Abstract
Background:
Limited data are available regarding the immunogenicity of high-dose influenza vaccine among persons with chronic lymphocytic leukemia (CLL) and monoclonal B cell lymphocytosis (MBL).
Methods:
A prospective pilot study of humoral immune responses to 2013–2014 and 2014–2015 high-dose trivalent influenza vaccine (HD IIV; Fluzone® High-Dose; Sanofi Pasteur) was conducted among individuals with MBL and previously untreated CLL. Serum hemagglutination inhibition (HAI) antibody titers were measured at baseline and Day 28 after vaccination; seroprotection and seroconversion rates were determined. Memory B cell responses were assessed by B-cell enzyme-linked immune absorbent spot assays.
Results:
Thirty subjects (17 CLL and 13 MBL) were included. Median age was 69.5 years. Day 28 seroprotection rates for the cohort were 19/30 (63.3%) for A/H1N1; 21/23 (91.3%) for A/H3N2; and 13/30 (43.3%) for influenza B. Those with MBL achieved higher day 28 HAI geometric mean titers (54.1 [4.9, 600.1] vs. 12.1 [1.3, 110.1]; p = 0.01) and higher Day 28 seroprotection rates (76.9% vs. 17.6%; p = 0.002) against the influenza B-vaccine strain virus than those with CLL.
Conclusions:
Immunogenicity of the HD IIV3 in patients with CLL and MBL is lower than reported in healthy adults. Immunogenicity to influenza B was greater in those with MBL than CLL.
Keywords: Influenza, Influenza vaccines, Leukemia, Lymphocytic, Chronic, B-cell, Monoclonal B cell lymphocytosis
1. Introduction
Persons with hematologic malignancies experiencing influenza infection are at high risk of serious complications [1]. Studies of acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25–37% [2-4]. Chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world, comprising 30% of all leukemia cases, and accounting for 11% of all hematologic malignancies [5]. Infection is the cause of death in 30–50% of persons with CLL [6-8]. Monoclonal B cell lymphocytosis (MBL), the precursor state to CLL, is defined as the presence of a small population of clonal B cells (<5 × 109/L) in the peripheral blood in the absence of lymphadenopathy, cytopenias, or autoimmune disease [9,10]. In most cases, the MBL immunophenotype is identical to that of CLL. MBL is typically categorized into “low count” (LC) MBL or “high count” (HC) MBL depending on whether the B-cell count is below or above 0.5 × 109/L, respectively. Population-based studies have demonstrated that low count MBL affects more than 5% of adults over age 40, and its prevalence increases with increasing age [9,11]. Only a fraction of MBL cases, typically those with high count MBL, come to clinical attention when patients are found to have mild lymphocytosis identified on complete blood count. Persons with both CLL and MBL have also been shown to have a higher risk for serious infection than the general population. A case-control study of individuals with clinical MBL and previously untreated CLL demonstrated that both groups have a three-fold higher risk of hospitalization for infection compared to a control population in multivariate analysis. Persons with MBL were four times more likely to be hospitalized for infection than to progress to CLL and require chemotherapy [12,13].
Increased susceptibility to infection among those with CLL occurs prior to initiation of immunosuppressive medications and is related to a complex immune dysregulation that includes hypogammaglobulinemia [7,14-16], B cell [17,18] and T cell dysfunction [19-23], and defects in innate immunity [24,25]. Although defects in immune competence in MBL have not been studied well to date, they are likely to be very similar to those of CLL given its precursor status to development of CLL [26,27].
One of the strategies to prevent select infections in patients with hematologic malignancies is vaccination. In the United States, annual influenza vaccination is recommended for all people 6 months of age or older, including those with CLL. Although the burden of influenza among persons with MBL and CLL has not been described, limited data suggests decreased influenza vaccine immunogenicity in persons with CLL. One study reported influenza vaccine seroconversion rates of 5–15% for influenza A and B in persons with CLL who received trivalent standard dose inactivated subunit influenza vaccine (SD IIV3) [28]. Older influenza vaccine studies among persons with CLL utilized whole virus vaccine and did not evaluate influenza vaccine immune response utilizing the currently accepted definitions for seroconversion; thus, these results are difficult to put into context [29-32]. In the United States, trivalent high-dose influenza vaccine (HD IIV3) is an option for persons aged ≥ 65 years. One study evaluating the response to HD and SD IIV3 vaccine among 19 persons with CLL who were on therapy with ibrutinib found seroconversion for at least 1 vaccine-strain virus in 5/19 (26%) of subjects [33]. This study did not specify the proportion of subjects who received the HD vaccine. Another study of 13 subjects with CLL and one with Waldenstrom’s macroglobulinemia who were treated with ibrutinib who were vaccinated with SD IIV3 reported that only 1/14 (7%) seroconverted to each vaccine-virus strain [34]. To our knowledge, there are no published data regarding immune responses to any vaccines among individuals with LC MBL and HC MBL. Given the prevalence of MBL and increased infection risk in this condition, characterizing vaccine responses in this population is important in patient management.
We conducted a pilot study of the 2013–2014 and 2014–2015 HD IIV3 vaccines in persons with HCMBL and previously untreated CLL at the Mayo Clinic in Rochester, Minnesota. The objective of this study was to characterize and compare the humoral immune response to HD IIV3 among persons with MBL and untreated CLL.
