Abstract
Low high density lipoprotein cholesterol (HDL-C) characterizes an atherogenic dyslipidemia that reflects adverse lifestyle choices, impaired metabolism, and increased cardiovascular risk. Low HDL-C is also associated with increased risk of inflammatory disorders, malignancy, diabetes, and other diseases. This epidemiologic evidence has not translated to raising HDL-C as a viable therapeutic target, partly because HDL-C does not reflect HDL function. Mendelian randomization analyses that have found no evidence of a causal relationship between HDL-C levels and cardiovascular risk have decreased interest in increasing HDL-C levels as a therapeutic target even further. HDLs comprise distinct subpopulations of particles of varying size, charge and composition that have several dynamic and context-dependent functions, especially with respect to acute and chronic inflammatory states. These functions include reverse cholesterol transport, inhibition of inflammation and oxidation, and anti-diabetic properties. HDLs can be anti-inflammatory, which may protect against atherosclerosis and diabetes, as well as pro-inflammatory, which may help clear pathogens in sepsis. In addition, the molecular regulation of HDLs is complex, as evidenced by their association with multiple proteins, as well as bioactive lipids and non-coding RNAs. Clinical investigations of HDL biomarkers (HDL-C, HDL particle number, and apolipoprotein A-I) have revealed non-linear relationships with cardiovascular outcomes, differential relationships by sex and ethnicity, and differential patterns with coronary versus non-coronary events. Novel HDL markers may also have relevance for heart failure, cancer, and diabetes. HDL function markers, namely cholesterol efflux capacity, are associated with coronary disease, but remain research tools thus far. Ultimately, therapeutics that manipulate aspects of HDL metabolism remain the holy grail. None thus far have proven to be successful, but most have targeted HDL-C, not metrics of HDL function. Future therapeutic strategies should focus on optimizing HDL function in the right patients at the optimal time in their disease course. We provide here a framework to help the research and clinical communities, as well as funding agencies and stakeholders, obtain insights into current thinking on these topics, and what we predict will be an exciting future for research and development on HDLs.
Keywords: HDL (high density lipoprotein), HDL function, lipoprotein, inflammation, cardiovascular, atherosclerosis, omics, biomarker
Introduction
Epidemiological evidence of an inverse association of plasma high density lipoprotein cholesterol (HDL-C) levels with atherosclerotic cardiovascular disease (ASCVD) risk has not translated into positive outcomes in large scale randomized controlled trials of interventions that raise HDL-C levels.1 Lack of a plausible causal link between HDL-C and ASCVD from Mendelian Randomization studies has led to a paradigm shift towards determining whether improving HDL function explains the cardioprotective effects of HDLs. Another recent major shift in the HDL area has been towards identifying broader roles beyond atheroprotection, including infections and autoimmune disorders, diabetes, chronic kidney disease, and cancer, all of which are driven by inflammation and perturbed metabolism.
We aim to summarize current understanding of the structure, function and metabolism of HDLs, as well as their clinical utility as a potential therapeutic. The main themes that recur through the document include: the diversity of HDL structure and function; the dynamic and context-dependent nature of HDL functions, especially with respect to acute and chronic inflammatory states; and the complexities of the molecular regulation of HDLs. These themes collectively highlight an increasing need for sophistication and validation in future studies. The hope is that new and evolving insights into the beneficial functions of HDLs will pave the way for their use in both cardiovascular (CV) and non-CV diseases. We provide here a framework for the research and clinical communities, as well as for funding agencies and stakeholders, to become more informed and obtain insights into what we predict will be an exciting future for research and development on HDLs.
I. HDL Structure and Function
As HDL structure and function are inextricably linked, it is essential to consider them both when investigating their roles in disease states.
HDL Structure
The HDLs in human plasma are predominantly spherical particles. They consist of several distinct HDL subpopulations of particles of varying size, surface charge and lipid and apolipoprotein composition. This heterogeneity is a reflection of the remodelling of individual HDL subpopulations by plasma factors such as the cholesterol esterifying enzyme, lecithin: cholesterol acyl transferase (LCAT), cholesteryl ester transfer protein (CETP), phospholipid transfer protein, hepatic lipase and endothelial lipase. Despite this heterogeneity, all HDLs have the same overall structure: a water insoluble, neutral lipid core (mainly cholesteryl esters and some triglycerides) surrounded by a surface monolayer (mainly phospholipids and some unesterified cholesterol) in which apolipoproteins are embedded. HDL apolipoproteins are highly α-helical. These helices have a hydrophobic face that drives association with lipid as well as a hydrophilic face that confers water solubility on the HDL particles.
Detailed insights into the structural organisation of spherical HDLs have emerged from studying homogeneous populations of reconstituted HDLs, and HDL subpopulations isolated from human plasma. Mass spectrometric analysis of these preparations indicate that most HDLs contain three copies of apolipoprotein A-I (apoA-I) that are organised on the particle surface as a trefoil,2 or as two copies in an anti-parallel orientation with the third apoA-I molecule localized separately in a U-shaped conformation.3 Whether these variations in the spatial organization of apoA-I on the HDL surface impact on HDL function is not known.
As discussed in detail in a later section, HDLs contain several other apolipoproteins in addition to apoA-I. The second most abundant HDL apolipoprotein is apoA-II, followed by apoA-IV, the C apolipoproteins, apoE and apoM. These apolipoproteins all contribute to HDL structural stability and, in some cases, HDL function. HDLs also transport a cargo of other proteins that potentially further impact on HDL function.
In addition to the aforementioned proteins that are predominantly associated with lipid metabolism, HDLs contain proteins that promote proteolysis (e.g. alpha-1-antitrypsin), hemostasis (alpha-2-HS-glycoprotein), immunity (e.g. the acute phase reactant SAA4), complement activation (e.g. complement C3), and inflammation (e.g. haptoglobin-related protein) (https://homepages.uc.edu/~davidswm/HDLproteome.html). As the concentration of most HDL-associated proteins is lower than the plasma concentration of HDL particles, each protein can associate only with specific HDL particle subsets.4 We do not know whether the association of these proteins with HDLs is regulated by the size or composition of the particles, or how HDL-associated proteins impact on HDL function. The development of innovative approaches to address these issues will provide important insights into the interrelationship of HDL structure, function and metabolism, and potentially identify targets that may boost the cardioprotective and other functions of HDL. Progress is being made in this direction with the clustering of specific HDL subpopulations into functional classes on the basis of their proteome.4
HDL Function:
HDLs protect the function and survival of organisms by multiple, overlapping mechanisms. For example, proteins and bioactive lipids that associate with HDLs directly activate signal transduction pathways. HDLs also function indirectly by effluxing cholesterol from cells and influencing cholesterol homeostasis. HDLs can detoxify potential hazards through enzymes such as paraoxonases (PON), or by delivering them to the liver for biotransformation and excretion by pathways that are shared with reverse cholesterol transport (RCT).
Cholesterol efflux and reverse cholesterol transport.
The efflux of excess cholesterol from macrophages and other cell types is the most extensively studied function of HDLs and apoA-I. Large, spherical HDLs accept the cholesterol that is exported from cells by the ATP binding cholesterol transporter, ABCG1, while the related transporter, ABCA1, exports cellular cholesterol to lipid-free apoA-I and small, dense HDLs. The importance of cholesterol efflux has emerged from human cohort studies that have revealed inverse associations between the capacity of plasma depleted of apoB-containing lipoproteins to accept macrophage-derived cholesterol and CV risk in most, but not all, studies.5
The efflux of cholesterol to HDLs and apoA-I also represents the first step in RCT the pathway whereby excess cholesterol from macrophages in the artery wall is acquired by apoA-I and HDLs and transported to the liver for excretion as a component of bile. Preclinical RCT studies have established that increasing cholesterol flux though the RCT pathway reduces atherosclerosis in animal models. Although clinical translation of these findings has been slow, a study using an integrated approach to quantify the entire RCT pathway in humans was recently published.6 This may pave the way for detailed investigations of the regulation of RCT and ASCVD by HDLs in humans.
Inhibition of inflammation by HDLs.
HDLs reduce inflammation in multiple cell types, including endothelial cells and macrophages. In endothelial cells HDLs inhibit inflammation by reducing activation of nuclear factor-κB (NF-κB) and 3β-hydroxysteroid-Δ24 reductase, by activating the cytoprotective enzyme, heme oxygenase-1 and by inhibiting inflammasome activation.7, 8 HDLs exert these effects by several mechanisms including the interaction of HDL-associated apoM/sphingosine-1-phosphate (S1P) with S1P receptors.9 They also reduce inflammation in monocytes and attenuate the binding of monocytes to adhesion molecules on the surface of activated endothelial cells.9, 10 Collectively, these findings highlight several targets with the potential to improve the anti-inflammatory properties of HDLs in endothelial cells. The role of HDLs in macrophage inflammation is addressed in the next section.
Anti-diabetic properties of HDLs.
HDLs and HDL apolipoproteins improve glycemic control in animal models of diabetes by enhancing pancreatic β-cell function and survival and improving insulin sensitivity.11 HDLs also inhibit β-cell apoptosis and protect β-cells from oxidation by low-density lipoproteins (LDLs).12, 13
Evidence that the anti-diabetic functions of HDLs are clinically relevant has emerged from a post-hoc meta-analysis of all cholesteryl transfer protein (CETP) inhibitor trials showing a 12% reduction in incident diabetes.14 A more detailed in analysis of dalcetrapib revealed that the reduced incidence in diabetes may be a consequence of the treatment-related increase in HDL-C (but not change in body mass index) and regression from diabetes to no diabetes.15 These studies provided the first direct evidence that increasing HDL levels may reduce cardiometabolic risk. Identification of specific HDL subpopulations that mediate these effects would enable this approach to be further developed via commercial production of relevant rHDLs.
Inhibition of oxidative stress by HDL.
HDLs reduce oxidative stress in LDLs and other atherogenic lipoproteins by accepting lipid hydroperoxides and detoxifying them into lipid hydroxides that are cleared from the circulation by the liver. Small HDLs inhibit oxidation more effectively than large HDLs.16 This can potentially negate, at least in part, the reduced cardioprotection that is associated with low plasma HDL levels. It also raises the possibility that treatment with HDL-raising agents that increase the level of large HDLs may not improve the antioxidant properties of HDLs.
Paraoxonase1 (PON1) and platelet-activating factor acetyl hydrolase (PAF-AH) contribute to the antioxidant properties of HDLs independent of reducing lipid hydroperoxides to hydroxides. Although PON1 knockout mice are atherosclerosis-prone, and overexpression of human PON1 reduces atherosclerosis in mice,17, 18 the mechanism by which PON1 inhibits oxidation, and its impact on atherosclerotic lesion development, is not well understood. The mechanism of the antioxidant properties of PAF-AH have, by contrast, been elucidated, and include the hydrolysis of oxidised fatty acid constituents in phospholipids. However, the precise contribution of PAF-AH to the antioxidant properties of HDLs requires further clarification. Interventions that exploit these cardioprotective functions of HDLs have yet to be developed.
Conclusion.
While insights into the protective functions of HDLs have progressed in recent years, little is known about the identity and structural characteristics of specific HDL subpopulations that mediate these effects. The development of innovative approaches to identify these subpopulations, their origins, and their regulation would enable specific HDL subpopulations to be targeted as a means of preserving, and possibly enhancing the cardioprotective functions of HDLs. Recent technological advances have increased the feasibility of reaching this milestone in the short- to medium-term and could ultimately have major long-term benefits for the clinical utility of HDL-based therapies.
