Supplemental Digital Content is available in the text
Keywords: backlog, consensus, coronavirus, Delphi, endoscopy, pandemic, pathways, recommendations, resources, resumption, surgery
Abstract
Objective:
The aim of this work is to formulate recommendations based on global expert consensus to guide the surgical community on the safe resumption of surgical and endoscopic activities.
Background:
The COVID-19 pandemic has caused marked disruptions in the delivery of surgical care worldwide. A thoughtful, structured approach to resuming surgical services is necessary as the impact of COVID-19 becomes better controlled. The Coronavirus Global Surgical Collaborative sought to formulate, through rigorous scientific methodology, consensus-based recommendations in collaboration with a multidisciplinary group of international experts and policymakers.
Methods:
Recommendations were developed following a Delphi process. Domain topics were formulated and subsequently subdivided into questions pertinent to different aspects of surgical care in the COVID-19 crisis. Forty-four experts from 15 countries across 4 continents drafted statements based on the specific questions. Anonymous Delphi voting on the statements was performed in 2 rounds, as well as in a telepresence meeting.
Results:
One hundred statements were formulated across 10 domains. The statements addressed terminology, impact on procedural services, patient/staff safety, managing a backlog of surgeries, methods to restart and sustain surgical services, education, and research. Eighty-three of the statements were approved during the first round of Delphi voting, and 11 during the second round. A final telepresence meeting and discussion yielded acceptance of 5 other statements.
Conclusions:
The Delphi process resulted in 99 recommendations. These consensus statements provide expert guidance, based on scientific methodology, for the safe resumption of surgical activities during the COVID-19 pandemic.
The rapid global spread of coronavirus disease 19 (COVID-19) presents an unprecedented crisis for the surgical and endoscopic community, which has forced to rapidly decrease or even halt elective surgical practices.1,2 As a consequence there is a backlog of patients needing surgical care, along with increased financial hardships for healthcare workers and hospital systems.3,4 There remains uncertainty about the duration of this pandemic and the extent of its consequences on surgical services and patients.5
The COVID19 pandemic presents immediate challenges to the surgical and endoscopic global community. Given the complexity of the growing issues, there is a need in healthcare communities for clear and structured guidance pertaining to when, where, and how to restart surgical practices amidst the COVID-19 pandemic.
Despite multiple position statements from prominent organizations with recommendations on when and how to resume elective surgical services,3,4 there is a paucity of evidence-based techniques used to formulate these processes. Additional limitations of the published position statements are that either they are not specifically made for surgical and endoscopic settings or they are not detailed enough to serve as an effective and comprehensive guide. The acute nature of the pandemic has made it difficult to employ traditional sources of high-level evidence and stringent consensus methodology.
The Coronavirus Global Surgical Collaborative (CVGSC) in conjunction with a group of international experts from four continents representing a wide range of surgical, anesthesia, and endoscopic societies sought to formulate consensus recommendations. The group also included policymakers and patient representatives, and sought to apply validated, rigorous scientific methodology to formulate pertinent recommendations.
The CVGSC was formed with the purpose of sharing experiences and disseminating information related to the COVID-19 pandemic. It is an initiative sponsored by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) in collaboration with representatives from the European Association for Endoscopic Surgery (EAES), Americas Hepato-Pancreato-Biliary Association (AHPBA), American Society of Anesthesiologist (ASA), the European-African Hepato-Pancreato-Biliary Association (E-AHPBA), the Endoscopic and Laparoscopic Surgeons of Asia (ELSA), the Enhanced Recovery after Surgery Society (ERAS-UK), French Surgical Association (AFC), the International Consortium of Minimally Invasive Pancreatic Surgery (IMIPS), the Korean Society of Endoscopic and Laparoscopic Surgeons (KSELS), the Spanish Association of Surgeons (AEC), Society for Surgery of the Alimentary Tract (SSAT), and other international leaders in surgery.
This work aims not to provide expertise in the management of the COVID-19 disease, but to take advantage of the knowledge gained by internationally renowned surgical leaders in the handling of the COVID-19 crisis affecting the surgical community and on the safe resumption of surgical and endoscopic activities.
