Abstract
Background.
Primary gallbladder neuroendocrine tumors (NETs) are rare, poorly understood cancers infrequently encountered at even the largest of tertiary referral centers. We therefore sought to identify a large cohort of patients with gallbladder NETs using a national database, with the aim of defining treatment modalities employed and survival associated with these uncommon malignancies.
Methods.
Patients with primary gallbladder NETs were identified in the National Cancer Database, and clinicopathologic characteristics were recorded. A univariate log-rank survival analysis was completed for patients who underwent resection. Parameters found to be significant were entered into a multivariate accelerated failure time analysis. For context, survival comparisons were included for patients who underwent resections for NETs at any gastrointestinal site and for gallbladder adenocarcinoma.
Results.
Overall, 754 patients with gallbladder NETs were identified. Patients were predominantly female (n = 518, 69%), White (n = 503, 67%), presented with stage IV disease (n = 295, 39%) and had high-grade lesions (n = 312, 41%). The majority underwent resection (n = 480, 64%), primarily simple cholecystectomy (n = 431, 90%), whereas a minority received multimodal therapy (n = 145, 21%). Among patients who underwent resection, older age (p = 0.001), large cell histology (p = 0.012), and positive margins (p = 0.030) were independently associated with worse overall survival. Patients with gallbladder NETs had improved survival relative to those with gallbladder adenocarcinoma (p = 0.001), but significantly worse survival than patients with NETs from other gastrointestinal sites (p<0.001).
Conclusions.
Primary gallbladder NETs are aggressive lesions that carry a worse prognosis than NETs of other gastrointestinal sites. Older age, positive margins, and large cell histology are associated with abbreviated survival after resection.
Gallbladder cancer is an aggressive malignancy with an annual incidence of 1.13 cases per 100,000 in the US and an overall 5-year survival rate of <20%.1 The vast majority of gallbladder cancers are adenocarcinomas.2,3 Primary gallbladder neuroendocrine tumors (NETs) are very rare, accounting for only 2–3% of primary gallbladder neoplasms.3,4
Due to their rarity, the literature on gallbladder NETs is limited to case reports and small case series, with no specific staging system for prognostication.5–7 Currently, gallbladder neuroendocrine carcinomas are classified using the same American Joint Committee on Cancer (AJCC) staging system as gallbladder adenocarcinomas, although it is known that NETs from other gastrointestinal sites generally have a more indolent clinical course than adenocarcinomas.8–10 Knowledge of the optimal management for gallbladder NETs is equally limited. The National Comprehensive Cancer Network (NCCN) and North American Neuroendocrine Tumor Society (NANETS) clinical guidelines do not address gallbladder NETs, and the treatment strategies employed for these tumors in case reports vary significantly.11–13 The most consistently practiced treatment for gallbladder NETs is complete resection, which is extrapolated from gallbladder adenocarcinoma management.4,5 However, little is known about patient outcomes after resection and the factors that have the greatest impact on survival.
The use of single-institution retrospective data to study gallbladder NETs is difficult due to prohibitively small sample sizes. Fortunately, large national datasets have made it possible to gain insight into these rare neoplasms by amassing data from thousands of hospitals nationwide. In this study, we use the National Cancer Database (NCDB) to evaluate gallbladder NETs, with three aims: (1) describe clinical characteristics and treatment patterns; (2) determine factors associated with survival in patients who undergo resection; and (3) compare the survival of patients undergoing resection of gallbladder NETs with that of patients undergoing resection for gallbladder adenocarcinoma and NETs from other gastrointestinal primary sites.
METHODS
Data Source
We performed a retrospective cohort study using the NCDB Participant User Files (PUFs) for gallbladder tumors. This study was exempt for review from our Institutional Review Board. The NCDB is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, and is a hospital-based registry with data from more than 1500 CoC-accredited hospitals. It includes information about patient demographics, comorbidities, disease stage, and the first course of treatment for 70% of newly diagnosed cancer cases in the US. The CoC and American Cancer Society have not verified and are not responsible for the analytic or statistical methodology used or for the conclusions drawn from these data.
