ABSTRACT
The Advisory Committee on Immunization Practices (ACIP) recommended catch-up 9-valent Human Papillomavirus (HPV) vaccination through age 26 years, and shared clinical decision-making for adults aged 27–45 years, compared with catch-up through age 26 years and 21 years for females and males, respectively (status quo; pre-June-2019 recommendations). This study assessed the public health impact and cost-effectiveness of expanded catch-up vaccination through age 45 years (expanded catch-up) compared with status quo. We used an HPV dynamic transmission infection and disease model to assess disease outcomes and incremental cost-effectiveness ratio (ICER) of expanded catch-up compared with status quo. Costs (2018 USD), calculated from a healthcare sector perspective, and quality-adjusted life years (QALY) were discounted at 3% annually. Historical vaccination coverage was estimated using NIS-TEEN survey data (NHANES data for sensitivity analysis). Alternative scenario analyses included restricting upper age of expanded catch-up through 26 years (June-2019 ACIP recommendation), 29 years, and further 5-year increments. Our results show expanded catch-up vaccination would prevent additional 37,856 cancers, 314,468 cervical intraepithelial neoplasia-2/3s, 1,743,461 genital warts, and 10,698 deaths compared with status quo over 100 years at cost of $141,000/QALY. With NHANES coverage, the ICER was $96,000/QALY. The June-2019 ACIP recommendation also provided public health benefits with an ICER of $117,000/QALY, compared with status quo. The ICER for expanded vaccination through age 34 years was $107,000/QALY. Expanding catch-up vaccination program through age 45 years-old in the US is expected to provide public health benefits, and cost-effectiveness improves with expanding catch-up through age 34.
KEYWORDS: Cost-effectiveness, disease transmission models, 9-valent human papillomavirus (HPV) vaccine, adult, United States
Introduction
Human papillomavirus (HPV) is a known cause of cervical, vaginal, vulvar, anal, oropharyngeal and penile cancer. There were 43,999 reported incident cases of HPV-associated cancers each year in the US during 2012–2016.1 HPV imposes substantial burden among persons aged 27–45 years, often referred to as mid-adult persons. An estimated 216,000 women were diagnosed with a cervical intraepithelial neoplasia 2 or worse (CIN2+), with half of the diagnosis occurring in women older than 28 years.2 The prevalence of anogenital warts among mid-adult males has increased since 2006.3 In the HPV in Men (HIM) study, the incidence for anal infection and genital warts was highest among men aged 31–44 years.4 Seroprevalence for any of the 9-valent HPV vaccine types was highest among women aged 30–39 years and men aged 40–49 years .5
The quadrivalent HPV vaccine (qHPV) (HPV types 6/11/16/18) has been shown to be efficacious among mid-adults in clinical studies .6–8 In another clinical study, 9 the 9-valent HPV vaccine (9vHPV) (HPV types 6/11/16/18/31/33/45/52/58) has been shown to generate noninferior antibody responses to the 9 HPV types targeted by the vaccine in mid-adult women (27–45 years of age) compared with young women (16–26 years of age; the population in which 9vHPV vaccine efficacy was established10,11). In October 2018, subsequent to a priority review, the FDA granted market authorization for use of 9vHPV vaccine through age 45 for both females and males, modifying the previous upper age-limit of 26 years.12 At the time of the FDA approval, the Advisory Committee on Immunization Practices (ACIP) recommendation included routine vaccination of both boys and girls 11–12-years-old (vaccination could begin at age 9- years-old), and catch-up vaccination of females through 26 years and for males through 21 years.13 Subsequently in June 2019, the ACIP recommended expanding catch-up vaccination of both females and males through 26 years and shared clinical decision-making for both females and males 27 through 45 years of age.14 Results from five health-economic models presented to the ACIP varied substantially.15 Given the unmet medical need among mid-adult persons and the current ACIP recommendation, it is essential to assess the economic value of mid-adult vaccination accurately.
The primary objective of this analysis was to assess the public health impact and cost-effectiveness of an expanded catch-up program of vaccinating 13–45-year-old females and males (expanded catch-up), compared with vaccinating 13–26 year-old females and 13–21-year-old males (status quo) with 9vHPV vaccine. We also evaluated cost-effectiveness of post-2019 ACIP recommendation with expanded catch-up through 26 years compared with status quo.
Methods
Population, perspective, and discounting
The target population for vaccination included both females and males 9–45 years in the US. Costs and benefits were computed from a healthcare sector perspective for the entire population over a 100 year time horizon (lifetime) beginning 2019 in 2018 US$ at 3% annual discount rate.
Strategies compared
In the base case analysis, we compared the following strategies:
Status quo (pre-June 2019 ACIP recommendations): routine vaccination 11–12-year-old, catch-up vaccination (13–26-year-old females and 13–21-year-old males)
Expanded catch-up: expanding the ACIP catch-up recommendation to include 13–45-year-old males and females.
Both strategies included cervical screening and treatment of diseases. We also evaluated the cost-effectiveness of June 2019 ACIP recommendation compared with status quo as one of the scenarios.
