ABSTRACT
Many countries are replacing meningococcal serogroup C (MenC) conjugate vaccines (MCCV) with quadrivalent conjugate (MenACWY) vaccines, such as MenACWY-TT (Nimenrix®). This review examined eight studies comparing MenC immune responses induced by MenACWY-TT and MCCV to determine if these data support these changes. MenC serum bactericidal antibody levels using human (hSBA) or rabbit complement (rSBA) were evaluated at ~1 month postvaccination. Overall, ≥98.4% of infants administered 2 + 1 MenACWY-TT or MCCV schedules had rSBA titers ≥1:8 postprimary and postbooster vaccination; hSBA titers ≥1:8 were similar. In toddlers administered single MenACWY-TT or MCCV doses, ≥97.3% had rSBA titers ≥1:8 postvaccination; percentages with hSBA titers ≥1:8 were higher post-MenACWY-TT. Of children and adolescents receiving primary and booster MenACWY-TT and MCCV, ≥98.6% had rSBA titers ≥1:8; all children receiving MenACWY-TT or MCCV booster had hSBA titers ≥1:8 postdosing. MenC immune responses induced by MenACWY-TT are robust and generally comparable/superior to MCCV, supporting changes to recommendations.
KEYWORDS: MenACWY-TT, immunogenicity, meningococcal serogroup C, clinical study, Europe
Introduction
Invasive meningococcal disease (IMD) is caused by infection with the bacterium Neisseria meningitidis.1 Clinically presenting most often as meningitis and/or septicemia, IMD is associated with significant levels of mortality; those who survive can experience disabling, long-term sequelae such as neurologic and hearing impairments and amputation.2–4 Disease incidence is highest in infants and toddlers, with a second peak often occurring among adolescents and young adults.1 Because of the sudden onset and rapid progression of the disease,2 preventive vaccination is considered the most effective strategy to protect against IMD.5
Of the 12 identified serogroups of N meningitidis, 5 have historically predominated as the main cause of IMD globally (i.e., A, B, C, W, and Y); all are currently preventable with available monovalent and polyvalent meningococcal vaccine formulations (Table 1).20,21 Specifically, several monovalent conjugate vaccines have been developed for protection against meningococcal serogroup C (MenC) disease, and a monovalent conjugate vaccine has been developed against serogroup A (MenA) disease.6–11 Vaccines that combine Haemophilus influenzae type b (Hib) and meningococcal antigens (i.e., serogroups Y and/or C) were/are available in some countries.14,15 Two vaccines based on subcapsular antigens are also available for the prevention of meningococcal serogroup B (MenB) disease.12,13,22 The availability of quadrivalent vaccines targeting meningococcal serogroups A, C, W, and Y (MenACWY) has led to broader serogroup coverage against disease-causing strains.23 The implementation of immunization programs using these various meningococcal vaccine formulations has substantially contributed to reductions in IMD disease burden.24,25
Table 1.
Meningococcal conjugate and recombinant protein vaccines used globally
| Vaccine | Type | Meningococcal serogroups (other antigens) | European indication, age* |
|---|---|---|---|
| Monovalent | |||
| MenAfricVac (PsA-TT)6,7 | TT conjugate | A | Not licensed in Europe |
| Menjugate (MenC-CRM197 adsorbed to aluminum hydroxide)8 | CRM197 conjugate | C | ≥2 mo |
| Meningitec (MenC-CRM197 adsorbed to aluminum phosphate)9,10 | CRM197 conjugate | C | Discontinued in Europe |
| NeisVac-C (MenC-TT)11 | TT conjugate | C | ≥2 mo |
| Bexsero (MenB-4C)12 | Recombinant protein | B | ≥2 mo |
| Trumenba (MenB-FHbp)13 | Recombinant protein | B | ≥10 y |
| Combination | |||
| MenHibrix (HibMenCY-TT)14 | TT conjugate | C, Y (Hib) | Not licensed in Europe |
| Menitorix (Hib-MenC-TT)15 | TT conjugate | C (Hib) | ≥2 mo |
| Quadrivalent | |||
| Nimenrix (MenACWY-TT)16 | TT conjugate | A, C, W, Y | ≥6 wk |
| Menveo (MenACWY-CRM197)17 | CRM197 conjugate | A, C, W, Y | ≥2 y |
| Menactra (MenACWY-D)18 | D conjugate | A, C, W, Y | Not licensed in Europe |
| MenQuadfi (MenACYW-TT)19 | TT conjugate | A, C, W, Y | Not licensed in Europe |
CRM197, diphtheria protein cross-reactive material 197; D, diphtheria toxin; Hib, Haemophilus influenzae type b; TT, tetanus toxoid.
*For currently available vaccines.
Four quadrivalent meningococcal vaccines are currently licensed and differ according to carrier protein, posology, and availability (Table 1).16–19 One such quadrivalent vaccine is MenACWY-TT (Nimenrix®, Pfizer Ltd, Kent, UK), which is conjugated to tetanus toxoid (TT) and is indicated for use in individuals from age 6 weeks.16 MenACWY-TT, the focus of this review, is administered to infants as a 2-dose (6 weeks–<6 months) or 1-dose (6–<12 months) primary series plus a 1-dose booster in the second year of life.16,26 In individuals aged 12 months and older, MenACWY-TT is given as a single dose.16 Booster dosing can be given to individuals from age 12 months who were previously vaccinated with a conjugated or plain polysaccharide meningococcal vaccine.
The distribution of meningococcal serogroups causing disease varies geographically and over time.27 Therefore, to ensure adequate protection against IMD, national vaccination strategies have adapted to temporal changes in epidemiology. This is exemplified by the experience with the introduction of MenC vaccination programs. During the late 1990s, the incidence of MenC disease increased in many European countries, mainly due to a hypervirulent ST-11 clone.28 In response, several countries added monovalent MenC vaccination to their routine infant immunization schedule, usually with catch-up programs in toddlers, children, and adolescents and, in some countries, young adults.28,29 Subsequent to the implementation of these strategies, decreases in MenC disease were observed.24,28 In contrast, during the past decade, the incidence of IMD caused by meningococcal serogroup W (MenW) and serogroup Y (MenY) has increased across multiple age groups in many countries within Europe.30 MenW cases have frequently been associated with a hypervirulent ST-11 strain and, more recently, an emergent ST-9316 strain predominantly affecting children aged <4 years.31–35 A proportion of these MenW cases have presented with atypical clinical features, such as septic arthritis, gastrointestinal symptoms, and severe respiratory tract infections, such as pneumonia, epiglottitis, and supraglottitis.31 MenY cases have shown variability in the most commonly affected age group; MenY cases have also shown increased manifestation as septicemia and decreased susceptibility to penicillin.35,36 In response to this serogroup shift, several countries introduced quadrivalent MenACWY vaccination to their immunization programs, in many instances as replacement for the existing monovalent MenC vaccine (Figure 1).31,37–59
Figure 1.
