Using voxel-based – and structural connectome – lesion-symptom mapping of the left hemisphere in chronic stroke survivors with aphasia, Bonilha et al. propose that the posterior lateral and inferior temporal cortices integrate auditory and conceptual processing that are crucial for auditory word comprehension.
Keywords: aphasia, comprehension, stroke, diffusion tensor imaging, connectome
Abstract
Auditory word comprehension is a cognitive process that involves the transformation of auditory signals into abstract concepts. Traditional lesion-based studies of stroke survivors with aphasia have suggested that neocortical regions adjacent to auditory cortex are primarily responsible for word comprehension. However, recent primary progressive aphasia and normal neurophysiological studies have challenged this concept, suggesting that the left temporal pole is crucial for word comprehension. Due to its vasculature, the temporal pole is not commonly completely lesioned in stroke survivors and this heterogeneity may have prevented its identification in lesion-based studies of auditory comprehension. We aimed to resolve this controversy using a combined voxel-based—and structural connectome—lesion symptom mapping approach, since cortical dysfunction after stroke can arise from cortical damage or from white matter disconnection. Magnetic resonance imaging (T1-weighted and diffusion tensor imaging-based structural connectome), auditory word comprehension and object recognition tests were obtained from 67 chronic left hemisphere stroke survivors. We observed that damage to the inferior temporal gyrus, to the fusiform gyrus and to a white matter network including the left posterior temporal region and its connections to the middle temporal gyrus, inferior temporal gyrus, and cingulate cortex, was associated with word comprehension difficulties after factoring out object recognition. These results suggest that the posterior lateral and inferior temporal regions are crucial for word comprehension, serving as a hub to integrate auditory and conceptual processing. Early processing linking auditory words to concepts is situated in posterior lateral temporal regions, whereas additional and deeper levels of semantic processing likely require more anterior temporal regions.
Introduction
Speech comprehension is accomplished by linking acoustic speech information with abstract concepts (Shim and Grabowski, 2010). While the most basic aspect of perception is the auditory processing of speech sounds, the subsequent steps involve a multi-layered and hierarchical organization from which information such as phonemes, syllables, words, syntax, meaning and context can be derived (Skeide and Friederici, 2016). Our goal in this study was to identify the neural structures that, when damaged by stroke, were associated with impairments in auditory word comprehension.
Speech comprehension is frequently impaired in individuals with neurological disorders, especially in patients with post-stroke aphasia (Damasio, 1992). Strokes affecting the posterior division of the middle cerebral artery frequently lead to speech comprehension problems (Kreisler et al., 2000). The first insights into the functional neuroanatomy of speech comprehension originated from the case descriptions by Karl Wernicke in the 19th century, inspired by neuropathologist Theodor Meynert (Whitaker and Etlinger, 1993). Post-mortem inspection revealed that Wernicke’s patients shared a common lesion location in the postero-lateral aspect of the temporal lobes, more specifically within the superior temporal region (Eggert, 1977). Subsequently, multiple in vivo studies using neuroimaging have refined the anatomical location of speech comprehension using lesion-symptom mapping (Kreisler et al., 2000; Bates et al., 2003; Newhart et al., 2007; Rogalsky et al., 2008), functional activation in normal individuals (Yarkoni et al., 2008; Price, 2010, 2012) and in stroke survivors (Thompson and den Ouden, 2008), and intraoperative neurophysiology (Pasley and Knight, 2013). In spite of relatively minor differences in the cortical regions identified as crucial for speech comprehension, most studies to date have confirmed the importance of the posterior aspects of the middle and superior temporal gyri, and of the superior temporal sulcus in speech comprehension (Hickok and Poeppel, 2007).
Nonetheless, these observations were recently challenged by a thought-provoking study by Mesulam et al. (2015), which evaluated crucial neural underpinnings of word comprehension in subjects with primary progressive aphasia (PPA). Controlling for object recognition (semantic knowledge), Mesulam and colleagues observed that auditory word comprehension was more strongly associated with anterior temporal lobe atrophy, instead of the postero-lateral temporal region, as would have been expected based on the classical location of Wernicke’s speech comprehension area. These findings contradicted much of the current knowledge about the functional anatomy of speech comprehension, including functional imaging studies as well as lesion-deficit association studies. Moreover, this study was also noteworthy for important additional conceptual reasons. First, lesion-based studies typically include stroke survivors with middle cerebral artery cortical and subcortical strokes. Even though the middle cerebral artery perfuses the superior aspect of the temporal pole, the proportion of patients with lesions in the temporal pole is smaller compared with patients with lesions in the frontal or temporal opercular regions. In general, only patients with large lesions display damage to the temporal poles. This difference in proportion leads to a higher statistical power to detect an association between speech comprehension and opercular regions, compared with temporal polar regions. Moreover, even in cases with stroke damage to the temporal pole, its inferior aspect may remain preserved and thus support comprehension. This limitation did not affect the aforementioned study by Mesulam et al., since PPA is associated with damage to the entire temporal poles.
Equally important, cerebrovascular lesions result in direct or secondary white matter damage (Bonilha et al., 2014a), which may cause functional disconnection of the temporal pole. For this reason, the cortex of the temporal pole may be dysfunctional and yet appear intact. When attempting to localize function to brain damage, lesion mapping may falsely localize it to the location of obvious structural damage, i.e. the posterior–lateral temporal cortex, and underestimate the importance of the temporal pole. In the context of PPA, the histopathological damage, whether tauopathy, TDP-43 proteinopathies, or Alzheimer’s disease pathology, is primarily localized to the cortical structures (Sonty et al., 2003), and it is unknown to what degree they lead to secondary white matter damage.
The findings by Mesulam et al. (2015) were also intriguing because recent neurophysiological studies using magnetoencephalography have indicated that the inferior aspect of the temporal pole is involved with comprehension of words (versus pseudowords). In fact, the cortex of the temporal pole is the location with maximal voltage sources during this contrast, becoming active ∼80 and 170 ms after auditory word presentation (MacGregor et al., 2012). Moreover, many functional MRI studies have indicated that the temporal pole is activated by tasks that involve manipulation of sematic information and sentence processing (Mazoyer et al., 1993; Humphries et al., 2001, 2005; Vandenberghe et al., 2002; Rogalsky and Hickok, 2009; Rogalsky et al., 2011). As comprehensively described by Binder et al. (2009) in a large meta-analysis, the semantic network involves several domain general regions, including the temporal pole.