II. HDL and inflammation
Inflammation is a key driver of chronic diseases such as ASCVD and diabetes as well as infections and malignancies. HDLs directly affect the inflammatory process and, vice versa, inflammation affects HDL function.8, 19–25 Approximately ~90-95% of the apoA-I in plasma is bound to HDL particles, but pro-inflammatory states can cause it to dissociate into the circulation in a lipid-free or lipid-poor form.26 The role of HDLs and apoA-I in macrophage inflammation, a key driver of atherosclerotic lesion progression, has been investigated extensively.8, 10, 19–25, 27 While early studies focused on the anti-inflammatory effects of HDLs,8, 10, 19, 20, 23–25, 27 more recent studies showing that HDLs and apoA-I can also be pro-inflammatory20–22 may be one explanation for the limited success of HDL-raising drugs in reducing ASCVD. However, the pro-inflammatory effects of HDLs could be beneficial in other diseases, such as sepsis, where enhanced inflammation may promote efficient clearance of bacteria.22 Understanding the mechanistic links between HDLs and their role in inflammation is vital for understanding the prognostic and therapeutic potential of this lipoprotein class.
Whether HDLs and apoA-I are pro- or anti-inflammatory depends on several structural/functional features, including HDL composition,20, 21 macrophage cholesterol content,20 and signalling pathways.19, 22 Macrophages produce pro- and anti-inflammatory cytokines. Production of pro-inflammatory cytokines occurs downstream of Toll-like receptors (TLRs) that are activated by components of viruses or bacteria. The best known examples of this is are lipopolysaccharide (LPS) that activates TLR4, and lipoteichoic acid (LTA) that activates TLR2.28 While HDLs and apoA-I suppress inflammation by binding directly to and neutralising LPS or LTA,28 they also affect TLR activation and downstream signalling pathways directly (Figure 1).
Figure 1 –
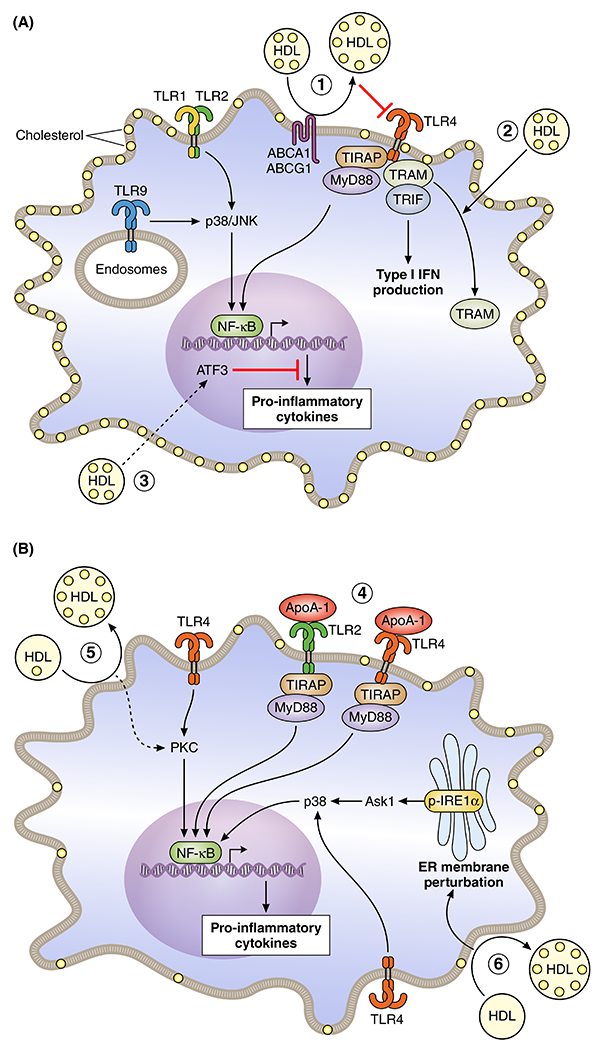
Anti-and pro-inflammatory effects of HDL in Macrophages
A. Anti-inflammatory effects. 1. HDL induces cholesterol efflux mediated by the cholesterol transporters ABCA1 and ABCG1, leading to a decreased TLR4 surface expression and decreased downstream MyD88 and TRIF signaling, suppressing the NF-κB and type I IFN response, respectively. 2. HDL stimulates the translocation of TRAM from the plasma membrane to intracellular compartments, reducing its availability for TRIF signaling and diminishing type I IFN production. 3. HDL induces Atf3 expression as such suppressing TLR9 or TLR1/2 induced inflammatory gene expression downstream of NF-κB. Dashed arrow indicates that the exact mechanism is unknown. Yellow dots indicate free cholesterol and in the case of HDL, particle free cholesterol enrichment because of cholesterol efflux. B. Pro-inflammatory effects. 4. ApoA-1 binds to TLR2 and TLR4, enhancing MyD88 signaling and NF-κB activation, and TRIF signaling (although not shown). 5. HDL induces plasma membrane cholesterol depletion, augmenting PKC signaling and downstream NF-κB activation induced by a TLR ligand. 6. HDL induces excessive cholesterol depletion, leading to ER membrane perturbation and enhanced IRE1a/ASK1/p38 MAPK signaling, which augments NF-κB activation in the presence of LPS (shown as TLR4 activation). Dashed arrows (in A and B) indicate that the exact mechanism is unknown.
Cholesterol efflux-dependent anti-inflammatory effects of HDLs.
HDLs suppress inflammation in macrophages by decreasing TLR4 signalling that is mediated by myeloid differentiation primary response 88 (MyD88)/nuclear factor-κB (NF-κB) or TIR-domain-containing adapter-inducing interferon-β (TRIF), which reflect early and late anti-inflammatory responses, respectively (Fig 1A, 1 and 2).20, 24, 25 These effects are partly related to the ability of HDLs to accept the cholesterol that effluxes from cells via ABCA1 and ABCG1 in processes that suppress TLR4 expression on cell surfaces.20, 24 The HDL-mediated suppression of pro-inflammatory responses in macrophages that occurs when LPS binds to TLR4 is partially dependent on expression of the Abca1 and Abcg1 genes (Fig 1A, 1).29 The ability of apoA-I, but not HDLs, to suppress pro-inflammatory monocyte activation by phorbol 12-myristate 13-acetate (PMA) in human monocytes also depends on ABCA1.10 Humans heterozygous for loss-of-function mutations in the ABCA1 gene have a ~50% decrease in plasma HDL-cholesterol levels, increased plasma pro-inflammatory cytokine levels and extensive vascular inflammation compared to healthy controls,30 suggesting clinical relevance.
Cholesterol efflux-independent anti-inflammatory effects of HDLs.
The anti-inflammatory effects of HDLs are related to suppression of LPS-induced gene expression downstream of TRIF/ and TRIF related adaptor molecule (TRAM).25 Mechanistically, HDLs reduce the availability of TRIF for signaling by translocating TRAM from the plasma membrane to intracellular compartments (Fig 1A, 2).25 HDLs also inhibit inflammation by increasing expression of activating transcription factor 3 (Atf3), which is induced by TLRs, but limits pro-inflammatory cytokine production downstream of NF-κB.19 None of these effects are related to cholesterol efflux (Fig 1A, 3).19 However, the Atf3 findings are somewhat controversial. Some studies have reported that HDLs do not increase Atf3 gene expression,8, 20, 22 while others have found that HDLs suppress Atf3 gene expression through TLR4 in a process that depends on cholesterol efflux (Fig 1A, 1).20 In contrast, cholesterol efflux does not affect TLR9 signaling.24 This may explain why ATF3 accounts for the anti-inflammatory effects of HDL when TLR9 is activated (Fig 1A, 3).
Pro-inflammatory effects of HDLs.
The apoA-I that dissociates from HDLs under pro-inflammatory conditions26 directly activates TLR2 and TLR4 (Fig 1B, 4).21 Although controversial,20 HDLs may also exert pro-inflammatory effects by augmenting protein kinase C (PKC) activation in response to TLR ligands (Fig 1B, 5).22 To a large extent, the pro-inflammatory effects of HDLs are due to excessive cellular cholesterol depletion. This activates inositol-requiring enzyme 1a (IRE1a)/apoptosis signal-regulating kinase 1 (ASK1)/p38 mitogen-activated protein kinase (p38 MAPK) signalling, which results in a pro-inflammatory endoplasmic reticulum (ER) stress response (Fig 1B, 6).20
HDLs and cholesterol efflux pathways suppress inflammasome activation.
Inflammasomes are intracellular complexes comprising the sensor molecule NOD-Like Receptor (NLR), the adaptor protein apoptosis-associated speck-like protein (ASC), and caspase-1.31 NLR Family Pyrin Domain Containing 3 (NLRP3), the most extensively characterized inflammasome, requires two signals for activation: (i) NF-κB activation, which increases transcription of all NLRP3 inflammasome components, called inflammasome priming, and (ii) other signals31 including, but not limited to, accumulation of free cholesterol or cholesterol crystals in lysosomes which leads to cleavage of caspase-132 and generates the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18.31
HDLs suppress inflammasome activation by decreasing LPS-induced inflammasome priming.8 ABCA1- and ABCG1-mediated cholesterol efflux also suppresses inflammasome activation in myeloid cells and dendritic cells, which reduces atherosclerosis and auto-immunity, respectively.27, 33 Patients with Tangier Disease who are homozygous for loss-of-function mutations in the ABCA1 gene have very low cholesterol efflux and plasma HDL-C levels and increased plasma levels of IL-1β and IL-18, indicating human relevance.27 Similarly, decreased cholesterol efflux to HDLs due to reduced gene expression of ABCA1/ABCG1 in blood monocytes of patients with poorly controlled diabetes mellitus,34 chronic kidney diseases,35 or rheumatoid arthritis,36 may contribute to inflammasome activation and increased systemic inflammation.
Conclusion.
HDLs and apoA-I have pro- and anti-inflammatory effects. The anti-inflammatory effects are beneficial in the context of atherosclerosis and diabetes, while the pro-inflammatory properties of HDLs and apoA-I may contribute to efficient clearance of bacteria in sepsis. Some of the effects of HDLs and apoA-I on inflammation are dependent on cholesterol efflux. Cholesterol efflux to HDLs and apoA-I is generally anti-inflammatory in monocytes and macrophages. However, excessive cholesterol depletion in macrophages by HDLs can induce pro-inflammatory effects.
