Abstract
Background:
Current literature lacks characterization of the post-recovery sequelae among COVID-19 patients. This review characterizes the course of clinical, laboratory, radiological findings during the primary infection period, and the complications post-recovery. Primary care findings are presented for long-COVID care.
Methods:
Adhering to PRISMA guidelines, 4 databases were searched (PubMed, Embase, CINAHL Plus, Scopus) through December 5, 2020, using the keywords “COVID-19 and/or recovered and/or cardiovascular and/or long-term and/or sequelae and/or sub-acute and/or complication.” We included published peer-reviewed case reports, case series, and cross-sectional studies providing the clinical course of COVID-19 infection, and cardiopulmonary complications of patients who recovered from COVID-19, while making healthcare considerations for primary care workers.
Results:
We identified 29 studies across 9 countries including 37.9% Chinese and 24.1% U.S. studies, comprising 655 patients (Mean Age = 45) with various ethnical backgrounds including Asian and European. Based on the WHO COVID-19 severity classification scale, initial disease severity was mild for 377 patients and severe for 52 patients. Treatments during primary infection included corticosteroids, oxygen support, and antivirals. The mean value (in days) for complication onset after acute recovery was 28 days. Complete blood counts and RT-PCR tests were the most common laboratory results described. In 22 of the studies, patients showed signs of clinical improvement and were prescribed medications such as anticoagulants or corticosteroids.
Conclusion:
Post-recovery infectious complications are common in long-COVID-19 patients ranging from mild infections to life-threatening conditions. International thoracic and cardiovascular societies need to develop guidelines for patients recovering from COVID-19 pneumonia, while focused patient care by the primary care physician is crucial to curb preventable adverse events. Recommendations for real-time and lab-quality diagnostic tests are warranted to establish point-of-care testing, detect early complications, and provide timely treatment.
Keywords: primary care, long COVID, cardiac, pulmonary, COVID-19
Introduction
Toward the end of December 2019, the COVID-19 outbreak resulted in massive fear worldwide. Although this outbreak initially occurred in Wuhan, a city in Hubei province, China, it rapidly spread throughout the world. The World Health Organization (WHO) announced that the COVID-19 pandemic as a health emergency of public concern on January 30, 2020. 1 The pandemic has drastically impacted all industries and sectors worldwide, placing an immense burden on the health care system. 2 COVID-19 has a wide array of clinical presentations ranging from non-specific symptoms such as fever, fatigue, and diarrhea to severe respiratory and cardiovascular complications, including acute respiratory distress syndrome (ARDS) and multiple organ failure leading to mortality. 3 As a result of the data gathered from numerous clinical trials conducted worldwide, there has been ongoing concern regarding the persistence of the SARS-CoV-2 virus in the post-acute period. 4 Due to the limited understanding of the clinical course of COVID-19, the possible long-term health impacts have not been elucidated. Our systematic review recognizes the sequelae of COVID-19 patients in the post-acute period to identify their persistent symptoms and clinical outcomes and to provide recommendations as the COVID-19 pandemic continues to spread.
Methods
For this systematic review, a literature search was performed from December 1, 2019, through December 5, 2020, to identify published articles that reported the outcomes of COVID-19 patients after recovery. A systematic literature search was conducted on PubMed, Embase, CINAHL Plus, and Scopus to retrieve articles under the Preferred Reporting Items for Systematic Reviews (PRISMA) checklist criteria. The following search terms were used: “COVID-19 and/or recovered and/or cardiovascular and/or long-term and/or sequelae and/or sub-acute and/or complication.” The search terms were kept broad to encompass all possibilities for applicable studies. There were no restrictions on the language of articles published.
Duplicates were removed using Endnote v9. After eliminating duplicates, 3 investigators (AS, ZS, and MKC) independently reviewed all titles and abstracts. The full texts of articles regarded as potentially eligible for consideration were extracted and screened for further analysis. Some records were also retrieved via cross-references from published papers (Umbrella review). Thereafter, eligible articles were selected for final analysis according to predefined inclusion and exclusion criteria. Disagreements between the authors were resolved through consensus and active discussion.
Only human studies with clearly defined recovery, survival, other outcomes from acute COVID-19 were included. The patient population had mild, moderate, severe, or critical COVID-19 disease and a delineated of persistent symptoms and clinical outcomes in the post-acute period was essential for inclusion. The exclusion criteria consisted of review articles; animal studies; and the absence or unclear reporting of recovery status of COVID-19. As proposed by the National Institutes of Health, we employed a uniform severity classification, ranging from mild, moderate, severe, and critical. 5
1. Mild Illness: Individuals who have any of the various signs and symptoms of COVID-19 (eg, fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste, and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging.
2. Moderate Illness: Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air at sea level.
3. Severe Illness : Individuals who have SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, respiratory frequency >30 breaths/minutes, or lung infiltrates >50%.
4. Critical Illness: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction.
The only exception to the severity scale employed is the paper by Wei 6 which relied on the “Diagnosis and Treatment Protocol of Novel Coronavirus issued by the National Health Commission of the People’s Republic of China.” Data were tabulated into a shared spreadsheet customized for this review. All tables were optimized before commencing data entry using 6 sample articles included in the review to ensure optimal presentation of findings. Articles selected for the final analysis were independently graded by 2 authors (AS and ZS) for quality using the Newcastle–Ottawa Scale (NOS).
No funding was obtained for this study.
Results
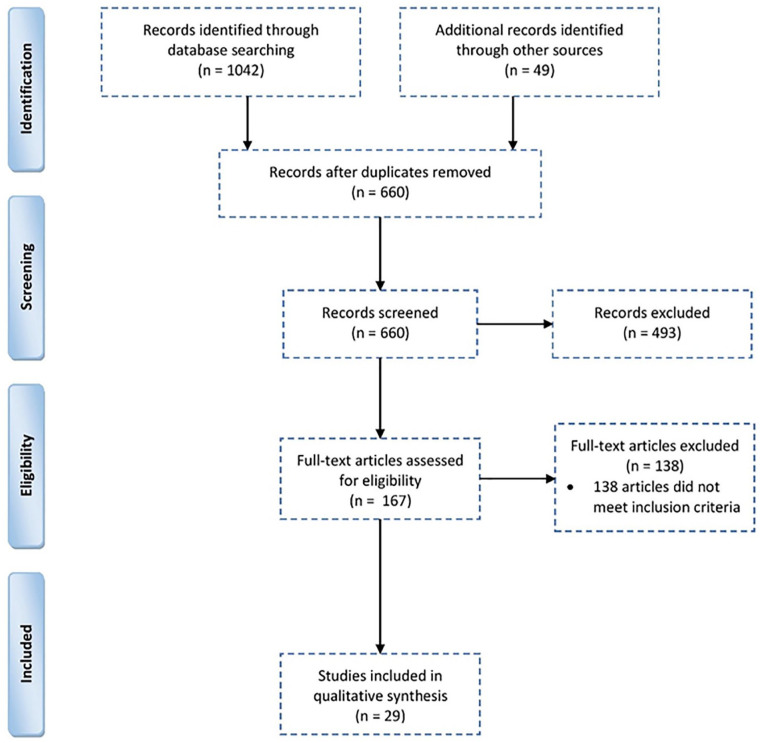
The search yielded 1091 studies. After removing 431 duplicates, 660 articles were reviewed for title and abstract. After the initial screening, only 167 articles met the inclusion criteria and underwent full-text evaluation. Records that consisted of commentaries, editorials, or reviews that did not meet our criteria were removed, generating a final list of 29 articles (Figure 1).
