Abstract
Growth faltering in early childhood is prevalent in many low resource countries. Poor maternal dietary diversity during pregnancy has been linked with increased risk of fetal growth failure and adverse birth outcomes but may also influence subsequent infant growth. Our aim is to assess the role of prenatal maternal dietary diversity in infant growth in rural Uganda. Data from 3291 women and infant pairs enrolled in a birth cohort from 2014 to 2016 were analysed (NCT04233944). Maternal diets were assessed using dietary recall in the second or third trimesters of pregnancy. Maternal dietary diversity scores (DDS) were calculated using the FAO Minimum Dietary Diversity for Women (MDD‐W). Cox regression models were used to evaluate associations of the DDS with the incidence of underweight, stunting and wasting in infants from 3 to 12 months, adjusting for confounding factors. The median DDS for women was low, at 3.0 (interquartile range 3.0–4.0), relative to the threshold of consuming five or more food groups daily. Infants of women in highest quartile of DDS (diverse diets) were less likely to be underweight (adjusted hazard ratio: 0.70, 95% confidence interval: 0.61, 0.80) compared with infants of women in Quartile 1 (p for trend <0.001) in models controlling for maternal factors. There was no significant association between DDS and stunting or wasting. Our findings suggest a relationship between higher maternal dietary diversity and lower risk of underweight in infancy. These findings suggest that programmes to improve infant growth could additionally consider strengthening prenatal dietary diversity to improve child outcomes globally.
Keywords: infant growth, maternal dietary diversity, MDD‐W, prenatal, stunting, Uganda, underweight, wasting
Abbreviations
- CC
community connector
- CI
confidence interval
- DDS
dietary diversity score
- FAO
Food and Agriculture Organization
- HAZ/LAZ
height for age/length for age Z score
- HIV
human immunodeficiency virus
- HR
hazard ratio
- IQR
interquartile range
- LBW
low birth weight
- LMICs
low and middle income countries
- MDD‐W
minimum dietary diversity for women
- SD
standard deviation
- SGA
small for gestational age
- UBCS
Uganda Birth Cohort Study
- UCCP
Uganda Community Connector Program
- WAZ
weight for age Z score
- WHZ/WLZ
weight for height/weight for length Z score
Key messages.
Infant growth faltering is prevalent in many low‐ and middle‐income countries.
Maternal dietary diversity during pregnancy has been linked with fetal growth and birth outcomes.
We found that maternal prenatal dietary diversity was low in rural Uganda and few women met the minimum dietary diversity threshold of five or more food groups out of 10.
In our study, higher maternal dietary diversity during pregnancy was associated with lower risk of infant underweight but not stunting or wasting.
1. INTRODUCTION
Childhood growth faltering, defined as a deficit in a child's length or weight for their age and sex compared with international benchmarks, is prevalent in many low resource countries (Roth et al., 2017; Victora, de Onis, Hallal, Blossner, & Shrimpton, 2010). Underweight, a form of growth faltering, affects 13.5% of the world's children under 5 years, whereas stunting affects 21.9%, and wasting affects 7.3% (United Nations Children's Fund [UNICEF], 2019). Growth faltering is a significant problem in Uganda, where 11% of the children under 5 years are underweight, 29% are stunted and 4% are wasted (Uganda Bureau of Statistics, 2018).
Consequences of poor child growth are severe. Approximately 45% of all child deaths under 5 years are attributed to undernutrition (Black et al., 2013). Children with underweight, stunting or wasting have increased risk of infections and mortality (Black et al., 2013; Olofin et al., 2013). Poor linear growth in childhood has been associated with increased risk of obesity and non‐communicable diseases in adulthood and has been associated with low educational achievement and wage income losses in later life (Black et al., 2013; Fink et al., 2016).
Studies of early child growth in sub‐Saharan Africa indicate that on average, infants begin life with normal weight‐for‐height (WHZ) and weight‐for‐age (WAZ) Z‐scores (Victora, de Onis, Hallal, Blossner, & Shrimpton, 2010), but lower than normal height‐for‐age (HAZ) (Roth et al., 2017). There is then a progressive lagging in growth, starting from early infancy and continuing into the first 2 years of life (Alderman & Headey, 2018; Roth et al., 2017). The period of infancy therefore offers an important window for intervening to prevent or mitigate poor growth. Importantly, addressing poor infant growth may also require intervening to address maternal factors during pregnancy that may influence fetal growth. Maternal diet quality during pregnancy is one such modifiable factor; however, its role in early child growth has not been well described.
Nutrient requirements for women are higher during pregnancy due to additional requirements for fetal growth and other pregnancy‐mediated physiological changes. Energy requirements increase by 13%, protein requirements are 54% higher, and demand for micronutrients, including iron and folate, also increases during pregnancy (Adu‐Afarwuah, Lartey, & Dewey, 2017; Dewey, 2016). Consumption of diversified diets during pregnancy increases the likelihood that a woman will meet her increased micronutrient needs for fetal growth (Arimond et al., 2010; Food and Agriculture Organization [FAO], 2016; Martin‐Prével et al., 2015). Poor dietary diversity and dietary patterns during pregnancy have been associated with increased risk of low birthweight (LBW), small for gestational age (SGA) and preterm births (Gresham, Byles, Bisquera, & Hure, 2014; Madzorera et al., 2020; Okubo et al., 2012; Saaka, 2012; Zerfu, Umeta, & Baye, 2016). Poor birth outcomes predispose children to poor growth, including underweight, stunting and wasting (Aryastami et al., 2017; Christian et al., 2013; Rahman, Howlader, Masud, & Rahman, 2016). In addition, supplementation with micronutrients during pregnancy has been associated with improved postnatal growth (Iannotti, Zavaleta, León, Shankar, & Caulfield, 2008; Villamor et al., 2005).
There is limited evidence on the influence of prenatal maternal diets on early child growth and their role in preventing poor infant growth in resource‐poor settings. One cross‐sectional study in Tanzania found an association between maternal dietary diversity and risk of wasting in children under 24 months of age (Huang et al., 2018). Given these associations, we hypothesized that diverse maternal diets, which provide a wide range of macro‐ and micro‐nutrients, may influence infant growth outcomes. We therefore evaluated the role of maternal dietary diversity during pregnancy on growth outcomes in infants of women enrolled in a birth cohort in Uganda.
2. METHODS
2.1. Context and study location
The study was implemented within the context of the Uganda Community Connector Program (UCCP), a 5‐year cluster‐randomized study of an integrated agriculture–nutrition intervention, designed with a goal of reducing malnutrition among vulnerable populations in Uganda. Study participants received interventions relating to a range of agricultural enterprises (such as promotion of fruit and vegetable cultivation) and nutrition, health and water, sanitation and hygiene (WASH) behaviours, as well as savings and fiscal literacy to improve disposable income. Details of the parent study are described elsewhere (Feed the Future Innovation Lab for Nutrition, 2018).
The study itself was called the Uganda Birth Cohort Study (UBCS; NCT04233944). It recruited participants in 12 districts/16 subcounties in northern and south‐western Uganda from 2014 through 2016. Districts in Uganda are composed of subcounties. The UBCS was composed of eight subcounties randomly selected from the UCCP intervention arm and matched to eight non‐intervention subcounties. The UBCS sample was drawn from women in the intervention and control clusters for the UCCP. Mother–infant pairs selected for the birth cohort were prospectively followed from the time of enrolment in the second and third trimesters of pregnancy, until infants reached 12 months of age. Study participants were pregnant women aged 15–49 years, who consented to the study and intended to stay in the study area throughout the study period.