Selection of the Study Population
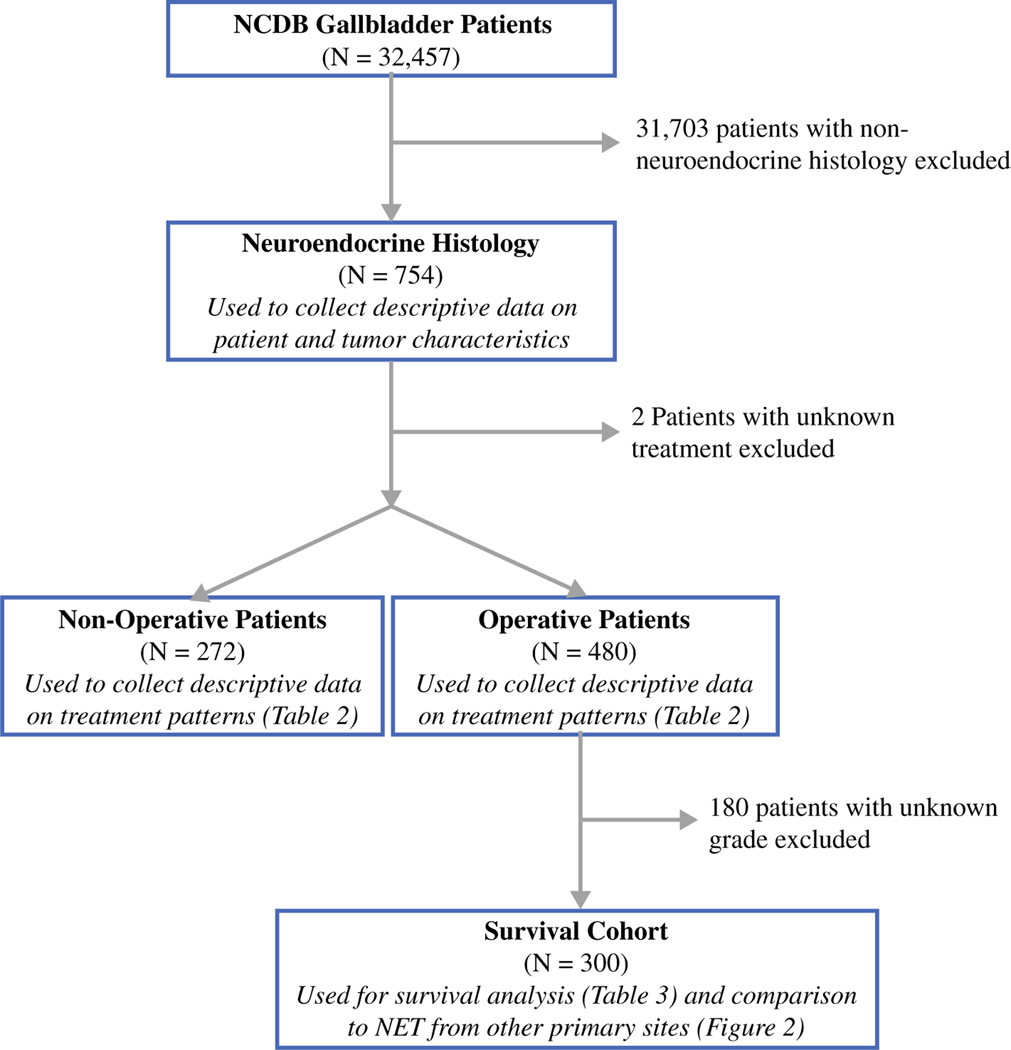
The study population included patients 18 years and older who were diagnosed with gallbladder NETs from 2004 to 2015 in the NCDB gallbladder PUFs (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] code C23.9). Patients with histologically confirmed neuroendocrine carcinoma not otherwise specified (NOS; 8246/3), small cell neuroendocrine carcinoma (8041/3), large cell neuroendocrine carcinoma (8013/3), and typical carcinoid (8240/3) were identified, and descriptive patient and tumor data were collected. Patients with a missing surgical status were excluded. The remaining patients were stratified into those managed operatively and those managed without an operation. Further survival analysis was then restricted to those patients who underwent an operation and had a reported histologic grade (Fig. 1).
FIG. 1.