2. Methods
2.1. Study design
A prospective pilot study of humoral immune responses to 2013–2014 and 2014–2015 HD IIV3 (Fluzone® High-Dose; Sanofi Pasteur) that was administered as part of routine clinical care for patients with HC MBL and previously untreated CLL was conducted at Mayo Clinic. The study was approved through the Mayo Clinic Institutional Review Board. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsiniki). The primary outcomes of interest were seroconversion and seroprotection, as measured by HAI (hemagglutination inhibition assay). Baseline clinical variables including age, sex, MBL diagnosis, CLL diagnosis and Rai stage, time since MBL or CLL diagnosis were captured prior to influenza vaccination. Samples for baseline complete blood count (CBC) with differential, quantitative immunoglobulins, and immunogenicity assays were collected at Day 0, prior to influenza vaccination, and at Day 28 after influenza immunization. Sera were obtained and stored at −80 °C for future batched HAI assays. Peripheral blood mononuclear cells (PBMCs) were obtained and were stored on liquid nitrogen for future batched B cell ELISpot and flow cytometry analyses. The 2013–2014 and 2014–2015 HD IIV3s contained the same influenza hemagglutinin antigens from the A/California/7/2009 (H1N1)-like virus and B/Massachusetts/2/2012-like (Yamagata lineage) virus. Data were combined for both years for the A/H1N1 and influenza B vaccine responses. Unique patients were enrolled in each year of the study. Responses to the A/H3N2 vaccine strain were measured only for the group who received the 2014–2015 HD IIV3.
2.2. Study population
Persons with clinical HC MBL and previously untreated CLL who presented for routine outpatient clinical care through the specialized MBL/CLL clinic within the Mayo Clinic Hematology Division in Rochester, MN and who were already enrolled in the MBL/CLL natural cohort study, for which they provided informed consent, were recruited for participation in this study. Inclusion criteria were age ≥ 18 years, diagnosis of MBL or CLL that was not previously treated with chemotherapy or immunotherapy, and no prior receipt of the seasonal influenza vaccine during the influenza season when they entered the study. None of the participants received IVIG within 3 months before or after the study immunization. Vaccination occurred between October 14, 2013–November 8, 2013 and October 13, 2014–November 4, 2014. Participants received a single 0.5 mL dose of HD IIV3 (Fluzone® High-Dose; Sanofi Pasteur) via intramuscular route in the deltoid muscle. Vaccines were stored according to package insert at 2–8 °C in temperature-monitored refrigerators. Cold chain was maintained until time of vaccine administration.
2.3. Laboratory methods
HAI assays were performed in triplicate at the Human Immunology Phenotyping Consortium Central Lab Core Site at Mt. Sinai School of Medicine for the influenza A/H1N1 and influenza B strains contained in the 2013–2014 and 2014–2015 Northern Hemisphere HD IIV3 vaccines: influenza A/California/7/2009 (H1N1)-like virus, and influenza B/Massachusetts/2/2012-like (Yamagata lineage) virus. These strains were unchanged in the 2013–2014 and 2014–2015 Northern Hemisphere vaccines. No testing was performed for the A/H3N2 strain contained in the 2013–2014 Northern Hemisphere vaccine for the 7 participants who received vaccine in this season. For the 23 who received HD IIV3 in 2014–2015, testing was performed using the influenza A/Texas/50/2012 (H3N2)-like virus. The HAI assays were performed using previously described methods [35]. Seroprotection was defined as HAI antibody titer ≥ 40. Seroconversion was defined as a pre-vaccination HAI antibody titer < 10 and a postvaccination HAI antibody titer ≥ 40 or a pre-vaccination HI titer ≥ 10 and a minimum four-fold rise in post-vaccination HAI antibody titer. We utilized these widely accepted definitions for seroprotection and seroconversion that are recommended for the licensure of influenza vaccines by the United States Food and Drug Administration.
Memory B cell ELISpot assays were performed for each vaccine strain using the Mabtech ELISpotPLUS kit for human IgG (Mabtech Inc, Cincinnati, OH) and manufacturer’s instructions with a few previously described modifications [36]. Further details are listed in the Supplementary Material.
Complete blood counts with differential and quantitative immunoglobulins for Day 0 samples were performed by Mayo Medical Laboratories and archived by us for use in this analysis and report. Flow cytometry on Day 0 samples was performed according to methods described in Supplementary Appendix.
3. Results
3.1. Baseline characteristics
Thirty subjects were recruited for the study. Of these, 17 had CLL (70% with Rai Stages 0/1 and 30% with Rai Stages ≥ 2), and 13 had MBL (Table 1). The median age was 69.5 years (range 49.0–82.0); 36.7% were female. The median number of years from the diagnosis of MBL or CLL was 5.4 years (range 0.1–14.8). Seven subjects were enrolled during the 2013–2014 influenza season, and 23 were enrolled during the 2014–2015 influenza season.
Table 1.
Clinical characteristics of cohort at baseline.