Figure 1.
PRISMA flow diagram.
Characteristics of Included Studies
All studies included in this review were published through December 5, 2020. Twenty studies were case reports, 5 were retrospective cohorts, 2 were prospective observational studies, 1 was a case-control, and 1 was a case series (Table 1). Nine countries were represented with the largest data derived from studies in China (37.9%) and the United States (24.1%). The total number of included participants was 655, with a mean age of 45, comprising of various ethnical backgrounds including Asian and European. A total of 419 patients with mild symptoms and 59 patients with severe symptoms were included. Patient outcomes were notably well due to recovery from infection in 22 of the studies where they recovered or were prescribed medications for underlying thromboembolic conditions including anticoagulants.
Table 1.
Characteristics of included studies.
| No. | Author | Type of study | Sample (n) | Region | Ethnicity | Age (years) | Gender or male (%) | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| 1 | Kosugi et al 7 | Case-control | 145 | Brazil | Not specified | Median: 36 (IQR 30-44) | Male 46.3% | 5.1% Asthma, 5.1% HTN |
| 2 | Brancatella et al 8 | Case report | 1 | Italy | European | 18 | Female | — |
| 3 | Alfano et al 9 | Case report | 1 | Italy | European | 72 | Male | CVD, HTN, DM, CKD, ICH, AF |
| 4 | Garg et al 10 | Case report | 1 | US | Not specified | 78 | Male | HTN, DM, CKD, AF CHA2DS2-VASc of 4, ILD |
| 5 | Hollingshead and Hanrahan 11 | Case report | 1 | US | Not specified | 50 | Male | — |
| 6 | He et al 12 | Case report | 1 | China | Chinese | 39 | Female | ICH |
| 7 | Sardari et al 13 | Case report | 1 | Iran | Iranian | 31 | Male | — |
| 8 | Cavalagli et al 14 | Case report | 1 | Italy | European | 69 | Male | CVD, CeVD |
| 9 | Beckman et al 15 | Case report | 1 | Sweden | European | 51 | Male | — |
| 10 | Liu et al 16 | Case report | 1 | China | Asian | 35 | Male | — |
| 11 | Li et al 17 | Case report | 1 | China | Asian | 41 | Male | — |
| 12 | Fujikura et al 18 | Case report | 1 | US | Not specified | 77 | Female | CeVD, HTN, DM, Breast Cancer |
| 13 | Pohlan et al 19 | Case report | 1 | US | Not specified | 64 | Female | — |
| 14 | Zhou et al 20 | Case report | 1 | China | Not specified | 40 | Male | — |
| 15 | Chen et al 21 | Case report | 1 | China | Not specified | 38 | Male | — |
| 16 | May 22 | Case report | 1 | UK | European | 12 | Male | — |
| 17 | Xia et al 23 | Case report | 2 | China | Not specified | 70, 42 | Male 50% | — |
| 18 | Dou et al 24 | Case report | 1 | China | Chinese | 34 | Male | DM |
| 19 | Abushahin et al 25 | Case report | 1 | USA | American | 49 | Male | DM |
| 20 | Insausti-García et al 26 | Case report | 1 | Spain | European | 40 | Male | — |
| 21 | Gervasio et al 27 | Case report | 2 | Italy | Not specified | 54, 43 | Male 100% | HTN 50%, DM 50% |
| 22 | Takeda 28 | Case series | 6 | Brazil | Not specified | Mean (SD): 46.2 (16) | Male 83.3% | HTN 33.3%, BA 16.7%, ICH 16.7% |
| 23 | Puntmann et al 29 | Prospective Observational cohort study | 100 | Germany | European | Median: 49 (IQR 45-53) | Male 53% | CVD 13%, HTN 22%, DM 18%, BA 21%, COPD 21% |
| 24 | Lu 30 | Prospective study | 99 | China | Chinese | Cases mean (SD): 44.1 (16), controls mean (SD): 45.88 (13.9) | Male cases 56.7%, controls 56.4% | HTN 29.3%, DM 7.1% |
| 25 | Mo et al 31 | Retrospective cohort | 110 | China | Asian | Mean (SD): 49.1 (14) | Male 50% | CVD 2.7%, CeVD 2.7%, HTN 23.6%, DM 8.2%, Ca 0.9%, BA 0.9%, COPD 2.7%, CLD 5.5%, CKD 1.8% |
| 26 | Zhao et al 32 | Retrospective cohort | 55 | China | Asian | Mean (SD): 47.74 (15.49) | Male 58.1% | CVD 3.64%, HTN 10.91%, DM 3.64% |
| 27 | Clark et al 33 | Retrospective cohort | 66 | US | N = 16, Non-Hispanic | Median: 20 (IQR 19-21) | Male 63.6% | — |
| 28 | Rajpal et al 34 | Retrospective cohort | 26 | US | Not specified | Mean (SD): 19.5 (1.5) | Male 57.7% | — |
| 29 | Huang et al 35 | Retrospective, observational | 26 | China | Asian | Median: 38 (IQR 32-45) | Male 38% | HTN 8% |
Abbreviations: AF, atrial fibrillation; BA, bronchial asthma; Ca, Cancer; CeVD, cerebrovascular disease; CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease: CVD, cardiovascular disease; DM, diabetes mellitus; HTN: hypertension; ICH, immuno-compromised host; ILD, interstitial lung disease.
Clinical Course and Pulmonary Complications of COVID-19 Recovered Patients
Table 2 summarizes the clinical course of SARS-CoV-2 infection in pulmonary cases, 8 out of these 14 studies had a male predominance. About 12 studies reported complete clinical remission, 4 of them reported intensive care unit admission (ICU), 9 mentioned hospital admission, and an average of 34 days from the primary infection to the presence of complication was reported across 11 studies. The major complication during hospital stay was a respiratory failure even though it was only reported in only 2 studies. Treatment was provided in detail in 12 studies, encompassing oxygen support, antivirals, antibiotics, corticosteroids, and anticoagulants. Most patients described shortness of breath, upper respiratory tract like symptoms, and dyspnea as main presenting features. RT-PCR tests and Computed Tomography (CT) Scans were the most commonly recommended tests (Table 3).
Table 2.