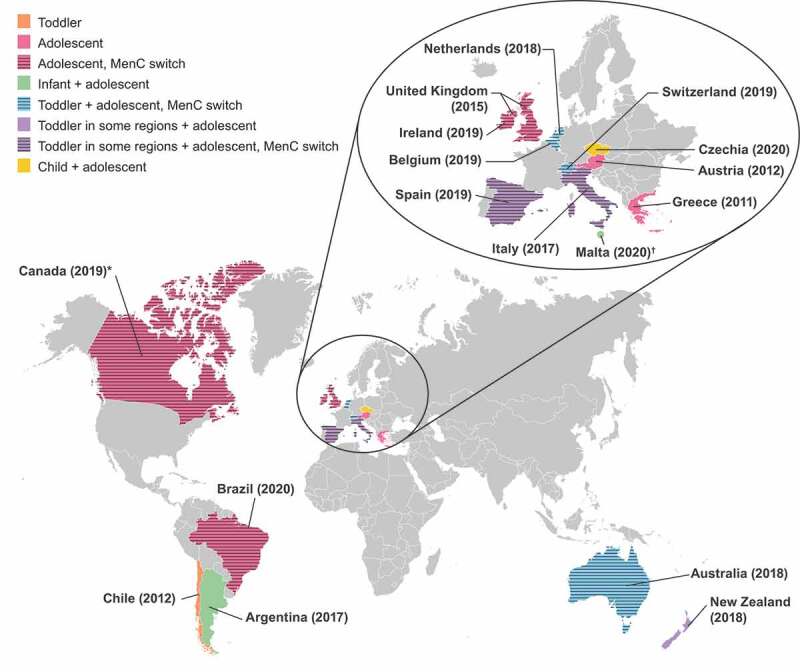
Countries with recent MenACWY vaccine recommendations.31,37–59
*In all provinces apart from Quebec. †Malta is not shown to scale or to shape.
To ensure optimal protection against MenC disease is maintained in countries switching to MenACWY vaccines, it is important that the immune response elicited to serogroup C is comparable to that achieved with monovalent MenC vaccines. This article reviews key clinical studies that compared MenC immune responses induced by MenACWY-TT with those of monovalent MenC conjugate vaccines.
Methods
Clinical studies evaluating the immunogenicity of MenACWY-TT were identified by searches of clinical trial registries for studies in which MenACWY-TT was compared with a MenC vaccine and in which serology was completed for the licensed posology. PubMed, ClinicalTrials.gov, and the EU Clinical Trials Register were searched using the keywords “MenACWY-TT”, “Nimenrix”, “ACWY-TT”, and “GSK134612”, without filters or limits. The GSK Study Register was searched using the same keywords and the limit of “Meningococcal Infections” as the condition/disease. The inclusion criteria were studies directly comparing the immunogenicity of MenACWY-TT with that of ≥1 monovalent MenC conjugate vaccine (MenC-TT, MenC-CRM197/Al(OH)3, or MenC-CRM197/AlPO4 [Table 1]). Immune responses in these studies were evaluated in serum bactericidal antibody assays using human (hSBA) or baby rabbit complement (rSBA).60–67 Immunogenicity assessments evaluated in the current review include percentage of subjects with rSBA titers ≥1:8, rSBA geometric mean titer (GMT), percentage of subjects with hSBA titers ≥1:8, and hSBA GMT at 1 month/42 days after primary or booster vaccination. Only data relevant to the licensed MenACWY-TT schedule are presented (i.e., 2 + 1 schedule in infants aged 6 weeks–<6 months; 1 + 1 schedule in infants aged 6–<12 months; single dose in individuals aged ≥12 months16). Preimmune and persistence data were not considered. This article is based entirely on previously published studies and does not contain data from any new studies using human or animal subjects.
Results
Studies included
Eight studies met the criteria for inclusion in the review, comprising one study in infants, four in toddlers, and three in children and adolescents. The designs of these studies are summarized in Table 2.60–67 No studies carried out in adults met the inclusion criteria.
Table 2.
Overview of included studies
| Study | Clinical trial registration number | Location | Study design | Total subjects, n | Vaccine comparison relevant to this review | Subjects of comparison, n |
|---|---|---|---|---|---|---|
| Merino Arribas 201760 | ClinicalTrials.gov NCT01144663 | Estonia, Germany, Spain | Phase 3, open, randomized, controlled | 2095 | MenACWY-TT MenC-TT MenC-CRM197/Al(OH)3 |
Healthy infants aged 6–12 wk at first vaccination, n = 1567 |
| Vesikari 201161 | ClinicalTrials.gov NCT00474266 | Finland | Phase 3, open, randomized, controlled | 1000 | MenACWY-TT MenC-CRM197/AlPO4 |
Healthy toddlers aged 12–23 mo, n = 499 |
| Vesikari 201267 | ClinicalTrials.gov NCT00427908 | Finland | Phase 2, open, randomized, controlled | 304 | MenACWY-TT MenC-CRM197/AlPO4 |
Healthy toddlers aged 12–23 mo, n = 304 |
| Knuf 201063 | ClinicalTrials.gov NCT00126984 | Germany, Austria | Phase 2, blind, randomized, controlled | 508 | MenACWY-TT MenC-CRM197/AlPO4 |
Healthy toddlers aged 12–14 mo, n = 96 |
| Knuf 201163 | ClinicalTrials.gov NCT00508261 | Austria, Germany, Greece | Phase 3, open, randomized, controlled | 793 | MenACWY-TT MenC-CRM197/AlPO4 |
Healthy toddlers aged 12–23 mo, n = 347 |
| Knuf 201364 | ClinicalTrials.gov NCT00674583 | Germany, France | Phase 3, open, randomized, controlled | 414 | MenACWY-TT MenC-CRM197/Al(OH)3 |
Healthy children aged 2–10 y, n = 414 |
| Vesikari 201565 | ClinicalTrials.gov NCT00955682 (extension study to NCT00474266) | Finland | Phase 3, open, controlled, persistence/booster extension | 293 | MenACWY-TT MenC-CRM197/AlPO4 |
Healthy children aged 60–69 mo who had previously received MenACWY-TT or MenC-CRM197/AlPO4 as toddlers, n = 293 |
| Van Ravenhorst 201766 | Clinicaltrialsregister.eu EudraCT 2013–001823-38 Trialregister.nl NTR4430 |
The Netherlands | Phase 4, open, randomized, controlled | 501 | MenACWY-TT MenC-TT |
Healthy adolescents aged 10, 12, and 15 y previously vaccinated with MenC-TT at 14 mo–3 y; n = 501 |
MenACWY-TT, meningococcal serogroups A, C, W, and Y vaccine conjugated to tetanus toxoid as a carrier protein; MenC-CRM197/Al(OH)3, meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum hydroxide; MenC-CRM197/AlPO4, meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum phosphate; MenC-TT, meningococcal serogroup C vaccine conjugated to tetanus toxoid.
Studies in infants
Only 1 infant study met the inclusion criteria. Conducted in Spain, Germany, and Estonia, this noninferiority study compared 1567 infants who received MenACWY-TT, MenC-TT, or MenC-CRM197/Al(OH)3 at ages 2, 4, and 12 months.60 All subjects also received routine vaccinations as recommended. Immunogenicity was assessed at 1 month after the primary series and at 1 month after the booster dose.
