Abstract
Objectives:
Non-pharmaceutical interventions (e.g. quarantine and isolation) are used to mitigate and control viral infectious disease, but their effectiveness has not been well studied. For COVID-19, disease control efforts will rely on non-pharmaceutical interventions until pharmaceutical interventions become widely available, while non-pharmaceutical interventions will be of continued importance thereafter.
Methods:
This rapid evidence-based review provides both qualitative and quantitative analyses of the effectiveness of social distancing non-pharmaceutical interventions on disease outcomes. Literature was retrieved from MEDLINE, Google Scholar, and pre-print databases (BioRxiv.org, MedRxiv.org, and Wellcome Open Research).
Results:
Twenty-eight studies met inclusion criteria (n = 28). Early, sustained, and combined application of various non-pharmaceutical interventions could mitigate and control primary outbreaks and prevent more severe secondary or tertiary outbreaks. The strategic use of non-pharmaceutical interventions decreased incidence, transmission, and/or mortality across all interventions examined. The pooled attack rates for no non-pharmaceutical intervention, single non-pharmaceutical interventions, and multiple non-pharmaceutical interventions were 42% (95% confidence interval = 30% – 55%), 29% (95% confidence interval = 23% – 36%), and 22% (95% confidence interval = 16% – 29%), respectively.
Conclusion:
Implementation of multiple non-pharmaceutical interventions at key decision points for public health could effectively facilitate disease mitigation and suppression until pharmaceutical interventions become available. Dynamics around R0 values, the susceptibility of certain high-risk patient groups to infection, and the probability of asymptomatic cases spreading disease should be considered.
Keywords: Quarantine, patient isolation, pandemics, COVID-19, influenza pandemic 1918–1919, non-pharmaceutical interventions, incidence, transmission, mortality
Introduction
In the absence of a vaccine, mitigation of the coronavirus disease 2019 (COVID-19) pandemic requires non-pharmaceutical interventions (NPIs), such as social distancing, increased hand hygiene, mask wearing, and surface decontamination, which have been implemented across the globe. 1
However, the effectiveness of NPIs is difficult to measure, especially for a rapidly evolving disease like COVID-19. To help understand the implications of using NPIs, singly or in combination with other NPIs or pharmaceutical interventions (PIs), epidemiologists are relying on evidence from retrospective studies of past outbreaks to manage the current COVID-19 pandemic. With emerging but limited current evidence around COVID-19, the effectiveness of NPIs to mitigate and control other viral diseases from retrospective studies may offer valuable information to improve pandemic preparedness and response.
Social distancing NPIs have historically decreased the spread of viral infectious diseases. These interventions lower the likelihood that a healthy individual will come in contact with an infected person, help limit disease spread, and promote suppression of new cases, such as in the 1918 influenza A pandemic,2,3 epidemics related to coronavirus (e.g. 2003 severe acute respiratory syndrome coronavirus (SARS-CoV)), 4 and various others. Studies have been recently conducted to show the observed impact of NPIs on a variety of pathogens, including influenza and norovirus, during the COVID-19 pandemic.5,6
The objective of this study is to identify and synthesize evidence regarding the effectiveness of social distancing NPIs on respiratory infectious viral disease outcomes. This rapid evidence-based review and meta-analysis focuses on studies describing the implementation and assessment of social distancing-related NPIs, including general distancing strategies, quarantine, and/or isolation using single or multiple interventions during respiratory viral epidemics or pandemics. The knowledge gained from this review could help public health policy makers, clinicians, researchers, and so on to strategically plan and implement these interventions in order to limit the spread and mitigation of COVID-19 or other future respiratory viral pandemics.
Methods
Study design
A rapid evidence-based review7,8 was conducted to identify studies examining the effectiveness of single or multiple social distancing NPIs on infectious viral disease (pandemic or epidemic) outcomes (e.g. incidence, transmission, and mortality) with comparisons to examine the effects of the intervention, its timing, and/or combination(s) of interventions. Included studies with the following social distancing NPIs were clustered into three main groups: (1) General: voluntary or mandatory steps taken to reduce face-to-face interactions among people in the community; (2) Quarantine: imposed separation or restriction of movement of persons who are exposed, who may or may not be infected but are not ill, and may become infectious to others; and (3) Isolation: the separation and confinement of individuals known or suspected to be infectious or ill with a contagious disease in order to prevent them from transmitting the disease to others. Primary or secondary studies published in English, conducted in humans, and with an abstract, were considered. Supplementary Tables I and II present the study methodology details.
Search strategy
A search of published literature in MEDLINE via PubMed, Google Scholar, and pre-print databases (BioRxiv.org, MedRxiv.org, and Wellcome Open Research) was conducted to identify references published or available online through 27 March 2020 (Supplementary Table I). The search strategy queried the terms (‘non pharmaceutical intervention*’ or ‘non-pharmaceutical intervention*’) or (‘social distancing’) in titles.
Screening, data extraction, qualitative synthesis, and quality assessment
Two-reviewer screening of both titles/abstracts and full-texts was performed independently against a priori inclusion criteria (Supplementary Table II), and conflicts were resolved with adjudication by a third reviewer. Inter-rater reliability (IRR) was determined by the kappa statistic. 9 Results were tracked in Microsoft Excel and EndNote®. Data were abstracted into standardized forms for synthesis and thematic analysis. Systematic reviews with the same inclusion criteria were included and evidence was abstracted from the primary study for qualitative synthesis. Study quality was assessed by dual review using the Oxford levels of evidence (Table 1). 10
Table 1.
Summary of study characteristics.
