ABSTRACT
This open-label, single-center, Phase 3 study (NCT03546842) assessed the immunogenicity and safety of the nine-valent human papillomavirus (9vHPV; HPV6/11/16/18/31/33/45/52/58) vaccine in Vietnamese males and females, with the aim to support 9vHPV vaccine licensure in Vietnam. Participants aged 9–26 years received three 9vHPV vaccine doses (Day 1, Month 2, Month 6). Serum samples were obtained on Day 1 (pre-vaccination) and at Month 7 (one month post-Dose 3) for the measurement of anti-HPV antibodies. Geometric mean titers (GMTs) and seroconversion percentages were obtained using the HPV-9 competitive Luminex immunoassay. Injection-site adverse events (AEs), systemic AEs, serious AEs (SAEs), and study discontinuations due to AEs were recorded. Of 201 participants enrolled, 200 (99.5%) received ≥1 vaccine dose. All participants who received the three-dose regimen (198/200, 98.5%) seroconverted for all 9vHPV vaccine types by Month 7. Robust anti-HPV GMT responses were also observed. Half of participants (50.5%) reported ≥1 AE; the majority were injection-site-related (45.0%) and mild (43.0%). There were no deaths, vaccine-related SAEs, or discontinuations due to AEs. Administration of three 9vHPV vaccine doses was highly immunogenic and resulted in acceptable seropositivity percentages for all vaccine HPV types. The 9vHPV vaccine was generally well tolerated among this study population.
Region of origin: Vietnam
Trial registration: clinicaltrials.gov Identifier NCT03546842
KEYWORDS: Human papillomavirus (HPV), immunogenicity, Vietnam, nine-valent human papillomavirus vaccine, safety
Introduction
Human papillomavirus (HPV) is the cause of nearly all cervical cancers, as well as a number of anal, vulvar, vaginal, penile, and oropharyngeal cancers1; annually, HPV causes 690,000 new cancer cases in women and men worldwide (based on 2018 data).2 Approximately half of the global HPV-related cancers occur in Asia, with 140,000 and 67,000 new cases in 2018 in East and Southeast Asia, respectively.2
In Vietnam, HPV-related cancers burden both women and men. An estimated 4177 women (crude incidence rate of 8.6 per 100,000 women per year) were diagnosed with cervical cancer in 2018 and 2420 died from the disease.3 Incidences of other HPV-related cancers have been reported: 0.2 (anal cancer; for both men and women), 0.3 (vulvar cancer), 0.1 (vaginal cancer), 0.6 (penile cancer), and 0.8 (oropharyngeal cancer; for men) and 0.2 (oropharyngeal cancer; for women) per 100,000 per year.3
Three prophylactic HPV vaccines are widely licensed and recommended for use in many countries worldwide for the prevention of HPV-related disease.4 The quadrivalent (qHPV; HPV6/11/16/18) and bivalent (HPV16/18) HPV vaccines (initially approved in 2006 and 2007, respectively)5 protect against infection and disease caused by HPV types 16 and 18, which are responsible for 70% of cervical cancers, 50% of CIN2/3 cervical pre-cancers, and 65%–85% of HPV-related vulvar, vaginal, and anal cancers worldwide.6–10 The qHPV vaccine also protects against HPV types 6 and 11, which cause approximately 90% of cases of genital warts.11 The nine-valent HPV (9vHPV) vaccine, first approved in 2014, was developed to provide protection against infection and disease caused by the four HPV types covered by the qHPV vaccine plus five additional high-risk oncogenic HPV types (HPV31/33/45/52/58).5 The 9vHPV vaccine has potential to prevent 90% of HPV-related cervical, vulvar, vaginal, and anal cancers, 70%–85% of cervical pre-cancers, and 90% of anogenital warts worldwide based on global epidemiological studies.6–12 In the international, pivotal 9vHPV vaccine immunogenicity, safety, and efficacy trial in young women 16 to 26 years of age, the 9vHPV vaccine demonstrated efficacy against HPV31/33/45/52/58-related persistent infection and disease, and generated antibody responses to HPV types 6, 11, 16, and 18 that were non-inferior to those elicited by the qHPV vaccine.13,14 Efficacy results observed in young women were extrapolated to girls and boys 9 to 15 years of age and young men 16 to 26 years of age based on immunogenicity bridging studies.15,16
While HPV16 and HPV18 are the most prevalent high-risk types globally and in Asia, HPV types 52 and 58 are relatively more prevalent in East Asia compared with other regions.17–21 Moreover, higher prevalences of HPV52- and HPV58 have been reported in cervical cancer cases in Asian populations (5.5% [95% confidence interval (CI): 5.2–5.9] of 17,552 cases and 7.4% [95% CI: 7.0–7.8] of 18,455 cases, respectively) compared with reported worldwide prevalences (3.5% [95% CI: 3.3–3.6] of 49,978 cases and 3.9% [95% CI: 3.8–4.1] of 50,814 cases, respectively).21,22 These findings suggest that East Asian populations could benefit from implementation of a 9vHPV vaccination program; the absence of Vietnamese participants from the global 9vHPV vaccine clinical development program suggests further investigation in this population is warranted.