Model
The analysis was conducted using a previously published, validated, non-linear, deterministic, mathematical model of the transmission dynamics of HPV infection (6/11/16/18/31/33/45/52/58) and disease development in an age-structured population. All major HPV-related cancers (cervical, vaginal, vulvar, anal, penile, and oropharyngeal) and HPV types were modeled independently. A separate model was used for recurrent respiratory papillomavirus (RRP), genital warts, and premalignant cervical lesions. We included HPV acquisition, transmission, recovery, re-infection, natural immunity, progression to pre-cancerous lesions and cancer as well as treatment, regression, screening, and vaccination. A detailed description of the model, along with all model equations, has been presented in previous publications.16,17 Additional details are available in Appendix 1 (model inputs) and Appendix 2 (validation and calibration).
Outputs
Epidemiological outcomes included total and incremental cases of HPV-related cervical, vaginal, vulvar, anal, oropharyngeal, and penile cancers; cases of CIN1, CIN2/3, vaginal intra-epithelial neoplasia (VaIN), genital warts, and RRP; cancer and RRP deaths. The cumulative age-distribution of HPV infection, and the median age at HPV infection defined as the age by which half of all HPV infections of all types will have occurred, was also predicted. Discounted costs, discounted quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICER) were estimated.
Cost and QALY calculations include only diagnosed disease and cancer cases. However, survival QALYs for both diagnosed and undiagnosed cancers are included in the total QALY calculations in the base-case analysis. We have also provided the base-case ICER when survival QALYs due to undiagnosed cancers are excluded.
Model inputs
Model inputs included data on demographics, screening, natural history of the disease, treatment patterns, cancer mortality, costs (Appendix 1), and vaccination coverage (Appendix 3). Cost values from various sources were converted to 2018 USD using the medical care component of the US consumer price index.18
The model follows other models of sexually transmitted infection (STIs) and defines risk of acquisition in terms of new sex partner.19,20 However, this data was not available for the US, instead, we used the number of partners in the previous year as a proxy (Appendix 1 section 1.1.2).
Historical HPV vaccine coverage
We modeled the impact of the historical HPV vaccination program in the US from 2007 through 2018. The comparator “status quo” scenario began in 2019 and was a continuation of the historical vaccination coverage trend. The expanded strategy was an incremental change to the status quo beginning in 2019 with the same historical coverage as the status quo.
We used raw National Immunization Survey-Teen (NIS-TEEN) coverage from 2007 to 2016 to estimate historical vaccination uptake.21 The calculations and uptake assumptions used were similar to Chesson et al 201822 and are covered in detail in Appendix 3.
Expanded HPV vaccination incremental uptake
For the expanded strategy we again followed Chesson et al.22 and assumed the same uptake as the status quo uptake for 19–26-year-old females and 19–21-year-old males, which resulted in 3.5% uptake for 27–45- year-old females and 2.8% for 22–45- year-old males.
Additional scenario analysis
June 2019 ACIP recommendation
We conducted an analysis to simulate the incremental impact of the current June 2019 ACIP recommendation (upper age limit of catch-up vaccination in both females and males harmonized to 26 years) compared with status quo.
Incremental increase of upper age limits of catch-up
We conducted analyses with four additional scenarios in which we incrementally increased the upper age limit of expanded catch-up through either 29, 34, or 39 years.
Sensitivity analysis
A sensitivity analysis considered used historical vaccination coverage data from National Health and Nutrition Examination Survey (NHANES), an annual survey of approximately 5,000 children and adults aged 1–74-year-olds23 The NHANES median 9–44-year-old overall predicted long-term coverage to be approximately 30% lower than NIS-Teen median coverage.
Other sensitivity analyses included varying vaccination coverage according to upper and lower bounds for the NIS-Teen historical coverage; modifying the discount rate (0%, 5%, and 1.5% as recently suggested by the Joint Committee on Vaccination and Immunization (JVCI) in the UK24); conducting one-way sensitivity analyses for treatment costs, and health utilities; and expanded catch-up of females only, or expanded catch-up of males only.
Results
Status quo and expanded vaccine coverage
Of the approximate 83 million mid-adults who lived in the United States in 2019, about 44% were age-eligible for HPV vaccination as adolescents and young adults. However, only about 12% were vaccinated based on modeled historical coverage. In the status quo, a total of 338.0 million persons were vaccinated with 655.8 million doses over the 100 year time horizon beginning in 2019. An additional 54.2 million persons were vaccinated with an additional 129.8 million doses in the expanded catch-up scenario, which resulted in a vaccination coverage of 76.9% of 9–45 year-olds over the 100 year time horizon (Figure 1).
Figure 1.