Selection of the study cohort. NCDB National Cancer Database, NET neuroendocrine tumor
Patient and Tumor Characteristics
Patient and tumor characteristics were examined, including age, sex, race, Charlson–Deyo score, tumor size, stage, grade, histologic subtype, and sites of metastasis. Race was classified as White, Black, or other, which included small numbers of Asian, Asian Indian, Middle Eastern, Pacific Islander, and Native American populations. Tumor size was stratified into <25 mm versus ≥ 25 mm for the purpose of multivariate analysis, with the threshold of 25 mm chosen because it was the median tumor size in this cohort. Stage was based on the AJCC guidelines for gallbladder adenocarcinoma. Nomenclature for histologic subtype has changed over the last 2 decades, and is also known to vary between institutions. The World Health Organization (WHO) and European Neuroendocrine Tumor Society (ENETS) systems of nomenclature state that high-grade NETs may be classified as neuroendocrine carcinomas, while the designation of typical carcinoid should be reserved for low-grade NETs. However, we noted that the majority of patients reported as having typical carcinoid in the NCDB had grade 3 tumors. To avoid confusion stemming from inconsistent nomenclature, the histologic subtypes of neuroendocrine carcinoma NOS and typical carcinoid were combined into one group called neuroendocrine carcinoma. A Kruskal–Wallis test was used to compare median tumor sizes of the three different histologies.
Treatment Characteristics
Surgical management was defined using surgery of the primary site codes, and was inclusive of cholecystectomy (considered simple or total surgical removal of the primary site [− 30, − 40]), radical cholecystectomy (− 60), debulking (− 50), and surgical resection NOS (− 90). Margins were classified as microscopically negative (R0) or microscopically positive (R1), both without gross residual tumor remaining after surgery. Debulking operations and grossly positive margins were classified as R2 resections. Receipt of neoadjuvant and adjuvant chemotherapy or radiation was also included in the analysis.
Survival Analysis
The primary outcome of the survival analysis was overall survival (OS), which was based on all-cause mortality and was calculated from the date of diagnosis to the date of death. Patients alive at the date of last contact were censored. Kaplan–Meier curves were created to explore differences in survival between study variables. Univariate analysis of the association between study variables and OS was completed using the log-rank test.
To evaluate the adjusted survival time accounting for variables that were significant on univariate analysis, a multivariate accelerated failure time (AFT) model with a log-normal distribution was used. Patients with missing data for factors of interest were excluded from the multivariate model.
Comparison with Neuroendocrine Tumors from Other Sites and Gallbladder Adenocarcinoma
Due to the paucity of data regarding outcomes of gallbladder NETs, a separate survival analysis was completed comparing patients with resected gallbladder NETs with those with resected NETs arising from other gastrointestinal primary sites and resected gallbladder adenocarcinoma. Patients were selected from the NCDB PUF files for pancreas (code C25.0–C25.9), small bowel (code C17.0–C17.9), appendix (code C18.1), colon (code C18.0, C18.2–C18.9), rectum (code C19.9, C20.9), and stomach (code C16.0–16.9) using the same criteria used to select patients for the survival analysis above (Fig. 1). Patients with adenocarcinoma histology (codes 8140/3, 8144/3, 8310/3, 8480/3, 8490/3) were also selected from the NCDB gallbladder PUFs using these selection criteria. OS was compared using Kaplan–Meier curves and the log-rank test.
All statistical tests were performed using SPSS® version 25.0 (IBM Corporation, Armonk, NY, USA) or R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and a p value <0.05 was considered statistically significant.
RESULTS
Patient and Tumor Characteristics
Overall, 754 patients with primary gallbladder NETs were identified, accounting for 2.3% of the 32,457 patients with gallbladder tumors in the database. The median patient age was 66 years. Patients were predominantly female (n = 518, 69%) and White (n = 503, 67%). The majority of patients had a Charlson–Deyo comorbidity score of 0 (n = 538, 71%). The median primary tumor size was 26 mm, and nearly half of the patients presented with stage IV disease (n = 295, 39%). Of these patients, the site of distant metastasis was documented in 148 cases (50%) and was most commonly the liver (n = 126). Of the cases reporting tumor grade, high-grade lesions (grade 3 and undifferentiated) were predominant (n = 312, 41%). The most common histologic subtype was neuroendocrine carcinoma (n = 464, 62%), followed by small cell neuroendocrine carcinoma (n = 217, 29%) and large cell neuroendocrine carcinoma (n = 67, 9%) (Table 1). The median size of large cell tumors (35 mm) and small cell tumors (45 mm) was greater than the median size of neuroendocrine carcinomas (12 mm) (p<0.001).
TABLE 1.