| CLL (n = 17) | MBL (n = 13) | Total (n = 30) | |
|---|---|---|---|
| Age in years | |||
| Median (range) | 69.0 (49.0–80.0) | 73.0 (60.0–82.0) | 69.5 (49.0–82.0) |
| Female | 7 (41.2%) | 4 (30.8%) | 11 (36.7%) |
| Race, White | 17 (100%) | 13 (100%) | 30 (100%) |
| Ethnicity, Not Hispanic or Latino | 17 (100%) | 13 (100%) | 30 (100%) |
| Years from Diagnosis to Study Entry | |||
| Median (range) | 5.0 (0.1–10.6) | 5.8 (0.6–14.8) | 5.4 (0.1–14.8) |
| Rai Stage for CLL | |||
| 0 | 10 (58.8%) | n/a | n/a |
| 1 | 2 (11.8%) | ||
| 2 | 2 (11.8%) | ||
| 3 | 2 (11.8%) | ||
| 4 | 1 (5.9%) | ||
| IGHV mutation | |||
| Missing data | 3 | 3 | 6 |
| Mutation | 10 (71.4%) | 7 (70.0%) | 17 (70.8%) |
| Unmutated | 4 (28.6%) | 3 (30.0%) | 7 (29.2%) |
| CD38 expression ≥ 30% | |||
| Missing data | 0 | 2 | 2 |
| No (CD38 negative) | 15 (88.2%) | 9 (81.8%) | 24 (85.7%) |
| Yes (CD38 positive) | 2 (11.8%) | 2 (18.2%) | 4 (14.3%) |
| FISH | |||
| Missing data | 2 | 2 | 4 |
| Normal | 7 (46.7%) | 3 (27.3%) | 10 (38.5%) |
| 13q- | 5 (33.3%) | 6 (54.5%) | 11 (42.3%) |
| Trisomy 12 | 3 (20.0%) | 1 (9.1%) | 4 (15.4%) |
| 11q- | 0 | 1 (9.1%) | 1 (3.9%) |
| 17p- | 0 | 0 | 0 |
| Days from baseline immunogenicity labs (pre-vaccine) to follow-up immunogenicity labs (post vaccine) | |||
| Median (IQR) | 30.0 (28.0, 33.0) | 27.0 (25.0, 30.0) | 28.5 (26.0, 31.8) |
CLL = chronic lymphocytic leukemia.
MBL = monoclonal B cell lymphocytosis.
IGHV = immunoglobulin heavy chain gene.
FISH = Fluorescence in situ hybridization.
IQR = interquartile range.
Baseline quantitative immunoglobulin levels for those with CLL and MBL are reported in Table 2. Subjects with MBL had higher median quantitative serum immunoglobulin (Ig) G levels than those with CLL (911 mg/dl vs 703 mg/dl; p = 0.03) (Table 2). Individuals with MBL also had higher IgG1 (p = 0.012), IgG2 (p = 0.018), IgG3 (p = 0.024) subclass levels than those with CLL, as well as higher serum IgA (173 vs 92 mg/dl; p = 0.010) and serum IgM (58 mg/dl vs 27 mg/dl; p = 0.037) levels (Table 2).
Table 2.
Immunologic characteristics of cohort at baseline.
| CLL (n = 17) Median (IQR) |
MBL (n = 13) Median (IQR) |
p-valuea | |
|---|---|---|---|
| Total serum immunoglobulin (Ig) G mg/dl | 703 (391, 815) | 911 (800, 1010) | 0.030 |
| Serum IgG1 mg/dl | 331 (225, 396) | 468 (380, 494) | 0.012 |
| Serum IgG2 mg/dl | 185 (101, 265) | 303 (245, 405) | 0.018 |
| Serum IgG3 mg/dl | 30 (15, 43) | 56 (47, 66) | 0.024 |
| Serum IgG4 mg/dl | 7 (3, 12) | 21 (7, 40) | 0.058 |
| Serum IgM mg/dl | 27 (19, 52) | 58 (52, 70) | 0.037 |
| Serum IgA mg/dl | 92 (57, 129) | 173 (121, 204) | 0.010 |
| Absolute neutrophil count × 109/L | |||
| Median (Q1, Q3) | 3.12 (2.49, 4.60) | 3.79 (2.77, 4.48) | 0.917 |
| Absolute lymphocyte count × 109/L | |||
| Median (Q1, Q3) | 23.20 (14.08, 39.80) | 5.15 (2.70, 8.90) | 1.95x10−4 |
| Absolute T-cell count × 109/L | |||
| Median (Q1, Q3) | 3.72 (2.50, 6.62) | 1.82 (1.48, 2.70) | 0.005 |
| Absolute CD4 + T-cell count × 109/L | |||
| Median (Q1, Q3) | 2.30 (1.17, 2.99) | 1.06 (0.73, 1.25) | 0.012 |
| Absolute CD8 + T-cell count × 109/L | |||
| Median (Q1, Q3) | 0.73 (0.47, 0.99) | 0.35 (0.24, 0.80) | 0.103 |
IQR = interquartile range.
Normal reference values: serum IgG 767–1590 mg/dl; serum IgG1 341–894 mg/dl; serum IgG2 171–632 mg/dl; serum IgG3 18.4–106.0 mg/dl; serum IgG4 2.4–121.0 mg/dl; serum IgM 37–286 mg/dl; serum IgA 61–356 mg/dl; absolute neutrophil count 1.56–6.45 × 109/L; absolute lymphocyte count 0.95–3.07 × 109/L.
Wilcoxon Rank Sum Test between CLL and MBL values.