III. Application of –omics to HDLs
Genomics:
Mendelian Randomization studies focusing primarily on the cholesterol content of HDLs have not found a causal link between HDL-C and ASCVD1 but have suggested a causal role for HDLs in chronic kidney disease (CKD) and infection.1
However, Mendelian Randomization studies are hampered by several major limitations. Firstly, they rely on linear associations between exposure and outcome. However, the associations of HDL-C with the risk of ASCVD, CKD, infection, and mortality are parabolic.1 Within the broad nadirs encompassing up to 40% of the population, differences in HDL-C are not associated with any change in risk.1 Second, the direction of associations are not consistent. Loss-of-function mutations in SCARB1 (the gene that encodes for SR-BI) and APOA1 increase and decrease HDL-C, respectively, but both increase the risk of ASCVD.37, 38 Third, the cholesterol in HDL is an inert surrogate marker of the number and size of HDL particles and is not responsible for any of the cardioprotective functions of HDLs. Thus, genomic approaches investigating markers of HDL function are likely to yield more biologically and clinically relevant insights than HDL-C levels. Targeting apoA-I may also directly link genes to function. However, a recent Mendelian Randomization study did not find a genetic association of apoA-I plasma levels with risk of ASCVD.39 Mendelian Randomization studies have linked plasma levels of apoC-III and risk of ASCVD, but this is typically interpreted as an adverse role of apoC-III in triglyceride-rich lipoprotein metabolism rather than an indicator of HDL dysfunction.40 Additional Mendelian Randomization studies on apoC-III levels in HDL or apoB-free plasma are needed to demonstrate if the association of apoC-III containing HDL with increased risk of ASCVD is causal.41
Proteomics:
Reports on the number of HDL-associated proteins vary from 9 to nearly 500.42 This reflects differences in how the HDLs were isolated, the sensitivity of the mass spectrometry method that was used to detect individual proteins and the absence or presence of disease.42
The method of HDL isolation will also affect the number of HDL-associated proteins. Isolating HDLs by sequential ultracentrifugation suffers from contamination with LDLs, exosomes and microvesicles. High resolution size exclusion chromatography combined with phospholipid affinity42 or anti-apoA-I immunoaffinity chromatography4,43 go some way towards reducing this risk. To date, 219 HDL-associated proteins have been validated by at least three independent labs (http://homepages.uc.edu/~davidswm/HDLproteome.html).44 The wide range of protein concentrations (<100 nmol/L for apoL1 and PLTP to >50 μmol/L for apoA-I relative to an average HDL particle concentration of ~20 μmol/L (Figure 2) suggests that HDL-associated proteins are non-randomly distributed among HDL particles. A recent approach utilizing two sequential immunoaffinity chromatography steps supports this concept, revealing sixteen HDL subclasses with distinct proteomes.4
Figure 2:
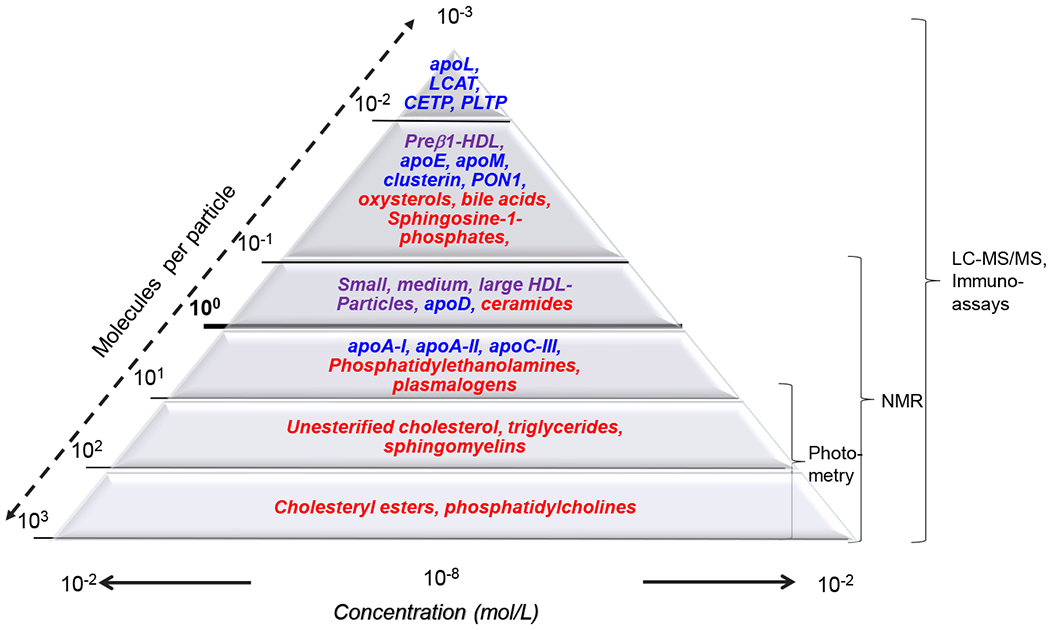
Concentration ranges of high-density lipoprotein (HDL) particles as well as the different proteins and lipids found in HDL. The width of the triangle’s baseline axis reflects the concentrations of HDL particles and components which range from more than 1 mmol/L (i.e. 10−3 mol/L for cholesterol) to the submicromolar range (i.e. 10−7 or even 10−8 mol/L for lipids such as sphingosine-1-phosphates and oxysterols or proteins such as apoL1 or phospholipid transfer protein (PLTP). The numbers at the left diagonal axis of the triangle shows the abundance of HDL subclasses or components relative to an average concentration of HDL particles of 20 μmol/L, which is highlighted with the bold line crossing the triangle at 100 = 1. The curly brackets on the right side of the triangle reflect the measuring ranges of analytical methods used for the characterization of HDL. HDL subclasses are denoted by purple font, lipids by red font, and proteins by blue font. MicroRNAs are not presented but their concentration of 10000 copies per μg HDL protein82 implies a relative abundance of about one molecule per 107 HDL particles.
Abbreviations: Apo, apolipoprotein; CETP, cholesteryl ester transfer protein; LCAT, lecithin:cholesterol acyltransferase; PLTP, phospholipid transfer protein.
Posttranslational modifications of HDL-associated proteins such as acylation and phosphorylation, and modifications such as oxidation, nitration, chlorination, and carbamylation that are caused by inflammation, hyperglycemia, and uremia also have a profound impact on the HDL proteome.42, 44 A recent analysis by high-resolution mass spectrometry identified nearly 1000 different posttranslational modifications of 54 HDL-associated proteins, with apoA-I most frequently affected. Some of these modifications interfere with HDL function, including cholesterol efflux and may serve as biomarkers of dysfunctional HDL.42, 44
In line with these observations, future investigations of the effects of HDL-associated proteins on HDL function will need to account for alterations of the HDL proteome in response to disease or treatment42 (Table 1).
Table 1:
Examples of HDL-associated proteins, lipids, and miRNAs associated with the presence or incidence of diseases or mortality
| Molecules | Function in HDL | Disease association |
|---|---|---|
| Particles | ||
| HDL particle number | Determines CEC45 | Inversely with risk of ASCVD46 |
| Small HDL | Determines CEC45 | Positively with diabetes, Inversely with risk of ASCVD45,47 |
| Large HDL | Determines CEC45 | Positively with diabetes45, 47 |
| Prebeta-HDL | HDL-precursor, determines CEC | Positively with the presence of ASCVD48 |
| Proteins | ||
| ApoA-I | Structural component Activator of LCAT Ligand of HDL receptor and ABCA1 Anti-oxidative | Total apoA-I: Inversely with incident ASCVD or mortality39, various posttranslational modifications of apoA-I are positively associated with presence of ASCVD42, 44 |
| apoC-III | Promotes apoptosis of endothelial cells and activation, inhibits cholesterol efflux49, 50 | Positively with incident ASCVD or diabetes39, 51 |
| apoE | Hepatic Removal of HDL,52 stimulation of cholesterol efflux anti-inflammatory activities | Inversely with risk of ASCVD and dementia1 |
| Serum amyloid A | Acute phase reactant inhibits cholesterol efflux and eNOS activation53 | Positively with presence of ASCVD and mortality of patients with ASCVD or ESRD53, 54 |
| Paraoxonase 1 | Inhibition of lipid-peroxidation | Inversely with presence of ASCVD or diabetes55 |
| Pulmonary surfactant protein B | Component of lung surfactant | Positively with mortality in patients with ESRD or heart failure54, 56 |
| Lipids | ||
| Cholesteryl esters | Core lipid determining size and shape | Inversely with presence and incidence of ASCVD, diabetes, and other diseases1 |
| Triglycerides | Core lipid | Positively with mortality in ASCVD patients57 |
| Phosphatidylcholines | Determine fluidity of HDL and thereby cholesterol efflux capacity;45, 58 substrate for the generation of lyso-phospholipids | Heterogeneous, depending on species45, 58 |
| PC- and PE-plasmalogens | Anti-oxidative58–60 | Inversely with presence of ASCVD or diabetes58–60 |
| Lysophosphatidylcholines (e.g. LPC18:1, LPC18:2) | Enzymatically produced from HDL-derived lipids. Signalling | Inversely with diabetes45, 61 |
| sphingomyelins | Determine rigidity of HDL and thereby cholesterol efflux capacity and anti-apoptotic activity towards endothelial cells;45 substrate for the generation of S1P and ceramides | Some species inversely with presence of diabetes or ASCVD45, 61, 62 |
| Sphingosine-1 phosphate | Agonist of five G-protein coupled S1P receptors; Multiple vasoprotective, anti-diabetic and anti-inflammatory actions63, 64 |
Inversely with presence of ASCVD or diabetes64 |
| microRNAs | ||
| miR-223 | Most abundant miRNA in HDL. Regulates VCAM expression in endothelial cells and cholesterol metabolism in liver65 | Increased in ACS and diabetes. Decreases upon weight loss66, 67 |
| miR-375-3p | Secreted by pancreatic beta cells to HDL68 | Increased levels in beta cell failure after islet transplantation69 |
HDL=high density lipoprotein; CEC=cholesterol efflux capacity; ASCVD=atherosclerotic cardiovascular disease; ApoA-I=apolipoprotein A-I; LCAT=lecithin–cholesterol acyltransferase; ABCA1=ATP-binding cassette transporter ABCA1; apoC-III=apolipoprotein C-III; apoE=apolipoprotein E; eNOS=endothelial nitric oxide synthase; ESRD=end-stage renaldisease; PC=phosphatidylcholine; PE=phosphatidylethanolamine; LPC= lysophosphatidylcholines; S1P=sphingosine-1-phosphate; miR=micro ribonucleic acid; VCAM=vascular cell adhesion molecule; ACS=acute coronary syndrome
Understanding of these complex changes in the HDL proteome with HDL function requires comprehensive high throughput proteomic HDL assays. This has led to the development of a proteomic score derived from apolipoproteins A-I, C-I, C-II, C-III and C-IV that correlates with cholesterol efflux capacity and independently associates with ASCVD and CV mortality.70 Another recent proteomic study found that associations with ASCVD events were modified by either enrichment or depletion of HDLs with several proteins. ApoC-III enrichment of HDLs: 1) interferes with their capacity to inhibit apoptosis of endothelial cells; 2) reduces their capacity to efflux cholesterol from macrophages; and 3) is a potent adverse ASCVD risk marker.41, 49, 70 This identifies apoC-III as an interesting HDL-targeted strategy for therapy beyond lowering of triglycerides.
Lipidomics:
The concentrations of lipid species in plasma and HDLs range from nanomolar to millimolar, with cholesterol being the most abundant (>100 molecules/ HDL particle) and other lipids such as S1P, oxysterols, and bile acids at extremely low abundance (1 molecule associated with <10% of HDL particles) (Table 1, Figure 2). Cholesteryl esters and triglycerides in the core influence HDL function indirectly by regulating particle size and being substrates for esterases. Lipids in the HDL surface influence HDL function directly: (i) the fatty acid acyl chains in phospholipids and sphingomyelin influence particle fluidity and rigidity, respectively, and thereby HDL function, including cholesterol efflux;45 (ii) S1P has vasoprotective and anti-diabetic functions;63, 64 and (iii) some HDL lipids are substrates for enzymes which generate biologically active lipids including lysophospholipids and polyunsaturated fatty acids.71 These functions are interconnected. For example HDLs promote the efflux of cholesterol and bioactive lipids such as S1P and oxysterols.12, 72 By providing substrates and inducing efflux, HDLs thus serve as a scaffold for paracrine regulation by bioactive lipids. Standard analysis of isolated HDLs may therefore underestimate the complexity and functional relevance of the HDL lipidome in vivo.
Variations in the structure and concentration of HDL lipids precludes, to some extent, the development of a comprehensive, unifying standardized method for measuring all lipid species. Nuclear magnetic resonance (NMR) spectrometry directly measures individual lipoprotein subclasses without prior fractionation and lipid extraction. However, its low sensitivity only allows measurement of lipid subclasses (cholesteryl esters, unesterified cholesterol, phosphatidylcholines, sphingomyelins) rather than individual lipid species. Nevertheless, NMR-derived HDL particle numbers predicts incident ASCVD events better than HDL-C.46 Broad NMR profiling of HDLs also correlates with cholesterol efflux capacity, but not other HDL functions.45 Interestingly, NMR studies found that type 2 diabetes is positively associated with small HDLs and inversely associated with large HDLs,47 whereas ASCVD is inversely associated with small and medium-sized HDLs.45 In contrast to NMR, mass spectrometry has higher sensitivity at low concentrations and detects more lipid species with potential functional as well as clinical relevance, for example lysophosphatidylcholines and sphingomyelins differing by the composition of O- and N-linked fatty acids (Table 1). 45, 61, 73 Several of them have been associated with the presence of acute or chronic ASCVD or diabetes and react to therapeutic interventions.58–60 Prospective studies are needed to investigate the prognostic performance of specific lipid species in HDL in addition to total plasma or serum.
