Abstract
Background:
Comparative effectiveness of different types of palliative homecare is sparsely researched internationally—despite its potential to inform necessary decisions in palliative care infrastructure development. In Germany, specialized palliative homecare delivered by multi-professional teams has increased in recent years and factors beyond medical need seem to drive its involvement and affect the application of primary palliative care, delivered by general practitioners who are supported by nursing services.
Aim:
To compare effectiveness of primary palliative care and specialized palliative homecare in reducing potentially aggressive interventions at the end-of-life in cancer and non-cancer.
Design:
Retrospective population-based study with claims data from 95,962 deceased adults in Germany in 2016 using multivariable regression analyses.
Settings/participants:
Patients having received primary palliative care or specialized palliative homecare (alone or in addition to primary palliative care), for at least 14 days before death, differentiating between cancer and non-cancer patients.
Results:
Rates of potentially aggressive interventions in most indicators were higher in primary palliative care than in specialized palliative homecare (p < 0.01), in both cancer and non-cancer patients: death in hospital (odds ratio (OR) 4.541), hospital care (OR 2.720), intensive care treatment (OR 6.749), chemotherapy (OR 2.173), and application of a percutaneous endoscopic gastrostomy (OR 4.476), but not for parenteral nutrition (OR 0.477).
Conclusion:
Specialized palliative homecare is more strongly associated with reduction of potentially aggressive interventions than primary palliative care in the last days of life. Future research should identify elements of specialized palliative homecare applicable for more effective primary palliative care, too. German Clinical Trials Register (DRKS00014730).
Keywords: palliative care, terminal care, homecare services, primary health care, quality of health care, retrospective studies, administrative claims, Germany
What is already known about the topic?
Palliative homecare is an important component of palliative care, and it has a positive impact on the quality of care at the end-of-life.
The effect of different types of palliative homecare on quality of care is sparsely researched.
Within palliative homecare, cancer patients still outweigh non-cancer patients.
What this paper adds?
We compared two types of palliative homecare in Germany: primary palliative care and specialized palliative homecare. Both reduced potentially aggressive interventions at the end-of-life.
The more comprehensive specialized palliative homecare was associated with less potentially aggressive interventions in terms of lower rates of hospital as the place of death, hospital care, intensive care treatment, chemotherapy, and application of a percutaneous endoscopic gastrostomy (PEG) within the last days of life. These results hold equally for cancer patients as well as non-cancer patients.
Only for parenteral nutrition, we found a possible indication of oversupply in cancer patients (excepting those with gastrointestinal cancer) within specialized palliative homecare.
Implications for practice, theory or policy
The potential of palliative homecare, particularly of specialized palliative homecare, to reduce potentially aggressive interventions at the end-of-life deserves more attention in healthcare and health politics.
Future studies should investigate which elements of specialized palliative homecare are effective and can be integrated into primary palliative care, where appropriate.
Introduction
Patients with life-shortening diseases have the option of receiving palliative care near the end-of-life. In many high-income countries, there are different types and levels of palliative homecare. 1 In Germany, the majority of patients receive primary palliative care, mainly provided as homecare by general practitioners with support from nursing or hospice services. For patients with very complex symptoms, primary palliative care alone might not be sufficient. These patients might be in need of a multi-professional team, including physicians and nurses with mandatory qualification in palliative care, providing specialized palliative homecare (alone or in addition to primary palliative care). In recent years, the uptake of specialized palliative homecare as the more comprehensive and more costly form of palliative homecare has increased.2,3 At the same time, it is evident that specialized palliative homecare does not only depend on the patient’s medical need (and demand) but also on surrounding factors that can impede the involvement of primary palliative care alone, such as the available palliative care infrastructure, as well as the high workload of general practitioners. 4 Therefore, it is valuable to compare the impact of primary palliative care and specialized palliative homecare in end-of-life care.
Systematic reviews and meta-analyses1,5 –7 as well as claims data analyses8 –10 show that palliative care in general as well as different types such as hospital-based and homecare have a positive effect on end-of-life care. However, the comparative effectiveness of different types of palliative homecare on potentially aggressive interventions at the end-of-life has been less studied so far. In the literature we found a subsequent analysis 11 of a meta-analysis, 6 that delivers an indirect comparison between specialized palliative care (not differentiating between hospital-based and homecare) and primary palliative care. As a result, both types of palliative care improve patients’ quality of life and clinical outcomes, such as symptom management. Another study 12 concludes that, in a mixed palliative care model, the prevention of potentially aggressive interventions at the end-of-life is suspected to be related to the large share of patients within primary palliative care—but again without explicitly distinguishing between the two types of palliative homecare in terms of outcomes. It should also be noted that most patients who receive palliative care are cancer patients, and that they are disproportionately overrepresented in the German specialized palliative homecare with up to 88%2,13,14 against 64% 2 in primary palliative care. This is relevant as non-cancer patients receive less palliative care and more potentially aggressive interventions.15,16 In addition to possibly different effects of primary palliative care and specialized palliative homecare on potentially aggressive interventions at the end-of-life, we also expect an impact of the unequal distribution of non-cancer patients.
Thus, our study aims to close an evidence-gap on the comparative effectiveness of primary palliative care and specialized palliative homecare on healthcare indicators at the end-of-life in cancer and non-cancer patients. The results can help to identify promising directions for further development of palliative homecare.
Methods
Study design and setting
As a subproject of the multi-methods study “SAVOIR—evaluation of specialised palliative homecare (SPHC) in Germany: outcomes, interactions, regional differences” 17 we conducted a retrospective population-based study using claims data.