| Reference number | Reference, year | Pathogen | Geography | Study design; Level of evidence a | Funding source(s) b | |
|---|---|---|---|---|---|---|
| Location | Continent | |||||
| 1 | Ali et al., 2013 11 | Influenza A (H1N1 subtype) | India | Asia | Modeling study; 2b | Academia; research council |
| 2 | Andradóttir et al., 2011 12 | Influenza A (H1N1 subtype) | North America | North America | Modeling study; 2b | Industry |
| 3 | Bolton et al., 2012 13 | Influenza A (H1N1 subtype) | Mongolia | Asia | Modeling study; 2b | Government |
| 4 | Bootsma et al., 2007 14 | Influenza A (H1N1 subtype) | The United States | North America | Modeling study; 2b | Government; research council |
| 5 | Caley et al., 2008 2 | Influenza A (H1N1 subtype) | Australia | Australia | Modeling study; 2b | Government; research council |
| 6 | Cowling et al., 2020 15 | COVID-19 | Hong Kong | Asia | Observational study; 2b | Government |
| 7 | Davey et al., 2008 3 | Influenza A (H1N1 subtype) | The United States | North America | Modeling study; 2b | Government |
| 8 | Esquivel-Gómez et al., 2018 16 | Non-specific | Mexico | North America | Modeling study; 2b | Government; research council |
| 9 | Ferguson et al., 2005 17 | Influenza A (H5N1 subtype) | Thailand | Asia | Modeling study; 2b | Government |
| 10 | Ferguson et al., 2020 1 | COVID-19 | The United Kingdom; The United States | Europe; North America | Modeling study; 2b | Academia; government; research council |
| 11 | Fong et al., 2020 18 | Various influenza strains and subtypes; seasonal and future | Multiple countries | Global | Systematic review; 2a | Academia; government; research council |
| 12 | Glass et al., 2006 19 | Influenza A (H2N2 subtype) | The United States | North America | Modeling study; 2b | Research council |
| 13 | Halloran et al., 2008 4 | Future influenza pandemic | The United States | North America | Modeling study; 2b | Government |
| 14 | Hatchett et al., 2007 20 | Influenza A (H1N1 subtype) | The United States | North America | Case-series; 4 | Government |
| 15 | He et al., 2013 21 | Influenza A (H1N1 subtype) | The United Kingdom | Europe | Modeling study; 2b | Government |
| 16 | Hellewell et al., 2020 22 | COVID-19; SARS-coronavirus | Non-specific | NA | Modeling study; 2b | Academia |
| 17 | Hens et al., 2009 23 | Future influenza pandemic | Europe | Europe | Modeling study; 2b | Government |
| 18 | Herrera-Valdez et al., 2011 24 | Influenza A (H1N1 subtype) | Mexico | North America | Modeling study; 2b | Academia |
| 19 | Jackson et al., 2014 25 | Influenza | Non-specific | NA | Systematic review; 2a | Government |
| 20 | Kelso et al., 2009 26 | Influenza A (H3N2 subtype) | Australia | Australia | Modeling study; 2b | Government; research council |
| 21 | Kelso et al., 2013 27 | Influenza A (H1N1, H3N2 and H5N1 subtypes) | Australia | Australia | Modeling study; 2b | Academia |
| 22 | Khazeni et al., 2014 28 | Influenza A (H1N1, H5N1 and H7N9 subtypes) | The United States | North America | Modeling study; 2b | Government |
| 23 | Koo et al., 2020 29 | COVID-19 | Singapore | Asia | Modeling study; 2b | Government |
| 24 | Maier and Brockmann, 2020 30 | COVID-19 | Mainland China | Asia | Modeling study; 3b | Academia; industry |
| 25 | Markel et al., 2007 31 | Influenza A (H1N1 subtype) | The United States | North America | Modeling study; 3b | Government |
| 26 | Prem et al., 2020 32 | COVID-19 | Mainland China | Asia | Modeling study; 2b | Government; research council |
| 27 | Teslya et al., 2020 33 | COVID-19 | The Netherlands | Europe | Modeling study; 2b | Government |
| 28 | Zhang et al., 2020 34 | COVID-19 | Mainland China | Asia | Modeling study; 3b | Government; research council |
NA: not applicable.
Adapted from Oxford Levels of Evidence: 10 Level 2a, systematic review with homogeneity of 2b or better studies; modeling studies were considered similar to economic and decision analysis study types; Level 2b, analysis based on clinically sensible costs or alternatives; limited review(s) of the evidence, or single studies, and including multi-way sensitivity analyses; Level 3b, analysis based on limited alternatives or costs, poor-quality estimates of data, but including sensitivity analyses incorporating clinically sensible variations; Level 4, case-series.
Funding source considerations: academia includes both government and private institutions; government; industry; and research councils include both for-profit and not-for-profit.
Meta-analysis
Meta-analyses were performed using a random-effects DerSimonian–Laird model 35 for proportions, using the inverse variance method with logit transformation to yield a pooled estimate and 95% confidence intervals (CIs) for attack rates. Separate analyses were conducted for ‘no intervention’, ‘single intervention’, and ‘multiple interventions’. Between study heterogeneity was assessed using the τ 2 statistic. Statistical heterogeneity was also assessed using the I 2 statistic (range = 0% – 100%), where higher values indicate a greater degree of variation. The I 2 statistic indicates the percentage of total variation that is attributable to study heterogeneity, rather than sampling error. Heterogeneity was tested using Cochran’s Q statistic. Forest plots were produced to display study-specific and pooled attack rates, along with 95% CIs. The statistical significance level was set to 0.05. All analyses were conducted in R version 4.0.1 using the package Meta.36,37
Results
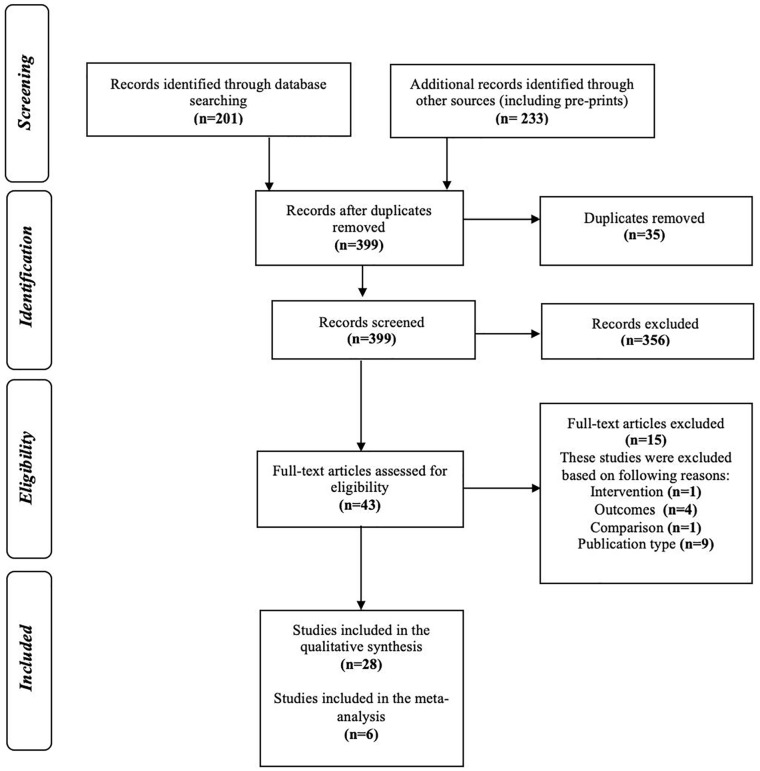
Literature searches identified 399 unique records from 434 references retrieved. Forty-three full-texts were reviewed (IRR = 89%), and 28 studies met inclusion criteria (Figure 1).
Figure 1.
Study selection process.
Summary of articles identified by search queries, and tracking of articles that were included and excluded across the study screening phases with reasons for exclusion of full-texts.
