Abstract
Epstein-Barr virus (EBV) normally infects B cells, but in some persons the virus infects T or NK cells. Infection of B cells can result in infectious mononucleosis, and the virus is associated with several B cell malignancies including Hodgkin lymphoma, Burkitt lymphoma, and diffuse large B cell lymphoma. Infection of T or NK cells with EBV is associated with extranodal NK/T cell lymphoma, aggressive NK-cell leukemia, systemic EBV-associated T-cell lymphoma, and chronic active EBV disease, which in some cases can include hydroa vacciniforme-like lymphoproliferative disease and severe mosquito bite allergy. While NK and T cell lymphoproliferative disease is more common in Asia and Latin America, increasing numbers of cases are being reported from the United States and Europe. This review focuses on classification, clinical findings, pathogenesis, and recent genetic advances in NK and T cell lymphoproliferative diseases associated with EBV.
Keywords: Aggressive NK-cell leukemia, chronic active EBV disease, extranodal NK/T cell lymphoma, hydroa vacciniforme-like lymphoproliferative disease, severe mosquito bite allergy, systemic EBV-positive T-cell lymphoma of childhood
Introduction
While Epstein-Barr virus (EBV) typically infects oropharyngeal epithelial cells and B cells, the virus has been reported to infect other cell types including T cells in healthy African children [1] and rare T and NK cells in the tonsils [2]. Persons with HIV [3] and various lymphoproliferative disorders (LPD) [4] can have peripheral blood T cells infected with EBV. A variety of EBV NK and T cell disorders have been described ranging from LPD to lymphoma and leukemia. These disorders are more common in Asia and Latin America, but also have been reported in the United States and Europe. They can differ widely in their prognosis. Recent studies have identified somatic mutations in some patients with these diseases.
On 28 July 2018 a meeting was held at the University of Wisconsin to update knowledge on EBV NK and T cell LPD. This review summarizes recent findings of these diseases with an emphasis on classification, differences in disease based on geography, and new findings of the genetics of the diseases.
Classification of EBV NK and T cell LPDs
Elaine S. Jaffe, MD, (National Cancer Institute), reported on classification of EBV LPD. B cells are the primary lymphoid target of EBV infection, and most EBV-related lymphomas and LPDs are of B-cell lineage. EBV-positive T-cell and NK-cell disorders are much less common and exhibit distinct clinical and epidemiological features. In the 2001 WHO classification of Tumors of the Hematopoietic and Lymphoid Tissues the following T/NK-cell disorders were recognized as being almost universally associated with EBV: aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma (ENKTL), nasal type, and a less well-defined spectrum of disorders seen most often in children, collectively referred to as EBV-positive T-cell LPD of childhood [5]. This latter group has seen significant evolution in our thinking, with noteworthy revisions included in the latest revision of the WHO classification published in 2017 [6] (Table 1).
Table 1.
Classification of Epstein-Barr virus NK and T cell lymphoproliferative disease.
| Disease | Clinical features | Immunophenotype | Prognosis |
|---|---|---|---|
| EBV positive HLH | HLH with fever, splenomegaly, cytopenias, elevated ferritin; often CNS abnormalities, elevated liver function tests | Activated CD8+, TIA1+ and CD56+ | HLH associated with genetic disease or CAEBV usually requires HSCT |
| Aggressive NK cell leukemia | Fever, hepatosplenomegaly; often lymphadenopathy, may have HLH, coagulopathy, multiorgan failure | CD3c+, CD56+, usually CD16+, TIA1+ | Fulminant disease, <2 year survival |
| Extranodal NK/T cell lymphoma, nasal type | Destructive lesion of nose and middle of face, epistaxis, nasal obstruction; may extend to sinuses, nasopharynx, orbit | CD3c+, CD56+, CD16−, TIA1+ | Disease localized to nose has good prognosis; extra-nasal disease has poor prognosis |
| Systemic chronic active EBV, T and NK cell types | Fever, lymphadenopathy, hepatosplenomegaly; often HLH, liver failure | T cell type: CD3+, CD56−; NK cell type: CD3c+, CD56+ | Indolent, high risk of progression to fulminant disease |
| Cutaneous chronic active EBV: Hydroa vacciniforme-like LPD | Papulovesicular rash with ulceration and scarring; classic form with disease localized to the skin; systemic form with fever, lymphadenopathy, splenomegaly | CD3+, usually CD8+, αβ > γδ, some CD56+, | Classic form-good prognosis may resolve; Systemic form-poor prognosis |
| Cutaneous chronic active EBV: Severe mosquito bite allergy | Fever, ulceration, necrosis, scarring | CD56+, TIA1+ | Indolent with progression to CAEBV, HLH, aggressive NK cell leukemia |
| Systemic EBV-positive T-cell lymphoma of childhood | Fever, then hepatosplenomegaly, liver failure; often lymphadenopathy, HLH, pancytopenia | CD3+, CD56−, TIA1+ | Fulminant, death usually within weeks of diagnosis |
HLH: hemophagocytic lymphohistiocytosis; CD3c+: cytoplasmic CD3 (positive in NK cells which are negative for surface CD3); HSCT: hematopoietic stem cell transplant; LPD: lymphoproliferative disorder: CAEBV: chronic active EBV.
Aggressive NK-cell leukemia is a systemic neoplastic proliferation of NK cells, nearly always associated with EBV, and associated with an aggressive clinical course [7,8]. Most patients have peripheral blood involvement with systemic manifestations involving the liver, spleen, bone marrow, and skin. As such, the term aggressive NK-cell leukemia/lymphoma has been used by some authors. Most patients are young to middle-aged, with a median age of 40. The disease is more prevalent among Asians, but sporadic cases occur on a worldwide basis in all races. Rare EBV-negative cases have been reported [9,10]. It had been suggested that EBV-negative cases might have a less aggressive clinical course [11,12], but more recent data have shown a universally aggressive course, with median survival of less than six months, and often a matter of weeks [10]. The clinical course is frequently complicated by coagulopathy and hemophagocytic syndrome. Recurrent genetic aberrations have been reported, with some differences from those seen in ENKTL, nasal type [13].
ENKTL was the first of the T/NK-cell lesions to be linked to EBV, and the first defined as a distinct entity [14]. It is more prevalent in Asians, and in indigenous populations of Mexico, Central and South America. It is always extranodal in presentation, with preferential sites of involvement including upper aerodigestive tract, skin, gastrointestinal tract, and testes [15,16]. There is considerable variation in cytological features, with some historical series suggesting that tumors composed of small cells have a better outcome [17]. However, stage is a more significant prognostic feature; patients with advanced stage disease (Stage III/IV) have an unfavorable clinical course.
More significant differences have occurred in the classification of EBV-associated T-cell and NK-cell lesions seen predominantly in the pediatric age group. These range from EBV-associated hemophagocytic lymphohistiocytosis (HLH), to systemic EBV-positive T-cell lymphoma of childhood. Diseases with intermediate prognosis include the systemic and cutaneous forms of chronic active EBV disease (CAEBV). All of these disorders share similar epidemiological features, being more common in Asians and Hispanic populations of indigenous origin.
EBV-HLH is seen mainly following acute EBV infection [18,19]. These patients present with the clinical stigmata of HLH and have high EBV viral loads. In situ staining of bone marrow, liver, or spleen for EBV encoded RNA (EBER), shows increased virus-positive lymphocytes, many of which are T or NK cells. Therapeutic intervention with an HLH-directed treatment protocol may lead to remission of disease, and if the EBV viral load remains low, complete remission may be sustained. However, recurrence of symptoms beyond several months may lead to a picture of systemic CAEBV infection.