Characteristics of patients with primary gallbladder NETs
| Characteristic | All patients |
|---|---|
| [N = 754] | |
| Median age, years | 66 |
| Sex | |
| Female | 518 (69) |
| Male | 236 (31) |
| Race | |
| White | 503 (67) |
| Black | 130 (17) |
| Other | 121 (16) |
| Charlson–Deyo score | |
| 0 | 538 (71) |
| 1 | 157 (21) |
| ≥ 2 | 59 (8) |
| Tumor histology | |
| Neuroendocrine carcinoma | 464 (62) |
| Small cell neuroendocrine carcinoma | 217 (29) |
| Large cell neuroendocrine carcinoma | 67 (9) |
| Median tumor size, mm | 26 |
| AJCC stagea | |
| I | 205 (27) |
| II | 77 (10) |
| III | 121 (16) |
| IV | 295 (39) |
| Positive regional nodesa | |
| Yes | 111 (15) |
| No | 72 (47) |
| Tumor gradea | |
| 1 | 93 (12) |
| 2 | 21 (3) |
| 3 or undifferentiated | 312 (41) |
| Site of metastasis | |
| Liver | 126 (17) |
| Bone | 10 (1) |
| Lung | 7 (1) |
| Brain | 5 (1) |
| Unspecified | 147 (19) |
Data are expressed as n (%)
NETs neuroendocrine tumors, AJCC American Joint Committee on Cancer
Missing data were omitted
Treatment Patterns
More than half of the patients in this cohort underwent resection of their disease (n = 480, 64%). The vast majority of these operations were documented as a simple cholecystectomy (n = 431, 90%), while a few patients underwent a radical cholecystectomy (n = 29, 6%). An R0 resection was accomplished in most cases (n = 323, 67%). Of the patients who underwent an operation, most (n = 335, 70%) did not receive any additional therapy. Of the 272 patients who did not undergo a resection, 52% (n = 141) were treated with chemotherapy alone and 11% (n = 30) were treated with chemotherapy and radiotherapy. Ninety-nine patients (36%) did not receive any documented treatment (Table 2).
TABLE 2.
Management of primary gallbladder NETs
| Management | Operative [N = 480] | Non-operative [N = 272] |
|---|---|---|
| Chemotherapy | 99 (21) | 141 (52) |
| Radiation | 8 (2) | 2 (1) |
| Chemotherapy and radiation | 38 (8) | 30 (11) |
| No medical management | 335 (70) | 99 (36) |
| Extent of operation | ||
| Cholecystectomy | 431 (90) | |
| Radical cholecystectomy | 29 (6) | |
| Debulking | 3 (1) | |
| Surgical resection, NOS | 17 (3) | |
| Margin status | ||
| R0 | 323 (67) | |
| R1 | 59 (12) | |
| R2 | 12 (3) | |
| Positive, NOS | 33 (7) | |
| Unknown | 53 (11) |
Data are expressed as n (%)
NETs neuroendocrine tumors, NOS not otherwise specified
Factors Associated with Survival
Three hundred patients underwent tumor extirpation and had a documented histologic grade. The median OS for this cohort was 25 months. On univariate log-rank analysis, race, Charlson–Deyo score, large and small cell histologies, tumor size (electronic supplementary Figure 1), AJCC stage, tumor grade, margin status, and receipt of multimodal therapy were all associated with significant differences in survival. These factors were entered into a multivariate AFT analysis, which demonstrated that older age (time ratio (TR) 0.95, 95% confidence interval [CI] 0.93–0.98), large cell histology (TR 0.36, 95% CI 0.16–0.80), and positive surgical margins (TR 0.43, 95% CI 0.20–0.92) were independently associated with decreased survival (Table 3).
TABLE 3.