2.2. Study procedures and follow‐up
A pregnancy surveillance system was established in study areas to screen and enrol women who met the eligibility criteria. Village Health Teams (VHTs) visited women of reproductive age in study areas at home every 3 months. A urine test was used to confirm pregnancy and women's eligibility for the study. Study research assistants visited consenting women at home every 3 months from the time of enrolment until their offspring were 12 months old. A dietary recall was administered to pregnant women at the first prenatal visit (see below).
Anthropometric measurements for infants were made at birth (+3 weeks), 3‐, 6‐, 9‐ and 12‐month postpartum scheduled visits by trained project staff. Length measurements were made to the nearest 0.1 cm using length boards, and weight measures were made to the nearest 100 g using digital Seca weighing scales. Weight and length measures for infants were made in triplicate, and mean measures were used in this analysis.
2.3. Maternal dietary diversity
The primary exposure of interest, maternal dietary diversity during pregnancy, was assessed using a single dietary recall administered to study women by project staff. Women were asked to recall the food they consumed in the previous day, from the time they woke up to the time they went to sleep. The women were probed to assist in their recall of foods consumed. Women's responses were recorded by research staff using a precoded form, which included a list of locally available, commonly consumed foods and food groups.
Recalled foods were categorized into 10 food groups based on Food and Agriculture Organization (FAO)'s Minimum Dietary Diversity for Women (MDD‐W) index (FAO, 2016). Food groups included were (1) grains, white roots and tubers and plantains; (2) legumes (beans, peas and lentils); (3) nuts and seeds; (4) dairy; (5) meats, poultry and fish; (6) eggs; (7) vitamin A‐rich dark green vegetables; (8) other vitamin A‐rich fruits and vegetables; (9) other vegetables; and (10) other fruits. If a food was eaten at least once in the previous day, it was included in the food group. Dietary diversity scores (DDS) were computed as the number of food groups consumed in the previous day based on the baseline prenatal dietary recall for women. The DDS were categorized into quartiles for analysis. In addition, a binary indicator for minimum dietary diversity was defined as consumption of five or more food groups in the previous 24 h as recommended by FAO (2016) in secondary analysis.
2.4. Study outcomes
Infants included in this analysis were aged 0–12 months. Growth outcomes of WAZ, LAZ and WLZ were computed using the WHO growth reference standards (Multicentre Growth Reference Study Group, 2006). Extreme observations determined based on WHO growth standards and infant measurements of WAZ < −6 or WAZ > 5; LAZ < −6 or LAZ > 6; or WLZ < −5 or WLZ > 5 were excluded from the analysis (WHO Child Growth Standards SAS igrowup package, 2006). Where values of infant length were outliers as defined by standardized residuals (≤−3 or ≥+3), in individual linear regressions, the implausible height measures were replaced with predicted height obtained from linear regression using weight and age as predictors to prevent undue influence of extreme values (Shi, Korsiak, & Roth, 2018; Yang & Hutcheon, 2016). Given the greater likelihood of measurement error for anthropometric measures in early infancy and concerns about accuracy and reliability of measures obtained at birth, we restricted our analyses to anthropometric measurements obtained from 3 months onwards.
The primary outcomes were underweight, stunting and wasting. Underweight was defined as WAZ < −2 standard deviations, stunting as LAZ < −2 standard deviations and wasting as WLZ < −2 standard deviations from the median based on WHO growth standards (Multicentre Growth Reference Study Group, 2006).
2.5. Statistical analysis
The analysis was restricted to women who met the cohort study eligibility criteria, had prenatal dietary assessment and infants with anthropometry at the third (±1) month and had at least one other visit. We excluded twin births and women who were HIV positive (self‐reported). Mean (±SD) was used to summarize continuous baseline characteristics, and n (%) was used for categorical variables. Differences between quartiles of dietary diversity were compared using the chi‐square test for categorical variables and the Wilcoxon rank‐sum test for continuous variables.
Times were categorized as the ‘scheduled’ follow‐up times, based on windows of roughly every 3 months, and only one observation per child was used in each window. Hazard ratios (HR) with respect to incident underweight, stunting and wasting were calculated using Cox regression models with the exact method for ties (Smith, Smith, & Ryan, 2003), treating child age as the time scale. The follow‐up time was from the 3‐month visit until infants had the outcome event (growth failure), were lost to follow‐up, died or reached the end of follow‐up at the 12‐month visit, whichever occurred first. The assumption of proportional hazards in the study was assessed by addition of an interaction term for DDS and infant age, and the significance of the interaction term was evaluated using the likelihood ratio test (LRT). If there was evidence of non‐proportional hazards, we included the interaction term with infant age in the final model and the relation of DDS with the outcome is presented separately for each age. All models adjust for clustering by subcounty due to the parent trial design. Tests for trend were conducted for multivariable models using median scores for DDS quartile.
2.6. Potential confounders
We considered the following covariates as potential confounders and explanatory variables: maternal age (<20 years, 20–29 years, ≥30 years), marital status (married/single), maternal education and paternal education (none and primary [0–6 years], secondary and higher [7+ years]), maternal height (continuous or binary [shortness <145 cm, yes/no]), infant sex (male/female), breastfeeding status at each visit (yes/no) and season at time of anthropometric measure (rainy/dry). We defined a household wealth index using principal component analysis based on household asset ownership, quality of housing building materials, fuel used and water and sanitation facilities. We classified households into tertiles based on the wealth index score. We assessed maternal nutrition knowledge in the study using a composite indicator derived from 24 questions on maternal knowledge on pregnancy nutrition, breastfeeding, complementary feeding and childcare practices. Women responded yes or no to the questions and received points for correct responses. A summary score was computed, with a maximum score of 24. We classified women into tertiles based on their maternal nutrition knowledge score. Potential confounding variables were selected for inclusion in adjusted models based on univariate association with the outcome using a significance criterion a of p < 0.20 (Greenland & Pearce, 2015).
All models adjusted for maternal height, child sex and region (north [Apac, Kole, Lamwo, Lira, Nebbi, Pader, Zombo]/south‐west [Kabale, Kabarole, Kamwenge, Kanungu, Rukungiri]) to account for regional, ethnic and other differences in the study sites. We also controlled for whether the woman/household was in a UCCP treatment or control district. The missing indicator method was used to adjust for all missing confounder data (Groenwold et al., 2012).
We also conducted sensitivity analysis with the inclusion of birthweight in fully adjusted models. This was to account for the fact that suboptimal birth outcomes could be potential confounders or mediators of subsequent infant growth. Additionally, we considered that child dietary diversity may have an influence on growth during infancy period. Dietary intake was assessed at all follow‐up visits for infants. We calculated child dietary diversity as the number of food groups consumed by infants in the previous day, from 8 groups: grains, roots and tubers; legumes and nuts; dairy; meats; eggs; fats and oils; vitamin A‐rich fruits and vegetables; and other fruit and vegetable groups. As a sensitivity analysis, we included child dietary diversity in adjusted models.
Finally, to explore whether our imputation of heights for outlier values affected our analysis, we analysed the data omitting all observations with missing or outlier values for the outcome. We also conducted analysis including the outlier height measures.