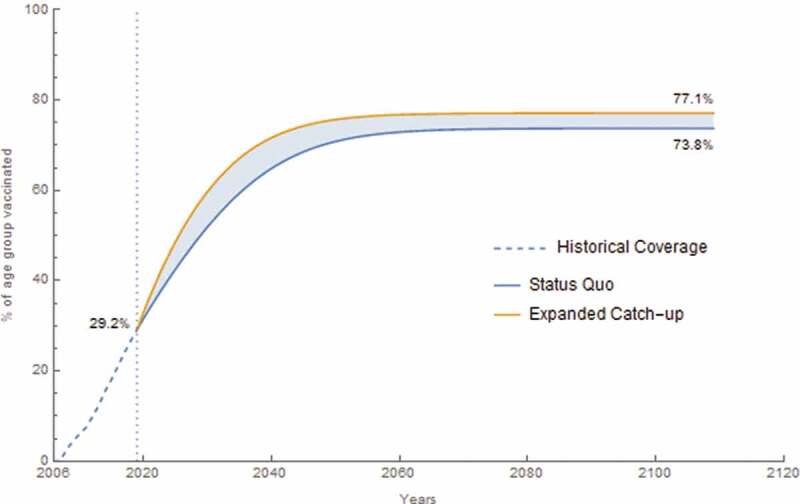
Vaccination coverage for 9–45-year-old females and males over historical and analytic time horizon. The dashed segment is the historical coverage for this age group from 2007 to 2019 where it reaches 29.2% coverage. The lower curve is the status quo coverage from 2019 to 2119 with a final coverage of 73.8%. The upper curve is the expanded catch-up program coverage from 2019 to 2119 with a final coverage of 77.1%
Base case epidemiological results
Under base case assumptions, expanded catch-up prevented an additional 37,856 cancer cases and 314,468 CIN2/3 cases over 100 years, relative to the status quo strategy. It also prevented an additional 8,688 RRP and 1,743,461 genital warts cases over 100 years, compared with the status quo strategy. Expanded catch-up prevented an additional 10,698 deaths over 100 years. Individual cancer, disease, and death cases avoided are shown in Table 1. The age group with median age of acquisition of HPV 16/18/31/33/45/52 or 58 infection among females was 25–26 years (Figure 2).
Table 1.
Cumulative HPV 6/11/16/18/31/33/45/52/58-related total diseases cases and deaths and cases averted over a 100-year time horizon, base case analysis
| Status-quob Number of casesa |
Expanded catch-upc Number of casesa |
Cases averted compared with status quo |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV-related disease | Female | Male | Total | Female | Male | Total | Female | Male | Total |
| Cervical cancer | 335,909 | - | 335,909 | 320,187 | - | 320,187 | 15,721 | - | 15,721 |
| Vulvar cancer | 128,215 | - | 128,215 | 126,161 | - | 126,161 | 2,054 | - | 2,054 |
| Vaginal cancer | 33,097 | - | 33,097 | 32,545 | - | 32,545 | 552 | - | 552 |
| Anal Cancer | 136,448 | 87,458 | 223,905 | 132,620 | 84,416 | 217,036 | 3,827 | 3,042 | 6,869 |
| Oropharyngeal cancer | 128,132 | 509,557 | 637,689 | 125,707 | 500,852 | 626,558 | 2,425 | 8,705 | 11,131 |
| Penile cancer | - | 54,501 | 54,501 | - | 52,972 | 52,972 | - | 1,529 | 1,529 |
| All cancers | 761,800 | 651,516 | 1,413,316 | 737,220 | 638,239 | 1,375,459 | 24,580 | 13,276 | 37,856 |
| CIN 1 | 5,357,575 | - | 5,357,575 | 5,426,836 | - | 5,426,836 | 171,472 | - | 171,472 |
| CIN 2/3 | 2,723,260 | - | 2,723,260 | 2,408,792 | - | 2,408,792 | 314,468 | - | 314,468 |
| VaIN 1 | 101,028 | - | 101,028 | 97,615 | - | 97,615 | 3,413 | - | 3,413 |
| VaIN 2/3 | 151,076 | - | 151,076 | 147,221 | - | 147,221 | 3,855 | - | 3,855 |
| Genital warts | 2,506,613 | 2,973,091 | 5,479,704 | 1,721,140 | 2,015,104 | 3,736,243 | 785,473 | 957,987 | 1,743,461 |
| RRP | 11,357 | 18,348 | 29,704 | 8,116 | 12,901 | 21,016 | 3,241 | 5,447 | 8,688 |
| HPV-related diseases deaths | 232,050 | 236,798 | 468,848 | 225,786 | 232,364 | 447,581 | 6,264 | 4,434 | 10,698 |
| Cervical cancer deaths | 107,657 | - | 107,657 | 103,835 | - | 174,690 | 3,821 | - | 3,821 |
| Vaginal cancer deaths | 13,311 | - | 13,311 | 13,100 | - | 10,955 | 211 | - | 211 |
| Vulvar cancer deaths | 30,605 | - | 30,605 | 30,135 | - | 22,132 | 471 | - | 471 |
| Anal cancer deaths | 19,359 | 12,885 | 32,243 | 18,842 | 232,364 | 34,264 | 516 | 424 | 940 |
| Oropharyngeal cancer deaths | 60,416 | 208,193 | 268,608 | 59,318 | 204,822 | 188,282 | 1,097 | 3,371 | 4,469 |
| Penile cancer deaths | - | 14,644 | 14,644 | - | 14,251 | 7,749 | - | 394 | 394 |
| RRP deaths | 702 | 1,076 | 1,779 | 555 | 831 | 9,510 | 147 | 245 | 392 |
a Case counts are based on a static population size of 322.899 million.
bStatus quo includes vaccination of 11–26 year females, and 11–21 year males
cExpanded catch-up is defined as additional catch-up vaccination for females 27–45 and males 22–45.
Abbreviations: CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; RRP, recurrent respiratory papillomatosis; VaIN, vaginal intraepithileal neoplasia
Figure 2.