In summary, even though Mesulam et al. (2015) did not support the classical concept of word comprehension as relying heavily on the postero-lateral aspects of the left middle and superior temporal lobe, their findings are in agreement with other, mostly non-lesion studies, suggesting that the temporal pole may be a critical hub for morphosyntatic and lexical-semantic categorization.
A possible explanation for conflicting evidence regarding the areas most critical to auditory word comprehension lies in the fact that the structural networks crucial for word comprehension have not been fully mapped. Lesion studies may have underestimated the importance of the temporal pole if it remained intact, but disconnected. Conversely, the temporal pole may appear functionally involved if its strong connectivity with the crucial posterior temporal cortex leads to secondary activation that cannot be discriminated by functional methods, particularly functional MRI.
The resolution of this discrepancy requires a method that can identify not only brain regions that are crucial (rather than merely involved with) word comprehension (i.e. a lesion impairment-based study), but can also assess lesions in terms of networks rather than isolated damage. By demonstrating which components of specific networks are associated with speech comprehension, it would be possible to dissociate the significance of distinct regions that are part of the same network, such as the temporal pole and posterior temporal regions. Importantly, our group recently confirmed that cortical dysfunction after stroke may arise from direct cortical damage, as well as from cortical disconnection (Fridriksson et al., 2007; Bonilha et al., 2014a,b). Therefore, assessing white matter networks extending beyond the location of cortical necrosis can provide a more comprehensive assessment of brain damage and its impact on language processing.
Connectome-lesion symptom mapping (CLSM) is a newly developed method that is optimally suited to address this problem (Yourganov et al., 2016). CLSM is based on the concept of the human brain connectome, providing a 3D map of all medium- and long-range white matter connections across the human brain (Sporns et al., 2005). Our group has optimized connectome mapping for lesioned brains after cerebrovascular injury; each individual’s connectome provides a map of the remaining white matter networks in the chronic period of stroke recovery, quantifying lost as well as spared networks. The connectome demonstrates not only the location of subcortical injury immediately adjacent to cortical damage, but also remote secondary white matter loss, and distant cortical deafferentation. It expands on conventional voxel-based lesion symptom mapping (VSLM) by mapping networks and disclosing damaged systems crucially associated with behaviour. For this reason, CLSM can be used to address whether the temporal pole is the location more strongly associated with word comprehension, or a component of a broader network located in more posterior temporal regions. Likewise, it can also address whether the temporal pole is part of the network associated with word comprehension, or if it is unrelated to this function, with its involvement possibly related to its secondary connectivity to other crucial posterior temporal regions.
In this study, we applied CLSM to data from a large group of chronic stroke survivors with a previous one-time stroke affecting the language dominant hemisphere. The participants exhibited various degrees of word comprehension problems, and we assessed the crucial anatomy underlying these deficits controlling for semantic knowledge and object recognition. We hypothesized that preservation of short-range networks linking the middle and superior posterior aspects of the temporal cortex would be critical for word comprehension. We also tested whether the temporal polar cortex was a subcomponent, a central hub, or unrelated to this network.
Materials and methods
Participants
Sixty-seven chronic stroke survivors participated in this study [45 males, 22 females, mean age = 59.39 years, standard deviation (SD) = 9.61]. All patients were right-handed prior to the stroke, and they all suffered a one-time ischaemic stroke at least 6 months prior to enrolling in this study. Recruitment was performed through local advertisement. None of the patients had a pre-morbid history of other neurological or psychiatric disorders; and all participants signed informed consent to participate in this study, which was approved by the Institutional Review Boards at the University of South Carolina and the Medical University of South Carolina.
Language and neuropsychological testing
Aphasia types and severity
The Western Aphasia Battery–Revised (WAB-R) (Kertesz, 2007) was applied to all subjects to determine if they had aphasia, and also to quantify its severity. The WAB-R was used to classify specific aphasia types.
Word comprehension
Word comprehension was assessed using the WAB-R. The word comprehension test of the WAB-R involves the participant pointing to a target item that corresponds to a spoken word presented by a clinician. The targets include items such as real objects, pictures of objects, numbers, colours, objects in the examination room, and body parts. A total of 60 words are included as target items on this task; therefore, participants may score from 0 to 60.
Object recognition
The Pyramids and Palm Trees Test (PPTT) (Howard and Patterson, 1992) was used to assess conceptual semantic processing, therefore serving as a control measure to probe lexical-semantic processing rather than amodal semantic knowledge. The PPTT was applied to 43 subjects from the overall group of 67 participants (35 males, eight females, mean age 59.5 years, SD = 7.75).
The PPTT involves matching two semantically-related pictures that are presented along with a distractor foil on a single sheet of paper. The target picture appears at the top of the sheet and the target match and the unrelated foil are presented as a pair towards the bottom of the sheet. The PPTT includes 52 stimulus pairs and, accordingly, a potential range of scores is 0–52.
Neuroimaging analyses
Imaging acquisition and preprocessing
All participants underwent high resolution T1 and T2 MRI scanning using a Siemens 3 T Trio System with a 12-channel head-coil located at the University of South Carolina. For each participant, T1-weighted images were obtained using an MP-RAGE sequence with 1 mm3 isotropic voxels, field of view matrix of 256 × 256 mm, 9° flip angle, and 192 sagittal slice sequence with repetition time = 2250 ms, inversion time = 925 ms, and echo time = 4.15 ms, with parallel imaging (GRAPPA = 2, 80 reference lines). T2-weighted images were obtained using sampling perfection with application optimized contrasts by using differing flip angle evolutions (SPACE) sequence for the purpose of demarcating lesions. The parameters for this T2 scan included a voxel size of 1 mm3, 256 × 256 mm field of view matrix, 160 sagittal slice sequence, variable flip angle, repetition time = 3200 ms, echo time = 352 ms, with no slice acceleration. Slice centre and angulation were identical to the T1 image sequence. All participants also underwent diffusion MRI using the following parameters: diffusion EPI scan using 30 directions with b = 1000 s/mm2 (60 volumes), b = 2000 s/mm2 (60) and b = 0 s/mm2 (11), repetition time = 6100 ms, echo time = 101 ms, 82 × 82 matrix, 222 × 222 mm field of view, with parallel imaging GRAPPA = 2, 45 contiguous 2.7 mm axial slices, acquisition time = 853 s. All images were converted from DICOM to NIfTI format using dcm2niix (Li et al., 2016), which transforms the diffusion gradients to image space for subsequent analyses.