3.2. Primary immunogenicity analyses
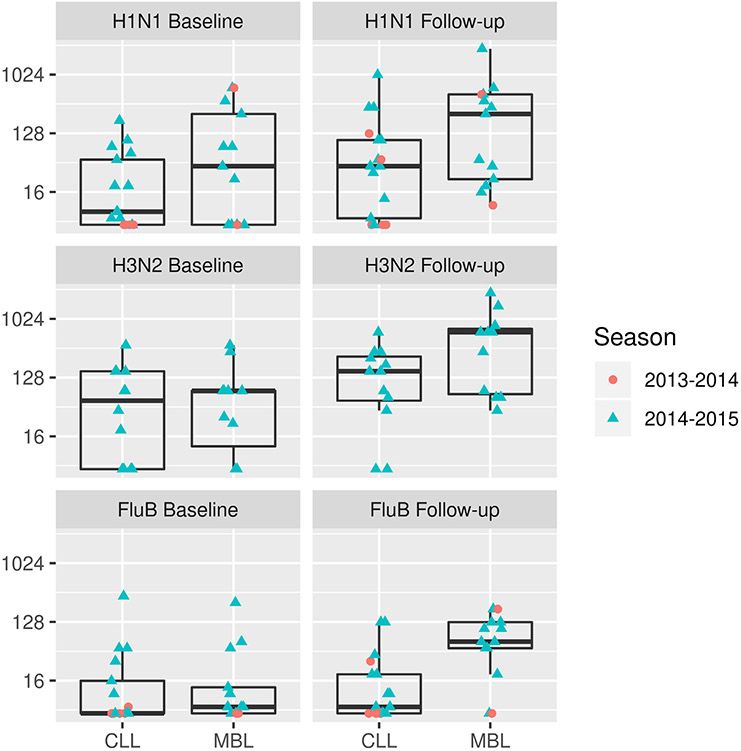
Geometric mean titers (GMTs) of serum HAI antibody for Day 0 and Day 28, seroprotection, and seroconversion rates are displayed in Table 3 for both influenza seasons, with the exception of responses to A/H3N2, which were only performed for the 23 subjects from the 2014–2015 influenza season. Fig. 1 depicts GMTs of HAI antibody by influenza season. Supplemental Tables 2 and 3 display results for each separate influenza season.
Table 3.
Geometric mean titers of Serum hemagglutination inhibition assay antibody, seroconversion, and seroprotection rates: combined data from 2013–2014 to 2014–2015 cohorts with exception of A/H3N2 (2014–2015 only).
| Vaccine strain |
Day post- vaccine |
Overall cohort (n = 30) |
CLL (n = 17) | MBL (n = 13) | p-value comparing CLL to MBL |
|
|---|---|---|---|---|---|---|
| HAI GMT (95% CI) | A/H1N1 | 0 | 23.0 (0.9, 606.5) | 14.8 (1.2, 184.4) | 40.7 (0.8, 1963.2) | 0.27d |
| 28 | 64.5 (2.0, 2117.3) | 38.4 (1.5, 1002.9) | 127.0 (4.0, 3995.7) | 0.09d | ||
| A/H3N2 | 0 | 38.4 (1.8, 843.2) | 36.3 (1.4, 911.3) | 40.8 (1.9, 895.7) | 0.98d | |
| 28 | 168.2 (7.3, 3876.7) | 93.3 (4.3, 2005.1) | 320.0 (19.1, 5373.0) | 0.08d | ||
| B | 0 | 11.3 (1.1, 121.2) | 10.6 (1.0, 115.0) | 12.4 (1.1, 141.3) | 0.31d | |
| 28 | 23.2 (1.6, 343.6) | 12.1 (1.3, 110.1) | 54.1 (4.9, 600.1) | 0.01d | ||
| Ratio of day 28/day 0 HAI GMT (95% CI) | A/H1N1 | 28/0 | 2.8 (0.3, 26.0) | 2.6 (0.3, 25.2) | 3.1 (0.3, 29.2) | 0.77d |
| A/H3N2 | 28/0 | 4.4 (0.2, 112.0)a | 2.6 (0.1, 58.0)b | 7.8 (0.3, 176.4)c | 0.06d | |
| B | 28/0 | 2.0 (0.1, 28.0) | 1.1 (0.2, 7.1) | 4.4 (0.3, 72.4) | 0.02d | |
| Seroconversion | A/H1N1 | 28/0 | 3 (10.0%) | 2 (11.8%) | 1 (7.7%) | 1.0e |
| A/H3N2 | 28/0 | 5/23 (21.7%)a | 2/12 (16.7%)b | 3/11 (27.3%)c | 0.64e | |
| B | 28/0 | 3 (10.0%) | 0 (0.0%) | 3 (23.1%) | 0.07e | |
| Seroprotection | A/H1N1 | 0 | 12 (40.0%) | 5 (29.4%) | 7 (53.8%) | 0.26e |
| 28 | 19 (63.3%) | 10 (58.8%) | 9 (69.2%) | 0.71e | ||
| A/H3N2 | 0 | 13/23 (56.5%) | 7/12 (58.3%) | 6/11 (54.5%) | 1.00e | |
| 28 | 21/23 (91.3%) | 10/12 (83.3%) | 11/11 (100%) | 0.48e | ||
| B | 0 | 6 (20.0%) | 3 (17.6%) | 3 (23.1%) | 1.0e | |
| 28 | 13 (43.3%) | 3 (17.6%) | 10 (76.9%) | 0.002e |
n = 23.
n = 12.
n = 11.
Kruskal-Wallis test.
Fisher’s exact test.
Fig. 1.
Geometric mean titers of serum hemagglutination inhibition assay antibody before and 28 days following immunization.
Day 28 seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1; 21/23 (91.3%) for A/H3N2; and 13/30 (43.3%) for influenza B. Individuals with MBL achieved higher day 28 seroprotection rates (76.9% vs. 17.6%; p = 0.002) against the influenza B-vaccine strain virus than those with CLL. Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B.
Those with MBL achieved higher day 28 HAI GMTs (54.1 [4.9, 600.1] vs. 12.1 [1.3,110.1]; p = 0.01) than those with CLL. There were no significant differences in GMTs, seroconversion, or seroprotection rates between individuals with MBL and CLL for influenza A/H1N1 or A/H3N2 vaccine-strains.
3.3. Influenza-virus specific memory B cell ELISpot responses
Influenza-virus specific memory B cell ELISpot responses at Day 28 post-vaccine are shown in Fig. 2. The MBL cohort demonstrated higher memory B cell ELISpot results in response to stimulation with each of the three influenza viruses compared to those with CLL. Fig. 3 displays the fold change in the B cell ELISpot response between Day 28 and Day 0. The MBL cohort demonstrated higher fold changes between Days 28 and 0 for influenza A/H1N2 and A/H3N2.