Our study population contained the total number of 95,962 people aged 19 years or older, who died in 2016, were insured with a large German social health insurance fund (BARMER) for at least 2 years before death and resided in a federal state of Germany. This corresponds to about 10.5% of the German population. 18
The two different types of palliative homecare were determined on the documentation of at least one corresponding service. The use of related fee schedule items to identify services (see eSupplement ) has already been applied in other claims data-based studies.2,19 To ensure that palliative care could have an impact on the indicators presented, palliative care had to be documented at least once before the observation period of 14 days before death. This observation period was chosen because palliative homecare is often initiated close to death.2,20 To enable comparison with more other studies, we also examined the observation period of 30 days before death.21,22 These results are presented and discussed in the eSupplement . The assignment of diseases (cancer or non-cancer) was based on documented diagnoses according to Murtagh et al. 23 (for ICD-10 codes, see eSupplement: Table 1).
Data source
The analyzed health claims data included demographic information, outpatient and hospital diagnoses and procedures, and outpatient drug prescriptions. For further details, access to data and data cleaning procedures see eSupplement .
Healthcare indicators
For both observation periods, the following healthcare indicators, which have already been used internationally,10,21,22,24 –26 were calculated: place of death as an indicator of palliative homecare until the end-of-life; a potentially aggressive intervention was assumed for hospital care, intensive care treatment, chemotherapy, parenteral nutrition, insertion or change of a percutaneous endoscopic gastrostomy (PEG). A detailed description is depicted in Table 1.
Table 1.
Definition of used healthcare indicators in end-of-life care.
| Healthcare indicator | In claims data identified by | Subpopulation |
|---|---|---|
| Place of death: hospital | Discharge type “death” in hospital case data 1 | All patients with palliative care |
| Hospital care | Begin or end of a hospital case without palliative care service (OPS 2 : 8-98e or 8-982) during the observation period | All patients with palliative care |
| Intensive care treatment | A corresponding hospital procedure code (OPS: 8-980 or 8-98f) during the observation period | All patients with palliative care |
| Chemotherapy | A chemotherapeutic treatment code (ATC 3 : L01 without L01CH or L01CP, PZN 4 : 09999092, GOP 5 : 86510, 86512, 86514, 86516, OPS: 8-54) during the observation period | Patients with palliative care and a diagnosis of cancer (ICD: C00 to C97) |
| Parenteral nutrition | A parenteral treatment code (ATC: B05BA, OPS: 8-016, OPS 8-018) during the observation period | Patients with palliative care and without gastrointestinal cancer or gastrointestinal metastases (ICD: C15 to C26, C78.4 to C78.8) |
| Insertion or change of a PEG 6 | An insertion or change of a PEG (GOP: 13412, OPS: 431.2, 5-431.2, 8-123.0, 5-431.2, 8-123.0) during the observation period | Patients with palliative care and without gastrointestinal cancer or gastrointestinal metastases (ICD: C15 to C26, C78.4 to C78.8) |
Other places of death were considered as “not in a hospital” and include all deaths in a domestic environment: at home, in a nursing home, hospice, or other “domestic” places.
OPS German version of the international classification of procedures in medicine.
ATC Anatomical Therapeutic Chemical Code.
PZN Central Pharmaceutical Number.
GOP fee schedule item.
PEG percutaneous endoscopic gastrostomy.
Statistical analysis
Statistical analysis was performed by Independent Samples t Test for continuous parameters (mean, mean differences [MD], standard deviation [SD]) and Chi-Squared Test of Independence for binary outcomes (change in percentage points [PP]). The group differences between primary palliative care and specialized palliative homecare and between cancer and non-cancer patients in terms of the healthcare indicators were presented with odds ratios (OR) calculated by multivariable logistic regression. To exclude other influences on measured outcomes, we examined age, gender, nursing home resident status, rural or urban geographic location, cancer or non-cancer diagnosis, and comorbidity measured by the Charlson Comorbidity Index [CCI]. 27 Statistical differences in these variables between the types of palliative homecare were included in the model as covariates. To assess the combined impact of the type of palliative homecare and the underlying disease, the interaction of both was tested for statistical significance. Detailed results are presented in the eSupplement . All analyses were exploratory, so there was no correction for multiple testing. The differences were reported with a 95% confidence interval (CI) and the statistical significance level was set to 5%. All statistical analyses were performed with SAS 9.4 and SAS Enterprise Guide 7.1 (SAS Institute, Inc.; Cary/NC). Design, performance and report of the claims data analyses were based on the recommendations of STROSA (consensus German reporting standard for secondary data analyses) 28 and RECORD (reporting of studies conducted using observational routinely-collected health data). 29
Results
Study population
From a total of 95,962 individuals deceased in 2016, 18,906 patients could be included in the analysis (Figure 1).
Figure 1.
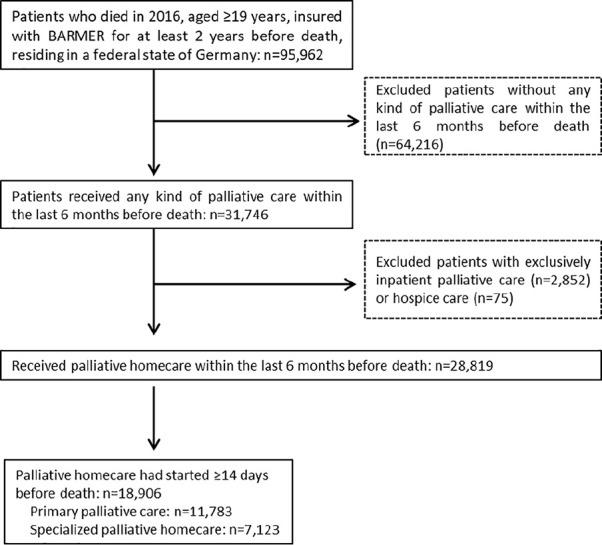
Flow-chart study population.