After primary vaccination, the percentage of subjects with rSBA titers ≥1:8 for MenC was high across the 3 vaccine groups, ranging from 98.7% (MenACWY-TT) to 100% (MenC-TT) (Figure 2A). The percentage of subjects with postbooster MenC rSBA titers ≥1:8 was also consistently high in all groups, varying from 98.4% in the MenC-CRM197/Al(OH)3 group to 99.8% and 100% in the MenACWY-TT and MenC-TT groups, respectively (Figure 2A). Comparisons of MenC rSBA GMT both after the primary series and the booster dose showed a <2-fold difference among vaccine groups (postprimary range, 612 − 1188; postbooster range, 1051 − 1960; Figure 2B). Postprimary and postbooster, the percentages of subjects with MenC hSBA titers ≥1:8 were 100% for the monovalent vaccines and ≥98.6% for MenACWY-TT (Figure 2C). MenC hSBA GMTs after primary vaccination were 1308 among MenACWY-TT recipients, 3188 among those who received MenC-CRM197/Al(OH)3, and 2627 among those who received MenC-TT. In contrast, after the booster vaccination, MenC hSBA GMTs ranged from 4992 to 5542 among the 3 vaccine groups (Figure 2D). These findings confirmed the noninferiority of an infant 2-dose primary series of MenACWY-TT with that of a 2-dose primary series of MenC-CRM197/Al(OH)3 or MenC-TT with respect to the immune response against MenC. Additionally, immune responses with a MenACWY-TT booster dose supported the induction of immune memory after receipt of a 2-dose primary infant series.
Figure 2.
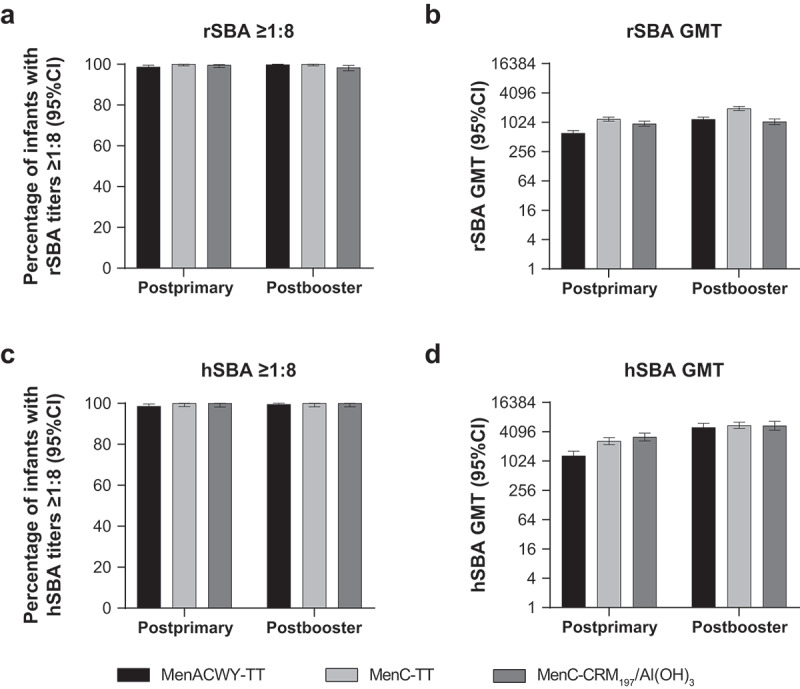
Serum bactericidal antibody measurements against serogroup C at 1 month postprimary and postbooster vaccination in infants vaccinated with MenACWY-TT, MenC-TT, or MenC-CRM197/Al(OH)3 at 2, 4, and 12 months of age.60 (a) Percentage of infants with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of infants with hSBA titers ≥1:8, and (d) hSBA GMTs.
Subjects had blood samples collected at 1 month (range, 21–48 days) after the second primary dose (primary ATP cohort) and the booster dose (booster ATP cohort). Panel A. Postprimary, n = 455–457; postbooster, n = 446–463. Panel B. Postprimary, n = 455–457; postbooster, n = 446–463. Panel C. Postprimary, n = 202–226; postbooster, n = 216–221. Panel D. Postprimary, n = 202–226; postbooster, n = 216–221. ATP = according to protocol; GMT = geometric mean titer; hSBA = serum bactericidal antibody using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y vaccine conjugated to tetanus toxoid as a carrier protein; MenC-CRM197/Al(OH)3 = meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum hydroxide; MenC-TT = meningococcal serogroup C vaccine conjugated to tetanus toxoid; rSBA = serum bactericidal antibody using baby rabbit complement.
Studies in toddlers
Four toddler studies were reviewed, all of which compared the immunogenicity of a single dose of MenACWY-TT with that of MenC-CRM197/AlPO4 in unprimed subjects.61–63,67 Three studies were conducted in toddlers aged 12 to 23 months61,63,67 and 1 in toddlers aged 12 to 14 months;62 overall, 1246 subjects in directly comparable groups were vaccinated with MenACWY-TT or MenC-CRM197/AlPO4.61–63,67 In all studies, immunogenicity against MenC was measured by the rSBA assay at 1 month/42 days postvaccination, whereas hSBA titers were assessed in 2 studies.
In 3 studies, the percentage of vaccinated subjects with MenC rSBA titers ≥1:8 was higher in the MenACWY-TT group compared with that in the MenC-CRM197/AlPO4 group (99.7%–100% vs 97.5%–98.5%);61,62,67 in the fourth study (toddlers aged 12–23 months), the percentages in the MenACWY-TT group were 97.3% and 98.2% in the MenC-CRM197/AlPO4 group63 (Figure 3A). Evaluation of MenC rSBA GMTs showed that postvaccination levels in 3 studies were ≥2-fold higher in toddlers vaccinated with MenACWY-TT compared with those in the corresponding MenC-CRM197/AlPO4 group (range, 212 − 984).61,62,67 In the remaining study, MenC rSBA GMTs were similar across both vaccine groups in toddlers 12 to 23 months of age, albeit slightly higher in those who received MenACWY-TT (MenACWY-TT recipients, 829; MenC-CRM197/AlPO4 recipients, 691; Figure 3B).63 Two of the toddler studies also used the hSBA assay to assess MenC immunogenicity.61,67 In both studies, the percentage of subjects with postvaccination MenC hSBA titers ≥1:8 was considerably higher in the MenACWY-TT group compared with the MenC-CRM197/AlPO4 group (98.5% vs 81.9% and 99.1% vs 72.1%, respectively; Figure 3C). Correspondingly, hSBA GMTs were approximately 5- and 10-fold higher in the MenACWY-TT groups compared with their MenC-CRM197/AlPO4 counterparts (196 vs 40 and 190 vs 21, respectively; Figure 3D).
Figure 3.