CAEBV was originally defined as a systemic EBV-positive LPD characterized by fever, lymphadenopathy, and splenomegaly developing after primary virus infection in patients without known immunodeficiency [20,21]. Affected patients have elevated EBV DNA in the blood, and histological evidence of organ damage by EBV-positive lymphocytes infiltrating the tissues (Figure 1(A–C)). The original diagnosis required persistence of symptoms for six months or more, but recent proposals have reduced that requirement to three months. The original concept envisioned that the affected lymphoid cell type was a B cell, but in recent years CAEBV is now considered mainly a disease of persistent EBV infection of T or NK cells [8,22,23]. Many patients with persistence of EBV in B cells upon genomic testing are shown to have an underlying immune deficiency disorder leading to failure of the immune system to fully handle EBV [24].
Figure 1.
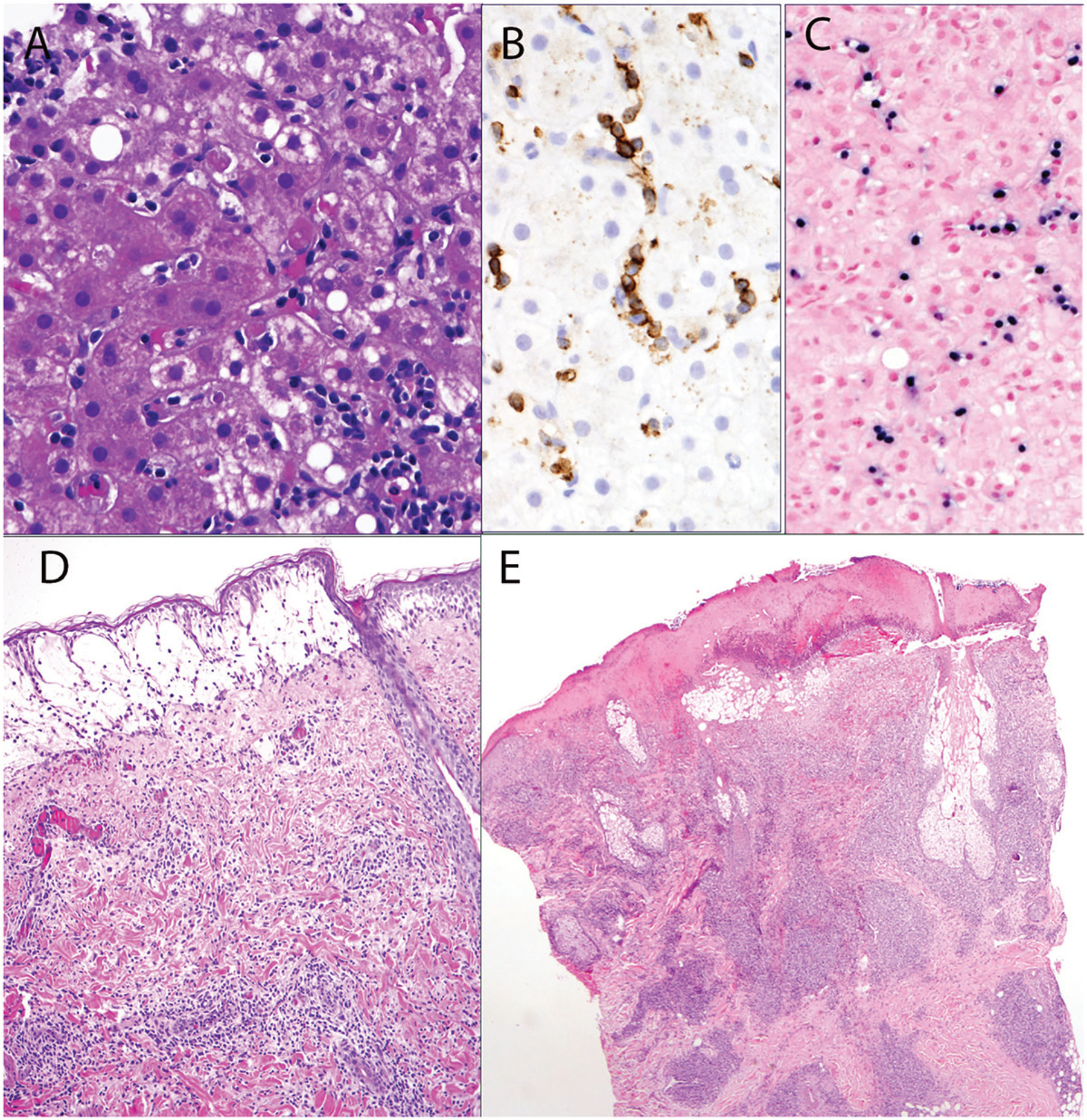
Chronic active EBV-infection of T-cell type. (A) Liver biopsy from a 23-year-old Hispanic female. A sinusoidal lymphoid infiltrate with focal single cell hepatocellular necrosis is present. (B) Infiltrating cells are positive for CD3. (C) Sinusoidal lymphocytes are positive for EBER by situ hybridization. (D) and (E) illustrate skin lesions from patients with hydroa vacciniforme-like lymphoproliferative disorder. (D) Superficial vesicle with patchy perivascular lymphoid infiltrate. (E) Skin biopsy shows more advanced lesion with necrosis of the overlying epidermis, and more dense dermal lymphoid infiltrate.
In the WHO classification, CAEBV is divided into two major forms: systemic CAEBV and cutaneous CAEBV; overlap occurs with some patients with cutaneous CAEBV exhibiting clinical and pathological evidence of systemic EBV infection [6]. Generally, patients with cutaneous forms of CAEBV have milder disease. The two main forms are hydroa vacciniforme-like LPD (HVLPD) and severe mosquito bite allergy (SMBA) [25–29]. The former is most often a result of EBV infection of T cells, which may be of αβ or γδ T cell derivation, while SMBA is usually a consequence of EBV-infection of NK cells.
The WHO classification formerly had a category of HV-like lymphoma [5]. However, currently HVLPD is viewed as a continuum ranging from very benign and self-limited disease in many patients, to more clinically aggressive EBV-associated disease (Figure 1(D,E)), such that a distinction between HV and HV-like lymphoma is not reliable [26]. Moreover, HV as originally reported was considered a benign photo-dermatitis presenting in childhood after sun exposure, leading to a vesicular eruption that healed after ulceration and crust formation. In the original reports, an association with EBV was not known. For some time, it was presumed that both EBV-positive and EBV-negative cases of HV existed. Recent data suggest that an EBV-negative form of HV probably does not occur, and thus all cases have a common link to EBV, with variations in the severity of the illness and the risk for systemic disease. Thus, HVLPD encompasses the full spectrum of clinical manifestations [30].
The last category of EBV-associated T-cell lymphoma in the revised WHO classification is systemic EBV-positive T-cell lymphoma of childhood (Figure 2). This disease was previously included under the term ‘systemic EBV-positive T-cell LPD of childhood’ [31]. That category was heterogeneous, and encompassed cases of systemic CAEBV and the more aggressive and often fulminant T-cell lymphomas [32]. Additionally, the WHO classification recognizes rare forms of EBV-positive peripheral T-cell lymphoma, often presenting with nodal disease [33]. These lesions are currently considered an EBV-positive variant of peripheral T-cell lymphoma, not otherwise specified. Data are limited about these cases, but at least some occur in a setting of decreased immune surveillance, including HIV infection and advanced age.
Figure 2.