Patient characteristics are shown in Table 2: Patients within primary palliative care were older (mean difference (MD) +6.67 years, p < 0.0001), more often female (percentage points difference (PP) +2.73, p = 0.0002), lived more often in nursing homes (PP +13.01, p < 0.0001), had fewer comorbidities (MD −1.07, p < 0.0001) and fewer had cancer diagnoses (PP −28.79, p < 0.0001).
Table 2.
Patients’ characteristics, 14 days before death.
| Primary palliative care |
Specialized palliative homecare |
Total |
Primary palliative care vs Specialized palliative homecare |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Non-cancer | Cancer | All | Non-cancer | Cancer | All | Non-cancer | Cancer | MD or PP | p-Value | |
| Patients receiving palliative homecare 14 days before death | |||||||||||
| n | 11,783 | 5141 | 6629 | 7123 | 1065 | 6058 | 18,906 | 6219 | 12,687 | <0.0001 | |
| % | 62.32 | 43.74 | 56.26 | 37.68 | 14.95 | 85.05 | 100.00 | 32.89 | 67.11 | ||
| Patients without gastrointestinal cancer or corresponding metastases | |||||||||||
| n | 8637 | 5141 | 3496 | 3704 | 1065 | 2639 | 12,341 | 6219 | 6135 | <0.0001 | |
| % of all | 73.30 | 59.52 | 40.48 | 52.00 | 28.75 | 71.25 | 65.28 | 50.39 | 49.61 | 21.30 | |
| Age in years | |||||||||||
| Mean (SD) | 81.06 (11.32) | 85.79 (9.32) | 77.38 (11.37) | 74.39 (12.25) | 81.24 (12.61) | 73.18 (11.78) | 78.54 (12.12) | 85.01 (10.10) | 75.37 (11.76) | 6.67 | <0.0001 |
| Female gender | |||||||||||
| n | 7197 | 3643 | 3554 | 4156 | 648 | 3472 | 11,353 | 4327 | 7026 | 0.0002 | |
| % | 61.08 | 50.62 | 49.38 | 58.35 | 16.46 | 83.54 | 60.05 | 38.11 | 61.89 | 2.73 | |
| Morbidity weight, CCI | |||||||||||
| Mean (SD) | 5.25 (3.74) | 3.68 (2.60) | 6.47 (4.03) | 6.32 (4.05) | 3.81 (2.75) | 6.76 (4.08) | 5.65 (3.89) | 3.71 (2.63) | 6.61 (4.06) | –1.07 | <0.0001 |
| Nursing home resident | |||||||||||
| n | 5164 | 3154 | 2010 | 2195 | 544 | 1651 | 7359 | 3698 | 3661 | <0.0001 | |
| % | 43.83 | 61.08 | 38.92 | 30.82 | 24.78 | 75.22 | 38.92 | 50.25 | 49.75 | 13.01 | |
| Residency, urban | |||||||||||
| n (missing: primary palliative care n = 452, specialized palliative homecare n = 270) | 8040 | 3628 | 4412 | 4871 | 817 | 4054 | 12,911 | 4445 | 8466 | 0.8661 | |
| % | 70.96 | 45.12 | 54.88 | 71.08 | 16.77 | 71.08 | 71.00 | 34.43 | 65.57 | –0.12 | |
CCI: Charlson comorbidity index; MD: mean difference; PP: percentage points difference; SD: standard deviation.
Healthcare indicators
The results are shown in Table 3.
Table 3.
Healthcare indicators, 14 days before death.
| Primary palliative care |
Specialized palliative homecare |
Total |
Primary palliative care vs Specialized palliative homecare |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Non-cancer | Cancer | All | Non-cancer | Cancer | All | Non-cancer | Cancer | PP | p-Value | |
| Patients receiving palliative homecare 14 days before death | |||||||||||
| n | 11,783 | 5141 | 6629 | 7123 | 1065 | 6058 | 18,906 | 6219 | 12,687 | ||
| % | 62.32 | 43.74 | 56.26 | 37.68 | 14.95 | 85.05 | 100.00 | 32.89 | 67.11 | ||
| Place of death, hospital | |||||||||||
| n | 4232 | 1219 | 3013 | 1338 | 90 | 1248 | 5570 | 1309 | 4261 | <0.0001 | |
| % | 35.92 | 23.65 | 45.45 | 18.78 | 8.45 | 20.60 | 29.46 | 21.05 | 33.59 | 17.14 | |
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 4.541 [4.178–4.936] | ||||||||||
| Odds ratio non-cancer, primary palliative care vs specialized palliative homecare [CI] | 5.446 [4.274–6.938] | 5.446 [4.274–6.938] | Interaction primary palliative care vs specialized palliative homecare and non-cancer vs cancer p = 0.1150 | ||||||||
| Odds ratio cancer, primary palliative care vs specialized palliative homecare [CI] | 4.435 [4.061–4.884] | 4.435 [4.061–4.884] | |||||||||
| Hospital care | |||||||||||
| n | 3036 | 951 | 2085 | 1114 | 93 | 1021 | 4150 | 1044 | 3106 | <0.0001 | |
| % | 25.77 | 18.45 | 31.45 | 15.64 | 8.73 | 16.85 | 21.95 | 16.79 | 24.48 | 10.13 | |
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 2.720 [2.499–2.961] | ||||||||||
| Odds ratio non-cancer, primary palliative care vs specialized palliative homecare [CI] | 3.060 [2.424–3.864] | 3.060 [2.424–3.864] | Interaction primary palliative care vs specialized palliative homecare and non-cancer vs cancer p = 0.2856 | ||||||||
| Odds ratio cancer, primary palliative care vs specialized palliative homecare [CI] | 2.673 [2.441–2.926] | 2.673 [2.441–2.926] | |||||||||
| Intensive care treatment | |||||||||||
| n | 516 | 192 | 324 | 61 | 11 | 50 | 577 | 203 | 374 | <0.0001 | |
| % | 4.38 | 3.73 | 4.89 | 0.86 | 1.03 | 0.83 | 3.05 | 3.26 | 2.95 | 3.52 | |
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 6.749 [5.110–8.912] | ||||||||||