METHODS
These recommendations were produced following modified Delphi methodology.6 The Executive Committee - EC (HA, NF, MAH, FK, DA) served as the organizers of the consensus and guided the Delphi process through the steps outlined below. The Steering Committee (SC) was composed of members of the CVGSC. The SC in conjunction with other international experts made up the Expert Group (EG). Selection of members of the EG was based on their peers’ recommendations and their leadership positions across different specialties and societies. Due to the global nature of the pandemic and broad range of topics discussed, diversity of participant background was essential (see supplemental table 2). EG members represented surgeons, anesthesiologists, gastroenterologists, governmental policymakers, and patient advocates/representatives from 15 different countries in North and South America, Europe, and Asia.
Identification of Topic Domains and Formulation of Questions
General domains and associated questions relevant to the pandemic's effect on the delivery of surgical services were initially proposed by the SC on April 27, 2020. Published position statements on how to resume surgical services were reviewed and used to help formulating the areas of interest and associated questions. These domains and questions were approved by members of the EC and further defined during a virtual meeting between the SC and EC. For standardization purposes, the domains and questions were formulated in the setting of three phases of the COVID-19 pandemic as defined by the CVGSC (Table 1). The answers to these questions would form the basis for the recommendations produced by the process.
TABLE 1.
CVGSC Classification of COVID 19 Pandemic Phases
| Phase 1 | Sustained human to human transmission |
| Phase 2 | Pandemic phase with widespread human infection and strain in healthcare resources |
| Phase 3 | Post peak phase, when pandemic disease levels with adequate surveillance would have dropped to below peak levels, and where pandemic activities seem to be decreasing but it is still uncertain if a recurrent outbreak will occur |
Formulation of Statements
The EG members were divided into subgroups organized by domain topics and led by a designated chair. Each subgroup formulated statements addressing the questions in their domains. The statements were produced taking into account the literature and guidelines that were available at the time the manuscript was drafted. Statements were then submitted to the EC, which did not participate in the formulation of these statements.
Voting
The EC compiled a synthesis of the statements received from each subgroup of experts. In some cases, the wording of the statements was modified by the EC to have more uniform syntax across the statements and to eliminate redundant proposals. Potentially conflicting statements were left as-is and highlighted during subsequent rounds of voting and discussion. The EC did not alter the content of the statements, and any concerns regarding the effect of adjusting statements were discussed with and approved by the subgroups that wrote them.
First Round of Voting (D1) – May 10, 2020
The statements were then distributed to all experts for a first round of Delphi voting (D1). EG members voted to agree or disagree with the statements, and thus qualify the statements as valid by expert opinion. A dichotomous polling method was chosen over a Likert process because the final goal was to assess if there was agreement with the statements or not. Using a Likert process may have added more variability of opinion to the subjects for which no science is available. The binary system would force the experts to be more definitive in their decision. Beside each statement, a section for comments was available. The authors of the statements and the resultant votes/comments remained anonymous. This approach was utilized to avoid bias created by undue influence of individuals or subgroups on others. The EC did not partake in voting.
Consensus was achieved when a statement reached at least 80% of votes in agreement. Statements with less than 80% agreement and related comments in D1 were returned to the expert subgroup that formulated them. Per methodology, statements that reached consensus in the first round were not sent for a second round because if they reached the 80% threshold they would not undergo any major modification that would require a second voting process. However, any minor concerns could be addressed in the virtual meeting. The subgroups had the option to revise statements that did not reach 80% agreement based on feedback, or to recommend discarding them based on excessive need for modification.
Second Round of Voting (D2) – May 16, 2020.
Revised statements were sent for a second round of Delphi voting (D2). The same process of anonymous submission, voting, and commenting was undertaken. Statements that did not reach 80% approval were marked for further review.
Virtual Meeting (VM3) – May 22, 2020
A virtual meeting with the entire group of experts was held for final discussion (VM3) of statements that did not reach approval in D2. This meeting also allowed experts to bring to attention any other issues or statements they felt required further consideration. Any adjusted statements were anonymously voted on after discussion, with the same 80% threshold for approval.