Fig. 2.
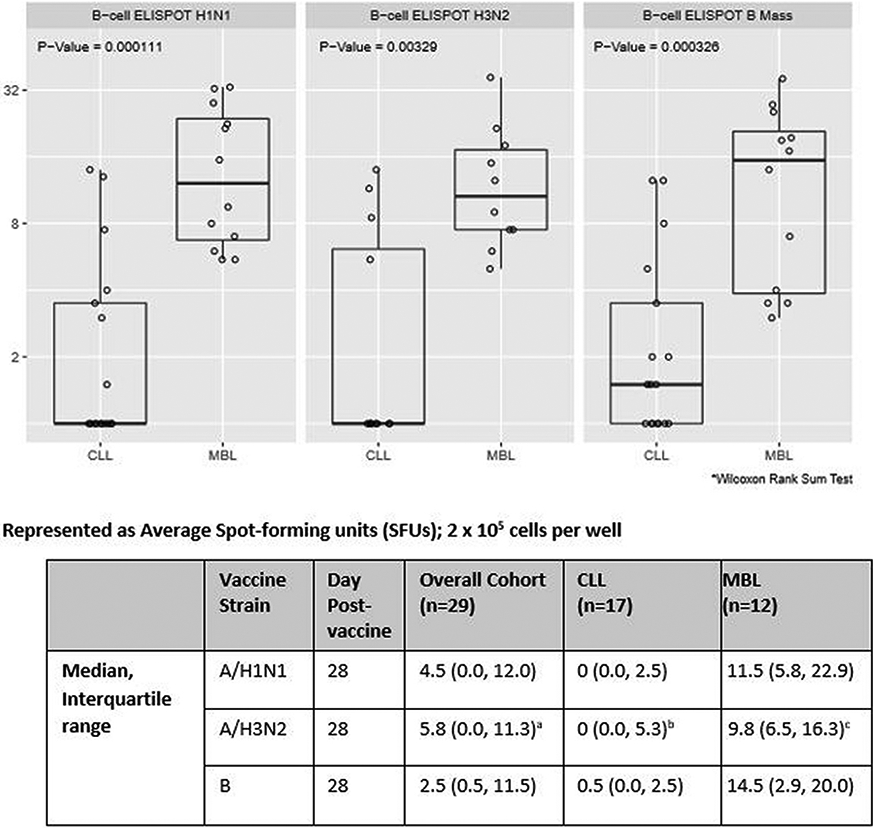
Influenza-virus specific memory B cell ELISpot counts at day 28 after immunization: combined data from 2013–2014 and 2014–2015 cohorts with exception of A/H3N2 (2014–2015 only).
an=22; bn=12; cn=10
Fig. 3.
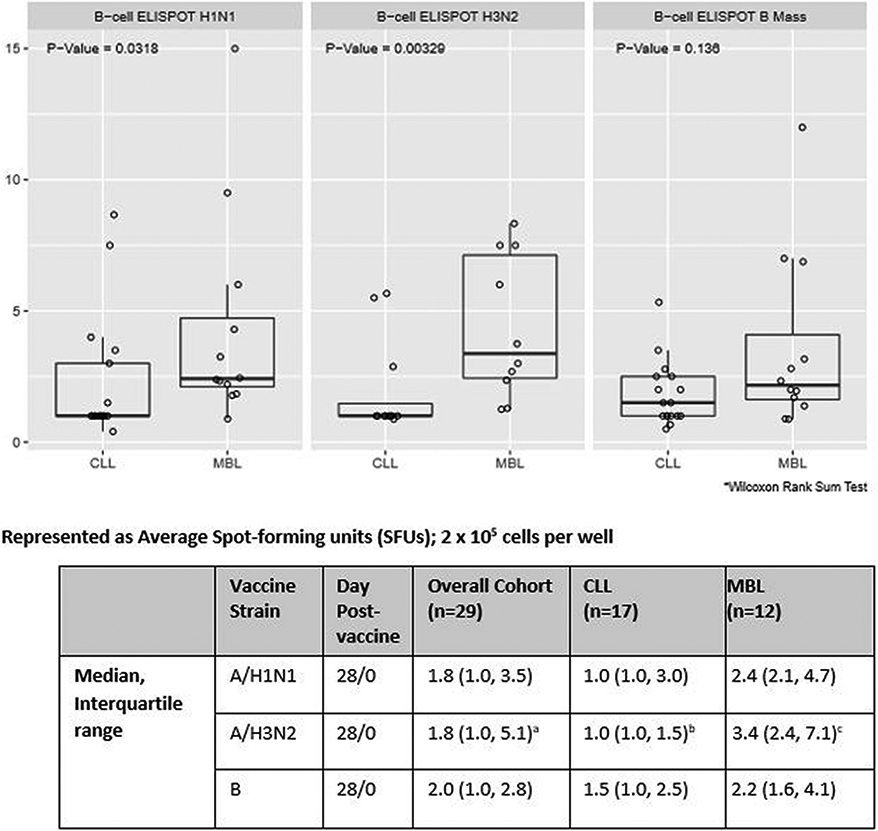
Fold change in B cell ELISpot response (day 28 vs day 0): combined data from 2013–2014 and 2014–2015 cohorts with exception of A/H3N2 (2014–2015 only).
an=22; bn=12; cn=10
4. Discussion
In this pilot study evaluating the humoral immune response to HD IIV3 among persons with MBL and previously untreated CLL, we observed that the majority of subjects demonstrated seroprotection at day 28 for each viral strain, with the exception of persons with CLL to influenza B. We also noted that a higher HAI GMTs in those with MBL and that a higher proportion of those with MBL developed seroprotection at Day 28 after vaccine than those with CLL. The majority of those with MBL developed seroprotection, with up to 100% developing seroprotection against A/H3N2. Those with CLL had low day 28 rates of seroprotection against influenza B (17.6%) but modest day 28 rates of seroprotection against A/H3N2 (83.3%) and A/H1N1 (58.8%).