| Odds ratio non-cancer, primary palliative care vs specialized palliative homecare [CI] | 5.005 [2.699–9.282] | 5.005 [2.699–9.282] | Interaction primary palliative care vs specialized palliative homecare and non-cancer vs cancer p = .3019 | ||||||||
| Odds ratio cancer, primary palliative care vs specialized palliative homecare [CI] | 7.190 [5.282–9.787] | 7.190 [5.282–9.787] | |||||||||
| Chemotherapy | |||||||||||
| Cancer patients | 6629 | 6058 | 12,687 | ||||||||
| n | 411 | 221 | 632 | <0.0001 | |||||||
| % | 6.20 | 3.65 | 4.98 | 2.55 | |||||||
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 2.173 [1.823–2.591] | ||||||||||
| Parenteral nutrition | |||||||||||
| Patients without gastrointestinal cancer or corresponding metastases | 8637 | 5141 | 3496 | 3704 | 1065 | 2639 | 12,341 | 6219 | 6135 | ||
| n | 48 | 20 | 28 | 75 | 4 | 71 | 123 | 24 | 99 | <0.0001 | |
| % | 0.56 | 0.39 | 0.80 | 2.02 | 0.38 | 2.69 | 1.00 | 0.39 | 1.61 | –1.46 | |
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 0.477 [0.323–0.705] | ||||||||||
| Odds ratio non-cancer, primary palliative care vs specialized palliative homecare [CI] | 1.553 [0.521–4.629] | 1.553 [0.521–4.629] | Interaction primary palliative care vs specialized palliative homecare and non-cancer vs cancer p = 0.0164 | ||||||||
| Odds ratio cancer other than gastrointestinal or corresponding metastases, primary palliative care vs specialized palliative homecare [CI] | 0.368 [0.233–0.581] | 0.368 [0.233–0.581] | |||||||||
| Insertion or change of a PEG | |||||||||||
| Patients without gastrointestinal cancer or corresponding metastases | 8637 | 5141 | 3496 | 3704 | 1065 | 2639 | 12,341 | 6219 | 6135 | ||
| n | 41 | 25 | 16 | 5 | 1 | 4 | 46 | 26 | 20 | 0.0045 | |
| % | 0.47 | 0.49 | 0.46 | 0.13 | 0.09 | 0.15 | 0.37 | 0.42 | 0.33 | 0.34 | |
| Odds ratio primary palliative care vs specialized palliative homecare [CI] | 4.476 [1.717–11.666] | ||||||||||
| Odds ratio non-cancer, primary palliative care vs specialized palliative homecare [CI] | 7.185 [0.961–53.715] | 7.185 [0.961–53.715] | Interaction primary palliative care vs specialized palliative homecare and non-cancer vs cancer p = 0.5715 | ||||||||
| Odds ratio cancer other than gastrointestinal or corresponding metastases, primary palliative care vs specialized palliative homecare [CI] | 3.716 [1.229–11.229] | 3.716 [1.229–11.229] | |||||||||
CI: confidence interval; PEG: percutaneous endoscopic gastrostomy; PP: percentage points difference; SD: standard deviation.
Place of death: Patients within primary palliative care had a higher probability of dying in hospital than patients within specialized palliative homecare (PP +17.14, p < 0.0001; OR 4.541 [CI 4.178–4.936]). This difference was also seen in subgroups of cancer and non-cancer patients, due to the non-significant interaction of disease and type of palliative homecare (OR non-cancer 5.446 [CI 4.274–6.938]; OR cancer 4.435 [CI 4.061–4.884]; interaction p = 0.1150).
Hospital care: The probability of receiving hospital treatment was higher for patients within primary palliative care compared to specialized palliative homecare (PP +10.13, p < 0.0001; OR 2.720 [CI 2.499–2.961]). Again, the difference remained in the subgroups of cancer and non-cancer patients (OR non-cancer 3.060 [CI 2.424–3.864]; OR cancer 2.673 [CI 2.441–2.926]; interaction p = 0.2856).
Intensive care treatment: The risk of receiving intensive care treatment was higher within primary palliative care than within specialized palliative homecare (PP +3.52, p < 0.0001; OR 6.749 [CI 5.110–8.912]). The difference remained in the subgroups of cancer and non-cancer patients (OR non-cancer 5.005 [CI 2.699–9.282]; OR cancer 7.190 [CI 5.282–9.787]; interaction p = 0.3019).
Chemotherapy: The incidence of cancer patients receiving chemotherapy was higher within primary palliative care than within specialized palliative homecare (PP +2.55, p < 0.0001; OR 2.173 [CI 1.823–2.591]).
Parenteral nutrition: Irrespective of the underlying disease (excluding gastrointestinal cancer or corresponding metastases), patients within primary palliative care had a lower probability of receiving parenteral nutrition than within specialized palliative homecare (PP −1.46, p < 0.0001; OR 0.477 [0.323–0.705]). Taking into account cancer and non-cancer patients, there was a significant interaction of disease and type of palliative homecare (OR non-cancer 1.553 [CI 0.521–4.629]; OR cancer 0.368 [CI 0.233–0.581]; interaction p = 0.0164). This means that the probability of receiving more parenteral nutrition in specialized palliative homecare than in primary palliative care only holds for cancer patients.