Voting for D1 and D2 was carried out through electronic questionnaires on the online platform SurveyGizmo.7 The video teleconference during VM3 was held on Zoom,8 and voting during that conference was through Poll Everywhere.9
Data analysis was based on percentage response rates for each statement in each round of voting. After completion of all voting and statement formulation, the manuscript was drafted with the recommendations and sent to all members for revision, input, and approval before submission for publication. A certification of reading and acknowledgment was electronically collected from all 44 authors. Voting in D1/D2 and manuscript review was mandatory for all EG members.
Throughout the process, 2 surgeon researchers (FK, DA) were involved in collecting and organizing data, communicating with experts/committee members, and creating and distributing the electronic questionnaires. These individuals did not partake in the selection of experts, organization of subgroups, formulation of questions or statements, or in voting.
RESULTS
A total of 10 domains pertinent to surgery and endoscopy during the global crisis were identified, and 12 general questions were jointly created by the Executive and Steering Committees within these domains. The questions pertaining to each domain were addressed by the 10 groups of experts in the form of 100 statements (Fig. 1).
FIGURE 1.

Flowchart of the Delphi process. Numbers between boxes reflect the number of statements carried on to be voted on the next stage. The Expert Group includes members of the Steering Committee but excludes members of the Executive Committee.
Eighty-three of the statements (83.0%) were approved during D1. Fifteen statements that were not approved were revised and submitted for voting in D2. Two statements that did not reach consensus were considered by the author subgroup to not warrant revision. A total of 440 comments were made during D1.
Eleven out of the 15 submitted statements (73.3%) were approved in D2. There were 74 comments submitted in D2. The large majority of the revised statements attained a significantly improved approval score after revision.
Twenty-nine experts attended VM3. Four unapproved statements from D2 were discussed and voted on. Further discussion was held about various other topics for better clarification and standardization. This process included revisiting one of the previously discarded statements from D1. This statement was also revised and submitted for voting. After detailed discussion and revision, all five statements voted on in VM3 were approved.
Overall, the Delphi process approved 99 statements (99.0%) for the expert consensus. The questions, final recommendations, and respective approval rates in each step of the Delphi processes (D1/D2/VM3) are depicted in supplemental table 1. Flowcharts for prompt visualization of the statements pertinent to patients and staff are depicted in Figures 2 and 3.
FIGURE 2.
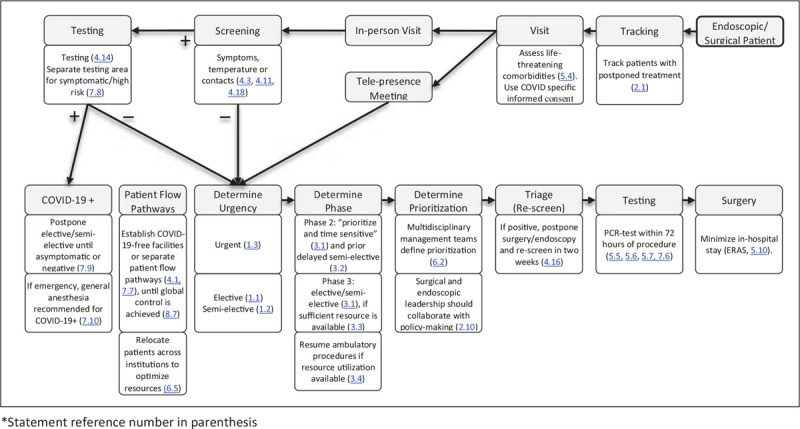
Flowchart for patient tracking, in-hospital screening, and preoperative testing (statement reference number in parenthesis).
FIGURE 3.