Clinical Course of Pulmonary COVID-19 Among Recovered Patients.
| No. | Author | Disease severity | Status | Clinically recovered (%) | ICU Admission (%) and length of ICU stay | Length of hospital stay (days) | Complications during stay | Treatments during hospital stay | Months of primary infection | Complication onset after primary infection (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kosugi et al 7 | Mild | Course of COVID 19 (+) patients with acute olfactory alterations | 52.6% | — | Median: 31 (IQR 10.5-39)* | — | — | — | — |
| 2 | Garg et al 10 | — | Presents to ER after 1 month of previous confirmed COVID 19 by nasopharyngeal RT-PCR (+) | 100% | 100%, 5 days | — | AF, deterioration, and respiratory distress | Vancomycin, cefepime, MPD, enoxaparin started with till INR of 3 | — | 30 days |
| 3 | Hollingshead and Hanrahan 11 | Mild | Presents to ER after 1 month of previous COVID 19 infection | 100% | — | — | — | Oxygen therapy | Late April 2020 | 30 days |
| 4 | Beckman et al 15 | Asymptomatic | Suspected COVID 19, not confirmed | 100% | — | 49 | — | Tinzaparin, LMWH | Beginning of April | 7 weeks |
| 5 | Liu et al 16 | Mild | Reoccurrence, change of status after RNA SARS-CoV-2 (−) test | 100% | — | 16 | — | LPV, INF α2b, AHG, MPD | Late January 2020 | 30 days |
| 6 | Li et al 17 | Severe | Reoccurrence of infection | 100% | — | 7 | — | INF-A, TCM, oxygen therapy | Late January 2020 | 25 days |
| 7 | Fujikura et al 18 | Severe | Development of thrombotic state caused by lack of adherence to recommend anticoagulant | 100% | — | 17 | — | Hydroxychloroquine, azithromycin, vancomycin, oxygen, heparin | — | 28 days |
| 8 | Pohlan et al 19 | Mild | Reoccurrence of infection | 100% | 100% | — | — | LWMH, sultamicillin | Beginning of April | 13 days after initial COVID onset |
| 9 | Zhou et al 20 | Severe | Reoccurrence of infection by RT-PCR assay due to insufficient antibody production | 100% | — | 18 | — | BiPAP ventilator, MPD, immunoglobulin | — | 27 days |
| 10 | Dou et al 24 | Severe | Reoccurrence of infection by RT-PCR assay in a diabetic patient | — | — | 39 | — | Arbidol, ribavirin, cefuroxime, chloroquine, AMP, INF-A | Beginning of February | 30 days after discharge |
| 11 | Abushahin et al 25 | Severe | Presents to ER after 21 days of previous COVID 19 infection | 100% | 100%, 8 days | 21 | Respiratory failure | Oxygen, piperacillin/tazobactam, vancomycin, azithromycin, hydroxychloroquine | — | 21 days |
| 12 | Takeda 28 | Mild | Recurrent clinical COVID 19 symptoms in health care workers after RT PCR confirmed cases | 100% | 16.70% | — | Sudden Hypoxemia | Oseltamivir, azithromycin, hydroxychloroquine, prednisone, levofloxacin, piperacillin–tazobactam, MPD, prophylactic anticoagulation, ivermectin | March, Beginning of April | Median (IQR): 56.5 (53-70) |
| 13 | Mo et al 31 | Mild 21.8%, Pneumonia 60.9%, Severe pneumonia 17.3% | Pulmonary testing at time of discharge, impairment of diffusion capacity, restrictive ventilatory defects | 100% | — | Mean (SD): 27 (9) | — | — | — | — |
| 14 | Zhao et al 32 | Mild 7.3%, cases of pneumonia 85.4%, severe pneumonia 7.3% | Pulmonary testing after 3 months of discharge | 100% | — | — | — | Oxygen therapy, MPD, TCM | — | 3 months |
Abbreviations: (+), positive; (−), negative; AHG, arbidol hydrochloride granules; AMP, adenosine monophosphate; BiPAP, bilevel positive airway pressure; INF-A, interferon alpha; LMWH, low molecular weight heparin; LPV, lopinavir; MPD, methylprednisolone; TCM, traditional Chinese medicine.
Follow up of survey data collection.
Table 3.
Pulmonary Complications in COVID-19 Patients.
| No. | Author | Signs | Symptoms | Laboratory findings | Radiological findings | Diagnosis | Days until recovery | Outcomes, discharge indications | Primary care considerations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kosugi et al 7 | — | 86.9% acute anosmia, 13.1% acute hyposmia | — | — | Sudden olfactory dysfunction | 15 (mean) | Recovered, follow up time of 31 days | Patients presented with hyposmia recovered in lesser time than those that presented with acute anosmia |
| 2 | Garg et al 10 | O2 90% on 4 L nasal cannula, irregular pulse with tachycardia | SOB, nonproductive cough | Nasopharyngeal PCR negative, PT 38, INR 6.3, PTT 36 | CXR Bilateral opacities, ECK AF, CT multiple segmental PE on right lung | RF with multiple segmental PE on warfarin | — | Recovered, apixaban on discharge | Thromboprophylaxis duration and scheme changes should be given in a case-by-case basis till there is more information in literature |
| 3 | Hollingshead and Hanrahan 11 | — | SOB | — | CT Diffuse GGO, 10 cm loculated posterior right pneumothorax | Pneumothorax | — | Improvement post chest tube placement | High grade of suspicion based on presentation |
| 4 | Beckman et al 15 | O2 93% | Exercise induced dyspnea (~4 weeks) and dyspnea at rest (~48 hours) | Troponin T 1200 mg/L, BNP 1200, CPR 15 | CT Bilateral segmental PE and GGOs. Two negative PCR | Widespread PE | — | Recovered, Apixaban for 6 months | Pulmonary sequelae can persist after acute infection; the presence of dyspnea could be key for the timely detection of patients at an early stage |
| 5 | Liu et al 16 | — | URTI like symptoms | RNA SARS-CoV-2 (+) on day 1, negative on days 14, 15; Positive again on day 30 | CT GGO and patchy hyperdense areas | Positive SARS-CoV-2 RNA recurs repeatedly in a case recovered from COVID-19: dynamic results from 108 days of follow-up | 95 | Patient recovered | The consequences or involvement of dyslipidemia and liver injury must be discerned in greater depth |
| 6 | Li et al 17 | Fever, O2 90% | Chest pain, cough | RT-PCR (nasal swabs, sputum, stool) all (+); RT-PCR throat swabs were (−), B cells increased, and NK cells decreased | CT: Scattered patches and ground-glass opacity on both lungs, Septal Line* | Reoccurrence of COVID-19 | >35 | Recovered, symptoms improved after 8 days | — |
| 7 | Fujikura et al 18 | Tachycardiac, O2 85% | Severe SOB | At first visit: LDH (804 U/L), D-dimer (>20.00 μg/mL), and CPR (11.7 mg/dL). Readmission: D-dimer 9.34 μg/mL | Large saddle PE. ECHO: McConnell’s sign. Sausage-like mass in RA, moderate TR, PASP 62 mm Hg. Venous duplex Doppler: Acute occlusive thrombosis | Late presentation of saddle PE and thrombus-in-transit straddle the patent foramen | — | Recovered, tachycardia resolved, discharged home with enoxaparin | Hypercoagulable state even after acute infection, may be of concern in follow-up patients |
| 8 | Pohlan et al 19 | Tachycardia, RR 30 | Severe SOB and right leg pain | NT-pro BNP 3936 ng/L, D dimer 18.3 mg/L | CT Massive PE, bilateral infiltrates. US DVT of right leg. ECHO: RV strain | PE and DVT | — | Angiographic aspiration thrombectomy, normalization of functional ECHO parameters | — |
| 9 | Zhou et al 20 | O2 < 80% | Dry cough, dyspnea, and diarrhea | Elevated inflammatory markers, reduced Lymphocytes count, (+) RNA test for SARS-CoV-2 again after 5 days discharge from hospital, lower levels of antibodies against SARS-CoV-2 | CT: Bilateral multiple irregular areas of GGO and consolidation | Recurrent pneumonia and probable immunodeficiency | — | Recovered, SARS-CoV-2 remained negative after 14 days of further isolation at home | Low tiers anti SARS COV 2 doesn’t exclude COVID 19 relapse in all patients |
| 10 | Dou et al 24 | — | No symptoms at recurrence | Increased inflammatory markers, after 2 consecutive negative results with SARS-CoV-2, RT-PCR assay sample was positive | CT Multiple GGO in the bilateral lungs, partially absorbed | Recurrence of positive SARS-CoV-2 (RNA) | 73 | Recovered, following second discharge, tested negative for SARS-CoV-2 on qRT-PCR for 5 weeks | High probability of severe COVID 19 stages when diabetes mellitus is present |
