Abstract
Eyes in a schematic face and arrows presented at fixation can each cue an upcoming lateralized target such that responses to the target are faster to a valid than an invalid cue (sometimes claimed to reflect “automatic” orienting). One test of an automatic process concerns the extent to which it can be interfered with by another process. The present experiment investigates the ability of eyes and arrows to cue an upcoming target when both cues are present at the same time. On some trials they are congruent (both cues signal the same direction); on other trials they are incongruent (the two cues signal opposite directions). When the cues are congruent a valid cue produced faster response times than an invalid cue. In the incongruent case arrows are resistant to interference from eyes, whereas an incongruent arrow eliminates a cueing effect for eyes. The discussion elaborates briefly on the theoretical implications.
Keywords: Automaticity, eye gaze
Introduction
Human eyes have attracted considerable attention in literature, art, and of course in the developmental and cognitive psychology literatures. It is well known that attention to eye direction appears very early in development, and has been argued to arise because of special purpose mechanisms that are innate (e.g., Hoehl et al., 2008). There are also seminal reports that eye gaze direction attracts attention in the absence of an intention or task set, can interfere with performance even when irrelevant to the task at hand, can cue an upcoming target, even when the cue-target stimulus onset asynchrony (SOA) is short, even when the cue carries no information, and even when the cues are counter-predictive. In short, eye direction, even in a schematic face, is often understood as eliciting “automatic” orienting (e.g., Driver et al., 1999; Friesen & Kingstone, 1998; Friesen et al., 2004; Frischen et al., 2007; Kuhn & Kingstone, 2009, among others).
To the contrary, the uniqueness of eyes, at least with respect to their ability to elicit automatic orienting, has been challenged by several reports that arrows too can act as cues for an upcoming target, even when there is a short SOA between cue and target and when the cues are uninformative (e.g., Galfano et al., 2012; Tipples, 2002).
In sum, both eyes and arrows are associated with some aspect of “automatic” orienting. It is, however, less clear whether these results require an explanation couched in terms of processes that are “automatic” beyond the claim that they can be seen in the absence of informational content, and at short cue-target SOAs. For example, putatively automatic processes are widely seen as being able to interfere with other processes, but are themselves widely seen as resistant to interference (e.g., as in the Stroop and semantic priming literatures). In that vein, we can ask whether eyes are more likely to elicit orienting than arrows or whether there are circumstances where the opposite is true.
How could this question be investigated? Mindful of Popper’s (1959) emphasis on falsifiability, we devised a simple test in which the task is timed letter identification, and eyes and arrows serve as spatial cues. Critically, in one block of trials arrows and eyes both appear on all trials, but on some trials, they point in the same direction (congruent), while on others they point in different directions (incongruent). Which of these cues will dominate in the incongruent case, or will neither affect performance?
Three hypotheses
Eyes dominate arrows
One hypothesis is that an eye direction cue is strongly dominant over the arrow direction cue. A cueing effect (valid faster than invalid) is expected when the two cues are congruent with respect to direction. The critical question is whether this effect will persist when the two cues are incongruent with respect to direction, and the eyes are coded as valid (the direction the eyes are pointing is the direction a target subsequently appears on half the trials). A significant difference between valid and invalid conditions in the incongruent condition would support the idea that this process is resistant to interference from the irrelevant arrow pointing in the opposite direction, and hence by that criterion is “automatic.”
Arrows dominate eyes
The inverse hypothesis is that arrow direction cues dominate eye direction cues. A cueing effect (valid faster than invalid) is again expected when the two cues are congruent with respect to direction. The critical question is whether this effect will persist when the two cues are incongruent with respect to direction, and the arrows are coded as valid (the direction the arrow is pointing in is the direction a target subsequently appears on half the trials). A significant difference between valid and invalid conditions in the incongruent condition would support the idea that this process is resistant to interference from the irrelevant eyes pointing in the opposite direction, and hence by that criterion is “automatic.”
A null effect of cueing in the incongruent condition
The third hypothesis is that neither eye nor arrow cues will elicit a cueing effect when these two cues point in opposite direction. 1 A null effect could be understood in several ways. For example, participants might simply give up orienting under this condition. Alternatively, the attempts to orient in the direction of a cue fail because of interference generated by the other cue pointing in the opposite direction. For present purposes the central point is that a null result supports the claim that both cues are sensitive to interference (albeit possibly for distinct reasons) and hence neither can be thought of as “automatic,” according to the resistance to interference criterion.