We observed low rates of seroconversion in the overall cohort to all three influenza vaccine viral strains, Low rates of seroconversion may have been impacted, in part, by pre-existing immunity against A/H1N1 and A/H3N2. At baseline, 12/30 (40.0%) of the cohort already had seroprotective antibody titers against A/ H1N1, and 13/23 (56.5%) of the cohort already had seroprotective antibody titers against A/H3N2.
A limitation of our study is that it did not include a healthy age matched control population. Published data from older adult populations who have received HD IIV3 should be examined to put our findings into context. Immunogenicity data varies by influenza season and vaccine viral strains. There are no published studies reporting immune responses of healthy older adults (≥65 years old) following immunization with 2013–2014 HD IIV3. One randomized controlled trial (RCT) evaluated immunogenicity of SD versus HD IIV3 in 2014–2015, but seroprotection or serconversion rates were not reported [37]. A RCT of adults ≥ 65 years old who received the 2012–2013 HD IIV3 demonstrated seroprotection rates of 98.8% % for influenza A/H1N1; 98.6% for A/H3N2; and 86.2% for influenza B [38]. There are caveats in comparing influenza vaccine response rates across influenza seasons, as vaccine response may be affected by pre-existing immunity and differences in the antigens contained in vaccines across seasons. With these caveats, we did note lower seroconversion and seroprotection rates than were reported for healthy older adults who received HD IIV3. Another limitation of the study is that we did capture documented influenza infection after vaccination to determine vaccine efficacy.
Our study demonstrated higher seroconversion rates than the 5–15% for influenza A and B in persons with CLL who received SDIIV [28]. With the exception of a recent study of persons with CLL on therapy with ibrutinib [33], no studies of influenza vaccine immunogenicity among persons with CLL have utilized the currently accepted definitions for HAI antibody seroprotection or seroconversion [28-31]. The one study that did report seroconversion rates, defined seroprotection against influenza A as a HAI antibody titer ≥ 100 and seroprotection against influenza B as a HAI antibody titer ≥ 200 [28]. The study of 19 persons with CLL receiving ibrutinib therapy utilized HD IIV3 in persons older than 65 years and either HD or SD IIV3 in those younger than 65 years. However, the authors did not report how many subjects received HD IIV3 or SD IIV3. This study was also conducted in the 2014–2015 influenza season and reported seroprotection rates of 42% for A/H1N1; 32% for A/H3N2; and 11% for B. Unlike our study, these patients were receiving treatment with ibrutinib [33]. We noted higher seroprotection rates for each of these viral strains among those with CLL than was reported in the ibrutinib study [33]. Further studies are needed on the role of ibrutinib plays in responses to vaccination. One study has suggested that ibrutinib may impair serologic responses to influenza vaccination [34]. However, recent reports also indicate enhancement and alternatively disruption of the non-B cell compartment of the immune system by ibrutinib [39-41].
Interestingly, none of the published studies of influenza vaccine immunogenicity in patients with CLL report any information regarding the receipt of IVIG within the months before vaccination or during the period of pre- and post-vaccination sample collection [28-33]. Receipt of IVIG within three months prior to serum collection for immunogenicity studies would alter the HAI antibody titers [42]. Thus, it is important to account for receipt of IVIG when conducting vaccine studies among persons who are being treated with IVIG. No individual included in our study received IVIG therapy.
To our knowledge, this publication reports the first data on vaccine immunogenicity for any vaccine type in persons with HC MBL. Our sample size was small and may not have been powered to detect differences between immune responses in the MBL and CLL groups. Nonetheless, we did observe higher HAI GMTs and seroprotection rates in those with MBL versus CLL for influenza B. We noted greater influenza-virus specific memory B cell response for the MBL group for each influenza vaccine strain. The differences in length of disease, leukemic burden and associated changes with key components of the immune system including functional B cell populations between these two groups may account for these findings.
Limitations of our study include small sample size, lack of an age-matched healthy adult population as a control, lack of immunogenicity data against the A/H3N2 vaccine virus from the cohort who participated in the 2013–2014 influenza vaccine season, and no data on vaccine effectiveness. Strengths of our study include consideration for recent administration of IVIG, characterization of the clinical characteristics and immunophenotype of our cohort, and detailed evaluation of memory B cell responses.
Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection. Previous reports suggesting poor immunogenicity among patients with CLL receiving SD IIV3 may, in some instances, diminish provider adherence due a concern for ineffectual vaccine response. In the present study, we demonstrate that the majority of persons with MBL and CLL developed seroprotection in response to HD IIV3. Although this study demonstrates that there is room to improve influenza vaccine immunogenicity in this population, given that we do detect meaningful vaccine responses, the study also supports the immunization of persons with MBL and CLL against influenza. Indeed, even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications [43-45]. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations.
Supplementary Material
Acknowledgements
The authors thank Randy Albrecht, Ph.D. at Icahn School of Medicine at Mount Sinai for performing HAI assays.
This work was financially supported by the National Institutes of Health U01 AI089859-04 and R01 CA197120.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JAW, DEG, KMG, and TGC have no conflicts of interest to disclose.