In a post hoc power analysis, assuming a two‐sided test with an α of 0.05, the analysis has at least 80% power to detect an HR of stunting of ≤0.82 or ≥1.23 and an HR of underweight of ≤0.80 or ≥1.30. For wasting, we would be able detect a true HR of ≤0.75. Statistical analysis was conducted using SAS Version 9.4.
2.7. Ethical considerations
Written informed consent was obtained from study women prior to participation in the study. Ethical approval was obtained from the Makerere University School of Public Health, the Uganda National Council for Science and Technology, Tufts University and the Harvard T.H. Chan School of Public Health.
3. RESULTS
The study recruited 5044 women who met eligibility criteria in the birth cohort study from November 2014 to June 2016. Information on maternal diets and pregnancy outcomes was collected for 4574 women. Women were excluded who did not have live births (N = 120), who reported being HIV positive (N = 227), who had twin births (N = 2 women, four infants) or whose infants had missing dates of birth or did not have anthropometric measures at the 3‐month visit (N = 869) (Figure S1). The final analysis included 3294 woman and infant pairs (Table 1).
TABLE 1.
Baseline socio‐demographic characteristics of pregnant women in the rural Uganda birth cohort
| Characteristics | Maternal dietary diversity score (DDS) a | |||
|---|---|---|---|---|
| Q1 N = 725 (0–2) | Q2 N = 1132 (3) | Q3 N = 844 (4) | Q4 N = 593 (5–9) | |
| N (%) | N (%) | N (%) | N (%) | |
| Maternal characteristics | ||||
| Woman is head of household | 72 (9.9) | 71 (6.3) | 47 (5.6) | 26 (4.4)*** |
| Married | 688 (94.9) | 10496 (92.8) | 785 (93.1) | 549 (92.6) |
| Age, years (mean, SD) | 25.4 ± 6.3 | 25.6 ± 6.2 | 25.6 ± 6.2 | 25.4 ± 5.9 |
| <20 years | 126 (17.5) | 187 (16.6) | 148 (17.6) | 99 (16.7) |
| 20–29 years | 416 (57.8) | 645 (57.3) | 468 (55.7) | 357 (6.2) |
| 30 years or more | 178 (24.7) | 294 (26.1) | 224 (26.7) | 137 (23.1) |
| Maternal education | ||||
| None or primary | 509 (70.2) | 779 (68.9) | 582 (69.0) | 324 (54.6)*** |
| Secondary school or higher | 216 (29.8) | 351 (31.1) | 261 (31.0) | 269 (45.4)*** |
| Maternal height | 158.8 (±6.1) | 150.0 (±6.1) | 159.2 (±6.3) | 159.0 (±6.0) |
| Other study characteristics | ||||
| Child birthweight (median, IQR) | 3.20 (2.90–3.50) | 3.20 (2.95–3.50) | 3.25 (2.98–3.53) | 3.30 (3.00–3.60)*** |
| Community connector participation | 443 (61.1) | 563 (49.8) | 376 (44.6) | 273 (46.0)*** |
| Paternal education | ||||
| None or primary | 363 (50.0) | 574 (50.4) | 401 (47.6) | 279 (40.5)*** |
| Secondary school or higher | 362 (50.0) | 556 (49.2) | 442 (52.4) | 353 (59.5)*** |
| Wealth index b | ||||
| Tertile 1 | 315 (43.5) | 479 (42.4) | 381 (45.2) | 183 (30.9)*** |
| Tertile 2 | 246 (33.9) | 377 (33.4) | 250 (29.7) | 187 (31.5)*** |
| Tertile 3 | 164 (22.6) | 274 (24.3) | 212 (25.2) | 223 (37.6)*** |
| Household fuel | ||||
| Wood | 692 (95.5) | 1062 (94.0) | 790 (93.7) | 523 (88.2)*** |
| Charcoal or other | 33 (4.6) | 66 (5.8) | 52 (6.2) | 68 (11.5)*** |
| Household has electricity | 7 (1.0) | 10 (0.9) | 15 (1.8) | 21 (3.5)*** |
| Household has running water | 17 (2.3) | 22 (2.0) | 34 (4.0) | 56 (9.4)*** |
| Improved pit latrine or flush toilet | 14 (1.9) | 36 (3.2) | 36 (4.3) | 43 (7.3)*** |
Note: Chi‐square p‐values reported for categorical/binary variables and the Wilcoxon test for continuous variables.
Abbreviations: DDS, diet diversity score for women; IQR, interquartile range.
Women's food consumption was assessed using 24‐h recall, and recalled foods were categorized into 10 food groups based on FAO's Minimum Dietary Diversity for Women (MDD‐W) index. Dietary diversity scores (DDS) were computed as the number of food groups consumed.
We defined a household wealth index using principal component analysis based on household asset ownership, quality of housing building materials, fuel used and water and sanitation facilities. We classified households into tertiles based on the wealth index score.
p < 0.05.
p < 0.01.
p < 0.001.
Women in the study population were on average 25.6 (SD ± 6.2) years old, and the majority had primary school education or lower (66.7%). There were socio‐demographic differences in baseline characteristics by quartiles of maternal diet diversity. Women in the highest quartile of diet diversity were more likely to have secondary or higher education, as did their partners, compared with women in the lowest quartile of diversity. Women with highest diet diversity were more likely to be in the highest tertile of wealth index and have electricity and running water in the household compared with women with the least diverse diets. Women in the lowest quartile of diet diversity were more likely to participate in the community connector intervention (61.1% vs. 46.0%) than women in the highest quartile. There was no difference in maternal age or marital status by diet diversity quartiles.
In the previous 24 h, 99% of women reported consuming grains, roots and tubers, 71% beans and peas, 44% other vegetables, 13% orange and yellow fruits and vegetables, 12% dairy and 2% eggs (Figure 1). Dietary diversity in the study population was low, with a median DDS of 3.0 (IQR 3.0–4.0) relative to the acceptable threshold internationally set at five or more food groups out of 10 (FAO, 2016). This standard was achieved by only 18% (N = 593) of the women.
FIGURE 1.
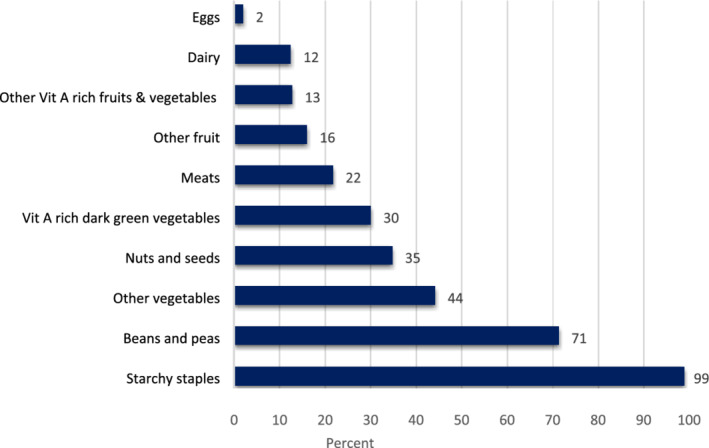
Consumption of food groups in the previous 24 h by pregnant women in the Uganda birth cohort
3.1. Underweight
The prevalence of underweight in infants was 6.4% at the 3‐month visit and increased to 8.5%, 10.1% and 12.1% at the 6‐, 9‐ and 12‐month visits, respectively. Infants of women in Quartile 4 of the DDS were less likely to be underweight at all time points (adjusted HR 0.70, 95% CI: 0.62, 0.80, p ≤ 0.001) compared with infants of women in Quartile 1 (Table 2). No associations were found between minimum dietary diversity (MDD‐W ≥ 5 food groups) and underweight in infants (results not shown).