Transcriptomics:
In addition to proteins and lipids, RNAs are highly abundant in HDLs, the majority of which are fragments from longer RNAs originating from bacteria and fungi and being of unclear clinical relevance.74 HDLs also transport microRNAs (miRs) that regulate cellular differentiation, proliferation, apoptosis, and metabolic homeostasis.65, 74 miR-223, the most abundant and best characterized miRNA in HDLs, is released from myeloid cells and delivered by HDL into endothelial cells, hepatocytes, smooth muscle cells, and monocytes.75 To date, the functional consequence of HDL-derived miRs remains controversial.76 The concentration of the most abundant HDL-associated miR-223 is 7 to 8 orders of magnitude lower than the plasma HDL concentration (20 μmol/L).66, 67, 77 To deliver sufficient amounts of specific miRNAs for posttranscriptional regulation, HDLs may shuttle miRs secreted by circulating blood cells or neighboring cells and thereby mediate autocrine or paracrine rather than endocrine regulation. Only small case-control studies have investigated the associations of HDL-associated miRNAs with disease and treatment.66–69 Future studies should provide mechanistic insights and observations in large human cohorts.
Conclusion.
In conclusion, hypothesis-free research strategies have led to the discovery of genes, proteins, lipid species, and miRs that regulate HDL metabolism and determine the physiological or pathological functions of HDLs. Some of these HDL constituents may serve as biomarkers to improve the identification, treatment stratification and monitoring of individuals at risk for different diseases. These multiple regulatory factors will necessitate the application of multiparametric -omics technologies to well characterized biobanks in order to validate the utility of biomarker candidates. The specific and comprehensive analyses of HDL constituents by methods combining high resolution and high throughput, however, remains the main technical challenge in this field.
IV. HDL: Clinical Implications
Clinical implications in ASCVD
Low levels of HDL-C, specifically below 40mg/dL, are strongly associated with increased risk of coronary and peripheral arterial disease and are characterized by an atherogenic dyslipidemia consisting of high levels of small, dense LDL particles, elevated triglycerides and increased insulin resistance. The remarkably consistent predictive information captured by low HDL-C, particularly among White populations, supports continued measurement of HDL-C clinically for diagnosing metabolic syndrome and as a guide for ASCVD risk prediction.
Despite the clinical utility of low HDL-C, the associations between HDL-C and ASCVD are not linear and vary according to race/ethnicity. In particular, there is a U-shaped association between HDL-C and ASCVD/mortality, with a linear inverse association preserved below 40mg/dL in men and below 50-58 mg/dL in women, no association across the normal range (40-96 mg/dL in men and 50-134 mg/dL in women), and a modest but increased ASCVD risk at HDL-C levels above 90 mg/dL in Asians, above 97 mg/dL in White male and above 135 mg/dL in White female populations.1, 78 Moreover, the links between HDL-C and ASCVD among Blacks may be attenuated or even trend in the opposite direction compared to Whites.46 Future studies may clarify consistency of these associations across non-White populations and in non-coronary vascular domains.
ApoA-I is mechanistically linked to atherosclerosis and is inversely associated with ASCVD risk. However, apoA-I levels do not improve risk prediction beyond non-HDL-C and HDL-C levels,79 limiting clinical utility as a prognostic biomarker. Other HDL apolipoproteins such as apoA-II and apoC-III may improve risk information,4, 41, 80 but will require validation in longitudinal cohorts and utility beyond non-HDL-C and other standard risk factors. Given the mechanistic role for apoA-I in non-atherosclerotic CVD, future studies should also assess whether apoA-I improves risk prediction of heart failure81 and arrhythmias82 and whether interventions targeting these diseases work, at least in part, by increasing apoA-I levels and/or function.
HDL-P reflects total HDL particle concentration and is currently measured commercially by NMR and ion mobility assays. HDL-P measured by NMR consistently outperforms HDL-C in associating with ASCVD in large cohorts.46 For HDL-P to gain traction as a clinical risk marker, studies are needed that directly assess risk prediction for the specific vascular endpoints of myocardial infarction, stroke, and peripheral arterial disease (PAD). It is also important to establish whether HDL-P is useful for risk prediction in non-White populations.
HDL function is increasingly being used in observational and interventional investigations. Studies assessing ex-vivo cholesterol efflux from macrophages to apoB-depleted serum have linked impaired efflux to a higher risk of incident and recurrent ASCVD in most, but not all, studies.5 Intriguingly, this risk may be specific to coronary atherosclerosis, especially thin cap fibroatheroma and non-calcified plaque,83–85 and have little to no relevance for cerebrovascular atherosclerosis and ischemic stroke.86, 87 A recent sub-study of the PREDIMED (Prevención con Dieta Mediterránea) trial suggests that assessing multiple aspects of HDL composition and function gives insights into the effects of an intervention and whether the intervention is a potentially bone fide therapeutic target.88 Currently, these assays are only research tools and need to be scaled up before they can be used clinically to improve risk prediction of incident CV events or for rapid, high throughput measurement of HDL function in specific populations based on risk status, gender, and ethnicity.
Given the dynamic nature of HDL structure-function relationships in the context of various diseases and therapies and the diversity of CV end-points, several factors should be considered in the design and analysis of future epidemiologic, translational, and intervention studies focused on ASCVD phenotypes and outcomes (Table 2).
Table 2.
Factors to Consider in Future Studies Investigating HDL markers with Respect to Study Populations and Cardiovascular End Points
| Type of Coronary Heart Disease (CHD) • represent a continuum of risk but also reflect varying pathology from chronic atherosclerotic and inflammatory processes to increased thrombotic and acute inflammatory perturbations |
•Stable CHD •Unstable Angina •NSTEMI •STEMI |
| Coronary vs. peripheral arterial disease | •MI •Cerebrovascular atherosclerosis/ ischemic (non-embolic) stroke • Lower extremity peripheral arterial disease |
| Atherosclerotic vs. non-atherosclerotic CVD | •Atherosclerotic CVD (ASCVD): • CHD, Cerebrovascular disease (atherosclerotic stroke, embolic stroke, hemorrhagic stroke), peripheral arterial disease •Non-atherosclerotic CVD: • Heart failure, arrhythmias, vascular stiffness, etc. |
| Accounting for cardiometabolic medications | •Lipid-modifying drugs •Glucose-lowering drugs •Blood pressure drugs (affecting vascular tone) |
HDL=high density lipoprotein; CHD=coronary heart disease; NSTEMI=non-ST elevation myocardial infarction; STEMI=ST elevation myocardial infarction; MI=myocardial infarction; CVD=cardiovascular disease;
Clinical Implications in Non-CV Diseases
HDL metabolism is directly relevant to non-CV diseases such as cancer and diabetes. Epidemiologic studies have linked lower HDL-C and apoA-I levels to increased risk of lung, liver, colorectal, breast, prostate and hematologic malignancies,89 as well as with increased disease progression and diminished therapeutic response.90 Higher HDL-C and apoA-I levels during treatment also predict improved response and survival.91 These studies are balanced by some reports that specific cancers may upregulate apoA-I, particularly in metastatic disease,92 perhaps reflecting increased cholesterol uptake by malignant cells during tumor progression. In line with these observations, SR-BI, which primarily mediates uptake of cholesteryl esters into cells, is upregulated in several cancers and prognostic for tumor progression and metastasis.93 Although pre-clinical studies suggest a role for apoA-I in suppressing tumors, it remains unknown whether increasing apoA-I improves cancer prognosis in humans.
Specific questions to clarify the role of HDL markers include which HDL markers best predict risk of incident cancer vs. progression vs. prognosis. How does chemotherapy, especially immune-checkpoint inhibitors, affect HDL function and are these effects beneficial or harmful? To what degree do changes in HDL markers reflect the cancers themselves, the effects of treatments, changes in cancer-related CV risk, or changes in diet/lifestyle and body composition that are related to cancer treatment and survival?
The prevention of diabetes and its complications is a clear unmet need given its increasing prevalence. As described above, a large body of preclinical data support a protective role of HDLs in preventing hyperglycemia.11 Intriguingly, therapies that increase HDL-C and apoA-I levels, such as CETP inhibitors and rHDL infusions, reduce the incidence and progression of diabetes.14 Although these specific therapies have not translated to clinical use, it is clearly worthwhile investigating whether interventions that target HDL metabolism may be used for the prevention and treatment of diabetes. The antioxidant and anti-inflammatory functions of HDLs are also decreased in patients with diabetes and they have a reduced ability to increase nitric oxide (NO) production, which may explain at least part of the increased CV risk in these patients.
Other non-CV diseases not covered here, but where there is emerging evidence of a role for HDLs, include infection, renal failure, auto-immune disorders, age-related macular degeneration, and pulmonary hypertension.1
Future Directions
Future studies assessing HDL-C as a risk marker should consider the potential for non-linearity as well as effects of ethnicity, prevalent disease status, and HDL functionality. The relationship of HDLs and apoA-I with non-atherosclerotic CVD diseases are poorly understood, reflecting a clear unmet need. Future studies on HDL function should focus on clinical effects of post translational and disease-specific modifications of apoA-I as well as the impact of other HDL-associated proteins and lipids. Total HDL particle concentration may give additional information on risk, but a lack of standardization limits the broader clinical utility of this approach. Use of composite end points (i.e. MI + stroke; all CVD) in epidemiologic and intervention studies may also blur associations, which highlights the importance of reporting end-point specific associations and effects. Use of apoA-I as a direct therapeutic to improve ASCVD, non-atherosclerotic CVD, and non-CVD is also worthy of additional investigation. The challenge will be to identify the specific subpopulations and disease states where HDL-related therapies are likely to have the most benefit and least harm. Pre-clinical data and observational data in humans support continued investigation of apoA-I as well as other mediators of HDL function in the development of new therapies for treating ASCVD, all other CVD, as well as non-CVDs in which HDLs and apoA-I are known to participate.
V. HDL Therapeutics
The rationale for developing therapeutic agents that modulate HDL metabolism is largely based on three considerations. First, even after the introduction of more effective LDL-C lowering therapies, such as PCSK9-inhibitors, there will likely still be significant residual ASCVD risk. Statins by themselves in the landmark clinical trials that led to their approval only reduced ASCVD events by approximately a third. This has generated great interest going back two decades in identifying other lipoprotein targets besides LDLs for drug development. The second factor is the consistent and long standing observation of increased ASCVD events in subjects with low HDL-C in large scale epidemiologic studies94. In fact, besides total cholesterol, the only other lipid parameter used in most ASCVD risk equations is HDL-C. This association, however, does not as noted above, necessarily indicate that HDL-C is causally related to the pathogenesis of atherosclerosis. There is, nevertheless, relatively good biologic plausibility for how HDL can be mechanistically related to atheroprotection, which is the third factor that has prompted efforts to develop HDL-based drugs. There are numerous experimental studies showing that HDLs have several potential anti-atherogenic functions94 such as an ability to accept cholesterol that effluxes from cells, thereby stimulating RCT.5 Compositionally, HDLs are more diverse than LDLs and transport a large number of proteins and lipids and even miRNAs that may mediate various functions.94 Pre-clinical animal models have also supported a direct role for HDLs in both blocking the progression of atherosclerosis and in promoting its regression95, but results from animal models, of course, do not always translate to humans.