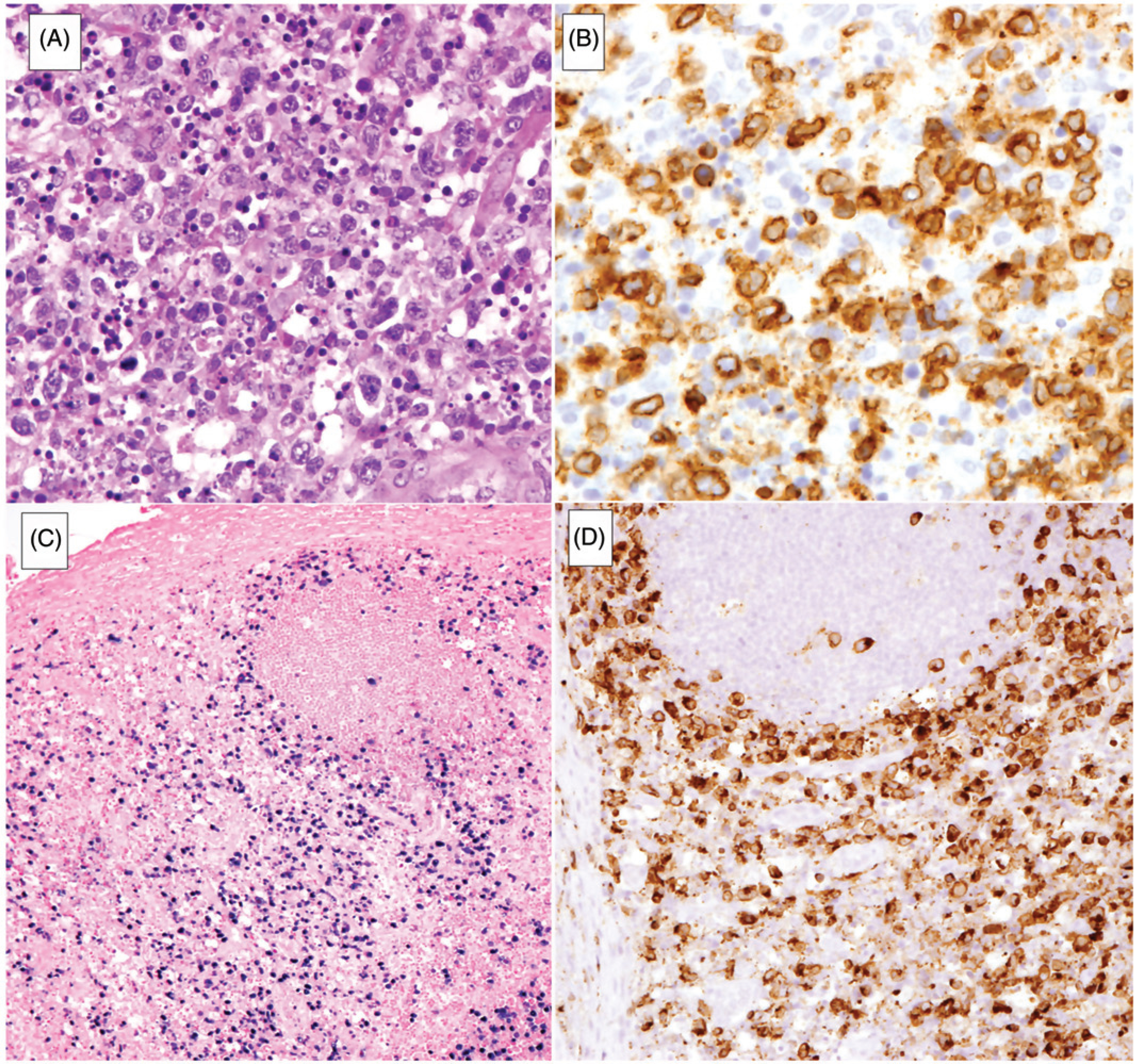
Systemic EBV + T-cell lymphoma of childhood. (A) Lymph node is diffusely infiltrated by atypical lymphoid cells with prominent single-cell necrosis and histiocytic reaction. (B) Atypical lymphoid cells are positive for CD3. (C) EBER in situ hybridization highlights the atypical cells which infiltrate the paracortex, sparing focal reactive follicles. (D) Neoplastic cells are positive for perforin.
EBV T/NK cell LPD in Asia
The frequency of EBV-positive LPD varies among countries in Asia. ENKTL, nasal-type, accounts for 15% of all cases of non-Hodgkin lymphoma in the southwest region of China, 6.1% in Korea, 2.6% in Japan, and 2.8% in Taiwan [34–37]. Among EBV-positive NK/T cell LPDs, ENKTL, nasal-type was the most common subtype in a Korean study, accounting for 83% of 107 EBV-positive T- or NK-cell type non-Hodgkin lymphoma cases, followed by aggressive NK-cell leukemia (8.4%) [38].
Young Hyeh Ko, MD (Sumsung Medical Center) reviewed a Korean series of EBV-positive T/NK cell LPDs [39]. HLH accounted for 26% of cases, CAEBV for 31%, systemic unclassifiable disease for 24%, and HVLPD for 19%. In patients with CAEBV in the Korean series, the onset of disease was between 2.2 and 42.8 years of age (median 15.9 years). Clinical findings included fever (92%), hepatosplenomegaly (68%), lymphadenopathy (52%), NK lymphocytosis (42%), skin lesions (54%), SMBA (28%), HVLPD-like rash (8%), and pneumonia (17%). Some patients presented with bowel perforation, IgA nephropathy, chorea, or stroke. At a mean follow-up of 25 months, 10 of 25 patients (40%) died of the disease. The causes of death were infection, organ failure, and EBV-positive lymphoma (the latter in 25% of patients) [8,39–41]. HLH was found in 25 to 60% of cases with T cell disease more commonly than NK cell disease; monoclonality was detected in 6 of 15 T cell disease cases (40%) [8,39–41]. The mean survival was 92 months.
In the Korean series, patients with HVLPD were divided into three groups. The first group was classic HVLPD without systemic symptoms and presented in childhood (n = 10). The onset of the disease was between 1 to 11 years of age (median 6.5 years). The median period from disease onset to diagnosis was 2.5 years (range, 0 to 15 years). Sites involved were the face, scalp, forearm, hands, and chest. All patients showed photosensitivity either by positive reaction to UV provocation or symptoms induced with sun exposure. All five patients in whom the viral load in blood was tested had high levels of EBV. The follow-up period was 1 to 18 years (median 3.5 years). Most patients experienced chronic recurrent or sporadic eruptions associated with sun exposure and healing with scarring without complications. However, two patients with chronic recurrent cutaneous disease died of EBV-positive T cell lymphoma or developed systemic CAEBV with worsening of skin lesions at 15 and 16 years after initial presentation, respectively. The second group was classic HVLPD presenting in adolescence and adulthood (n = 4). The onset of the disease was 18–66 years (median 33.5 years). All patients had high viral loads in the blood. Three patients developed EBV-positive systemic or cutaneous T cell lymphoma at 3–19 years after initial presentation. The third group had systemic HVLPD associated with systemic symptoms (n = 4). The age at presentation ranged from 8 to 18 years (median 14.5 years). Two of four patients developed EBV-positive systemic T cell lymphoma 3 years after diagnosis. In summary, HVLPD in the Korean series was not confined to children and had diverse clinical features. Classic HVLPD was the most common type. HVLPD with early onset had a favorable prognosis, while systemic HVLPD had a poor prognosis [8,41–46].
Koichi Ohshima, MD, (Kurume University) and colleagues reported on a study of adult-onset CAEBV [47]. The median period from CAEBV disease onset to diagnosis was approximately 12–24 months (range, 3–120 months). The median age at diagnosis was 39 years (range, 16 to 86 years). Lymphadenopathy was significantly more frequent for the patients with T-cell CAEBV, whereas skin lesions were common with NK-cell CAEBV. Patients with adult-onset CAEBV had less fever and more frequently had skin lesions than those with pediatric-onset CAEBV. The adult-onset group had a lower frequency of SMBA and HVLPD, but a higher frequency of elevated serum liver enzymes and HLH [47]. As indicators of CAEBV disease prognosis, thrombocytopenia, EBV nuclear antigen (EBNA) antibody titer ≥40, and the presence of HLH at initial diagnosis were all associated with a poor prognosis. However, the type of infected cell was not a prognostic factor and there were no differences in prognosis among the three histological classifications [32].
