to be of low to very low certainty, according to GRADE criteria.
The study reported no data on pneumonia and other serious adverse events, quality of life, lung function test indices or disease‐specific adverse events. The risk of bias was largely low or unclear for the majority of domains, though the performance bias was a high risk, as the study was not blinded.
Authors' conclusions
Evidence identified by this review is insufficient to determine whether treatment with PPIs is a potential option for COPD. The sample size of the included trial is small, and the evidence is low to very low‐certainty. The efficacy and safety profile of PPIs for people with COPD remains uncertain. Future large‐scale, high‐quality studies are warranted, which investigate major clinical outcomes such as COPD exacerbation rate, serious adverse events and quality of life.
Plain language summary
Proton pump inhibitors for chronic obstructive pulmonary disease
What is the aim of this review?
The review authors want to know whether proton pump inhibitors (PPIs) are effective in (a) reducing chronic obstructive pulmonary disease (COPD) exacerbations, and (b) improving quality of life for people with COPD. We searched for randomised trials to answer this question and found only one study, with 103 participants.
Key message
Giving PPIs to people with COPD may reduce the frequency of COPD exacerbations. However, further high‐quality studies are needed to be more certain. Future studies should include different types of PPIs.
What was studied in the review?
This study specifically looked at COPD. COPD is a common respiratory disease, characterised by cough with mucus and breathlessness. COPD is one of the leading causes of death worldwide, and reduces quality of life. COPD exacerbation is associated with hospitalisation and death, placing a large burden on both society and the economy. COPD exacerbation is caused by different conditions that require different therapies.
Gastroesophageal reflux disease (GERD) is one cause of COPD exacerbation. GERD is a common gastrointestinal disease caused by reflux of stomach acid into the oesophagus (food pipe) and lungs. This gives people the symptoms of heartburn and cough. When people have GERD, they are given PPIs to treat it. These work by reducing the amount of stomach acid. While PPIs are effective for treating symptoms of GERD, it is unclear whether adding PPIs to usual care reduces the frequency of COPD exacerbations or improves the quality of life for people with COPD.
What are the main results of the review?
We found only one relevant study. It was from a university hospital and three city hospitals in Japan. This study compared the effects of a PPI plus usual care against usual care alone in people with COPD who had no history or symptoms of GERD. The researchers investigated changes in the frequency of COPD exacerbations and the common cold over a 12‐month period. This study used a 15 mg daily dose of a PPI called lansoprazole. There was low‐certainty evidence of a reduction in the number of people on lansoprazole who had COPD exacerbations compared with people who had usual care, and very low‐certainty evidence that similar numbers of people in each group had at least one common cold.
The review authors did not find any studies that described the effects of PPIs on pneumonia and serious adverse events, quality of life, lung function, or disease‐specific adverse events.
How up‐to‐date is this review?
We ran the latest search for studies on 22 May 2020.
Summary of findings
Summary of findings 1. PPI plus usual care versus usual care alone or chronic obstructive pulmonary disease.
| Usual care plus PPI compared to Usual care plus alone for chronic obstructive pulmonary disease | ||||||
| Patient or population: chronic obstructive pulmonary disease Setting: hospital outpatients in Japan (three secondary care hospitals and one tertiary care hospital) Intervention: Usual care plus PPI Comparison: Usual care plus alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Usual care plus alone | Risk with Usual care plus PPI | |||||
| Exacerbation rate (participants with one or more) study duration: 12 months |
Study population | RR 0.46 (0.26 to 0.81) | 100 (1 RCT) | ⊕⊕⊝⊝ LOW ab | The frequency of exacerbations improved with intervention. | |
| 520 per 1,000 | 239 per 1,000 (135 to 421) | |||||
| Pneumonia and other serious adverse events | — | — | — | — | Not reported | |
| Quality of life | — | — | — | — | Not reported | |
| Lung function indices | — | — | — | — | Not reported | |
| Acute respiratory infections study duration: 12 months |
Study population | RR 0.96 (0.67 to 1.39) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW abc | No clear benefit or harm from PPI | |
| 540 per 1,000 | 518 per 1,000 (362 to 751) | |||||
| Disease‐specific adverse events | — | — | — | — | Not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; PPI: proton pump inhibitor; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for imprecision: single small trial describes this result
bDowngraded by one level for study limitations: no blinding of participants and personnel
cDowngraded by one level for study limitations: the rate and number of common colds using outcomes that tend to be subjective
Background
Description of the condition
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline defines chronic obstructive pulmonary disease (COPD) as a common, preventable and treatable disease that has a significant detrimental impact on quality of life, and poses a substantial and growing economic and social burden (GOLD 2020). As a leading cause of global mortality, approximately three million deaths are attributed to COPD every year (WHO 2018). People with COPD have a high frequency of respiratory complications and systemic comorbidities. These comorbidities, including cardiovascular diseases, osteoporosis, depression and gastroesophageal reflux disease (GERD), may potentiate the severity of COPD, leading to an increase in acute exacerbations (Wedzicha 2013).
The latest GOLD guidelines define COPD exacerbation as "an acute worsening of respiratory symptoms that results in additional therapy" (GOLD 2020). The economic impact of COPD exacerbations is substantial, since COPD exacerbations are linked to mortality, morbidity, and hospitalisations (Perera 2012). Therefore, effective COPD management should aim to relieve presenting symptoms, prevent future exacerbations and delay disease progression (Woodruff 2015), with an emphasis on a comprehensive care plan for COPD and associated comorbidities (Barnes 2013; Wedzicha 2013).
GOLD specifies the diagnostic criteria for COPD as a post bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of less than 0.70 (GOLD 2020). However, defining COPD by these lung function test indices alone may imply that COPD is a homogenous condition. In fact, COPD is a heterogeneous group of conditions with a wide array of symptoms and therapeutic responses towards conventional treatment, and prognoses and comorbidities associated with COPD vary. This heterogeneity cannot be explained by lung function alone, and groups of people with COPD who express particular characteristics can be regarded as having different phenotypes (Han 2010). The ultimate goal of identifying COPD phenotypes is to develop effective and people‐oriented treatment regimens, in order to improve clinical outcomes.
People experiencing two or more exacerbations of COPD per year are regarded as having frequent exacerbations (Hurst 2010); people who experienced frequent exacerbations in the past often have a progressive decline in lung function and a poorer prognosis (Seemungal 1998). The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study has shown that the strongest predictor of frequent COPD exacerbations in the future is the number of exacerbations experienced in the previous year (Hurst 2010).
In addition, people with frequent exacerbations tend to be more susceptible to viral infections (Wedzicha 2013). In other words, a management strategy that includes virus infection as a treatment target may be effective for people who repeatedly experience COPD exacerbations. Furthermore, some studies suggest that GERD, a disease comprising symptoms, end‐organ effects and complications related to the reflux of gastric contents into the oesophagus, oral cavity or the lung (Katz 2013), is an independent predictor of frequent COPD exacerbations (Hurst 2010; Ingebrigtsen 2015; Martinez 2014; Terada 2008). It is worth highlighting that the relative risk (RR) of an exacerbation over two years in people with COPD and GERD is 1.93 (95% confidence interval (CI): 1.32 to 2.84), i.e. the risk of exacerbation is almost twice as high for people who have GERD (Terada 2008).
As a common condition, the diagnosis of GERD is often made by general practitioners and gastroenterologists in outpatients departments (Shaheen 2006). Prevalence of GERD is rising worldwide, and the associated disease burden is also increasing (El‐Serag 2014). For instance, cumulative incidence of pneumonia has been reported to be significantly higher in people with GERD than in people without GERD (Hsu 2017). Microaspiration of gastric acid tends to occur in people with GERD, and it has been suggested that this condition is pathologically connected to the development of lung diseases (Morehead 2009). Hsu 2017 reported that the use of proton pump inhibitors (PPIs), a pharmacological treatment for GERD, for more than four months increased the risk of pneumonia in people with GERD. A potential explanation of the relationship between PPI and pneumonia is that an increase in pH due to gastric acid suppression might promote bacterial colonization in the oral cavity, which subsequently leads to pneumonia (Gulmez 2007). However, the precise mechanism of the development of pneumonia when using PPIs is still unclear.
The cause of GERD is complex, but there are several known factors. First, gastric reflux occurs when the gradient of pressure between the stomach and the lower oesophageal sphincter is lost, due to low or absent lower oesophageal sphincter pressure and anatomic disruptions of the oesophagogastric junction, such as hiatal hernia (Orlando 2001). Second, oesophageal peristaltic dysfunction (dysfunction of oesophageal contraction movement which eliminates oesophageal contents to the stomach) and prolonged oesophageal acid clearance (a dysfunction of eliminating contents in the oesophagus by the oesophageal contraction movement, secretions from the oesophagus glands or swallowed saliva) can feature in gastroesophageal reflux (Kahrilas 1988; Sugiura 2001). Amongst people with COPD who smoke, nicotine might lead to changes in lower oesophageal sphincter pressure and abnormal oesophageal peristalsis (Pandolfino 2000). Third, certain pharmacological therapies for COPD, including theophylline, beta2‐agonists, anticholinergics, and corticosteroids, may alter oesophageal sphincter tone and respiratory mechanics, leading to worsened symptoms of GERD (Phulpoto 2005). GERD has been reported to be a frequent comorbidity of COPD, and prevalence of GERD has been found to be higher in people with COPD compared with healthy controls (Casanova 2004; Mokhlesi 2001). Fourth, the prevalence of GERD symptoms tends to be higher in people with severe airway obstruction (Mokhlesi 2001). Lung hyperinflation correlates with COPD severity and requires more inspiratory effort to inhale. This altered mechanism of breathing may increase the transdiaphragmatic pressure gradient on the chest and abdomen, and lead to GERD (Del Grande 2016). Thus, it may also explain the correlation between severe COPD and GERD. Other factors, such as obesity and comorbidities, may also be associated with the increased prevalence of GERD found in people with COPD (Herbella 2010).