Despite the promise of HDLs as a target for new cardiovascular drugs, the results so far have been disappointing. Recent genome wide association studies (GWAS) and Mendelian Randomization studies have questioned whether HDLs are causally related to atherosclerosis (as discussed above). An alternative plausible explanation for its inverse association with ASCVD is that HDL-C is just a marker for triglyceride-rich lipoproteins (TRLs), which unlike HDL-C have clearly been implicated in genetic studies to cause ASCVD96. Increased triglyceride (TG) in TRL particles are transferred to HDL by CETP. Subsequent lipolysis of HDL-associated TG generates small HDL particles with less cholesterol97. This process of CETP-mediated lipid exchange also accelerates the catabolism of HDLs, which along with the reduction in size, leads to an inverse association between HDL-C and TG. HDL-C, however, is a better ASCVD risk marker than TG, but this may because of its lower biological variability, thus allowing it to serve as a more stable marker of TG metabolism.94
As further described below, most of the efforts to date have used HDL-C as the main metric for monitoring the effect of new drugs on HDL metabolism. We now know that some of the functional properties of HDLs may be better predictors of their atheroprotective properties than HDL-C. This assumption may have hindered our efforts so far to develop effective HDL-based drugs. The most persuasive example of the importance of HDL function are the numerous studies showing that the cholesterol efflux capacity (CEC) of HDLs is a better negative ASCVD risk factor than HDL-C.5 From an evolutionary perspective, it has been suggested that a predominantly anti-atherosclerotic role for HDLs was unlikely because this condition is largely a disease of modern society and even today it frequently does not become clinically manifest until after child-bearing age. It may be that one or more of the other pathophysiologic processes that HDLs modulate, such as inflammation,8 are more relevant to its “true” biologic role. Given, however, the pleiotropic structural role of cholesterol in cell membranes that affects many biologic processes, including inflammation,8 the connection between the CEC capacity of HDL and atherosclerosis may still be relevant for developing future HDL-based drugs.
In Table 3, the various types of drugs that raise HDL that have been approved or are being developed are described98. One of the first ASCVD drugs ever used is niacin. It raises HDL-C by 30-50%, and lowers TG and Lp(a)99. Two large randomized clinical trials (AIM-HIGH [Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride: Impact on Global Health Outcomes] and HSP2Thrive [Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events]) of niacin when used on top of statins in patients with relatively well controlled LDL-C have failed to show any benefit in reducing clinical events despite increasing HDL-C. Whether niacin may still be useful in other clinical settings, such as for patients with hypertriglyceridemia or with elevated lipoprotein (a), is not known. Fibrates, another old class of drugs, can also raise HDL-C by acting as PPAR agonists, but they also have many other potential beneficial effects, particularly in lowering TRL particles. Randomized clinical trials of fibrates on top of statins have failed so far to show benefit but there are several ongoing studies that are examining their possible utility specifically in patients with elevated TG. If these clinical trials are successful, it will likely not be clear, however, whether their modulation of HDL-C played any role.
Table 3 –
Drugs affecting HDL Metabolism
| Drugs | % HDL-C Elevation | Mechanism of Action | Stage of Development |
|---|---|---|---|
| Niacin | 30-50 | Multifactorial effects | Approved |
| Fibrates (Fenofibrate) |
5-15 | Multifactorial effects by acting as PPAR agonists | Approved |
| CETP- Inhibitors (Anacetrapib, Dalcetrapib) |
25-135 | Inhibit transfer of cholesteryl esters away from HDL | Phase III (completed) |
| BET Inhibitor (Apabetalone) |
5-10 | Epigenetic modification altering apoA-I transcription | Phase III (completed) |
| HDL Infusion Therapy (CSL-112) |
50-200 | Direct infusion of exogenous HDL | Ongoing Phase III (AEGIS-II: NCT03473223) |
| Recombinant LCAT (MEDI6012) |
50-100 | Increase esterification of cholesterol on HDL | Ongoing Phase II ((REAL-TIMI 63B: NCT03578809)) |
HDL=high density lipoprotein; PPAR=peroxisome proliferator-activated receptor; CETP=cholesteryl ester transfer protein; BET=bromodomain and extraterminal; apoA-I=apolipoprotein A-I; AEGIS=ApoA-I Event reducing in Ischemic Syndromes; LCAT=lecithin-cholesterol acyltransferase;
CETP-inhibitors were the first drugs specifically designed for increasing HDL-C. By blocking the equilibration of cholesteryl esters and TG between the various lipoprotein classes, which is the main function of CETP, HDL-C is increased97. Several CETP-inhibitors have been investigated, but have mostly failed to show ASCVD benefit from raising HDL-C in clinical trials when used in conjunction with statins.100–102 Anacetrapib did modestly lower ASCVD events in a large Phase III study, but this was attributed to its ability to also lower LDL-C.103 This is consistent with a recent Mendelian randomization study that has revealed that genetic variants with decreased CETP function are associated with lower apoB and LDL-C levels and decrease ASCVD events.104 Dalcetrapib in a large Phase III clinical trial also failed to show benefit102, but it is still being investigated, because in post hoc analysis patients with a relatively common adenylyl cyclase type 9 (ADCY9) genotype may have had less clinical events on drug105. In a recent study with anacetrapib, however, there was no benefit in subjects with the potentially beneficial ADCY9 genotype106. Considering the recent progress that has been made using other non-statin drugs for lowering LDL-C, and in light of numerous clinical trials demonstrating that raising HDL-C with CETP-inhibitors does not contribute to ASCVD event reduction, there does not appear to be a role for the combined use of CETP-inhibitors with statins, although the potential clinical utility of anacetrapib is not entirely known.
RVX-208 (Apabetalone) represents another class of drugs that raises HDL-C modestly by epigenetically inhibiting the bromodomain and extraterminal (BET) family of proteins107. BETs are a large class of proteins that recognize histone acetylation and have wide ranging effects on gene expression. They have been mostly studied in an oncogenesis setting. Their wide-ranging effects makes it challenging to develop drugs based on this target, but at the same time it raises the possibility that they may have multiple beneficial effects. In fact, RVX-208 not only raises HDL-C but also decreases CRP and improves insulin sensitivity107, 108. In Phase II trials mixed results were found,108 and in a recent Phase III trial (BETonMACE) in acute coronary syndrome patients with Type 2 diabetes,109 RVX-208 did not reduce clinical events compared to placebo.
Promising animal studies showing that a small number of intravenous infusions of HDLs can rapidly reverse atherosclerotic plaque95 have stimulated efforts by several drug companies to test rHDLs as a therapy. Either purified or recombinant apoA-I is combined with phospholipids in these different rHDL preparations. The rationale for acute therapy is that once a patient presents with a myocardial infarction they are at high risk of having a second event, but it typically takes more than a year or more to see a significant reduction in clinical events after statin treatment. The expectation is that acute treatment with rHDLs over several weeks and concurrently starting statin therapy could rapidly stabilize patients. The early stage clinical trials that were largely based on intravascular ultrasound imaging (IVUS) of plaque in coronary vessels were encouraging95, but later larger Phase II clinical trials also based on IVUS did not show evidence for significant improvement.110 One formulation of rHDL that uses apoA-I purified from plasma called CSL112111 is still under evaluation for cardiovascular outcomes in a Phase III clinical trial of acute coronary syndrome patients (AEGIS-II [ApoA-I Event reducing in Ischemic Syndrome II]: NCT03473223). Small synthetic apoA-I mimetic peptides that share many of the same biologic functions of apoA-I but have several potential advantages in terms of drug development have also been used alone or complexed with lipid in rHDL112. These apolipoprotein mimetic peptides have also been tested in early stage clinical trials mostly for safety, but their further development will likely depend on the success of CSL112.
Another strategy for raising HDL-C involves treatment with recombinant LCAT, which esterifies cholesterol in HDLs and to a lesser degree in LDLs. Patients with a genetic defect in LCAT have Familial LCAT Deficiency (FLD) and present with low HDL-C, corneal opacities from cholesterol deposition and anemia. Their main clinical problem is renal failure due to deposition of LpX, an abnormal multilamellar vesicle rich in phospholipid and free cholesterol.113 Despite their low HDL-C, FLD patients do not appear to have increased atherosclerosis based on carotid intima media thickness studies,114 possibly because they also have low LDL-C. Nevertheless, recombinant LCAT is being considered as an intravenous infusion drug therapy to raise HDL-C for the acute treatment of patients with myocardial infarction. In a Phase I study, an early formulation of recombinant LCAT was shown to be safe and raised HDL-C by ~50% for a week115. A new formulation (MEDI6012) of recombinant LCAT with better pharmacokinetic and pharmacodynamic parameters is now being tested on infarct size in a Phase II study in acute coronary syndrome patients (REAL-TIMI 63B: NCT03578809). Recombinant LCAT may also be a useful therapy for preventing renal disease in FLD patients and was shown to nearly correct the abnormal lipoprotein profile in one FLD patient116. There are also early efforts for activating LCAT with small molecules,117 which may be a more attractive approach that could be developed into a chronic therapy, if the early studies on recombinant LCAT turn out to be successful.
In summary, several different types of drugs for modulating HDL have been tested for preventing and treating ACSVD, but none have proven to be successful. It may be that HDLs are not causally related to ASCVD, making these efforts futile and/or the impact of HDLs in the face of effective LDL-C lowering may be too minimal to be clinically meaningful. More research related to HDL function may uncover other metrics of HDLs besides HDL-C that are causally linked to the development of atherosclerosis, enabling the development of successful HDL-based drugs for ASCVD or possibly other disorders.
Conclusion
This document summarizes critical aspects of HDL structure, function, and metabolism. Implications for clinical utility will likely hinge on several key points: 1) clarifying the dynamic and context-dependent nature of HDL function in multiple disease states; 2) improved understanding of the regulation of HDL function at the molecular level; 3) extending and further validating -omics analyses of HDL-associated proteins, lipids, and other constituents; 4) specifying CVD phenotype, acute vs. chronic background illness, and concurrent medical therapy in human studies; and 5) focusing on therapeutic strategies that optimize HDL functions in the right patients at the optimal time in their disease course. We recommend moving away from HDL-C levels as a focus of investigation. Instead, we propose that increased use of deep phenotyping approaches (-omics) in diverse populations will reveal specific HDL subpopulations that convey specific functions. Investigation of multiple functions within the same populations will likely yield metabolic networks that more precisely elucidate the role of HDLs in cardiovascular disease as well as cancer, infection, diabetes, renal disease, and other disorders. Determining the role of inflammation and reverse cholesterol transport on these HDL function-disease relationships will be critical for translation of HDLs as diagnostic or therapeutic targets (Figure 3).
Figure 3.
