Abstract
Background
Coexistence of coronary artery fistulas and atherosclerotic coronary artery disease (CAD) is rare.
Case summary
We present a unique case of a patient initially presenting with an anterior ST-elevation myocardial infarction, subsequently found to have two-vessel CAD and an aneurysmal left coronary-to-right pulmonary artery fistula.
Discussion
After discussion with the patient and a multidisciplinary discussion with the heart team, consisting of cardiovascular surgery, interventional cardiology, and vascular surgery, a percutaneous approach was chosen. He underwent successful multivessel percutaneous coronary intervention followed by fistula embolization.
Keywords: Coronary artery fistula, STEMI, Fistula embolization, Case report
Learning points
Discuss therapeutic options in a patient presenting with severe multivessel coronary disease and ST-elevation myocardial infarction, complicated by a large, aneurysmal coronary artery fistula (CAF).
Emphasize that large CAFs may lead to clinically significant ischaemia.
Demonstrate that a transcatheter-based approach with percutaneous coronary intervention and coiling is a safe and effective option for high-risk patients with concomitant coronary artery disease and CAF.
Introduction
A coronary artery fistula (CAF) is an anomalous connection between a coronary artery and a cardiac chamber, or systemic vasculature, or pulmonary vasculature. Coronary artery fistulas are rare, with an incidence of <1.5% of patients undergoing coronary angiography.1–3 While primarily congenital in nature, CAFs can also be acquired from chest trauma, Kawasaki’s vasculitis, or iatrogenic causes (e.g. myocardial biopsy).1,4 Coronary artery fistulas are often discovered incidentally, and a majority arise from the left anterior descending artery (LAD) or right coronary artery (RCA),4 with drainage into the right ventricle or pulmonary artery (PA).5 In symptomatic patients, the most common complaint is angina, reported to occur in up to 57% of patients with CAF.5 In the absence of obstructive coronary disease, this may be attributable to coronary steal, when oxygenated blood is shifted from the high-pressure coronary vasculature to a lower resistance cavity. Blood flow diverted away from the distal coronary vascular bed results in ischaemia beyond the origin of the fistula.2 Complications of CAFs include endocarditis, thrombosis, rupture, pulmonary hypertension, ischaemia, and sudden cardiac death.4 Angiography is the gold standard for diagnosis, although magnetic resonance imaging or computed tomography (CT) angiography are useful adjunctive modalities.1,4
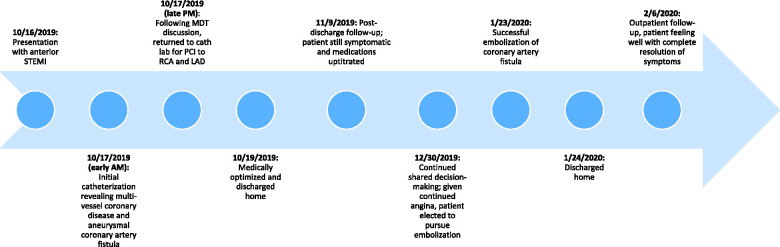
Timeline
Case presentation
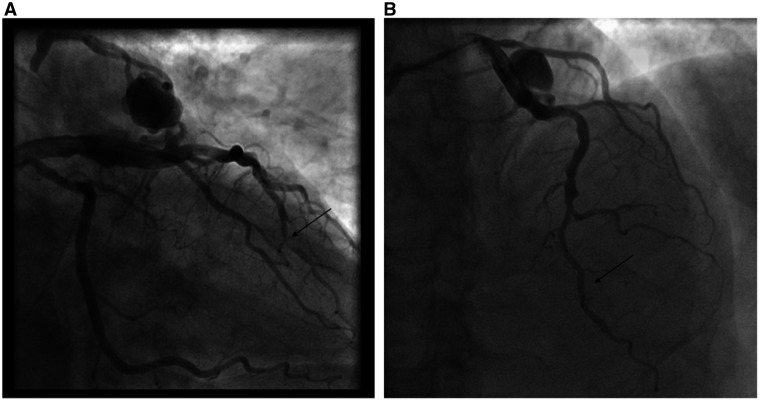
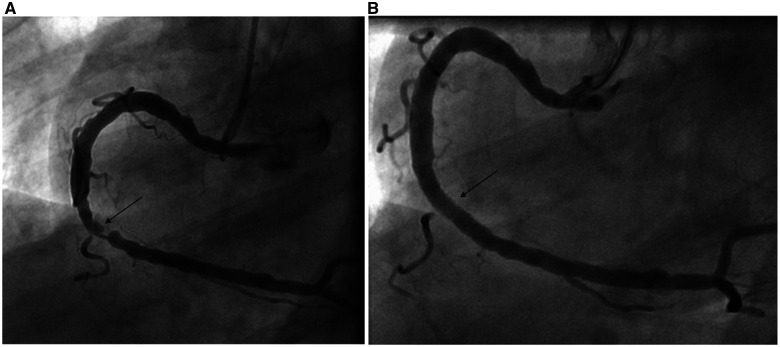