Univariate comparisons and multivariate accelerated failure time survival analysis of patients [n = 300] who underwent resection of gallbladder NETs
| Variable | Operative patients (%) | Median OS (months) | 95% CI | Univariate p value | Time ratio | 95% CI | Multivariate p value |
|---|---|---|---|---|---|---|---|
| Median age | 64 | – | – | – | 0.95 | 0.93–0.98 | 0.001 |
| Sex | 0.648 | ||||||
| Female | 197 (66) | 27 | 16–37 | – | – | – | |
| Male | 103 (34) | 24 | 10–38 | ||||
| Race | 0.040 | ||||||
| White | 212 (71) | 24 | 15–32 | Reference | |||
| Black | 44 (15) | 16 | 9–24 | 0.91 | 0.36–2.31 | 0.835 | |
| Other | 44 (15) | NR | – | 3.87 | 1.27–11.81 | 0.017 | |
| Charlson–Deyo score | 0.001 | ||||||
| 0 | 200 (67) | 34 | 19–48 | Reference | |||
| 1 | 77 (26) | 17 | 5–29 | 1.05 | 0.51–2.15 | 0.899 | |
| ≥2 | 23 (8) | 6 | 2–9 | 0.31 | 0.09–1.13 | 0.076 | |
| Tumor histology | <0.001 | ||||||
| Neuroendocrine carcinoma | 196 (65) | 51 | 26–75 | Reference | |||
| Small cell neuroendocrine carcinoma | 56 (19) | 11 | 7–15 | 0.54 | 0.24–1.19 | 0.124 | |
| Large cell neuroendocrine carcinoma | 48 (16) | 11 | 5–16 | 0.36 | 0.16–0.8 | 0.012 | |
| Tumor size, mma | <0.001 | ||||||
| <25 | 114 (38) | NR | – | Reference | |||
| ≥25 | 113 (38) | 13 | 9–18 | 0.94 | 0.42–2.09 | 0.878 | |
| AJCC stagea | <0.001 | ||||||
| I | 94 (31) | NR | – | Reference | |||
| II | 51 (17) | 41 | 9–73 | 3.40 | 0.63–18.40 | 0.156 | |
| III | 56 (19) | 24 | 16–32 | 3.77 | 0.65–21.94 | 0.139 | |
| IV | 84 (28) | 9 | 6–11 | 1.17 | 0.23–6.10 | 0.850 | |
| Tumor grade | <0.001 | ||||||
| 1 | 91 (30) | NR | – | Reference | |||
| 2 | 19 (6) | 51 | 0–113 | 0.23 | 0.05–1.09 | 0.064 | |
| 3 or undifferentiated | 190 (63) | 14 | 9–18 | 0.32 | 0.07–1.60 | 0.167 | |
| Positive marginsa | <0.001 | ||||||
| No | 186 (62) | NR | – | Reference | |||
| Yes | 83 (28) | 9 | 6–12 | 0.43 | 0.20–0.92 | 0.030 | |
| Multimodal therapy | 0.003 | ||||||
| No | 186 (62) | 43 | 25–61 | Reference | |||
| Yes | 114 (38) | 17 | 13–21 | 0.68 | 0.33–1.41 | 0.300 |
NETs neuroendocrine tumors, OS overall survival, CI confidence interval, AJCC American Joint Committee on Cancer, NR not reached
Missing data were omitted
Survival Comparison with Other Neuroendocrine Tumors and Adenocarcinoma
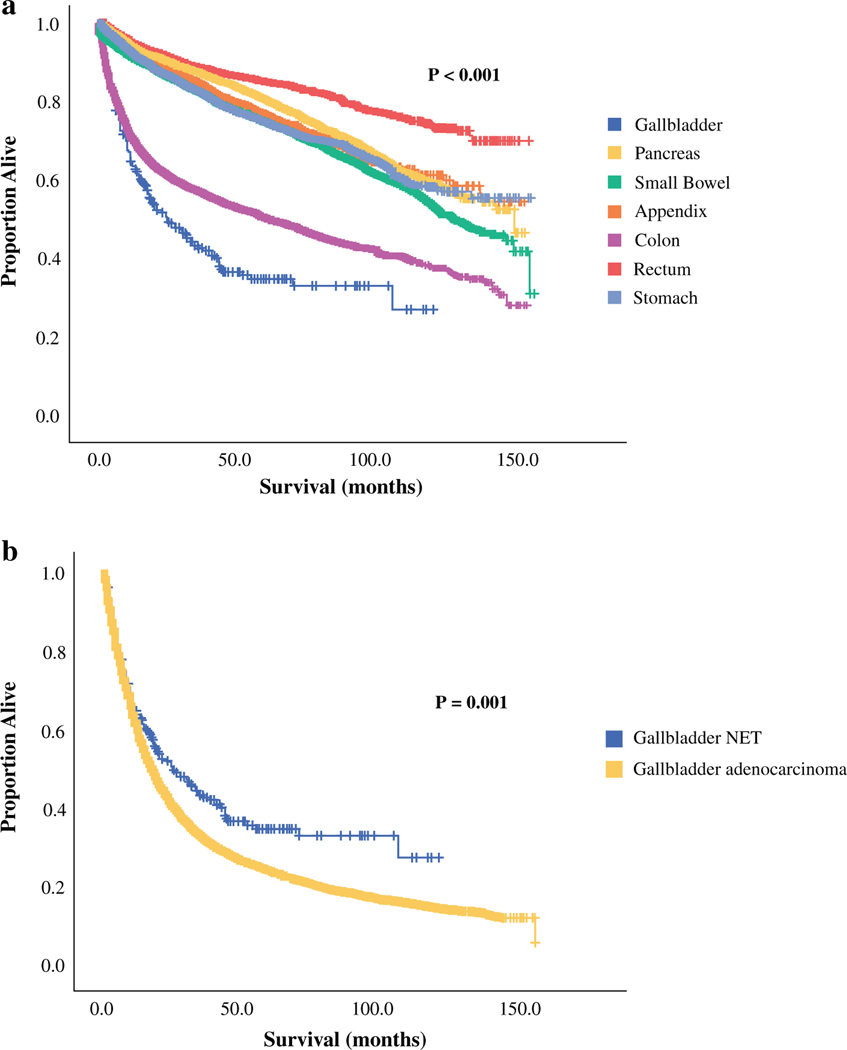
Patients with NETs arising in other abdominal organs, as well as patients with gallbladder adenocarcinoma, were selected using the same criteria used to identify the survival cohort (Fig. 1). Patients with NETs arising in the pancreas (n = 8611), small bowel (n = 19,275), appendix (n = 5221), colon (n = 4881), rectum (n = 6625), and stomach (n = 3297) were included, as well as patients with gallbladder adenocarcinoma (n = 16,228). The median OS of patients with gallbladder NETs (25 months) was found to be significantly shorter than that of patients with NETs from any of the other abdominal primary sites (p<0.001) (Fig. 2a). Conversely, survival with gallbladder NETs was significantly longer than survival with gallbladder adenocarcinoma (25 vs. 17 months; p = 0.001) (Fig. 2b).
FIG. 2.