Insertion or change of a percutaneous endoscopic gastrostomy (PEG): Looking at the same subgroup of patients without gastrointestinal cancer or corresponding metastases, patients within primary palliative care had a higher probability of insertion or change of a PEG than those within specialized palliative homecare (PP +0.34, p = 0.0045; OR 4.476 [CI 1.717–11.666]). The difference remained in the subgroups of cancer and non-cancer patients (OR non-cancer 7.185 [CI 0.961–53.715]; OR cancer 3.716 [CI 1.229–11.229]; interaction p = 0.5715).
Discussion
Main findings
In this retrospective claims data study, we compared primary palliative care and specialized palliative homecare in terms of healthcare indicators at the end-of-life to identify promising directions for further development of palliative homecare. Primary palliative care was more associated with potentially aggressive interventions at the end-of-life than specialized palliative homecare regarding the indicators of hospital care, death in hospital, intensive care treatment, chemotherapy, and insertion or change of a PEG. The benefit of specialized palliative homecare was evident in both subgroups of cancer and non-cancer patients. Hints of more potentially aggressive interventions within specialized palliative homecare than within primary palliative care were only evident in parenteral nutrition.
Primary palliative care versus specialized palliative homecare: comparison with international and national literature
Comparisons of our results with findings from other national and international studies were only possible for aggregated types of palliative care or for populations without palliative care. For an overview, see Table 4. In summary, our findings of potentially aggressive interventions compared reasonably well to international studies, confirming the plausibility of the results. Compared to no palliative care, both types of palliative homecare seem to reduce potentially aggressive interventions at the end-of-life. According to our results, specialized palliative homecare was the more effective of these.
Table 4.
Overview of comparing our results with findings form other studies.
| Healthcare indicator | Our results (Table 3) | Results from international studies | Results from other German studies |
|---|---|---|---|
| Place of death: hospital | Primary palliative care 35.9%; Specialized palliative homecare 18.8%; together: 29.5% | Palliative care: 30.3% 8 , 39.0% 9 ; no palliative care: 74.8% 9 , 80.3% 8 | Overall, including palliative care and no palliative care: 38.1% to 42.0%25,30,31 |
| Hospital care | Primary palliative care 25.8%; Specialized palliative homecare 15.6%; together 22.0% | Palliative care: 27.4% 10 (last 14 days) to 76% 8 (last 180 days); no palliative care: 60.8%, 10 98.5% 8 | Overall, including palliative care and no palliative care: 44.8% 25 (last 30 days) |
| Intensive care treatment | Primary palliative care 4.4%; Specialized palliative homecare 0.9%; together 3.0% | Palliative care: 4.6% 8 (last 180 days), 18.3% 10 (last 14 days); no palliative care: 14.3%, 8 40.4% 10 | Overall, including palliative care and no palliative care: 3.5% 25 (last 30 days) |
| Chemotherapy | Primary palliative care 6.2%; Specialized palliative homecare 3.7%; together 5.0% | Overall, including palliative care and no palliative care: 11.3% 32 (last 14 days); 4.8%–12.7% 25 (last 30 days) | Overall, including palliative care and no palliative care; only outpatient chemotherapy: 10.5% 25 (last 30 days) |
| Parenteral nutrition | Primary palliative care 0.6%; Specialized palliative homecare 2.0%; together 1.0% | Overall, including palliative care and no palliative care; subsumed as artificial nutrition: 2.9% 33 (last 7 days); 0.7% 22 (last 14 days) | |
| Insertion/change of a PEG | Primary palliative care 0.5%; Specialized palliative homecare 0.1%; together 0.4% | Specialized palliative homecare: 1.5% to 3.7% 2 (last 90 days in patients with dementia) |
Comparing our results with other German studies that examined the same types of palliative homecare is limited because of different populations and observation periods. We found that rates of patients dying in hospital in our study were much higher than reported in two existing case studies, however, these assessed only one specialized palliative homecare team each.13,20
It should be noted that two indicators—parenteral nutrition, and the insertion or change of a PEG—showed remarkably low frequencies, despite our large study population (about 10% of the deceased Germans in 2016). These findings should, therefore, be interpreted with caution. Furthermore, parenteral nutrition requires a more detailed reflection, since it was the only indicator without advantage for specialized palliative homecare. It is already known that younger age and male gender are associated with higher rates of artificial nutrition. 33 However, even after adjustment for these parameters, patients within specialized palliative homecare showed a higher probability for parenteral nutrition. This possible overprovision within specialized palliative homecare should be analyzed in future studies.
Palliative care for cancer and non-cancer patients
It is already known that non-cancer patients receive palliative care less frequently. 16 This was in line with our results: we observed that there were more cancer patients within specialized palliative homecare than within primary palliative care. There is also evidence that non-cancer patients receive lower quality palliative care. 15 An explanation for this may be the more difficult predictability of disease progression 34 and the associated more complex homecare 35 in non-cancer patients. Thus, we suspected similar results from our analysis. However, the results contradicted this: in purely descriptive numbers, we depicted a pattern of slightly lower rates of potentially aggressive interventions in non-cancer patients within primary palliative care, even more so within specialized palliative homecare. Adjusted by logistic regression and tested for interaction, the benefit of specialized palliative homecare remained in the subgroups cancer and non-cancer patients. To find explanations for this result, we consulted the literature again. The question of whether cancer patients require a different palliative care approach than non-cancer patients is an important issue in current palliative care research.15,16,36 –38 As there might be little to no difference in the severity of symptoms, end-of-life care seems to be more influenced by the severity of the life-limiting disease, by the attitudes toward the disease, and by the social support the patient receives than by the category cancer versus non-cancer. 38 We therefore derive that presumably effective elements of specialized palliative homecare (such as keeping a team on standby 24/7, training in palliative care, and coordination of care) 39 also display effect in the complex care of non-cancer patients. Further research should explore the single factors/elements that produce positive effects in terms of avoiding potentially aggressive interventions at the end-of-life.