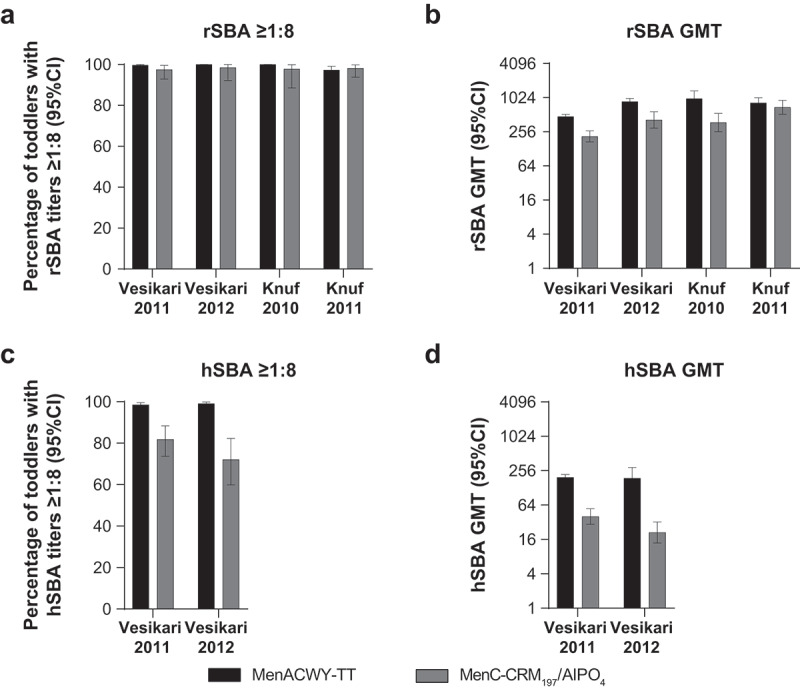
Serum bactericidal antibody measurements against serogroup C at 1 month/42 days postvaccination in toddlers administered a single dose of MenACWY-TT or MenC-CRM197/AlPO4 vaccine.61–63,67 (a) Percentage of toddlers with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of toddlers with hSBA titers ≥1:8, and (d) hSBA GMTs.
Subjects had blood samples collected at 1 month (range, 21–48 days) after the second primary dose (primary ATP cohort) and the booster dose (booster ATP cohort). Panel A. Postprimary, n = 455–457; postbooster, n = 446–463. Panel B. Postprimary, n = 455–457; postbooster, n = 446–463. Panel C. Postprimary, n = 202–226; postbooster, n = 216–221. Panel D. Postprimary, n = 202–226; postbooster, n = 216–221. ATP = according to protocol; GMT = geometric mean titer; hSBA = serum bactericidal antibody using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y vaccine conjugated to tetanus toxoid as a carrier protein; MenC-CRM197/Al(OH)3 = meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum hydroxide; MenC-TT = meningococcal serogroup C vaccine conjugated to tetanus toxoid; rSBA = serum bactericidal antibody using baby rabbit complement.
Studies in children and adolescents
Three studies investigating the immune response to MenACWY-TT in subjects aged ≥2 years were included in the review.64–66 A study conducted in 414 children aged 2 to 10 years compared a single dose of MenACWY-TT and MenC-CRM197/Al(OH)3.64 Two studies assessed the vaccines as a booster: 501 adolescents aged 10, 12, or 15 years who were previously vaccinated with a single dose of MenC-TT as toddlers received a booster dose of MenACWY-TT or MenC-TT;66 293 children aged 50 to 69 months who had previously received 1 primary dose of MenACWY-TT or MenC-CRM197/AlPO4 as toddlers were administered the same vaccine as a booster dose.65 The percentage of subjects with postvaccination MenC rSBA titers ≥1:8 was high across all 3 studies (Figure 4A). In both the primary vaccination and the booster study conducted in children, all subjects in the MenACWY-TT and MenC-CRM197 groups had rSBA titers ≥1:8 at 1 month.64,65 In the adolescent study, postbooster rSBA titers ≥1:8 against MenC were observed in all subjects aged 12 and 15 years and in 98.6% and 100% of subjects vaccinated with MenACWY-TT and MenC-TT, respectively, aged 10 years.66 Overall, MenC rSBA GMTs were also similar in MenACWY-TT and monovalent vaccine groups (Figure 4B). In both studies conducted in children, there was a <2-fold difference in GMT between the MenACWY-TT and MenC-CRM197 groups,64,65 and in the adolescent study, postbooster GMTs were comparably high across all ages and vaccines.66 A single study reported hSBA titers.65 All children administered a booster dose of MenACWY-TT or MenC-CRM197/AlPO4 after receiving the same vaccine as toddlers had MenC hSBA titers ≥1:8 at 1 month (Figure 4C), whereas the postbooster hSBA GMT in subjects vaccinated with MenACWY-TT was approximately 2-fold higher than that in subjects vaccinated with MenC-CRM197/AlPO4 (Figure 4D).
Figure 4.
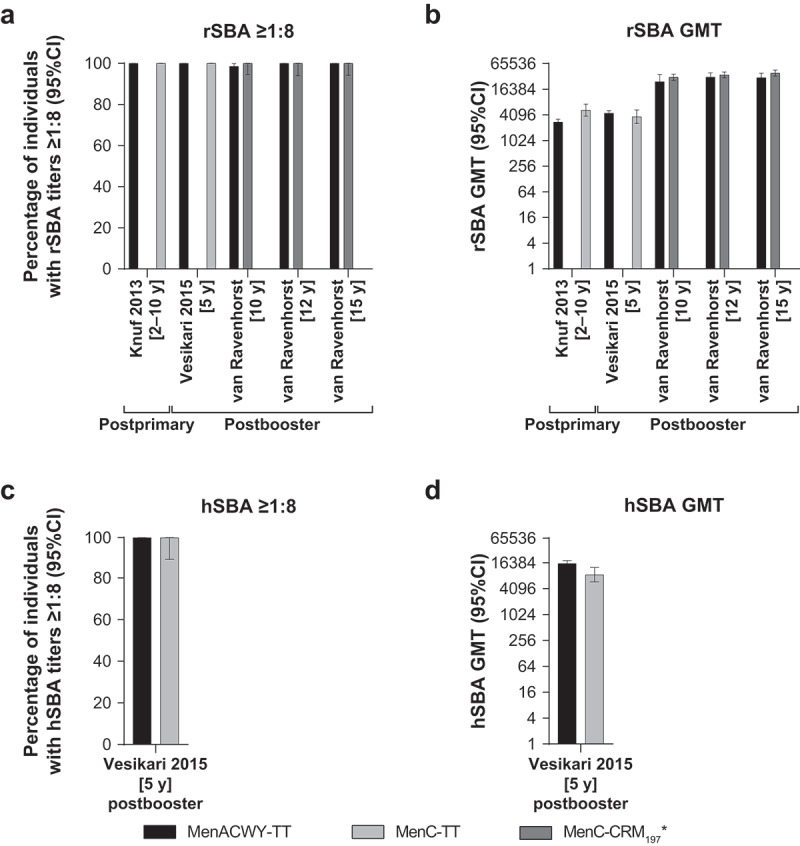
Serum bactericidal antibody measurements against serogroup C at 1 month postvaccination in individuals aged ≥2 years administered a single or booster dose of MenACWY-TT, MenC-CRM197/Al(OH)3, MenC-TT, or MenC-CRM197/AlPO4 vaccine.64–66 (a) Percentage of individuals with rSBA titers ≥1:8, (b) rSBA GMTs, (c) percentage of individuals with hSBA titers ≥1:8, and (d) hSBA GMTs.