The post-stroke chronic lesions were manually delineated on the T2-weighted images by a neurologist (L.B.) who was blinded to the behavioural scores at the time of the lesion drawing. To define the magnitude of damage to specific cortical regions, we used the John’s Hopkins University (JHU) brain Atlas (Mori et al., 2008; Faria et al., 2012), which is a map in standard stereotaxic space dividing the grey matter into 108 regions. Supplementary Table 1 provides a detailed description of the anatomical regions of interest included in this atlas and used in this study. Using SPM12 and open source MATLAB scripts developed in-house (Rorden et al., 2012) the stroke lesions were spatially normalized to standard space through the following steps: (i) the T2 image was linearly co-registered onto the T1-weighted scan and this transformation was applied to the lesion mask; (ii) the resliced lesion maps were smoothed with a 3 mm full-width at half-maximum Gaussian kernel to remove uneven edges associated with manual drawing; (iii) an enantiomorphic approach (Nachev et al., 2008) using SPM12’s unified segmentation-normalization (Ashburner and Friston, 2005) was applied to normalize the T1-weighted images onto the standard space, using a chimeric T1-weighted image where the area corresponding to the stroke lesion was replaced by the mirrored equivalent region in the intact (right side) hemisphere; and (iv) only voxels with a probability >50% of corresponding to the lesion mask were maintained in the final normalized lesion mask. Once the lesion masks were placed in standard space, they were overlaid onto the anatomical parcellation atlas to determine: (i) overall normalized lesion size; and (ii) the percentage of each cortical region damaged in each individual.
Structural brain connectivity
The structural white matter whole-brain brain connectome was reconstructed from all individuals using probabilistic diffusion tensor imaging. All connectome preprocessing steps were performed in diffusion space. Therefore, we computed a reverse-normalization to warp the template (in standard space) to the individual’s native diffusion image. To accomplish this, we used SPM12’s ‘oldnorm’ function to compute a non-linear transform from the individual’s enantiomorphically normalized scalp-stripped (using the segmentation estimates) T1 scan to their own intensity-normalized, scalp-stripped diffusion-based fractional anisotropy image. The transformation parameters were then used to normalize the probabilistic maps and the stroke lesion into the diffusion MRI space. The probabilistic grey and white matter maps were obtained during SPM12’s unified segmentation-normalization steps described above. Of note, prior to transformation into diffusion space, the probabilistic grey map was segmented into regions of interest based on the same parcellation atlas described above, by obtaining the reverse of the normalization matrix from T1 to standard space and by applying this matrix to the atlas in standard space. The atlas in T1 space was overlaid onto the probabilistic grey matter map and grey matter voxels corresponding to each region of interest were marked accordingly.
Once the images were in native diffusion space, pair-wise connectivity between all possible grey matter regions of interest was obtained using probabilistic tractography with a graphics-card accelerated (Hernandez et al., 2013) version of FSL FDT’s method (Behrens et al., 2007). FDT’s Bedpost was used to build default distributions of diffusion parameters at each voxel followed by probabilistic tractography using FDT’s probtrackX (parameters: 5000 individual pathways drawn through the probability distributions on principle fibre direction, curvature threshold set at 0.2, 200 maximum steps, step length 0.5 mm and distance correction). For all probabilistic tractography estimations, the probabilistic white matter map excluding the stroke lesion was used as a waypoint mask. The number of streamlines arriving in one region of interest when another region of interest was seeded was computed for each possible pair of regions of interest. Since tractography is not dependent on directionality, the connectivity between two regions of interest (ROI) was defined as the average between (i) the number of streamlines arriving in ROIi when ROIj was seeded; and (ii) the number of streamlines arriving in ROIj when ROIi was seeded. The weighted connectivity between regions of interest was corrected based on the distance travelled by the streamlines (‘distance correction’ built into probtrackX) and further divided by the sum of volume of the regions of interest. This process resulted, for each study participant, in a 108 × 108 square adjacency matrix, symmetrical along its diagonal, where each entry was the weighted connectivity between the corresponding regions of interest, i.e. the connectome edge. We did not track fibres within the lesion site. As such, links whose fibres were not tracked (either from being in the lesion site, or from being related to non-existing fibres elsewhere) were input as zero weight in the adjacency matrix.
Statistical analyses
VLSM and CLSM statistical analyses were carried out using the software package NiiStat (https://github.com/neurolabusc/NiiStat). In the case of VLSM, language scores were assessed in relationship with voxel-based lesions, where for each voxel a t-test was performed to compare the average language scores in subjects with a lesion involving voxel versus those whose lesions did not involve that voxel. Univariate analyses were performed and the statistical threshold was corrected for multiple comparisons using permutation analyses (4000).
The relationship between language scores and the integrity of structural connectome pathways was performed using CLSM. CLSM evaluated the linear relationship (regression) between the dependent behavioural measure and the connectome pair-wise edge weight. Each edge (connection between two grey matter regions of interest, as explained above) is assessed independently through univariate analyses and the level of statistical significance of the behavioural-connectome edge relationship is also corrected based on the number of multiple comparisons using permutation threshold. This process is similar to univariate VLSM, except that instead of voxels, CLSM uses connectome edges (connections) as independent variables. Whenever indicated, analyses were carried to regress out the influence of one (or more) factors using a Freedmann Lane test, also implemented in NiiStat (Winkler et al., 2014). All statistical analyses including PPTT scores were performed using the data from 43 subjects, since 43 of 67 participants had PPTT scores, as explained above. Otherwise, all other statistical analyses involving WAB-R scores included 67 participants.
To focus the CLSM analyses on language-related brain regions and to improve statistical power, a subnetwork of the entire brain connectome was used on all CLSM analyses. Since the JHU atlas contains 108 grey matter regions, each subject’s connectome is composed of 5778 unique connections (the adjacency matrix is symmetrical along its diagonal). We restricted CLSM to include only connections between language-specific and domain general regions in the left hemisphere, as proposed by Fedorenko et al. (2012), expanded to include all temporal pole regions, i.e. the pole of the superior, middle and inferior temporal gyri. These regions are summarized in Supplementary Table 2. Nineteen regions were included in total with 171 possible connections being evaluated as independent measures.