Implications for further development of palliative homecare
The better overall results for specialized palliative homecare may enforce the conclusion to recommend the expansion of specialized palliative homecare capacities. However, there are far more aspects to consider than those we have studied. Firstly, there are repercussions of specialized palliative homecare for the provision of palliative as well as primary care in general. For example, this has been described as fragmentation of primary care and a lack of responsibility of all other than specialized palliative homecare providers. 40 Secondly, there is an economic necessity to take associated costs of organizing palliative homecare into account. Specialized palliative homecare may avoid potentially aggressive interventions at the end-of-life, which spares financial resources, but this might be balanced out by higher resources. Related cost-effectiveness ratios are not known yet. Therefore, the already available evidence for cost savings in palliative homecare in general 41 should also be investigated for primary palliative care versus specialized palliative homecare.
In conclusion, effective specialized palliative homecare should be maintained, identifying the driving factors and making these applicable to primary palliative care. Besides the already mentioned factors like keeping a team on standby 24/7, training in palliative care, and coordination of care, these may involve time for care and attention for patients. Both seem to be limited within primary palliative care to ensure the intended care at the end-of-life.42,43 To strengthen primary palliative care, possible approaches, like training in palliative skills and better interconnectedness within a supporting infrastructure (for example nursing services and hospices), have already been identified.4,44 In more general terms, it seems important to empower a greater interaction and cooperation, rather than the coexistence of primary palliative care and specialized palliative homecare. Then, two aims can be reached equally: providing access to specialized palliative homecare and strengthening primary palliative care.35,40
Strengths and limitations
Up to now, no investigation on effectiveness of primary palliative care and specialized palliative homecare has been carried out with explicit distinction between cancer and non-cancer patients. We were able to analyze the effects of palliative homecare provided 14 days before death in over 90,000 deceased patients, covering about 10% of those who died in Germany in 2016. 18 In addition to the well-known advantages and disadvantages of claims data analyses, we have to address the following limitations: first, we conducted a retrospective population-based study, which—compared to prospective studies—is not able to consider the unknown time of death in actual care decisions. 45 Second, evaluating end-of-life care at a population level using claims data can only address potentially aggressive interventions. Patient reported outcomes like symptom severity, patient satisfaction and social support, which are vitally important in each individual situation or care decision, are either not validly documented or not available in claims data. We are therefore aware that using claims data based indicators rather means describing the provision of healthcare than evaluating quality of palliative care. Third, we were only able to adjust for known and available confounding factors. Fourth, by using the assignment rule “documentation of at least one corresponding fee schedule item,” we did not account for probable differences in the continuity or intensity of care hiding behind billing. Fifth, we compared primary palliative care-only with specialized palliative homecare (with or without simultaneous primary palliative care). We did not analyze the outcomes of a simultaneous delivery of primary palliative care and specialized palliative homecare (which accounted for about 7% of our population) separately and we did not include “no palliative care” into the comparison. Finally, the transferability of results to other high-income countries may be restricted due to differences in health systems and palliative care delivery. However, the general observation of specialized palliative homecare having advantages over primary palliative care in avoiding potentially aggressive interventions at the end-of-life might also hold for countries other than Germany.
Conclusion
We found that specialized palliative homecare was associated with lower rates of potentially aggressive interventions at the end-of-life than primary palliative care in cancer and non-cancer patients. From these findings, we conclude that further research should investigate the single elements of specialized palliative homecare that produce both positive and negative effects. This could be used to inform and optimize primary palliative care, and to avoid negative specialized palliative homecare effects in the future. The potential of palliative homecare, particularly of specialized palliative homecare, in reducing potentially aggressive interventions at the end-of-life, deserves more attention in healthcare and politics.
Supplemental Material
Supplemental material, sj-pdf-1-pmj-10.1177_02692163211013666 for Effectiveness of two types of palliative home care in cancer and non-cancer patients: A retrospective population-based study using claims data by Markus Krause, Bianka Ditscheid, Thomas Lehmann, Maximiliane Jansky, Ursula Marschall, Winfried Meißner, Friedemann Nauck, Ulrich Wedding and Antje Freytag in Palliative Medicine
Acknowledgments
SAVOIR Study Group: Anna Bauer, Lia Bergmann, Bianka Ditscheid, Cornelia Eichhorn, Antje Freytag, Michaela Hach, Ulrike Hammer, Aicko Helbig, Beata Hennig, Maximiliane Jansky, Michelle Kaufmann, Markus Krause, Sabine Krauss, Thomas Lehmann, Helmut L’hoest, Srikanth Maddela, Ursula Marschall, Martial Mboulla, Winfried Meißner, Heiner Melching, Florian Mühler, Cornelia Nageler, Friedemann Nauck, Judith Rothaug, Joachim Saam, Werner Schneider, Sven Schulz, Kathleen Stichling, Horst C. Vollmar, Julia von Hayek, Ulrich Wedding. Undergraduate assistants: Marie-Luise Völker, Vivienne Kley, Jana Feustel, Ketura Herklotz.
Footnotes
Authorship: MK and AF drafted the paper. AF, MK and BD designed the study. BD and MK managed and prepared data. MK, TL, AF and BD conceptualized, MK, TL and BD performed the statistical analyses. MK, AF, TL and BD interpreted the data. UW, WM, UM, MJ and FN contributed valuable comments on result interpretation. AF planned, headed and supervised the whole study. WM and AF applied for funding. WM led the overall project SAVOIR of which this study forms part. All authors revised the manuscript for intellectual content, read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research project SAVOIR was funded by the German Innovations Fund of the Federal Joint Committee in Germany (G-BA) (grant number: 01VSF16005). The funder did not influence the design of the study, the writing of the manuscript and did not influence the collection, analysis and interpretation of data.