*MenC-CRM197 vaccine = MenC-CRM197/Al(OH)3 in Knuf 201364 and MenC-CRM197/AlPO4 in Vesikari 2015.65 All analyses were performed on ATP immunogenicity cohorts. Knuf 2013: Subjects aged 2–10 years administered a single vaccine dose; MenACWY-TT, n = 293; MenC-CRM197/Al(OH)3, n = 97; blood samples collected at 1 month postvaccination (range, 21–48 days).64 Vesikari 2015: Subjects aged 5 years (60–69 months) who had previously received 1 dose of MenACWY-TT or MenC-CRM197/AlPO4 as toddlers were administered a booster dose of the same vaccine. MenACWY-TT, n = 209–215; MenC-CRM197/AlPO4, n = 33–43; blood samples collected at 1 month postbooster (range, 21–48 days).65 van Ravenhorst 2017: Subjects aged 10, 12, or 15 years who had previously received a primary dose of MenC-TT as toddlers were administered a booster vaccine dose; MenACWY-TT, n = 78–79; MenC-TT, n = 78–89; blood samples collected at 1 month postbooster (ATP blood sampling window not available).66 ATP = according to protocol; GMT = geometric mean titer; hSBA = serum bactericidal antibody using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y vaccine conjugated to tetanus toxoid as a carrier protein; MenC-CRM197/Al(OH)3 = meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum hydroxide; MenC-CRM197/AlPO4 = meningococcal serogroup C vaccine conjugated to the nontoxic form of diphtheria protein cross-reactive material 197 and adsorbed onto aluminum phosphate; MenC-TT = meningococcal serogroup C vaccine conjugated to tetanus toxoid; rSBA = serum bactericidal antibody using baby rabbit complement.
Discussion
The current review of clinical studies comparing MenC immunogenicity between MenACWY-TT and monovalent MenC conjugate vaccines consistently showed that rSBA and hSBA immune response to MenC elicited by MenACWY-TT is comparable to that of monovalent MenC conjugate vaccines. The reviewed studies measured the percentage of subjects with rSBA titers ≥1:8 at approximately 1 month postvaccination.60–67
The dynamic nature of meningococcal disease, which varies geographically and temporally,28 has necessitated that vaccination programs adapt to provide rational recommendations for vaccines that address current epidemiologic trends in a given region. As shown in several countries, recent changes in the recommendations were modified from use of monovalent MenC vaccines to MenACWY vaccines in response to increased risk of MenW and MenY disease (Figure 1).31,37–59 Although quadrivalent vaccines provide broader serogroup coverage than monovalent vaccines against disease-causing strains,23 including against increasingly prevalent MenW and MenY disease,30 it is important to confirm that optimal protection against MenC is maintained. This is particularly pertinent as vaccination programs using monovalent MenC vaccines have shown sizable decreases in the number of cases of MenC disease.24,28
In the infant study, criteria based on the percentage of subjects with rSBA titers ≥1:8 at 1 month after a 2-dose primary schedule showed the noninferiority of MenACWY-TT to the monovalent vaccines MenC-TT and MenC-CRM197/Al(OH)3.60 The booster response after a further MenACWY-TT dose at age 12 months was similarly robust, with 99.8% of subjects reaching the ≥1:8 threshold. Notably, although hSBA and rSBA GMTs were lower in the MenACWY-TT group at 1 month after primary vaccination, declines during the post-primary period were sharper in infants who received MenC-TT or MenC-CRM197/Al(OH)3 so that GMTs were similar among all 3 vaccine groups by the prebooster time point.
In the 4 toddler studies, the percentage of subjects vaccinated with MenACWY-TT with rSBA titers ≥1:8 at 1 month (42 days for 1 study) after a single primary dose was comparable to, and in some studies potentially higher than, that observed in the corresponding MenC-CRM197/AlPO4 group.61–63,67 Two of these toddler studies assessed the noninferiority of MenACWY-TT to MenC-CRM197/AlPO4 for MenC immunogenicity based on group differences in the percentages of subjects with MenC rSBA titers ≥1:8; in both studies, noninferiority of MenACWY-TT was confirmed.61,67 A strong immune response against MenC was observed in children aged 2 to 10 years after a single primary MenACWY-TT or MenC-CRM197/Al(OH)3 dose, with all subjects reaching the ≥1:8 rSBA threshold.64 Noninferiority of MenACWY-TT to MenC-CRM197/Al(OH)3 for immunogenicity against MenC was confirmed by comparison of a predefined vaccine response based on rSBA titer increases.
The booster response to MenACWY-TT in older children and adolescents was compared with monovalent MenC vaccines in two studies.65,66 When children aged 5 years received a booster dose with the same vaccine used for primary vaccination as toddlers, 100% of subjects in the MenACWY-TT and MenC-CRM197/AlPO4 groups had an rSBA titer ≥1:8 at 1 month postbooster.65 In the adolescent study, subjects vaccinated with MenC-TT as toddlers received a booster dose of either MenC-TT or MenACWY-TT; booster rSBA responses to both vaccines were robust, with 98.6% to 100% of subjects aged 10 years and all subjects aged 12 and 15 years showing an rSBA titer ≥1:8 at 1 month.66
The reviewed studies also reported rSBA GMTs.60–67 Within each study, the magnitude of rSBA GMT against serogroup C in the MenACWY-TT compared with monovalent MenC vaccine groups varied by <3-fold. There was no apparent pattern regarding which vaccine type gave rise to higher MenC rSBA GMTs: in 5 studies, levels were higher in the MenACWY-TT–vaccinated groups (i.e., all toddler studies and the study in 5-year-olds),61–63,65,67 and in 3 studies, levels were higher in the monovalent vaccinated groups (i.e., the infant study, the adolescent study, and the study in 2–10-year-olds).60,64,66 The study in adolescents used MenC rSBA GMT ratio to assess noninferiority of MenACWY-TT to MenC-TT at 1 month; noninferiority was shown for the 12-year-olds (GMT ratio, 0.88 [95% CI: 0.67–1.15]) but not for the 10- (GMT ratio, 0.80 [95% CI: 0.54–1.18]) or 15-year (GMT ratio, 0.81 [95% CI: 0.61–1.06]) age group.66 However, these differences are minor and not likely to be of clinical significance.
The hSBA assessments were reported in four of the reviewed studies,60,61,65,67 supporting the similar trends observed with MenC rSBA assessments. In the infant study, the percentage of subjects with MenC hSBA titers ≥1:8 after primary vaccination generally reflected the corresponding rSBA percentages, whereas that of postbooster hSBA titers ≥1:8 appeared to be slightly more uniform across vaccine groups.60 Similarly, the pattern of variation in hSBA GMTs among infant vaccine groups at the postprimary time point was consistent with that observed in rSBA GMTs, whereas postbooster hSBA GMTs were more uniform than their rSBA counterparts. In both toddler studies that assessed hSBA titers, the magnitude of difference between MenACWY-TT and MenC-CRM197/AlPO4 in the percentages of subjects with titers ≥1:8 was notably larger in hSBA compared with rSBA assays (hSBA 16.6% vs rSBA 2.2%, and hSBA 27.0% vs rSBA 1.5%, respectively).61,67 However, the ranking of vaccine groups did not differ between SBA assay types; in each study, both assays consistently resulted in higher percentages for the MenACWY-TT cohorts.