To support licensure of the 9vHPV vaccine in Vietnam, we report immunogenicity and safety data from a local registration study of the 9vHPV vaccine in males and females 9 to 26 years of age in Vietnam.
Methods
Study V503-017 (NCT03546842) was an open-label, single-center, Phase 3 study assessing the immunogenicity and safety of the 9vHPV vaccine in Vietnamese males and females 9 to 26 years of age conducted in Vietnam between June 29, 2018 (first participant visit) and January 29, 2019 (last participant visit). The enrollment of approximately 200 participants was planned; participants were stratified by age and gender. Age stratification included the following age strata, in a 1:1 ratio: 9 to 15 years of age and 16 to 26 years of age. Gender stratification included a female:male ratio of 2:1 within each age strata.
Participants 9 to 15 years of age were included if they were not sexually active and did not plan on becoming sexually active during the vaccination period, and those 16 to 26 years of age who had not had Papanicolaou (Pap) testing (cervical or anal) or had only normal Pap test results, had no history of HPV-related anogenital lesions, and had 0–4 lifetime male and/or female sexual partners. Females 16 to 26 years of age were not pregnant and effective contraception was used from Day 1 of the study through Month 7.
The study conformed with Good Clinical Practice requirements and applicable country and/or local statutes and regulations regarding independent ethical committee review, informed consent, and the protection of the rights and welfare of human participants in biomedical research; all participants and/or legally acceptable representatives provided informed consent.
The 9vHPV vaccine was administered as a series of three, 0.5-mL intramuscular injections at Day 1, Month 2, and Month 6, the dosing regimen used in the pivotal studies of the 9vHPV vaccine program.13–16 Blood samples were collected at Day 1 (prior to vaccine administration) and at Month 7 for immunogenicity testing. Antibodies to HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 were measured using the HPV-9 competitive Luminex immunoassay (cLIA).23 The original version of the cLIA was used through 2015 for testing samples in 9vHPV vaccine clinical trials. A new version was used starting in 2016. The newer version of the assay was bridged to the earlier version to ensure comparable antibody measurements between the two versions. A participant was defined to be seropositive for HPV6, 11, 16, 18, 31, 33, 45, 52, or 58 if his or her anti-HPV serum level was ≥50, ≥29, ≥41, ≥59, ≥29, ≥22, ≥15, ≥20, or ≥15 milliMerck units (mMU/mL), respectively. Serostatus cutoffs were defined as the antibody level above the assay’s lower limit of quantitation that reliably distinguished between “positive” and “negative” samples (for this assessment, samples were classified “positive” or “negative” based on clinical likelihood of HPV infection and positive or negative status using previous versions of the cLIA). No minimum level of antibody that predicts protection against infection or disease has been defined.24 Therefore, seropositivity does not necessarily indicate protection. Seropositivity to the vaccine HPV types at Day 1 was not a reason for exclusion from the study; however, the results of this screening were used as a criterion to define the per-protocol immunogenicity (PPI) population for each of the relevant HPV types.
Participants were monitored for at least 30 minutes after each study vaccination for any adverse events (AEs) including allergic reactions. Injection-site AEs and systemic AEs were collected for 15 days following each vaccination, including the day of vaccination, using vaccination report cards (VRCs). Injection-site AEs of erythema, pain, and swelling, as well as oral temperatures, were solicited on the VRC for 5 days following each vaccination. Serious AEs (SAEs) were collected for the entire duration of the study regardless of causality.