SAP: Research funding has been provided to the institution from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, Bristol Myers Squibb, AbbVie, and Ascentage Pharma for clinical studies in which Sameer A. Parikh is a principal investigator. Sameer A. Parikh has also participated in Advisory Board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie. (He was not personally compensated for his participation.)
TDS: Research funding has been provided to the institution from Genentech, Pharmacyclics, Abbvie, GlaxoSmithKline, and Merck.
NEK: Research funding from: Acerta Pharma, Bristol Meyer Squib, Celgene, Pharmacyclics, Tolero Pharmaceuticals, MEI Pharma, Sunesis,and Abbvie. DSMC (Data Safety Monitoring Committee) for Agios Pharm, AstraZeneca, Celgene, Cytomx Therapeutics, Morpho-sys and Rigel. Advisory Board for: Cytomx Therapy, Pharmacyclics, Dava oncology, Juno Theraputics, Astra Zeneca and Oncotracker.
RBK has received research funding from Merck Research Laboratories to study waning immune responses to mumps vaccine. GAP and RBK hold a patent related to vaccinia peptide vaccines.
WD: research funding from Merck, DTRM pharma, Astrazenica, Abbvie and Mundi Pharma in which WD is a PI for these research studies. WD has participated Advisory board of Alexion, Merck, Octapharma and MEI pharma (no personal compensation for participation).
SSK is inventor on patents in the field of CART cell therapy that are licensed to Novartis, Humanigen, and Mettefoge. SSK has received research funding from Novartis, Kite, Gilead, Juno, Celgene, Tolero, Humanigen, Sunesis, Morphosys, and Lentigen. SSK has participated on advisory boards for Humanigen, Kite, and Calibr.
GAP is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. GAP offers consultative advice on vaccine development to Merck & Co. Inc., Avianax, Adjuvance, Valneva, Medicago, Sanofi Pasteur, GlaxoSmithKline, Dynavax, and Emergent Biosolutions. GAP and RBK hold a patent related to vaccinia peptide vaccines.
These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.01.001.
References
- [1].Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 2009;9:493–504. 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons discussion 25-6. Am J Med 1997;102:2–9. 10.1016/s0002-9343(97)00003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yousuf HM, Englund J, Couch R, Rolston K, Luna M, Goodrich J, et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis 1997;24:1095–9. 10.1086/513648. [DOI] [PubMed] [Google Scholar]
- [4].Elting LS, Whimbey E, Lo W, Couch R, Andreeff M, Bodey GP. Epidemiology of influenza A virus infection in patients with acute or chronic leukemia. Support Care Cancer 1995;3:198–202. [DOI] [PubMed] [Google Scholar]
- [5].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [6].Williams AM, Baran AM, Meacham PJ, Feldman MM, Valencia HE, Newsom-Stewart C, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma 2018;59:625–32. 10.1080/10428194.2017.1347931. [DOI] [PubMed] [Google Scholar]
- [7].Francis S, Karanth M, Pratt G, Starczynski J, Hooper L, Fegan C, et al. The effect of immunoglobulin VH gene mutation status and other prognostic factors on the incidence of major infections in patients with chronic lymphocytic leukemia. Cancer 2006;107:1023–33. 10.1002/cncr.22094. [DOI] [PubMed] [Google Scholar]
- [8].Strati P, Jain N, O’Brien S. Chronic lymphocytic leukemia: diagnosis and treatment. Mayo Clin Proc 2018;93:651–64. 10.1016/j.mayocp.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [9].Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia 2010;24:512–20. 10.1038/leu.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rawstron AC, Shanafelt T, Lanasa MC, Landgren O, Hanson C, Orfao A, et al. Different biology and clinical outcome according to the absolute numbers of clonal B-cells in monoclonal B-cell lymphocytosis (MBL). Cytometry B Clin Cytom 2010;78(Suppl 1):S19–23. 10.1002/cyto.b.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rawstron AC, Green MJ, Kuzmicki A, Kennedy B, Fenton JA, Evans PA, et al. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood 2002;100:635–9. 10.1182/blood.v100.2.635. [DOI] [PubMed] [Google Scholar]
- [12].Moreira J, Rabe KG, Cerhan JR, Kay NE, Wilson JW, Call TG, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia 2013;27:136–41. 10.1038/leu.2012.187. [DOI] [PubMed] [Google Scholar]
- [13].Shanafelt TD, Kay NE, Parikh SA, Achenbach SJ, Lesnick CE, Hanson CA, et al. Risk of serious infection among individuals with and without low count monoclonal B-cell lymphocytosis (MBL). Leukemia 2020. 10.1038/s41375-020-0799-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Freeman JA, Crassini KR, Best OG, Forsyth CJ, Mackinlay NJ, Han P, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma 2013;54:99–104. 10.3109/10428194.2012.706285. [DOI] [PubMed] [Google Scholar]
- [15].Molica S Infections in chronic lymphocytic leukemia: risk factors, and impact on survival, and treatment. Leuk Lymphoma 1994;13:203–14. 10.3109/10428199409056283. [DOI] [PubMed] [Google Scholar]
- [16].Griffiths H, Lea J, Bunch C, Lee M, Chapel H. Predictors of infection in chronic lymphocytic leukaemia (CLL). Clin Exp Immunol 1992;89:374–7. 10.1111/j.1365-2249.1992.tb06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull 2008;87:49–62. 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- [18].Foon KA, Rai KR, Gale RP. Chronic lymphocytic leukemia: new insights into biology and therapy. Ann Intern Med 1990;113:525–39. 10.7326/0003-4819-113-7-525. [DOI] [PubMed] [Google Scholar]