Kaplan–Meier survival curves comparing patients with resected gallbladder NETs with a patients with resected NETs from other gastrointestinal primary sites, and b patients with resected gallbladder adenocarcinoma. Median OS for patients with gallbladder NETs (n = 300) was 25 months, and median OS for patients with resected small bowel (n = 19,275) and colon (n = 4881) NETs was 130 months and 65 months, respectively. Median OS for NETs of the pancreas, appendix, rectum, and stomach was not reached, while median OS for resected gallbladder adenocarcinoma (n = 16,228) was 17 months. NETs neuroendocrine tumors, OS overall survival
DISCUSSION
Primary gallbladder NETs are rare, accounting for 2.3% of gallbladder cancers in the NCDB. The existing literature on these tumors is limited to case reports and single-institution series, the largest of which includes 25 patients [5,6, 14–19]. In this study, we evaluated a cohort of 754 patients with primary gallbladder NETs and found that patients frequently present with metastatic disease and high-grade histology. Of the patients undergoing resection, which was almost always simple cholecystectomy, large cell histology and positive margins predict a particularly abbreviated OS. In order to frame the survival of patients undergoing a resection for gallbladder NETs into a familiar context for the practicing clinician, we documented and compared the survival of patients undergoing resection for gastrointestinal NETs and gallbladder adenocarcinoma using the same national database. In doing so, we demonstrated that gallbladder NETs have the worst survival of all gastrointestinal NETs, albeit superior to patients with gallbladder adenocarcinoma.
Due to their rarity, there is no specific staging system or clinical guideline to inform prognostication and management of gallbladder NETs. The AJCC 8th edition includes individual staging criteria for NETs of the stomach, ampulla, small bowel, appendix, large bowel, and pancreas, while gallbladder NETs are staged using the same schema as gallbladder adenocarcinoma.10 Similarly, the NCCN and NANETS guidelines discuss the management of gastric, small bowel, pancreatic, and large bowel NETs as distinct entities, but do not address the management of gallbladder NETs.11,12 Given the difficulty of accumulating enough patients for a meaningful single- or even multi-institutional retrospective review, large nationwide datasets such as the NCDB are ideally suited for the investigation of rare malignancies such as gallbladder NETs.20,21 In the study of gallbladder adenocarcinoma, which itself is an uncommon disease, the NCDB has been used to describe trends in treatment and management,22 validate changes to the AJCC staging system,23 and, most recently, to elucidate the efficacy of multimodal therapy for locally advanced disease.24,25 Similarly, NETs of the colon and rectum, small bowel, and appendix have been investigated using the NCDB and the Surveillance, Epidemiology, and End Results (SEER) database, providing information that has informed the recommendations of the NCCN.11,26–28 While our data lack the necessary power to construct a new staging system or propose clinical guidelines for gallbladder NETs, they do provide a valuable reference to help clinicians estimate prognosis and evaluate management options when this rare disease is encountered.