Methods
Participants
Fifty-two undergraduate students from the University of Waterloo participated in exchange for course credit. Participants all had normal or corrected to normal vision.
Stimuli
The set of four schematic faces from O’Malley and Besner (2014) were used in Block 2 (Figure 1; left) and Block 3 (see Figure 1; left and right), along with four unidimensional schematic faces created for Block 1 (Figure 2). Each face was 3.8 cm in diameter and consisted of a black outlined circle on a white background. The eyes consisted of two circles midway between the centre line of the circle and the top, while each circle contained a black “pupil” that indicated left or right. At the midline of the circle there was an arrow that would indicate either right or left. Finally, a line was placed below the arrow to indicate a mouth and complete the schematic face. In two of the schematic faces, the arrows and eyes each indicated the same direction (congruent), whereas in the other two they appeared in opposition to each other (incongruent). After the appearance of the face cue, one of two letters (the capital letters A or Y in 36 pt. Consolas font) appeared in the cued location (valid) or opposite the cued location (invalid). Incongruent trials presented conflicting cue information being valid for one dimension while invalid for the other.
Figure 1.
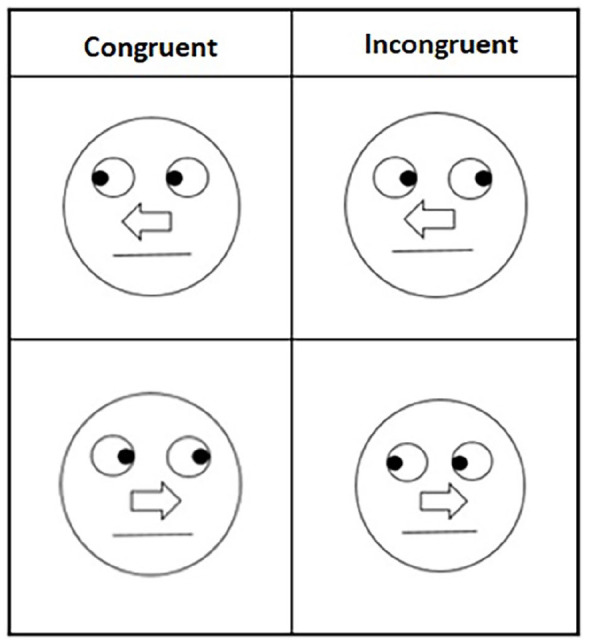
Block 2 (left panels) and Block 3 (all panels) stimuli.
Figure 2.
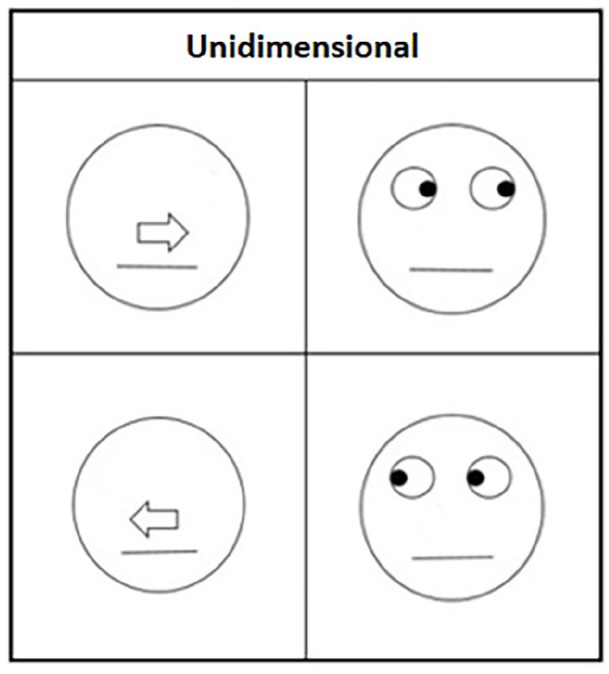
Block 1 stimuli.
Apparatus
Participants were tested in a large multi-station, dimly lit lab, with groups of up to six. They sat stationed individually in cubicles, and viewed a computer monitor from a distance of approximately 60 cm. Stimuli were presented on a 60-cm widescreen LCD monitor slaved to a Windows 10-based computer system. All timing and stimulus presentation used E-Prime version 3 at a resolution of 1,920 × 1,080 pixels and 120-Hz refresh rate with responses collected by the keyboard.