Allogeneic hematopoietic stem cell transplantation (HSCT) was the most effective treatment for improving survival of the CAEBV patients. There was no difference in overall survival between patients ≥50 years and those <50 years of age. In a univariate analysis, age (>60 years), a high-risk Eastern Cooperative Oncology Group performance status, the type of infected cell, an elevated lactate dehydrogenase level, and the number of EBV DNA copies in the peripheral blood or EBER-positive cells per high-power field in tissue were not prognostic factors. Conversely, thrombocytopenia, a high EBNA titer, and not receiving HSCT were independent poor prognostic factors [47]. Patients with adult-onset CAEBV had a poorer overall survival than patients with pediatric-onset CAEBV and ENKTL, nasal type. However, the overall survival for patients with ENKTL, non-nasal-type and for those with adult-onset CAEBV was comparable. Patients with nodal EBV-positive peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) and those with adult-onset CAEBV with nodal lesions showed no statistical difference in prognosis or overall survival (Figure 3). Of the patients with adult-onset CAEBV in this study, 18 (33.3%) were diagnosed with a malignant lymphoma (aggressive NK cell leukemia, ENKTL, and EBV-positive PTCL-NOS) at the time of CAEBV diagnosis.
Figure 3.
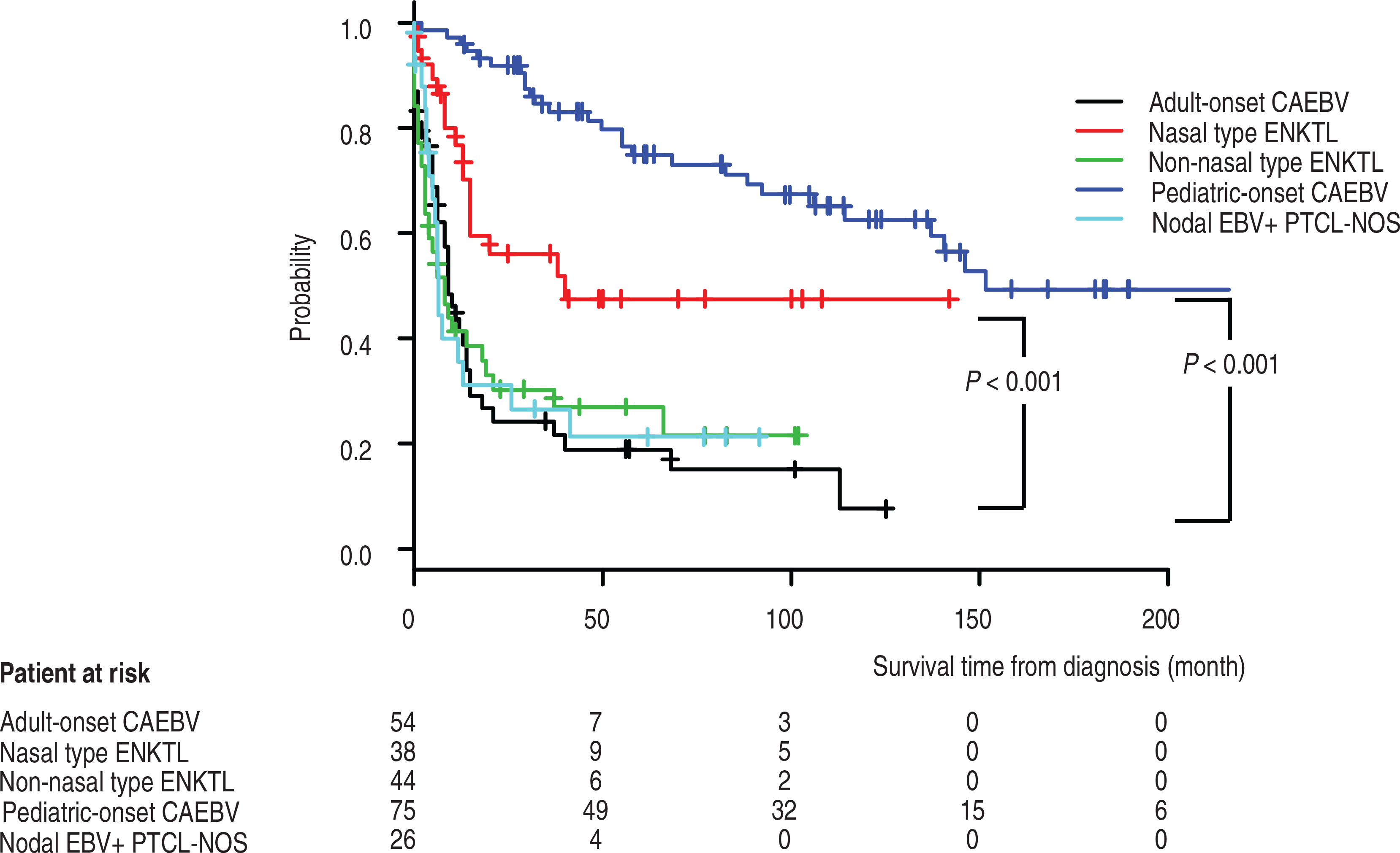
Comparison of overall survival of patients with NK- and T-cell lymphoproliferative diseases. Kaplan-Meier survival curves of patients with adult or pediatric onset chronic active EBV (CAEBV), extranodal NK T cell lymphoma (ENKTL), nasal or non-nasal type, and nodal EBV-positive peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). Significant differences in survival are seen for comparison of pediatric-onset with adult-onset CAEBV and with ENKTL, nasal-type with adult onset CAEBV (adapted from [47] with permission).
Keiji Iwatsuki, MD, (Okayama University) reported on a Japanese study [48] in which 50 patients were classified into four clinical subtypes: 1) classic HVLPD, 2) systemic HVLPD, 3) SMBA alone, and 4) SMBA with HVLPD (Figure 4) based on prior criteria [49]. Patients with classic HVLPD and SMBA have increased percentages of EBV-positive γδ cells (≥5% of lymphocytes) and EBV-positive NK cells (>30% of lymphocytes), respectively, in the peripheral blood [49]. Systemic HVLPD is further divided into two groups: γδ T-cell and αβ T-cell-dominant types; the former is observed in younger patients and shows a favorable prognosis, while the latter may occur in adults and often has a fatal outcome [48]. In the study, patients ranged in age from 1 to 74 years old. EBV DNA loads in peripheral blood mononuclear cells were elevated in the four patient groups (mean, 67,420 copies/ug of DNA) compared with healthy persons (<100 copies/ug of DNA), but the levels were not significantly different among the four groups. In the study, BZLF1 mRNA was detected in skin lesions of patients with systemic HVLPD, SMBA alone, and SMBA with HVLPD; however, BZLF1 mRNA was not detected in classic HVLPD. Thirty of the 50 patients in the study were included in a follow-up study; 37% of those with systemic HVLPD, SMBA alone, and SMBA with HVLPD died, while none with classic HV died although two patients progressed to CAEBV [50]. Poor prognostic indicators included the clinical subtypes of systemic HVLPD and SMBA, onset age over 9 years, expression of EBV BZLF1 mRNA in skin lesions, αβ T-cell-dominant HVLPD, and NK-cell-dominant SMBA [48,50].
Figure 4.