- [19].Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma 2003;44:383–9. 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- [20].Kay NE. Abnormal T-cell subpopulation function in CLL: excessive suppressor (T gamma) and deficient helper (T mu) activity with respect to B-cell proliferation. Blood 1981;57:418–20. [PubMed] [Google Scholar]
- [21].Ziegler HW, Kay NE, Zarling JM. Deficiency of natural killer cell activity in patients with chronic lymphocytic leukemia. Int J Cancer 1981;27:321–7. [DOI] [PubMed] [Google Scholar]
- [22].Briggs PG, Kraft N, Atkins RC. T cells and CD45R expression in B-chronic lymphocytic leukemia. Leuk Res 1990;14:155–9. [DOI] [PubMed] [Google Scholar]
- [23].Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008;118:2427–37. 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ntoufa S, Vardi A, Papakonstantinou N, Anagnostopoulos A, Aleporou-Marinou V, Belessi C, et al. Distinct innate immunity pathways to activation and tolerance in subgroups of chronic lymphocytic leukemia with distinct immunoglobulin receptors. Mol Med 2012;18:1281–91. 10.2119/molmed.2011.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barcellini W, Imperiali FG, Zaninoni A, Reda G, Consonni D, Fattizzo B, et al. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: relationship with infections, autoimmunity and disease progression. Leuk Lymphoma 2014;55:1768–73. 10.3109/10428194.2013.856426. [DOI] [PubMed] [Google Scholar]
- [26].Hauswirth AW, Almeida J, Nieto WG, Teodosio C, Rodriguez-Caballero A, Romero A, et al. Monoclonal B-cell lymphocytosis (MBL) with normal lymphocyte counts is associated with decreased numbers of normal circulating B-cell subsets. Am J Hematol 2012;87:721–4. 10.1002/ajh.23214. [DOI] [PubMed] [Google Scholar]
- [27].Glancy E, Siles R. Monoclonal B-cell lymphocytosis and hypogammaglobulinaemia. Br J Haematol 2016;173:316–7. 10.1111/bjh.13585. [DOI] [PubMed] [Google Scholar]
- [28].van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med 2001;12:420–4. [DOI] [PubMed] [Google Scholar]
- [29].Marotta G, Bucalossi A, Galieni P, Bigazzi C, Nuti S, Valenzin PE, et al. CD4+/ CD45RA+ ‘naive’ T cells and immunological response to influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol 1998;99:18–21. 10.1159/000040709. [DOI] [PubMed] [Google Scholar]
- [30].Bucalossi A, Marotta G, Galieni P, Bigazzi C, Valenzin PE, Dispensa E. Immunological response to influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol 1995;93:56. 10.1159/000204095. [DOI] [PubMed] [Google Scholar]
- [31].Jurlander J, de Nully BP, Skov PS, Henrichsen J, Heron I, Obel N, et al. Improved vaccination response during ranitidine treatment, and increased plasma histamine concentrations, in patients with B cell chronic lymphocytic leukemia. Leukemia 1995;9:1902–9. [PubMed] [Google Scholar]
- [32].Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol 1994;91:115–8. 10.1159/000204315. [DOI] [PubMed] [Google Scholar]
- [33].Sun C, Gao J, Couzens L, Tian X, Farooqui MZ, Eichelberger MC, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol 2016;2:1656–7. 10.1001/jamaoncol.2016.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica 2017;102:e397–9. 10.3324/haematol.2017.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang S, Taaffe J, Parker C, Solorzano A, Cao H, Garcia-Sastre A, et al. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J Virol 2006;80:11628–37. 10.1128/JVI.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Painter SD, Haralambieva IH, Ovsyannikova IG, Grill DE, Poland GA. Detection of influenza A/H1N1-specific human IgG-secreting B cells in older adults by ELISPOT assay. Viral Immunol 2014;27:32–8. 10.1089/vim.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Merani S, Kuchel GA, Kleppinger A, McElhaney JE. Influenza vaccine-mediated protection in older adults: Impact of influenza infection, cytomegalovirus serostatus and vaccine dosage. Exp Gerontol 2018;107:116–25. 10.1016/j.exger.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371:635–45. 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- [39].Fiorcari S, Maffei R, Audrito V, Martinelli S, Ten Hacken E, Zucchini P, et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 2016;7(65968–81). 10.18632/oncotarget.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Parry HM, Mirajkar N, Cutmore N, Zuo J, Long H, Kwok M, et al. Long-term ibrutinib therapy reverses CD8(+) T cell exhaustion in B cell chronic lymphocytic leukaemia. Front Immunol 2019;10:2832. 10.3389/fimmu.2019.02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ibrutinib may boost efficacy of CAR T cells. Cancer Discov 2019;9:OF3. doi: 10.1158/2159-8290.CD-NB2018-167. [DOI] [PubMed] [Google Scholar]
- [42].Onodera H, Urayama T, Hirota K, Maeda K, Kubota-Koketsu R, Takahashi K, et al. Neutralizing activities against seasonal influenza viruses in human intravenous immunoglobulin. Biologics 2017;11:23–30. 10.2147/BTT.S123831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol 2009;333:43–82. 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- [44].Dean AS, Moffatt CR, Rosewell A, Dwyer DE, Lindley RI, Booy R, et al. Incompletely matched influenza vaccine still provides protection in frail elderly. Vaccine 2010;28:864–7. 10.1016/j.vaccine.2009.03.024. [DOI] [PubMed] [Google Scholar]
- [45].Kelly HA, Sullivan SG, Grant KA, Fielding JE. Moderate influenza vaccine effectiveness with variable effectiveness by match between circulating and vaccine strains in Australian adults aged 20–64 years, 2007–2011. Influenza Other Respir Viruses 2013;7:729–37. 10.1111/irv.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.