Design and procedure
The study consisted of three blocks, completed by all participants. The purpose of Block 1 was to show that the two dimensions (eyes/arrows), on their own, are equally discriminable. Besner et al. (2020) report the same experiment as in Block 1. That experiment yielded a null difference (eyes were responded to 3 ms faster than to an arrow; BF01 = 3.05). Following the recommendation of Jeffreys (1961), currently widely adopted, a Bayes factor (BF) value of the range 3–10 may be considered as moderate evidence for the null (BF01) or for the alternative (BF10). We report this same experiment here as Besner et al. (2020), because their experiment has yet to be published. Block 1 thus consisted of the unidimensional stimuli that indicated either left or right and the participant responded using the “Q” and “P” respectively.
Each trial began with a 500-ms blank, followed by a square fixation box that touched the four sides of the schematic face. This was presented for 1,000 ms followed by a 250-ms blank period, and finally the target image was displayed until the participant responded by pressing either the P or the Q key. There were a total of 160 trials preceded by 20 practice trials (Figure 3).
Figure 3.
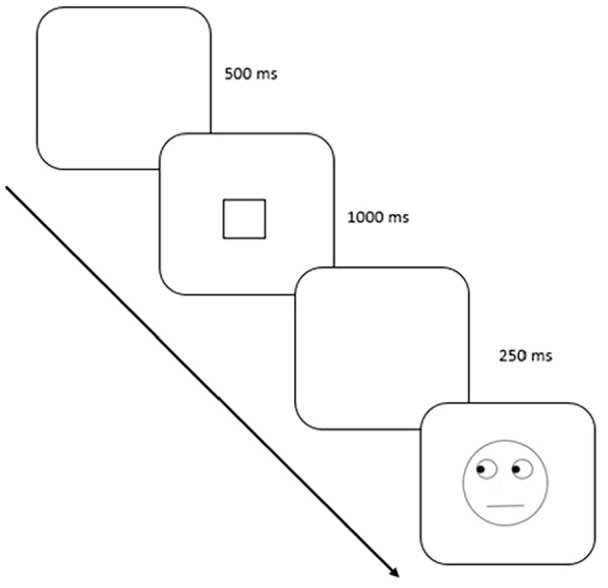
Block 1: a typical trial sequence with events running from top-left to bottom-right. Stimuli are not depicted to scale.
The purpose of Block 2 was to show that the two cues, when shown together, can produce a spatial cueing effect when a target letter is identified. Block 2 consisted of all congruent faces; half were valid cues for the location of the upcoming target and half were invalid cues, where the target appeared in the location opposite to the cue.
The trial sequence was modified to ensure that the attention was focused on the centre of the screen where the schematic face would appear (Figure 4). The sequence consisted of a 500-ms blank screen followed by the fixation square fixation (as seen in Block 1), displayed for 300 ms. Next, a second blank screen appeared for 100 ms and then the fixation box returned for 300 ms followed by a 100-ms blank screen and then the schematic face cue was presented for 150 ms, followed by a blank screen for 50 ms, and then the target. The target letter was approximately 0.7 cm in maximum width and height and were presented 0.3 cm from the edge of the schematic face to the nearest edge of the target letter on either the right or left.
Figure 4.
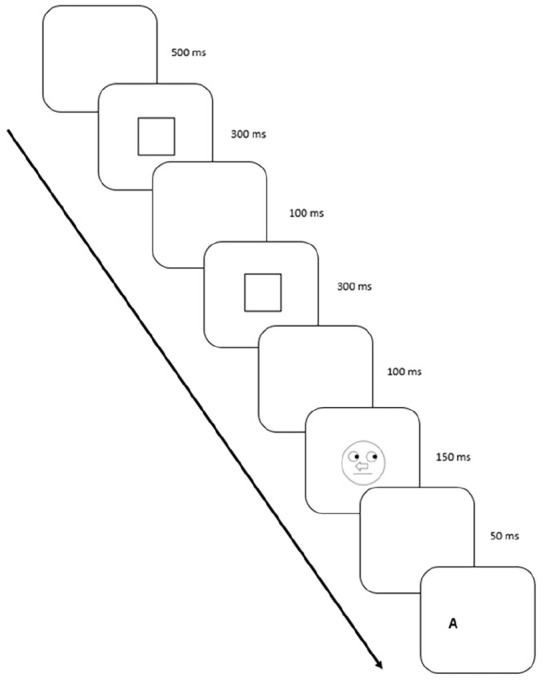
Blocks 2 and 3: a trial sequence with successive events running from top-left to bottom-right. Stimuli are not depicted to scale.