Anatomical display of VLSM and CLSM results
VLSM results were anatomically displayed on a brain surface template, illustrating voxels with a statistically significant relationship with the dependent behavioural measure. CLSM results were also demonstrated by displaying the neural networks statistically associated with the behavioural measure. However, white matter networks harbour two important elements of information: (i) the configuration of the crucial network (i.e. which regions are connected with which regions, and the strength of the relationship between these connections and the behavioural variable); and (ii) anatomical pathways traversed by involved connections. Therefore, to illustrate CLSM results to their full extent, they were displayed in two formats: (i) a diagrammatic network with crucial connectome connections represented by lines linking connectome nodes illustrated by spheres in the region of interest’s centre of mass; and (ii) a reconstruction of the crucial connectome connections through white matter tractography streamlines indicating the pathways traversed by the connectome links. To demonstrate crucial connectome connections through white matter tractography, the whole brain connectome was reconstructed using deterministic tractography and the JHU atlas from 59 healthy controls (45 female, mean age 54.7 ± 8.3 years) (DTI parameters: twice refocused echo-planar imaging sequence with diffusion weightings of b = 0, 1000, 30 diffusion encoding directions, repetition time = 8500 ms, echo time = 98 ms, field of view = 222 × 222 mm2, matrix = 74 × 74, 3 mm slice thickness, and 40 axial slices; using DSI studio with parameters: source images were calculated using FSL FDT’s method, fibre reconstruction was performed using Q-Space Diffeomorphic Reconstruction (Yeh and Tseng, 2011), 1.25 diffusion length sampling ratio, 2 mm output resolution). All JHU regions of interest pair-wise connections were saved as a connectome link tractography file for each control subject. Each connectome link was transferred to stereotaxic space using DSI studio (7, 9, and 7 transformation parameters, Fourier basis) and then combined across subjects. The resulting bundle was interpolated into 100 segments and the centre of mass of for each segment was calculated. Streamlines whose segment-wise deviation from the centre of mass was within 0.5 SD were maintained, yielding an anatomically representative connectome link. Those connections indicated by CLSM results were then combined and displayed in stereotaxic space.
Results
Language and neuropsychological testing
Aphasia types and severity
Among all 67 participants, four (6% of the all participants) were classified as having global aphasia, four (6%) as having Wernicke’s aphasia, 10 (14.9%) conduction aphasia, 11 (16.4%) anomic aphasia, and 17 (25.4%) Broca’s aphasia. Twenty-one participants (31.3%) did not have aphasia. Across all participants, the average WAB-R aphasia quotient (WAB–AQ) was 72.2 (SD = 27.4), with an average of 60.3 (SD = 25.3) among patients with aphasia. Within the group of 43 patients who were tested for object recognition (PPTT), six (13.9%) had anomic aphasia, 13 (30%) Broca’s, four (9.3%) Wernicke’s, three (6.9%) global, 10 (23.2%) conduction, and seven (16.3%) did not have aphasia.
Word comprehension and object recognition
The average word comprehension score was 53.6 (89% correct) (SD = 10) (all subjects) and 50.7 (85% correct) (SD = 10.9) among patients with aphasia. Average PPTT scores were 48.2 (93% correct) (SD = 2.7) (all subjects) and 47.8 (92% correct) (SD = 2.7) (patients with aphasia).
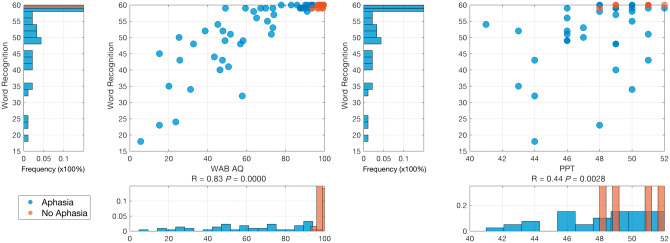
Lower scores in word comprehension were associated with worse aphasia severity (WAB–AQ) (R = 0.83, P < 0.001) and lower scores in word comprehension were correlated with lower scores on the PPTT (R = 0.44, P < 0.0028 all participants). Figure 1 demonstrates the relationships between word comprehension, aphasia severity, and object recognition.
Figure 1.
The relationships between word comprehension, aphasia severity, and object recognition.
Neuroimaging analyses
VLSM
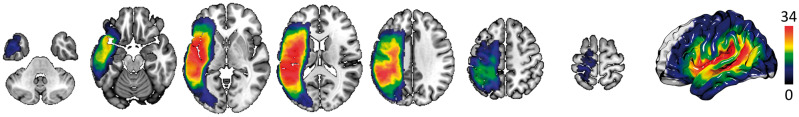
The voxel-based overlay of all binary stroke lesions in stereotaxic space is shown in Fig. 2. Considering cortical regions with at least 5% of post-stroke lesion, the most common location of damage was the supramarginal gyrus (42/67 subjects, 63%) followed by the angular gyrus (41/67 subjects, 61%), the precentral gyrus (41/67, 61%), superior temporal gyrus (40/67, 60%), and the posterior superior temporal gyrus (39/67, 58%). Thirty-four of 67 subjects (50%) had insular lesions. Thirty-four of 67 subjects (50%) had lesions involving the pole of the superior temporal gyrus and 17/67 (25%) involving the pole of the middle temporal gyrus. Among the 43 subjects with PPTT scores, the most common lesion location was the superior temporal gyrus (30/43 subjects, 70%), followed by the posterior superior temporal gyrus (29/43, 67%), precentral gyrus (29/43, 67%), supramarginal (29/43, 67%), and angular gyrus (28/43, 65%). Twenty-six of 43 subjects (60%) had lesions involving the pole of the superior temporal gyrus and 14/43 (33%) involving the pole of the middle temporal gyrus. A detailed description of lesion location and behavioural scores is given in Supplementary Table 3.
Figure 2.
Lesion overlay. The scale bar indicates colours representing the number of subjects with a lesion in a given voxel.
There was a strong relationship between WAB-AQ and lesion location, with worse WAB-AQ associated with cortical lesions involving the insula, the superior and middle temporal gyri, the frontal operculum, the angular gyrus, and the supramarginal gyrus, as demonstrated in Fig. 1. The voxel cluster with the highest statistical association (Z = −7.79) was centred in the posterior superior temporal gyrus. Supplementary Table 4 and Supplementary Fig. 1 provide a detailed list of the anatomical regions whose lesions were statistically associated with worse WAB-AQ.