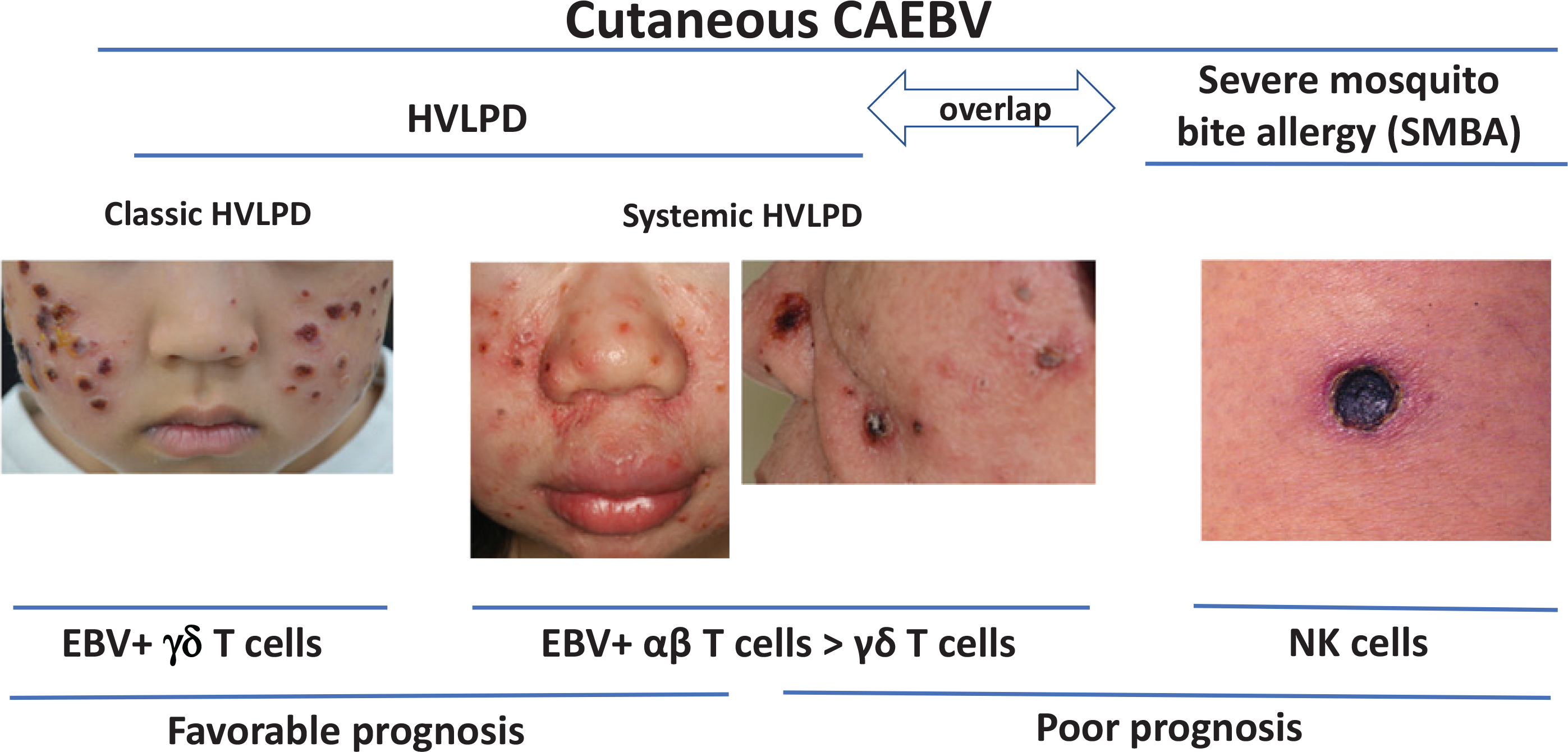
Photographs of patients and prognosis for cutaneous forms of chronic active EBV (CAEBV), including classic or systemic hydroa vacciniforme-like lymphoproliferative disease (HVLPD) and severe mosquito bite allergy (SMBA).
EBV T/NK cell LPD in Latin America
Leticia Quintanilla-Martinez, MD, (University Hospital Tübingen), reported that EBV-associated T and NK cell LPDs are prevalent in Latin American countries including Mexico, Guatemala, Peru, Ecuador and Bolivia, indicating a strong racial predisposition [51]. ENKTL, the prototype of the EBV-associated lymphomas represents around 23% of all T-cell lymphomas in Latin America (up to 40% in Mexico [52] and 66% in Guatemala [51]), compared with 4% to 5% in Europe and the United States [53]. The clinical presentation is similar in all geographic regions; however, patients in Latin America tend to present with more advanced clinical stages [52,54].
Systemic EBV T-cell lymphoma of childhood is a rare disorder prevalent in Taiwan [55,56] and Japan [7,57] with few cases reported in Korea [41] and Mexico [31]. There are no data available from other Latin American countries. The fulminant course of this disease after primary EBV infection suggests a genetically determined susceptibility, possibly based on certain HLA types that result in an abnormal response to primary EBV infection. A probably related disorder presenting mainly with lymphadenopathy and high lactate dehydrogenase levels has been reported in children from Peru [58]. In addition to the characteristic features of acute onset with fever, weight loss and hepatosplenomegaly, these children have peripheral, mediastinal and intraabdominal lymphadenopathy. The disease progresses rapidly causing death with a median survival of 7 months. The infiltrating cells in systemic EBV-positive T-cell lymphoma of childhood are predominantly CD8+ cytotoxic αβ T cells. Molecular analysis of the TCR genes show a monoclonal T-cell proliferation in all cases [31,59].
CAEBV is also prevalent in Latin America. Whereas HVLPD and SMBA are well recognized in Mexico [26,60], Peru [25,61] and Bolivia [62,63], the systemic form of CAEBV is less well documented. HVLPD was first described in Mexico as edematous scarring vasculitic panniculitis to separate it from the indolent ‘classic’ form of HVLPD [64]. In this first report, the severity of the disease, the presence of systemic symptoms in many cases, and the risk to progress to a T- or NK-cell lymphoma were highlighted. The clinical presentation is characterized by edema of the face and hands with recurrent vesicles and crust formation that leave scars. Prominent periorbital swelling was observed in a series of patients from Bolivia [63]. Systemic symptoms like fever, lymphadenopathy and/or hepatosplenomegaly are common [26,30]. These lesions occur in sun-exposed as well as unexposed areas. In the acute phase an intraepidermal spongiotic vesicle is observed. The lymphoid infiltrate predominates in the dermis around adnexa and blood vessels and sometimes extends to the deep subcutaneous tissue. The infiltrating cells are predominantly T cells (αβ or γδ), and less often NK cells or a mixture of both types [26,65]. In contrast to Japanese series where increased numbers of EBV-infected γδ T cells in peripheral blood have been reported, in series reported from Mexico only rarely have tissue infiltrating γδ T cells been demonstrated [26,60].
T/NK cell LPD in the United States and Europe
Jeffrey Cohen, MD, (National Institute of Allergy and Infectious Diseases) reviewed their experience with 19 patients with CAEBV in the United States [66]. Sixty percent of patients were Caucasian with the remainder predominantly Asian or Hispanic. The most common signs were lymphadenopathy, splenomegaly, fever, hepatitis, hypogammaglobulinemia, and pancytopenia; fever and hepatitis were less common (<50% of patients) than reported in Japan (≥90%) and unlike Asians, none of the patients from the United States had SMBA [22]. Patients had EBV in T, NK, or B cells. These latter patients often had hypogammaglobulinemia with progressive loss of B cells. Unlike reports from Asia, many patients in the United States had reduced numbers of NK cells. Only one patient had germline mutations (in perforin) that were responsible for their disease. Serum levels of IFN-γ, TNF-α, IL-6, and IL-10 were higher in CAEBV patients than controls. Chemotherapy and immunosuppressive therapy resulted in temporary responses; only HSCT was curative.