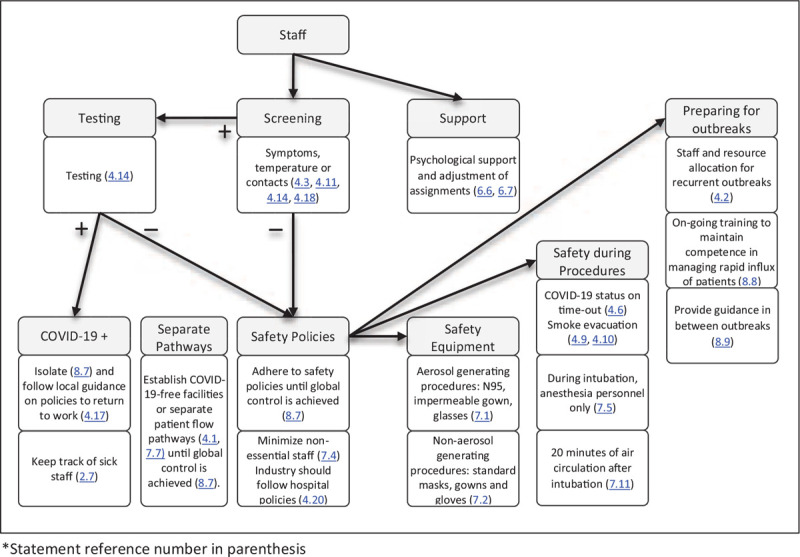
Flowchart and safety measures for staff in the perioperative setting (statement reference number in parenthesis).
DISCUSSION
The CVGSC recommendations represent a cohesive international effort to provide guidance on the resumption of hospital surgical and endoscopic activities taking into account the serious burden on our healthcare systems and society caused by the COVID-19 pandemic. At the time the recommendations were drafted, over 5.4 million cases of COVID-19 infections had been reported worldwide, leading to more than 340,000 related deaths and a significant burden on hospital admissions.10 Both the volume of critically ill patients and the uncertainty about characteristics specific to COVID-19 present unprecedented challenges that have left healthcare systems disoriented worldwide.11–13 This was exacerbated by the significant need to reallocate healthcare resources, which has led to a profound disruption in surgical and endoscopic services.14 A recent estimate notes that over 28 million patients are awaiting treatment, a number which continues to grow in the setting of new restrictions on delivery of care and a pandemic that is still evolving15 As this progression continues, it is clear that ongoing changes in procedure-based specialties must include safety, economic, logistic, and ethical considerations.16–19
The same considerations are central to strategies for managing the backlog of patients awaiting surgery. Many countries in quarantine are evaluating ways to ease social restrictions.20–22
Given the lack of evidence to guide the surgical community on how to safely resume surgical activities amid the COVID-19 pandemic, the CVGSC recommendations were developed with rigorous adherence to Delphi methodology of establishing expert consensus. This methodology overcomes limitations inherent to group pooling and discussion by virtue of its structure and element of anonymity.23 These shortcomings include undue influence by certain individuals, pressure to conform to the group, and noncontributory discussions that deviate from stated objectives. The structured nature of the Delphi process facilitates controlled feedback, reiteration of concept and reassessment of opinion, and the ability to apply statistical analysis techniques.6,24
A threshold of ≥80% of votes in agreement was used to qualify a statement as having reached group consensus. In Delphi methodology, there is no validated level of agreement to be attained.25 Given the lack of evidence in this field, investigators aimed to achieve a strong consensus by choosing a higher agreement threshold than those often employed (around 70%–75%).
It was important to begin the Delphi process by ensuring agreement on the nomenclature that is related to the urgency of care. The definitions established in Statement 1.1 to 1.3 (“urgent,” “semi-elective,” “elective”) serve to differentiate between procedures based on the consequence of their being delayed. The complexity of surgical diseases includes the consideration of the many factors that affect outcomes, from patient comorbidities to the availability of treatment options, to patient preference. It is therefore difficult to define the procedure's urgency. The definitions do not represent further nuances of the disease process, such as whether a malignancy is present or not.
Consensus was established for all three definitions in D1. The topic was revisited during discussion of Statement 3.1 in VM3. The final wording of Statement 3.1 reflects the position that the procedures with most flexibility in rescheduling should be those procedures with least expected negative consequence after a delay, independent of the diagnosis. Other factors remain an important part of decision making, such as the phase of the pandemic and available medical supplies.