Study characteristics
Study characteristic are provided in Table 1. The following results are reported as number of studies (n) and the corresponding percent of studies identified, for example, (n, %). The majority of included publications were modeling studies (24, 85%; Supplementary Tables III and IV).1–4,11–14,16,17,19,21–24,26–34 The remaining studies were systematic reviews with similar inclusion criteria (2, 7%),18,25 observational (1, 4%), 15 or case-series (1, 4%). 20 Studies were conducted in North America (10, 38%),3,4,12,14,16,19,20,24,28,31 Asia (8, 29%),11,13,15,17,29,30,32,34 Europe (3, 11%),21,23,33 Australia (3, 11%),2,26,27 and Europe combined with North America (1, 4%). 1 Two studies (2, 7%)22,25 did not specify geography, and one study (1, 7%)1,18 was conducted globally. The pathogen (i.e. causative viral agent), intervention type and duration varied across the studies, and both children and/or adults were targeted (Table 2). Influenza A (19, 68%)2–4,11–14,17–21,23–28,31 was most frequently examined, with hemagglutinin (H) and neuraminidase (N) subtypes including H1N1, H3N2, H5N1, and H7N9, across multiple years of outbreaks from 1918 to 2014. Coronavirus-related diseases including SARS 2003 (1, 4%) 22 and/or COVID-19 caused by SARS-CoV-2 (8, 29%)1,15,22,29,30,32–34 were included. Three influenza studies (14%)4,18,23 did not provide viral subtype, and one study (4%) considered general infectious diseases. 16 The majority of studies were completely funded or funded in part by government agencies (21, 75%).1–4,13–18,20,21,23,25,26,28,29,31–34
Table 2.
Summary of outcome-based evidence from social distancing non-pharmaceutical interventions identified.
| Type of social distancing NPI | Subtype NPI | Number of studies a | Study designs included | Outcome-based evidence for consideration in decision-making related to the effectiveness of social distancing NPIs on disease outcomes b |
|---|---|---|---|---|
| General c | Non-specific | 11 | Modeling | INCIDENCE: ◦ Low influenza incidence linked to policies enforcing public social distancing interventions. 2 ◦ As efficacy of the general social distancing interventions increased, COVID-19 attack rates decreased, especially where there is a slow rate of disease awareness spread. 33 ◦ Combining multiple social distancing interventions were more effective to reduce influenza attack rate than treating with antivirals alone. 13 ◦ General sustained, social distancing measures (in combination) were effective to decrease cumulative incidence of a moderate COVID-19 epidemic. 32 ◦ Effectiveness of social distancing interventions rapidly declined once the frequency of reported influenza cases increased. 13 TRANSMISSION: ◦ Early and short-term general social distancing interventions can only delay the timing of peak COVID-19 infections—it did not impact incidence nor transmission. 33 ◦ Stronger isolation plus quarantine policies require less general social distancing interventions to effectively lower R0 in COVID-19 settings. 30 MORTALITY: ◦ The largest impact on reduction in COVID-19-related mortality is reported with implementation of age-dependent social distancing, quarantine, and isolation. 1 OVERALL: ◦ Entire population-wide social distancing had the largest impact on COVID-19 suppression (i.e. reverse R0 and achieve R0 <1). 1 ◦ Early implementation of generalized social distancing (or school closures) resulted in better mitigation (i.e. reduce R0 but not <1) than the very early implementation of travel restrictions in pandemic influenza. 13 |
| Mass gathering cancelations/avoiding crowding | 11 | Case-series; modeling; observational | INCIDENCE: ◦ Mass gathering cancelations had a moderate impact on COVID-19 attack rate; however, the effect was less than non-specific social distancing (e.g. age specific >70 years or for the entire population). 1 ◦ Combining other NPIs with mass gathering cancelation resulted in stronger effects in COVID-19 settings. 1 TRANSMISSION: ◦ Multiple systematic reviews reported the effectiveness of other NPIs, including avoiding crowding, on disease transmission during influenza pandemics. 18 |
|
| School closure | 20 | Case-series; modeling; observational | INCIDENCE: ◦ Closing schools and keeping both children and teenagers at home substantially reduced influenza attack rate. 19 ◦ School closure had the highest impact on influenza attack rate with greater impacts on peak attack rate than cumulative attack rate when: 25 • R0 <2, • Immediate implementation occurred following an outbreak, and • Attack rates were higher in children than in adults. ◦ School closure is insufficient as a single intervention in COVID-19 settings. 1 Combination of general social distancing NPIs including school closure, reducing community contacts, and implementing workplace policies with case isolation was most effective to decrease mean influenza attack rate. 26 ◦ In influenza pandemics, only continuous and additive general social distancing NPIs (e.g. limit community contact, and invoking school closures and workplace policies) have comparable impact to combined intervention of fixed duration using general social distancing policies with provision of antiviral prophylaxis and treatment. 27 TRANSMISSION: ◦ The addition of workplace policies to limit contact to school closures lowered transmissibility of influenza and COVID-19. 15 ◦ An association was identified between timing and duration of school holidays and decreased transmission rates during a mild-to-moderate influenza pandemic. 11 ◦ Multiple systematic reviews examined and reported the effectiveness of other NPIs (including school closure) in lowering influenza transmission. 18 MORTALITY: ◦ Combinations of social distancing NPIs (especially school closure and public gathering bans) had the largest effect on influenza-related mortality rate than single social distancing NPIs. 31 OVERALL: ◦ School closure was a more effective strategy for influenza epidemic suppression than mitigation. 11 ◦ Early implementation of school closures or generalized social distancing resulted in better influenza mitigation than the very early implementation of travel restrictions. 13 |
|
| Travel restriction | 4 | Modeling; observational | INCIDENCE: ◦ Travel restriction decreased mean influenza attack rates. 13 OVERALL: ◦ Early implementation of school closures or generalized social distancing resulted in better influenza mitigation than the very early implementation of travel restrictions. 13 |
|
| General c | Workplace policy | 11 | Case-series; modeling | INCIDENCE: ◦ Workplace and general crowd avoidance have moderate impact on influenza attack rates. 18 ◦ Effects are strengthened by other interventions in pandemic influenza. 18 Combinations of interventions that include school closure and workplace policies, reducing community contacts, isolation, and increase effectiveness (e.g. reduce mean influenza attack rate) over those with school closure alone.3,26 ◦ Only continuous and additive general social distancing interventions (reduced community contact, school closures, and workplace policies) have comparable impact to combined intervention of fixed duration general social distancing with antiviral prophylaxis and treatment to mitigate influenza. 27 ◦ For COVID-19, workplace, quarantine, and general distancing should be prioritized over school closure since symptomatic children have higher withdrawal rates from school than do symptomatic adults from work. 29 TRANSMISSION: ◦ Effectiveness of workplace policies improves with increasing ID, as adult contacts become responsible for a larger share of influenza transmission. 3 ◦ Multiple systematic reviews concluded NPIs (including workplace policies) were effective at reducing influenza transmission. 18 |