The primary immunogenicity objective was to demonstrate that the 9vHPV vaccine is immunogenic; the primary hypothesis was that the 9vHPV vaccine induces seroconversion percentages >90% at Month 7 for the nine HPV types covered by the vaccine (HPV6, 11, 16, 18, 31, 33, 45, 52, and 58). The secondary immunogenicity objective was to summarize GMTs to HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 at Month 7. Immunogenicity assessment was conducted at Month 7 (1 month post-Dose 3), which was the primary immunogenicity timepoint for the pivotal studies of the 9vHPV vaccine program.13–16 Primary and secondary immunogenicity analyses were performed in the PPI populations, which included participants who: (1) received all three vaccinations with the correct dose of 9vHPV vaccine within acceptable day ranges, (2) were seronegative at Day 1 for the relevant HPV type(s), (3) provided a serum sample within 21 to 49 days post-Dose 3, and (4) had no protocol violations that could interfere with the immunogenicity evaluation.
For the primary immunogenicity analysis, point estimates were calculated as the percentage of participants who seroconverted (i.e., a participant who was anti-HPV seronegative at Day 1 and became seropositive at Month 7). Corresponding 95% CI estimates were derived based on exact binomial distribution. The statistical criterion for acceptable anti-HPV seroconversion required the lower limit of the 95% CI of the percentage of participants who seroconverted to be >90% for each HPV type. The hypothesis testing for each HPV type was conducted at a 1-sided Type 1 error level of 0.025 and no adjustments for multiplicity were required. With 200 participants, the study had 97% power at an overall 1-sided 2.5% alpha-level to establish that the 9vHPV vaccine induces >90% seroconversion to the nine HPV types covered by the vaccine.
To summarize anti-HPV GMTs, point estimates were calculated by exponentiating the mean estimates of the natural logarithm-transformed anti-HPV titers. Two-sided 95% CI estimates were calculated by exponentiating the 95% CI estimates for the means of the natural logarithm-transformed anti-HPV titers based on the t-distribution.
Safety analyses were performed in the ‘all participants as treated’ population, consisting of all participants who received at least one vaccine dose and provided safety data at any point during the study. Safety assessments were descriptive in nature and included counts and percentages of participants experiencing AEs, injection-site AEs, systemic AEs, vaccine-related AEs, SAEs, and study discontinuations due to AEs following any 9vHPV vaccination. Oral temperatures were summarized.
Results
Participant disposition is shown in Figure 1. A total of 201 participants were enrolled in the study, of whom 198 (98.5%) completed the three-dose regimen. Three participants discontinued from the study (protocol deviation, n = 1; participant withdrawal, n = 2). Baseline demographic characteristics and HPV serostatus are presented in Table 1.
Figure 1.
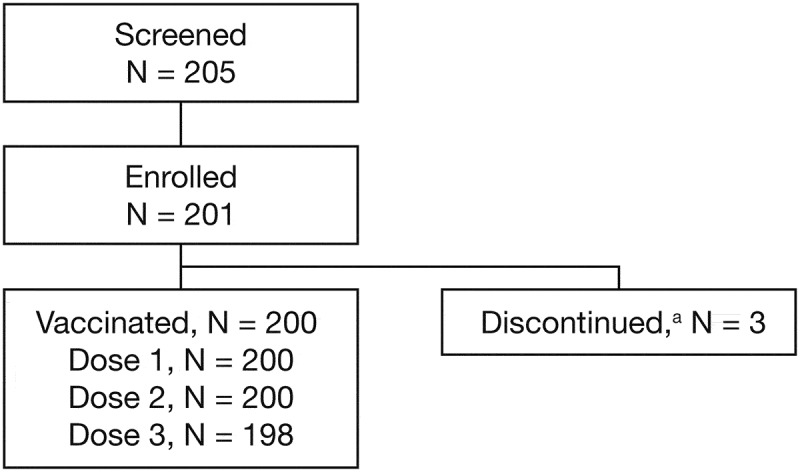
Participant disposition. aOne participant discontinued due to a protocol deviation (the participant was randomized in error [failed to satisfy eligibility criteria] and was discontinued from the study prior to receiving the first 9vHPV vaccine dose) and two participants withdrew consent. 9vHPV, nine-valent human papillomavirus
Table 1.