Review of published case reports suggests that the management of gallbladder NETs is highly inconsistent, ranging from simple cholecystectomy to radical resection with varying combinations of systemic agents and radiation extrapolated from regimens for biliary tract adenocarcinomas and gastrointestinal NETs.4–6,13,17, 18,29–31 These treatment options are nearly impossible to study in a prospective fashion due to the scarcity of these tumors. One of our aims was to simply describe how these tumors are currently being managed in the large cohort made available by the NCDB. We found that the majority of patients with gallbladder NETs were treated surgically, primarily with a simple cholecystectomy. A small minority underwent radical cholecystectomy, and most did not receive multimodal therapy. Of those who were treated non-operatively, most received systemic chemotherapy alone. Unfortunately, the NCDB does not contain data regarding the types of systemic agents used. Existing case reports suggest that these tumors are frequently treated with combinations of a platinum agent with gemcitabine or etoposide, with occasional partial responses to both regimens.6, 13
Our survival analysis revealed that large cell histology portends a particularly poor prognosis for patients with gallbladder NETs. Large cell NETs are characterized by a high mitotic rate (>10 mitoses/2 mm2), polygonal cells that are approximately three times larger than those of small cell NETs, irregular nuclei, cellular palisading, and, frequently, large areas of necrosis.4,5 In a 2010 literature review evaluating 29 case reports of poorly differentiated gallbladder NETs, Iype et al. found that large cell histology was associated with worse prognosis and reduced responsiveness to chemotherapy.13 While a finding of large cell histology is likely to be made after resection, it may be informative to the treating oncologist if identified during metastatic work-up, especially considering the targetable ALK rearrangement identified in a fraction of pulmonary large cell NETs.32
While this study offers valuable insight into gallbladder NETs, it does have significant limitations. As is the case with nationwide database reviews, our study was limited by the quality and granularity of the data reported. Several potentially important parameters, such as depth of tumor penetration into the gallbladder wall, extent of lymph node evaluation, and lymph node status, could not be included in the survival analysis because the majority of patients did not have these variables reported. Furthermore, it was not possible to determine whether the diagnosis of gallbladder NET was made incidentally during cholecystectomy, a scenario that could impact the treatment rendered and, potentially, the clinical outcome. The lack of data on specific systemic therapies also made it impossible to identify chemotherapeutic regimens that may have been more effective than others. Finally, the NCDB also does not document genetic syndromes such as von Hippel–Lindau disease, neurofibromatosis, or multiple endocrine neoplasia, which are associated with a significant number of NETs and may affect prognosis.
CONCLUSIONS
Primary gallbladder NETs are rare, aggressive neoplasms that are often high-grade and present at an advanced stage. Complete tumor extirpation should be pursued when feasible. Older age, positive margins, and large cell histology are independently associated with poor survival among patients undergoing resection.
Supplementary Material
Acknowledgments
FUNDING This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1245/s10434-019-07440-6) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2017 Special Section: Rare Cancers in Adults. 2017. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017-special-section-rare-cancers-in-adults.pdf. Accessed 5 Nov 2018.
- 2.Yadav R, Jain D, Mathur SR, Sharma A, Iyer VK. Gallbladder carcinoma: An attempt of WHO histological classification on fine needle aspiration material. Cytojournal. 2013;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98(7):485–9. [DOI] [PubMed] [Google Scholar]
- 4.Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 2010;44(10):687–95. [DOI] [PubMed] [Google Scholar]
- 5.Soin S, Pannu BS, Myint PT, Dhillon AS. Large cell neuroendocrine carcinoma and adenocarcinoma of gallbladder with concomitant hepatitis C infection. BMJ Case Rep. 2018. 10.1136/bcr-2018-225141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanetkar AV, Patkar S, Khobragade KH, Ostwal V, Ramaswamy A, Goel M. Neuroendocrine carcinoma of gallbladder: a step beyond palliative therapy, experience of 25 cases. J Gastrointest Cancer. 2019;50(2):298–303. [DOI] [PubMed] [Google Scholar]
- 7.Adachi T, Haraguchi M, Irie J, et al. Gallbladder small cell carcinoma: a case report and literature review. Surg Case Rep. 2016;2(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav S, Sharma P, Zakalik D. Comparison of demographics, tumor characteristics, and survival between pancreatic adenocarcinomas and pancreatic neuroendocrine tumors: a population-based study. Am J Clin Oncol. 2018;41(5):485–91. [DOI] [PubMed] [Google Scholar]
- 9.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th ed. Chicago: Springer; 2018. [Google Scholar]
- 11.NCCN practice guidelines in oncology: neuroendocrine and adrenal tumors. 2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed 31 Oct 2018.