| Quarantine d | Border control | 2 | Modeling | INCIDENCE: ◦ Border control could be highly effective in reducing median influenza attack rates in lower R0 values. 18 MORTALITY: ◦ Border control could be effective in lowering excess influenza-related death rates with lower R0 values. 18 |
| Close contacts/household | 11 | Modeling; observational | INCIDENCE: ◦ Attack rates of multiple social distancing interventions are similar to those found with quarantine interventions in moderate-to-severe influenza epidemic models in a low-income region. 13 ◦ Policies to limit workplace influenza infections by household quarantine if member of family was infected were effective to reduce infections, but the likelihood of a household contact (concurrently quarantined with an isolated individual) becoming a secondary case increases with each day of quarantine. 18 ◦ Adding quarantine interventions to social distancing does not reduce influenza infection rates in low compliance settings. 3 ◦ The combination of general, quarantine, and isolation social distancing was the most effective at reducing the number of infections infection rates and in a moderate-to-severe COVID-19 pandemic (with higher ID).3,29 TRANSMISSION: ◦ Isolation and quarantine have high impact on limiting COVID-19 disease transmission. 30 ◦ Quarantine had a moderate impact on reducing influenza transmission. 18 ◦ Stronger isolation plus quarantine policies require less general social distancing interventions to effectively lower R0 in a severe coronavirus epidemic. 30 ◦ Multiple systematic reviews the examined and reported the effectiveness of other NPIs (including contact tracing and quarantine) in reducing influenza transmission. 18 MORTALITY: ◦ A combination of school closures, quarantine, and isolation or personal protection interventions had the largest effect on excess influenza-related death rates. 31 ◦ The largest impact on reduction in COVID-19-related death was reported with implementation of age-dependent social distancing, quarantine, and isolation. 1 OVERALL: ◦ Individuals avoiding contact with others can only mitigate not prevent a moderate-to-severe epidemic. 33 ◦ Workplace quarantine and social distancing should be prioritized over school closure during COVID-19, since symptomatic children have higher withdrawal rates from school than do symptomatic adults from work. 29 |
|
| Onboard | 1 | Modeling | TRANSMISSION: ◦ Onboard quarantine was ineffective at preventing disease influenza transmission, but supplemented public health monitoring of cases. 18 |
|
| Voluntary self-protection | 3 | Modeling | INCIDENCE: ◦ Self-imposed measures lead to larger reductions in the peak number of viral disease diagnoses and in the attack rate very little effect on peak timing. 16 ◦ Entire population-wide social distancing has the largest impact on COVID-19 suppression and combining that intervention with voluntary home quarantine and school closures can rapidly reduce case incidence to suppress transmission to R0 <1. 1 TRANSMISSION: ◦ Self-protection by quarantine is an effective measure to reduce viral disease transmission. 16 ◦ The effectiveness of quarantine to contain viral disease spread is increased when social distancing interventions are combined. 16 ◦ Quarantine had a moderate impact on reducing influenza transmission. 18 MORTALITY: ◦ The largest impact on reduction in COVID-19-related death was reported with implementation of age-dependent social distancing, quarantine, and isolation. 1 |
|
| Quarantine d | Zones (city) | 1 | Modeling | TRANSMISSION: ◦ The most effective strategy for influenza transmission elimination combines all interventions—social distancing, zone quarantine and radial (⩽10 km) geographic targeted antiviral prophylaxis. 17 ◦ The effectiveness of quarantine to contain viral disease spread is increased when social distancing interventions are combined. 16 ◦ Blanket prophylaxis of an entire region would be able to eliminate pandemic influenza with high R0 values (⩽3.6) that is often not feasible. Targeted strategy (social vs geographical) is a more practical approach (minimal drug usage with maximum impact) when combined with quarantine interventions. 17 MORTALITY: ◦ Earlier epicenter lockdown would increase the number of COVID-19 infections and related deaths in the epicenter while reducing the number of infections and deaths in the rest of the areas. 34 |
| Isolation e | Cases f | 13 | Modeling; observational | INCIDENCE: ◦ The combination of general, quarantine, and isolation social distancing was the most effective at reducing the number of infections infection rates and in a moderate-to-severe COVID-19 pandemic (with higher ID).3,29 ◦ Combination of general social distancing interventions including school closure, reducing community contacts, and implementing workplace policies with case isolation was most effective to reduce mean influenza attack rate. 26 ◦ If applied early, isolation plus other NPIs can reduce total influenza attack rate. 18 TRANSMISSION: ◦ The larger the R0, the larger percentage case isolation required. If R0 >0.5, case isolation will not contain the influenza epidemic. Isolation has moderate impact in reducing transmission. 18 ◦ Isolation and quarantine have high impact on limiting COVID-19 transmission. 30 ◦ Multiple systematic reviews the examined and reported the effectiveness of other NPIs (including isolation) in reducing influenza transmission. 18 ◦ Isolation and contact tracing decreased COVID-19 transmission, but if R0 are high then outbreak containment will require very high levels achievement in the intervention. 22 MORTALITY: ◦ If applied early, isolation plus other NPIs can also reduce influenza-related mortality rate but these values are dependent upon the intervention delay time. 18 ◦ Social distancing interventions were associated with decreased influenza-related mortality, but case isolation did not impact mortality significantly. 20 ◦ The largest impact on reduction in COVID-19 death was reported with implementation of age-dependent social distancing, quarantine, and isolation. 1 OTHER: ◦ Effectiveness of interventions improved with earlier implementation in moderate-to-severe influenza pandemic. 26 ◦ The delay between symptom onset and isolation had the largest role in determining whether a COVID-19 outbreak was controllable when R0 was 1.5. 22 ◦ Case isolation effectiveness to prevent an influenza epidemic must occur within 3 weeks. 18 ◦ For an influenza epidemic with an R0 value of 1.5, the only single intervention measure capable of preventing an epidemic was the 90% case isolation measure, and only if applied within 3 weeks. 26 ◦ Government-enforced isolation with strict quarantine monitoring requires less general social distancing interventions to effectively lower R0 in a severe coronavirus epidemic. 30 |
| Hospitalized patients | 2 | Modeling; observational | MORTALITY: ◦ With higher numbers of asymptomatic COVID-19 patients, NPIs were less effective—resulting in a need for case management, treatment, and vaccination. 29 |
COVID-19: coronavirus disease 2019; ID: disease infectivity factor; NPIs: non-pharmaceutical interventions; R0: reproduction number.
Details provided in abstraction Supplementary Table III.
Multiple subtypes of social distancing NPIs were used in studies.
Substantial heterogeneity was noted in outcome measures employed to report each outcome, that is, disease incidence (e.g. infection rate or incident rate, incidence proportion or attack rate); disease transmission (e.g. R0, disease infectivity (ID) factor), and disease mortality (e.g. case fatality proportion, peak excess death rates, and mortality rates).
General social distancing (i.e. reduced interactions between potentially infectious individuals—but not diagnosed—in a broader community).