Monovalent MenC conjugate vaccines were initially licensed based on strong postvaccination immune responses shown in the rSBA assay.68,69 Subsequent postlicensure data from the United Kingdom indicated that the percentages of subjects with rSBA titers ≥1:8 were more consistent with observed effectiveness than the percentages with titers ≥1:128.70 Accordingly, rSBA titers ≥1:8 have been widely accepted as correlate of protection for MenC conjugate vaccines.71
Although studies have generally found that higher titers are measured by rSBA compared with hSBA assays, reasonable correlations between rSBA and hSBA titers have been shown for MenC vaccines.68,72 However, the licensure of MenACWY-TT was primarily based on rSBA assay results, although some hSBA assay data were included.73 Data, including that from postlicensure effectiveness studies, support the use of rSBA for this vaccine,73–75 and data from recent MenACWY-TT clinical studies suggest that hSBA assays may be less relevant.76–78
The strengths of our review include the large number of studies, with >4000 subjects, and the range of age groups assessed. However, our review was limited to comparing SBA assay results within studies. As aspects of the protocols varied, comparison of SBA assay results across different laboratories is difficult. Even when a standardized method exists, such as the widely adopted rSBA assay standard for serogroups A and C published in 1997, interlaboratory variation remains significant.79 Additionally, this review did not consider the immunogenicity of the other serogroups in the vaccine (i.e., A, W, and Y). In the included studies, robust immune responses to serogroups A, W, and Y were observed following primary MenACWY-TT vaccination;60–64,67 similar to the serogroup C response, rSBA and hSBA antibody levels against A, W, and Y declined during the post-primary period and responded strongly post-booster.60,65 Differences in immune responses based on 1- versus 2-dose schedules were also not considered. Of note, MenACWY-TT was chosen for this comparison because of the availability of clinical data across age groups; however, this review did not consider comparative MenC responses to other quadrivalent vaccines.
Only short-term MenC antibody responses were considered in this current analysis. However, long-term antibody persistence following MenACWY-TT and MenC-CRM vaccination has been recently reported.80,81 Ten years after primary vaccination with 1 dose of MenACWY-TT or MenC-CRM as toddlers,67 57% of MenACWY-TT recipients and 86% of MenC-CRM recipients had MenC rSBA titers ≥1:8.81 In the same study, subjects with a suboptimal serogroup C response to primary MenACWY-TT or MenC-CRM vaccination received a booster dose of MenC-CRM by Year 5; in these subjects, percentages with MenC rSBA titers ≥1:8 at Year 10 were 98% and 90%, respectively. Overall, the percentage of subjects with a MenC rSBA titer ≥1:8 at Year 10 was 83% in the MenACWY-TT primary vaccine group and 88% in the MenC-CRM primary vaccine group. Notably, all 4 sub-groups showed a robust response to booster vaccination with MenACWY-TT at Year 10, with 100% of subjects having a MenC rSBA titer ≥1:8 at 1 month post-booster. In another persistence study,80 6 years after booster vaccination of 5-year-old children with MenACWY-TT or MenC-CRM65 (i.e., 10 years after primary vaccination with the same vaccine60), 72% of MenACWY-TT recipients and 65% of MenC-CRM recipients had MenC rSBA titers ≥1:8.80
In conclusion, MenC immune responses induced by MenACWY-TT are robust and generally comparable to monovalent MenC conjugate vaccines, supporting changes from monovalent MenC to MenACWY vaccination recommendations. The broad serogroup protection provided by MenACWY-TT, as well as its licensure from 6 weeks of age, together suggest that MenACWY-TT is a suitable option to provide protection against many of the common disease-causing meningococcal serogroups across at-risk age-based populations.82,83
Acknowledgments
Editorial/medical writing support was provided by Tricia Newell, PhD, at ICON plc (North Wales, PA) and was funded by Pfizer Inc.
Funding Statement
This study was sponsored by Pfizer Inc.
Disclosure of potential conflicts of interest
LS, JF, and KY are employees of Pfizer Inc and may have stock or stock options.
MK is a principal investigator for clinical studies and has been an advisor to and made presentations during industry symposia for GSK, Pfizer Inc, Baxter, Novartis, AstraZeneca, MedImmune, SPMSD, Sanofi, MSD, and Jansen.
FM-T has received compensation from GSK, Pfizer Inc, Sanofi Pasteur, MSD, Seqirus, and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. FM-T has also acted as principal investigator in randomized controlled trials of the above-mentioned companies as well as Ablynx, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution. FM-T received support for research activities from the Instituto de Salud Carlos III (Proyecto de Investigación en Salud, Acción Estratégica en Salud): Fondo de Investigación Sanitaria (FIS; PI070069/PI1000540/PI1601569/PI1901090) del plan nacional de I+D+I and ‘fondos FEDER’, and 2016-PG071 Consolidación e Estructuración REDES 2016GI-1344 G3VIP (Grupo Gallego de Genética Vacunas Infecciones y Pediatría, ED341D R2016/021).
References
- 1.European Centre for Disease Prevention and Control . Fact sheet about meningococcal disease. [accessed 2020. January 13] https://www.ecdc.europa.eu/en/meningococcal-disease/factsheet.
- 2.Pace D, Pollard AJ.. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(suppl 2):B3–B9. doi: 10.1016/j.vaccine.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 3.Stinson C, Burman C, Presa J, Abalos M.. Atypical presentation of invasive meningococcal disease caused by serogroup W meningococci. Epidemiol Infect. 2020;148:e12. doi: 10.1017/S0950268819002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59(2 Suppl):S12–20. doi: 10.1016/j.jadohealth.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meer HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. [accessed 2020 July 2]. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm [PubMed] [Google Scholar]
- 6.World Health Organization . Immunization standards: meningococcal A conjugate 10 dose presentation. World Health Organization. [accessed 2020. May 28] https://www.who.int/immunization_standards/vaccine_quality/PQ_197_MenAconjugate_10dose_SII/en/
- 7.MenAfriVac (meningococcal A conjugate vaccine) . Full Prescribing Information. Pune (India): Serum Institute of India; 2015.
- 8.Menjugate 10 micrograms suspension for injection (Meningococcal group C conjugate vaccine) . Summary of Product Characteristics, Siena (Italy): GSK Vaccines S.r.l.; 2018.