Participant baseline demographics and characteristics
| Total participants (N = 201) | |
|---|---|
| Age | |
| Mean (SD), years | 15.8 (4.4) |
| Median (range), years | 15.0 (9–26) |
| 9 to 12 years, n (%) | 50 (24.9) |
| 13 to 15 years, n (%) | 51 (25.4) |
| 16 to 26 years, n (%) | 100 (49.8) |
| Gender, n (%) | |
| Female | 135 (67.2) |
| Male | 66 (32.8) |
| Height, cm | |
| Mean (SD) | 155.22 (10.86) |
| Median (range) | 157.00 (120–177) |
| Weight, kg | |
| Mean (SD) | 48.21 (11.69) |
| Median (range) | 47.50 (25–92) |
| Sexual history at Day 1,a n (%) | |
| Has not had sexual debut | 91 (91.0) |
| Has had sexual debut | 9 (9.0) |
| Age at first sexual intercourse among non-virgins,b years | |
| Mean (SD) | 20.3 (2.3) |
| Median (range) | 21.0 (16–23) |
| Lifetime number of male or female sexual partners among non-virgins,b n (%) | |
| 1 | 8 (8.0) |
| 3 | 1 (1.0) |
| Median | 1 |
| HPV seropositivity status at Day 1, n (%) | |
| Anti-HPV6 | 4 (2.0) |
| Anti-HPV11 | 1 (0.5) |
| Anti-HPV16 | 8 (4.0) |
| Anti-HPV18 | 5 (2.5) |
| Anti-HPV31 | 7 (3.5) |
| Anti-HPV33 | 1 (0.5) |
| Anti-HPV45 | 2 (1.0) |
| Anti-HPV52 | 3 (1.5) |
| Anti-HPV58 | 5 (2.5) |
aSexual history was collected from participants 16 to 26 years of age only (N = 100).
b9 participants were non-virgins period
cm, centimeters; HPV, human papillomavirus; kg, kilograms; SD, standard deviation.
Following completion of the 9vHPV vaccination regimen, all PPI-eligible participants seroconverted for each of the vaccine HPV types by Month 7 (Table 2) across genders and age groups. The lower limit of the 95% CI of the percentage seroconversion was ≥98.0% for each HPV type, meeting the pre-defined statistical criterion (lower limit of the 95% CI to be >90%) for acceptable anti-HPV seroconversion. Robust anti-HPV GMTs were observed for all 9vHPV vaccine HPV types by Month 7 post-Dose 3 (Table 2). In a subgroup analysis stratified by age, a trend towards higher GMTs was observed in younger participants (9 to 15 years of age) compared with older participants (16 to 26 years of age) (Table 2). GMTs in older participants (16 to 26 years of age) were similar (with overlapping 95% CIs) to historic GMTs in the 9vHPV vaccine arm of the pivotal efficacy study of the 9vHPV vaccine for HPV6, 11, 33, 52, and 58, and trended higher (with non-overlapping 95% CIs) compared with the 9vHPV vaccine arm of the pivotal efficacy study for HPV16, 18, 31, and 45 (Table 2).
Table 2.
Summary of anti-HPV seroconversion percentages and GMTs at Month 7 in the Vietnam registration study and historic seroconversion percentages and GMTs at Month 7 in the pivotal efficacy study of the 9vHPV vaccine (PPI population)
| Registration study of 9vHPV vaccine in Vietnam | |||||
|---|---|---|---|---|---|
| Assay (cLIA) | Study population | N | n | % seroconversion (95% CI)a | GMT (95% CI)b |
| Anti-HPV6 | All participants | 200 | 190 | 100 (98.1–100) | 1008.2 (921.9–1102.6) |
| Participants 9–15 years of age | 100 | 98 | 100 (96.3–100) | 1203.1 (1076.0–1345.2) | |
| Participants 16–26 years of age | 100 | 92 | 100 (96.1–100) | 835.2 (731.1–954.1) | |
| Anti-HPV11 | All participants | 200 | 190 | 100 (98.1–100) | 796.3 (722.2–878.0) |
| Participants 9–15 years of age | 100 | 98 | 100 (96.3–100) | 970.1 (863.2–1090.2) | |
| Participants 16–26 years of age | 100 | 92 | 100 (96.1–100) | 645.3 (555.8–749.2) | |
| Anti-HPV16 | All participants | 200 | 187 | 100 (98.0–100) | 4605.4 (4163.7–5093.9) |
| Participants 9–15 years of age | 100 | 98 | 100 (96.3–100) | 5579.0 (4915.4–6332.1) | |