TABLE 2.
Cox proportional hazards models for incidence of underweight for children aged 3–12 months by prenatal DDS in the Uganda birth cohort, 2014–2016
| Maternal diet diversity score (DDS) | |||||
|---|---|---|---|---|---|
| Q1 (0–2) | Q2 (3) | Q3 (4) | Q4 (5–9) | p for trend | |
| Underweight a | |||||
| n/N | 66/720 | 61/1128 | 50/842 | 34/589 | |
| Univariate HR (95% CI) | |||||
| HR (95% CI) | 1 | 0.63 (0.56, 0.70)*** | 0.64 (0.57, 0.73)*** | 0.65 (0.56, 0.74)*** | <0.001*** |
| Multivariable model HR (95% CI) b | |||||
| HR (95% CI) | 1 | 0.65 (0.57, 0.72)*** | 0.67 (0.59, 0.75)*** | 0.70 (0.62, 0.80)*** | <0.001*** |
| Multivariable model controlling for birthweight c | |||||
| HR (95% CI) | 1 | 0.66 (0.59, 0.74)*** | 0.67 (0.60, 0.76)*** | 0.70 (0.61, 0.80)*** | <0.001*** |
Note: Women's food consumption was assessed using 24‐h recall. Dietary diversity scores (DDS) were computed as the number of food groups consumed. Test for trend conducted using median DDS for diet quartiles.
Abbreviations: 95% CI, 95% confidence interval; DDS: dietary diversity scores; HR: hazard ratio.
We estimated the hazard ratio (HR) of underweight using Cox regression models. HR below 1 implies that the incidence of the outcome was lower among infants of women with more diverse diets. For underweight models, the interaction of maternal diet diversity with child age was not significant, and the proportional hazards assumption is not violated. Models for underweight do not include an interaction term, and we do not present HR by child age.
The multivariable model for underweight adjusts for region (north/south‐west), child sex (male/female), maternal height, breastfeeding status (yes/no), paternal education status (0–6 years/7 or more years), marital status (married/not married), season of dietary intake (rainy/dry) and household wealth index (tertiles). The model also adjusts for participation in the community connector programme (treatment/control) and for clustering by subcounty.
Multivariable model controls for confounders above and also controls for birthweight in sensitivity analysis.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Stunting
Stunting was 27.1% at the 3‐month visit and increased to 29.7%, 35.6% and 39.4% at 6‐, 9‐ and 12‐month visits, respectively (Table 3). Because the assumption of proportional hazards was violated, an interaction term for maternal diets and infant age was included in the models for stunting. Comparing infants of women in Quartile 4 of the DDS with offspring of women in Quartile 1, there was no significant association between DDS and stunting at 3, 6, 9 and 12 months (Table 3). Being a male child was significantly associated with being stunted, whereas taller women had a lower risk of having a stunted infant. Further, infants with higher birthweight were also less likely to be stunted, as shown in sensitivity analysis.
TABLE 3.
Cox regression models for incidence of stunting for children aged 3–12 months by prenatal DDS in the Uganda birth cohort, 2014–2016
| Outcomes | Maternal diet diversity score (DDS) | p for trend | |||
|---|---|---|---|---|---|
| Q1 (0–2) | Q2 (3) | Q3 (4) | Q4 (5–9) | ||
| Stunting a | |||||
| n/N | 188/707 | 301/1106 | 240/824 | 144/583 | |
| Univariate HR (95% CI) | |||||
| 3 months | 1 | 0.95 (0.87, 1.04) | 1.02 (0.93, 1.12) | 0.99 (0.89, 1.10) | 0.02* |
| 6 months | 1 | 0.91 (0.85, 0.97)* | 0.93 (0.86, 1.00)* | 0.95 (0.88, 1.04) | |
| 9 months | 1 | 0.86 (0.79, 0.95)* | 0.84 (0.76, 0.94)* | 0.92 (0.82, 1.03) | |
| 12 months | 1 | 0.82 (0.71, 0.95)* | 0.77 (0.66, 0.91)* | 0.89 (0.75, 1.06) | |
| Multivariable model HR (95% CI) b | |||||
| 3 months | 1 | 0.94 (0.86, 1.03) | 1.01 (0.91, 1.11) | 0.98 (0.88, 1.09) | 0.21 |
| 6 months | 1 | 0.91 (0.85, 0.98)* | 0.94 (0.87, 1.01) | 0.97 (0.89, 1.05) | |
| 9 months | 1 | 0.89 (0.81, 0.98)* | 0.88 (0.79, 0.98)* | 0.95 (0.85, 1.07) | |
| 12 months | 1 | 0.86 (0.74, 1.00)* | 0.82 (0.70, 0.96)* | 0.94 (0.79, 1.11) | |
| Multivariable model HR (95% CI) controlling for birthweight c | |||||
| 3 months | 1 | 0.94 (0.86, 1.03) | 0.99 (0.91, 1.10) | 1.00 (0.90, 1.11) | 0.14 |
| 6 months | 1 | 0.91 (0.85, 0.97)* | 0.93 (0.86, 1.00)* | 0.96 (0.89, 1.05) | |
| 9 months | 1 | 0.87 (0.80, 0.96)* | 0.86 (0.78, 0.96)* | 0.93 (0.83, 1.04) | |
| 12 months | 1 | 0.84 (0.73, 0.98)* | 0.80 (0.68, 0.94)* | 0.89 (0.75, 1.07) | |
Note: Women's food consumption was assessed using 24‐h recall. Dietary diversity scores (DDS) were computed as the number of food groups consumed. Test for trend conducted using median DDS for diet quartiles.
Abbreviations: 95% CI, 95% confidence interval; DDS, dietary diversity scores; HR, hazard ratio.
We estimated the hazard ratio (HR) of stunting using Cox regression models. HR below 1 implies that the incidence of the outcome was less among infant of women with more diverse diets. We evaluated for proportional hazards using interactions with child age. For stunting, the interaction of maternal diet diversity with child age was significant, thus models include an interaction term. We present HR of stunting by child age.
Multivariable models for child stunting adjust for region (north/south‐west), child sex (male/female), maternal height, breastfeeding status (yes/no), paternal education status (0–6 years/7 or more years), maternal age (<20 years, 20–29 years, > 30 years), season of dietary intake (rainy/dry), nutrition knowledge (tertiles) and household wealth index (tertiles). The model also adjusts for participation in the community connector programme (treatment/control) and for clustering by subcounty.
Multivariable model controls for confounders above and also control for birthweight as a sensitivity analysis.
p < 0.05.
3.3. Wasting
The prevalence of wasting increased from the 3‐month visit (4.2%) to the 12‐month visit (5.3 %) (Table 4). Because the assumption of proportional hazards was violated, an interaction term for maternal diets and infant age was included in models for wasting. Comparing infants of women in Quartile 4 of the DDS with offspring of women in Quartile 1, there was no significant association between DDS and wasting at 3, 6, 9 or 12 months. Being a male child was significantly associated with a higher risk of wasting, whereas being of higher socio‐economic status and maternal height were negatively associated with wasting.