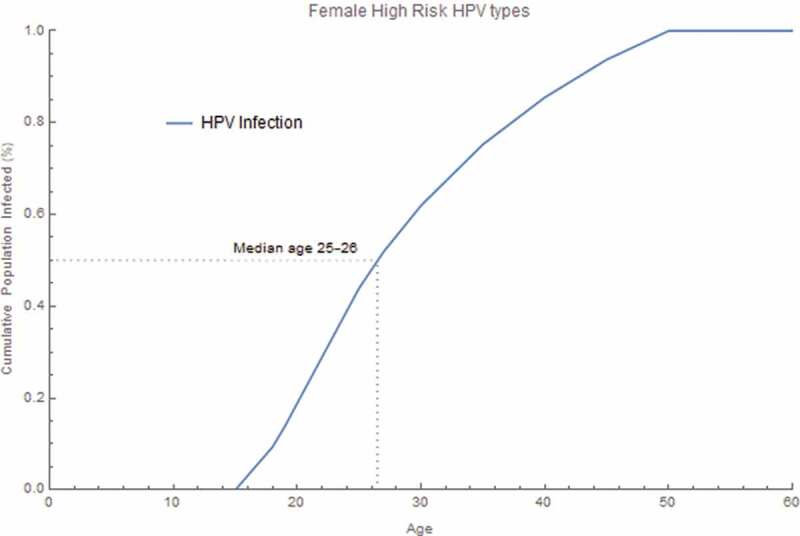
Pre-vaccine era cumulative proportion of the age at acquisition of HPV infection. The cumulative proportion of HPV infection was calculated for all female high-risk HPV infection acquired by age. The proportion was calculated by summing all new incident HPV 16/18/31/33/45/52/58 infections within each age group, dividing by the total infections over all age groups and accumulating through each age group
Base case cost-effectiveness results
The cost-effectiveness results for the base case analysis in terms of costs, QALYs and ICERs are presented in Table 2. Compared with status quo, the expanded catch-up strategy of vaccination through age 45 for both females and males provided better population health outcomes at an additional cost of 38.65 USD per capita at an ICER of 141,000 USD/QALY.
Table 2.
Cost-effectiveness results for base case analysisab.
| Strategy | Treatment costs ($) | Screening costs ($) | Vaccine costs ($) | Total costs ($) | Total QALYs | Incremental cost-effectiveness ratio (ICER)c ($/QALY)b |
|---|---|---|---|---|---|---|
| Status quo | 287.83 | 1,003.43 | 140.57 | 1,431.83 | 27.6097625 | - |
| Expanded catch-up | 280.15 | 1,003.34 | 187.00 | 1,470.49 | 27.6100365 | 141,000d |
aCosts and QALYs are per capita accumulated over a 100-year time horizon with 3% annual discount rate. b Base case analysis compared two strategies: the status quo and expanded catch up. Status quo includes vaccination of 11–26 year females and 11–21 year males. Expanded catch-up is defined as additional catch-up vaccination for females 27–45 and males 22–45.
bResults include impact of cervical, vaginal, vulvar, penile, anal, oropharyngeal cancers and precancers, genital warts, and RRP. ICER is rounded to nearest 1000.
cICER is Incremental costs/Incremental QALYs
dIncludes survival QALYs due to undiagnosed cancer deaths, when these QALYs are excluded the ICER is $142,000/QALY (see Appendix 4 for additional information)
Abbreviations: ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years
Additional scenarios
Expanded HPV vaccination:June 2019 ACIP recommendation
Expanding the upper age limit of catch-up through age 26 years also resulted in public health benefits (Table 3) and had an ICER of 117,000 USD/QALY when considered in the absence of other upper age limit choices. This represented the ICER of June 2019 ACIP recommendation, compared with pre-June 2019 ACIP recommendation.
Table 3.
Cost-effectiveness for expanded HPV Vaccination: incremental increase of upper age limit of expanded catch-upa.
| Strategy | Treatment costs ($) | Screening costs ($) | Vaccine costs ($) | Total costs ($) | Total QALYs (QALY) | ICER ($/QALY) |
|---|---|---|---|---|---|---|
| Status quob | 287.83 | 1,003.43 | 140.57 | 1,431.83 | 27.6097625 | – |
| Expanded catch-up for 13–26 year-olds | 286.60 | 1,003.42 | 146.68 | 1,436.70 | 27.6098038 | Weakly Dominated ($117,000c) |
| Expanded catch-up for 13–29 year-olds | 285.37 | 1,003.41 | 152.07 | 1,440.85 | 27.6098501 | 103,000d |
| Expanded catch-up for 13–34 year-olds | 283.21 | 1,003.38 | 162.70 | 1,449.29 | 27.6099291 | 107,000e |
| Expanded catch-up for 13–39 year-olds | 281.42 | 1,003.35 | 174.47 | 1,459.24 | 27.6099926 | 157,000f |
| Expanded catch-up for 13–45 year-olds | 280.15 | 1,003.34 | 187.00 | 1,470.49 | 27.6100365 | 256,000g |
aCosts and QALYs are per capita accumulated over a 100-year time horizon with 3% annual discount rate.