| 11 | Abushahin et al 25 | Hypoxemia, tachypnea | Chest discomfort, dry cough, SOB | COVID-19 was confirmed by RT-PCR testing from nasopharyngeal swab | CXR large left-sided pneumothorax and residual infiltrates | COVID-19, pneumothorax | — | Recovered after pigtail chest insertion | — |
| 12 | Takeda 28 | — | URTI like symptoms, 1 individual presented hypoxemia | Two discharged patients had negative (RT)-PCR tests, but reverted to being positive | Patient 3 presented a (HRCT) with slight scattered and bilateral GGO, more evident in peripheral regions (less than 50% extension | Recurrent clinical symptoms of COVID-19 | — | All improved without hospitalization except one who improved after NIV | Phenoms of reoccurrence, viral reactivation or reinfection must be investigated when recurrent clinical symptoms are present |
| 13 | Mo et al 31 | — | — | Anomalies: DLCO abnormalities 47.2%, TLC pred 25%, FEV1 pred 13.6%, FVC pred 9.1%, FEV1/FVC 4.5%, and small airway function 7.3% | — | Impaired diffusing capacity: 30.4% (mild illness), 42.4% (pneumonia), and 84.2% (severe pneumonia) | — | All patients were discharged | Prevailing of impaired diffusing lung capacity at time of discharge |
| 14 | Zhao et al 32 | — | GI (30.9%), headache (18.2%), fatigue (16.4%), exertional dyspnea (16.4%), and cough and sputum (1.8%) | Patients with abnormal CT had low serum albumin, high serum sodium, elevated D-dimer and high BUN levels. DLCO impairment (n = 9) | Abnormal CT (n = 39): pure GGO (n = 7), interstitial thickening (n = 15), crazy paving (n = 3) | Radiographic and physiological abnormalities in Covid-19 recovered patients | — | All patients were discharged | Higher D-dimer levels on admission could be predictors for impaired DLCO 3 months post discharge |
Pulmonary complications in COVID-19 patients.
Clinical Course and Complications of Cardiovascular COVID-19 Patients
Table 4 summarizes the clinical course and complications of cardiovascular COVID 19 patients. During our search, only 5 studies were classified in the cardiovascular section, with 1 study reported to have a severe COVID-19 population. Chest pain, shortness of breath, and chest pain were noted, with patients in these groups aged 50 years or younger. Complete blood count (CBC) and inflammatory cardiac markers were recurrently elevated and 60% of the cases presented lesions visible through Cardiac Magnetic Resonance (CMR), diagnosed with myocarditis in 3 studies. An approximate period of a month was described from the period of primary infection to the onset of a complication. Treatments during hospital stay were not reported, except for Huang et al 35 which reported antiviral, antibiotic, and oxygen support management during the hospital stay (not shown in the table).
Table 4.
Clinical Course and Complications of Cardiovascular COVID-19 Patients.
| No. | Author | Disease severity | Symptoms | Laboratory findings | Radiological findings | Diagnosis | Complication onset after primary infection (days) | Outcomes, discharge indications | Primary care considerations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sardari et al 13 | — | Dyspnea on exertion, low grade fever | CRP 3.3 mg/L, Troponin T <0.03 ng/mL; RT– PCR negative for SARS-CoV-2 | TTE: Mild left ventricular (LV) dysfunction. CMR: T2-weighted showed edema/inflammation in the mid inferoseptal and inferior wall, subepicardial fibrosis in the mid inferior wall | Active myocarditis | 21 days from previous hospital admission | Started with bisoprolol and lisinopril | Cardiovascular follow up could be important if sufficient data is gathered regarding cardiovascular complications such as myocarditis, even in mild disease |
| 2 | Puntmann et al 29 | Mild | — | — | — | COVID-19 | 14 days | Lower left ventricular ejection fraction, higher left ventricle volumes, higher left ventricle mass, and raised native T1 and T2; CMR revealed cardiac involvement (78%) and ongoing myocardial inflammation (60%) | Cardiovascular involvement after infection could be present independently to severity of disease |
| 3 | Clark et al 27 | Mild 77%, asymptomatic 23% | — | Troponin I < 99% for age (82%); No alterations in BNP or CRP | CMR: Late gadolinium enhancement found in 9% of cases; ECO: Median LVEF of 59% LVEDVi 94 mL/m2, RVEF 52% | 9% patients with myocardial inflammation or fibrosis; 5% met formal criteria for myocarditis | — | All were improved before CMR was done (a median of 52 days after SARS-CoV-2 infection) | Cardiovascular compromise may be missed if there is no opportunity to perform specific cardiac studies (ie, ECG, Ti, and strain echocardiography) |
| 4 | Rajpal et al 34 | Mild 26.9%, Asymptomatic 53.8% | About 2 of 4 athletes with myocardial inflammation had SOB | Normal troponin I | CMR: 15% had myocarditis based on Lake Louise Criteria. About 7.5% had pericardial effusion. Mean (SD) T2 in those with suspected myocarditis was 59(3) ms; Negative findings on electrocardiogram, normal range TTE | 15% with myocarditis | — | Competitive athletes: all patients recovered | CMR could use as a stratification tool for patients at high risk, such as competitive athletes |
| 5 | Huang et al 35 | Moderate 85%, Severe 15% | Chest pain, palpitation, and chest distress | Normal blood work and inflammatory median markers | — | COVID-19 | Median (IQR): 47 (36-58) | 58% with abnormal CMR findings: myocardial edema 54%, LGE 31%; Decreased right ventricle functional parameters in patients with positive conventional CMR findings. Global native T1, T2, and ECV were significantly elevated in patients with positive conventional CMR findings; CMR manifestation included myocardial edema, fibrosis, and impaired right ventricle function | The presence of cardiac tissue involvement at this stage could be early in course, more literature is needed to discern the correct follow up duration |
Complications and Clinical Course of COVID-19 Patients with Specific Characteristics
The complications and clinical course of all included COVID-19 patients with specific characteristics such as endocrine, hematological, ophthalmological, and neurological details are described in Supplemental Table 1. Of all the aforementioned patients, 70% of them were hospitalized, and 30% of them were admitted to the intensive care unit (ICU). The patients were treated mainly with oxygen supportive therapy, steroids, antibacterial, and antivirals. All patients were confirmed to have COVID-19 with a positive PCR test, initial complete blood work was followed, in addition to inflammatory markers and special panels according to each underlying comorbidity. Lung CT scans were followed in 60% of cases; 70% of the patients develop resolution of the acute clinical syndrome, even though a longer follow up study is suggested to observe the full resolution of symptoms or complete basal return. In 4 (28.6%) of the pulmonary studies, pulmonary embolism (PE) was present. In 2 (14.3%) of studies, pneumothorax was noted, and recurrence of infection was observed in 5 (35.7%) of the studies. Of the cardiovascular studies, 4 (80%) required an additional test to detect abnormal functionality (Tables 3 and 4). Relevant important points for primary care were extracted and described for cardiovascular and pulmonary cases (Tables 3 and 4). Despite the heterogeneity of the included articles, the need for guidelines, and the demand for follow up studies to describe duration or clinical course evolution is significant.