Quarantine (i.e. involves the restriction of movement of presumably infectious individuals as they may have been exposed to disease).
Isolation (i.e. separation of diagnosed individuals).
Included suspected and undiagnosed cases.
Types of interventions and outcomes
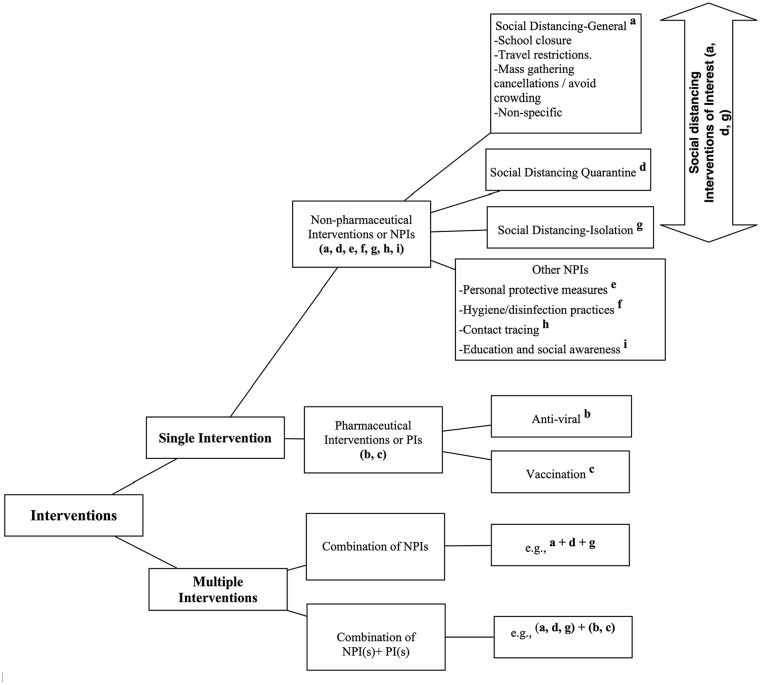
Social distancing NPIs of interest included general social distancing (referred to as general), quarantine, and isolation (for intervention definition, see Supplementary Table III; Figure 2).
Figure 2.
Study intervention hierarchy for data analysis. This graphic visualizes the study details regarding data collection concerning interventions to mitigate or control viral pandemics or epidemics. Single (non-pharmaceutical interventions (NPIs) or pharmaceutical interventions (PIs)) interventions and/or multiple interventions with combinations of exclusive social distancing NPIs, social distancing NPIs plus other NPIs, social distancing NPIs plus PIs, and/or social distancing NPIs plus other NPIs and PIs.
Superscripts denote the labeling of specific interventions used to categorize study results provided in the data abstraction.
Social distancing NPIs were used as a single intervention (19, 68%)1,3,11–13,16–22,24–26,31–34 and/or multiple interventions (24, 86%)1–4,12–18,20–22,24,26–34 with other NPIs and/or PIs including antiviral treatment and prophylaxis, and/or vaccination. Infectious viral disease outcomes were limited to disease incidence, transmission, and mortality (Figure 3, Supplementary Tables III and IV).
Figure 3.
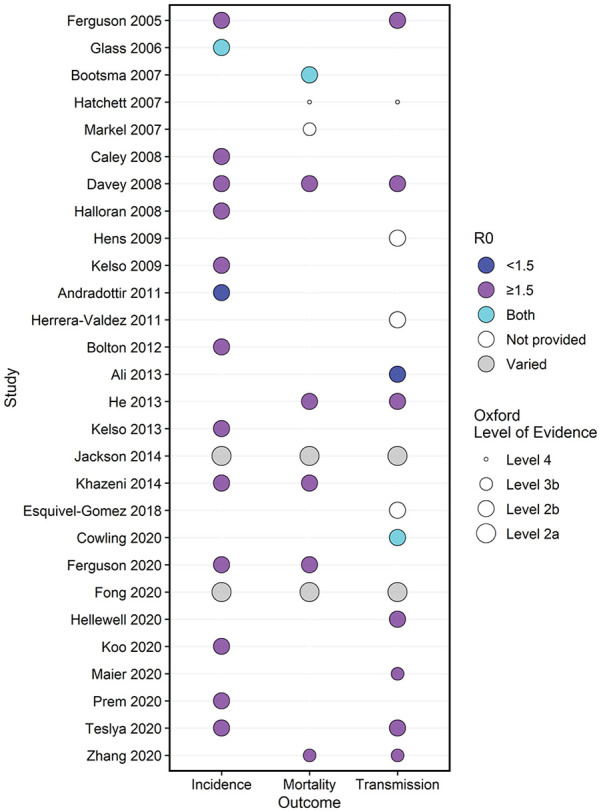
Summary of outcomes by study with respect to basic reproduction number (R0) and quality assessment. The bubble plot lists included studies by year (y-axis) and outcome (x-axis; disease incidence, mortality, and/or disease transmission). The size of the circle represents the quality assessment provided by the corresponding Oxford Level of Evidence, 10 whereby smaller circles indicate low-quality (i.e. Level 3b or 4, case series) and larger circles denote moderate-quality (i.e. Level 2a, systematic review with homogeneity of 2b or better studies and Level 2b, modeling studies) evidence. The color of the circle represents the reproduction number (R0) whereby blue indicates R0 <1.5, purple denotes R0 >1.5, turquoise represents studies with range of 1.5 > R0 < 1.5 (both), white shows R0 was not provided, and gray for systematic reviews that had a varied range of R0 values.