bStatus quo includes vaccination of 9–26 year females, and 9–21 year males
cThe ICER of current ACIP recommendations (harmonization) compared with status quo is $117,000/QALY
dICER for expanded catch-up for 13–29 years, compared with status quo
eICER for expanded catch-up for 13–34 years, compared with vaccinating 13–29 years
fICER for expanded catch-up for 13–39 years, compared with vaccinating 13–34 years
gICER for expanded catch-up for 13–45 years, compared with vaccinating 13–39 years
Abbreviations: ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years
Expanded HPV vaccination: incremental increase of upper age limit of expanded catch-up
The June 2019 Recommendation scenario was weakly-dominated by expanding the upper age limit to 29 years (ICER compared to status quo of 103,000 USD/QALY). Expanding upper age limit through 34, 39, and 45 years resulted in ICERs of 107,000 USD/QALY, 157,000 USD/QALY, 256,000 USD/QALY respectively, when compared with catch-up for the previous 5-year age group.
Sensitivity analyses
Results of sensitivity analysis are presented in Table 4. ICERs were most sensitive to variations in health utility and discount rate assumptions. ICERs were also very sensitive to differential vaccination between females and males. The results were sensitive to historical coverage assumptions. Assuming NHANES historical coverage resulted in an ICER of 96,000 USD/QALY. However, small variations around the NIS-Teen coverage (95% CI of coverages) led to a relatively small variation in ICER. ICER was not very sensitive to variations in treatment costs or variations in the expanded catch-up uptake rates when female and male rates were either increased or decreased together.
Table 4.
Cost-effectiveness results for sensitivity analysisa.
| Strategy | Total costs ($) | Total QALYs (QALY) | ICER ($/QALY) | |
|---|---|---|---|---|
| Discount rate | ||||
| 0% | Status quo | 4,137.56 | 86.1119756 | - |
| Expanded catch-up | 4,202.94 | 86.1132341 | 52,000 | |
| 1.5% | Status quo | 2,243.69 | 44.7814782 | - |
| Expanded catch-up | 2,291.35 | 44.7820234 | 87,000 | |
| 3% (base case) | Status quo | 1,431.83 | 27.6097625 | - |
| Expanded catch-up | 1,470.49 | 27.6100365 | 141,000 | |
| 5% | Status quo | 936.83 | 17.5092661 | - |
| Expanded catch-up | 968.57 | 17.5094005 | 236,000 | |
| Costs | ||||
| Lower limit values | Status quo | 1,069.87 | 27.6097625 | – |
| Expanded catch-up | 1,110.48 | 27.6100365 | 148,000 | |
| Upper limit values | Status quo | 1,737.76 | 27.6097625 | – |
| Expanded catch-up | 1,773.58 | 27.6100365 | 131,000 | |
| Health utilities | ||||
| Lower limit values | Status quo | 1,432.87 | 27.6035336 | – |
| Expanded catch-up | 1,471.52 | 27.6040961 | 69,000 | |
| Upper limit values | Status quo | 1,432.87 | 27.6151270 | – |
| Expanded catch-up | 1,471.52 | 27.6152287 | 380,000 | |
| Varying mid-adult uptake rates by gender | ||||
| Status quo | 1,431.83 | 27.6097625 | – | |
| Both Low (female = 1.76%; male = 1.42%) | 1,453.74 | 27.6099179 | 141,000b,c | |
| Both High (female = 5.27%; male = 4.27%) | 1,483.96 | 27.6101325 | 141,000b,c | |
| Female only (female = 3.5%; male = 0.0%) | 1,445.27 | 27.6099200 | 85,000b | |
| Male only (female = 0.0%; male = 2.8%) | 1,456.67 | 27.6098911 | 193,000b | |
| NIS-Teen Historical Coverage | ||||
| Lower 95% CI NIS-Teen Coverage | Status quo | 1,434.02 | 27.6095228 | - |
| Expanded catch-up | 1,472.56 | 27.6098096 | 134,000 | |
| Upper 95% CI NIS-Teen Coverage | Status quo | 1,429.67 | 27.6099702 | - |
| Expanded catch-up | 1,468.19 | 27.6102279 | 149,000 | |
| NHANES as historical vaccination coverage | ||||
| Status quo | 1,416.32 | 27.6079577 | - | |
| Expanded catch-up | 1,469.51 | 27.6085115 | 96,000 | |
aCosts and QALYs are per capita accumulated over a 100-year time horizon with 3% annual discount rate. ICERS are rounded to the nearest $1000/QALY
bICER relative to status quo,
cDifferences between ICERs are <$50/QALY
Abbreviations: CI, confidence interval; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratio; QALYs, quality adjusted life-years.
Discussion
We assessed the public health and economic impact of expanding the current program of catch-up vaccination for 13–26 year-old females and 13–21 year-old males with 9vHPV vaccine to catch-up vaccination for 13–45 year-old females and males with 9vHPV vaccine in the US. Overall, the expanded catch-up strategy averted significant disease compared with the current strategy, with an ICER of 141,000 USD/QALY.