The participant responded using their left hand which was positioned on the keyboard where they would press either the “W” key for the target “A” or the “S” key for the target “Y.” 2 Participants completed 20 practice trials with feedback about accuracy, and all trials were limited to 900 ms for a response. Failures to respond in the allotted time period (900 ms) were signalled to the participant by the appearance of a red X at central fixation throughout Block 2. After completing the 20 practice trials, participants completed 160 trials with no feedback about accuracy. Half the trials, randomly, for each participant, were cued validly and the other half invalidly.
Block 3 followed the same sequence and timing as Block 2; however, incongruent faces were now intermixed with the congruent faces and formed 50% of the trials. After completing 40 practice trials, participants completed a further 240 trials (congruent cues and 120 incongruent cues randomly intermixed, with half of each being valid and half being invalid).
Results
Response time (RT) data for the experiment was first subjected to an error removal (resulting in the loss of four subjects who produced more than the cut-off of 20% errors in any one condition). The remaining RT data were subjected to an outlier analysis in which RTs falling 3 SDs above or below the mean correct RT score for each participant in each condition were discarded (<3%). One additional subject was lost because their mean RT lay 3 SDs beyond the mean in one of the blocks when a grand mean across conditions was calculated for each subject in each block, and subjects were treated as trials in an outlier analysis. Correct mean RTs and percentage errors are reported in each of the tables. None of the error differences in any of the blocks are significant and are not discussed further.
The first order of business is to verify that, when only an arrow or a pair eyes are presented on a trial, the two dimensions are equally discriminable. In Block 1, participants were equally fast to identify the direction of eyes or an arrow, on separate trials, by pressing a key on the left or right of the keyboard. There was no significant difference between the two dimensions, t (46) = 1.05, SEM = 3.11, p = .301, replicating the null reported by Besner et al. (2020). The associated BF (BF01 = 3.8) means there is 3.8 times as much evidence for the null as there is for the alternative hypothesis. 3 These data can be seen in Table 1.
Table 1.
Block 1: unidimensional trials (mean RTs [ms] and errors [%]).
| RTs | Errors | ||
|---|---|---|---|
| Eye |

|
410 | 1.0% |
| Arrow |
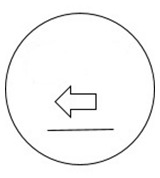
|
407 | 1.0% |
RTs: response times.
It could be objected that the unidimensional stimuli used in Block 1 fails to address the possibility that one dimension might be less discriminable in the presence of the other dimension. In response to this possibility, we note that O’Malley and Besner (2014) report an experiment whose stimuli are identical to those used here in Block 3. In their experiment the eyes and an arrow were presented on every trial, and depending on the status of an auditory cue, participants were asked to determine in which direction the relevant dimension was pointing to. O’Malley and Besner reported (twice, in their Experiment 1) that the Arrow task was anecdotally slower than the Eye task. Furthermore, the Congruency effect for judgements about eye direction, when the arrow was to be ignored, were equal in magnitude to the Congruency effect when the judgement was about arrow direction, and the eyes were to be ignored. These results should allay concern that the two cues are not equally discriminable when both dimensions are present.
The data from Block 2 can be seen in Table 2. In this block both cues were presented on every trial, and always pointed in the same direction; in half the trials the cues pointed left, and in the remaining trials they pointed right. Block 2 produced a significant cueing effect; valid trials were faster than invalid trials, t(46) = 3.80, SEM = 2.08, p < .001, BF10 = 62.8.
Table 2.
Block 2: all congruent trials (mean RTs [ms] and errors [%]).
| RTs | Errors | ||
|---|---|---|---|
| Valid (e.g., target appears on the left) |

|
427 | 6.4% |
| Invalid (e.g., target appears on the left) |

|
435 | 7.1% |
RTs: response times.
In Block 3, a validity effect was again seen when both cues pointed in the same direction (Table 3), t(46) = 2.48, SEM = 2.06, p < .02, but this effect was anecdotal BF10 = 2.48.
Table 3.
Block 3: congruent trials (mean RTs [ms] and errors [%]).
| RTs | Errors | ||
|---|---|---|---|
| Valid (e.g., target appears on the left) |

|
430 | 6.7% |
| Invalid (e.g., target appears on the left) |

|
435 | 5.8% |
RTs: response times.
Incongruent trials
Most critically, half the trials in Block 3 consisted of incongruent trials where the two cues pointed in opposite directions. This difference was not significant, t(46) = 1.41, SEM = 2.25, p = .167. This null result, in the first instance, suggests that neither cue can be considered to be automatic insofar as it was resistant to interference from the other cue when it pointed in the opposite direction.