Thematic analysis stratified results related to the effectiveness of general, isolation, and quarantine social distancing NPIs (Table 2). General social distancing was most frequently observed (27, 96%),1–4,11–21,23–34 with school closures identified as a common subtype (20, 71%).1–4,11–15,17–21,23–33 Other types of general social distancing identified included non-specific (7, 25%),1,13,17,26,27,30,33 avoidance of contact, crowding, or mass gatherings (7, 25%),1,4,18,20,31–33 workplace policies to limit contact (12, 43%),4,17,18,20,23,26–30,32,33 and travel restrictions (3, 11%).13,15,18 Isolation and quarantine were similarly identified (both: 16, 57%).1–4,13–20,22,26,28–31,33,34 Case isolation was identified in 13 studies (13, 46%)2,4,14–16,19,20,22,29–31,34 but only two studies (2, 7%) described hospitalization.15,18 Household quarantine (11, 39%)2,3,4,13,18,20,28–31,34 was most common, with fewer studies examining other quarantine conditions, including border control (2, 7%),18,15 geographic region by city or zone (1, 4%), 17 onboard (e.g. airline or ship) (1, 4%), 18 and voluntary self-protection (3, 11%).1,16,18
If R0 increased, then multiple NPIs’ effectiveness on attack rate improved (R0 = 1.5, − 29.3%; R0 = 2.5, − 43.8%) at 3 weeks delay, but there was no relative change in attack rate if applied at 8 weeks. 26 Another combination of social distancing NPIs (e.g. general, quarantine, and isolation) plus a PI (e.g. antiviral treatment and prophylaxis) could decrease influenza attack rate by 39.5%–46.6% in a similar R0 range with 60%–80% compliance. 4
School closure predicted age-specific reductions in percent cumulative attack rate comparing school-age children (21%–22%) to adults >53 years (40%) and adults <53 years (12%) by child-to-child community and household transmission. 25 Early implementation of school closure or generalized social distancing may result in better mitigation than the very early implementation of public travel restrictions.13,18
General social distancing plus quarantine and isolation NPIs substantially decreased 15 or predicted decrease30,34 of COVID-19 R0 (pre-intervention R0 range = 1.28–6.2; post-intervention R0 range = 0.72 – 3.22).15,30,34 Government-enforced isolation with monitored quarantine predicted that fewer general social distancing interventions were required to effectively lower R0 in severe (R0 >1.5) COVID-19 settings. 30
Timing and duration of school holidays played a critical role in limiting COVID-19 and influenza transmission rates in observational and modeling studies.11,15 Combinations of these general social distancing NPIs (e.g. changes in population behavior to limit public contact) with quarantine and isolation had a greater effect on decreasing COVID-19 transmissibility when the intervention duration was extended beyond holiday-related school closures (holiday only, 14% – 15%; extension beyond holiday, 33% – 44%). 15 A detailed summary of data abstraction for included studies is provided in Supplementary Table III.
Effectiveness summarization
All studies (28, 100%) reported some degree of effectiveness for each social distancing NPI examined across mild-to-moderate (R0 <1.5) and/or moderate-to-severe (R0 ⩾1.5) epidemics or pandemics based on provided R0 (Figure 3). Four studies20,23,24,31 did not provide R0, and two studies18,25 had ranges of R0. Overall, combined social distancing NPIs were generally more effective in improving disease outcomes when compared to single interventions. Due to intervention heterogeneity and outcome reporting, it was generally not possible to provide valid head-to-head effectiveness comparisons across studies; however, a meta-analysis was conducted to examine the effect of social distancing NPIs on one outcome of interest, disease incidence.
Disease incidence including meta-analysis on attack rate
Disease incidence was examined in 16 studies (57%)1–4,12,13,17–19,25–29,32,33 with primarily percent attack rate, and secondarily, infection rate reported (Figure 3 and Supple-mentary Table III) and had mixed results when comparing the effectiveness of single versus combined NPIs.
Combining social distancing NPIs may be more effective in decreasing attack rate than single NPIs, in both COVID-19 and non-COVID-19 settings.1,3,13,26,32 As shown in the modeling studies, timeliness of and compliance with multiple interventions and the R0 values may influence effectiveness. Delay in implementing multiple, general social distancing NPIs (e.g. workplace policies, school closure, and limiting group interactions) with case isolation is predicted to limit influenza attack rate change (week delay: three, −29.3%; eight, −14.3%)18,26 and would have similar effectiveness to earlier implemented single, general social distancing NPIs (3 weeks: range = −18.3% to −8.3%). 26
Modeling studies examining single social distancing NPIs’ effectiveness suggest wide variation in general viral attack rate change (range = 0% – 99%), and greater effects on influenza when the frequency of new cases remains low. 13 Simulation of influenza outbreaks revealed school closure was a common single NPI and may substantially lower attack rate (typically 20% – 60%), 25 but had the highest impact when implemented in combination NPIs, 1 especially early in pandemics 25 and when continued for adequate duration. 27 The remaining general social distancing subtypes of mass gathering prohibitions, contact avoidance, and workplace policies to limit contact had similar effectiveness with a moderate (<20% decrease) effect on COVID-19 attack rate; 1 however, when used in combination NPIs, the impact was larger (>20% decrease). 1 Case isolation may be less effective than other single general social distancing NPIs on influenza attack rate, but changes in attack rate could improve with early implementation (3 weeks, −25.3%; 8 weeks, −8.3%). 18
A meta-analysis was conducted on six studies3,12,13,19,26,27 that reported influenza attack rates and the population at-risk for both no intervention and social distancing NPIs as a proxy (Supplementary material, Figures I and II). Some studies provided attack rate estimates assuming different model parameters (e.g. R0) and interventions (Supplementary material, Figure II). Attack rates for no intervention ranged from 10% to 73% and the pooled attack rate was 42% (95% CI = 30% – 55%) with significant heterogeneity (p < 0.0001). Ranges of attack rates varied greatly for social distancing NPIs (single NPIs, 6% – 65%; multiple NPIs, 4% – 73%). The pooled attack rate for single NPIs and multiple NPIs was 29% (95% CI = 23% – 36%) and 22% (95% CI = 16% – 29%), respectively, with significant heterogeneity (p < 0.0001). The random-effects model demonstrated substantial statistical heterogeneity (I 2 = 100%) ensuing from various possible resources within each analysis, such as different modeling methods, different parameters for the simulations, and R0 values.
Disease transmission
Disease transmission was examined in 15 (54%) studies3,11,15–18,20–25,30,33,34 as percent or effective decrease in R0, or scaled disease infectivity (ID) factor as a proxy for transmissibility (Figure 3). A few studies examined single NPIs, predicting decreased influenza transmission following school closures (range = 14%–100%),11,17,21 household quarantine (20%), 18 and vaccination (15%). 24 Modeling of case isolation predicted decreases in COVID-19 effective R0 from 1.5 to 1.25 and 2.5 to 2.1. 22
Generally, combinations of social distancing NPIs predicted18,24 or changed18,20 viral transmissibility in both COVID-19 and non-COVID-19 settings (range = −14% to −54%),18,15,24 but compliance affected their success. One modeling study noted that there was greater than 90% probability of obtaining 0% influenza transmission rates with a combination of general social distancing NPIs; 90% achievement with socially targeted antiviral treatment and prophylaxis and 80% with reduced movement in affected zones following geographic targeting of quarantine interventions. 17 Isolation15,17,18,22,30,34 and quarantine16,18,30 are both effective in limiting influenza and COVID-19 disease transmission when used in combination NPIs. Modeling of isolation combined with contact tracing decreased COVID-19 transmission (pre-intervention R0, 1.5; post-intervention R0, 0.5 – 0.9 based on 20% – 100% contact tracing achievement). 22 This study noted the delay between symptom onset and isolation had the largest role in determining whether a COVID-19 outbreak (R0 = 1.5) was controllable, and higher achievement of contact tracing was required as R0 increased. 22 However, the effectiveness of quarantine on general viral disease could be increased when general social distancing interventions are combined.16,17 Risk of secondary influenza transmission decreased when quarantine (adjusted odds ratio (OR) = 1.25; 95% CI = 1.06 – 1.47; p = 0.0008) was combined with antiviral prophylaxis and treatment (adjusted OR = 0.042; 95% CI = 0.004 – 0.434; p = 0.0008) in simulation. 18
Intervention timing influenced NPI effectiveness on limiting transmission. Early and short-term (e.g. 3 months) general social distancing NPIs could delay the timing of peak infections by as much as 7 months, but would have a modest impact on COVID-19 transmission. 33 Modeling effectiveness of workplace policies improved with increasing ID, as adult contacts contribute largely to influenza transmission. 3 In addition, mask wearing and handwashing, combined with social distancing NPIs, limited COVID-19 and influenza transmissibility in both observational and modeling studies.18,15
Mortality
Mortality was examined in 10 studies (36%)1,3,14,18,20,21,25,28,31,34 as were case fatality proportion, mortality rate, peak excess death rate, and total deaths (i.e. all-cause mortality) (Figure 3). Single and multiple social distancing NPI modeling had large variability on influenza-related mortality rate (ranges of single: range = 3% – 78%; multiple = 6% – 97%). 31 A COVID-19 modeling study determined mortality could be further decreased by 34%–49% by adding age-dependent (>70 years) general social distancing interventions to quarantine and isolation. 1 However, general social distancing NPIs plus PIs could further lessen mortality in predictive models; using general social distancing NPIs, influenza-related mortality could be decreased by 93%, but by providing antiviral prophylaxis and treatment (e.g. adamantanes and neuraminidase inhibitors) to 2% of the affected region, mortality could be decreased by 97%. 3 The number of general social distancing NPIs was associated with lower peak excess influenza death rates (0.002 > p < 0.047), 20 whereby more interventions predicted lower death rates.