A 73-year-old man presented to a rural emergency department with 4 h of chest pain. Electrocardiogram revealed anterior ST-elevation myocardial infarction (STEMI). Given the distance from a percutaneous coronary intervention (PCI)-capable centre, he received aspirin, clopidogrel, heparin, and fibrinolysis prior to transfer. He had a remote history of bladder cancer, prostate cancer, and hypertension. The patient arrived haemodynamically stable, chest pain free and with resolution of his anterior ST elevations. Angiography revealed a 95% stenosis in the distal LAD with Thrombolysis in Myocardial Infarction (TIMI)-2 flow (Figure 1A and Video 1A), a dominant RCA with a 90% distal stenosis (Figure 2A) and a prominent CAF between the LAD and right PA with large aneurysmal dilatation (Figure 1A). Echocardiogram estimated a left ventricular ejection fraction of 69% with apical hypokinesis. There was no evidence of anomalous flow into the PA by echocardiography. Troponin T was elevated at 0.72 ng/mL.
Figure 1.
Angiography showing coronary artery fistula and distal left anterior descending artery culprit on presentation (A) and post-percutaneous coronary intervention (B).
Figure 2.
Right coronary artery angiogram pre-percutaneous coronary intervention (A) and post-percutaneous coronary intervention (B).
Given the patient’s clinical stability post-fibrinolysis, rescue PCI was deferred, glycoprotein IIB/IIIA inhibitor and heparin infusions initiated, and urgent multidisciplinary Heart Team consultation conducted to determine the optimal management strategy. The TIMI-2 flow was likely multifactorial, due to both slow flow from the fistula and aneurysm as well as distal plaque rupture and angiographically significant stenosis. The patient was deemed high surgical risk due to age, frailty, and an unfavourable distal target for a bypass graft to the LAD. A minimally invasive strategy was pursued after shared decision-making with the patient. He underwent successful PCI of the LAD with a 2.25 mm × 16 mm drug-eluting stent (DES) and of the RCA with a 4.00 mm × 20 mm DES (Figure 1B, 2B and Videos 1B).
After PCI, haemodynamic assessment of the LAD (distal to the fistula takeoff) was performed using instantaneous wave-free ratio (iFR). Instantaneous wave-free ratio measured 0.88, suggesting potential coronary steal physiology. Notably, although overall TIMI flow was improved to the distal vessel post-revascularization, flow distal to the fistula remained slow. Additionally, a saturation run revealed a significant step up at the level of the right PA (superior vena cava 69%, right PA 77%, left PA 68%), confirming an LAD-to-right PA fistula. Calculated shunt ratio was 1.5. Medical therapies were optimized, the patient was discharged home, and outpatient follow-up scheduled.
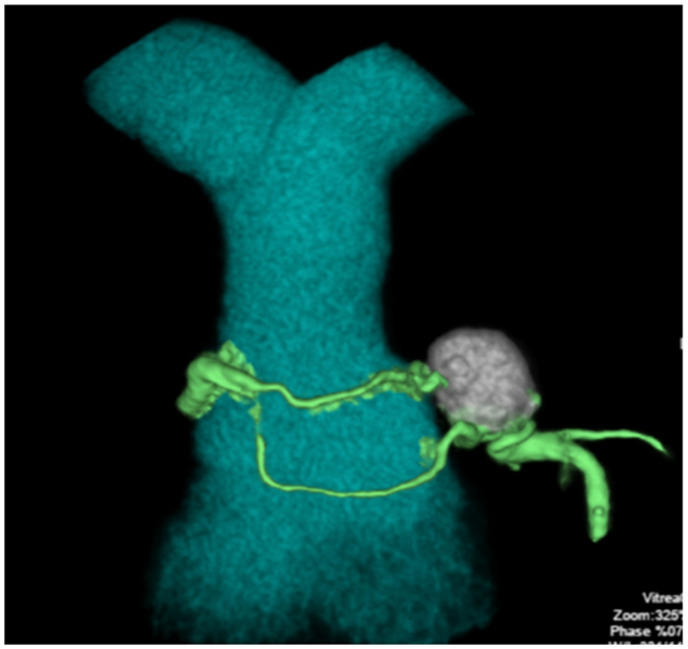
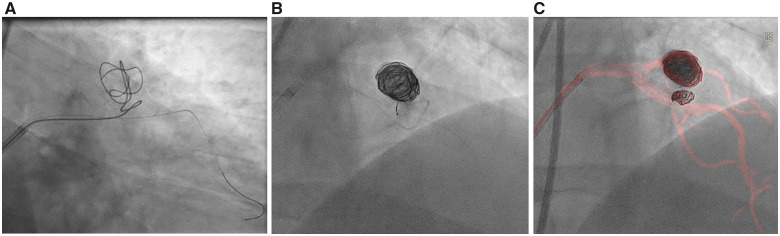