Word comprehension deficits were also strongly associated with lesion location, with worse performance being observed in participants with lesions involving the superior, middle and inferior temporal gyri, as well as the posterior aspects of the insula and the medial temporal cortex. Interestingly, the centre of the voxel cluster with maximal statistical association (Z = −7.23) was located in the pole of the superior temporal gyrus. Supplementary Fig. 2 demonstrates the anatomical location of the statistical maps, which are described in detail in Supplementary Table 5.
However, when controlling for object amodal semantic processing of objects (PPTT scores), the posterior inferior temporal gyrus was the lesion location that best predicted word comprehension (Z = −5.64). These results are demonstrated in Fig. 3 and described in further detail in Supplementary Table 6.
Figure 3.
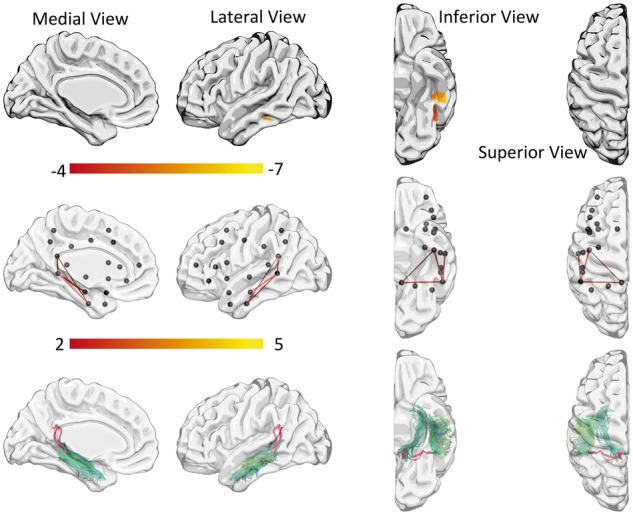
Word comprehension results controlling for object recognition (PPTT). VLSM results are shown in the top row and CLSM results in the middle and bottom rows.
CLSM
CLSM analyses revealed damage to an extensive network associated with WAB-AQ, with lower scores in the WAB-AQ being correlated with reduced connectivity weight involving 61 connections. The connections, when damaged, that most accurately predicted lower WAB-AQ were between the angular gyrus and the supramarginal gyrus, the middle and the inferior temporal gyrus, the pars opercularis and the pars triangularis, and the superior temporal gyrus and the angular gyrus. This network is demonstrated in Supplementary Fig. 1 and described in detail in Supplementary Table 7.
A connectome network, when damaged, was also associated with poorer word comprehension. Using the sample of 67 subjects, without controlling for other variables, damage to 37 connectome connections was associated with worse word comprehension performance. The connections with the strongest association were between the inferior and the middle temporal gyri, the middle and the superior temporal gyri, and the superior temporal and the angular gyri (Supplementary Fig. 2 and Supplementary Table 8A). Six connections involving the temporal pole were associated with word comprehension, namely between the middle temporal pole and the superior temporal gyrus, middle temporal gyrus, insula, and superior parietal gyrus; and between the superior temporal gyrus pole and the supramarginal gyrus and the superior parietal gyrus.
When controlling for amodal semantic processing (PPTT scores), the crucial network associated with word comprehension was localized to connections involving the posterior superior middle temporal gyrus and the inferior temporal gyrus. Specifically, connections between the posterior superior middle temporal gyrus and the inferior temporal gyrus, the posterior superior middle temporal gyrus and the middle temporal gyrus, the posterior superior middle temporal gyrus and the posterior cingulate, and between the inferior temporal gyrus and posterior cingulate (Fig. 3 and Supplementary Table 9). The CLSM analyses did not indicate that the temporal pole was part of the network crucially associated with word comprehension when controlling for semantic processing (PPTT scores).
To assess whether the lack of temporal pole connections was due to reduced statistical power from the smaller sample size (43 subjects, instead of 67), or due to the addition of object recognition as a control variable, we re-computed the CLSM analysis evaluating the networks associated with word comprehension (without controlling for object recognition) this time using only the data from the 43 subjects with PPTT scores. This analysis revealed 19 connections associated with word comprehension and, importantly, four connections including the temporal pole were statistically associated with word comprehension (connections between the temporal pole and the superior parietal gyrus, the supramarginal gyrus, the insula, and the superior temporal gyrus) (Supplementary Table 8B). These results corroborate that the lack of association between temporal pole regions and word comprehension when controlling for object recognition was due the regression of PPTT and not due to reduced statistical power from the smaller sample size.
Expanding CLSM based on VLSM results
CLSM was originally performed using the language-specific and domains general regions of interest as described by Fedorenko et al. (2012). Nonetheless, the VLSM analyses described above indicated a small but significant contribution of the fusiform cortex in word comprehension (along with, or as an extension of the posterior inferior temporal gyrus). Thus, we repeated the CLSM analyses including the fusiform gyrus to evaluate whether connections to this region would independently predict word comprehension. In addition to the left fusiform gyrus, this analysis included all previously included regions of interest (i.e. the 19 language-specific and domain general regions) and was performed in a similar fashion as before, i.e. Freedmann Lane test to regress the influence on the PPTT on word comprehension, adjusting the P-value based on permutation analyses to correct for multiple comparisons. Interestingly, the results of the this CLSM analysis did not change from the results described above, except that, with more connections, the number of statistical comparisons was higher and the threshold for corrected statistical significance was, therefore, also higher (z > 2.76901) so that the link with the lowest Z-value (ITG_L–PCC_L, Z = 2.4481436) was no longer significant. The fusiform was not identified in this post hoc analysis.
Discussion
In this study, we evaluated the necessary brain structures for word comprehension in a large cohort of individuals with chronic post-stroke aphasia. By adding CLSM to VLSM, we assessed cortical and subcortical networks located beyond the left middle cerebral artery perfusion areas. To assess brain areas involved in lexical-semantic processing, rather than amodal semantic knowledge, we evaluated the relationship between brain structure and word comprehension controlling for object recognition. In summary, after factoring out object recognition, word comprehension difficulties were associated with damage to the inferior temporal gyrus, to the fusiform gyrus and to a white matter network including the left posterior temporal region and its connections to the middle temporal gyrus, inferior temporal gyrus, and cingulate cortex.