TABLE 4.
Cox regression models for incidence of wasting for children aged 3–12 months by prenatal DDS in the Uganda birth cohort, 2014–2016
| Outcomes | Maternal diet diversity score (DDS) | ||||
|---|---|---|---|---|---|
| Q1 (0–2) | Q2 (3) | Q3 (4) | Q4 (5–9) | p for trend | |
| Wasting a | |||||
| n/N | 39/676 | 43/1061 | 30/805 | 18/556 | |
| Univariate HR (95% CI) | |||||
| 3 months | 1 | 0.63 (0.50, 0.80)* | 0.60 (0.46, 0.77)* | 0.86 (0.66, 1.12) | 0.62 |
| 6 months | 1 | 0.69 (0.59, 0.81)* | 0.74 (0.62, 0.88)* | 0.93 (0.77, 1.12) | |
| 9 months | 1 | 0.76 (0.63, 0.92)* | 0.91 (0.74, 1.12) | 1.00 (0.80, 1.25) | |
| 12 months | 1 | 0.83 (0.61, 1.12) | 1.13 (0.83, 1.53) | 1.08 (0.78, 1.50) | |
| Multivariable model HR (95% CI) b | |||||
| 3 months | 1 | 0.62 (0.50, 0.78)* | 0.61 (0.47, 0.79)* | 0.90 (0.69, 1.19) | 0.68 |
| 6 months | 1 | 0.69 (0.59, 0.81)* | 0.75 (0.63, 0.89)* | 0.97 (0.80, 1.17) | |
| 9 months | 1 | 0.76 (0.63, 0.92)* | 0.91 (0.74, 1.12) | 1.04 (0.83, 1.29) | |
| 12 months | 1 | 0.84 (0.63, 1.13) | 1.12 (0.82, 1.52) | 1.11 (0.80, 1.54) | |
| Multivariable model HR (95% CI) controlling for birthweight c | |||||
| 3 months | 1 | 0.63 (0.50, 0.80)* | 0.62 (0.48, 0.80)* | 0.90 (0.69, 1.19) | 0.78 |
| 6 months | 1 | 0.70 (0.59, 0.82)* | 0.77 (0.65, 0.93)* | 1.00 (0.82, 1.22) | |
| 9 months | 1 | 0.78 (0.64, 0.95)* | 0.96 (0.78, 1.18) | 1.11 (0.89, 1.39) | |
| 12 months | 1 | 0.86 (0.63, 1.17) | 1.20 (0.87, 1.64) | 1.23 (0.88, 1.72) | |
Note: Women's food consumption was assessed using 24‐h recall. Dietary diversity scores (DDS) were computed as the number of food groups consumed. Test for trend conducted using median DDS for diet quartiles.
Abbreviations: 95% CI, 95% confidence interval; DDS: dietary diversity scores; HR, hazard ratio.
We estimated the hazard ratio (HR) of wasting using Cox regression models. HR below 1 implies that the incidence of the outcome was less among infant of women with more diverse diets. We evaluated for proportional hazards using interactions with child age. For wasting, the interaction of maternal diet diversity with child age was significant; thus, models include an interaction term. We present HR of wasting by child age.
Models for wasting adjusts for region (north/south‐west), child sex (male/female), maternal height, paternal education status (0–6 years/7 or more years), maternal age (<20 years, 20–29 years, > 30 years), marital status (married/not married), season of dietary intake (rainy/dry) and household wealth index (tertiles). The model also adjusts for participation in the community connector programme (treatment/control) and for clustering by subcounty.
Multivariable model controls for confounders above and also control for birthweight as a sensitivity analysis.
p < 0.05.
In sensitivity analyses, we controlled for birthweight in all fully adjusted models for birth outcomes (underweight, stunting and wasting). Infants with higher birthweight were less likely to be underweight, stunted or wasted. Controlling for birthweight did not change the observed relationships to prenatal diet diversity (Tables 2, 3 and 4). For the binary exposure of minimum dietary diversity (MDD‐W ≥ 5 food groups), we no found significant association with outcomes of stunting and wasting in infants.
Finally, we considered, as a sensitivity analysis, complete case analysis where we excluded observations for infants with outliers for height defined as standardized residuals ≤−3 or ≥+3 from the analysis. We also conducted analysis including the outlier height measures. Our results from both analyses are consistent with reported findings of no association of prenatal maternal diets with stunting and wasting.
4. DISCUSSION
In this prospective study, we evaluated the association of prenatal maternal dietary diversity with the incidence of underweight, stunting and wasting among infants in rural Uganda. We found that diversified maternal diets during pregnancy, measured using the DDS, were associated with a significantly lower risk of underweight in infants after controlling for maternal education, wealth and other factors.
Only limited studies have prospectively evaluated the association of prenatal diets with infant growth. One study conducted in the United States evaluated associations between the consumption of the alternative Mediterranean diet and adherence to the Alternative Healthy Eating Index (AHEI) during pregnancy with growth (WLZ scores) in infants aged 3–6 months and found no association (K Poon, Yeung, Boghossian, S Albert, & Zhang, 2013). Another study, also from the United States, evaluated maternal consumption of unhealthy diets, composed of red and processed meats, and fried foods, and found a negative association with body mass index (BMI‐for‐age) in infants at birth and a positive association at 6 and 12 months of age in unadjusted models, compared with a healthier diet (Martin et al., 2016). These studies were conducted in a different context of a high‐income country where maternal over‐nutrition is a key factor affecting infant growth; however, results from the latter study suggest that maternal diets in pregnancy may have a role in infant growth.
Our findings are similar to the only study that we reviewed that was conducted in a low‐income context, a cross‐sectional study that was conducted in Tanzania. This study found that postnatal maternal dietary diversity (MDD‐W) was positively associated with WHZ and WAZ and lower risk of wasting in children under 24 months of age (Huang et al., 2018). However, this study did not evaluate maternal diets during pregnancy. Finally, other studies have shown that maternal undernutrition prior to or during pregnancy may influence early child growth. For example, maternal underweight has been associated with greater prevalence of underweight, stunting and wasting in young children (Gewa & Yandell, 2012). The limitations of these studies are that they did not evaluate maternal diets during pregnancy and cross‐sectional study designs.
In this study, we found an association between maternal diets and underweight in infants but not with other growth outcomes. All three outcomes share causal factors, such as poor breastfeeding and complementary feeding practices and infectious diseases, however we believe that the magnitude of the effects may be different for the outcomes. Stunting reflects long‐term exposures and their cumulative influence on child growth, whereas wasting reflects an acute response to poor nutrition, and underweight is a composite indicator that reflects both long‐term and acute deprivation (Briend, Khara, & Dolan, 2015; Saaka & Galaa, 2016; Schoenbuchner et al., 2019; Stewart, Iannotti, Dewey, Michaelsen, & Onyango, 2013). Underweight is a complex indicator that represents children who are stunted or wasted, or else who have either both outcomes or neither (Myatt et al., 2018). Therefore, its relationship with maternal diets requires further evaluation.