The authors acknowledge that the true impact of this pandemic will be extensive and long-lasting but it was felt that measuring the impact of COVID-19 was an essential step to adjust current recommendations, and to prepare for potential future major disruptions in healthcare. Recommendations are provided in Domain 2 on how to gather information that can be used to assess this impact, immediately and prospectively. This approach involves a multifaceted analysis including data from screening radiology and endoscopy, cancer stage at presentation, and trends in case volume. Furthermore, there is emphasis on tracking patients whose plans for intervention were altered, which will avoid losing patients to follow-up.
The authors agree that the leadership role of surgeons, endoscopists, and other interventional providers extend far beyond their procedural rooms. Physician leaders are encouraged to actively participate in the decision-making that can shape local, regional, and global policies. At the same time, the global extent of COVID-19 and its effect across medical specialties necessitates a collaborative mindset. Statements in Questions 2B, 6, 9, and others highlight the importance of multidisciplinary communication and shared decision-making.
Protection of patients and staff was another important domain that underpinned several statements in this study reporting on measures that support infection prevention and control. This is achieved through adequate protection of COVID-19-negative healthcare workers and patients, and successful isolation of COVID-19-positive individuals. The pathways outlined aim to minimize exposure to the virus by strictly controlling the risk of transmission throughout the perioperative pathway. Domain 4 elaborates on important measures necessary to protect both patients, visitors and staff as hospitals consider returning to more active surgical/endoscopic schedules. Of note, it was recommended that visitors should not be allowed in the hospital during periods when the local burden of cases is high.
These recommendations are in overall agreement with the principles and guidelines previously published by the American College of Surgeons (ACS), American Center for Disease Control and Prevention (CDC), and European Centre for Disease Prevention and Control (ECDC).3,26,27 However, they provide detailed information encompassing a wide variety of subjects on a single document and elaborate on how to address education, training, and research during the pandemic.
Much discussion was generated in the final round regarding the screening and testing for COVID-19 among staff and patients. This is a complex issue for many reasons: no tests are well-validated with concurrent high sensitivity/specificity,28,29 signs and symptoms vary widely,30 there is no good evidence on optimal protocols, and the current data is part of a dynamic and constantly evolving field.
According to a symptom-based approach to testing, the CDC recommends that staff should return to work at least 10 days after the beginning and 3 days after resolution of symptoms.27 The ECDC recommends 8 days after onset and 3 days after resolution26 of symptoms, and the United Kingdom National Health Service (NHS) recommends self-isolation until resolution of fever, at least 7 days after symptom onset.31 The World Health Organization (WHO) recommends self-isolation for 14 days after onset.32 There are likewise discrepancies between recommendations for test-based approaches offered by these major organizations.
The differences between guidelines on this important topic reflect the difficulty in delineating the best testing strategies. During the discussions in VM3, much debate revolved around the timing of testing before a procedure. It was decided that, ideally, the testing should take place as near to the procedure as possible. For practical reasons, however, it was agreed that it would be acceptable to perform screening of patients within 72 hours of the procedure. The debate surrounding this topic was the basis for the discussion and revision of a statement in D1 (Statement 5.7) that was initially discarded. It was approved after further discussion in VM3. The authors furthermore felt that it was beyond their area of expertise to give more extensive recommendations on screening and testing of healthcare staff. Thus, awareness and adherence to local policies is recommended (Statement 4.17).
The recommendations acknowledged the need for a patient-centered approach during different phases of the pandemic. Recommendations that underscore this approach include the designation of patient advocates (Statement 6.8), establishment of proper communication pathways (Statement 2.11), and consent forms updated with risks specific to the pandemic (Statement 5.9). The involvement of patient representatives in the consensus process was essential to ensure that patients’ views were incorporated into decision-making.
Weaknesses inherent to these recommendations include the reliance on expert opinion and discussion to formulate recommendations. These recommendations were also drafted in the setting of a rapidly evolving pandemic of unprecedented proportions. There is a lack of empirical data to support many of the underlying statements.