These results are in accordance with the conventional anatomical localization of speech and word comprehension (Eggert, 1977; Hickok and Poeppel, 2007; Mesulam et al., 2015). They corroborate the importance of the middle and posterior temporal regions in speech comprehension in general, as demonstrated by VLSM studies (Bates et al., 2003; Dronkers et al., 2004), or by the combined VLSM and voxel-based morphometry (VBM) study by Geva et al. (2012). In fact, the VLSM results in Geva et al. demonstrated crucial areas localized in more posterior temporal regions compared with VBM, possibly related to the VLSM anatomical bias towards higher statistical power in areas more commonly lesioned after strokes. More specifically concerning studies of word comprehension, the results presented here are in accordance with previous studies by Cloutman et al. (2009), who observed errors in a word comprehension picture matching test in subjects with acute strokes and hypoperfusion of the superior temporal gyrus. However, Cloutman et al. also observed that 50% of subjects with ischaemia in the left temporal pole made errors in word comprehension. Interestingly, our results also replicated findings from Faria et al. (2014) who evaluated individuals with PPA and observed that word comprehension was associated with volume of middle temporal and posterior inferior temporal regions in the left hemisphere.
The discrepancy between the aforementioned studies, along with the results described here, versus the results indicated by Mesulam et al. (2015) is possibly best explained by the CLSM analyses. CLSM provides a more comprehensive description of crucial neuronal networks associated with behaviour, demonstrating that the temporal poles are part of a broader network associated with semantic interpretation, but that when the effect of object recognition is factored out, only the core of that network, mostly involving the middle and inferior temporal areas, is necessary for word comprehension.
PPA is associated with temporal cortex atrophy, notably with a preferential target to the temporal poles (Sonty et al., 2003). The temporal poles, in turn, are directly connected to the middle and posterior temporal regions, as demonstrated by the temporal lobe subnetwork associated with uncontrolled word comprehension. Thus, when assessing word comprehension in PPA, there is an indirect association between comprehension and the temporal poles (through their mutual relationship with the posterior aspects of the temporal lobe and the middle temporal gyrus). If the posterior temporal cortex damage is relatively spared, a statistical relationship between the temporal pole and word comprehension can emerge, without necessarily determining whether the pole is crucial for comprehension. Of note, it is important to highlight that one of the limitations of the present study (and of most lesion studies including stroke survivors in general) is the fact that it did not include individuals with complete lesions of the temporal pole, and thus we did not demonstrate that patients with completely lesioned left temporal pole had intact word comprehension.
The association between the temporal pole and semantic knowledge is unambiguous. Using the virtual meta-analytic engine NeuroSynth (Yarkoni et al., 2011), it is possible to pool neuroimaging data from multiple studies, and assess commonalities in functional brain activation in relationship with a behavioural measure. Based on the NeuroSynth database at the time of preparation of this manuscript, 59 functional activation studies focused on semantic knowledge (Supplementary Table 10). The global maximum of this association map is located in the inferior aspect of the temporal pole (Fig. 4A, approximately around the coordinate x = 28, y = −4, z = −48). More importantly, the NeuroSynth engine also enables the evaluation of brain regions with co-activation with the temporal poles, i.e. regions that are also reported to be active across studies when the temporal pole was activated (based on seeding a 6 mm sphere centred on the coordinate listed above). The meta-analytic co-activation map demonstrates a strong association between activation in the temporal poles and activation in the middle temporal and superior temporal gyri, suggesting a robust functional association between these regions (Fig. 4B).
Figure 4.
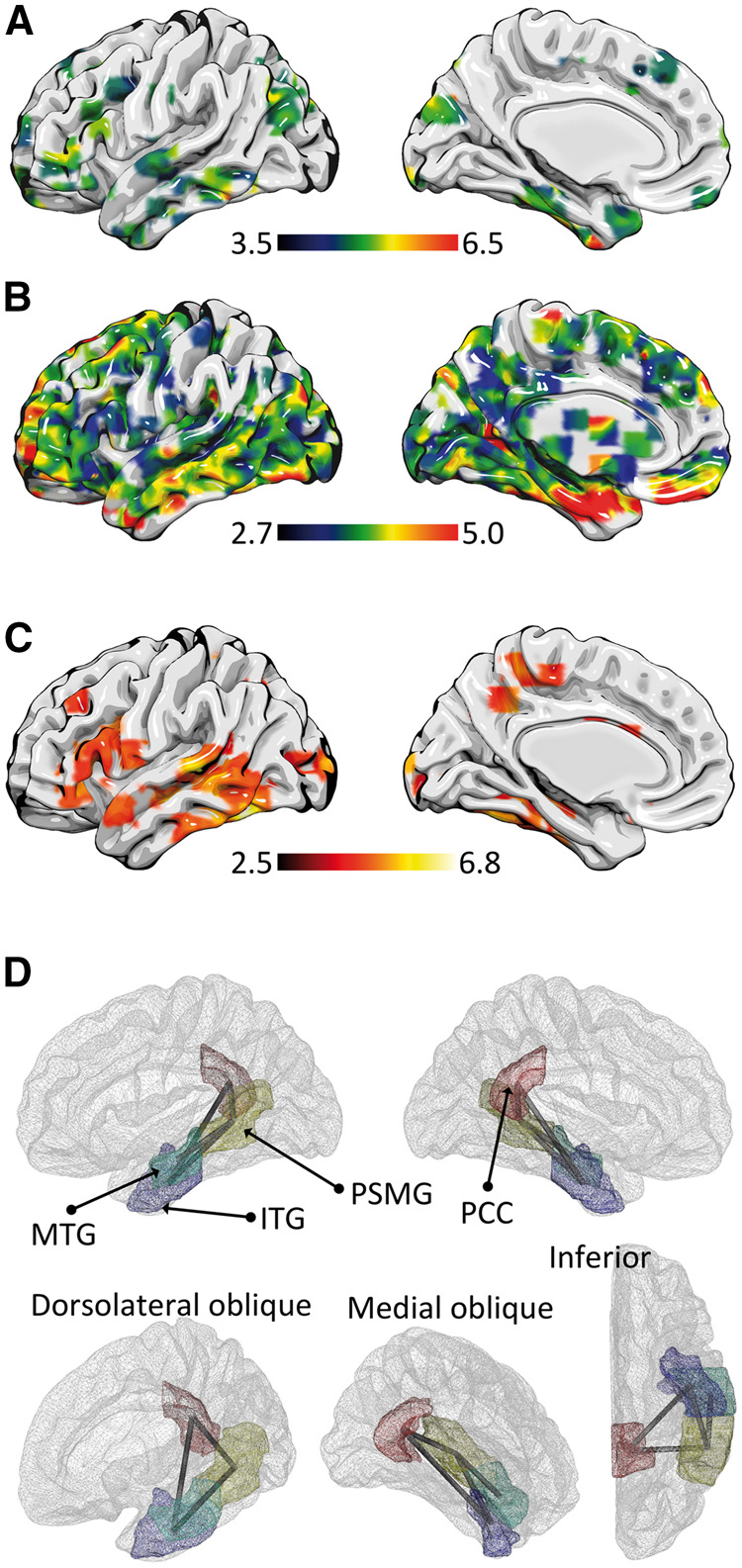
Brain networks associated with word comprehension. (A) The meta-analytical functional map obtained from NeuroSynth related to 59 functional MRI studies evaluating semantic knowledge (listed in Supplementary Table 10). The global maximum (Z = 6.92) is located in the pole of the left temporal lobe. (B) Co-activation maps associated with temporal pole, demonstrating a widespread functional network, with a noteworthy involvement of the left middle temporal gyrus. (C) Also using NeuroSynth’s database, this panel demonstrates the pooled results from 74 studies assessing word recognition (Supplementary Table 11). (D) The neuronal network observed in this study associated with word comprehension, controlling for object identification, was centred on the posterior middle temporal gyrus (PSMG), from which connections linked the middle temporal gyrus (MTG), inferior temporal gyrus (ITG) and the posterior cingulate cortex (PCC). The ITG was also directly linked with the PCC.