The fact that we did not find a significant association with stunting may be further explained in part by the fact that there may be other factors other than prenatal maternal diets that are important for infant stunting. The influence of maternal diets may be smaller than that of maternal height, a known predictor of infant growth (Addo et al., 2013; Negash, Whiting, Henry, Belachew, & Hailemariam, 2015). Maternal height has been shown to have a strong influence on stunting but may be less influential on underweight and wasting (Addo et al., 2013; Ali, Saaka, Adams, Kamwininaang, & Abizari, 2017; Subramanian, Ackerson, Davey Smith, & John, 2009). In this study, we adjusted for maternal height, and this may explain the non‐significant association with stunting. We also did not find an association between maternal diets and wasting in infants, and we posit that maternal prenatal diets may not be a key determinant of wasting in infants in this study.
We hypothesized that maternal diets are associated with infant growth outcomes mainly through their effect on birth outcomes. One previous study found that maternal supplementation with zinc in the later postpartum period was associated with longer femur length (Merialdi et al., 2004). As a sensitivity analysis, we adjusted for birthweight, as maternal prenatal diets have been linked to birthweight (Quansah & Boateng, 2020), and birth measures may predict subsequent child growth (Ali, Saaka, Adams, Kamwininaang, & Abizari, 2017). The inclusion of birthweight in the model did not alter our finding that there is an association with diet. This suggests that pregnancy diet or postpartum diets (of the mother and the child) may be important factors. We considered that maternal diets in the study may reflect the importance of infant diets, as maternal diets are positively associated with child diets (Amugsi, Mittelmark, & Oduro, 2015; Nguyen et al., 2013). Mothers and infants in the same household share the same family resources and will most likely eat from the same family pot (Amugsi, Mittelmark, & Oduro, 2015). When we adjusted for child dietary diversity in sensitivity analysis, our findings were however unchanged.
We also considered that maternal diets during pregnancy are likely to be correlated with postnatal diets. In this study, in addition to prenatal diets, we also collected dietary intake for women at 6 and 12 months' postpartum. We found that on average, maternal dietary diversity at all the time points (prenatal and postnatal) was comparable (mean DDS = 3.4 at pregnancy and 3.1 at all other time points). When we adjusted for postnatal diets (at birth for infants at age 3 and 6 months and at 6 months for infants aged 9 and 12 months) in addition to prenatal diets, we found that this did not improve model fit for underweight and wasting (results not shown). We however found that adjusting for postnatal diets improved model fit for stunting, although the observed null associations between prenatal diets and stunting remained unchanged (results also not shown).
We hypothesize that another mechanism through which postnatal diets may be associated with infant growth may be through their influence on breast milk. Studies show that postnatal maternal diets that are high in selenium, omega‐3 and omega‐6 fatty acids and vitamins A, B6 and B12 have been associated with higher content of these nutrients in breast milk (Dal Pont et al., 2016; Dorea, 2002; Innis, 2014). Associations have been shown between child vitamin A status and child growth, and short‐chain fatty acids from breast milk have been associated with weight gain in infants (Prentice et al., 2019; Yakoob & Lo, 2017). We controlled for the infants' breastfeeding status as a sensitivity analysis and observed associations persisted after this adjustment. However, our study is limited by the lack of data on breast milk quality; therefore, our ability to elaborate on this pathway is limited.
An alternative hypothesis is maternal dietary diversity and nutrient deficiencies during pregnancy may predispose infants to nutrient deficiencies that can affect their growth (Allen, 2012). For example, maternal vitamin D status in pregnancy may influence infant vitamin D levels, and vitamin D has been associated with infant growth (Perumal, Al Mahmud, Baqui, & Roth, 2017; Roth et al., 2018). We also anticipated that maternal diet diversity could also be a reflection of family wealth and social status. In multivariable models, we account for these factors and observed associations persist. However, residual confounding could still be a factor as in all cohort studies.
The strengths of our study include that we had a large sample size and we present a prospective analysis with anthropometric data measured at several time points during infancy. The limitations of our study include that maternal diet quality was assessed with the DDS, an index that has been evaluated for associations with micronutrient adequacy, but not other aspects of diet quality (Arimond et al., 2010). We used a single dietary measure for women, measured in the second or third trimester; thus, our study may have missed the effects of early pregnancy diets. Further, our study also experienced loss to follow‐up among infants, with the sample size decreasing at the 12‐month follow‐up period. However, loss to follow‐up in the study was due to the end of project follow‐up, and this is not likely to be a differential cause of bias, as censoring is likely to be non‐informative and independent of the outcome.
This study's generalizability is limited to the rural areas of sub‐Saharan Africa that are similar to the study areas of Northern and South‐west Uganda. In this study, we have excluded birth measures of weight and length from the analysis and include children with pre‐existing underweight, stunting or wasting at 3 months (baseline). As sensitivity analyses, we adjusted for birthweight, and our findings are unchanged, suggesting associations beyond the influence of birth outcomes.
Our findings have implications for global efforts to improve child growth. Because we know that poor child growth in infancy lays the foundation for poor growth in childhood, this study further suggests that maternal dietary intake during pregnancy may also have an important role in determining growth later in infancy and therefore warrants attention. This is the first time to our knowledge that maternal prenatal dietary diversity has been linked with infant growth in LMICs.
5. CONCLUSION
This study suggests a significant relationship between maternal diet diversity and the risk of underweight infants. Further research is required to assess this association and the role of maternal diets during pregnancy on other infant growth outcomes in diverse locations with different dietary patterns. These findings, if confirmed, will have significant implications for policy and programme approaches to improve dietary diversity for women during pregnancy and to decrease child undernutrition in LMICs.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CONTRIBUTIONS
IM conceived the study, conducted the data analysis and drafted the article. CD was a coprincipal investigator for the parent study, conceived the study, contributed to study design, interpreted the data and guided revisions of the manuscript. PW and SG were coprincipal investigators for the parent study, contributed to study design, interpreted the data and guided revisions of the draft manuscript. BB, EA and FT were coprincipal investigators for the parent study, participated in the study implementation and field supervision, interpreted the data and guided revisions of the manuscript. MW, WF, SI, EH and GN contributed to study design, interpreted the data and guided revisions of the draft manuscript. All authors contributed to the editing of the final version of the manuscript.
Supporting information
Data S1 Supporting information
ACKNOWLEDGMENTS
This paper is supported by the Feed the Future Innovation Lab for Nutrition at Tufts University in Boston, MA, and by the United States Agency for International Development (award AID‐OAA‐L‐10‐00006). CD was supported in part by NIH grants K24DK104676 and 2P30 DK040561.