Temporal relationships with mortality were similarly identified whereby duration and timing impacted the effectiveness of social distancing NPIs. Influenza-related mortality could be further decreased by extending intervention durations;14,31 one modeling study noted that a substantial decrease in mortality could be obtained by extending a combination of NPIs (e.g. general social distancing, handwashing, mask wearing, and case isolation) by 5 months (no extension, 6% – 43%; with extension, 38% – 92%). 14 Quarantine by controlling movement within infected geographies with lower R0 (e.g. R0 >1) predicted lower excess influenza death rates. 18 A model predicted that earlier epicenter lockdown would have reduced the number COVID-19 deaths overall (up to 99.3%), but would increase the number of deaths in the epicenter. 34 Moreover when added to general social distancing and personal preventive measures (e.g. handwashing and mask wearing), quarantine would reduce the influenza mortality rate by 63%. 28 Case isolation had mixed effects on influenza-related mortality;17,20 however, total mortality burden was predicted to be lower when isolation was used early (p = 0.008) and with increased duration (p = 0.005), in combination with quarantine and general social distancing NPIs. 18 Similarly, early implemented general social distancing NPIs predicted lower peak excess influenza death rates. 20 Late interventions, regardless of duration or type of intervention, had worse predicted mortality-related outcomes in influenza outbreaks. 31
Study quality
Using Oxford levels of evidence, 10 two (7%) studies provided Level 2a evidence;18,25 22 (79%) studies were assessed as Level 2b;1–4,11–17,19,21–24,26–29,32,33 three (7%) were Level 3b;30,31,34 and one (4%) 20 provided Level 4 evidence. The quality of the evidence is moderate as the majority of studies were Level 2 quality (descriptions in Table 1 and Figure 3).
Discussion
NPIs are important public health measures, implemented either as a single measure or in combination with other NPIs, to help decrease incidence, transmission, and mortality of viral and other infectious diseases. Early, sustained, and combined application of various NPIs could mitigate and control primary outbreaks and prevent more severe secondary or tertiary outbreaks,20,24,34 provided decision-makers consider dynamic R0 values, the propensity of getting infected among certain high-risk groups (e.g. increasing age and underlying comorbidities), and asymptomatic cases that may be as infectious as symptomatic patients.
Retrospective observational and modeling studies suggest the effectiveness of social distancing NPIs depends on disease severity as well as intervention timing and adherence (e.g. implementation delays, duration, and population compliance/coverage). Thus, an outbreak could be effectively suppressed through strict and early implementation of an intervention (single or in combination) for a pre-determined duration. The more widespread the infectious disease and/or the longer the delay in implementation of a measure, the more limited the effectiveness of the intervention.3,12,17,18,26,33
Effective mitigation requires combined social distancing interventions (e.g. school closure, quarantine, or isolation), supplemented by other NPIs (e.g. mask wearing and handwashing) and PIs (e.g. antiviral treatment and/or vaccinations). Several studies suggested that multiple interventions are more effective than a single intervention.1,16,18,29,30 Timely and continuous but evolving adoption of evidence-based social distancing strategies could substantially mitigate and control the pandemic until an efficacious treatment and or/matched vaccine become available.28,33
The majority of included studies implemented modeling to predict the effects of social distancing NPIs on viral disease outbreaks. Further work is needed to model case fatality rates, mortality, and costs, and to predict disease transmission, effects of differing vaccination rates, and case severity and their correlation with NPIs.
Public health implications
This study provides both qualitative and quantitative evidence related to the implications of using NPIs to contain infectious diseases, mostly from past studies, supported by current experiences. Public health policy makers, educators, clinicians, and researchers could better understand the factors that could facilitate more favorable outcomes resulting from implementations of NPIs. These include matching the right intervention type to specific community circumstances such as the stage of the curve or type of venue (school closures, mass gathering cancelations, contact tracing, etc.) and/or strategizing implementation time, duration, and intensity (early, prolonged and strict lock down, prioritization of protecting the high-risk patient population first, for example, elderly having other comorbidities).
Strengths and limitations
To our knowledge, this is a novel review employing a rapid methodology to provide both qualitative and quantitative synthesis of the evidence related to the effects of social distancing NPIs on respiratory viral disease outcomes including incidence, transmission, and mortality. Our results should be interpreted in the context of the limitations of this study. Studies with a search cutoff of 27 March 2020 were limited from online searches of MEDLINE, Google Scholar, and pre-print databases. In addition, more relevant studies may be identified from other databases and those published after the search cutoff date. Handsearching of included studies or conference proceedings was not performed. In addition, articles were limited to English language with an abstract. Meta-analyses were limited to influenza attack rate and results had high statistical heterogeneity. These quantitative findings conflict with the narrative results relating to the effectiveness of single versus combined NPIs on disease incidence. Potential explanations could be the high study heterogeneity (i.e. different models, varying parameters for the models, variable R0, and different compliance) and a limited number of studies each of the analyses. Because of the limited number of studies, meta regression analysis with multiple covariates was not recommended. Despite these limitations, the visual representations derived from the meta-analysis provide insights into attack rate comparisons by type of intervention (e.g. none, single, and combined). Confounders such as weather changes (e.g. temperature and humidity levels) were not considered. In addition, a large number of modeling studies were used to derive this evidence as opposed to epidemiological findings. This evidence can facilitate decision-making to better recognize and implement measures that support mitigation and suppression of viral infectious disease during outbreaks, and successful NPIs can provide time for the development and evaluation of effective treatments and vaccines.