Research ethics and patient consent: Ethical approval was obtained from the local ethics committee of the Jena University Hospital (no. 5317-10/17, 23.10.2017). Access to data of statutory health insurance funds for research purposes was possible in accordance with the German Social Law (SGB V § 287). Patient consent was not necessary. The study was registered with the German Clinical Trials Register, 30.05.2018 (DRKS00014730, www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00014730).
Trial registration: German Clinical Trials Register (DRKS): DRKS00014730 (30.05.2018). https://www.drks.de/drks_web/setLocale_EN.do
Prior publication: Congress contribution: Krause M, Ditscheid B, Hennig B, Jansky M, Lehmann T, Maddela S, Meißner W, Schulz S, Stichling K, Vollmar HC, Wedding U, Freytag A, for the SAVOIR Study Group. Quality of End-of-Life Care—Comparing Claims Data Based Quality Indicators between Different Types of Palliative Care in Germany. 16th World Congress of the European Association for Palliative Care (EAPC); 23.05.-25.05.219; Berlin.
Data management and sharing: Access to data is only provided through data use agreement and via protected gateway to the scientific data warehouse of the health insurance fund BARMER. Further information about the data and access regulations are available from the corresponding author on request.
ORCID iDs: Markus Krause  https://orcid.org/0000-0002-9614-3326
https://orcid.org/0000-0002-9614-3326
Antje Freytag  https://orcid.org/0000-0001-8288-2729
https://orcid.org/0000-0001-8288-2729
Supplemental material: Supplemental material for this article is available online.
References
- 1. Gonzalez-Jaramillo V, Fuhrer V, Gonzalez-Jaramillo N, et al. Impact of home-based palliative care on health care costs and hospital use: a systematic review. Palliat Support Care 2020: 1–14. [DOI] [PubMed] [Google Scholar]
- 2. Radbruch L, Andersohn F, Walker J. Palliativversorgung: Überversorgung kurativ – Unterversorgung palliativ? Analyse ausgewählter Behandlungen am Lebensende. Faktencheck Gesundheit, Bertelsmann Stiftung, 2015. [Google Scholar]
- 3. Ditscheid B, Krause M, Jansky M, et al. German perspectives on palliative care at the end-of-life: who receives what type of care in Germany? - A claims data analysis. In: EAPC Abstracts: SAGE Publications Ltd STM, 2019. [Google Scholar]
- 4. Stichling K, Krause M, Ditscheid B, et al. Factors influencing GPs’ perception of specialised palliative homecare (SPHC) importance – results of a cross-sectional study. BMC Palliative Care 2020; 19: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shepperd S, Gonçalves-Bradley DC, Straus SE, et al. Hospital at home: home-based end-of-life care. Cochrane Database Syst Rev 2016; 2: CD009231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kavalieratos D, Corbelli J, Di Zhang, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016; 316: 2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev 2013; 6: CD007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palma R de, Fortuna D, Hegarty SE, et al. Effectiveness of palliative care services: a population-based study of end-of-life care for cancer patients. Palliat Med 2018; 32: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 9. Schelin ME, Sallerfors B, Rasmussen BH, et al. Quality of care for the dying across different levels of palliative care development: a population-based cohort study. Palliat Med 2018; 32: 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maetens A, Beernaert K, Schreye R de, et al. Impact of palliative home care support on the quality and costs of care at the end of life: a population-level matched cohort study. BMJ Open 2019; 9: e025180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ernecoff NC, Check D, Bannon M, et al. Comparing specialty and primary palliative care interventions: analysis of a systematic review. J Palliat Med 2020; 23: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boddaert MS, Pereira C, Adema J, et al. Inappropriate end-of-life cancer care in a generalist and specialist palliative care model: a nationwide retrospective population-based observational study. BMJ Support Palliat Care 2020: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bretschneider K, Kasprick L, Luderer C. Elisabeth Mobil mbH“ – die spezialisierte ambulante Palliativversorgung im Raum Halle (Saale) – eine wissenschaftliche Auswertung. Palliativmedizin 2012; 13: 36–46. [Google Scholar]
- 14. Heckel M, Stiel S, Frauendorf T, et al. Retrospektive datenanalyse von patienten in der spezialisierten ambulanten palliativversorgung (SAPV) - Vergleich zwischen Stadt und Landkreis: Comparison of patients and their care in urban and rural specialised palliative home care – A single service analysis. Gesundheitswesen 2016; 78: 431–437. [DOI] [PubMed] [Google Scholar]
- 15. Penders YW, Onwuteaka-Philipsen B, Moreels S, et al. Differences in primary palliative care between people with organ failure and people with cancer: an international mortality follow-back study using quality indicators. Palliat Med 2018; 32: 1498–1508. [DOI] [PubMed] [Google Scholar]
- 16. Schreye R de, Smets T, Deliens L, et al. Appropriateness of end-of-life care in people dying from COPD. Applying quality indicators on linked administrative databases. J Pain Symptom Manage 2018; 56: 541–550. [DOI] [PubMed] [Google Scholar]
- 17. Freytag A, Krause M, Bauer A, et al. Study protocol for a multi-methods study: SAVOIR - evaluation of specialized outpatient palliative care (SAPV) in Germany: outcomes, interactions, regional differences. BMC Palliative Care 2019; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Statistisches Bundesamt. Sterbefälle: Fallzahlen nach Tagen, Wochen, Monaten, Altersgruppen und Bundesländern für Deutschland, https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Sterbefaelle-Lebenserwartung/Tabellen/sonderauswertung-sterbefaelle-pdf.pdf?__blob=publicationFile (2020, accessed 11 June 2020).