The prevalence of GERD in people with COPD has been reported to range from 17% to 78% (Lee 2015). The mechanism underlying GERD that leads to COPD exacerbations is thought to be related to pulmonary microaspiration of gastric acid into airways (Javorkova 2008; Lee 2015), and this mechanism is also thought to be a risk factor for pneumonia (Gaude 2009; Terada 2008). The inflammatory response triggered by GERD may cause the contraction of airways via vagal reflex or further stimulate acid sensitive receptors in the oesophageal wall, both of which may lead to COPD exacerbations (Mansfield 1981; Schan 1994; Tuchman 1984). Furthermore, GERD is considered to be one of the causes of chronic cough, which is a common symptom of COPD (Irwin 2000).
Description of the intervention
PPIs are one of the most effective medications for reducing gastric acid secretion. Since their introduction in the late 1980s, PPIs remain one of the most commonly prescribed medications worldwide (Bashford 1998). Currently, seven PPIs are available (lansoprazole, omeprazole, pantoprazole, rabeprazole, esomeprazole, dexlansoprazole, and vonoprazan), and some are available as over‐the‐counter medications. Vonoprazan is a novel PPI and has been licensed for use to treat GERD in Japan since 2015. In the USA, for example, lansoprazole, omeprazole and esomeprazole do not require prescription, and omeprazole can be purchased in high‐street supermarkets. However, pantoprazole, rabeprazole, and dexlansoprazole are all prescription‐only medications (Chen 2019). In Japan, all PPIs require a prescription. Therefore, consumer usage of PPIs varies from country to country. Nevertheless, PPIs are one of the most prescribed and most consumed medications worldwide.
The family of PPIs has dramatically improved many conditions, such as peptic ulcer disease, as well as being used in the treatment and prevention of gastroduodenal ulcers associated with the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) (Agrawal 2000; Rostom 2002). They are also included in regimens that aim to eradicate Helicobacter pylori (Hu 2017). In the longer‐term, PPIs can prevent pulmonary microaspiration of gastric contents by reducing the production of stomach acid (Lee 2015). PPIs also promote the healing of oesophageal erosion produced by the reflux of gastric acid into the oesophagus (Dekel 2004).
Treatment with PPIs remains the mainstay approach to the management of GERD (Freedberg 2017; Katz 2013). Given that GERD is an independent risk factor for frequent exacerbations of COPD (Hurst 2010), Baumeler 2016 hypothesised that administration of PPIs for GERD might improve the symptoms and frequency of acute exacerbations of COPD. However, this study eventually showed that annual COPD exacerbation rates and severity were higher amongst participants in the PPI group than in those participants who were not receiving PPI. Moreover, in the PPI group, the incidence rates of hypertension, heart failure, diabetes mellitus and dementia were higher and the quality of life was lower than in the non‐PPI group (Baumeler 2016).
The following reasons might partly explain why the effects of PPI on COPD are different from Baumeler's hypothesis that PPI might reduce COPD exacerbations (Baumeler 2016); (1) the effect of PPIs on bacterial infection; (2) the influence of confounding factors; and (3) other effects of PPIs.
First, as gastric acid secretion plays a protective role against infectious agents, prolonged PPI‐induced low acid status can increase the risk of bacterial overgrowth in the stomach and oesophagus (Eom 2011). This bacterial overgrowth caused by long‐term PPI use may heighten the risk of bacterial aspiration (Gaude 2009). It may also be associated with an increased risk of community‐acquired pneumonia (CAP) (Laheij 2004). CAP is a type of pneumonia caused by organisms found regularly outside of hospital settings, such as Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma, as opposed to hospital‐acquired pneumonia (Metlay 2019).
Observational studies have shown a positive association between PPI usage and CAP (Gulmez 2007; Laheij 2004). In a large‐scale population‐based cohort study derived from the Clinical Practice Research Datalink, Braeken 2017 indicated that people with COPD were at a four‐hold increased risk of developing CAP (hazard ratio 4.51, 95% confidence interval (CI): 4.27 to 4.77).
Several meta‐analyses have suggested that short duration of PPI usage, particularly within the first month, may associated with an increased risk of CAP (Giuliano 2012; Johnstone 2010; Lambert 2015). However, it is not yet clear why short‐duration usage of PPIs correlates with increased risk of CAP. In addition, people with COPD and CAP were found to have longer hospital stays and mortality rates when compared to people with CAP alone (Braeken 2015). Thus, it is likely that the increased risk of CAP associated with PPI treatment could eventually lead to further COPD exacerbations.
Second, there are few intervention studies on this topic, and association of confounding factors may be unclear and controversial. As stated previously, studies have suggested that PPI usage may increase the risk of CAP (Gulmez 2007; Laheij 2004). By contrast, other studies have suggested that this associated risk may be due to significant unmeasured confounding (e.g. comorbidities and baseline characteristics) (Jena 2013; Othman 2016). Othman 2016 examined 160,000 new PPI users to investigate whether there was a change in the risk of CAP before and after the prescription of PPIs. This cohort study initially showed that the risk of CAP was higher in those prescribed PPI (RR 1.67, 95% CI 1.55 to 1.79). However, after adjustment for confounding factors, PPI use was associated with a lower risk of CAP (RR 0.91, 95% CI 0.83 to 0.99). Thus, the association between PPI usage and risk of CAP may be due to confounding factors.
Third, PPI may have effects other than acid suppression. Meijvis 2011 suggested that PPIs may not increase either the gastrointestinal or oropharyngeal bacteria that cause CAP (Meijvis 2011). One possible explanation for COPD exacerbation, besides bacterial infections, could be the effects of PPIs on viral infections.
As well as bacterial infections, several viruses (such as the human rhinovirus) have been reported to be associated with exacerbations of COPD (Falsey 2006; Hurst 2005). In previous studies, causes of COPD exacerbations were shown to be mostly bacterial infections, and viral infections were considered to be the cause of only 14% to 18% of COPD exacerbations (Buscho 1978; Smith 1980). However, with the widespread use of pneumococcal vaccines worldwide, the general microbial pathogenesis pathway is likely to have changed and as such, awareness of virus infections in the prognosis of people with COPD is increasing (Metlay 2019). Virus detection during COPD exacerbation has been found to vary from 22% to 60% (Gunawardana 2014; Sethi 2008). Rhinoviruses are the most reported virus in many studies, with influenza and respiratory syncytial viruses also commonly detected. Other existing data also support a causal link between viral infections and exacerbations of COPD (Beasley 2012; Quint 2010; Wedzicha 2013). Sasaki 2009 suggested a possible preventive effect of PPIs against viral infection, and an association with a reduction in COPD exacerbations. This inhibitory effect of PPIs on viral infection could not explain why Baumeler's hypothesis was not completely supported. The efficacy and safety of PPIs in relation to viral infection is still unclear.
Therefore, the effects of PPIs on acute exacerbations of COPD are not yet clear and remain controversial (Filion 2014).
Usual therapy for COPD
Updated GOLD guidelines and recently published clinical recommendations by the American Thoracic Society (ATS)/European Respiratory Society (ERS) have emphasised the benefits of smoking cessation and pharmacological therapy, including β2‐agonists, anticholinergics, and corticosteroids (GOLD 2020; Wedzicha 2017). The most appropriate pharmacological therapies should be selected for each person, according to their severity of COPD and symptoms. Asymptomatic people with mild airflow limitation can be treated with on‐demand short‐acting bronchodilators, such as inhalation of short‐acting beta2‐agonists (SABA) or short‐acting muscarinic antagonists (SAMA). If the symptoms do not improve, long‐acting beta‐agonists (LABA), long‐acting muscarinic antagonists (LAMA), and inhaled corticosteroids are also used. If LABA or LAMA monotherapy cannot control the symptoms, administration of two or more medications may be effective (GOLD 2020). Furthermore, non‐pharmacological therapies for COPD are also considered, including education and self‐management, smoking cessation, nutritional guidance, pulmonary rehabilitation, immunisations, and long‐term oxygen therapy (GOLD 2020). To prevent acute exacerbation of COPD, it is also necessary to combine pharmacological approaches with non‐pharmacological therapies. GOLD guidelines suggest that the therapeutic goal in terms of COPD exacerbation is to minimise the adverse effects on physical symptoms, respiratory function, and quality of life associated with acute exacerbations (GOLD 2020). Among several COPD clinical phenotypes, the adverse impact of frequent exacerbations is substantial. Due to this, the management strategy for acute exacerbations of COPD should be guided by phenotype (Agusti 2016).