| Participants 16–26 years of age | 100 | 89 | 100 (95.9–100) | 3728.7 (3209.6–4331.7) | |
| Anti-HPV18 | All participants | 200 | 190 | 100 (98.1–100) | 1621.6 (1441.2–1824.5) |
| Participants 9–15 years of age | 100 | 98 | 100 (96.3–100) | 2115.1 (1833.9–2439.3) | |
| Participants 16–26 years of age | 100 | 92 | 100 (96.1–100) | 1221.8 (1025.7–1455.4) | |
| Anti-HPV31 | All participants | 200 | 188 | 100 (98.1–100) | 1137.9 (1017.2–1273.0) |
| Participants 9–15 years of age | 100 | 97 | 100 (96.3–100) | 1458.4 (1271.2–1673.2) | |
| Participants 16–26 years of age | 100 | 91 | 100 (96.0–100) | 873.5 (740.5–1030.4) | |
| Anti-HPV33 | All participants | 200 | 194 | 100 (98.1–100) | 507.8 (458.5–562.4) |
| Participants 9–15 years of age | 100 | 100 | 100 (96.4–100) | 641.2 (564.2–728.6) | |
| Participants 16–26 years of age | 100 | 94 | 100 (96.2–100) | 396.2 (342.0–459.0) | |
| Anti-HPV45 | All participants | 200 | 193 | 100 (98.1–100) | 579.2 (511.7–655.6) |
| Participants 9–15 years of age | 100 | 99 | 100 (96.3–100) | 752.4 (636.3–889.7) | |
| Participants 16–26 years of age | 100 | 94 | 100 (96.2–100) | 439.7 (371.4–520.5) | |
| Anti-HPV52 | All participants | 200 | 192 | 100 (98.1–100) | 500.8 (450.5–556.7) |
| Participants 9–15 years of age | 100 | 100 | 100 (96.4–100) | 632.4 (551.0–725.8) | |
| Participants 16–26 years of age | 100 | 92 | 100 (96.1–100) | 388.6 (335.2–450.6) | |
| Anti-HPV58 | All participants | 200 | 190 | 100 (98.1–100) | 701.8 (628.5–783.7) |
| Participants 9–15 years of age | 100 | 98 | 100 (96.3–100) | 888.9 (771.3–1024.5) | |
| Participants 16–26 years of age |
100 |
92 |
100 (96.1–100) |
545.6 (466.3–638.6) |
|
| Historic results from the pivotal efficacy study of the 9vHPV vaccine (9vHPV vaccine arm)c | |||||
| Assay (cLIA) |
Study population |
N |
n |
% seroconversion (95% CI)a |
GMT (95% CI)b |
| Anti-HPV6 | Participants 16–26 years of age | 6792 | 3993 | 99.8 (99.6–99.9) | 893.1 (871.7–915.1) |
| Anti-HPV11 | Participants 16–26 years of age | 6792 | 3995 | 100 (99.9–100) | 666.3 (649.6–683.4) |
| Anti-HPV16 | Participants 16–26 years of age | 6792 | 4032 | 100 (99.9–100) | 3131.1 (3057.1–3206.9) |
| Anti-HPV18 | Participants 16–26 years of age | 6792 | 4539 | 99.8 (99.7–99.9) | 804.6 (782.7–827.1) |
| Anti-HPV31 | Participants 16–26 years of age | 6792 | 4466 | 99.8 (99.6–99.9) | 658.4 (636.7–680.9) |
| Anti-HPV33 | Participants 16–26 years of age | 6792 | 4702 | 99.7 (99.5–99.9) | 415.9 (405.6–426.4) |
| Anti-HPV45 | Participants 16–26 years of age | 6792 | 4792 | 99.6 (99.4–99.8) | 252.8 (246.2–259.6) |
| Anti-HPV52 | Participants 16–26 years of age | 6792 | 4455 | 99.8 (99.6–99.9) | 379.7 (371.6–388.0) |
| Anti-HPV58 | Participants 16–26 years of age | 6792 | 4486 | 99.8 (99.6–99.9) | 482.5 (469.9–495.3) |
N = number of participants who received at least one 9vHPV vaccine dose.
n = number of participants contributing to the analysis.
aSeroconversion = 100*(number of participants who seroconverted/number of participants included in the analysis).
bUnits in mMU/mL.
cResults from Huh et al.14
The PPI population included participants who received three vaccinations within acceptable day ranges, were seronegative at Day 1 for the relevant HPV type(s), provided a serum sample within 21 to 49 days post-Dose 3, and had no protocol violations that could interfere with the immunogenicity evaluation. In addition, for the PPI population in the historic pivotal efficacy study, participants also had to be PCR-negative from Day 1 to Month 7 for the relevant HPV type(s).