The association between the temporal poles and semantic processing is further corroborated by the clinical observation of conceptual object recognition deficits in individuals with the semantic variant of PPA (svPPA), which predominantly affects the temporal poles (Sonty et al., 2003). Nonetheless, the association between semantic deficits and other neurological diseases with temporal pole damage is less clear, notably among patients with temporal lobe epilepsy (and post-surgical anterior temporal lobectomy), who typically exhibit considerably milder deficits compared with svPPA (Lambon Ralph et al., 2012). Data from temporal lobe epilepsy patients and stroke patients with damage to left temporal pole without semantic deficits (Tsapkini et al., 2011) indicate that semantic processing is more broadly and redundantly distributed beyond the left temporal pole or in bilateral temporal poles. In a PET study, Mummery et al. (1999) reported that individuals with svPPA demonstrated less activation in the left posterior inferior temporal gyrus while performing a semantic decision task, but their atrophy pattern include the temporal poles and not the posterior inferior temporal gyrus, suggesting a dysfunction of posterior temporal regions in svPPA.
The results presented here provide confirmatory evidence that the neuronal network associated with word comprehension involves the middle, inferior and posterior temporal regions as the most important regions. The left temporal pole is strongly connected, both functionally and structurally, to the middle temporal gyrus. While these connections may lead to indirect findings indicating a role of left temporal pole in word comprehension, the temporal pole is likely not a crucial structure, i.e. early processing linking auditory words to concepts is situated in posterior lateral temporal regions, whereas additional and deeper levels of semantic processing likely require more anterior temporal regions.
These results are also in accordance with the majority of functional imaging studies assessing word comprehension. Using the NeuroSynth database, 74 studies assessed the relationship between word comprehension and neuroimaging (Supplementary Table 11). Across these studies, the regions most strongly activated were the posterior aspect of the middle temporal gyrus and the boundary between the inferior temporal gyrus and the fusiform gyrus (Fig. 4C).
Based on the aggregate functional literature review, on previous clinical and experimental lesion studies, and on our VLSM and CLSM results, we propose that the posterior and inferior aspects of the temporal lobe serve as an initial hub for word comprehension. Considering that speech comprehension involves speech perception, interpreting, and comprehending, the posterior aspect of the middle temporal gyrus possibly exerts the role of bridging speech perception with subsequent comprehension. This is likely accomplished by linking auditory signals processed in the primary auditory cortex to the posterior superior middle temporal gyrus, which subsequently disseminates them to more anterior aspects of the middle temporal gyrus, inferior temporal gyrus and posterior cingulate. A diagram representing this pattern is demonstrated in Fig. 4D. Within this network, the inferior temporal gyrus serves as the connection to the temporal pole, from which additional semantic integration may be performed. The temporal pole is also likely to be essential in recognizing objects—an essential process required early in the task of matching spoken words to pictures or objects.
It is also important to highlight the differential contribution of the CSLM and VLSM results. Specifically, a component of the fusiform gyrus was identified by VLSM as significantly associated with word comprehension controlling for object recognition, but fusiform gyrus links were not identified by CLSM. This difference is related to important methodological aspects that should be taken into account when interpreting the results of this study. First, VLSM and CLSM are complementary methods; they are both related to structural lesion patterns, but CLSM assesses the degree of primary and secondary white matter damage, which may extend beyond the necrotic and gliotic lesion. As such, CLSM is sensitive to cortical damage that may occur as a result of direct damage or disconnection. This explains why more pervasive networks were identified with CLSM. Second, the VLSM results described here were based on voxel-wise analyses, whereas CLSM was based on regions of interest. Thus, if a component of the region of interest was associated with behaviour, but not the entire region of interest (for example, the segment of the fusiform gyrus that is adjacent to the posterior inferior temporal gyrus), these voxels may be identified by VLSM only. Indeed, hypometabolism in the anterior portion of the fusiform gyrus has been identified as a predictor of semantic scores in individuals with frontotemporal dementia (Mion et al., 2010), and the left fusiform gyrus has been associated with object recognition (Wierenga et al., 2009; Stevens et al., 2015). As such, portions of the fusiform gyrus may be important as an interface between word and object recognition. Likewise, CLSM may not identify this relationship since CLSM is based on entire regions of interest, neglecting the effect of parts of the region of interest. Third, complex fibre anatomy in the inferior portion of the temporal lobe may prevent a more thorough assessment of fine-grained cortical folding. Similarly, the absence of the middle temporal gyrus in the VLSM results corresponds to the same phenomenon in reverse: the middle temporal gyrus may appear intact in some individuals and yet be lesioned through disconnection. Thus, VSLM can neglect the relationship between damage caused by disconnection when cortical gliosis/necrosis is milder. This is the central advantage of CLSM. For these reasons, it is important to interpret CLSM and VLSM as complementary tests.
In summary, we observed that damage to the inferior temporal gyrus, to the fusiform gyrus and to a white matter network including the left posterior temporal region and its connections to the middle temporal gyrus, inferior temporal gyrus, and cingulate cortex, was associated with word comprehension difficulties after factoring out object recognition. These findings support the view that posterior lateral and inferior aspects of the temporal cortex are crucial for word comprehension and may serve as a hub to integrate auditory and conceptual information necessary to recognize words. The results also explain why previous studies suggested an association between the temporal pole in word comprehension, since the temporal pole is functionally and structurally connected to the middle temporal gyrus. Thus, when the pole is disproportionally affected, an indirect knock-on effect may lead to a statistical association with poor word comprehension.