How the intervention might work
It has been suggested that GERD could be an independent risk factor of COPD exacerbation (Hurst 2010). The acid suppression effects of PPIs might play a role in COPD management based on the following mechanisms. Gastric acid secretion is a complex process regulated by at least three types of receptors on the parietal cells (histamine, gastrin, and acetylcholine). Usually, PPIs reduce gastric acid production by irreversibly blocking the enzymes responsible for hydrogen potassium ATPase (proton pump) in parietal cells (Akazawa 2016; Scott 2015). However, vonoprazan has a somewhat unique action, which involves reversibly blocking hydrogen potassium ATPase through competing with potassium ions (Hunt 2015). Thus, vonoprazan is a faster‐acting acid suppressive agent than conventional PPIs. Although vonoprazan differs somewhat from the existing PPIs, it is still classified as a PPI because it inhibits the proton pump and suppresses gastric acid production. PPIs, including vonoprazan, make it possible to reduce gastric acid secretion and to prevent central reflex and pulmonary microaspiration of stomach acid (Lee 2015). However, PPIs can cause gastric and oesophagus bacterial overgrowth by excessive acid suppression (Eom 2011), and may lead to COPD exacerbation (Giuliano 2012; Laheij 2004; Lambert 2015). The same safety concerns have also been discussed regarding vonoprazan (Sugano 2018). In addition, excessive suppression of gastric acid increases the likelihood of developing CAP (Gulmez 2007; Laheij 2004), which may lead to worsening of COPD. Therefore, the relationship between the acid suppression effects of PPIs and COPD exacerbations remains controversial.
In addition to acid suppression, PPIs have been reported to have an impact on viral infections, for example rhinovirus infections (Long 2015; Sasaki 2005). Rhinoviruses are commonly identified viruses in people who experience frequent COPD exacerbations (Hurst 2005). A rhinovirus can amplify the expression of intercellular adhesion molecule‐1 (ICAM‐1) and cytokine (Yu 2017). ICAM‐1 is the major rhinovirus infection receptor and increases viral susceptibility on respiratory epithelial cells (George 2014). Lansoprazole and omeprazole have been reported to have suppressing effects on the expression of ICAM‐1 (Ohara 1999; Watanabe 2001). Researchers found that both lansoprazole and omeprazole were able to suppress viral infections by inhibiting vacuolar hydrogen potassium ATPase, thereby increasing endosomal pH and inhibiting the expression of ICAM‐1 (Sasaki 2005). Other studies have also suggested that PPIs may possess systemic anti‐inflammatory effects (Becker 2006; Sasaki 2011). Viral infections induce inflammatory mediators, including various cytokines (Sethi 2008). The anti‐inflammatory effect of PPIs is probably due to their effect of inhibiting the production of proinflammatory cytokines (Sasaki 2011). However, these antiviral and anti‐inflammatory effects of PPIs are primarily in vitro observations. Thus, evidence based on human studies is currently lacking. People who experience frequent COPD exacerbations may have a high sensitivity towards respiratory viral infections or have poor ability to prevent viral replications (George 2014), which makes PPIs a viable treatment option for COPD.
Why it is important to do this review
COPD exacerbations are associated with substantial impact at both the individual level and for overall society (Barnes 2013; Wedzicha 2013). Therefore, effective management of existing COPD symptoms and prevention of exacerbations is a major therapeutic target. Given that the frequent‐exacerbation phenotype of COPD is known to be associated with a history of gastroesophageal reflux or heartburn (Hurst 2010), PPIs may have the potential to influence COPD exacerbations. Since PPIs are widely available, with millions of users worldwide, it is necessary to evaluate the role of PPIs on people with COPD in this context.
We have identified a randomised controlled trial (RCT) (Sasaki 2009), and some other studies on this topic. To our knowledge, there is no existing systematic review that explores the precise role of PPIs in the management of COPD, so this remains to be ascertained. Therefore, a systematic review is necessary to determine whether PPIs are useful to treat people with COPD.
Objectives
To evaluate the efficacy and safety of PPI administration for people with COPD, focusing on COPD‐specific outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which participants were randomly assigned at the individual or cluster level. We included studies reported as full‐text articles, as abstracts/conference proceedings only, and unpublished data. We excluded cross‐over trials.
Types of participants
We included male and female adults aged 18 years or above with a diagnosis of COPD. We imposed no restrictions on clinical stability, i.e. people with stable COPD as well as those with exacerbated COPD were all eligible. We excluded participants who were prescribed PPIs within one month of study commencement. We also excluded studies that only included participants with asthma. For the purpose of this review, the definition of asthma was that defined by the study investigators (e.g. lack of substantial smoking history and significant bronchodilator reversibility). However, we acknowledged that certain studies might include participants with asthma‐COPD overlap syndrome (ACOS) i.e., people with chronic asthma that causes chronic airflow limitation (Postma 2015a). Such asthma cases would be difficult to distinguish from COPD, so we decided to include studies of people with ACOS as well as studies that enrolled participants who were diagnosed with asthma as a COPD comorbidity.
Diagnosis
We included studies that enrolled participants diagnosed with COPD according to established criteria, such as the American Thoracic Society and GOLD criteria (GOLD 2020). However, we recognised that the definition of any condition might change over time. For older studies, we examined the directness of the evidence by applying GRADE criteria to the diagnosis of the study participants, as determined by the investigators. If we identified trials in which only a subset of the participants had COPD, we included them on the basis that they reported disaggregated data by baseline diagnosis (COPD or asthma), or if the study investigators provided such data on request.
Comorbidities
As long as the comorbidity itself was not the main focus of the study, we included studies that enrolled people with COPD and comorbid chronic physical conditions (e.g. hypertension, cardiovascular disease, GERD, asthma). We excluded participants with the following prespecified comorbidities: bronchiectasis or genetic diseases, such as cystic fibrosis or primary ciliary dyskinesia.
Settings
All types of healthcare settings were eligible for inclusion: primary, secondary, and tertiary care.
Types of interventions
The optimal ("ideal") dosage regimen of oral PPIs has not been clarified. Standard dosage regimens of PPIs vary depending on the country/geographical location, but reference ranges have been reported as follows (Iwakiri 2016; Zhang 2017).
-
Standard dose of PPIs per day:
lansoprazole, 15 mg to 30 mg;
omeprazole, 10 mg to 40 mg;
pantoprazole, 40 mg;
rabeprazole, 10 mg to 20 mg;
esomeprazole, 10 mg to 20 mg;
dexlansoprazole, 30 mg to 60 mg; and
vonoprazan, 10 mg to 20 mg.
Eligible comparators were: placebo, usual care, or low‐dose PPI.
We planned to consider the following comparisons.
PPI plus usual COPD care versus placebo plus usual care.
PPI plus usual COPD care versus usual care alone.
PPI plus usual care versus lower‐dose PPI plus usual care.
Our a priori definition of usual care for COPD was comprehensive respiratory care that aimed to support self‐management through drug treatment, regular exacerbation monitoring, lifestyle guidance on issues such as smoking cessation, vaccinations, nutritional advice, exercise therapy, and appropriate management of comorbidities.
As it could take a few days for PPIs to exhibit their acid‐suppressive effects (Andersson 2005), and since chronic cough associated with GERD might take at least two to three months to develop (Irwin 2006), we included studies of at least two months' duration. We recorded and compared the intervention period and follow‐up duration.
Types of outcome measures
Primary outcomes
-
Exacerbations of COPD, reported as exacerbation rate or as time to first exacerbation after administration of PPIs
We extracted the definition used for an COPD exacerbation from the included study. If we had found the definition across studies to be inconsistent, we would have considered the impact on the overall result when applying GRADE ratings (Higgins 2017).
-
Pneumonia and other serious adverse events (we planned to report pneumonia and other serious events separately)
Our definition of a serious adverse event follows that of established criteria, which is an untoward medical incident that leads to death, is life‐threatening, requires hospitalisation or prolongs existing hospitalisation, results in persistent or significant disability, a birth defect, or any important medical event that might jeopardise the participant or requires intervention to prevent its effects (ICH 2016).
Secondary outcomes
Quality of life (measured by a validated generic or disease‐specific tool for COPD)
Lung function test indices: change from baseline in trough FEV1 and FVC
Acute respiratory infections (participants experiencing at least one episode)
Disease‐specific adverse events (participants with at least one event)
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Register of Trials (airways.cochrane.org/trials-register), which is a database maintained by the Group's Information Specialist. This register contains RCTs and quasi‐RCTs identified from several sources, including the following.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL Issue 5, 2020) through Cochrane Register of Studies;
Weekly searches of MEDLINE (Ovid SP) 1946 to present;
Weekly searches of Embase (Ovid SP) 1974 to present;
Monthly searches of PsycINFO (Ovid SP) 1967 to present;
Monthly searches of CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO) 1937 to present;
Monthly searches of AMED (Allied and Complementary Medicine) (EBSCO) from inception to present.
Our literature search dated from database inception to 22 May 2020, with no restriction on language or publication status. Search strategies developed for the Cochrane Airways Register of Trials are illustrated in Appendix 1. See Appendix 2 for search terms we used to identify studies for this review.
We also searched the following trial registries on 22 May 2020.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch).
Searching other resources
We performed handsearching of the conference proceedings of major conferences in the field of respiratory medicine (Appendix 1).
We checked the reference lists of all primary studies and relevant narrative review articles for additional references. We searched relevant manufacturers' websites for study information.
We also monitored for errata or retractions of the included study on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (SK and YT) independently screened the titles and abstracts of the search results and coded them as either 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We obtained full‐text study reports of all potentially eligible studies and two review authors (SK and HI) independently screened them for inclusion, recording the reasons for exclusion during the process. We resolved any disagreement through discussion or by consulting a third author (YT or TT). We identified and omitted duplicated reports and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We illustrated our selection process using a PRISMA flow diagram (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which was piloted for use on one study. One review author (SK) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number (N), mean age, age range, gender, severity of COPD, diagnostic criteria, follow‐up duration, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparator, concomitant medications, excluded medications, and dosage of the intervention.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding source(s), any notable conflicts of interest of trial authors and other information where available.