- 12.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iype S, Mirza TA, Propper DJ, Bhattacharya S, Feakins RM, Kocher HM. Neuroendocrine tumours of the gallbladder: three cases and a review of the literature. Postgrad Med J. 2009;85(1002):213–8. [DOI] [PubMed] [Google Scholar]
- 14.Nemenqani DM, Fuloria J, Karam RA, Hammadi H. Gallbladder neuroendocrine neoplasms: a case report of gallbladder small cell carcinoma. J Gastrointest Cancer. 2016;47(4):432–5. [DOI] [PubMed] [Google Scholar]
- 15.Kutting F, Schmidt M, Waldschmidt D, Curth H, Schramm C, Steffen HM. Neuroendocrine carcinoma of the gallbladder masquerading as a klatskin tumor in a 74-year-old male. J Gastrointest Cancer. 2016;47(1):118–22. [DOI] [PubMed] [Google Scholar]
- 16.Buscemi S, Orlando E, Damiano G, et al. “Pure” large cell neuroendocrine carcinoma of the gallbladder. Report of a case and review of the literature. Int J Surg. 2016;28(Suppl 1):S128–32. [DOI] [PubMed] [Google Scholar]
- 17.Kamboj M, Gandhi JS, Gupta G, et al. Neuroendocrine carcinoma of gall bladder: a series of 19 cases with review of literature. J Gastrointest Cancer. 2015;46(4):356–64. [DOI] [PubMed] [Google Scholar]
- 18.Furrukh M, Qureshi A, Saparamadu A, Kumar S. Malignant neuroendocrine tumour of the gallbladder with elevated carcinoembryonic antigen: case report and literature review. BMJ Case Rep. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deehan DJ, Heys SD, Kernohan N, Eremin O. Carcinoid tumour of the gall bladder: two case reports and a review of published works. Gut. 1993;34(9):1274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AC, Ethun CG, Liu Y, et al. Studying a rare disease using multi-institutional research collaborations vs big data: Where lies the truth? J Am Coll Surg. 2018;227(3):357–66.e3. [DOI] [PubMed] [Google Scholar]
- 22.Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989–1995. Cancer. 1998;83(12):2618–28. [DOI] [PubMed] [Google Scholar]
- 23.Fong Y, Wagman L, Gonen M, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the national cancer database. Ann Surg. 2006;243(6):767–71; discussion 771–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasumova GG, Tabatabaie O, Najarian RM, et al. Surgical management of gallbladder cancer: simple versus extended cholecystectomy and the role of adjuvant therapy. Ann Surg. 2017;266(4):625–31. [DOI] [PubMed] [Google Scholar]
- 25.Mantripragada KC, Hamid F, Shafqat H, Olszewski AJ. Adjuvant therapy for resected gallbladder cancer: analysis of the national cancer data base. J Natl Cancer Inst. 2017;109(2). [DOI] [PubMed] [Google Scholar]
- 26.Chagpar R, Chiang YJ, Xing Y, et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20(4):1170–8. [DOI] [PubMed] [Google Scholar]
- 27.Kim MK, Warner RR, Roayaie S, et al. Revised staging classification improves outcome prediction for small intestinal neuroendocrine tumors. J Clin Oncol. 2013;31(30):3776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landry CS, Woodall C, Scoggins CR, McMasters KM, Martin RC 2nd. Analysis of 900 appendiceal carcinoid tumors for a proposed predictive staging system. Arch Surg. 2008;143(7):664–70; discussion 670. [DOI] [PubMed] [Google Scholar]
- 29.Yoon KW, Park CH, Lee WS, et al. A case of primary neuroendocrine carcinoma of the gallbladder associated with anomalous union of the pancreaticobiliary duct. Gut Liver. 2009;3(3):231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanakala V, Kasaraneni R, Smith DA, Goulbourne IA. Primary neuroendocrine neoplasm of the gallbladder. BMJ Case Rep. 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Hwang S, Lee SG, et al. Neuroendocrine tumors of the gallbladder: twelve cases in a single institution. Hepatogastroenterology. 2010;57(102–103):1064–8. [PubMed] [Google Scholar]
- 32.Shimizu N, Akashi Y, Fujii T, et al. Use of ALK Immunohisto-chemistry for Optimal Therapeutic Strategy of Pulmonary Large-cell Neuroendocrine Carcinoma and Identification of a Novel KIF5B-ALK Fusion Oncokinase. Anticancer Res. 2019;39(1):413–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.