9vHPV, nine-valent human papillomavirus; CI, confidence interval; cLIA, competitive Luminex immunoassay; GMT, geometric mean titer; HPV, human papillomavirus; PCR, polymerase chain reaction; PPI, per-protocol immunogenicity; mMU, milli-Merck unit.
A summary of AEs experienced by participants within 15 days following any vaccination is shown in Table 3. Approximately half of participants (50.5%) reported ≥1 AE at any time during the study.
Table 3.
AE summary (‘all participants as treated’ population)
| 9vHPV vaccine (N = 200) | |
|---|---|
| Participants with ≥1 AE, n (%)a | 101 (50.5) |
| Injection-site AEs, n (%)b | 90 (45.0) |
| Injection-site pain | 89 (44.5) |
| Mild | 86 (43.0) |
| Moderate | 3 (1.5) |
| Injection-site swelling | 11 (5.5) |
| Mild (0 to 2.5 cm) | 9 (4.5) |
| Moderate (2.5 to 5.0 cm) | 1 (0.5) |
| Severe (>5.0 cm) | 1 (0.5) |
| Injection-site erythema | 4 (2.0) |
| Mild (0 to 2.5 cm) | 4 (2.0) |
| Systemic AEs, n (%)c | 34 (17.0) |
| Headache | 7 (3.5) |
| Dizziness | 5 (2.5) |
| Nasopharyngitis | 5 (2.5) |
| Vaccine-relatedd systemic AEs, n (%)c | 2 (1.0) |
| SAEs, n (%)a | 1 (0.5) |
| Vaccine-relatedd SAEs, n (%) | 0 (0.0) |
| Deaths, n (%) | 0 (0.0) |
| Participants who discontinuede due to an AE,a n (%) | 0 (0.0) |
| Maximum temperature (oral) <37.8°Cb | 200 (100) |
N = number of participants who had ≥1 dose of the 9vHPV vaccine and had ≥1 follow-up visit for an AE.
aAt any time during the study.
bDays 1 to 5 following any vaccination.
cDays 1 to 15 following any vaccination.
dAs determined by the reporting investigator.
eStudy vaccination withdrawn.
Injection-site and systemic AEs shown are those with a frequency ≥2%. Participants were counted once for each applicable AE.
9vHPV, nine-valent human papillomavirus; AE, adverse event; SAE, serious adverse event.
The most common injection-site AEs were injection-site pain (44.5%), injection-site swelling (5.5%), and injection-site erythema (2.0%) (Days 1 to 5 following vaccination; Table 3). Most AEs of injection-site pain (n = 86/89 participants, 96.6%), swelling (n = 9/11, 81.8%), and erythema (n = 4/4, 100%) were considered to be mild. One participant experienced severe injection-site swelling.
Headache (3.5%), dizziness (2.5%), and nasopharyngitis (2.5%) were the most frequently reported systemic AEs. Vaccine-related systemic AEs were reported for two participants (1.0%): muscle fatigue (n = 1 participant), and dizziness and headache (n = 1 participant), both of which were mild in intensity and resolved without interruption to the dosing schedule.
No participant had an oral temperature of ≥37.8°C from Days 1 to 5 following administration of any vaccination dose. No deaths were reported during the course of the study, and there were no discontinuations due to AEs. There were no SAEs considered to be vaccination-related by the investigator during the study. One SAE was reported during the study: subcutaneous abscess of the left cheek at 12 days post-Dose 3; this AE was considered moderate in intensity and fully resolved.
Discussion
To support licensure of the 9vHPV vaccine in Vietnam, this local registration study evaluated the immunogenicity and safety of the 9vHPV vaccine in Vietnamese males and females 9 to 26 years of age.