Two review authors (SK and HI) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if the study authors did not report outcome data in a usable format. We resolved disagreements by consensus or by involving a third review author (YT). One review author (SK) then transferred data into the Review Manager software for further processing (Review Manager 2014). We checked that the data were entered correctly by undertaking double data entry and a second review author (HI) performed spot‐checks to assure accuracy of data extraction and management.
Assessment of risk of bias in included studies
Two review authors (SK and HI) independently assessed the risk of bias in included studies using Cochrane's 'Risk of bias' tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion or by involving a third review author (YT). We assessed the risk of bias according to the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other sources of bias
We judged each potential source of bias as either 'high', 'low', or 'unclear', and provided a quote from the study report with a justification for our judgement in the 'Risk of bias' table for each study. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for pneumonia or serious adverse events may be very different than that for a patient‐reported quality of life scale). Where information on risk of bias related to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting this review
We conducted the review according to a published Cochrane protocol (Kikuchi 2018), and highlighted any deviations from it in the 'Differences between protocol and review' section.
Measures of treatment effect
We planned to only undertake meta‐analysis when it was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for data pooling. Since only one study was included, we reported our findings narratively. For transparency of data presentation, we also presented individual study findings as risk ratios (RRs) and means and standard deviations (SDs) or dichotomous and continuous data, respectively.
For future updates of this review that include additional eligible studies, we plan to also calculate standardised mean differences (SMDs) for outcome data that are measured by different metric scales (for example, quality of life measured by different assessment tools). If we combine data from rating scales in such a meta‐analysis, we will ensure that we enter them with a consistent direction of effect (e.g. lower scores always indicate improvement). We will conduct an 'as reported' and intention‐to‐treat (ITT) analysis of the primary outcomes; for secondary outcomes we will use an 'as reported' analysis.
For subsequent updates, we plan to describe skewed data narratively (for example, as medians and interquartile ranges for each group).
Unit of analysis issues
We used participants, rather than events, as the unit of analysis (i.e. number of participants admitted to hospital, rather than number of admissions per participant). If a study reported outcomes at multiple time points, we used the last time point measured.
Where multiple trial arms are reported in an included study of future updates of this review, we will include only the relevant arms; similarly, if two comparisons (e.g. drug A versus placebo and drug B versus placebo) are combined in the same meta‐analysis, we will either combine the active arms or divide the control group into halves to avoid double‐counting.
We had planned to calculate risk ratios (for example, for the outcome of COPD exacerbation rates) should sufficient data be available, but we only identified one eligible study. For future updates of this review, we will analyse data on this basis, allowing for the inclusion of more than one event in a participant over the time of the trial.
We also planned to analyse data from cluster‐RCTs if the trials had adjusted the data to account for clustering, or if we were able to adjust it ourselves. However, we did not identify any eligible cluster‐RCTs. Should future updates of this review include any cluster‐RCTs, we will manage data from these studies on this basis.
Dealing with missing data
Where possible, we contacted investigators or study sponsors in order to verify/obtain key study characteristics or any missing outcome data. We did not conduct a meta‐analysis for this review because we had insufficient studies.
For future updates, we intend to deal with missing participants using an ITT analysis, assuming that missing participants had failed treatment. In the case of dichotomous data that compared treatment response, we will include the total number of participants randomised to each comparison group (as the denominator). In the analyses of treatment response, we will only include data from trials that reported a group size prior to withdrawals. For continuous outcome measures, we plan to include summary statistics derived from mixed‐effect models, the last observation carried forward (LOCF), and observed cases' summary statistics.
LOCF method is a standard methodology in many clinical fields for imputing incomplete data. However, LOCF can lead to an underestimation of standard errors, which increases the likelihood of finding a false positive result (Mavridis 2019). Should additional studies be included in future updates of this review and a meta‐analysis performed, we also plan to investigate the impact of imputation by performing sensitivity analyses for each outcome. We can use a sensitivity analysis to check the robustness of results against departures from LOCF assumptions.
Where missing data are thought to introduce serious bias, we will take this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
Meta‐analysis was not feasible for this current review due to insufficient data. For future updates, we will use the Chi2 test (P value of less than 0.10 to be indicative of statistical heterogeneity) and the I2 statistic to assess statistical heterogeneity across studies. We will explore statistical diversity by estimates of treatment effect using forest plots. According to the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), statistical heterogeneity might not be important when the observed value of I2 is between 0% and 40%; there might be moderate heterogeneity when I2 is between 30% and 60%; substantial heterogeneity when I2 is between 50% and 90%; and considerable heterogeneity when I2 is greater than 75%. If we identify substantial heterogeneity in future updates of the review, we will report it and explore the possible causes by prespecified subgroup analysis (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
If we were able to include more than 10 studies, we had planned to create a funnel plot of effect estimates against their standard errors to explore possible small study and publication biases. We had planned to consider possible explanations if we found asymmetry of the funnel plot.
However, since we only included one study in this review, we did not assess reporting biases.
Data synthesis
We planned to combine the results from similar studies by undertaking a meta‐analysis with a random‐effects model, using Review Manager 2014. If substantial or considerable unexplained statistical heterogeneity (I2 > 50%) had been present, we had planned to omit meta‐analysis and instead report our findings narratively (Assessment of heterogeneity). Since we only included one study, meta‐analysis was not possible. We will pursue the stated plan in future updates of this review.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Baseline severity of COPD using GOLD spirometric assessment (GOLD 2020)
GOLD 1: mild (FEV1 ≥ 80% predicted);
GOLD 2: moderate (50% ≤ FEV1 < 80% predicted);
GOLD 3: severe (30% ≤ FEV1 < 50% predicted);
GOLD 4: very severe (FEV1 < 30% predicted).
Type of PPI (lansoprazole or omeprazole or other PPIs). Lansoprazole and omeprazole have been reported to inhibit cytokine production of proinflammatory cytokines and are expected to reduce the frequency of catching common colds (Sasaki 2009).
Baseline GERD symptoms: dichotomised as either 'yes' or 'no', according to inclusion criteria.
Trial funding sources (financial sponsorship from the pharmaceutical industries or from non‐industry sponsors).
We planned to use the formal test for subgroup interactions in Review Manager 2014.
We also planned to use the following outcomes in subgroup analyses.
Exacerbations
Pneumonia and other serious adverse events
Quality of life
Acute respiratory infections
We did not undertake these subgroup analyses for the current review because we had insufficient data, but we will include them in future updates.
Sensitivity analysis
We planned to undertake sensitivity analyses for the primary outcomes, to assess the robustness of our conclusions. We planned to remove studies at high risk of bias in at least two of the following domains: allocation concealment, blinding of outcome assessors, or incomplete outcome data, based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
We also planned to compare the results from a meta‐analysis based on a fixed‐effect model versus those from a random‐effects model.
However, given that we included only one study, we did not pursue the planned sensitivity analyses. If we identify sufficient data for future updates of this review, we will undertake the planned sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes.
Primary outcomes: exacerbations, pneumonia and other serious adverse events.
Secondary outcomes: quality of life, lung function indices, acute respiratory infections, disease‐specific adverse events.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it related to the study that contributed data for the prespecified outcomes. We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017; Schünemann 2017), using the GRADE working group's software (GRADEpro GDT). We justified all decisions to downgrade the quality of the evidence in the footnotes and also provided further remarks to aid reader's understanding of the our assessment where necessary.
Results
Description of studies
Results of the search
We identified 99 records through comprehensive literature searching. Of these, we excluded 86 clearly irrelevant records after screening titles and abstracts. We examined the full‐text articles of 13 records and excluded a further nine records; the remaining four records reported findings from a single study, which we included in the review. Our study selection process is illustrated in Figure 1 in the format of a PRISMA flow diagram.
1.
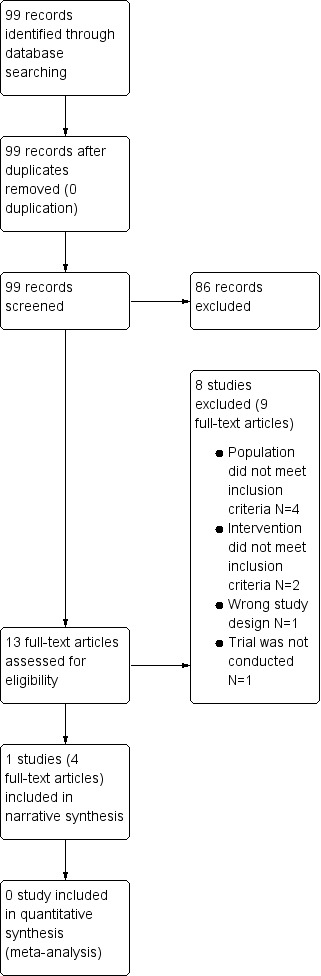
A PRISMA flow diagram illustrating our study selection process
Included studies
We identified one study that was eligible for inclusion (Characteristics of included studies). The study was reported as one full‐text peer‐reviewed journal article and three conference abstracts. The study was a single‐blind, multicentre RCT of 103 people with COPD, conducted over a 12‐month period in secondary and tertiary care hospitals in Japan (Sasaki 2009). The study compared the effects of lansoprazole (15 mg/day) plus usual care versus usual care alone on the prevention of exacerbations. Sasaki 2009 excluded people with GERD. Unfortunately, since a long period of time has passed since the end of the study, we were unable to obtain any raw data.