Two months later, the patient complained of persistent angina despite maximally tolerated medical therapy. He elected to pursue percutaneous exclusion of his aneurysm. Pre-procedural CT angiography (Figure 3) delineated a 20 mm × 15 mm aneurysm arising from a superiorly directed branch of the LAD. His procedure was performed via a transfemoral approach with an 8-Fr EBU 4.0 guide catheter. A Boston Scientific Renegade STC microcatheter (Natick, MA, USA) was advanced over a 0.014″ wire through the fistula and into the aneurysm sac. Seven Boston Scientific Interlock coils (Natick, MA, USA) ranging in size from 8 to 50 cm were delivered, resulting in successful embolization of the aneurysm sac as well as the vessel limb feeding into the aneurysm. Dynamic roadmap and rotational scanning (Philips Healthcare, Best, Netherlands) facilitated three-dimensional mapping of the anomaly and coil delivery (Video 2A and B and Figure 4A–C). Completion angiography revealed TIMI-3 flow in the LAD and no residual communication between the LAD and PA (Video 3A and B). Repeat iFR of the LAD distal to the excluded fistula measured 0.98, and repeat shunt run showed a normalized Qp:Qs ratio of 0.99 (complete resolution of prior step up observed: superior vena cava 71%, right PA 71%, left PA 70%). Post-procedure, the patient’s angina resolved.
Figure 3.
Computed tomography angiography of the coronary artery fistula.
Figure 4.
Imaging of stepwise coil insertions into the aneurysm (A and B), including dynamic coronary roadmap (C).
The patient was discharged home on dual antiplatelet therapy with aspirin and clopidogrel. He returned to clinic one and four months later with complete resolution of angina.
Discussion
Coronary artery fistulas are rare anomalies that are often found incidentally. Often, they do not require any intervention or treatment. However, surgical or percutaneous closure of a CAF may be considered when patients develop symptoms (angina, heart failure, or syncope), a pulmonary-to-systemic flow (Qp:Qs) >1.5:1, or aneurysmal degeneration.1,6 The risks of open heart surgery should be weighed against the risks of percutaneous intervention by a multidisciplinary Heart Team. The preferred treatment approach is dependent on the characteristics of the lesion, local expertise, and patient preferences.2
Currently, there are no guidelines supporting the use of routine anticoagulation for CAFs and/or aneurysms. However, case reports and small reviews have suggested that anticoagulation (with a direct oral anticoagulant or warfarin) may be acceptable in certain clinical scenarios, for example, when aneurysmal disease has resulted in thrombosis or embolization.7 However, in the absence of high-risk features, some experts recommend antiplatelet therapy for 1 year after intervention or indefinitely if observation is chosen as the preferred regimen for medium-to-large fistulas.8 Therefore, with this patient, we recommended dual antiplatelet therapy for 1 year following his coil embolization.
There are a number of transcatheter devices available for use in fistula closure including detachable coils, detachable balloons, covered stents, vascular plugs, umbrella devices, and vascular occlusion devices.4,9,10 The risks associated with transcatheter techniques include distal embolization, coronary or fistula dissection, or rupture of the aneurysm itself. Given the rarity of this condition, there is little data available to compare open vs. minimally invasive strategies.