The selection of experts is another critical aspect within consensus statements development. The group of experts involved in this research was all recommended by their peers as international leaders in their fields and were distributed across four continents. The experts represented a wide range of opinion leaders, policymakers, government advisors in health policy, in addition to multispecialty clinical team leaders to ensure generalizability and validity of the results in this study. The inclusion of patients’ representatives adds relevance to this patient-centered collaborative project. At the time the manuscript was drafted, COVID-19 had mainly affected the countries from where the expert representatives were included. The explanations for the initial preferential spreading pattern of COVID-19 to high-income countries include higher connectivity, colder climate, age profile and body habitus.33,34
The response rate among the participants in D1 and D2 was 100%. The entire process, from formulation of questions to finalizing statements, took less than four weeks (April 27–May 22). Both of these factors reflect hard work and commitment on behalf of the group of experts, underscoring the importance of the topics discussed.
The recommendations formulated by this international expert consensus group create a framework for resumption of surgical, endoscopic, and other procedural activities significantly impacted by the COVID-19 pandemic. The statements have the potential for wide application across different healthcare systems globally. The participation of leaders from a variety of surgical and endoscopic organizations in the creation of this manuscript gives an opportunity for a wider, systematic, distribution of these recommendations by the supporting societies.
The recommendations presented here give hospitals a stepwise approach to the COVID-19 crisis, serving as a reference on how to resume surgical and endoscopic activities contingent on the status of disease burden. It seems clear that the COVID-19 pandemia will have multiple recurrent outbreaks. The recommendations outlined in this manuscript will remain relevant at each of the recurrent outbreaks. Given the dynamic nature of the current global crisis, these statements will likely require re-evaluation as more objective information becomes available.
CONCLUSIONS
The recommendations formulated by this international expert consensus group create a framework for resumption of surgical, endoscopic, and other procedural activities in the era of the COVID-19 pandemic. The statements have the potential for wide global application in clinical services, education, and research across different healthcare systems.
DEFINITIONS
Standard PPE: surgical masks, gowns, head covers, and gloves.
Supplementary Material
Supplementary Material
REFERENCES
- 1. AHA Letter to Surgeon General Re: Elective Surgeries and COVID-19 | AHA. Am. Hosp. Assoc. Available at: https://www.aha.org/lettercomment/2020-03-15-aha-letter-surgeon-general-re-elective-surgeries-and-covid-19. Accessed May 26, 2020. [Google Scholar]
- 2. Evans M, Mathews AW. Hospitals Push Off Surgeries to Make Room for Coronavirus Patients - WSJ. Available at: https://www.wsj.com/articles/hospitals-push-off-surgeries-to-make-room-for-coronavirus-patients-11584298575. Accessed May 26, 2020. [Google Scholar]
- 3. Joint Statement: Roadmap for Resuming Elective Surgery after COVID-19 Pandemic. Am. Coll. Surg. Available at: https://www.facs.org/covid-19/clinical-guidance/roadmap-elective-surgery. Accessed May 18, 2020. [Google Scholar]
- 4. CMS Issues Recommendations to Re-Open Health Care Systems in Areas with Low Incidence of COVID-19 | CMS. Cent. Medicare Medicaid Serv. Available at: https://www.cms.gov/newsroom/press-releases/cms-issues-recommendations-re-open-health-care-systems-areas-low-incidence-covid-19. Accessed May 26, 2020. [Google Scholar]
- 5.Abu Hilal M, Besselink MG, Lemmers DHL, et al. Early look at the future of healthcare during the COVID-19 pandemic. Br J Surg 2020; 107:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci 1963; 9:458–467. [Google Scholar]
- 7. Enterprise Online Survey Software & Tools - SurveyGizmo. Surveygizmo. Available at: https://www.surveygizmo.com/. Accessed May 17, 2020. [Google Scholar]
- 8. Video Conferencing, Web Conferencing, Webinars, Screen Sharing. Zoom Video. Available at: https://zoom.us/. Accessed May 26, 2020. [Google Scholar]
- 9. Poll Everywhere. Poll Everywhere. Available at: https://pollev.com/ Accessed May 26, 2020. [Google Scholar]