Excluded studies
We excluded eight studies (Boeree 1998; Kiljander 2000; Liu 2018; Miller 2015; NCT00214552; NCT00523367; Sun 2016; UMIN000002056). These are listed in the 'Characteristics of excluded studies' table, with reasons for exclusion. For three studies, the reason for exclusion was the type of participants (a study of people with asthma only (NCT00214552); a study enrolling healthy participants only (Miller 2015); a study of people with chronic cough (Kiljander 2000)). Participants in two studies received not only PPIs but also an oral prokinetic (mosapride) as part of the acid‐suppressive treatment, so we excluded them (Liu 2018; Sun 2016). One study was not an RCT, and had a single‐group assignment design (NCT00523367). One study was previously registered in a trial registry but, through personal communication with the research director of the responsible institute, we found out that the study had not been executed, so we excluded it (UMIN000002056).
We also excluded Boeree 1998. The study was double‐blind and conducted in a tertiary care hospital in the Netherlands, enrolling a total of 36 people with asthma or COPD with airway hyperresponsiveness. The study compared the effects of omeprazole at a daily dose of 80 mg with placebo on pulmonary symptoms and functions over three months (Boeree 1998). For this study, there was no clear definition of asthma and COPD. We contacted the study authors for further clarification on the definitions of asthma and COPD, and were informed that they applied a so‐called “Dutch hypothesis” definition of asthma and COPD. The Dutch hypothesis is based on the theory asthma and COPD have common origins and clinical expressions, and is suggested to be determined both by endogenous factors such as heredity, sex and age, and exogenous factors such as smoking, air pollution, viruses and allergens (Bleecker 2004; Postma 2015b). It has been adopted by many researchers to identify heterogeneity in asthma and COPD, and to better define these diseases by phenotype to optimise their treatment (Postma 2015b).
Participants in Boeree 1998 met the recent COPD diagnostic criteria of (FEV1 < 70%) by GOLD 2020. However, the included group had extremely high FEV1 as percentage predicted compared to group of people who would fit in COPD definition, even in the irreversible group. Moreover, the study included only people who were treated with a high dose of inhaled corticosteroid and a positive metacholine test. Although this study might also have included people with COPD, the majority of participants met the definition for asthma. For this reason, we determined that there were not enough numbers to reach a conclusion about the impact of PPI on COPD, and we decided to exclude Boeree 1998 from this review.
Risk of bias in included studies
The 'Characteristics of included studies' table presents further details of 'Risk of bias' assessments and supporting evidence. Figure 2 provides a summary of 'Risk of bias' assessments by domain. We considered included study's risk of bias was largely low or unclear for the majority of domains, although there was a high risk of performance bias because there was no blinding.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.
Allocation
Sasaki 2009 provided details of the method of random sequence generation, so we judged the study to be at low risk of bias for this domain. However, there was insufficient information on how allocation concealment was performed, so we assigned 'unclear risk of bias' for this domain.
Blinding
Sasaki 2009 was described as an observer‐blind RCT; participants and study personnel were unblinded and we judged the domain of performance bias to be at 'high risk'. Furthermore, there was no description of the role of the observers, so we assessed the risk of detection bias to be unclear.
Incomplete outcome data
The study reports documented the rates of participant withdrawal. The withdrawal rates were low and balanced between groups. Thus, we judged Sasaki 2009 to be at 'low risk' for attrition bias.
Selective reporting
Sasaki 2009 was not preregistered on appropriate clinical trials registries, and we did not identify a study protocol. Thus, we assessed the study to be at 'unclear risk' of reporting bias.
Other potential sources of bias
We considered whether Sasaki 2009 had other potential sources of bias. Data analysis as reported in the study indicated that, of the 103 randomised participants, three were lost to follow‐up and were excluded from the analysis. This suggests that the study did not use an intention‐to‐treat (ITT) analytical approach. In addition, there appeared to be a baseline imbalance in COPD severity, where the people in the usual care (control) group had a more advanced stage of COPD, based on baseline lung function; however, there were no statistical comparisons available. We are aware that this baseline imbalance would tend to skew the results in favour of PPI use, resulting in a potential overestimation of the treatment effects. However, the randomisation method was appropriate and it was unlikely to have a significant effect on the results. Consequently, we judged Sasaki 2009 to be at 'low risk' for other bias.
Effects of interventions
See: Table 1
Table 1 presents an overview of the results and a summary of our certainty of the evidence. Only one study met the inclusion criteria for this review, so we are reporting the results narratively.
PPI plus usual care versus usual care alone
In people with COPD but no history or symptoms of GERD, Sasaki 2009 reported that 12 out of 50 people in the PPI plus usual care group experienced one or more exacerbations, compared to 26 out of 50 in the usual care alone group (RR 0.46, 95% CI 0.26 to 0.81; 100 participants; low‐certainty evidence; Analysis 1.1). The frequency of COPD exacerbations per person in a year was also lower in the PPI plus usual care group than in the usual care alone group(0.34 ± 0.72 vs 1.18 ± 1.40; P < 0.001). However, using GRADE criteria, we judged the evidence for this outcome to be low certainty, as it was from a single small study and there was no blinding.
1.1. Analysis.

Comparison 1: PPI plus usual care vs usual care alone, Outcome 1: People with one or more exacerbations
Sasaki 2009 also provided information on acute respiratory infections, as the number of people with COPD who experienced one or more episodes of common colds. At the end of 12 month's treatment, the number of participants who experienced one or more common colds (upper respiratory tract infections) per person was slightly lower in the PPI plus usual care group (26 out of 50) than in the usual care alone group (27 out of 50), but the confidence interval was wide (RR 0.96, 95% CI 0.67 to 1.39; 100 participants; very low‐certainty evidence; Analysis 1.2; Table 1). Using GRADE criteria, we judged the evidence for this outcome to be very low certainty.
1.2. Analysis.

Comparison 1: PPI plus usual care vs usual care alone, Outcome 2: Acute respiratory infections
We did not identify any evidence on pneumonia and other serious adverse events, quality of life, lung function test indices or disease‐specific adverse events.
Discussion
Summary of main results
We included only one study, which randomised a total of 103 participants, and we assessed the evidence to be of low to very low certainty. There is currently insufficient evidence to support the use of PPIs in people with COPD, in particular on outcomes such as pneumonia or serious adverse events.
Sasaki 2009 investigated the effects of lansoprazole plus usual care versus usual care alone in people with COPD but no history or symptoms of GERD. The study measured frequencies of the common cold and COPD exacerbation for 12 months. Findings indicated that lansoprazole plus usual care led to a reduced COPD exacerbation rate compared to usual care alone. Sasaki 2009 reported data on the common cold (upper respiratory tract infections), but found no difference between groups. The study did not report any data for our other prespecified primary and secondary outcomes, such as pneumonia or serious adverse events, quality of life, lung function indices and disease‐specific adverse events.
Overall completeness and applicability of evidence
We could not conduct a meta‐analysis because this review contained only one study. Instead, we narratively reported the findings. Caution should be used when extrapolating these results to similar populations or other settings, as there was a lack of data and the study did not use a placebo. We could not identify any studies that used PPIs other than lansoprazole, or studies that used low‐dose PPIs as a comparison.
There is a lack of evidence to indicate whether treatment with PPIs might play a role in managing COPD, in terms of exacerbations and adverse events. There is also insufficient evidence to clarify the effects of PPIs on quality of life, respiratory function or disease‐specific adverse events. Thus, the evidence for the benefits and harms of PPI interventions for people with COPD is incomplete. Evidence for the treatment's applicability to clinical practice is limited.
Quality of the evidence
Only one study contributed data to this review. Our assessment of its risk of bias indicated a high risk of performance bias due to unblinded participants and personnel. We were unable to pursue clinically meaningful subgroup analysis because we only included one study. We recognised the limitations and methodological shortcomings of the study, and evaluated the certainty of evidence to be low to very low, using the GRADE approach.
Potential biases in the review process
We received support from Cochrane Airways' information specialist to pursue a comprehensive and systematic literature search. Two reviewers independently selected eligible studies and evaluated the included studies following standard Cochrane methodology to minimise bias. We also made every effort to obtain missing study characteristics/data where appropriate by contacting the original study authors. As with any systematic review, we are aware of the potential publication bias in this Cochrane Review. Since we only included one study, we did not explore publication bias. For future updates, if the number of included studies exceeds 10, we will follow recommended methods to test for publication bias.
Agreements and disagreements with other studies or reviews
We were unable to identify another systematic review investigating the efficacy and safety of PPIs in people with COPD. To our knowledge, this is the first attempt at systematically identifying, appraising and synthesising evidence on the benefits and harms of PPIs for COPD. The inclusion of one small‐scale study of low to very low quality indicates that, at the moment, no concrete conclusions can be drawn regarding the effectiveness PPI as a treatment option for COPD.
Sasaki 2009 suggested that larger studies should be conducted to determine the efficacy and safety of PPI in people with COPD. Due to the limited data available from the review, it is not possible to assess whether PPIs have a positive or negative effect on COPD exacerbation and quality of life of people with COPD. However, planned future studies may help to resolve the uncertainty of PPI interventions for people with COPD.
Authors' conclusions
Implications for practice.
Findings of this review are based on only one low quality randomised controlled trial (RCT), for which reporting of clinically significant results was limited. The evidence was limited by various factors, including the study being potentially underpowered, and limited reporting of important outcomes of clinical benefit and harm. There is currently insufficient evidence to guide meaningful clinical practice decisions as to the potential benefits and risks of proton pump inhibitors (PPIs) for people with chronic obstructive pulmonary disease (COPD).
Implications for research.