EBV HVLPD, like CAEBV, is much less common in the United States and Europe than in Asia or Latin America. A study of 16 patients with EBV HVLPD in the United States and Great Britain over 11 years found that most patients had normal numbers of CD4, CD8, and B cells; 70% had high numbers of Ki67 (proliferating) T cells, 60% had low numbers of NK cells, and 40% had high numbers of γδ T cells [67]. Half of the patients had T cell clones in the blood. The mean EBV DNA copy number in blood was 1,159,000 copies/ml. 70% of patients had EBV predominantly in T cells, and the rest had EBV predominantly in NK cells or at similar levels in T and NK cells or in T and B cells. Serum cytokine levels in patients with HVLPD were more similar to those in healthy controls than those with CAEBV.
Ten of 16 patients with HVLPD were Caucasians and with one exception, all of the Caucasians had classic disease; the one patient with systemic disease had spontaneous resolution of his gastrointestinal disease [67]. Two of the Caucasian patients had complete remission of their cutaneous disease and the EBV viral level declined. In contrast, two-thirds of the non-Caucasians required HSCT. The Caucasian patients presented at a younger age, had lower levels of EBV in the blood, had levels of NK cells in the blood closer to the normal level, less often had abnormal T cell clones in the blood, and less often had abnormally high levels of cytokines in the blood than the non-Caucasians. Thus, most Caucasians with HVLPD had a good prognosis and required no treatment.
ENKTL, nasal type is less common in the United States and Europe than in Asia and Latin America. A review of ENKTL, nasal type in the United States and Europe showed that the disease is less common in Caucasians than in Hispanics and Asian-Pacific Islanders [68]. In the United States and Europe there was less variation in signs and symptoms at presentation of disease and patients were often diagnosed later than in Asia, likely due to decreased awareness of the disease. Like EBV HVLPD, the prognosis of ENKTL, nasal type appears to better in Caucasians than in non-Caucasians.
Genetics of HVLPD: germline mutations
Irini Manoli, MD, PhD, (National Human Genome Research Institute) and Stefania Pittaluga, MD, (National Cancer Institute) noted that while there are rare reports of familial cases of HVLPD in the older literature [69–72], they precede the establishment of the association of the disease with EBV-positive T and/or NK LPD [73,74] and the revised diagnostic classification [6,21,50]. Recent large case series of classic or severe HVLPD from different ethnic backgrounds consist of sporadic cases [6,27,48,61].
In a study of patients with HVLPD in the United States and Great Britain [67], one patient was included with severe HVLPD with HLH and GATA2 deficiency due to an intronic variant affecting the binding of a GATA2 enhancer resulting in reduced transcription [75,76]. Germline GATA2 heterozygous mutations [76,77] have been associated with numerous diseases including (a) primary immunodeficiency 21 (IMD21 or MONOMAC) characterized by profoundly decreased or absent monocytes, B cells, NK cells, and circulating and tissue dendritic cells, and predisposition to disseminated nontuberculous mycobacterial infections, viral and fungal infections, and (b) familial predisposition to acute myeloid leukemia or myelodysplastic syndrome. Screening for GATA2 mutations in the remaining patients with HVLPD was negative. One subject with HVLPD also had Moebius syndrome, presenting with facial palsy and the inability to move the eyes laterally, due to underdevelopment of the facial and abducens cranial nerves. GATA2 is located in a candidate locus for autosomal dominant hereditary congenital facial palsy type 1, in 3q21–22, identified by linkage analysis in large pedigrees [78]. Another gene in that same locus, PLXND1, was recently described to carry de novo mutations in 3 subjects with Moebius-like syndrome [79]. The patient with HVLPD and Moebius syndrome had no pathogenic variants in PLXND1 or GATA2 or any copy number variants in the 3q21–22 region.
Whole exome sequencing studies in the HVLPD patients and their parents identified no significant copy number variants or pathogenic variants in single genes or genes along a similar pathway that were shared amongst more than two families in our cohort. Although analysis of the sequencing data is ongoing, current data suggest that HVLPD is not caused by germline mutations in a single gene across affected subjects. It is possible that HVLPD is a heterogenous disease entity with different genes/pathways resulting in overlapping phenotypes, and/or that it has a digenic/polygenic inheritance pattern, or that it is caused by somatic mutations in lymphocytes (like other forms of CAEBV), or that it is associated with epigenetic changes or environmental factors.
Genetics of CAEBV: somatic mutations
Hiroshi Kimura, MD, (Nagoya University) noted that after EBV infection of some persons, the presence of viral oncogenes might enable infected T or NK cells to proliferate and escape apoptosis, resulting in the development of CAEBV. In the long term, the accumulation of genetic mutations and epigenetic modifications leads to the development of overt lymphomas or leukemias [80]. However, it is unclear why only some people develop the disease and which genes drive the development of the malignancies. To clarify the pathogenesis of CAEBV, Kimura and colleagues conducted a comprehensive genetic study of 80 patients with systemic CAEBV, SMBA, and HVLPD [81]. EBV-infected and uninfected cells in peripheral blood were isolated and whole exome sequencing and whole EBV sequencing was performed. The results revealed that germline mutations are rare in patients with CAEBV, suggesting that inherited immunodeficiency is unlikely in most cases. However, somatic driver mutations (such as DDX3D and KMT2D) were frequently found in EBV-infected cells, indicating that CAEBV should be considered a neoplastic disease. Different cell lineages with EBV infection shared identical driver mutations, suggesting that EBV infects the common ancestor of lymphoid cells.
The EBV genome in patients with CAEBV harbored intragenic deletions that were also common in EBV-associated lymphomas, such as ENKTL and diffuse large B-cell lymphoma [81]. However, similar deletions were not found in patients with infectious mononucleosis or post-transplant LPD. These deletions frequently affected EBV BART microRNA clusters, which regulate proliferation, differentiation, apoptosis, and the cell cycle of infected cells to establish latent infection and produce viral progeny [82]. Interestingly, humanized mice infected with BART-deleted EBV developed lymphomas more rapidly and showed upregulated expression of EBV BZLF1 compared to mice infected with wild-type EBV [83]. Taken together, these results indicate that the deletion of the BART clusters results in upregulation of expression of lytic genes and promotion of lymphomagenesis.
Somatic driver mutations and EBV intragenic deletions were detected in patients with SMBA, and their prognosis was poor, similar to patients with systemic CAEBV. By contrast, no somatic driver mutation or EBV intragenic deletion was found in patients with HVLPD. Their prognosis was favorable, particularly in those with γδ T cell infection. Therefore, the etiology of HVLPD may be different from that of other forms of CAEBV.
Conclusion
Recent advances in classification and studies of pathogenesis, genetics, and prognosis of EBV NK and T cell disorders have furthered our understanding of these diseases. While these diseases are more common in persons from Asia and Central or South America, and differences in HLA alleles between these populations and Caucasians have been reported, specific HLA polymorphisms have not been correlated with EBV NK and T cell diseases. Although many EBV B cell LPDs are associated with immune suppression or germline mutations in genes important for immune surveillance, somatic driver mutations in virus-infected cells are more often associated with EBV NK and T cell LPD. Most of these diseases require radiation therapy, chemotherapy, or HSCT; however, some patients with HVLPD, particularly Caucasians, may not require treatment. Additional research in immunology, genetics, virology, animal models, and clinical trials of these diseases is needed to find less toxic and more effective therapies.
Funding
This work was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, and the National Human Genome Research Institute. Additional funding was from a generous gift from the Roth Fellowship for CAEBV and Hydroa Vacciniforme.