Funding
This study was supported by research grants from the National Institutes of Health / National Institute on Deafness and Other Communication Disorders (NIH-NIDCD): DC014021 (PI: Bonilha), DC011739 (PI: Fridriksson), DC014664 (PI: Fridriksson); DC05375 (PI: Hillis), and from the American Heart Association: SFDRN26030003 (PI: Bonilha)
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- CLSM
connectome-lesion symptom mapping
- PPA
primary progressive aphasia
- PPTT
Pyramids and Palm Trees Test
- VSLM
voxel-based lesion symptom mapping
- WAB-R
Western Aphasia Battery-Revised
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003; 6: 448–50. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain?. Neuroimage 2007; 34: 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies Cereb Cortex 2009; 19: 2767–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Rorden C, Fillmore P, Ratnayake RP, Fridriksson J. Mapping remote subcortical ramifications of injury after ischemic strokes. Behav Neurol 2014a; 2014: 215380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke 2014b; 45: 988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman L, Gottesman R, Chaudhry P, Davis C, Kleinman JT, Pawlak M, et al. Where (in the brain) do semantic errors come from? Cortex 2009; 45: 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. N Engl J Med 1992; 326: 531–9. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004; 92: 145–77. [DOI] [PubMed] [Google Scholar]
- Eggert GH. Wernicke's works on aphasia : a sourcebook and review. The Hague; New York, NY: Mouton; 1977. [Google Scholar]
- Faria AV, Joel SE, Zhang Y, Oishi K, van Zjil PC, Miller MI, et al. Atlas-based analysis of resting-state functional connectivity: evaluation for reproducibility and multi-modal anatomy-function correlation studies. Neuroimage 2012; 61: 613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Sebastian R, Newhart M, Mori S, Hillis AE. Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: potential clinical significance. Aphasiology 2014; 28: 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca's area. Curr Biol 2012; 22: 2059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Rorden C. Severe Broca's aphasia without Broca's area damage. Behav Neurol 2007; 18: 237–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva S, Baron JC, Jones PS, Price CJ, Warburton EA. A comparison of VLSM and VBM in a cohort of patients with post-stroke aphasia. Neuroimage Clin 2012; 1: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Guerrero GD, Cecilia JM, Garcia JM, Inuggi A, Jbabdi S, et al. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One 2013; 8: e61892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007; 8: 393–402. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The pyramids and palm trees test: a test of semantic access from words and pictures. London: Harcourt Assessment; 1992. [Google Scholar]
- Humphries C, Love T, Swinney D, Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum Brain Mapp 2005; 26: 128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Willard K, Buchsbaum B, Hickok G. Role of anterior temporal cortex in auditory sentence comprehension: an fMRI study. Neuroreport 2001; 12: 1749–52. [DOI] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery—revised. New York, NY: Grune & Stratton; 2007. [Google Scholar]
- Kreisler A, Godefroy O, Delmaire C, Debachy B, Leclercq M, Pruvo JP, et al. The anatomy of aphasia revisited. Neurology 2000; 54: 1117–23. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Ehsan S, Baker GA, Rogers TT. Semantic memory is impaired in patients with unilateral anterior temporal lobe resection for temporal lobe epilepsy. Brain 2012; 135 (Pt 1): 242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods 2016; 264: 47–56. [DOI] [PubMed] [Google Scholar]
- MacGregor LJ, Pulvermuller F, van Casteren M, Shtyrov Y. Ultra-rapid access to words in the brain. Nat Commun 2012; 3: 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, et al. The cortical representation of speech. J Cogn Neurosci 1993; 5: 467–79. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain 2015; 138 (Pt 8): 2423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain 2010; 133: 3256–68. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008; 40: 570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain 1999; 122 (Pt 1): 61–73. [DOI] [PubMed] [Google Scholar]
- Nachev P, Coulthard E, Jager JR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage 2008; 39: 1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis AE. Neural networks essential for naming and word comprehension. Cogn Behav Neurol 2007; 20: 25–30. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Knight RT. Decoding speech for understanding and treating aphasia. Prog Brain Res 2013; 207: 435–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 2010; 1191: 62–88. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 2012; 62: 816–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Hickok G. Selective attention to semantic and syntactic features modulates sentence processing networks in anterior temporal cortex. Cereb Cortex 2009; 19: 786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Pitz E, Hillis AE, Hickok G. Auditory word comprehension impairment in acute stroke: relative contribution of phonemic versus semantic factors. Brain Lang 2008; 107: 167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Rong F, Saberi K, Hickok G. Functional anatomy of language and music perception: temporal and structural factors investigated using functional magnetic resonance imaging. J Neurosci 2011; 31: 3843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage 2012; 61: 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Grabowski TJ. Comprehension. Continuum 2010; 16 (4 Behavioral Neurology): 45–58. [DOI] [PubMed] [Google Scholar]
- Skeide MA, Friederici AD. The ontogeny of the cortical language network. Nat Rev Neurosci 2016; 17: 323–32. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol 2003; 53: 35–49. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol 2005; 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Tessler MH, Peng CS, Martin A. Functional connectivity constrains the category-related organization of human ventral occipitotemporal cortex. Hum Brain Mapp 2015; 36: 2187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, den Ouden DB. Neuroimaging and recovery of language in aphasia. Curr Neurol Neurosci Rep 2008; 8: 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain 2011; 134 (Pt 10): 3094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci 2002; 14: 550–60. [DOI] [PubMed] [Google Scholar]
- Whitaker HA, Etlinger SC. Theodor Meynert's contribution to classical 19th century aphasia studies. Brain Lang 1993; 45: 560–71. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Perlstein WM, Benjamin M, Leonard CM, Rothi LG, Conway T, et al. Neural substrates of object identification: functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. J Int Neuropsychol Soc 2009; 15: 169–81. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 2011; 8: 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Speer NK, Zacks JM. Neural substrates of narrative comprehension and memory. Neuroimage 2008; 41: 1408–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Tseng WY. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 2011; 58: 91–9. [DOI] [PubMed] [Google Scholar]
- Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J Neurosci 2016; 36: 6668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.