Key aims of the care pathway for people with COPD are to reduce the rate of COPD exacerbation and to improve overall quality of life. Existing research has suggested that gastroesophageal reflux disease (GERD) is a comorbidity of COPD. However, the precise effects of PPIs on COPD are yet to be comprehensively evaluated. This is an emerging and important research direction, given that PPIs are commonly prescribed and readily available. In order to fully understand whether PPIs are a viable treatment option for COPD, researchers in the field of respiratory and gastroenterology medicine should seek to define the study population carefully, assess and report important outcomes, such as COPD exacerbations (using clear diagnostic criteria), pneumonia and other serious adverse events, quality of life, and pulmonary function.
We recommend that people with COPD who have a history or symptoms of GERD should be excluded from the study population of future RCTs. The biggest reason is ethical issues. GERD can affect quality of life, and sometimes causes bleeding. Since PPIs are well established as first‐line drugs in the management of GERD (van Pinxteren 2003), we think that if we include people with GERD in the RCTs, we should use PPIs for those in the control group as well. However, excluding people with GERD from studies might reduce adverse events such as community‐acquired pneumonia (CAP). Therefore, it might be better to consider conducting a systematic review that includes non‐randomised studies of PPIs for people with COPD who have a history or symptoms of GERD.
The diagnosis of GERD is made by a combination of several diagnostic modalities, including symptom presentation, response to antacid therapy, upper gastrointestinal endoscopy, histological examination, and oesophageal pH monitoring (Katz 2013; Vakil 2006). However, in clinical practice, diagnosis of GERD is usually based on typical symptoms alone, and empirical PPI therapies are recommended as a reasonable approach to confirm GERD (Katz 2013; Vakil 2006). Oesophageal pH monitoring is a unique test used to check the frequency of reflux and the association of symptoms with reflux episodes. Although it has high sensitivity and specificity in the diagnosis of GERD, it is not a simple and quick test. A guideline recommends its use in certain situations, such as non‐erosive reflux disease, disease that is refractory to PPI therapy, and when the diagnosis of GERD is in question (Katz 2013). Considering the implications for practice, we recommend using typical symptoms and empirical PPI therapy rather than oesophageal pH monitoring for the diagnosis of GERD in future studies.
Moreover, future studies should employ a robust design of adequate power and methodological considerations (such as transparent reporting of randomisation methods, allocation concealment and blinding) to ensure that their are of high quality and relevance. Further evidence from robust, large‐scale trials investigating clinically relevant and patient‐centred outcomes such as COPD exacerbations and adverse events is warranted.
History
Protocol first published: Issue 8, 2018 Review first published: Issue 8, 2020
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
We acknowledge Dr Emma Dennett for her role in refining our research question, and Ms Elizabeth Stovold for developing the search strategy.
The authors and Airways Editorial Team,are grateful to the following peer reviewers for their time and comments: Toni Kiljander, Terveystalo Hospital, Finland and Prof Emma H Baker, St George's University's Hospitals NHS Foundation Trust, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid SP) | 1946 onwards | Weekly |
| EMBASE (Ovid SP) | 1974 onwards | Weekly |
| PsycINFO (Ovid SP) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE (Ovid) search strategy used to identify studies for the Cochrane Airways Trials Register
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases.
Appendix 2. Cochrane Airways Trials Register (via the Cochrane Register of Studies (CRS)) search strategy
| #1 | MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All |
| #2 | MeSH DESCRIPTOR Bronchitis, Chronic |
| #3 | (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) |
| #4 | COPD:MISC1 |
| #5 | (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | MESH DESCRIPTOR Proton Pump Inhibitors EXPLODE ALL |
| #8 | Proton Pump Inhibitor* or "Proton‐Pump Inhibitor*" or PPI or PPIs |
| #9 | lansoprazole |
| #10 | omeprazole |
| #11 | pantoprazole |
| #12 | rabeprazole |
| #13 | esomeprazole |
| #14 | dexlansoprazole |
| #15 | vonoprazan |
| #16 | MESH DESCRIPTOR Gastroesophageal Reflux EXPLODE ALL |
| #17 | MESH DESCRIPTOR Laryngopharyngeal Reflux EXPLODE ALL |
| #18 | ((gastroesophageal or gastro‐esophageal or gastro‐oesophageal or gastrooesophageal) NEAR3 reflux) |
| #19 | GERD or GORD |
| #20 | acid NEAR2 reflux |
| #21 | heartburn or pyrosis |
| #22 | {OR #7‐#21} |
| #23 | #22 AND #6 |
Data and analyses
Comparison 1. PPI plus usual care vs usual care alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 People with one or more exacerbations | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Acute respiratory infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sasaki 2009.
| Study characteristics | ||
| Methods |
Design: single‐blinded, multicentre randomised controlled trial Study duration: 12 months (October 2005 to March 2007) Number of study centres and location: four; Japan Setting: hospital outpatients (three secondary care hospitals and one tertiary care hospital) Withdrawals: PPI plus usual care: n = 1; usual care alone: n = 2 |
|
| Participants |
Number randomised: 103 participants with COPD (PPI plus usual care: n = 51; usual care alone: n = 52) Mean age (SD), age range: PPI plus usual care: 74.9 (8.9), range not reported; usual care alone: 74.8 (7.5), range not reported Gender, n (%) female: PPI plus usual care: 3 (6); usual care alone: 2 (4) Severity of COPD (Grade 1/2/3/4): PPI plus usual care: 16/14/17/3; usual care alone: 11/18/20/1 Diagnostic criteria: American Thoracic Society (ATS) for COPD Follow‐up duration: 12 months Baseline lung function:
Smoking history (mean pack years): PPI plus usual care: 56; usual care alone: 50 Inclusion criteria: not stated Exclusion criteria: People with obvious bronchial asthma, bronchiectasis, or diffuse panbronchiolitis were excluded from the trial. People with active gastric or duodenal ulcers and GERD, and with symptoms of these diseases, were also excluded. People with QUEST scores of more than 6 points were defined as positive for GERD symptoms, and were excluded from this study. |
|
| Interventions |
Intervention: PPI (lansoprazole) plus usual care* Comparison: usual care* alone Concomitant medications: not stated Excluded medications: not stated Dosage of the intervention: 15 mg daily *Usual care: participants were treated with bronchodilators, including sustained release theophylline, beta‐2 agonists, inhaled anticholinergic agents, and smoking cessation. Some of the participants with Stage III and IV COPD received inhaled corticosteroids because of frequent exacerbations of COPD. |
|
| Outcomes |
Outcomes:
Time point: 12 months |
|
| Notes |
Funding: Ministry of Health, Labour and Welfare, Japan; Ministry of Education, Science, Culture, Sports, Science and Technology (19590690; 19790455); Japanese Foundation for Aging and Health Notable author conflicts of interest: Dr Yamaya was partly supported by Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare (H20nannchiippann35) and the Respiratory Failure Research Group from the Ministry of Health, Labour and Welfare, Japan. Dr Nakayama was partly supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, Science and Technology (19590690) and a grant from the Japanese Foundation for Aging and Health. Dr Sasaki was partly supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, Science and Technology (19790455). This work was supported by these grants. The other co‐authors do not have any potential conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed using a random number table, and the list was held independently of the investigators." Since it was not clearly stated whether the concealment was maintained we judged it as an unclear risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and study personnel remained unblinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as observer‐blinded, but details about the exact role of the observers were not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | During trial: 1/51 withdrew from the intervention group; 2/52 withdrew from the control group. Although a total of 103 people were randomised, 3 losses at follow‐up were excluded from the analysis and no details were given. We contacted the author and confirmed that it was done with per‐protocol rather than ITT analysis. However, we thought that the dropout rate was small so the impact on the results was small and the risk of bias was low. |
| Selective reporting (reporting bias) | Unclear risk | The trial was not preregistered on any appropriate clinical trial registries; study protocol not available. |
| Other bias | Low risk | Baseline imbalances in disease severity: participants in the intervention group appeared to have less severe disease (COPD grade I/II/III/IV: Intervention group: 16/14/17/3; control group: 11/18/20/1). However, randomisation method was appropriate and it was unlikely to have a significant effect on the results, thus the risk of other bias was low. |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GERD: gastroesophageal disease; PPI: proton pump inhibitor; QUEST: Quality of Upper Extremity Skills Test; SD: standard deviation; ITT: intention‐to‐treat.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boeree 1998 | The majority of participants were people with asthma, and the rate of people with COPD was considered to be very low. |
| Kiljander 2000 | Type of study population does not meet inclusion criteria: people with chronic persistent cough for two months or longer. |
| Liu 2018 | Type of Intervention does not meet inclusion criteria: antireflux treatment administered was a combination of oral antacids (omeprazole) and mosapirde, a gastroprokinetic agent. |
| Miller 2015 | Types of population and intervention do not meet inclusion criteria; oral danirixin in healthy male subjects. |
| NCT00214552 | Type of population does not meet inclusion criteria: people with asthma only. |
| NCT00523367 | Non‐RCT: single‐arm study |
| Sun 2016 | Type of intervention does not meet inclusion criteria: combination of oral antacid (esomeprazole) with oral mosapride and tiotropium bromide. |
| UMIN000002056 | The trial was registered but not conducted. |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial
Differences between protocol and review
Due to limited data, we could not perform analyses of the following primary and secondary outcomes: pneumonia and other serious adverse events; quality of life; disease‐specific adverse events. Consequently, we could not conduct any of the prespecified subgroup and sensitivity analyses.