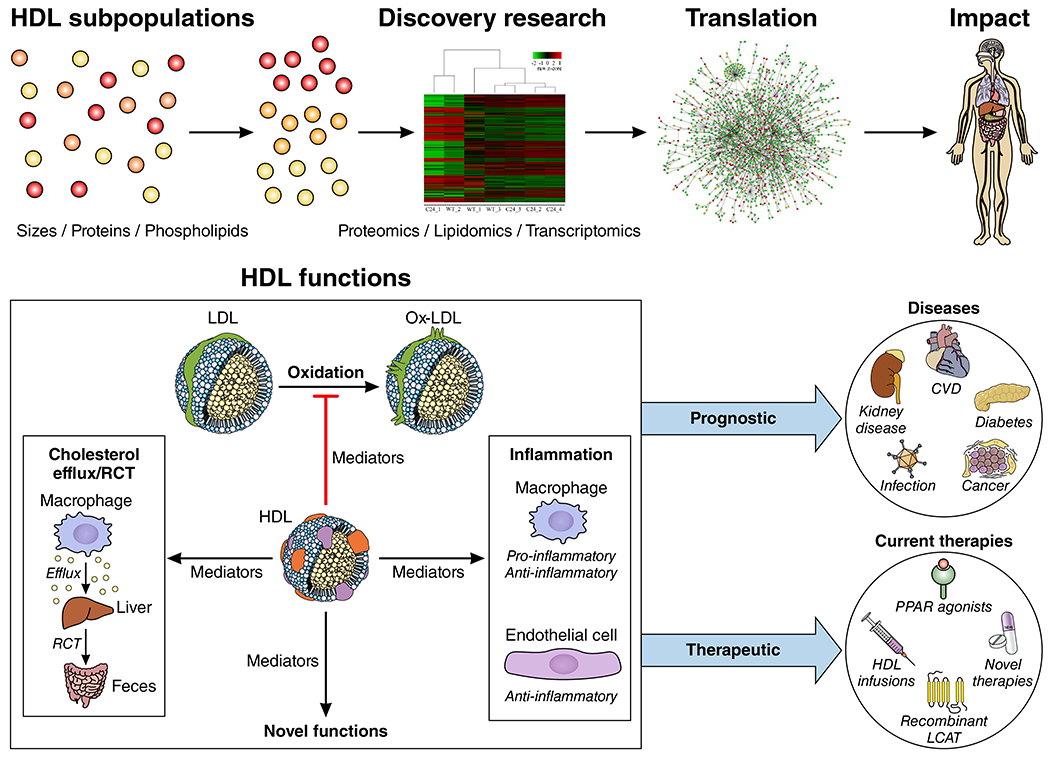
A Conceptual Framework for Investigating the Translational and Clinical Impact of HDLs. HDLs comprise multiple subpopulations with diverse functions that are context dependent. Use of -omics approaches in diverse human cohorts with and without disease will help identify these context-dependent functions of specific HDLs to improve risk prediction and therapeutic strategies for both cardiovascular and non-cardiovascular diseases.
Funding:
Anand Rohatgi: NIH/NHLBI R01HL136724 and NIH/NHLBI K24HL146838. Marit Westerterp: VIDI (The Innovational Research Incentives Scheme Vidi) grant 917.15.350 from the Netherlands Organization of Sciences (NWO) (to M. Westerterp); and a Rosalind Franklin Fellowship from the University Medical Center Groningen (to M. Westerterp). Arnold von Eckardstein: Swiss National Science Foundation ((31003A-160216 and 310030_166391/1), Swiss Heart Foundation, Systems X (MRD 2014/267). Alan Remaley: Research by AR is supported by intramural NHLBI funds from the NIH. Kerry-Anne Rye: New South Wales Cardiovascular Capacity Program H20/28248, National Heart Foundation of Australia Vanguard Grant 102845; National Health and Medical Research Council of Australia Grant APPP1148468
Non-standard Abbreviations and Acronyms:
- HDL
high-density lipoprotein
- ASCVD
atherosclerotic cardiovascular disease
- CV
cardiovascular
- LCAT
lecithin: cholesterol acyl transferase
- CETP
cholesteryl ester transfer protein
- ApoA-I
apolipoprotein A-I
- SAA
serum amyloid albumin
- PON
paraoxonase
- RCT
Reverse cholesterol transport
- ABCA1/G1
ATP binding cassette A1/G1
- S1P
sphingosine-1-phosphate
- LDL
low-density lipoprotein
- CETP
cholesteryl transfer protein
- PAF-AH
platelet-activating factor acetyl hydrolase
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- PMA
phorbol 12-myristate 13-acetate
- TRAM
TRIF related adaptor molecule
- Atf3
activating transcription factor 3
- PKC
protein kinase C
- NLR
NOD-Like Receptor
- NLRP3
NLR Family Pyrin Domain Containing 3
- IL
interleukin
- CKD
chronic kidney disease
- SR-BI
scavenger receptor B1
- NMR
Nuclear magnetic resonance
- RNA
ribonucleic acid
- miR
microRNA
- PAD
peripheral arterial disease
- PREDIMED
Prevención con Dieta Mediterránea)
- NO
nitric oxide
- MI
myocardial infarction
- TRL
triglyceride-rich lipoproteins
- TG
triglyceride
- CEC
cholesterol efflux capacity
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride: Impact on Global Health Outcomes
- HPS2-THRIVE
Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events
- ADCY9
adenylyl cyclase type 9
- BET
bromodomain and extraterminal
- rHDL
recombinant HDL
- IVUS
intravascular ultrasound
- AEGIS-II
ApoA-I Event reducing in Ischemic Syndrome II
- FLD
Familial LCAT deficiency
Footnotes
Disclosures: Marit Westerterp: None. Anand Rohatgi: Merck, research grant, significant; CSL Limited, consultant, modest. Arnold von Eckardstein: none. Kerry-Anne Rye: None.
References:
- 1.Madsen CM, Varbo A and Nordestgaard BG. Novel Insights From Human Studies on the Role of High-Density Lipoprotein in Mortality and Noncardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41:128–140. [DOI] [PubMed] [Google Scholar]
- 2.Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, Kontush A, Jerome WG, Rye KA and Davidson WS. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, Gogonea V, Lee X, May RP, Pipich V, Wagner MA, Undurti A, Tallant TC, Baleanu-Gogonea C, Charlton F, Ioffe A, DiDonato JA, Rye KA and Hazen SL. The low resolution structure of ApoA1 in spherical high density lipoprotein revealed by small angle neutron scattering. J Biol Chem. 2011;286:12495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furtado JD, Yamamoto R, Melchior JT, Andraski AB, Gamez-Guerrero M, Mulcahy P, He Z, Cai T, Davidson WS and Sacks FM. Distinct Proteomic Signatures in 16 HDL (High-Density Lipoprotein) Subspecies. Arterioscler Thromb Vasc Biol. 2018;38:2827–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anastasius M, Luquain-Costaz C, Kockx M, Jessup W and Kritharides L. A critical appraisal of the measurement of serum ‘cholesterol efflux capacity’ and its use as surrogate marker of risk of cardiovascular disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:1257–1273. [DOI] [PubMed] [Google Scholar]
- 6.Cuchel M, Raper AC, Conlon DM, Pryma DA, Freifelder RH, Poria R, Cromley D, Li X, Dunbar RL, French B, Qu L, Farver W, Su CC, Lund-Katz S, Baer A, Ruotolo G, Akerblad P, Ryan CS, Xiao L, Kirchgessner TG, Millar JS, Billheimer JT and Rader DJ. A novel approach to measuring macrophage-specific reverse cholesterol transport in vivo in humans. J Lipid Res. 2017;58:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ and Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3beta-hydroxysteroid-Delta24 reductase expression and inducing heme oxygenase-1. Circ Res. 2013;112:278–88. [DOI] [PubMed] [Google Scholar]
- 8.Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman LA, Sviridov DO, Demosky SJ Jr. and Remaley AT. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology. 2016;149:306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz M, Frej C, Holmer A, Guo LJ, Tran S and Dahlback B. High-Density Lipoprotein-Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118–129. [DOI] [PubMed] [Google Scholar]
- 10.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D and Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–7. [DOI] [PubMed] [Google Scholar]
- 11.Manandhar B, Cochran BJ and Rye KA. Role of High-Density Lipoproteins in Cholesterol Homeostasis and Glycemic Control. J Am Heart Assoc. 2020;9:e013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yalcinkaya M, Kerksiek A, Gebert K, Annema W, Sibler R, Radosavljevic S, Lutjohann D, Rohrer L and von Eckardstein A. HDL inhibits endoplasmic reticulum stress-induced apoptosis of pancreatic beta-cells in vitro by activation of Smoothened. J Lipid Res. 2020;61:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abderrahmani A, Niederhauser G, Favre D, Abdelli S, Ferdaoussi M, Yang JY, Regazzi R, Widmann C and Waeber G. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia. 2007;50:1304–14. [DOI] [PubMed] [Google Scholar]
- 14.Masson W, Lobo M, Siniawski D, Huerin M, Molinero G, Valero R and Nogueira JP. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab. 2018;44:508–513. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Leiter LA, Ballantyne CM, Barter PJ, Black DM, Kallend D, Laghrissi-Thode F, Leitersdorf E, McMurray JJV, Nicholls SJ, Olsson AG, Preiss D, Shah PK, Tardif JC and Kittelson J. Dalcetrapib Reduces Risk of New-Onset Diabetes in Patients With Coronary Heart Disease. Diabetes Care. 2020;43:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontush A, Chantepie S and Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:1881–8. [DOI] [PubMed] [Google Scholar]
- 17.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM and Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–7. [DOI] [PubMed] [Google Scholar]
- 18.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ and Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–90. [DOI] [PubMed] [Google Scholar]
- 19.De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, Vogelhuber J, Kraut M, Ulas T, Kerksiek A, Krebs W, Bode N, Grebe A, Fitzgerald ML, Hernandez NJ, Williams BR, Knolle P, Kneilling M, Rocken M, Lutjohann D, Wright SD, Schultze JL and Latz E. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nature immunology. 2014;15:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotakis P, Kothari V, Thomas DG, Westerterp M, Molusky MM, Altin E, Abramowicz S, Wang N, He Y, Heinecke JW, Bornfeldt KE and Tall AR. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler Thromb Vasc Biol. 2019;39:e253–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smoak KA, Aloor JJ, Madenspacher J, Merrick BA, Collins JB, Zhu X, Cavigiolio G, Oda MN, Parks JS and Fessler MB. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell Metab. 2010;11:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Vorst EPC, Theodorou K, Wu Y, Hoeksema MA, Goossens P, Bursill CA, Aliyev T, Huitema LFA, Tas SW, Wolfs IMJ, Kuijpers MJE, Gijbels MJ, Schalkwijk CG, Koonen DPY, Abdollahi-Roodsaz S, McDaniels K, Wang CC, Leitges M, Lawrence T, Plat J, Van Eck M, Rye KA, Touqui L, de Winther MPJ, Biessen EAL and Donners M. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-kappaB/STAT1-IRF1 Signaling. Cell Metab. 2017;25:197–207. [DOI] [PubMed] [Google Scholar]
- 23.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK and Tall AR. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30:1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N and Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki M, Pritchard DK, Becker L, Hoofnagle AN, Tanimura N, Bammler TK, Beyer RP, Bumgarner R, Vaisar T, de Beer MC, de Beer FC, Miyake K, Oram JF and Heinecke JW. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122:1919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR and de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, Welch CL, Reilly MP, Stroes ES, Moore KJ and Tall AR. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation. 2018;138:898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin EE, Guo L, Schwendeman A and Li XA. HDL in sepsis - risk factor and therapeutic approach. Frontiers in pharmacology. 2015;6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yvan-Charvet L, Matsuura F, Wang N, Bamberger MJ, Nguyen T, Rinninger F, Jiang XC, Shear CL and Tall AR. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol. 2007;27:1132–8. [DOI] [PubMed] [Google Scholar]
- 30.Bochem AE, van der Valk FM, Tolani S, Stroes ES, Westerterp M and Tall AR. Increased Systemic and Plaque Inflammation in ABCA1 Mutation Carriers With Attenuation by Statins. Arterioscler Thromb Vasc Biol. 2015;35:1663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinon F, Burns K and Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–26. [DOI] [PubMed] [Google Scholar]
- 32.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA and Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14:812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, Wang N, Randolph GJ, D’Agati VD, Yvan-Charvet L and Tall AR. Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab. 2017;25:1294–1304 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauldin JP, Nagelin MH, Wojcik AJ, Srinivasan S, Skaflen MD, Ayers CR, McNamara CA and Hedrick CC. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation. 2008;117:2785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganda A, Yvan-Charvet L, Zhang Y, Lai EJ, Regunathan-Shenk R, Hussain FN, Avasare R, Chakraborty B, Febus AJ, Vernocchi L, Lantigua R, Wang Y, Shi X, Hsieh J, Murphy AJ, Wang N, Bijl N, Gordon KM, de Miguel MH, Singer JR, Hogan J, Cremers S, Magnusson M, Melander O, Gerszten RE and Tall AR. Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with CKD. Journal of molecular and cellular cardiology. 2017;112:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragoljevic D, Kraakman MJ, Nagareddy PR, Ngo D, Shihata W, Kammoun HL, Whillas A, Lee MKS, Al-Sharea A, Pernes G, Flynn MC, Lancaster GI, Febbraio MA, Chin-Dusting J, Hanaoka BY, Wicks IP and Murphy AJ. Defective cholesterol metabolism in haematopoietic stem cells promotes monocyte-driven atherosclerosis in rheumatoid arthritis. European heart journal. 2018;39:2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Mannisto S, Amouyel P, Arveiler D, Ferrieres J, Muller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ, Consortium CHDE, Consortium CAE and Global Lipids Genetics C. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanoni P and von Eckardstein A. Inborn errors of apolipoprotein A-I metabolism: implications for disease, research and development. Curr Opin Lipidol. 2020;31:62–70. [DOI] [PubMed] [Google Scholar]