- 10. COVID-19 Map. Johns Hopkins Coronavirus Resour. Cent. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 26, 2020. [Google Scholar]
- 11.Aminian A, Safari S, Razeghian-Jahromi A, et al. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg 2020; 272:e27–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg 2020; 107:937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ives J, Huxtable R. Surgical ethics during a pandemic: moving into the unknown? Br J Surg 2020; 107:1089–1090. [DOI] [PubMed] [Google Scholar]
- 14.Søreide K, Hallet J, Matthews JB, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg 2020; 107:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nepogodiev D, Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. 2020;107:1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; 382:2049–2055. [DOI] [PubMed] [Google Scholar]
- 17.Khullar D, Bond AM, Schpero WL. COVID-19 and the Financial Health of US Hospitals. JAMA 2020; 323:2127–2128. [DOI] [PubMed] [Google Scholar]
- 18.Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 2020; 107:785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brindle ME, Doherty G, Lillemoe K, et al. Approaching surgical triage during the COVID-19 pandemic. Ann Surg 2020; 272:e40–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coronavirus: Trump unveils plan to reopen states in phases. BBC News. 2020; published online April 17. Available at: https://www.bbc.com/news/world-us-canada-52314866. Accessed May 26, 2020. [Google Scholar]
- 21. Ng A. Countries in the Middle East are easing coronavirus restrictions. Here's what experts have to say. CNBC. 2020; published online May 8. Available at: https://www.cnbc.com/2020/05/08/coronavirus-middle-east-countries-ease-restrictions.html. Accessed May 26, 2020. [Google Scholar]
- 22. Australia sets plan to end most COVID-19 restrictions by July - Reuters. Reuteurs. Available at: https://www.reuters.com/article/us-health-coronavirus-australia/australia-sets-plan-to-end-most-covid-19-restrictions-by-july-idUSKBN22J3HS. Accessed May 26, 2020. [Google Scholar]
- 23.Hsu C-C, Sandford B. The Delphi technique: making sense of consensus. Pract Assess Res Eval 2007; 12:1–8. [Google Scholar]
- 24.Helmer O, Rescher N. On the epistemology of the inexact science. Manag Sci. 1959;6:25. [Google Scholar]
- 25.Covvey JR, Ryan M. Use of a modified Delphi process to determine course objectives for a Model Global Health Course in a pharmacy curriculum. Am J Pharm Educ 2018; 82:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subcommittee Finds Significant Problems with Administration's Handling of Serology Testing | House Committee on Oversight and Reform. Available at: https://oversight.house.gov/news/press-releases/subcommittee-finds-significant-problems-with-administration-s-handling-of. Accessed May 26, 2020. [Google Scholar]
- 27. How Reliable Are COVID-19 Tests? Depends Which One You Mean. NPR.org. Available at: https://www.npr.org/sections/health-shots/2020/05/01/847368012/how-reliable-are-covid-19-tests-depends-which-one-you-mean. Accessed May 26, 2020. [Google Scholar]
- 28.Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Allergy Rhinol 2020; 10:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coronavirus Disease 2019 (COVID-19). Cent. Dis. Control Prev. 2020; published online Feb 11. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed May 20, 2020. [Google Scholar]
- 30.European Centre for Disease Prevention and Control. Infection prevention and control for COVID-19 in healthcare settings – Third update. ECDC Stockh 2020; published online May 13. [Google Scholar]
- 31. Stay at home: guidance for households with possible coronavirus (COVID-19) infection. GOV.UK. Available at: https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidance-for-households-with-possible-coronavirus-covid-19-infection. Accessed May 21, 2020. [Google Scholar]
- 32. Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts. World Health Organ. Available at: https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Accessed May 21, 2020. [Google Scholar]
- 33. Reed PKG and T. The effects of the coronavirus pandemic in emerging market and developing economies. Brookings. 2020; published online June 25. Available at: https://www.brookings.edu/bpea-articles/the-effects-of-the-coronavirus-pandemic-in-emerging-market-and-developing-economies/. Accessed Aug 27, 2020. [Google Scholar]
- 34.Cash R, Patel V. Has COVID-19 subverted global health? Lancet Lond Engl 2020; 395:1687–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