Discussion
This systematic review analyzes the cardio-pulmonary sequelae in recovered COVID-19 patients while making considerations for primary care. With the data gathered from numerous COVID-19 clinical studies conducted across the world, researchers have been able to demonstrate that despite apparent complete recovery from COVID-19, patients may suffer from post-recovery sequelae. However, there has been no consensus on the defining characteristics of post-acute COVID-19 syndrome. Commonly reported symptoms in the post-acute COVID-19 phase are fatigue, dyspnea, joint pain, and chest pain. 36 Cardiopulmonary consequences have been reported in the recovery phases, and have considerable implications for long-term health consequences.29,37 A survey conducted by the Centers for Disease Control and Prevention (CDC) identified the contribution of 3 or more underlying medical conditions and age greater than 50 years as important predictors of not achieving baseline health until 3 weeks after testing COVID-19 RT-PCR positive. Nevertheless, 20% of young individuals have not returned to baseline health after a median time of 16 days since testing positive for COVID-19. 38 A critical discussion of post-acute COVID-19 syndrome is imperative as sequelae are observed among patients along the infection severity scale (ie, mild to critical).
Pulmonary
Wu et al 39 report ARDS in 40% of patients infected with SARS-CoV-2 infection, with over 20% in the older age groups. Burnham et al 40 find that persistent inflammation of alveolar epithelium and hypercytokinemia in SARS-CoV-2 infection leads to fibroproliferative changes which include fibroblast accumulation and deposition of extracellular matrix collagen in the lung parenchyma. The resulting disruption of the balance between profibrotic and antifibrotic factors leads to pulmonary fibrosis which is observed in a subset of patients as protracted sequelae to ARDS. 40 Pulmonary fibrosis is characterized by the presence of an unusual interstitial pneumonia-like fibrotic abnormality on high-resolution computed tomography (HRCT) and this is linked to a more increased incidence of rapid progression of the disease.41-44
A prospective study was conducted by Liu et al including 149 patients who were discharged from various hospitals in Wuhan, China following recovery from COVID-19 pneumonia. Patients were followed up during the first, second, and third-weeks post-discharge. The results showed that patients younger than 44 years had a significantly higher percentage of complete radiological resolution than patients who were older than 44 years. Out of the various lesions observed, the most predominant were ground-glass opacity in 83.9% of patients followed by a fibrous stripe in 54.4% and only 22.1% showed thickening of the adjacent pleura. The number of patients testing positive for each lesion decreased gradually over 3 weeks and the radiological resolution was more evident in patients with ground-glass opacity and fibrous stripe in the first and third weeks, respectively. Most residual lung abnormalities were absorbed throughout recovery, suggesting the importance of radiological assessment 2 weeks post-discharge. 30 The importance of determining the timing and standpoints of radiological resolution in COVID-19 patients can provide further insight into deciding which patient groups need long term monitoring.
Mo et al 31 observed that COVID-19 survivors who had been discharged from the hospital demonstrated pulmonary function abnormalities, the most common among those being diffusion capacity impairment, followed by restrictive ventilatory defects. Zhao et al 32 demonstrated both physiologic and radiographic abnormalities in a significant number of COVID-19 patients in discharged COVID 19 patients after a 3-month follow-up period. In the INBUILD trial, Kolb and Vašáková 45 note increased rates of forced vital capacity (FVC) decline and mortality over the 52 weeks in patients with usual interstitial pneumonia (UIP) like lesions when compared to those patients with other patterns of fibrosis. Several factors are involved in the extent to which pulmonary complications present in clinically recovered patients; Salehi et al 46 document the most significant being patients’ age, presence of comorbidities, and type of medications administered during COVID-19 management. Another point of contention is those patients with long-term sequelae who did not have either ARDS or PE during acute infection. While there is a paucity of pulmonary sequelae among patients who did not have either ARDS or PE during the acute infection, Han et al 47 conducted a 6-month follow-up of chest CT findings after severe COVID-19 pneumonia. Patients without ARDS were less likely to be associated with post-COVID radiological and functional changes. 47 There is a lack of detailed knowledge on the pathophysiology of the disease, taking into consideration the available studies, following up on discharged COVID-19 patients, assessing them using radiological and pulmonary tests, and managing potential issues are all necessary precautionary measures.