Footnotes
Disclosure statement
Each of the authors reviewed and approved the summary of the work. The authors report no conflict of interest.
References
- [1].Coleman CB, Daud II, Ogolla SO, et al. Epstein-Barr virus type 2 infects t cells in healthy Kenyan children. J Infect Dis. 2017;216:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hudnall SD, Ge Y, Wei L, et al. Distribution and phenotype of Epstein-Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18: 519–527. [DOI] [PubMed] [Google Scholar]
- [3].Bekker V, Scherpbier H, Beld M, et al. Epstein-Barr virus infects B and non-B lymphocytes in HIV-1-infected children and adolescents. J Infect Dis. 2006; 194:1323–1330. [DOI] [PubMed] [Google Scholar]
- [4].Calattini S, Sereti I, Scheinberg P, et al. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders by using Immuno-FISH. Blood. 2010;116:4546–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jaffe ES, Harris NL, Stein H, et al. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon (France): IARC Press; 2001. [Google Scholar]
- [6].Swerdlow SH, Campo E, Harris NL, et al. , editors. WHO classification of tumours of haematopoietic and lymphoid tissues revised. 4th ed. Lyon (France): International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- [7].Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocom-promised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–686. [DOI] [PubMed] [Google Scholar]
- [8].Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. 2013;47:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao J, Behdad A, Ji P, et al. EBV-negative aggressive NK-cell leukemia/lymphoma: a clinical and pathological study from a single institution. Mod Pathol. 2017;30:1100–1115. [DOI] [PubMed] [Google Scholar]
- [10].Nicolae A, Ganapathi KA, Pham TH, et al. EBV-negative aggressive NK-cell leukemia/lymphoma: clinical, pathologic, and genetic features. Am J Surg Pathol. 2017;41:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ko YH, Park S, Kim K, et al. Aggressive natural killer cell leukemia: is Epstein-Barr virus negativity an indicator of a favorable prognosis? Acta Haematol. 2008; 120:199–206. [DOI] [PubMed] [Google Scholar]
- [12].Park JA, Jun KR, Nam SH, et al. Favorable outcome in a child with EBV-negative aggressive NK cell leukemia. Int J Hematol. 2013;97:673–676. [DOI] [PubMed] [Google Scholar]
- [13].Nakashima Y, Tagawa H, Suzuki R, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosom Cancer. 2005;44:247–255. [DOI] [PubMed] [Google Scholar]
- [14].Jaffe ES, Chan JK, Su IJ, et al. Report of the Workshop on Nasal and Related Extranodal Angiocentric T/Natural Killer Cell Lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996; 20:103–111. [DOI] [PubMed] [Google Scholar]
- [15].Chan JKC, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997;89:4501–4513. [PubMed] [Google Scholar]
- [16].Hsiao CH, Lee WI, Chang SL, et al. Angiocentric T-cell lymphoma of the intestine: a distinct etiology of ischemic bowel disease. Gastroenterology. 1996;110: 985–990. [DOI] [PubMed] [Google Scholar]
- [17].Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. [DOI] [PubMed] [Google Scholar]
- [18].Smith MC, Cohen DN, Greig B, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. 2014;7: 5738–5749. [PMC free article] [PubMed] [Google Scholar]
- [19].Kogawa K, Sato H, Asano T, et al. Prognostic factors of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in children: report of the Japan Histiocytosis Study Group. Pediatr Blood Cancer. 2014; 61:1257–1262. [DOI] [PubMed] [Google Scholar]
- [20].Straus SE. Acute progressive Epstein-Barr virus infections. Annu Rev Med. 1992;43:437–449. [DOI] [PubMed] [Google Scholar]
- [21].Cohen JI, Kimura H, Nakamura S, et al. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98:280–286. [DOI] [PubMed] [Google Scholar]
- [23].Wang RC, Chang ST, Hsieh YC, et al. Spectrum of Epstein-Barr virus-associated T-cell lymphoproliferative disorder in adolescents and young adults in Taiwan. Int J Clin Exp Pathol. 2014;7:2430–2437. [PMC free article] [PubMed] [Google Scholar]
- [24].Cohen JI. Primary immunodeficiencies associated with EBV disease. Curr Top Microbiol Immunol. 2015;390: 241–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barrionuevo C, Anderson VM, Zevallos-Giampietri E, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol. 2002;10:7–14. [DOI] [PubMed] [Google Scholar]
- [26].Quintanilla-Martinez L, Ridaura C, Nagl F, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV + lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122: 3101–3110. [DOI] [PubMed] [Google Scholar]
- [27].Liu Y, Ma C, Wang G, et al. Hydroa vacciniforme-like lymphoproliferative disorder: Clinicopathologic study of 41 cases. J Am Acad Dermatol. 2019;81:534–540. [DOI] [PubMed] [Google Scholar]
- [28].Ishihara S, Yabuta R, Tokura Y, et al. Hypersensitivity to mosquito bites is not an allergic disease, but an Epstein-Barr virus-associated lymphoproliferative disease. Int J Hematol. 2000;72:223–228. [PubMed] [Google Scholar]
- [29].Tokura Y, Ishihara S, Tagawa S, et al. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol. 2001;45:569–578. [DOI] [PubMed] [Google Scholar]
- [30].Quintanilla-Martinez L, Fend F. Deciphering hydroa vacciniforme. Blood. 2019;133:2735–2737. [DOI] [PubMed] [Google Scholar]
- [31].Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443–451. [PubMed] [Google Scholar]
- [32].Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008;58:209–217. [DOI] [PubMed] [Google Scholar]
- [33].Attygalle AD, Cabecadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the lymphoma workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64: 171–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee MY, Tan TD, Feng AC, et al. Clinicopathological analysis of malignant lymphoma in Taiwan, defined according to the World Health Organization classification. Haematologica. 2005;90:1703–1705. [PubMed] [Google Scholar]
- [35].Lymphoma Study Group of Japanese Pathologists. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int. 2000;50:696–702. [DOI] [PubMed] [Google Scholar]
- [36].Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: report of the Third Nationwide Study. Korean J Pathol. 2011;45:254–260. [Google Scholar]
- [37].Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cho EY, Kim KH, Kim WS, et al. The spectrum of Epstein-Barr virus-associated lymphoproliferative disease in Korea: incidence of disease entities by age groups. J Korean Med Sci. 2008;23:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paik JH, Choe JY, Kim H, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58:53–63. [DOI] [PubMed] [Google Scholar]
- [40].Lee TH, Ko YH. Chronic active EBV infection: the experience of the Samsung Medical Center in South Korea. Bol Med Hosp Infant Mex. 2016;73:10–17. [DOI] [PubMed] [Google Scholar]
- [41].Park S, Kim K, Kim WS, et al. Systemic EBV + T-cell lymphoma in elderly patients: comparison with children and young adult patients. Virchows Arch. 2008; 453:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huh SY, Choi M, Cho KH. A case of Epstein-Barr virus-associated hydroa vacciniforme. Ann Dermatol. 2009; 21:209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cho KH, Lee SH, Kim CW, et al. Epstein-Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol. 2004;151:372–380. [DOI] [PubMed] [Google Scholar]