Incidental discovery of a CAF in a patient with acute coronary syndrome is uncommon, and recommendations for management are based on expert opinion. One case published by Lee et al.11 reported a patient with CAF and adjacent unstable atherosclerotic plaque leading to non-STEMI. In this case, the lesion and the fistula existed in close anatomical proximity and thus treated together with a single covered stent. In another case, a patient presented with unstable angina from a subtotal LAD occlusion and concomitant CAF. This subject underwent coil embolization followed by DES placement, thus demonstrating safety and feasibility of a percutaneous strategy for this clinical scenario.12
We present a unique case in both presentation (anterior STEMI) and fistula anatomy. The patient possessed severe multivessel coronary disease and a large LAD-to-PA fistula that was haemodynamically significant and aneurysmal. Collectively, the unusual congenital anomaly with two-vessel coronary artery disease (CAD), presentation at a rural non-primary PCI hospital, and administration of lytic and P2Y12 therapies offered several decision-making challenges. Guided by the Heart Team, we conducted a stepwise treatment approach. We addressed the acute coronary syndrome and his non-culprit disease with PCI for complete revascularization. After a trial of outpatient medical therapy, we elected to treat his fistula percutaneously. Although iFR has not been validated for the evaluation or management of coronary steal syndrome, it may be reasonable to consider its use from a physiologic standpoint. The presence of a fistula diverts blood flow towards the fistula, and thus, leads to a decrement in blood flow in the distal vessel, that may potentially be reflected in an abnormal iFR measurement. In this case, the initially haemodynamically significant iFR measurement normalized after coil embolization, and also correlated with a marked decrease in coronary diastolic pressure with resolution of the patient’s anginal symptoms. Additionally, shunt measurements also normalized after coil embolization. It may be reasonable to consider the strategies detailed above in similar clinical scenarios.
Overall, CAFs are rare anomalies, and their coexistence with severe coronary disease is an even rarer entity, particularly in the setting of ACS. In such scenarios, it is reasonable to pursue a stepwise strategy for treatment of both disease processes, guided by a Heart Team and shared decision-making with the patient.
Lead author biography

Jennifer Frampton is currently an interventional cardiology fellow at Dartmouth-Hitchcock Medical Center (DHMC) in Lebanon, NH. She completed her internal medicine residency training and cardiology fellowship training at Dartmouth-Hitchcock Medical Center as well. She has a focused interested in complex CAD and acute coronary syndrome.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
Nathan Crain, PA (Dartmouth-Hitchcock Medical Center, Section of Cardiovascular Medicine).
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.Mangukia CV.Coronary artery fistula. Ann Thorac Surg 2012;93:2084–2092. [DOI] [PubMed] [Google Scholar]
- 2.Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G.. Coronary arteriovenous fistulae: a review. Int J Angiol 2014;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buccheri D, Chirco PR, Geraci S, Caramanno G, Cortese B.. Coronary artery fistulae: anatomy, diagnosis and management strategies. Heart Lung Circ 2018;27:940–951. [DOI] [PubMed] [Google Scholar]
- 4.Raju MG, Goyal SK, Punnam SR, Shah DO, Smith GF, Abela GS.. Coronary artery fistula: a case series with review of the literature. J Cardiol 2009;53:467–472. [DOI] [PubMed] [Google Scholar]
- 5.Said SM, Burkhart H, Schaff HV, Connolly HM, Phillips SD, Suri RM. et al. Late outcome of repair of congenital coronary artery fistulas—a word of caution. J Thorac Cardiovasc Surg 2013;145:455–460. [DOI] [PubMed] [Google Scholar]
- 6.Loukas M, St. Germain AS, Gabriel A, John A, Tubbs RS, Spicer D.. Coronary artery fistula: a review. Cardiovasc Pathol 2015;24:141–148. [DOI] [PubMed] [Google Scholar]
- 7.Yan Q, Ning L, Jian Y, Yang W, Yuan Q, Du Z.. Could the novel oral anticoagulants be used for coronary artery aneurysm? Case Rep Med 2020;2020:5073814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowda ST, Forbes TJ, Singh H, Kovach JA, Prito L, Latson LA. et al. Remodeling and thrombosis following closure of coronary artery fistula with review of management: large distal coronary artery fistula—to close or not to close? Catheter Cardiovasc Interv 2013;82:132–142. [DOI] [PubMed] [Google Scholar]
- 9.Niizeki T, Daidouji H, Ootaki Y, Kaneko K, Ito M, Oguma M. et al. Transcatheter coil embolization of coronary artery fistulas. J Cardiol Cases 2010;2:e55–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZG, Xu XD, Bai Y, Zhang XL, Tan HW, Zhu YF. et al. Transcatheter closure of medium and large congenital coronary artery fistula using wire-maintaining technique. J Cardiol 2015;66:509–513. [DOI] [PubMed] [Google Scholar]
- 11.Lee WC, Fang HY, Fang CY.. Covered stent for large coronary arterial fistula and adjacent atherosclerotic plaque with stenosis in a patient with non-ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2016;9:1512–1513. [DOI] [PubMed] [Google Scholar]
- 12.Lee WC, Fang HY, Wu CJ.. How to treat the combination of coronary artery fistula and occluded coronary artery. Medicine 2018;97:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.