Following administration of a three-dose 9vHPV vaccination regimen, all participants in the PPI population seroconverted to the relevant vaccine HPV type by Month 7. The seroconversion percentages (100%) observed for each vaccine HPV type at Month 7 were similar to those previously observed in the 9vHPV vaccine arm of the pivotal efficacy study of the 9vHPV vaccine (≥99.6% at Month 7).13,14
A trend in higher anti-HPV GMT response in participants 9 to 15 years of age compared with participants 16 to 26 years of age was observed in the current study in Vietnam. This trend has consistently been observed in several studies in the global clinical trials program, including sub-analyses of Asian participants in a global study.16,25,26
Anti-HPV GMTs across HPV types observed in participants 16 to 26 years of age in the current study in Vietnam were similar or higher compared with historic GMTs observed in the 9vHPV vaccine arm of the global pivotal 9vHPV vaccine efficacy study. Taken together, these results suggest that the 9vHPV vaccine will elicit sufficient protection against HPV6/11/16/18/31/33/45/52/58-related infection and disease in participants from Vietnam.
In the pivotal efficacy study of the 9vHPV vaccine, persistent efficacy up to 6 years and a robust immunogenicity profile for up to 60 months were observed following 9vHPV vaccination in women 16 to 26 years of age.14 The broadly similar immunogenicity profile observed at Month 7 between participants of the global clinical study program and the Vietnamese participants of the current study supports the potential for sustained efficacy.
The 9vHPV vaccine was generally well tolerated among study participants. Injection-site AEs were the most frequently reported safety events in the current study, the majority of which were mild in intensity. Lower proportions of Vietnamese participants reported injection-site AEs and systemic AEs compared with males and females 9 to 26 years of age who received the 9vHPV vaccine in the global clinical trial program (injection-site AEs: 45.0% vs 84.8%, respectively; systemic AEs: 17.0% vs 51.9%, respectively).27 Consistent with the lower rates of AEs observed in this study in Vietnam, lower frequencies of injection-site AEs and systemic AEs were observed in the sub-analysis of Asian participants compared to participants in the global study of the 9vHPV vaccine (injection-site AEs: 72.4% vs 90.7%, respectively; systemic AEs: 27.1% vs 55.8%, respectively).25
Study limitations include a relatively small sample size and short duration of follow-up; in addition, the study did not include participants older than 26 years of age. However, the consistency of the immunogenicity and safety results in this study with the findings in previous global studies and sub-analyses of Asian clinical trial participants suggest that results from the global studies can be extended to Vietnam. Although efficacy analyses were not performed in our study, the HPV antibody responses elicited by the 9vHPV vaccine in the Vietnamese participants appear sufficient to induce high-level protective efficacy against HPV6/11/16/18/31/33/45/52/58-related infection and disease.
In summary, administration of a three-dose 9vHPV vaccination regimen in Vietnamese males and females 9 to 26 years of age was highly immunogenic and resulted in acceptable seroconversion percentages to the vaccine HPV types. The 9vHPV vaccine was generally well tolerated in this population. These data are broadly consistent with the results from global 9vHPV vaccine studies conducted outside of Vietnam. Overall, these results support broad implementation of the 9vHPV vaccine in Vietnam to help reduce the burden of HPV-related infection and disease in this population.
Acknowledgments
Dr. Pham Huu Thang, Head of Infectious Diseases Control and Prevention, Center for Diseases Control and Prevention (Thai Binh province, Vietnam) contributed to the organization of the study in Thai Binh province. The authors thank all study participants, investigators, and contributors. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). Medical writing assistance, under the direction of the authors, was provided by Adele Blair, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by MSD.