- 9.Meningitec (meningococcal serogroup C conjugate vaccine) . Full Prescribing Information. West Ryde (NSW, Australia): Pfizer Australia Pty Ltd; 2011. [Google Scholar]
- 10.Serogroup C meningococcal conjugate vaccines: new preparations. Effective from age two months. Prescrire Int. 2003;12(64):43–46. [PubMed] [Google Scholar]
- 11.NeisVac-C (Meningococcal Group C Polysaccharide Conjugate Vaccine Adsorbed) . Summary of Product Characteristics. Sandwich (Kent, UK): Pfizer Limited; 2019. [Google Scholar]
- 12.Bexsero (Meningococcal group B Vaccine [rDNA, component, adsorbed]) . Summary of Product Characteristics. Uxbridge (Middlesex, UK): GlaxoSmithKline UK; 2019. [Google Scholar]
- 13.Trumenba (MenB-FHbp) . Summary of Product Characteristics. Sandwich (Kent, UK): Pfizer Ltd; 2018. [Google Scholar]
- 14.MENHIBRIX (Hib-MenCY-TT) . Full Prescribing Information. Rixensart (Belgium): GlaxoSmithKline; 2013. [Google Scholar]
- 15.Breton MC, Huang L, Snedecor SJ, Cornelio N, Fanton-Aita F. Cost-effectiveness of alternative strategies for vaccination of adolescents against serogroup B IMD with the MenB-FHbp vaccine in Canada. Can J Public Health. 2020;111(2):182–92. doi: 10.17269/s41997-019-00275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIMENRIX . Summary of Product Characteristics. Sandwich (Kent, UK): Pfizer Limited; 2020. [Google Scholar]
- 17.Menveo (Meningococcal Group A, C, W135 and Y conjugate vaccine) . Summary of Product Characteristics. Sovicille (Italy): GSK vaccines S.r.l.; 2014. [Google Scholar]
- 18.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MenQuadfi (MenACWY-TT) . Full Prescribing Information. Swiftwater (PA): Sanofi Pasteur Inc; 2020. [Google Scholar]
- 20.Purmohamad A, Abasi E, Azimi T, Hosseini S, Safari H, Nasiri MJ, Imani Fooladi AA. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;134:103571. doi: 10.1016/j.micpath.2019.103571. [DOI] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control . Prevention and control measures for meningococcal disease. [accessed 2020. April 3] https://www.ecdc.europa.eu/en/meningococcal-disease/prevention-and-control
- 22.Rivero-Calle I, Raguindin PF, Gomez-Rial J, Rodriguez-Tenreiro C. Meningococcal group. B vaccine for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroup B. Infect Drug Resist. 2019;12:3169–88. doi: 10.2147/IDR.S159952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy PC, Sharyan A, Sheikhi Moghaddam L. Meningococcal vaccines: current status and emerging strategies. Vaccines (Basel). 2018;6(1). doi: 10.3390/vaccines6010012. Epub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(suppl 2):S3–S11. doi: 10.1016/j.jadohealth.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, Bai X, Lucidarme J, Borrow R, Ramsay ME. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–17. doi: 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- 26.Martinon-Torres F, Serra L, Safadi MAP. Protecting the most vulnerable age group: a review of MenACWY-TT immunogenicity and safety in infants. Expert Rev Vaccines. 2020:1–13. doi: 10.1080/14760584.2020.1745070. [DOI] [PubMed] [Google Scholar]
- 27.Parikh S, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–98. doi: 10.1016/j.jinf.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 28.Whittaker R, Dias JG, Ramliden M, Kodmon C, Economopoulou A, Beer N, Pastore Celentano L, Kanitz E, Richter L, Mattheus W. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine. 2017;35(16):2034–41. doi: 10.1016/j.vaccine.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31(1):101–07. doi: 10.1111/j.1574-6976.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 30.European Centre for Disease Prevention and Control . Disease data from ECDC surveillance atlas for meningococcal disease. [accessed 2019. March 20] https://ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas.
- 31.Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15(2):470–80. doi: 10.1080/21645515.2018.1532248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skoczynska A, Wasko I, Kuch A, Ronkiewicz P, Golebiewska A, Wrobel I, Kiedrowska M, Hryniewicz W. Invasive Meningococcal disease in Poland [PO-004-13211]. Paper presented at: 15th Congress of the, European Meninogococcal and Haemophilus Disease Society; 2019. May 27-30;Lisbon (Portugal). [Google Scholar]
- 33.Wasko I, Golebiewska A, Kiedrowska M, Ronkiewicz P, Wrobel I, Kuch A, Skoczynska A.. Emerging meningococci representing ST-9316 in Poland. Paper presented at: 15th Congress of the EMGM European Meninogococcal and Haemophilus Disease Society; 2019. May 27- 30; Lisbon (Portugal). [Google Scholar]
- 34.Reinsert Skoczynska A, Wasko I, Kuch A, Ronkiewicz P, Golebiewska A, Wrobel I, Kiedrowska M, Hryniewicz W . Current status of serogroup W meningococcal disease in Poland [OC-13214]. Paper presented at: 15th Congress of the European Meninogococcal and Haemophilus Disease Society; 2019. May 27-30; Lisbon, Portugal. [Google Scholar]
- 35.Skoczynska A, Wasko I, Kuch A, Ronkiewicz P, Golebiewska A, Wrobel I, Kiedrowska M, Hryniewicz W . Current status of serogroup W meningococcal disease in Poland [OC-13214]. Paper presented at: 15th Congress of the European Meninogococcal and Haemophilus Disease Society: 2019. May 27–30; Lisbon, Portugal. [Google Scholar]
- 36.Deghmane A, Hong E, Taha M-K. The emergence of a new genetic lineage (ST-9316) of Neisseria meningitidis serogroup W in North France. Paper presented at: 15th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society; 2019. May 27-30; Lisbon, Portugal. [Google Scholar]
- 37.Fazio C, Neri A, Renna G, Vacca P, Antonetti R, Barbui AM, Daprai L, Lanzafame P, Rossi L, Santino I, et al. Persistent occurrence of serogroup Y/sequence type (ST)-23 complex invasive meningococcal disease among patients aged five to 14 years, Italy, 2007 to 2013. Eurosurveillance. 2015;20(45):30061. 10.2807/1560-7917.ES.2015.20.45.30061. [DOI] [PubMed] [Google Scholar]
- 38.Broker M, Emonet S, Fazio C, Jacobsson S, Koliou M, Kuusi M, Pace D, Paragi M, Pysik A, Simoes MJ, et al. Meningococcal serogroup Y disease in Europe: continuation of high importance in some European regions in 2013. Hum Vaccin Immunother. 2015;11(9):2281–86. doi: 10.1080/21645515.2015.1051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Public Health England . Meningococcal ACWY conjugate vaccination (MenACWY). [accessed 2020. July 2] https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/437901/150622_ACWY_bipartite_letter.pdf.
- 40.Knol MJ, Ruijs WL, Antonise-Kamp L, de Melker HE, van der Ende A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Euro Surveill. 2018;23(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signorelli C, Guerra R, Siliquini R, Ricciardi W. Italy’s response to vaccine hesitancy: an innovative and cost effective national immunization plan based on scientific evidence. Vaccine. 2017;35(33):4057–59. doi: 10.1016/j.vaccine.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Ministero della Salute Italia . Piano Nazionale Prevenzione Vaccinale: PNPV 2017-2019. Rome (Italy): Ministero della Salute Italia; 2017. [Google Scholar]
- 43.Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8(3):307–33. doi: 10.1007/s40121-019-0254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Health Protection Surveillance Centre . Bacterial Meningitis/Meningococcal disease. [accessed 2020. March 12] http://www.hse.ie/eng.
- 45.Spanish Ministry of Health . Recomendaciones de vacunación frente a la enfermedad meningocócica invasiva. [accessed 2019. July 12] https://www.mscbs.gob.es/en/profesionales/saludPublica/prevPromocion/vacunaciones/docs/Recomendaciones_Vacunacion_Meningococo.pdf.
- 46.Leon JDCY. Actualización del calendario de vacunación infantil 2019 vacunación frente a meningococo. 2018.
- 47.Sáfadi MA, McIntosh EDG, Safadi MA, McIntosh ED. Epidemiology and prevention of meningococcal disease: a critical appraisal of vaccine policies. Expert Rev Vaccines. 2011;10(12):1717–30. doi: 10.1586/erv.11.159. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization . WHO vaccine-preventable diseases: monitoring system. 2019 global summary. [accessed 2019. October 15] http://apps.who.int/immunization_monitoring/globalsummary/schedules.
- 49.Superior Health Council Belgium . Advisory 9485 meningococcal vaccination. [accessed 2020. January 28] https://www.health.belgium.be/nl/advies-9485-vaccinatie-tegen-meningokokken.