- 39.Karjalainen MK, Holmes MV, Wang Q, Anufrieva O, Kahonen M, Lehtimaki T, Havulinna AS, Kristiansson K, Salomaa V, Perola M, Viikari JS, Raitakari OT, Jarvelin MR, Ala-Korpela M and Kettunen J. Apolipoprotein A-I concentrations and risk of coronary artery disease: A Mendelian randomization study. Atherosclerosis. 2020;299:56–63. [DOI] [PubMed] [Google Scholar]
- 40.Wulff AB, Nordestgaard BG and Tybjaerg-Hansen A. APOC3 Loss-of-Function Mutations, Remnant Cholesterol, Low-Density Lipoprotein Cholesterol, and Cardiovascular Risk: Mediation- and Meta-Analyses of 137 895 Individuals. Arterioscler Thromb Vasc Biol. 2018;38:660–668. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MK, Aroner SA, Mukamal KJ, Furtado JD, Post WS, Tsai MY, Tjonneland A, Polak JF, Rimm EB, Overvad K, McClelland RL and Sacks FM. High-Density Lipoprotein Subspecies Defined by Presence of Apolipoprotein C-III and Incident Coronary Heart Disease in Four Cohorts. Circulation. 2018;137:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao B and Heinecke JW. Quantifying HDL proteins by mass spectrometry: how many proteins are there and what are their functions? Expert Rev Proteomics. 2018;15:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collier TS, Jin Z, Topbas C and Bystrom C. Rapid Affinity Enrichment of Human Apolipoprotein A-I Associated Lipoproteins for Proteome Analysis. J Proteome Res. 2018;17:1183–1193. [DOI] [PubMed] [Google Scholar]
- 44.Goetze S, Frey K, Rohrer L, Radosavljevic S, Krützfeldt J, Landmesser U, Bueter M, Pedrioli PGA, von Eckardstein A and Wollscheid B. Sensitive and reproducible determination of clinical HDL proteotypes. bioRxiv. Preprint posted online July 10, 2020, doi: 10.1101/2020.07.09.191312. [DOI] [PubMed] [Google Scholar]
- 45.Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, Hartung J, Shemet A, Krankel N, Radosavljevic S, Keel M, Othman A, Karsai G, Hornemann T, Claassen M, Liebisch G, Carreira E, Ritsch A, Landmesser U, Krutzfeldt J, Wolfrum C, Wollscheid B, Beerenwinkel N, Rohrer L and von Eckardstein A. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh K, Chandra A, Sperry T, Joshi PH, Khera A, Virani SS, Ballantyne CM, Otvos JD, Dullaart RPF, Gruppen EG, Connelly MA, Ayers CR and Rohatgi A. Associations Between High-Density Lipoprotein Particles and Ischemic Events by Vascular Domain, Sex, and Ethnicity: A Pooled Cohort Analysis. Circulation. 2020;142:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE and Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asztalos BF, Horvath KV and Schaefer EJ. High-Density Lipoprotein Particles, Cell-Cholesterol Efflux, and Coronary Heart Disease Risk. Arterioscler Thromb Vasc Biol. 2018;38:2007–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Luscher TF and Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. [DOI] [PubMed] [Google Scholar]
- 50.Zvintzou E, Lhomme M, Chasapi S, Filou S, Theodoropoulos V, Xapapadaki E, Kontush A, Spyroulias G, Tellis CC, Tselepis AD, Constantinou C and Kypreos KE. Pleiotropic effects of apolipoprotein C3 on HDL functionality and adipose tissue metabolic activity. J Lipid Res. 2017;58:1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aroner SA, Furtado JD, Sacks FM, Tsai MY, Mukamal KJ, McClelland RL and Jensen MK. Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2019;62:981–992. [DOI] [PubMed] [Google Scholar]
- 52.Valanti EK, Chroni A and Sanoudou D. The future of apolipoprotein E mimetic peptides in the prevention of cardiovascular disease. Curr Opin Lipidol. 2019;30:326–341. [DOI] [PubMed] [Google Scholar]
- 53.Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, Lehmann M, Kopecky C, Saemann MD, Lepper PM, Silbernagel G, Scharnagl H, Ritsch A, Thorand B, de las Heras Gala T, Wagenpfeil S, Koenig W, Peters A, Laufs U, Wanner C, Fliser D, Speer T and Marz W. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. European heart journal. 2015;36:3007–16. [DOI] [PubMed] [Google Scholar]
- 54.Kopecky C, Genser B, Drechsler C, Krane V, Kaltenecker CC, Hengstschlager M, Marz W, Wanner C, Saemann MD and Weichhart T. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin J Am Soc Nephrol. 2015;10:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khalil A, Fulop T and Berrougui H. Role of Paraoxonase1 in the Regulation of High-Density Lipoprotein Functionality and in Cardiovascular Protection. Antioxid Redox Signal. 2021;34:191–200. [DOI] [PubMed] [Google Scholar]
- 56.Emmens JE, Jones DJL, Cao TH, Chan DCS, Romaine SPR, Quinn PA, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M, de Boer RA, Voors AA and Ng LL. Proteomic diversity of high-density lipoprotein explains its association with clinical outcome in patients with heart failure. Eur J Heart Fail. 2018;20:260–267. [DOI] [PubMed] [Google Scholar]
- 57.Silbernagel G, Scharnagl H, Kleber ME, Delgado G, Stojakovic T, Laaksonen R, Erdmann J, Rankinen T, Bouchard C, Landmesser U, Schunkert H, Marz W and Grammer TB. LDL triglycerides, hepatic lipase activity, and coronary artery disease: An epidemiologic and Mendelian randomization study. Atherosclerosis. 2019;282:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meikle PJ, Formosa MF, Mellett NA, Jayawardana KS, Giles C, Bertovic DA, Jennings GL, Childs W, Reddy M, Carey AL, Baradi A, Nanayakkara S, Wilson AM, Duffy SJ and Kingwell BA. HDL Phospholipids, but Not Cholesterol Distinguish Acute Coronary Syndrome From Stable Coronary Artery Disease. J Am Heart Assoc. 2019;8:e011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan AA, Mundra PA, Straznicky NE, Nestel PJ, Wong G, Tan R, Huynh K, Ng TW, Mellett NA, Weir JM, Barlow CK, Alshehry ZH, Lambert GW, Kingwell BA and Meikle PJ. Weight Loss and Exercise Alter the High-Density Lipoprotein Lipidome and Improve High-Density Lipoprotein Functionality in Metabolic Syndrome. Arterioscler Thromb Vasc Biol. 2018;38:438–447. [DOI] [PubMed] [Google Scholar]
- 60.Orsoni A, Therond P, Tan R, Giral P, Robillard P, Kontush A, Meikle PJ and Chapman MJ. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J Lipid Res. 2016;57:2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stahlman M, Fagerberg B, Adiels M, Ekroos K, Chapman JM, Kontush A and Boren J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: impact on small HDL particles. Biochim Biophys Acta. 2013;1831:1609–17. [DOI] [PubMed] [Google Scholar]
- 62.Chew WS, Torta F, Ji S, Choi H, Begum H, Sim X, Khoo CM, Khoo EYH, Ong WY, Van Dam RM, Wenk MR, Tai ES and Herr DR. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight. 2019;5:e126925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cartier A and Hla T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science. 2019;366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurano M, Tsukamoto K, Shimizu T, Kassai H, Nakao K, Aiba A, Hara M and Yatomi Y. Protection Against Insulin Resistance by Apolipoprotein M/Sphingosine-1-Phosphate. Diabetes. 2020;69:867–881. [DOI] [PubMed] [Google Scholar]
- 65.Michell DL and Vickers KC. Lipoprotein carriers of microRNAs. Biochim Biophys Acta. 2016;1861:2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabet F, Cuesta Torres LF, Ong KL, Shrestha S, Choteau SA, Barter PJ, Clifton P and Rye KA. High-Density Lipoprotein-Associated miR-223 Is Altered after Diet-Induced Weight Loss in Overweight and Obese Males. PLoS One. 2016;11:e0151061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simionescu N, Niculescu LS, Carnuta MG, Sanda GM, Stancu CS, Popescu AC, Popescu MR, Vlad A, Dimulescu DR, Simionescu M and Sima AV. Hyperglycemia Determines Increased Specific MicroRNAs Levels in Sera and HDL of Acute Coronary Syndrome Patients and Stimulates MicroRNAs Production in Human Macrophages. PLoS One. 2016;11:e0161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedgeman LR, Beysen C, Ramirez Solano MA, Michell DL, Sheng Q, Zhao S, Turner S, Linton MF and Vickers KC. Beta cell secretion of miR-375 to HDL is inversely associated with insulin secretion. Sci Rep. 2019;9:3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanak MA, Takita M, Shahbazov R, Lawrence MC, Chung WY, Dennison AR, Levy MF and Naziruddin B. Evaluation of MicroRNA375 as a Novel Biomarker for Graft Damage in Clinical Islet Transplantation. Transplantation. 2015;99:1568–73. [DOI] [PubMed] [Google Scholar]
- 70.Natarajan P, Collier TS, Jin Z, Lyass A, Li Y, Ibrahim NE, Mukai R, McCarthy CP, Massaro JM, D’Agostino RB Sr., Gaggin HK, Bystrom, Penn MS and Januzzi JL Jr. Association of an HDL Apolipoproteomic Score With Coronary Atherosclerosis and Cardiovascular Death. J Am Coll Cardiol. 2019;73:2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curcic S, Holzer M, Pasterk L, Knuplez E, Eichmann TO, Frank S, Zimmermann R, Schicho R, Heinemann A and Marsche G. Secretory phospholipase A2 modified HDL rapidly and potently suppresses platelet activation. Sci Rep. 2017;7:8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutter I, Park R, Othman A, Rohrer L, Hornemann T, Stoffel M, Devuyst O and von Eckardstein A. Apolipoprotein M modulates erythrocyte efflux and tubular reabsorption of sphingosine-1-phosphate. J Lipid Res. 2014;55:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lone MA, Hulsmeier AJ, Saied EM, Karsai G, Arenz C, von Eckardstein A and Hornemann T. Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:15591–15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, Shyr Y, Sethupathy P, Linton MF, Graf GA, Sheng Q and Vickers KC. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles. 2018;7:1506198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuesta Torres LF, Zhu W, Ohrling G, Larsson R, Patel M, Wiese CB, Rye KA, Vickers KC and Tabet F. High-density lipoproteins induce miR-223-3p biogenesis and export from myeloid cells: Role of scavenger receptor BI-mediated lipid transfer. Atherosclerosis. 2019;286:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, Palmisano BT, Tabet F, Cui HL, Rye KA, Sethupathy P and Remaley AT. MicroRNA-223 coordinates cholesterol homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, Zeiher AM, Landmesser U and Dimmeler S. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:1392–400. [DOI] [PubMed] [Google Scholar]