Funding Statement
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure of potential conflicts of interest
VD Thiem, ND Quang, and NH Tuan are employees of the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam; they declare no conflicts of interest. K Cheon, N Gallagher, C Badshah, T Group, and A Luxembourg are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock/stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S.. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM.. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 3.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Vietnam. Summary Report. 2019. [accessed 2020 June30]. https://hpvcentre.net/statistics/reports/VNM.pdf
- 4.Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros-Balta AX, Low N, Henschke N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV‐related disease in females and males. Cochrane Database Syst Rev. 2019;2019(11):CD013479. doi: 10.1002/14651858.CD013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luxembourg A, Moeller E. 9-valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev Vaccines. 2017;16(11):1119–39. doi: 10.1080/14760584.2017.1383158. [DOI] [PubMed] [Google Scholar]
- 6.Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997–2008. doi: 10.1158/1055-9965.EPI-14-0410. [DOI] [PubMed] [Google Scholar]
- 7.Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, Bosch F, de Sanjosé S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7(1):38. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, Joura EA, Maldonado P, Laco J, Bravo IG, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49(16):3450–61. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Alemany L, Saunier M, Tinoco L, Quirós B, Alvarado-Cabrero I, Alejo M, Joura EA, Maldonado P, Klaustermeier J, Salmerón J, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: A worldwide study in 597 samples. Eur J Cancer. 2014;50(16):2846–54. doi: 10.1016/j.ejca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin H-R, Pirog EC, Guimerà N, Hernandez-Suarez G, Felix A, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt R, Joura E. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 12.Serrano B, de Sanjosé S, Tous S, Quiros B, Muñoz N, Bosch X, Alemany L. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51(13):1732–41. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 14.Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–59. doi: 10.1016/S0140-6736(17)31821-4. [DOI] [PubMed] [Google Scholar]
- 15.Castellsague X, Giuliano AR, Goldstone S, Guevara A, Mogensen O, Palefsky JM, Group T, Shields C, Liu K, Maansson R, et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33(48):6892–901. doi: 10.1016/j.vaccine.2015.06.088. [DOI] [PubMed] [Google Scholar]
- 16.Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–39. doi: 10.1542/peds.2014-3745. [DOI] [PubMed] [Google Scholar]
- 17.Baloch Z, Li Y, Yuan T, Feng Y, Liu Y, Tai W, Liu L, Wang B, Zhang A-M, Wu X, et al. Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infect Dis. 2016;16(1):228. doi: 10.1186/s12879-016-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma Y, Kusumoto-Matsuo R, Takeuchi F, Uenoyama A, Kondo K, Tsunoda H, Nagasaka K, Kawana K, Morisada T, Iwata T, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin Oncol. 2014;44(10):910–17. doi: 10.1093/jjco/hyu112. [DOI] [PubMed] [Google Scholar]
- 19.So KA, Hong JH, Lee JK. Human papillomavirus prevalence and type distribution among 968 women in South Korea. J Cancer Prev. 2016;21(2):104–09. doi: 10.15430/JCP.2016.21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H-C, You S-L, Hsieh C-Y, Schiffman M, Lin C-Y, Pan M-H, Chou Y-C, Liaw K-L, Hsing AW, Chen C-J, et al. Prevalence of genotype-specific human papillomavirus infection and cervical neoplasia in Taiwan: a community-based survey of 10,602 women. Int J Cancer. 2011;128(5):1192–203. doi: 10.1002/ijc.25685. [DOI] [PubMed] [Google Scholar]
- 21.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J,Bosch FX, de Sanjosé S. ICO/IARC information centre on HPV and cancer (HPV information centre). Human Papillomavirus and Related Diseases in Asia. Summary Report. 2019. [accessed 2020 June30]. http://www.hpvcentre.net/statistics/reports/XSX.pdf
- 22.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J,Bosch FX, de Sanjosé S. ICO/IARC information centre on HPV and cancer (HPV information centre). Human Papillomavirus and Related Diseases in the World. Summary Report. 2019. [accessed 2020 June30]. http://www.hpvcentre.net/statistics/reports/XWX.pdf.
- 23.Roberts C, Green T, Hess E, Matys K, Brown MJ, Haupt RM, Luxembourg A, Vuocolo S, Saah A, Antonello J, et al. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum Vaccin Immunother. 2014;10(8):2168–2174. https://www.tandfonline.com/doi/pdf/10.4161/hv.29205?needAccess=true [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley M, Pinto LA, Trimble C. Human Papillomavirus Vaccines – immune Responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 25.Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, Yang YC, Chu TY, Twu NF, Samakoses R, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian countries. J Infect Dis. 2018;218(1):95–108. doi: 10.1093/infdis/jiy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen LK, Restrepo J, Moreira ED Jr., Iversen O-E, Pitisuttithum P, Van Damme P, Joura EA, Olsson S-E, Ferris D, Block S, et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine – A combined analysis of five phase III clinical trials. Papillomavirus Res. 2017;3:105–15. doi: 10.1016/j.pvr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira ED Jr., Block SL, Ferris D, Giuliano AR, Iversen O-E, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 Phase III clinical trials. Pediatrics. 2016;138(2):e20154387. doi: 10.1542/peds.2015-4387. [DOI] [PubMed] [Google Scholar]