However, this putatively null result is ambiguous because the BF value for the null of 2.52 is only anecdotal. A t test is a sufficient test of the three alternative theoretical hypotheses considered here, but it is not the only possible test, nor the most robust. Additional analyses are reported below in which the Bayesian analysis provides stronger support for the hypothesis that an arrow dominates the eyes when the cues are incongruent.
We computed a pair of analyses of variance (ANOVAs). In one, Congruency and Validity are factors, and arrow direction is coded as valid/invalid in the incongruent condition (Table 4). This ANOVA yielded a main effect of Congruency, F(1, 46) = 5.72, MSE = 117.64, p < .05, but the BF provided only anecdotal support for the alternative hypothesis; BF10 = 2.2. There was a main effect of Validity, F(1, 46) = 7.82, MSE = 103.09, p = .008, BF10 = 4.1; this result supports the alternative hypothesis. Critically, the interaction of these two factors was not significant, F(1, 46) = .385, MSE = 115.95, p = .538. This null interaction is BF01 = 5.62 (there is 5.62 times more evidence for the null interaction than there is for the alternative). Put differently, the validity effect for the arrows in the incongruent condition in Block 3 is the same size as when the eyes and arrows are congruent.
Table 4.
Block 3: incongruent trials: arrows coded as valid (mean RTs [ms] and errors [%]).
| RTs | Errors | ||
|---|---|---|---|
| Valid arrow (e.g., target appears on left) Invalid eye |

|
434 | 6.5% |
| Invalid arrow (e.g., target appears on left) Valid eye |

|
438 | 6.6% |
RTs: response times.
In the second ANOVA, Congruency and Validity are factors, and eye direction is coded as valid/invalid in the incongruent condition (Table 5). This analysis yielded a main effect of Congruency, F(1, 46) = 5.72, MSE = 117.64, p < .05; but the BF10 of 2.2 provides only anecdotal support for the alternative hypothesis. There was no effect of Validity, F(1, 46) = .385, MSE = 115.95, p = .538. The BF01 value of 5.34 supports the null hypothesis over the alternative hypothesis. Critically, the interaction of these two factors is significant, F(1, 46) = 7.82, MSE = 103.09, p < .005; the Bayes value supports the alternative hypothesis (BF10 = 5.1). This interaction reflects the cross-over of a validity effect in the congruent case, and its elimination in the incongruent case.
Table 5.
Block 3: incongruent trials: eyes coded as valid (mean RTs [ms] and errors [%]).
| RTs | Errors | ||
|---|---|---|---|
| Valid eye (e.g., target appears on left) Invalid arrow |

|
438 | 6.6% |
| Invalid eye (e.g., target appears on left) Valid arrow |

|
434 | 6.5% |
Discussion
These data are compatible with two conclusions. First, empirically speaking, a cueing effect for eyes (i.e., when eye direction is coded as valid/invalid) is eliminated in the incongruent cues condition where the two cues are pitted against one another. This is consistent with the theoretical conclusion that eyes as cues do not reflect an automatic process in terms of an interference criterion. Second, empirically speaking, when arrow direction is coded as valid/invalid), a cueing effect was observed (valid trials were faster than invalid trials) and this effect was not qualified by Congruency, nor was the interaction of Congruency by Validity. This is consistent, theoretically, with the conclusion that arrows as cues produce automatic orienting in the narrow sense that they are resistant to interference from the eyes in the incongruent condition.
Put differently, if one were committed to an “automatic” processing perspective, as seen in various writings (e.g., Friesen & Kingstone, 1998; Galfano et al., 2012) then the present results show, at least for the claim that eyes elicit automatic orienting in the present context, that such an account is problematic. In the case of arrows, we would not wish to claim that arrows as cues are “automatic” in an across-the-board sense. We think it prudent to suppose instead that context dependency may apply (e.g., carrying a novel visuospatial memory load on each trial might eliminate the cueing effect when arrow direction is coded as valid/invalid).
To be sure, a number of researchers have also concluded that their results imply that spatial cueing with eyes (and sometimes with arrows) arrows are “context dependent” (e.g., Pereira et al., 2020; Ricciardelli et al., 2013). The present results, based on a novel experimental design that involves the direct comparison of effects within a trial of the two cues, add to these previous findings. It is, nonetheless, a useful reminder that “context dependency” is not a theory as such, but rather a claim that context matters in ways hitherto not widely considered (e.g., like in the present case). This inevitably means that the way(s) forward needs to acknowledge and treat these nuances (e.g., goals; intentions; local conditions) as important features inherent to understanding the underlying mental processes in the determination of eye and arrow direction. Most generally, too much emphasis on all or none “automaticity” is likely to hinder rather than deepen our understanding of mental processing.