Conclusion
This review provides evidence that a proper deployment of strategically combined social distancing NPIs as a public health measure, along with other non-pharmaceutical and PIs, could mitigate and control disease by decreasing viral disease incidence, transmission, and mortality.
Supplemental Material
Supplemental material, sj-doc-1-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-tiff-3-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-tiff-4-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Acknowledgments
The authors thank Winnie Felix and Courtney VanHouten for helping with searching for relevant papers, and Dr Van C. Willis for his critical reading and support for the completion of the revisions.
Footnotes
Availability of data and materials: All supporting data has been provided in additional files.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research study was supported by IBM® Watson Health® (no grant number to disclose).
ORCID iD: Rubina F Rizvi  https://orcid.org/0000-0001-9432-3086
https://orcid.org/0000-0001-9432-3086
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ferguson NM, Laydon D, Nedjati-Gilani G, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. Imperial College COVID-19 Response Team, 2020, https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-9-impact-of-npis-on-covid-19/ [DOI] [PMC free article] [PubMed]
- 2. Caley P, Philp DJ, McCracken K. Quantifying social distancing arising from pandemic influenza. J R Soc Interface 2008; 5: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davey VJ, Glass RJ, Min HJ, et al. Effective, robust design of community mitigation for pandemic influenza: a systematic examination of proposed US guidance. PLoS ONE 2008; 3: e2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A 2008; 105: 4639–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kraay AN, Han P, Kambhampati AK, et al. Impact of nonpharmaceutical interventions for severe acute respiratory syndrome coronavirus 2 on Norovirus outbreaks: an analysis of outbreaks reported by 9 US states. J Infect Dis 2021; 2021: jiab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun J, Shi Z, Xu H. Non-pharmaceutical interventions used for COVID-19 had a major impact on reducing influenza in China in 2020. J Travel Med 2020; 27: taaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qureshi AI, Janardhan V, Hanel RA, et al. Comparison of endovascular and surgical treatments for intracranial aneurysms: an evidence-based review. Lancet Neurol 2007; 6(9): 816–825. [DOI] [PubMed] [Google Scholar]
- 8. Gabarron E, Årsand E, Wynn R. Social media use in interventions for diabetes: rapid evidence-based review. J Med Int Res 2018; 20: e10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012; 22: 276–282. [PMC free article] [PubMed] [Google Scholar]
- 10. Oxford Centre for Evidence-based Medicine Levels of Evidence (March 2009)—CEBM, https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (2009, accessed 27 March 2020).
- 11. Ali ST, Kadi AS, Ferguson NM. Transmission dynamics of the 2009 influenza A (H1N1) pandemic in India: the impact of holiday-related school closure. Epidemics 2013; 5(4): 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andradottir S, Chiu W, Goldsman D, et al. Reactive strategies for containing developing outbreaks of pandemic influenza. BMC Public Health 2011; 11(Suppl. 1): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolton KJ, McCaw JM, Moss R, et al. Likely effectiveness of pharmaceutical and non-pharmaceutical interventions for mitigating influenza virus transmission in Mongolia. Bull World Health Organ 2012; 90: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bootsma MC, Ferguson NM. The effect of public health measures on the 1918 influenza pandemic in U.S. Cities. Proc Natl Acad Sci U S A 2007; 104: 7588–7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health 2020; 5(5): e279–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esquivel-Gomez JJ, Barajas-Ramirez JG. Efficiency of quarantine and self-protection processes in epidemic spreading control on scale-free networks. Chaos 2018; 28(1): 013119. [DOI] [PubMed] [Google Scholar]
- 17. Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437: 209–214. [DOI] [PubMed] [Google Scholar]
- 18. Fong MW, Gao H, Wong JY, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg Infect Dis 2020; 26(5): 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glass RJ, Glass LM, Beyeler WE, et al. Targeted social distancing design for pandemic influenza. Emerg Infect Dis 2006; 12(11): 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A 2007; 104: 7582–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He D, Dushoff J, Day T, et al. Inferring the causes of the three waves of the 1918 influenza pandemic in England and Wales. Proc Biol Sci 2013; 280: 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020; 8(4): e488–e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hens N, Ayele GM, Goeyvaerts N, et al. Estimating the impact of school closure on social mixing behaviour and the transmission of close contact infections in eight European countries. BMC Infect Dis 2009; 9: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrera-Valdez MA, Cruz-Aponte M, Castillo-Chavez C. Multiple outbreaks for the same pandemic: local transportation and social distancing explain the different “waves” of A-H1N1pdm cases observed in Mexico during 2009. Math Biosci Eng 2011; 8: 21–48. [DOI] [PubMed] [Google Scholar]
- 25. Jackson C, Mangtani P, Hawker J, et al. The effects of school closures on influenza outbreaks and pandemics: systematic review of simulation studies. PLoS ONE 2014; 9(5): e97297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelso JK, Milne GJ, Kelly H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 2009; 9: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelso JK, Halder N, Postma MJ, et al. Economic analysis of pandemic influenza mitigation strategies for five pandemic severity categories. BMC Public Health 2013; 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khazeni N, Hutton DW, Collins CI, et al. Health and economic benefits of early vaccination and nonpharmaceutical interventions for a human influenza A (H7N9) pandemic: a modeling study. Ann Intern Med 2014; 160: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis 2020; 20(6): 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maier BF, Brockmann D. Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China. Science 2020; 368: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA 2007; 298: 644–654. [DOI] [PubMed] [Google Scholar]
- 32. Prem K, Liu Y, Russell TW, et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health 2020; 5(5): e261–e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teslya A, Pham TM, Godijk NG, et al. Impact of self-imposed prevention measures and short-term government-imposed social distancing on mitigating and delaying a COVID-19 epidemic: a modelling study. PLoS Med 2020; 117(7): e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Jiang B, Yuan J, et al. The impact of social distancing and epicenter lockdown on the COVID-19 epidemic in mainland China: a data-driven SEIQR model study. Medrxiv, 2020, https://www.medrxiv.org/content/10.1101/2020.03.04.20031187v1
- 35. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 36. Wang N. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial, 2018, https://www.researchgate.net/publication/325486099_How_to_Conduct_a_Meta-Analysis_of_Proportions_in_R_A_Comprehensive_Tutorial
- 37. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-tiff-3-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine
Supplemental material, sj-tiff-4-smo-10.1177_20503121211022973 for Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis by Rubina F Rizvi, Kelly J Thomas Craig, Rezzan Hekmat, Fredy Reyes, Brett South, Bedda Rosario, William J Kassler and Gretchen P Jackson in SAGE Open Medicine