Contributions of authors
SK: selected studies for inclusion; extracted data from the studies and assessed the risk of bias; entered data into Review Manager 5 and performed the analyses; drafted the final review and had overall responsibility for the review. HI: extracted data from the studies and assessed the risk of bias; entered data into Review Manager 5 and performed the analyses; drafted the final review. YT: selected studies for inclusion. TT: drafted the final review. NW: drafted the final review.
Contributions of editorial team
Chris Cates (Co‐ordinating Editor) checked the data entry prior to the full write up of the review, edited the review; advised on methodology; approved the review prior to publication.
Wouter van Geffen (Contact Editor): edited the review; advised on methodology, interpretation and content.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review and edited the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
-
All authors, Japan
The authors declare that no such funding was received for this systematic review.
External sources
-
All authors, Japan
The authors declare that no such funding was received for this systematic review.
Declarations of interest
SK has no known conflicts of interest. HI has no known conflicts of interest. YT has no known conflicts of interest. TT has no known conflicts of interest. NW has research funds from the Japanese Ministry of Health, Labor and Welfare, and the Japanese Ministry of Education, Science, and Technology. He has also received royalties from Sogensha and Medical View for writings. The results in the review are completely independent of the intention of these grants.
New
References
References to studies included in this review
Sasaki 2009 {published data only}
- Sasaki T, Katsutoshi N, Hiroyasu Y, Toshihiro N, Mutsuo Y. The proton pump inhibitor, lansoprazole, reduces frequency of exacerbations in patients with chronic obstructive pulmonary disease. In: Chest 2011 Annual Meeting; 2011 Oct 22-26; Honolulu. 2011.
- Sasaki T, Nakayama K, Yasuda H, Yoshida M, Asamura T, Ohrui T, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. Journal of the American Geriatrics Society 2009;57(8):1453-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sasaki T, Nakayama K, Yasuda H, Yoshida M, Inoue D, Yamaya M. A proton pump inhibitor lansoprazole reduces frequency of exacerbations in patients with chronic obstructive pulmonary disease (COPD). In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008.
- Sasaki T, Nakayama K, Yasuda H, Yoshida M, Inoue D, Yamaya M. The proton pump inhibitor, lansoprazole, reduces frequency of exacerbations in patients with chronic obstructive pulmonary disease. In: Chest 2011 Annual Meeting; 2011 Oct 22-26; Honolulu. 2011.
References to studies excluded from this review
Boeree 1998 {published data only}
- Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. Effect of high-dose omeprazole on airway hyperresponsiveness and pulmonary function in patients with obstructive lung disease. Netherlands Journal of Medicine 1995;47:A3 (A1-A42). [Google Scholar]
- Boeree MJ, Peters FT, Postma DS, Kleibeuker JH. No effects of high-dose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro-oesophageal reflux. European Respiratory Journal 1998;11(5):1070-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kiljander 2000 {published data only}
- Kiljander TO, Salomaa ER, Hietanen EK, Terho EO. Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. European Respiratory Journal 2000;16(4):633-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2018 {published data only}
- Liu H. Assessment of anti-reflux treatment on pulmonary ventilation function and inflammatory cytokines in patients with stable chronic obstructive pulmonary disease combined with gastroesophageal reflux. Experimental and Therapeutic Medicine 2018;15(6):5528-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller BE, Mistry S, Smart K, Connolly P, Carpenter DC, Cooray H, et al. The pharmacokinetics and pharmacodynamics of danirixin (GSK1325756) - a selective CXCR2 antagonist - in healthy adult subjects. BMC Pharmacology and Toxicology 2015;16:18. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00214552 {unpublished data only}
- NCT00214552. Evaluate the effects on asthma control of rabeprazole given twice daily in subjects with asthma [A double-blind placebo controlled clinical trial to evaluate the effects on asthma control of rabeprazole given twice daily in subjects with asthma]. clinicaltrials.gov/ct2/show/NCT00214552 (first received 22 September 2005).
NCT00523367 {unpublished data only}
- NCT00523367. COPD patients diagnosed with GERD, COPD exacerbations after treatment with high dose PPI (GERD/COPD) [COPD patients diagnosed with gastroesophageal reflux disease have decreased rates of COPD exacerbations after treatment with high dose proton pump inhibitor therapy (Esomeprazole or Lansoprazole)]. clinicaltrials.gov/ct2/show/NCT00523367 (first received 31 August 2007).
Sun 2016 {published data only}
- Sun ZF, Zhang MX, Qu GH, Lan FJ, Li M, Sun SH. Clinical efficacy of antireflux therapy combined with tiotropium bromide in COPD patients with GERD. World Chinese Journal of Digestology 2016;9:1412-6. [Google Scholar]
UMIN000002056 {unpublished data only}
- UMIN000002056. Effects of rabeprazole on acute exacerbation in chronic obstructive pulmonary disease (COPD) patients with acid reflux symptoms. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000002502 (first received 8 June 2009).
Additional references
Agrawal 2000
- Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. Archives of Internal Medicine 2000;160(10):1455-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agusti 2016
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. The European Respiratory Journal 2016;47(2):410-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Akazawa 2016
- Akazawa Y, Fukuda D, Fukuda Y. Vonoprazan-based therapy for Helicobacter pylori eradication: experience and clinical evidence. Therapeutic Advances in Gastroenterology 2016;9(6):845-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersson 2005
- Andersson K, Carlsson E. Potassium-competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacology and Therapeutics 2005;108(3):294-307. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barnes 2013
- Barnes N, Calverley PM, Kaplan A, Rabe KF. Chronic obstructive pulmonary disease and exacerbations: patient insights from the global Hidden Depths of COPD survey. BMC Pulmonary Medicine 2013;13:54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bashford 1998
- Bashford JN, Norwood J, Chapman SR. Why are patients prescribed proton pump inhibitors? Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database. BMJ 1998;317(7156):452-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumeler 2016
- Baumeler L, Papakonstantinou E, Milenkovic B, Lacoma A, Louis R, Aerts JG, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology 2016;21(5):883-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beasley 2012
- Beasley V, Joshi PV, Singanayagam A, Molyneaux PL, Johnston SL, Mallia P. Lung microbiology and exacerbations in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:555-69. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2006
- Becker JC, Grosser N, Waltke C, Schulz S, Erdmann K, Domschke W, et al. Beyond gastric acid reduction: proton pump inhibitors induce heme oxygenase-1 in gastric and endothelial cells. Biochemical and Biophysical Research Communications 2006;345(3):1014-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bleecker 2004
- Bleecker ER. Similarities and differences in asthma and COPD. The Dutch hypothesis. Chest 2004;126(2 Suppl):93S-5S. [DOI] [PubMed] [Google Scholar]
Braeken 2015
- Braeken DC, Franssen FM, Schutte H, Pletz MW, Bals R, Martus P, et al. Increased severity and mortality of CAP in COPD: results from the German Competence Network, CAPNETZ. Journal of the COPD Foundation 2015;2(2):131-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Braeken 2017
- Braeken DC, Rohde GG, Franssen FM, Driessen JH, Staa TP, Souverein PC, et al. Risk of community-acquired pneumonia in chronic obstructive pulmonary disease stratified by smoking status: a population-based cohort study in the United Kingdom. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:2425-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buscho 1978
- Buscho RO, Saxtan D, Shultz PS, Finch E, Mufson MA. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. Journal of Infectious Diseases 1978;137(4):377-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Casanova 2004
- Casanova C, Baudet JS, Valle Velasco M, Martin JM, Aguirre-Jaime A, Torres JP, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. European Respiratory Journal 2004;23(6):841-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2019
- Chen J, Brady P. Gastroesophageal reflux disease: pathophysiology, diagnosis, and treatment. Gastroenterology Nursing 2019;42(1):20-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Dekel 2004
- Dekel R, Morse C, Fass R. The role of proton pump inhibitors in gastro-oesophageal reflux disease. Drugs 2004;64(3):277-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Del Grande 2016
- Del Grande LM, Herbella FA, Bigatao AM, Abrao H, Jardim JR, Patti MG. Pathophysiology of gastroesophageal reflux in patients with chronic pulmonary obstructive disease Is linked to an increased transdiaphragmatic pressure gradient and not to a defective esophagogastric barrier. Journal of Gastrointestinal Surgery 2016;20(1):104-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Serag 2014
- El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63(6):871-80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eom 2011
- Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. Canadian Medical Association Journal 2011;183(3):310-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falsey 2006
- Falsey AR, Formica MA, Hennessey PA, Criddle MM, Cullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2006;173(6):639-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filion 2014
- Filion KB, Chateau D, Targownik LE, Gershon A, Durand M, Tamim H, et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut 2014;63(4):552-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedberg 2017
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017;152(4):706-15. [DOI: 10.1053/j.gastro.2017.01.031] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gaude 2009
- Gaude GS. Pulmonary manifestations of gastroesophageal reflux disease. Annals of Thoracic Medicine 2009;4(3):115-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2014
- George SN, Garcha DS, Mackay AJ, Patel AR, Singh R, Sapsford RJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. European Respiratory Journal 2014;44(1):87-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Giuliano 2012
- Giuliano C, Wilhelm SM, Kale-Pradhan PB. Are proton pump inhibitors associated with the development of community-acquired pneumonia? A meta-analysis. Expert Review of Clinical Pharmacology 2012;5(3):337-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
GOLD 2020
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020. goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed prior to 1 May 2020).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 27 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gulmez 2007
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Archives of Internal Medicine 2007;167(9):950-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunawardana 2014
- Gunawardana N, Finney L, Johnston SL, Mallia P. Experimental rhinovirus infection in COPD: implications for antiviral therapies. Antiviral Research 2014;102:95-105. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2010
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. American Journal of Respiratory and Critical Care Medicine 2010;182(5):598-604. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbella 2010
- Herbella FA, Patti MG. Gastroesophageal reflux disease: from pathophysiology to treatment. World Journal of Gastroenterology 2010;16(30):3745-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hsu 2017
- Hsu WT, Lai CC, Wang YH, Tseng PH, Wang K, Wang CY, et al. Risk of pneumonia in patients with gastroesophageal reflux disease: a population-based cohort study. PLoS ONE 2017;12(8):e0183808. [DOI: 10.1371/journal.pone.0183808] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2017
- Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Frontiers in Cellular and Infection Microbiology 2017;7:168. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2015
- Hunt RH, Scarpignato C. Potassium-Competitive Acid Blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clinical and Translational Gastroenterology 2015;6:e119. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurst 2005
- Hurst JR, Donaldson GC, Wilkinson TM, Perera WR, Wedzicha JA. Epidemiological relationships between the common cold and exacerbation frequency in COPD. European Respiratory Journal 2005;26(5):846-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
ICH 2016
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). Current Step 4 version dated 9 November 2016. //database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 5 July 2018).