To be sure, other outcomes are possible, and admit of other interpretations. For example, there could be an interaction between validity and congruency such that the size of the validity effect for the incongruent trials is smaller than for congruent trials, but equal in size for both cues. This outcome is compatible with the hypothesis that only a single cue at a time carries the day on an incongruent trial (i.e., winner takes all notion).
The positioning of the target letter with respect to the schematic face was manipulated to encourage the subjects to attend globally, and hence there were two possible horizontal locations on each side where the target letter could appear. These appeared in one of two possible horizontal locations, one in line with the central axis of the arrow and the other in line with the eyes. There was less than 1-ms difference overall between the two locations, indicating that target position was not a moderating factor, and hence this factor was ignored in all subsequent analyses. In a previous experiment with only one location of the target per side and that location being at the centre of the stimulus, the same pattern of effects was seen, and the same statistical outcomes were observed.
Bayes factors (BF) for t tests and analyses of variance (ANOVAs) were calculated using the Jamovi software with r set to the default value (.707; see The Jamovi Project, 2020). BF10 means the extent to which the evidence supports the alternative hypothesis over the null hypothesis, whereas BF01 means the extent to which the evidence supports the null hypothesis over the alternative hypothesis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) to D.B.
ORCID iD: David McLean  https://orcid.org/0000-0003-4345-5083
https://orcid.org/0000-0003-4345-5083
References
- Besner D., McLean D., Young T. (2020). On the determination of eye gaze direction: Automaticity reconsidered. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- Driver J., IV, Davis G., Ricciardelli P., Kidd P., Maxwell E., Baron-Cohen S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6(5), 509–540. [Google Scholar]
- Friesen C. K., Kingstone A. (1998). The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review, 5(3), 490–495. 10.3758/BF03208827 [DOI] [Google Scholar]
- Friesen C. K., Ristic J., Kingstone A. (2004). Attentional effects of counter-predictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30(2), 319–329. [DOI] [PubMed] [Google Scholar]
- Frischen A., Bayliss A. P., Tipper S. P. (2007). Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin, 133(4), 694–724. 10.1037/0033-2909.133.4.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfano G., Dalmaso M., Marzoli D., Pavan G., Coricelli C., Castelli L. (2012). Eye gaze cannot be ignored (but neither can arrows). Quarterly Journal of Experimental Psychology, 65(10), 1895–1910. 10.1080/17470218.2012.663765 [DOI] [PubMed] [Google Scholar]
- Hoehl S., Reid V., Mooney J., Striano T. (2008). What are you looking at? Infants’ neural processing of an adult’s object-directed eye gaze. Developmental Science, 11, 10–16. 10.1111/j.1467-7687.2007.00643.x [DOI] [PubMed] [Google Scholar]
- The Jamovi Project. (2020). Jamovi (Version 1.2). https://www.jamovi.org
- Jeffreys H. (1961). Theory of probability (3rd ed.). Oxford University Press. [Google Scholar]
- Kuhn G., Kingstone A. (2009). Look away! Eyes and arrows engage oculomotor responses automatically. Attention Perception & Psychophysics, 71(2), 314–327. [DOI] [PubMed] [Google Scholar]
- O’Malley S., Besner D. (2014). Is eye gaze direction always determined without intent? Psychonomic Bulletin & Review, 21(6), 1495–1500. [DOI] [PubMed] [Google Scholar]
- Pereira E. J., Birmingham E., Ristic J. (2020). The eyes do not have it after all? Attention is not automatically biased towards faces and eyes. Psychological Research, 84, 1407–1423. 10.1007/s00426-018-1130-4 [DOI] [PubMed] [Google Scholar]
- Popper K. R. (1959). The logic of scientific discovery. Science Editions. [Google Scholar]
- Ricciardelli P., Carcagno S., Vallar G., Bricolo E. (2013). Is gaze following purely reflexive or goal-directed instead? Revisiting the automaticity of orienting attention by gaze cues. Experimental Brain Research, 224(1), 93–106. [DOI] [PubMed] [Google Scholar]
- Tipples J. (2002). Eye gaze is not unique: Automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review, 9(2), 314–318. [DOI] [PubMed] [Google Scholar]