- [44].Cho KH, Choi WW, Youn CS, et al. Skin is the frequent site for involvement of peripheral T-cell and natural killer cell lymphomas in Korea. J Dermatol. 2000;27: 500–507. [DOI] [PubMed] [Google Scholar]
- [45].Cho KH, Kim CW, Kwon OS, et al. Epstein-Barr virus-associated lymphoproliferative eruption with progression to large granular lymphocytic leukaemia. Br J Dermatol. 1997;137:426–430. [PubMed] [Google Scholar]
- [46].Cho KH, Kim CW, Lee DY, et al. An Epstein-Barr virus-associated lymphoproliferative lesion of the skin presenting as recurrent necrotic papulovesicles of the face. Br J Dermatol. 1996;134:791–796. [PubMed] [Google Scholar]
- [47].Kawamoto K, Miyoshi H, Suzuki T, et al. A distinct subtype of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorder: adult patients with chronic active Epstein-Barr virus infection-like features. Haematologica. 2018;103:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miyake T, Yamamoto T, Hirai Y, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol. 2015;172:56–63. [DOI] [PubMed] [Google Scholar]
- [49].Hirai Y, Yamamoto T, Kimura H, et al. Hydroa vacciniforme is associated with increased numbers of Epstein-Barr virus-infected γδ cells. J Invest Dermatol. 2012;132:1401–1408. [DOI] [PubMed] [Google Scholar]
- [50].Iwatsuki K, Miyake T, Hirai Y, et al. Hydroa vacciniforme: a distinctive form of Epstein-Barr virus-associated T-cell lymphoproliferative disorders. Eur J Dermatol. 2019;29:21–28. [DOI] [PubMed] [Google Scholar]
- [51].Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood. 2012;120: 4795–4801. [DOI] [PubMed] [Google Scholar]
- [52].Aviles A Nasal NK/T-cell lymphoma. A comparative analysis of a Mexican Population with the other populations of Latin America. Mediterr J Hematol Infect Dis. 2015;7:e2015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26: 4124–4130. [DOI] [PubMed] [Google Scholar]
- [54].Quintanilla-Martinez L, Kremer M, Keller G, et al. P53 mutations in nasal natural Killer/T-cell lymphoma from Mexico. Association with large cell morphology and advanced disease. Am J Pathol. 2001; 159: 2095–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Su IJ, Chen RL, Lin DT, et al. Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol. 1994;144:1219–1225. [PMC free article] [PubMed] [Google Scholar]
- [56].Chen RL, Su IJ, Lin KH, et al. Fulminant childhood hemophagocytic syndrome mimicking histiocytic medullary reticulosis. An atypical form of Epstein-Barr virus infection. Am J Clin Pathol. 1991;96:171–176. [DOI] [PubMed] [Google Scholar]
- [57].Kikuta H, Sakiyama Y, Matsumoto S, et al. Fatal Epstein-Barr virus-associated hemophagocytic syndrome. Blood. 1993;82:3259–3264. [PubMed] [Google Scholar]
- [58].Rodríguez-Pinilla SM, Barrionuevo C, García J, et al. Epstein-Barr virus-positive systemic NK/T-cell lymphomas in children: report of six cases. Histopathology. 2011;59:1183–1193. [DOI] [PubMed] [Google Scholar]
- [59].Coffey AM, Lewis A, Marcogliese AN, et al. A clinicopathologic study of the spectrum of systemic forms of EBV-associated T-cell lymphoproiferative disorders of childhood: A single tertiary care pediatric institution experience in North America. Pediatr Blood Cancer. 2019;66:e27798. [DOI] [PubMed] [Google Scholar]
- [60].Magaña M, Massone C, Magaña P, et al. Clinicopathologic features of hydroa vacciniforme-like lymphoma: a series of 9 patients. Am J Dermatopathol. 2016;38:20–25. [DOI] [PubMed] [Google Scholar]
- [61].Rodríguez-Pinilla SM, Barrionuevo C, Garcia J, et al. EBV-associated cutaneous NK/T-cell lymphoma: review of a series of 14 cases from Peru in children and young adults. Am J Surg Pathol. 2010;34:1773–1782. [DOI] [PubMed] [Google Scholar]
- [62].Sangueza M, Plaza JA. Hydroa vacciniforme-like cutaneous T-cell lymphoma: clinicopathologic and immunohistochemical study of 12 cases. J Am Acad Dermatol. 2013;69:112–119. [DOI] [PubMed] [Google Scholar]
- [63].Plaza JA, Sangueza M. Hydroa vacciniforme-like lymphoma with primarily periorbital swelling: 7 cases of an atypical clinical manifestation of this rare cutaneous T-cell lymphoma. Am J Dermatopathol. 2015; 37:20–25. [DOI] [PubMed] [Google Scholar]
- [64].Ruiz-Maldonado R, Parrilla FM, Orozco-Covarrubias ML, et al. Edematous, scarring vasculitic panniculits: a new multisystemic disease with malignant potential. J Am Acad Dermatol. 1995;32:37–44. [DOI] [PubMed] [Google Scholar]
- [65].Doeden K, Molina-Kirsch H, Perez E, et al. Hydroa-like lymphoma with CD56 expression. J Cutan Pathol. 2008;35:488–494. [DOI] [PubMed] [Google Scholar]
- [66].Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117:5835–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cohen JI, Manoli I, Dowdell KC, et al. Hydroa vacciniforme-like lymphoproliferative disorder: an EBV disease with a low risk of systemic illness in whites. Blood. 2019;133:2753–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Haverkos BM, Pan Z, Gru AA, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016;11:514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gupta G, Mohamed M, Kemmett D. Familial hydroa vacciniforme. Br J Dermatol. 1999;140:124–126. [DOI] [PubMed] [Google Scholar]
- [70].Annamalai R Hydroa vacciniforme in three alternate siblings. Arch Dermatol. 1971;103:224–225. [PubMed] [Google Scholar]
- [71].Wheeler CE, Cawley EP, Whitmore CW. Hydroa aestivale in identical twins. Arch Dermatol. 1960;82: 590–594. [DOI] [PubMed] [Google Scholar]
- [72].Gupta G, Man I, Kemmett D. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42:208–213. [DOI] [PubMed] [Google Scholar]
- [73].Iwatsuki K, Xu Z, Takata M, et al. The association of latent Epstein-Barr virus infection with hydroa vacciniforme. Br J Dermatol. 1999;140:715–721. [DOI] [PubMed] [Google Scholar]
- [74].Iwatsuki K, Satoh M, Yamamoto T, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol. 2006;142:587–595. [DOI] [PubMed] [Google Scholar]
- [75].Cohen JI, Dropulic L, Hsu AP, et al. Association of GATA2 deficiency with severe primary Epstein-Barr virus (EBV) infection and EBV-associated cancers. Clin Infect Dis. 2016;63:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cohen JI. GATA2 deficiency and Epstein-Barr virus disease. Front Immunol. 2017;8:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Michielse CB, Bhat M, Brady A, et al. Refinement of the locus for hereditary congenital facial palsy on chromosome 3q21 in two unrelated families and screening of positional candidate genes. Eur J Hum Genet. 2006;14:1306–1312. [DOI] [PubMed] [Google Scholar]
- [79].Tomas-Roca L, Tsaalbi-Shtylik A, Jansen JG, et al. De novo mutations in PLXND1 and REV3L cause Mobius syndrome. Nat Commun. 2015;6:7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kimura H EBV in T-/NK-Cell tumorigenesis. Adv Exp Med Biol. 2018;1045:459–475. [DOI] [PubMed] [Google Scholar]
- [81].Okuno Y, Murata T, Sato Y, et al. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat Microbiol. 2019;4:404–413. [DOI] [PubMed] [Google Scholar]
- [82].Klinke O, Feederle R, Delecluse HJ, et al. Genetics of Epstein-Barr virus microRNAs. Semin Cancer Biol. 2014;26:52–59. [DOI] [PubMed] [Google Scholar]
- [83].Lin X, Tsai MH, Shumilov A, et al. The Epstein-Barr virus BART miRNA cluster of the M81 strain modulates multiple functions in primary B cells. PLoS Pathog. 2015;11:e1005344. [DOI] [PMC free article] [PubMed] [Google Scholar]