Cardiovascular
Myocardial injury is defined by laboratory testing, namely by an increased troponin level, which has been documented as a severe COVID-19 sequela. 48 A median of 71 days post COVID-19 diagnosis, cardiac involvement was reported by Puntmann et al 29 in 78% patients, and 60% revealed myocardial inflammation on cardiac magnetic resonance imaging in a Germany study. Long et al 49 reported common cardiovascular sequelae in COVID-19 as myocarditis, thromboembolic disease, and arrhythmias. The alterations in normal cardiovascular physiology, coagulation state, and basal metabolism observed, including those with RNA-sequencing analysis, reveal increased levels of angiotensin-converting enzyme-2 (ACE-2) in cardio-myocytes thereby increasing the possibility of direct myocardial tissue infection in high-risk patients.47,48 As per Siripanthong et al, 50 the ACE-2 receptor on the myocardium may be used by SARS-CoV-2 to gain entry into cells, triggering the release of massive amounts of pro-inflammatory cytokines, immune hyper-activation, and myocardial tissue damage. Cardiac tropism, an impaired cardio-protective function of ACE2 downregulated by SARS-CoV-2, and elevated proinflammatory cytokine release are potential pathological mechanisms.51-53
Sardari et al 13 write that myocarditis may occur due to the residual myocardial inflammation in COVID-19. Evidence of SARS-CoV-2 mRNA in the myocardium has been observed by Tavazzi et al 54 and Wenzel et al 55 on autopsy and endomyocardial biopsies, suggestive of myocarditis. Underlying cardiovascular disease (CVD) may predispose patients to SARS-CoV-2 myocarditis. 55 Further, cardiovascular complications caused due to COVID-19 or prevalent before infection must be addressed. A meta-analysis finds that the pooled incidence of acute cardiovascular complications during the COVID-19 pandemic is 1.4%. However individuals with COVID-19 who presented with concomitant stroke were more likely to have pre-existing cardiovascular comorbidities not limited to arrhythmias, myocarditis, and thromboembolism, linked to severe COVID-19 infection. The patterns of large vessel occlusions and multi-territory infracts suggests that thromboembolism and/or cerebral thrombosis may potentially be causative pathways for COVID-19 disease. 56 The mortality and morbidity due to cardiovascular complications in COVID-19 patients are higher when there is evidence of myocardial involvement, as implicated by Mitrani et al 57 In the setting of myocarditis, myocyte lysis releases proteins with abnormal epitopes and with a similar structure to those present in the virus. Błyszczuk 58 and Gangaplara et al 59 state that these can be recognized by major histocompatibility complex and seemingly add autoimmune components to cardiac injury. Chapman et al 60 presume that these mechanisms of tissue damage cause long-term complications in COVID-19 patients. Varga et al 61 form an association between endothelitis and multi-organ failure with evidencing direct viral infection of the endothelium. Additionally, possible cytopathic effects and increased myocardial cell death after inducible SARS-CoV-2 on induced pluripotent stem cells (iPSC) have also been identified by Bose and McCarthy. 62
Multiple studies have shown an increased rate of arrhythmias in patients recovering from viral myocarditis. A study by Blagova et al 63 revealed that among 19 cases of “idiopathic” arrhythmias, 78.9% were identified as immune-mediated inflammatory after the endomyocardial biopsy was performed, finding cardiotropic viruses as etiologic agents. Since the beginning of the COVID-19 pandemic, thrombotic complications have been reported in critically ill patients. The term MicroCLOTS (microvascular COVID-19 Lung Vessels Obstructive Thromboinflammatory syndrome) due to microvascular pulmonary thrombosis is currently being used in literature to describe manifestations of the disease. The high incidence of micro thrombosis in the lungs was noted in 80% autopsies of COVID-19 cases by Dolhnikoff et al 64 Klok et al 65 report the incidence of venous thromboembolism as 31%, leading to pulmonary embolism (80%) and arterial thrombosis (3.7%). Further studies are needed to specifically describe cardiovascular complications post COVID-19, especially screening for the incidence of heart failure. Close monitoring of patients is pertinent, even in cases with no signs of cardiovascular impairment.
Considerations for Primary Care
Although the impact of long-term COVID-19 sequelae is still unclear, a large number of patients will likely experience persistent symptoms, especially in those geographical regions where large outbreaks occurred. Various strategies are being evaluated not only to treat COVID-19 patients but also to prevent as many detrimental complications as possible. Greenhalgh et al describe post-acute COVID-19 as “Long COVID,” which is a multisystem disease. 66 Notably, cardio-pulmonary complications may occur after mild infections as well, requiring attention by primary care providers. 66 For a general practitioner, it is essential to note improvements over 3 weeks and subsequent weeks/months. The symptoms may manifest in any bodily organ, however, the most common ones include cough (dry versus productive), fever, fatigue during long COVID. Uncommon symptomatology worth considering by the primary care practitioner includes mood swings, palpitations, anxiety, hypotension, and/or skin rashes (ie, maculopapular). The nonspecific (ie, lung burn, brain fog) versus the specific complications (ie, stroke, pulmonary embolism, myocarditis, heart failure) must be distinguished clinically upon a visit to a primary care practitioner and specific presentations recommended to specialist centers. A clinical insight for long COVID is the fact that the presentation during acute COVID does not directly relate to the severity of findings 6 months later (ie, if a patient had severe pneumonia on the acute onset, the sequelae may be less severe as compared to those who had less severe acute complications). Based on the limited evidence in published literature so far, patients with long COVID may have no access to specialist care. To improve patient outcomes, it is essential to adopt a community-based, interprofessional rehabilitation pattern of care by promoting remote technologies, telehealth appointments with a primary care physician, and adopting otherwise locally relevant solutions.
Recommendations
The implementation of a multi-disciplinary approach to identify, and prevent the impact of long-term health outcomes of COVID-19 across different organs, including cardiopulmonary, as well as the well-being of recovered patient groups is necessary to elucidate the potential classification of complications in post-acute COVID-19. 67 An example of how rehabilitation can successfully be applied to post-COVID-19 patient management is offered by Shan et al, 68 who describes the case of a woman at about 80 years of age gradually returning to her premorbid independent condition after severe COVID-19 complicated by acute respiratory distress syndrome (ARDS) following 10 days of post-acute inpatient pulmonary rehabilitation. Interestingly, current knowledge on the long-term impact of the SARS-CoV-2 infection to an important degree comes from previous epidemics, caused by severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS). However, deficits associated with the current pandemic are expected to be more critical, given the magnitude of the pandemic.
A practical approach is to follow up on all hospitalized patients with chest radiography and oxygen saturation measurements, as recommended by the British Thoracic Society. 69 However, the American Thoracic Society/European Respiratory Society does not make any suggestion in favor of or against routine pulmonary function tests (PFTs), chest CT scans, transthoracic echocardiography (TTE), and cardiopulmonary exercise testing (CPET) within 30 to 60 days. 70 Preliminary evidence suggests the development of respiratory sequelae as early as 2 weeks after discharge, especially among patients with elevated inflammatory markers and D-dimer levels.31,32 Data suggests the frequent occurrence of pulmonary fibrosis, arrhythmias, and cardiomyopathies among survivors over 2 months after recovery. 29 All patients requiring further investigation may be referred to as pulmonologists and cardiologists for further evaluation of pulmonary (PFTs and chest CT scans) and cardiac (TTE and CPET) assessment within 2 to 3 months of recovery. It is also necessary to establish a baseline time to follow recovery. Admittedly, resource-limited settings may not be able to conduct such tests. Consequently, evidence suggests routine testing of pulmonary and cardiac sequelae in COVID-19 is beneficial within 30 to 90 days of discharge in all previously hospitalized patients.
Limitations
In the United States, 3 different phases of the pandemic have been documented. At the onset, in April, 2020 there was limited testing. During the second phase in July, 2020, social distancing among other factors contributed to infectivity. However, testing was widely available to most of the public. The third phase at the onset of 2021 was potentially connected with seasonal respiratory viruses. These are uncontrollable limitations in post-infection pulmonary-cardiovascular tests that are presented with external factors contributing to varied test outcomes. The other limitation of our study relates to its retrospective nature. Many of the included studies were case reports and, therefore, findings may not be generalizable and the causality within those may not be fully proven. As the majority of the studies were from China, some of the presentations could be influenced by the ethnicity-related characteristics of the region.
Conclusion
With limited insight on the sequelae of COVID-19 infection, a holistic approach is required that incorporates primary care. Complications are frequently observed in patients recovering from COVID-19, ranging from mild to life-threatening conditions. Patients who presented with ARDS, PE, arrhythmia, myocarditis, or other cardiopulmonary complications during the acute phase necessitate multi-speciality care and follow-up for long-COVID. It is currently unclear whether ARDS or other complications due to COVID-19 or other causative factors will have different long-term outcomes. Follow-up of clinically recovered patients with cardiopulmonary imaging studies could be crucial for the evaluation of adverse events caused by SARS-CoV-2 infection. Primary health care forms a critical foundation for gatekeeping timely and effective support during the COVID-19 pandemic. Through primary care services, it is possible to provide rehabilitation and support to recovering patients in light of a greater volume of patients requiring rehabilitation in the post-acute COVID-19 infectious phase. Physicians across all healthcare levels should be alert on and monitor patients for possible adverse events after recovery from SARS-CoV-2 infection. International societies ought to develop guidelines for patients recovering from COVID-19 and recommend the routine monitoring of these patients to establish cardiopulmonary testing, detect early complications, and provide timely treatment. Importantly, observational studies and clinical trials reporting data on acute and post-acute infectious phases will help elucidate the health outcomes, attributable to COVID-19 and underlying medical conditions.