- 50.Diana A, Iten A, Landry P, Bouvier Gallacchi M. [Update of the 2019 Swiss immunization schedule 7 new recommendations and review of practical implications for health professionals]. Rev Med Suisse. 2019;15:1521–25. [PubMed] [Google Scholar]
- 51.Federal Ministry of Health (Austria) . Impfplan Österreich 2012.
- 52.ECDC Vaccine Scheduler . Vaccine schedules in all countries in the European Union. Czech Republic: Recommended vaccinations. [accessed 2020. May 27] https://vaccine-schedule.ecdc.europa.eu/.
- 53.Ministers Department of Health . Quad-strain meningococcal vaccine to be added to national immunisation program. [accessed 2019. June 3] http://www.health.gov.au/internet/ministers/publishing.nsf/Content/97678EE0888F2877CA258227007CCBFF/$File/GH016.pdf.
- 54.Australian Government Department of Health . National immunisation program. [accessed 2020. May 27] https://www.health.gov.au/initiatives-and-programs/national-immunisation-program.
- 55.New Zealand Ministry of Health . Immunisation Handbook. 2017. 2nd March 2018. Wellington, New Zealand: Ministry of Health. 2018. [Google Scholar]
- 56.New Zealand Ministry of Health . Meningococcal vaccines: eligibility, recommendations and supply. [accessed 2020. May 27] https://www.health.govt.nz/our-work/diseases-and-conditions/meningococcal/meningococcal-vaccines-eligibility-recommendations-and-supply.
- 57.Ministerio de Salud (Republica Argentina) . Fundamentos de la introducción de la vacuna tetravalente (ACYW) conjugada contra meningococo al Calendario Nacional de Inmunizaciones. [accessed 2019. June 3] http://www.msal.gob.ar/images/stories/bes/graficos/0000000927cnt-2016-12_lineamientos-meningo.pdf.
- 58.Salvadori M, Bortolussi R. Meningococcal vaccines in Canada: an update. Paediatr Child Health. 2011;16(8):485–86. doi: 10.1093/pch/16.8.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Government of Canada . Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada. [accessed 2020. May 27] https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html.
- 60.Government of Malta . National immunisation schedule (Immunisation schedule for ages 0-16 years). [accessed 2020. July 21] https://deputyprimeminister.gov.mt/en/phc/pchyhi/Pages/National-Immunisation-Schedule.aspx.
- 61.Programa Nacional de Imunizações. Informe Técnico . Orientações técnico-operacionais para a Vacinação dos Adolescentes com a Vacina Meningocócica ACWY (conjugada). 2020.
- 62.Merino Arribas JM, Carmona Martinez A, Horn M, Perez Porcuna XM, Otero Reigada MD, Mares Bermudez J, Centeno Malfaz F, Miranda M, Mendez M, Garcia Cabezas MA, et al. Safety and immunogenicity of the quadrivalent meningococcal serogroups A, C, W and Y tetanus toxoid conjugate vaccine coadministered with routine childhood vaccines in European infants: an open, randomized trial. Pediatr Infect Dis J. 2017;36(4):e98–e107. doi: 10.1097/INF.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 63.Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: an open, randomized controlled trial. Vaccine. 2011;29(25):4274–84. doi: 10.1016/j.vaccine.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 64.Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, Poolman J, Boutriau D. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine. 2010;28(3):744–53. doi: 10.1016/j.vaccine.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 65.Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine. 2011;29(25):4264–73. doi: 10.1016/j.vaccine.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Knuf M, Romain O, Kindler K, Walther U, Tran PM, Pankow-Culot H, Fischbach T, Kieninger-Baum D, Bianco V, Baine Y, et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2-10-year-old children: results of an open, randomised, controlled study. Eur J Pediatr. 2013;172(5):601–12. doi: 10.1007/s00431-012-1924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34(12):e298–307. doi: 10.1097/INF.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 68.van Ravenhorst MB, van der Klis FRM, van Rooijen DM, Sanders EAM, Berbers GAM. Adolescent meningococcal serogroup A, W and Y immune responses following immunization with quadrivalent meningococcal A, C, W and Y conjugate vaccine: optimal age for vaccination. Vaccine. 2017;35(36):4753–60. doi: 10.1016/j.vaccine.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–903. doi: 10.4161/hv.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borrow R, Andrews N, Goldblatt D, Miller E, Burns DL. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69(3):1568–73. doi: 10.1128/IAI.69.3.1568-1573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(suppl 1):S58–67. doi: 10.1016/S0264-410X(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 72.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–86. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 74.Gill CJ, Ram S, Welsch JA, Detora L, Anemona A. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine. 2011;30(1):29–34. doi: 10.1016/j.vaccine.2011.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.European Medicines Agency . Assessment report: Nimenrix. [accessed 2018. October 23] https://www.ema.europa.eu/documents/assessment-report/nimenrix-epar-public-assessment-report_en.pdf.
- 76.Campbell H, Edelstein M, Andrews N, Borrow R, Ramsay M, Ladhani S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015-2016. Emerg Infect Dis. 2017;23(7):1184–87. doi: 10.3201/eid2307.170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villena R, Santolaya ME.. Chilean experience with serogroup W outbreak and meningococcal ACWY conjugate vaccines. Paper presented at: 14th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society; 2017. September 18–21;Prague, Czech Republic. [Google Scholar]
- 78.Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence to meningococcal serogroups A, C, W and Y in toddlers two years after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate (MenACWY-TT) vaccine as measured by bactericidal antibody assays using rabbit or human complement. Trials Vaccinology. 2014;3:121–26. [Google Scholar]
- 79.Cutland CL, Nolan T, Halperin SA, Kurugol Z, Ahmed K, Perrett KP, Richmond P, Marshall HS, Ceyhan M, Kolhe D, et al. Immunogenicity and safety of one or two doses of the quadrivalent meningococcal vaccine MenACWY-TT given alone or with the 13-valent pneumococcal conjugate vaccine in toddlers: a phase III, open-label, randomised study. Vaccine. 2018;36(14):1908–16. doi: 10.1016/j.vaccine.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 80.Dbaibo G, Tinoco Favila JC, Traskine M, Jastorff A, Van der Wielen M. Van der Wielen M. Immunogenicity and safety of MenACWY-TT, a meningococcal conjugate vaccine, co-administered with routine childhood vaccine in healthy infants: a phase III, randomized study. Vaccine. 2018;36(28):4102–11. doi: 10.1016/j.vaccine.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 81.Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4(2):156–67. doi: 10.1128/CDLI.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vesikari T, Forsten A, Laudat F, Li P, Van Der Wielen M, Hezareh M, Perez JL, Webber C. Long-term antibody persistence after a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in healthy 5-year-old children. Vaccine. 2020;38(22):3902–08. doi: 10.1016/j.vaccine.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 83.Vesikari T, Peyrani P, Webber C, Van Der Wielen M, Cheuvart B, De Schrevel N, Aris E, Cutler M, Li P, Perez JL. Ten-year antibody persistence and booster response to MenACWY-TT vaccine after primary vaccination at 1-10 years of age. Hum Vaccin Immunother. 2020;16(6):1280–91. doi: 10.1080/21645515.2020.1746110. [DOI] [PMC free article] [PubMed] [Google Scholar]