Madzorera I, Ghosh S, Wang M, et al. Prenatal dietary diversity may influence underweight in infants in a Ugandan birth‐cohort. Matern Child Nutr. 2021;17:e13127. 10.1111/mcn.13127
REFERENCES
- Addo, O. Y. , Stein, A. D. , Fall, C. H. , Gigante, D. P. , Guntupalli, A. M. , Horta, B. L. , … Consortium on Health Orientated Research in Transitional Societies, G . (2013). Maternal height and child growth patterns. The Journal of Pediatrics, 163(2), 549–554. 10.1016/j.jpeds.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah, S. , Lartey, A. , & Dewey, K. G. (2017). Meeting nutritional needs in the first 1000 days: A place for small‐quantity lipid‐based nutrient supplements. Annals of the New York Academy of Sciences, 1392(1), 18–29. 10.1111/nyas.13328 [DOI] [PubMed] [Google Scholar]
- Alderman, H. , & Headey, D. (2018). The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS ONE, 13(4), e0195904–e0195904. 10.1371/journal.pone.0195904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, Z. , Saaka, M. , Adams, A.‐G. , Kamwininaang, S. K. , & Abizari, A.‐R. (2017). The effect of maternal and child factors on stunting, wasting and underweight among preschool children in Northern Ghana. BMC Nutrition, 3(1), 31. 10.1186/s40795-017-0154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, L. H. (2012). B vitamins in breast milk: relative importance of maternal status and intake, and effects on infant status and function. Advances in Nutrition, 3(3), 362–369. 10.3945/an.111.001172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amugsi, D. A. , Mittelmark, M. B. , & Oduro, A. (2015). Association between maternal and child dietary diversity: An analysis of the Ghana demographic and health survey. PLoS ONE, 10(8), e0136748–e0136748. 10.1371/journal.pone.0136748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimond, M. , Wiesmann, D. , Becquey, E. , Carriquiry, A. , Daniels, M. C. , Deitchler, M. , … Torheim, L. E. (2010). Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource‐poor settings. The Journal of Nutrition, 140(11), 2059S–2069S. 10.3945/jn.110.123414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryastami, N. K. , Shankar, A. , Kusumawardani, N. , Besral, B. , Jahari, A. B. , & Achadi, E. (2017). Low birth weight was the most dominant predictor associated with stunting among children aged 12–23 months in Indonesia. BMC Nutrition, 3(1), 16. 10.1186/s40795-017-0130-x [DOI] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Briend, A. , Khara, T. , & Dolan, C. (2015). Wasting and stunting‐‐Similarities and differences: Policy and programmatic implications. Food and Nutrition Bulletin, 36(1 Suppl), S15–S23. 10.1177/15648265150361s103 [DOI] [PubMed] [Google Scholar]
- Christian, P. , Lee, S. E. , Donahue Angel, M. , Adair, L. S. , Arifeen, S. E. , Ashorn, P. , … Hu, G. (2013). Risk of childhood undernutrition related to small‐for‐gestational age and preterm birth in low‐ and middle‐income countries. International Journal of Epidemiology, 42(5), 1340–1355. 10.1093/ije/dyt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Pont, A. , Ferraroni, M. , Bravi, F. , Decarli, A. , Agostoni, C. , & Wiens, F. (2016). Impact of maternal nutrition on breast‐milk composition: A systematic review. The American Journal of Clinical Nutrition, 104(3), 646–662. 10.3945/ajcn.115.120881 [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. (2016). Reducing stunting by improving maternal, infant and young child nutrition in regions such as South Asia: Evidence, challenges and opportunities. Maternal & Child Nutrition, 12(S1), 27–38. 10.1111/mcn.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorea, J. G. (2002). Selenium and breast‐feeding. British Journal of Nutrition, 88(5), 443–461. 10.1079/BJN2002692 [DOI] [PubMed] [Google Scholar]
- Feed the Future Innovation Lab for Nutrition . (2018). Semi Annual report Year 6: Tufts University. Tisch Library. [Google Scholar]
- Food and Agriculture Organization . (2016). Minimum dietary diversity for women: A guide to measurement. Retrieved from Feed the Future. (2018). Retrieved from Innovation Lab for Nutrition
- Fink, G. , Peet, E. , Danaei, G. , Andrews, K. , McCoy, D. C. , Sudfeld, C. R. , … Fawzi, W. W. (2016). Schooling and wage income losses due to early‐childhood growth faltering in developing countries: National, regional, and global estimates. The American Journal of Clinical Nutrition, 104(1), 104–112. 10.3945/ajcn.115.123968 [DOI] [PubMed] [Google Scholar]
- Gewa, C. A. , & Yandell, N. (2012). Undernutrition among Kenyan children: Contribution of child, maternal and household factors. Public Health Nutrition, 15(6), 1029–1038. 10.1017/s136898001100245x [DOI] [PubMed] [Google Scholar]
- Greenland, S. , & Pearce, N. (2015). Statistical foundations for model‐based adjustments. Annual Review of Public Health, 36, 89–108. 10.1146/annurev-publhealth-031914-122559 [DOI] [PubMed] [Google Scholar]
- Gresham, E. , Byles, J. E. , Bisquera, A. , & Hure, A. J. (2014). Effects of dietary interventions on neonatal and infant outcomes: A systematic review and meta‐analysis. The American Journal of Clinical Nutrition, 100(5), 1298–1321. 10.3945/ajcn.113.080655 [DOI] [PubMed] [Google Scholar]
- Groenwold, R. H. H. , White, I. R. , Donders, A. R. T. , Carpenter, J. R. , Altman, D. G. , & Moons, K. G. M. (2012). Missing covariate data in clinical research: When and when not to use the missing‐indicator method for analysis. CMAJ, 184(11), 1265–1269. 10.1503/cmaj.110977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Sudfeld, C. , Ismail, A. , Vuai, S. , Ntwenya, J. , Mwanyika‐Sando, M. , & Fawzi, W. (2018). Maternal dietary diversity and growth of children under 24 months of age in rural Dodoma, Tanzania. Food and Nutrition Bulletin, 39(2), 219–230. 10.1177/0379572118761682 [DOI] [PubMed] [Google Scholar]
- Iannotti, L. L. , Zavaleta, N. , León, Z. , Shankar, A. H. , & Caulfield, L. E. (2008). Maternal zinc supplementation and growth in Peruvian infants. The American Journal of Clinical Nutrition, 88(1), 154–160. 10.1093/ajcn/88.1.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis, S. M. (2014). Impact of maternal diet on human milk composition and neurological development of infants. The American Journal of Clinical Nutrition, 99(3), 734S–741S. 10.3945/ajcn.113.072595 [DOI] [PubMed] [Google Scholar]
- K Poon, A. , Yeung, E. , Boghossian, N. , S Albert, P. , & Zhang, C. (2013). Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth (vol. 2013). [DOI] [PMC free article] [PubMed]
- Madzorera, I., Isanaka, S., Wang, M., Msamanga, G. I., Urassa, W., Hertzmark, E., ⋯ Fawzi, W. W. (2020). Maternal dietary diversity and dietary quality scores in relation to adverse birth outcomes in Tanzanian women. The American Journal of Clinical Nutrition, 112(3), 695–706. 10.1093/ajcn/nqaa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C. L. , Siega‐Riz, A. M. , Sotres‐Alvarez, D. , Robinson, W. R. , Daniels, J. L. , Perrin, E. M. , & Stuebe, A. M. (2016). Maternal dietary patterns during pregnancy are associated with child growth in the first 3 years of life. The Journal of Nutrition, 146(11), 2281–2288. 10.3945/jn.116.234336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Prével, Y. , Allemand, P. , Wiesmann, D. , Arimond, M. , Ballard, T. , Deitchler, M. , Dop, M.C. , Kennedy, G. , Lee, W.T. & Mousi, M. (2015). Moving forward on choosing a standard operational indicator of women's dietary diversity. Retrieved from Rome:
- Merialdi, M. , Caulfield, L. , Zavaleta, N. , Figueroa, A. , Costigan, K. , Dominici, F. , & Dipietro, J. (2004). Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. The American Journal of Clinical Nutrition, 79, 826–830. 10.1093/ajcn/79.5.826 [DOI] [PubMed] [Google Scholar]
- Multicentre Growth Reference Study Group, W. H. O., & de Onis, M . (2006). WHO child growth standards based on length/height, weight and age. Acta Paediatrica, 95(Supplement 450), 76–85. 10.1080/08035320500495548 [DOI] [Google Scholar]
- Myatt, M. , Khara, T. , Schoenbuchner, S. , Pietzsch, S. , Dolan, C. , Lelijveld, N. , & Briend, A. (2018). Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Archives of Public Health, 76(1), 28. 10.1186/s13690-018-0277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash, C. , Whiting, S. J. , Henry, C. J. , Belachew, T. , & Hailemariam, T. G. (2015). Association between maternal and child nutritional status in Hula, rural Southern Ethiopia: A cross sectional study. PLoS ONE, 10(11), e0142301–e0142301. 10.1371/journal.pone.0142301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, P. H. , Avula, R. , Ruel, M. T. , Saha, K. K. , Ali, D. , Tran, L. M. , … Rawat, R. (2013). Maternal and child dietary diversity are associated in Bangladesh, Vietnam, and Ethiopia. The Journal of Nutrition, 143(7), 1176–1183. 10.3945/jn.112.172247 [DOI] [PubMed] [Google Scholar]
- Okubo, H. , Miyake, Y. , Sasaki, S. , Tanaka, K. , Murakami, K. , Hirota, Y. , … Ohya, Y. (2012). Maternal dietary patterns in pregnancy and fetal growth in Japan: The Osaka maternal and child health study. The British Journal of Nutrition, 107(10), 1526–1533. 10.1017/s0007114511004636 [DOI] [PubMed] [Google Scholar]
- Olofin, I. , McDonald, C. M. , Ezzati, M. , Flaxman, S. , Black, R. E. , Fawzi, W. W. , … Danaei, G. (2013). Associations of suboptimal growth with all‐cause and cause‐specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE, 8(5), e64636. 10.1371/journal.pone.0064636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal, N. , Al Mahmud, A. , Baqui, A. H. , & Roth, D. E. (2017). Prenatal vitamin D supplementation and infant vitamin D status in Bangladesh. Public Health Nutrition, 20(10), 1865–1873. 10.1017/s1368980015003092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice, P. M. , Schoemaker, M. H. , Vervoort, J. , Hettinga, K. , Lambers, T. T. , Van Tol, E. A. , … Koulman, A. (2019). Human milk short‐chain fatty acid composition is associated with adiposity outcomes in infants. The Journal of Nutrition, 149(5), 716–722. 10.1093/jn/nxy320 [DOI] [PubMed] [Google Scholar]
- Quansah, D. Y. , & Boateng, D. (2020). Maternal dietary diversity and pattern during pregnancy is associated with low infant birth weight in the Cape Coast metropolitan hospital, Ghana: A hospital based cross‐sectional study. Heliyon, 6(5), e03923. 10.1016/j.heliyon.2020.e03923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, M. S. , Howlader, T. , Masud, M. S. , & Rahman, M. L. (2016). Association of low‐birth weight with malnutrition in children under five years in Bangladesh: Do mother's education, socio‐economic status, and birth interval matter? PLoS ONE, 11(6), e0157814–e0157814. 10.1371/journal.pone.0157814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, D. E. , Krishna, A. , Leung, M. , Shi, J. , Bassani, D. G. , & Barros, A. J. D. (2017). Early childhood linear growth faltering in low‐income and middle‐income countries as a whole‐population condition: Analysis of 179 demographic and health surveys from 64 countries (1993‐2015). The Lancet Global Health, 5(12), e1249–e1257. 10.1016/S2214-109X(17)30418-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, D. E. , Morris, S. K. , Zlotkin, S. , Gernand, A. D. , Ahmed, T. , Shanta, S. S. , … Al Mahmud, A. (2018). Vitamin D supplementation in pregnancy and lactation and infant growth. New England Journal of Medicine, 379(6), 535–546. 10.1056/NEJMoa1800927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaka, M. (2012). Maternal dietary diversity and infant outcome of pregnant women in Northern Ghana. Int. J. Child Health Nutr.. 10.6000/1929-4247.2012.01.02.6 [DOI] [Google Scholar]
- Saaka, M. , & Galaa, S. Z. (2016). Relationships between wasting and stunting and their concurrent occurrence in Ghanaian preschool children. Journal of Nutrition and Metabolism, 2016, 4654920. 10.1155/2016/4654920, 1, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbuchner, S. M. , Dolan, C. , Mwangome, M. , Hall, A. , Richard, S. A. , Wells, J. C. , … Moore, S. E. (2019). The relationship between wasting and stunting: A retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. The American Journal of Clinical Nutrition, 110(2), 498–507. 10.1093/ajcn/nqy326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Korsiak, J. , & Roth, D. E. (2018). New approach for the identification of implausible values and outliers in longitudinal childhood anthropometric data. Annals of Epidemiology, 28(3), 204–211.e203. 10.1016/j.annepidem.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T. , Smith, B. , & Ryan, M. A. (2003). Survival analysis using Cox proportional hazards modeling for single and multiple event time data. Retrieved from Cary, NC, USA: Paper presented at the Proceedings of the Twenty‐eighth Annual SAS Users Group International Conference, SAS Institute, Inc., paper. [Google Scholar]
- Stewart, C. P. , Iannotti, L. , Dewey, K. G. , Michaelsen, K. F. , & Onyango, A. W. (2013). Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & Child Nutrition, 9(Suppl 2), 27–45. 10.1111/mcn.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S. V. , Ackerson, L. K. , Davey Smith, G. , & John, N. A. (2009). Association of maternal height with child mortality, anthropometric failure, and anemia in India. JAMA, 301(16), 1691–1701. 10.1001/jama.2009.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics, U., & Icf . (2018). Uganda demographic and health survey 2016. Retrieved from Kampala, Uganda. http://dhsprogram.com/pubs/pdf/FR333/FR333.pdf [Google Scholar]
- United Nations Children's Fund (UNICEF), W. H. O., International Bank for Reconstruction and Development/The World Bank . (2019). Levels and trends in child malnutrition: Key findings of the 2019 edition of the joint child malnutrition estimates. Retrieved from Geneva: World Health Organization. [Google Scholar]
- Victora, C. G. , de Onis, M. , Hallal, P. C. , Blossner, M. , & Shrimpton, R. (2010). Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics, 125(3), e473–e480. 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- Villamor, E. , Saathoff, E. , Bosch, R. J. , Hertzmark, E. , Baylin, A. , Manji, K. , … Fawzi, W. W. (2005). Vitamin supplementation of HIV‐infected women improves postnatal child growth. The American Journal of Clinical Nutrition, 81(4), 880–888. 10.1093/ajcn/81.4.880 [DOI] [PubMed] [Google Scholar]
- WHO Child Growth Standards SAS igrowup package . (2006). In.
- Yakoob, Y. M. , & Lo, W. C. (2017). Nutrition (micronutrients) in child growth and development: A systematic review on current evidence, recommendations and opportunities for further research. Journal of Developmental & Behavioral Pediatrics, 38(8), 665–679. 10.1097/DBP.0000000000000482 [DOI] [PubMed] [Google Scholar]
- Yang, S. , & Hutcheon, J. A. (2016). Identifying outliers and implausible values in growth trajectory data. Annals of Epidemiology, 26(1), 77–80.e802. 10.1016/j.annepidem.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfu, T. A. , Umeta, M. , & Baye, K. (2016). Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. The American Journal of Clinical Nutrition, 103(6), 1482–1488. 10.3945/ajcn.115.116798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information