Supplemental Material
Supplemental material, sj-pdf-1-jpc-10.1177_21501327211023726 for Cardio-Pulmonary Sequelae in Recovered COVID-19 Patients: Considerations for Primary Care by Zouina Sarfraz, Azza Sarfraz, Alanna Barrios, Radhika Garimella, Asimina Dominari, Manish KC, Krunal Pandav, Juan C. Pantoja, Varadha Retnakumar and Ivan Cherrez-Ojeda in Journal of Primary Care & Community Health
Acknowledgments
All authors acknowledge Jack Michel MD (Founder, Larkin Health System, South Miami, FL, USA) for his active support during the course of drafting this paper. All authors are indebted to Larkin Community Hospital, South Miami, FL, USA for being a driving force in promoting COVID-19 scholarly activities.
Appendix
List of Abbreviations
1C first case
2C second case
ABGq arterial blood gases
AD axial diffusivity
ALT aminotransferase
AHG arbidol hydrochloride granules
BPM breaths per minute
CMR cardiac magnetic resonance
CP convalescent plasma
CRP C-reactive protein
CT computed tomography
DLCO diffusing capacity of the lung for carbon monoxide
DTI diffusion tensor imaging
DVT deep vein thrombosis
ECHO echocardiography
ECMO extracorporeal membrane oxygenation
ELISA enzyme-linked immunoassay
ESR erythrocyte sedimentation rate
FA fractional anisotropy
FEV1 FORCED expiratory volume in 1 s
FO foramen ovale
FT3 free triiodothyronine
FT4 qfree thyroxine
FVC forced vital capacity
GGOs ground glass opacity
GMV bilateral gray matter volumes
Hb hemoglobin
HRCT high-resolution chest tomography
Hs-CRP high-sensitivity C-reactive protein
Hs-cTnI High-sensitivity cardiac troponin I
IL6 inerfertin-6
INR international normalized ratio
INF interferon
IV Ig intravenous immunoglobulin
LDH lactate dehydrogenase
LMWH low molecular weight heparin
LPV lopinavir
LVEDVi LV end diastolic volume index
LVEF left ventricular ejection fraction
LYMPH lymphocytes
MCH mean corpuscular hemoglobin
MCV mean corpuscular volume
MD mean diffusivity
ms milliseconds
MPD methylprednisolone
MV mechanical ventilation
NIV non-invasive ventilation
NT-pro BNP amino-terminal pro-brain natriuretic peptide
SAT subacute thyroiditis
OCT optical coherence tomography
PASP pulmonary artery systolic pressure
PCO2 carbon dioxide partial pressure
PE pulmonary embolism
PEEP positive end-expiratory pressure
PO2 partial pressure oxygen (Po2)
PT prothrombin time
PTE pulmonary thromboembolism
PTTa activated plasma thrombin time
RA right atrium
RD radial diffusivity
RR respiratory rate
RT-PCR reverse transcriptase-polymerase chain reaction
RV right ventricular
RVEF right ventricular ejection fraction
SOB shortness of breath
Tg thyroglobulin
TgAb thyroglobulin antibodies
TLC total lung capacity
TTE transthoracic echocardiogram
TTE transthoracic echocardiography
US ultrasonography
WBC white cell blood count
Footnotes
Author Contributions: The authors contributed equally to all aspects of the article. AS and ZS are co-guarantors of the paper.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Zouina Sarfraz  https://orcid.org/0000-0002-5132-7455
https://orcid.org/0000-0002-5132-7455
Azza Sarfraz  https://orcid.org/0000-0001-8206-5745
https://orcid.org/0000-0001-8206-5745
Alanna Barrios  https://orcid.org/0000-0001-8332-8682
https://orcid.org/0000-0001-8332-8682
Radhika Garimella  https://orcid.org/0000-0002-0726-6923
https://orcid.org/0000-0002-0726-6923
Asimina Dominari  https://orcid.org/0000-0002-4023-9767
https://orcid.org/0000-0002-4023-9767
Manish KC  https://orcid.org/0000-0003-1693-6068
https://orcid.org/0000-0003-1693-6068
Krunal Pandav  https://orcid.org/0000-0002-5451-7115
https://orcid.org/0000-0002-5451-7115
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71-76. doi: 10.1016/j.ijsu.2020.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185-193. doi: 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roy S. COVID-19 reinfection: myth or truth? SN Compr Clin Med. Published online May 29, 2020. doi: 10.1007/s42399-020-00335-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Clinical management of COVID-19 interim guidance. Internet Publication. https://apps.who.int/iris/handle/10665/332196 [Google Scholar]
- 6. Wei P-F. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial version 7). Chin Med J (Engl). 2020;133:1087-1095. doi: 10.1097/CM9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kosugi EM, Lavinsky J, Romano FR, et al. Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol. 2020;86:490-496. doi: 10.1016/j.bjorl.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105:2367-2370. doi: 10.1210/clinem/dgaa276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alfano G, Perrone R, Fontana F, et al. Long-term effects of COVID-19 in a patient on maintenance dialysis. Hemodial Int. 2020;24:E50-E54. doi: 10.1111/hdi.12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg A, Goyal S, Patel P. A case of COVID-19 infection with delayed thromboembolic complication on warfarin. Cureus. 2020;12:e8847. doi: 10.7759/cureus.8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hollingshead C, Hanrahan J. Spontaneous pneumothorax following COVID-19 pneumonia. IDCases. 2020;21:e00868. doi: 10.1016/j.idcr.2020.e00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He F, Luo Q, Lei M, et al. Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin Rheumatol. 2020;39:2803-2810. doi: 10.1007/s10067-020-05230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sardari A, Tabarsi P, Borhany H, Mohiaddin R, Houshmand G. (2021). Myocarditis detected after COVID-19 recovery. Eur Heart J Cardiovasc Imaging. 22(1):131-132. doi:10.1093/ehjci/jeaa166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cavalagli A, Peiti G, Conti C, Penati R, Vavassori F, Taveggia G. Cranial nerves impairment in post-acute oropharyngeal dysphagia after COVID-19: a case report. Eur J Phys Rehabil Med. 2020;56:853-857. doi: 10.23736/S1973-9087.20.06452-7 [DOI] [PubMed] [Google Scholar]
- 15. Beckman M, Nyrén S, Kistner A. A case-report of widespread pulmonary embolism in a middle-aged male seven weeks after asymptomatic suspected COVID 19 infection. Thromb J. 2020;18:19. doi: 10.1186/s12959-020-00235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu F, Cai ZB, Huang JS, et al. Positive SARS-CoV-2 RNA recurs repeatedly in a case recovered from COVID-19: dynamic results from 108 days of follow-up. Pathog Dis. 2020;78:ftaa031. doi: 10.1093/femspd/ftaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li XJ, Zhang ZW, Zong ZY. A case of a readmitted patient who recovered from COVID-19 in Chengdu, China. Crit Care. 2020;24:152. doi: 10.1186/s13054-020-02877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujikura K, Fontes JD, Taub CC. Saddle pulmonary embolism and thrombus-in-transit straddling the patent foramen ovale 28 days after COVID symptom onset. Echocardiography. 2020;37:1296-1299. doi: 10.1111/echo.14796 [DOI] [PubMed] [Google Scholar]