The cost-effectiveness improved when the upper age limit of catch-up vaccination was gradually increased in 5-year increments. Implementing the June 2019 ACIP recommendation (Expanding the upper age limit of catch-up vaccination through age 26 years) also averted disease compared with status quo, and could be considered cost-effective based on American College of Cardiology (ACC) and American Heart Association (AHA) intermediate value ICER range between 50,000 USD/QALY and 150,000 USD/QALY.25 Vaccination through ages 29 or 34 years weakly dominated vaccination through age 26 years. Thus, expanding catch-up through either 29 or 34 years might make more economic sense than restricting catch-up to age 26. Catch-up vaccination when evaluated in 5-year increments was near cost-effective through age 34 years. ICERs were greater than 150,000 USD/QALY for older upper age limits.
Health economic analysis of mid-adult vaccination from five different models were presented at the 2019 ACIP.15 All the models have been extensively peer-reviewed and cited, and have historically yielded similar cost effectiveness results in younger cohorts and for pre-2019 AICP scenarios.26 However, the models varied in their estimates of ICER for the base case of expanding catch-up through age 45 years, compared with status quo. The recently published analysis using the HPV-ADVISE model had the highest ICER ($1.47 million/QALY), followed by the simplified model ($417,200/QALY), CISNET-Harvard ($363,800/QALY), CISNET-Policy1-Cervix, ($199,300/QALY), and our study ($141,000/QALY).15,27 This variation could be due to differences in structural design and assumptions regarding the natural history of HPV infection, sexual behavior, and transmission of HPV. Overall, our model applies some assumptions regarding the cost and quality of life impact of HPV-associated disease that are more favorable to HPV vaccination than the assumptions in the other available models of mid-adult vaccination. However, these differences in models’ inputs only partially account for the magnitude of differences in results between the models. The differences in model results may be difficult to resolve without a detailed cross-validation exercise.
One construct that could help understand the differences in models is the age-distribution of causal HPV infection by age. Since our model is a compartmental model, we are able to estimate age-distribution of any HPV infection but cannot estimate causal infection. The median age at HPV infection estimated by our model of 25–26 years is consistent with the median ages at causal infection of 25.1, 25.4, 27.9 years for the UMN-HPV CA, Harvard, Policy 1-Cervix models, and is more conservative than the 49.9 years in the MISCAN-Cervix model studied by Burger et al. (2019) under assumptions of imperfect compliance with the US screening guidelines.28 The age-distribution of HPV infection implies that close to half of causal HPV infections originate during mid-adulthood and ~25% of infections occur after age 34 years; this demonstrates that mid-adult vaccination is an unmet medical need.
The median age at causal infection predicted by the HPV-ADVISE model, 19.9 years, is substantially lower than the median age of causal infection predicted by all the other models.27 Consequently, the ICERs predicted by this model in almost all scenarios are very high. A lower median age in the HPV-ADVISE model raises the possibility of low sexual activity, less susceptibility and low incident infection among mid-adults in this model compared with others. However, evidence from NSFG and NHANES shows that median number of lifetime partners increases with age, implying mid-adults continue to acquire new partners, a known risk factor for incident HPV infection.29–31 In the clinical trials of 4vHPV (Protocol V501-013/15 and V501-019) in women up to 45 years of age, 18 participants (~70%) tested negative for HPV DNA by PCR for 14 HPV types.32 No women tested positive for all 9 types in the 9vHPV at baseline, and approximately 21% had an anogenital infection with at least 1 HPV type targeted by the 9vHPV vaccine. Most HPV infections in adult women involved only one HPV type and very few had more than 3 HPV types, implying that women remain susceptible to new infection. Studies of mid-adult cohorts have also shown that mid-adults get infected with HPV31,33–36 . HPV infections among women aged 15–25 years in the placebo arm of the PATRICIA bivalent HPV vaccine trial progressed to CIN2+ and CIN1+ at a rate similar to that of women aged ≥25 years in the VIVIANE bivalent HPV vaccine trial .37
The economic value of vaccination also varies by gender. Sensitivity analyses show that vaccinating women only through age 45 years can be considered cost-effective. However, harmonization across both genders may create less provider-confusion and is likely to achieve better coverage and possibly better public health benefit. However, harmonization across genders may result in more vulnerable women not receiving sufficient protection. A case could be made for cost-effectiveness in the context of ACC/AHA intermediate value ICER range instead of reducing the age of catch-up.
The ICER was sensitive to assumptions of historical coverage; higher historical coverage results in more herd protection, and less potential for expanded catch-up to provide benefit. NHANES coverage inputs resulted in 30% less coverage compared with NIS- Teen coverage. However, NHANES data has similar sensitivity and specificity to NIS- Teen data among those who had a provider response,23 and has coverage comparable to estimates from claims data.38 Our estimate of expanded catch-up benefit is conservative, as coverage could be lower compared with NIS-Teen coverage.