Ingebrigtsen 2015
- Ingebrigtsen TS, Marott JL, Vestbo J, Nordestgaard BG, Hallas J, Lange P. Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology 2015;20(1):101-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Irwin 2000
- Irwin RS, Madison JM. The diagnosis and treatment of cough. New England Journal of Medicine 2000;343(23):1715-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Irwin 2006
- Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest 2006;129(1 Suppl):80S-94S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Iwakiri 2016
- Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. Journal of Gastroenterology 2016;51(8):751-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Javorkova 2008
- Javorkova N, Varechova S, Pecova R, Tatar M, Balaz D, Demeter M, et al. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterology and Motility 2008;20(2):119-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jena 2013
- Jena AB, Sun E, Goldman DP. Confounding in the association of proton pump inhibitor use with risk of community-acquired pneumonia. Journal of General Internal Medicine 2013;28(2):223-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnstone 2010
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Alimentary Pharmacology and Therapeutics 2010;31(11):1165-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kahrilas 1988
- Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 1988;94(1):73-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Katz 2013
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. American Journal of Gastroenterology 2013;108(3):308-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laheij 2004
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. Journal of the American Medical Association 2004;292(16):1955-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lambert 2015
- Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE 2015;10(6):e0128004. [DOI: 10.1371/journal.pone.0128004] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1935-49. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2015
- Long J, Wright E, Molesti E, Temperton N, Barclay W. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Research 2015;4:30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansfield 1981
- Mansfield LE, Hameister HH, Spaulding HS, Smith NJ, Glab N. The role of the vague nerve in airway narrowing caused by intraesophageal hydrochloric acid provocation and esophageal distention. Annals of Allergy 1981;47(6):431-4. [PMID: ] [PubMed] [Google Scholar]
Martinez 2014
- Martinez CH, Okajima Y, Murray S, Washko GR, Martinez FJ, Silverman EK, et al. Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respiratory Research 2014;15:62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2019
- Mavridis D, Salanti G, Furukawa TA, Cipriani A, Chaimani A, White IR. Allowing for uncertainty due to missing and LOCF imputed outcomes in meta-analysis. Statistics in medicine 2019;38(5):720-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijvis 2011
- Meijvis SC, Cornips MC, Voorn GP, Souverein PC, Endeman H, Biesma DH, et al. Microbial evaluation of proton-pump inhibitors and the risk of pneumonia. The European Respiratory Journal 2011;38(5):1165-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Metlay 2019
- Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. American Journal of Respiratory and Critical Care Medicine 2019;200(7):e45-67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokhlesi 2001
- Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest 2001;119(4):1043-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morehead 2009
- Morehead RS. Gastro-oesophageal reflux disease and non-asthma lung disease. European Respiratory Review 2009;18(114):233-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohara 1999
- Ohara T, Arakawa T. Lansoprazole decreases peripheral blood monocytes and intercellular adhesion molecule-1-positive mononuclear cells. Digestive Diseases and Sciences 1999;44(8):1710-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Orlando 2001
- Orlando RC. Overview of the mechanisms of gastroesophageal reflux. American Journal of Medicine 2001;111(Suppl 8A):174S-7S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Othman 2016
- Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: population based study. BMJ 2016;355:i5813. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandolfino 2000
- Pandolfino JE, Kahrilas PJ. Smoking and gastro-oesophageal reflux disease. European Journal of Gastroenterology and Hepatology 2000;12(8):837-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perera 2012
- Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD 2012;9(2):131-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phulpoto 2005
- Phulpoto MA, Qayyum S, Rizvi N, Khuhawar SM. Proportion of gastroesophageal reflux symptoms in patients with chronic obstructive pulmonary disease. Journal of the Pakistan Medical Association 2005;55(7):276-9. [PMID: ] [PubMed] [Google Scholar]
Postma 2015a
- Postma DS, Rabe KF. The asthma-COPD overlap syndrome. New England Journal of Medicine 2015;373(13):1241-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Postma 2015b
- Postma DS, Weiss ST, den Berge M, Kerstjens HA, Koppelman GH. Revisiting the Dutch hypothesis. The Journal of Allergy and Clinical Immunology 2015;136(3):521-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Quint 2010
- Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest 2010;137(4):812-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rostom 2002
- Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD002296. [DOI: 10.1002/14651858.CD002296] [DOI] [PubMed] [Google Scholar]
Sasaki 2005
- Sasaki T, Yamaya M, Yasuda H, Inoue D, Yamada M, Kubo H, et al. The proton pump inhibitor lansoprazole inhibits rhinovirus infection in cultured human tracheal epithelial cells. European Journal of Pharmacology 2005;509(2-3):201-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sasaki 2011
- Sasaki T, Nakayama K, Yasuda H, Yamaya M. A new strategy with proton pump inhibitors for the prevention of acute exacerbations in COPD. Therapeutic Advances in Respiratory Disease 2011;5(2):91-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schan 1994
- Schan CA, Harding SM, Haile JM, Bradley LA, Richter JE. Gastroesophageal reflux-induced bronchoconstriction. An intraesophageal acid infusion study using state-of-the-art technology. Chest 1994;106(3):731-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Scott 2015
- Scott DR, Munson KB, Marcus EA, Lambrecht NW, Sachs G. The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+ -ATPase. Alimentary Pharmacology and Therapeutics 2015;42(11-2):1315-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seemungal 1998
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(5 Pt 1):1418-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sethi 2008
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(22):2355-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shaheen 2006
- Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, et al. The burden of gastrointestinal and liver diseases, 2006. American Journal of Gastroenterology 2006;101(9):2128-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 1980
- Smith CB, Golden CA, Kanner RE, Renzetti AD Jr. Association of viral and Mycoplasma pneumoniae infections with acute respiratory illness in patients with chronic obstructive pulmonary diseases. American Review of Respiratory Disease 1980;121(2):225-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sugano 2018
- Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Therapeutic Advances in Gastroenterology 2018;11:1756283X17745776. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugiura 2001
- Sugiura T, Iwakiri K, Kotoyori M, Kobayashi M. Relationship between severity of reflux esophagitis according to the Los Angeles classification and esophageal motility. Journal of Gastroenterology 2001;36(4):226-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Terada 2008
- Terada K, Muro S, Sato S, Ohara T, Haruna A, Marumo S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax 2008;63(11):951-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tuchman 1984
- Tuchman DN, Boyle JT, Pack AI, Scwartz J, Kokonos M, Spitzer AR, et al. Comparison of airway responses following tracheal or esophageal acidification in the cat. Gastroenterology 1984;87(4):872-81. [PMID: ] [PubMed] [Google Scholar]
Vakil 2006
- Vakil N, Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. The American Journal of Gastroenterology 2006;101(8):1900-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Pinxteren 2003
- Pinxteren B, Numans ME, Lau J, Wit NJ, Hungin AP, Bonis PA. Short-term treatment of gastroesophageal reflux disease. Journal of General Internal Medicine 2003;18(9):755-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2001
- Watanabe T, Higuchi K, Tominaga K, Fujiwara Y, Arakawa T. Acid regulates inflammatory response in a rat model of induction of gastric ulcer recurrence by interleukin 1beta. Gut 2001;48(6):774-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wedzicha 2013
- Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Medicine 2013;11:181. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wedzicha 2017
- Wedzicha JA (ERS Co-Chair), Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. The European Respiratory Journal 2017;49(3):1600791. [DOI: 10.1183/13993003.00791-2016.] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. Chronic respiratory diseases. www.who.int/respiratory/en/ (accessed 22 January 2018).
Woodruff 2015
- Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet 2015;385(9979):1789-98. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2017
- Yu LY, Sun LN, Zhang XH, Li YQ, Yu L, Yuan ZQ, et al. A review of the novel application and potential adverse effects of proton pump inhibitors. Advances in Therapy 2017;34(5):1070-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2017
- Zhang C, Kwong JS, Yuan RX, Chen H, Xu C, Wang YP, et al. Effectiveness and tolerability of different recommended doses of PPIs and H2RAs in GERD: network meta-analysis and GRADE system. Scientific Reports 2017;7:41021. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kikuchi 2018
- Kikuchi S, Naoki Y, Tajiri T, Watanabe N. Proton pump inhibitors for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD013113. [DOI: 10.1002/14651858.CD013113] [DOI] [PMC free article] [PubMed] [Google Scholar]