- 78.Hirata A, Sugiyama D, Watanabe M, Tamakoshi A, Iso H, Kotani K, Kiyama M, Yamada M, Ishikawa S, Murakami Y, Miura K, Ueshima H, Okamura T and Evidence for Cardiovascular Prevention from Observational Cohorts in Japan Research G. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J Clin Lipidol. 2018;12:674–684 e5. [DOI] [PubMed] [Google Scholar]
- 79.Emerging Risk Factors C, Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, Butterworth AS, Sarwar N, Wormser D, Saleheen D, Ballantyne CM, Psaty BM, Sundstrom J, Ridker PM, Nagel D, Gillum RF, Ford I, Ducimetiere P, Kiechl S, Koenig W, Dullaart RP, Assmann G, D’Agostino RB Sr., Dagenais GR, Cooper JA, Kromhout D, Onat A, Tipping RW, Gomez-de-la-Camara A, Rosengren A, Sutherland SE, Gallacher J, Fowkes FG, Casiglia E, Hofman A, Salomaa V, Barrett-Connor E, Clarke R, Brunner E, Jukema, Simons LA, Sandhu M, Wareham NJ, Khaw KT, Kauhanen J, Salonen JT, Howard WJ, Nordestgaard BG, Wood AM, Thompson SG, Boekholdt SM, Sattar N, Packard C, Gudnason V and Danesh J. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melchior JT, Street SE, Andraski AB, Furtado JD, Sacks FM, Shute RL, Greve EI, Swertfeger DK, Li H, Shah AS, Lu LJ and Davidson WS. Apolipoprotein A-II alters the proteome of human lipoproteins and enhances cholesterol efflux from ABCA1. J Lipid Res. 2017;58:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amin R, Muthuramu I, Aboumsallem JP, Mishra M, Jacobs F and De Geest B. Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. Int J Mol Sci. 2017;18:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Den Ruijter HM, Franssen R, Verkerk AO, van Wijk DF, Vaessen SF, Holleboom AG, Levels JH, Opthof T, Sungnoon R, Stroes ES, Kuivenhoven JA and Coronel R. Reconstituted high-density lipoprotein shortens cardiac repolarization. J Am Coll Cardiol. 2011;58:40–4. [DOI] [PubMed] [Google Scholar]
- 83.Salahuddin T, Natarajan B, Playford MP, Joshi AA, Teague H, Masmoudi Y, Selwaness M, Chen MY, Bluemke DA and Mehta NN. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. European heart journal. 2015;36:2662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kini AS, Vengrenyuk Y, Shameer K, Maehara A, Purushothaman M, Yoshimura T, Matsumura M, Aquino M, Haider N, Johnson KW, Readhead B, Kidd BA, Feig JE, Krishnan P, Sweeny J, Milind M, Moreno P, Mehran R, Kovacic JC, Baber U, Dudley JT, Narula J and Sharma S. Intracoronary Imaging, Cholesterol Efflux, and Transcriptomes After Intensive Statin Treatment: The YELLOW II Study. J Am Coll Cardiol. 2017;69:628–640. [DOI] [PubMed] [Google Scholar]
- 85.Zimetti F, Freitas WM, Campos AM, Daher M, Adorni MP, Bernini F, Sposito AC, Zanotti I and Brazilian Study on Healthy A. Cholesterol efflux capacity does not associate with coronary calcium, plaque vulnerability, and telomere length in healthy octogenarians. J Lipid Res. 2018;59:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mutharasan RK, Thaxton CS, Berry J, Daviglus ML, Yuan C, Sun J, Ayers C, Lloyd-Jones DM and Wilkins JT. HDL efflux capacity, HDL particle size, and high-risk carotid atherosclerosis in a cohort of asymptomatic older adults: the Chicago Healthy Aging Study. J Lipid Res. 2017;58:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, Heinecke JW, Yvan-Charvet L and Tall AR. Cholesterol Mass Efflux Capacity, Incident Cardiovascular Disease, and Progression of Carotid Plaque. Arterioscler Thromb Vasc Biol. 2019;39:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hernaez A, Castaner O, Elosua R, Pinto X, Estruch R, Salas-Salvado J, Corella D, Aros F, Serra-Majem L, Fiol M, Ortega-Calvo M, Ros E, Martinez-Gonzalez MA, de la Torre R, Lopez-Sabater MC and Fito M. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation. 2017;135:633–643. [DOI] [PubMed] [Google Scholar]
- 89.Georgila K, Vyrla D and Drakos E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers (Basel). 2019;11:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou P, Li B, Liu B, Chen T and Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin Chim Acta. 2018;477:94–104. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Wang ZQ, Wang FH, Lei XF, Yan SM, Wang DS, Zhang F, Xu RH, Wang LY and Li YH. Predictive value of chemotherapy-related high-density lipoprotein cholesterol (HDL) elevation in patients with colorectal cancer receiving adjuvant chemotherapy: an exploratory analysis of 851 cases. Oncotarget. 2016;7:57290–57300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi F, Wu H, Qu K, Sun Q, Li F, Shi C, Li Y, Xiong X, Qin Q, Yu T, Jin X, Cheng L, Wei Q, Li Y and She J. Identification of serum proteins AHSG, FGA and APOA-I as diagnostic biomarkers for gastric cancer. Clin Proteomics. 2018;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu GH, Lou N, Shi HC, Xu YC, Ruan HL, Xiao W, Liu L, Li X, Xiao HB, Qiu B, Bao L, Yuan CF, Zhou YL, Hu WJ, Chen K, Yang HM and Zhang XP. Up-regulation of SR-BI promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma. BMC Cancer. 2018;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karathanasis SK, Freeman LA, Gordon SM and Remaley AT. The Changing Face of HDL and the Best Way to Measure It. Clin Chem. 2017;63:196–210. [DOI] [PubMed] [Google Scholar]
- 95.Krause BR and Remaley AT. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–6. [DOI] [PubMed] [Google Scholar]
- 96.Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Nicholls SJ, Bhatt DL, Sabatine MS and Catapano AL. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants With Risk of Coronary Heart Disease. JAMA. 2019;321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowaniec Z and Skoczynska A. Plasma lipid transfer proteins: The role of PLTP and CETP in atherogenesis. Adv Clin Exp Med. 2018;27:429–436. [DOI] [PubMed] [Google Scholar]
- 98.Estrada-Luna D, Ortiz-Rodriguez MA, Medina-Briseno L, Carreon-Torres E, Izquierdo-Vega JA, Sharma A, Cancino-Diaz JC, Perez-Mendez O, Belefant-Miller H and Betanzos-Cabrera G. Current Therapies Focused on High-Density Lipoproteins Associated with Cardiovascular Disease. Molecules. 2018;23:2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma A and Madan N. Role of niacin in current clinical practice. Minerva Med. 2019;110:79–83. [DOI] [PubMed] [Google Scholar]
- 100.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B and Investigators I. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357:2109–22. [DOI] [PubMed] [Google Scholar]
- 101.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE and Investigators A. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. The New England journal of medicine. 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS and dal OI. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367:2089–99. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, Zhang Q, Wang Y, Gao P and Chen D. The effect and safety of anacetrapib in the treatment of dyslipidemia: a systematic review and meta-analysis. Postgrad Med. 2018;130:129–136. [DOI] [PubMed] [Google Scholar]
- 104.Ference BA, Kastelein JJP, Ginsberg HN, Chapman MJ, Nicholls SJ, Ray KK, Packard CJ, Laufs U, Brook RD, Oliver-Williams C, Butterworth AS, Danesh J, Smith GD, Catapano AL and Sabatine MS. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA. 2017;318:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pfeffer MA, Dube MP and Tardif JC. Randomized Clinical Trial Needed to Confirm Whether Dalcetrapib Improves Outcomes for Specific ADCY9 Genotype. JAMA Cardiol. 2018;3:897. [DOI] [PubMed] [Google Scholar]
- 106.Hopewell JC, Ibrahim M, Hill M, Shaw PM, Braunwald E, Blaustein RO, Bowman L, Landray MJ, Sabatine MS, Collins R and Group HTRC. Impact of ADCY9 Genotype on Response to Anacetrapib. Circulation. 2019;140:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nikolic D, Rizzo M, Mikhailidis DP, Wong NC and Banach M. An evaluation of RVX-208 for the treatment of atherosclerosis. Expert Opin Investig Drugs. 2015;24:1389–98. [DOI] [PubMed] [Google Scholar]
- 108.Siebel AL, Trinh SK, Formosa MF, Mundra PA, Natoli AK, Reddy-Luthmoodoo M, Huynh K, Khan AA, Carey AL, van Hall G, Cobelli C, Dalla-Man C, Otvos JD, Rye KA, Johansson J, Gordon A, Wong NC, Sviridov D, Barter P, Duffy SJ, Meikle PJ and Kingwell BA. Effects of the BET-inhibitor, RVX-208 on the HDL lipidome and glucose metabolism in individuals with prediabetes: A randomized controlled trial. Metabolism. 2016;65:904–14. [DOI] [PubMed] [Google Scholar]
- 109.Ray KK, Nicholls SJ, Buhr KA, Ginsberg HN, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Sweeney M, Schwartz GG, Investigators BE and Committees. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 2020;323:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicholls SJ, Andrews J, Kastelein JJP, Merkely B, Nissen SE, Ray KK, Schwartz GG, Worthley SG, Keyserling C, Dasseux JL, Griffith L, Kim SW, Janssan A, Di Giovanni G, Pisaniello AD, Scherer DJ, Psaltis PJ and Butters J. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018;3:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gille A, D’Andrea D, Tortorici MA, Hartel G and Wright SD. CSL112 (Apolipoprotein A-I [Human]) Enhances Cholesterol Efflux Similarly in Healthy Individuals and Stable Atherosclerotic Disease Patients. Arterioscler Thromb Vasc Biol. 2018;38:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sviridov D and Remaley AT. High-density lipoprotein mimetics: promises and challenges. Biochem J. 2015;472:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ossoli A, Neufeld EB, Thacker SG, Vaisman B, Pryor M, Freeman LA, Brantner CA, Baranova I, Francone NO, Demosky SJ Jr., Vitali C, Locatelli M, Abbate M, Zoja C, Franceschini G, Calabresi L and Remaley AT. Lipoprotein X Causes Renal Disease in LCAT Deficiency. PLoS One. 2016;11:e0150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oldoni F, Baldassarre D, Castelnuovo S, Ossoli A, Amato M, van Capelleveen J, Hovingh GK, De Groot E, Bochem A, Simonelli S, Barbieri S, Veglia F, Franceschini G, Kuivenhoven JA, Holleboom AG and Calabresi L. Complete and Partial Lecithin:Cholesterol Acyltransferase Deficiency Is Differentially Associated With Atherosclerosis. Circulation. 2018;138:1000–1007. [DOI] [PubMed] [Google Scholar]
- 115.Shamburek RD, Bakker-Arkema R, Auerbach BJ, Krause BR, Homan R, Amar MJ, Freeman LA and Remaley AT. Familial lecithin:cholesterol acyltransferase deficiency: First-in-human treatment with enzyme replacement. J Clin Lipidol. 2016;10:356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shamburek RD, Bakker-Arkema R, Shamburek AM, Freeman LA, Amar MJ, Auerbach B, Krause BR, Homan R, Adelman SJ, Collins HL, Sampson M, Wolska A and Remaley AT. Safety and Tolerability of ACP-501, a Recombinant Human Lecithin:Cholesterol Acyltransferase, in a Phase 1 Single-Dose Escalation Study. Circ Res. 2016;118:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freeman LA, Demosky SJ Jr., Konaklieva M, Kuskovsky R, Aponte A, Ossoli AF, Gordon SM, Koby RF, Manthei KA, Shen M, Vaisman BL, Shamburek RD, Jadhav A, Calabresi L, Gucek M, Tesmer JJG, Levine RL and Remaley AT. Lecithin:Cholesterol Acyltransferase Activation by Sulfhydryl-Reactive Small Molecules: Role of Cysteine-31. J Pharmacol Exp Ther. 2017;362:306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]