Limitations
The current analysis has several limitations. HPV-related cancers affect women and men of working age and therefore affect their productivity. We did not consider the indirect costs related to productivity losses. We assumed that sexual behavior among adults has not changed significantly since 2002. In addition, we calculate risk of HPV transmissible sexual contacts using the annual number of sexual partners for each age group. The CDC’s Progression and Transmission of HIV/AIDS (PATH 2.0) model follows a similar approach39 for modeling the impact of sexual behavior on STI transmission. Other models assume this risk is only present for “new” sexual partners with no added risk of transmission from existing sex partners.27,40 Our approach may overestimate the risk of HPV transmission, especially among older populations. Analysis of the 2002 and 2011–2013 National Survey of Family Growth, showed that while there were no changes in median numbers of sexual partners (past year and lifetime), there is evidence of having more sexual partners at an older age, and an increase in the number of sexual partners among the most sexually active top 5% and top 20% of the population, especially men. As a result, the estimates for ICER presented in this study are likely to be conservative. Our model results are based on the single best fitting set of natural history parameters (described in Appendix 2). However, there are many sets of natural history parameters that can fit the data well but lead to different results. For example, HPV-Advise model considers 50 sets of reasonable natural history parameters which results in a wide range of ICERs, demonstrating the sensitivity of outcomes to natural history parameter choices. The model does not account for recent updates to pre-vaccine era estimates of cervical cancer and other disease incidences,41 nor does the model account for changes in background cancer incidence For example, cervical cancer incidence has been falling since 2005 and oropharyngeal cancer incidence has increased.42 This model does not take into account differences in natural history and transmission of HPV among the 5 additional 9vHPV types 31/33/45/52/58.41 The impact of these on the ICER is likely to be minimal. Finally, the current analysis assumes that the death rate for undiagnosed cancer is significantly lower than that of diagnosed cancers. Consequently, the model predicts that approximately 5% of all cancer deaths are due to undiagnosed cancer. There is some uncertainty about the expected number of deaths due to cancers that are not diagnosed and some evidence that it may be more than 5%.43–46 Because the QALYs gained due to deaths avoided includes deaths due to both diagnosed and undiagnosed cancer, the benefit of vaccination may be underestimated.
Despite these limitations, the findings regarding the cost-effectiveness of an extended vaccination program is robust to variation in key model inputs. The major strength of this analysis is its basis on the dynamic transmission model that simulates several important HPV-related diseases, includes a realistic representation of the currently known natural history of HPV and disease progression, and includes herd protection effects. All details of the model including differential equations are in the public domain.16,17
Conclusion
Our model-based results show that expanded catch-up of males and females aged 9–45 years would provide public health benefits among all age groups and could be considered cost-effective for catch-up vaccination of men and women through age 34 years, and for women through age 45 years. Vaccination as per the current ACIP guidelines that include catch-up vaccination through age 26 could also be considered near cost-effective. Given heterogeneity in risk and that mid-adults are susceptible to HPV infection which progresses to disease that could have been prevented through vaccination, mid-adult vaccination indisputably provides value among certain groups. Our results support catch-up vaccination through age 34 years and shared clinical decision making through age 45 years.
Supplementary Material
Acknowledgments
We wish to thank Aurélie Schmidt and Nadia El Mouaddin from ICON for providing writing assistance, and for their help in a literature review to determine model inputs.
Funding Statement
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; Merck Sharp and Dohme.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human papillomavirus–attributable cancers — United States, 2012–2016. Centers Dis Control Prev. 2019;68(33):724–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClung NM, Gargano JW, Park IU.. Estimated number of cases of high-grade cervical lesions diagnosed among women — United States, 2008 and 2016. Centers Dis Control Prev. 2019;68:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006-2014. Am J Public Health. 2018;108:112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudenga SL, Nyitray AG, Torres BN, Silva R, Villa L, Lazcano-Ponce E, Abrahamsen M, Baggio ML, Salmeron J, Quiterio M, et al. Comparison of anal HPV natural history among men by country of residence: Brazil, Mexico, and the United States. J Infect. 2017;75(1):35–47. doi: 10.1016/j.jinf.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu G, Markowitz LE, Hariri S, Panicker G, Unger ER. Seroprevalence of 9 human papillomavirus types in the United States, 2005–2006. J Infect Dis. 2016;213(2):191–98. doi: 10.1093/infdis/jiv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luna J, Plata M, Gonzalez M, Correa A, Maldonado I, Nossa C, Radley D, Vuocolo S, Haupt RM, Saah A, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of gardasil™ in adult women. PLOS One. 2013;8(12):e83431. doi: 10.1371/journal.pone.0083431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellsague X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105(1):28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muñoz N, Manalastas R, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 9.Joura E, Vandermeulen C, Peters K, Perino A, Ulied A, Seppä I, Jotterand V, Cheon K, Rawat S, Luxembourg A, et al., Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared with young women 16-26 years of age: an open-label phase 3 trial. in EUROGIN International Multidisciplinary HPV Congress, Monaco. 2019. [Google Scholar]
- 10.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 11.Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–59. doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration . FDA approves expanded use of gardasil 9 to include individuals 27 through 45 years old. Washington, D.C.: FDA News Release; 2018. [Google Scholar]
- 13.Centers for Disease Control and Prevention . HPV Vaccine recommendations. 2016. 13/12/2018. [accessed 2018 Dec 13]. Available from: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html.
- 14.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesson H. Overview of health economic models for HPV vaccination of mid-adults, C.f.D.C.a. Prevention, Editor. Atlanta, GA; 2019. [Google Scholar]
- 16.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–67. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Bureau of Labor Statistics . CPI-All Urban Consumers (Current Series) - Medical care in U.S. city average, all urban consumers, not seasonally adjusted. 2018.. [accessed 2018 April]. https://data.bls.gov/pdq/SurveyOutputServlet.