- 19. Walter J, Tufman A, Leidl R, et al. Rural versus urban differences in end-of-life care for lung cancer patients in Germany. Support Care Cancer 2018; 26: 2275–2283. [DOI] [PubMed] [Google Scholar]
- 20. Grabenhorst U. Specialized ambulatory palliative care: (SAPV) 5-year results of a multi-professional care model by HomeCare linker Niederrhein gGmbH (HC) in the Lower Rhine region. Ann Oncol 2017; 28 (Supplement 5): V500. [Google Scholar]
- 21. Langton JM, Blanch B, Drew AK, et al. Retrospective studies of end-of-life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliat Med 2014; 28: 1167–1196. [DOI] [PubMed] [Google Scholar]
- 22. Schreye R de, Smets T, Annemans L, et al. Applying quality indicators for administrative databases to evaluate end-of-life care for cancer patients in Belgium. Health Aff (Millwood) 2017; 36: 1234–1243. [DOI] [PubMed] [Google Scholar]
- 23. Murtagh FEM, Bausewein C, Verne J, et al. How many people need palliative care? A study developing and comparing methods for population-based estimates. Palliat Med 2014; 28: 49–58. [DOI] [PubMed] [Google Scholar]
- 24. Barbera L, Seow H, Sutradhar R, et al. Quality indicators of end-of-life care in patients with cancer: what rate is right? J Oncol Pract 2015; 11: e279–e287. [DOI] [PubMed] [Google Scholar]
- 25. Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA 2016; 315: 272–283. [DOI] [PubMed] [Google Scholar]
- 26. Setoguchi S, Glynn RJ, Stedman M, et al. Hospice, opiates, and acute care service use among the elderly before death from heart failure or cancer. Am Heart J 2010; 160:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 28. Swart E, Bitzer EM, Gothe H, et al. A consensus German reporting standard for secondary data analyses, Version 2 (STROSA-STandardisierte BerichtsROutine für SekundärdatenAnalysen). Gesundheitswesen 2016; 78: e145–e160. [DOI] [PubMed] [Google Scholar]
- 29. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12: e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinhold I, Claus F, Karmann A, et al. Hospizstudie 2017: Standorte und demografische Rahmenbedingungen zur Hospiz- und Palliativversorgung im Freistaat Sachsen, Wissenschaftliches Institut für Gesundheitsökonomie und Gesundheitssystemforschung GmbH (WIG2 Institut), 7 June 2018. [Google Scholar]
- 31. Pinzón LCE, Claus M, Zepf KI, et al. Symptom prevalence in the last days of life in Germany: the role of place of death. Am J Hosp Palliat Med 2012; 29: 431–437. [DOI] [PubMed] [Google Scholar]
- 32. Rochigneux P, Raoul JL, Beaussant Y, et al. Use of chemotherapy near the end of life: what factors matter? Ann Oncol 2017; 28: 809–817. [DOI] [PubMed] [Google Scholar]
- 33. Baumstarck K, Boyer L, Pauly V, et al. Use of artificial nutrition near the end of life: results from a French national population-based study of hospitalized cancer patients. Cancer Med 2020; 9: 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA 2003; 289: 2387–2392. [DOI] [PubMed] [Google Scholar]
- 35. Ferroni E, Avossa F, Figoli F, et al. Intensity of integrated primary and specialist home-based palliative care for chronic diseases in northeast Italy and its impact on end-of-life hospital access. J Palliat Med 2016; 19:1260–1266. [DOI] [PubMed] [Google Scholar]
- 36. Bannon M, Ernecoff NC, Dionne-Odom JN, et al. Comparison of palliative care interventions for cancer versus heart failure patients: a secondary analysis of a systematic review. J Palliat Med 2019; 22: 966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moens K, Higginson IJ, Harding R. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014; 48: 660–677. [DOI] [PubMed] [Google Scholar]
- 38. Steinhauser KE, Arnold RM, Olsen MK, et al. Comparing three life-limiting diseases: does diagnosis matter or is sick, sick? J Pain Symptom Manage 2011; 42: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014; 348: g3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quill TE, Abernethy AP. Generalist plus specialist palliative care - creating a more sustainable model. N Engl J Med 2013; 368: 1173–1175. [DOI] [PubMed] [Google Scholar]
- 41. Emmert M, Pohl-Dernick K, Wein A, et al. Palliative treatment of colorectal cancer in Germany: cost of care and quality of life. Eur J Health Econ 2013; 14: 629–638. [DOI] [PubMed] [Google Scholar]
- 42. Carduff E, Johnston S, Winstanley C, et al. What does 'complex' mean in palliative care? Triangulating qualitative findings from 3 settings. BMC Palliat Care 2018; 17: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giezendanner S, Jung C, Banderet H-R, et al. General practitioners' attitudes towards essential competencies in end-of-life care: a cross-sectional survey. PLoS One 2017; 12: e0170168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ewertowski H, Hesse AK, Schneider N, et al. Allgemeine Palliativversorgung in der hausärztlichen Praxis: Entwicklung von Strategien zur Verbesserung struktureller, rechtlicher und finanzieller Rahmenbedingungen. Z Evid Fortbild Qual Gesundhwes 2019; 149: 32–39. [DOI] [PubMed] [Google Scholar]
- 45. Setoguchi S, Earle CC, Glynn R, et al. Comparison of prospective and retrospective indicators of the quality of end-of-life cancer care. J Clin Oncol 2008; 26: 5671–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pmj-10.1177_02692163211013666 for Effectiveness of two types of palliative home care in cancer and non-cancer patients: A retrospective population-based study using claims data by Markus Krause, Bianka Ditscheid, Thomas Lehmann, Maximiliane Jansky, Ursula Marschall, Winfried Meißner, Friedemann Nauck, Ulrich Wedding and Antje Freytag in Palliative Medicine