- 19. Pohlan J, Kamel SN, Torsello GF, et al. Successful aspiration thrombectomy in a patient with submassive, intermediate-risk pulmonary embolism following COVID-19 pneumonia. Radiol Case Rep. 2020;15:1764-1768. doi: 10.1016/j.radcr.2020.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou X, Zhou J, Zhao J. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect Dis. 2020;20:500. doi: 10.1186/s12879-020-05231-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen W, Yang B, Li Z, Wang P, Chen Y, Zhou H. Sudden severe thrombocytopenia in a patient in the recovery stage of COVID-19. Lancet Haematol. 2020;7:e624. doi: 10.1016/s2352-3026(20)30175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi: 10.1136/bcr-2020-237056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia J, Feng Y, Li M, et al. Increased physiological dead space in mechanically ventilated COVID-19 patients recovering from severe acute respiratory distress syndrome: a case report. BMC Infect Dis. 2020;20:637. doi: 10.1186/s12879-020-05360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dou C, Xie X, Peng Z, et al. A case presentation for positive SARS-CoV-2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID-19. Diabetes Res Clin Pract. 2020;166:108300. doi: 10.1016/j.diabres.2020.108300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abushahin A, Degliuomini J, Aronow WS, Newman T. A case of spontaneous pneumothorax 21 days after diagnosis of coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep. 2020;21:e925787-1–e925787-4. doi: 10.12659/ajcr.925787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, López Vázquez Á, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: inflammation and hypercoagulable state. Eur J Ophthalmol. Published online July 30, 2020. doi: 10.1177/1120672120947591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gervasio CF, Averono G, Robiolio L, et al. Tracheal stenosis after tracheostomy for mechanical ventilation in COVID-19 pneumonia – a report of 2 cases from Northern Italy. Am J Case Rep. 2020;21:1-5. doi: 10.12659/ajcr.926731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandes Valente Takeda C, Moura de Almeida M, Gonçalves de Aguiar Gomes R, et al. Case report: recurrent clinical symptoms of COVID-19 in healthcare professionals: a series of cases from Brazil. Am J Trop Med Hyg. 2020;103:1993-1996. doi: 10.4269/ajtmh.20-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu D, Zhang W, Pan F, et al. The pulmonary sequalae in discharged patients with COVID-19: A short-term observational study. Respir Res. 2020;21, 125. doi: 10.1186/s12931-020-01385-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. EClinicalMedicine. 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark DE, Parikh A, Dendy JM, et al. COVID-19 Myocardial Pathology Evaluated Through scrEening Cardiac Magnetic Resonance (COMPETE CMR). Preprint. Posted Online Sep 2, 2020. medRxiv Prepr Serv Heal Sci. 2020. doi: 10.1101/2020.08.31.20185140 [DOI] [Google Scholar]
- 34. Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021; 6(1):116-118. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330-2339. doi: 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. doi: 10.1136/bmj.m3001 [DOI] [PubMed] [Google Scholar]
- 38. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993-998. doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burnham EL, Janssen WJ, Riches DWH, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276-285. doi: 10.1183/09031936.00196412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh SLF, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216-222. doi: 10.1136/thoraxjnl-2013-203843 [DOI] [PubMed] [Google Scholar]
- 42. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2017;69:542-549. doi: 10.1002/art.39971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salisbury ML, Gu T, Murray S, et al. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest. 2019;155:699-711. doi: 10.1016/j.chest.2018.08.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adegunsoye A, Oldham JM, Bellam SK, et al. Computed tomography honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann Am Thorac Soc. 2019;16:580-588. doi: 10.1513/annalsats.201807-443oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kolb M, Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res. 2019;20:57. doi: 10.1186/s12931-019-1022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salehi S, Reddy S, Gholamrezanezhad A. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J Thorac Imaging. 2020;35:W87-W89. doi: 10.1097/RTI.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 47. Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177-E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723. doi: 10.1001/jama.2020.19719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504-1507. doi: 10.1016/j.ajem.2020.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463-1471. doi: 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618-625. doi: 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;42:68-78. doi: 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 53. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407. doi: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911-915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wenzel P, Kopp S, Göbel S, et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661-1663. doi: 10.1093/cvr/cvaa160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137-149. doi: 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984-1990. doi: 10.1016/j.hrthm.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Błyszczuk P. Myocarditis in humans and in experimental animal models. Front Cardiovasc Med. 2019;6:64. doi: 10.3389/fcvm.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gangaplara A, Massilamany C, Brown DM, et al. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-α-reactive CD4 T cells in A/J mice. Clin Immunol. 2012;144:237-249. doi: 10.1016/j.clim.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 60. Chapman AR, Shah ASV, Lee KK, et al. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236-1245. doi: 10.1161/circulationaha.117.031806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. doi: 10.1016/s0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bose RJC, McCarthy JR. Direct SARS-CoV-2 infection of the heart potentiates the cardiovascular sequelae of COVID-19. Drug Discov Today. 2020;25:1559-1560. doi: 10.1016/j.drudis.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Blagova OV, Nedostup AV, Kogan EA, et al. Myocardial biopsy in “idiopathic” atrial fibrillation and other arrhythmias: nosological diagnosis, clinical and morphological parallels, and treatment. J Atr Fibrillation. 2017;9(5):1414. doi: 10.4022/jafib.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dolhnikoff M, Duarte-Neto AN, Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517-1519. doi: 10.1111/jth.14844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klok FA, Kruip MJ, van der Meer NJ, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148-150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 67. Lew HL, Lew HL, Oh-Park M, et al. The war on COVID-19 pandemic: role of rehabilitation professionals and hospitals. Am J Phys Med Rehabil. 2020;99:571-572. doi: 10.1097/PHM.0000000000001460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shan MX, Tran YM, Vu KT, Eapen BC. Postacute inpatient rehabilitation for COVID-19. BMJ Case Rep. 2020;13:e237406. doi: 10.1136/bcr-2020-237406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. British Thoracic Society. British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. British Thoracic Society; 2020. [Google Scholar]
- 70. Bai C, Chotirmall SH, Rello J, et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur Respir Rev. 2020;29:200287. doi: 10.1183/16000617.0287-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jpc-10.1177_21501327211023726 for Cardio-Pulmonary Sequelae in Recovered COVID-19 Patients: Considerations for Primary Care by Zouina Sarfraz, Azza Sarfraz, Alanna Barrios, Radhika Garimella, Asimina Dominari, Manish KC, Krunal Pandav, Juan C. Pantoja, Varadha Retnakumar and Ivan Cherrez-Ojeda in Journal of Primary Care & Community Health