- 19.Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci. 1993;342:137–59. [DOI] [PubMed] [Google Scholar]
- 20.Garnett GP, Anderson RM. Balancing sexual partnership in an age and activity stratified model of HIV transmission in heterosexual populations. IMA J Math Appl Med Biol. 1994;11(3):161–92. doi: 10.1093/imammb/11.3.161. [DOI] [PubMed] [Google Scholar]
- 21.Walker TY, Elam-Evans LD, Yankey D. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. Centers Dis Control Prev. 2019;68:718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26 years in the United States. Vaccine. 2018;36(29):4362–68. doi: 10.1016/j.vaccine.2018.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis RM, Markowitz LE. Human papillomavirus vaccination coverage among females and males, national health and nutrition examination survey, United States, 2007–2016. Vaccine. 2018;36(19):2567–73. doi: 10.1016/j.vaccine.2018.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joint Committee on Vaccination and Immunization . Statement on HPV vaccination. UK. GOV;2018. [Google Scholar]
- 25.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association task force on performance measures and task force on practice guidelines. Circulation. 2014;129(22):2329–45. doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 26.Brisson M, Bénard É, Drolet M, Bogaards JA, Baussano I, Vänskä S, Jit M, Boily M-C, Smith MA, Berkhof J, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Glob Health. 2016;1(1):e8–e17. doi: 10.1016/S2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laprise J-F, Chesson HW, Markowitz LE, Drolet M, Martin D, Bénard É, Brisson M. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann Intern Med. 2020;172(1):22–29. doi: 10.7326/M19-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger EA, de Kok Imcm, Groene E, Killen J, Canfell K, Kulasingam S, Kuntz KM, Matthijsse S, Regan C, Simms K, et al. Estimating the natural history of cervical carcinogenesis using simulation models: A CISNET comparative analysis. J Natl Cancer Inst; 2020;112(9):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper CR, Dittus PJ, Leichliter JS, Aral SO. Changes in the distribution of sex partners in the United States: 2002 to 2011-2013. Sex Transm Dis. 2017;390(10108):96–100. doi: 10.1097/OLQ.0000000000000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryser MD, Rositch A, Gravitt PE. Modeling of US Human Papillomavirus (HPV) seroprevalence by age and sexual behavior indicates an increasing trend of HPV infection following the sexual revolution. J Infect Dis. 2017;216(5):604–11. doi: 10.1093/infdis/jix333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70(21):8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merck & Co . Data on file. 2020. [Google Scholar]
- 33.Munoz N, Mendez F, Posso H, Molano M, van den Brule AJ, Ronderos M, Meijer C, Munoz A. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 34.Winer RL, Hughes JP, Feng Q, Stern JE, Xi LF, Koutsky LA. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25–65 years. J Infect Dis. 2016;214(5):665–75. doi: 10.1093/infdis/jiw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shvetsov YB, Hernandez B, McDuffie K, Wilkens L, Zhu X, Ning L, Killeen J, Kamemoto L, Goodman M. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clin Infect Dis. 2009;48(5):536–46. doi: 10.1086/596758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman MT, Shvetsov Y, McDuffie K, Wilkens L, Zhu X, Thompson P, Ning L, Killeen J, Kamemoto L, Hernandez B, et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. J Infect Dis. 2010;201(9):1331–39. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skinner SR, Wheeler CM, Romanowski B, Castellsagué X, Lazcano-Ponce E, Rowena Del Rosario-Raymundo M, Vallejos C, Minkina G, Pereira Da Silva D, McNeil S, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138(10):2428–38. doi: 10.1002/ijc.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gargano JW, Zhou F, Stokley S, Markowitz LE. Human papillomavirus vaccination in commercially-insured vaccine-eligible males and females, United States, 2007–2014. Vaccine. 2018;36(23):3381–86. doi: 10.1016/j.vaccine.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Gopalappa C, Farnham PG, Chen YH, Sansom SL. Progression and transmission of HIV/AIDS (PATH 2.0): a new. Agent-Based Model to Estimate HIV Transmissions in the United States. Med Decis Making. 2016;37(2):224–33. [DOI] [PubMed] [Google Scholar]
- 40.Choi YH, Jit M, Gay N, Cox A, Garnett GP, Edmunds WJ. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine. 2010;28(24):4091–102. doi: 10.1016/j.vaccine.2009.09.125. [DOI] [PubMed] [Google Scholar]
- 41.Merck . Data on file. 2009. [Google Scholar]
- 42.Dyne EAV, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus–associated cancers — United States, 1999–2015. MMWR. 2018;67(33):918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.German RR, Fink AK, Heron M, Stewart SL, Johnson CJ, Finch JL, Yin D. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35(2):126–31. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Kleina RF, Sperga I, Lutinska MD. The analysis of undiagnosed malignancies. Pap Anthropol. 2011;20:199–208. [Google Scholar]
- 45.Roulson J, Benbow EW, Hasleton PS. Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review. Histopathology. 2005;47(6):551–59. doi: 10.1111/j.1365-2559.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 46.Mieno MN, Tanaka N, Arai T, Kawahara T, Kuchiba A, Ishikawa S, Sawabe M. Accuracy of death certificates and assessment of factors for misclassification of underlying cause of death. J Epidemiol. 2016;26(4):191–98. doi: 10.2188/jea.JE20150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


