Abstract
Purpose:
(1) To investigate the effect of internal localized movement on 3DMR intracranial vessel wall imaging and (2) to develop a novel motion-compensation approach combining volumetric navigator (vNav) and self-gating (SG) to simultaneously compensate for bulk and localized movements.
Methods:
A 3D variable-flip-angle turbo spin-echo (ie, SPACE) sequence was modified to incorporate vNav and SG modules. The SG signals from the center k-space line are acquired at the beginning of each TR to detect localized motion-affected TRs. The vNavs from low-resolution 3D EPI are acquired to identify bulk head motion. Fifteen healthy subjects and 3 stroke patients were recruited in this study. Overall image quality (0-poor to 4-excellent) and vessel wall sharpness were compared among the scenarios with and without bulk and/or localized motion and/or the proposed compensation strategies.
Results:
Localized motion reduced wall sharpness, which was significantly mitigated by SG (ie, outer boundary of basilar artery: 0.68 ± 0.27 vs 0.86 ± 0.17; P = .037). When motion occurred, the overall image quality and vessel wall sharpness obtained with vNav-SG SPACE were significantly higher than those obtained with conventional SPACE (ie, basilarartery outer boundary sharpness: 0.73 ± 0.24 vs 0.94 ± 0.24; P = .033), yet comparable to those obtained in motion-free scans (ie, basilarartery outer boundary sharpness: 0.94 ± 0.24 vs 0.96 ± 0.31; P = .815).
Conclusion:
Localized movements can induce considerable artifacts in intracranial vessel wall imaging. The vNav-SG approach is capable of compensating for both bulk and localized motions.
Keywords: intracranial vessel wall, motion compensation, self-gating, vessel wall imaging, volumetric navigators
1 |. INTRODUCTION
Stroke is a leading cause of death and disability worldwide and arises from various intracranial vessel wall abnormalities, including atherosclerosis, vasculitis, and Moyamoya syndrome.1,2 Accurate identification of these pathologies may help elucidate stroke etiology and allow for prompt delivery of appropriate treatment.3 Currently, evaluations of stroke patients’ vessel-related pathologies rely exclusively on assessments of the degree of luminal stenosis using lumenography-based imaging methods, which are, however, inadequate for differentiating diverse intracranial pathologic processes of the vessel wall.4,5
MR intracranial vessel wall imaging (VWI) is a noninvasive modality that can directly visualize structure and characterize pathologic changes within the vessel wall.6 Three-dimensional variable-flip-angle turbo spin-echo is currently the method of choice for intracranial VWI.7–9 However, the relatively long scan times and submillimeter spatial resolutions render this technique inherently susceptible to motion. The 3D encoding strategy further exacerbates the problem.10 Resultant blurring or ghosting artifacts may lead to image-quality degradation and inaccurate qualitative and quantitative wall lesion assessment, or even completely unusable exams.11–13
Motion artifacts typically observed in intracranial VWI may be caused by either bulk head motion or localized movement of internal anatomic structures. Bulk head motion implies rigid changes in the patient’s head pose, such as nodding and rotation in the scanner.14 Internal localized movement includes sudden and transient involuntary movements, induced by coughing and yawning, and semiregular movements, such as swallowing.15 Although foam cushions are used commonly to minimize bulk motion, subtle head position changes may still be critical, given the fine vessel wall structure (0.5 ± 0.1 mm) as well as the demanding spatial resolution.16–18 While image quality deterioration caused by bulk head motion is well documented, the effects of localized movement on intracranial VWI are underexplored.
An effective motion-compensation strategy that can mitigate the effects of both bulk head motion and internal localized motion is highly desirable for 3D intracranial VWI. Navigators are the traditional means of tracking head positions during brain MRI.19 Tisdall et al developed an EPI-based volumetric navigator (vNav) approach to periodically collect low-resolution volumetric images of the head and prospectively realign the imaging coordinates.20 This approach is effective at mitigating image artifacts due to bulk head motion in conventional brain imaging20; however, localized motions are not resolved by vNavs. A self-gating (SG) motion-compensation strategy, which uses a one-dimensional projection of the imaging volume to detect the object’s motion, was previously developed to reduce swallowing-related motion artifacts in carotid VWI.10 This could be a promising approach to mitigate localized movement effects on intracranial VWI.
The aim of this study is 2-fold. First, we investigated the effect of internal localized movement on intracranial VWI quality and demonstrated the effectiveness of the SG motion-gating scheme in mitigating resultant motion artifacts in healthy subjects. Second, we developed a motion-robust intracranial VWI technique by incorporating a combined vNav-SG strategy into 3D variable-flip-angle turbo spin-echo (ie, SPACE). Demonstrated in healthy subjects and stroke patients, the developed vNav-SG SPACE sequence proved to be more robust for intracranial VWI than the conventional SPACE sequence when motion occurred.
2 |. METHODS
2.1 |. Motion-compensation strategy and sequence design
The motion-compensation strategy is built on the T1-weighted SPACE sequence in which each TR consists of a long train of variable-flip-angle nonselective refocusing RF pulses and a gap for magnetization recovery.21 The schematic of the prototype vNav-SG SPACE sequence is illustrated in Figure 1.
FIGURE 1.
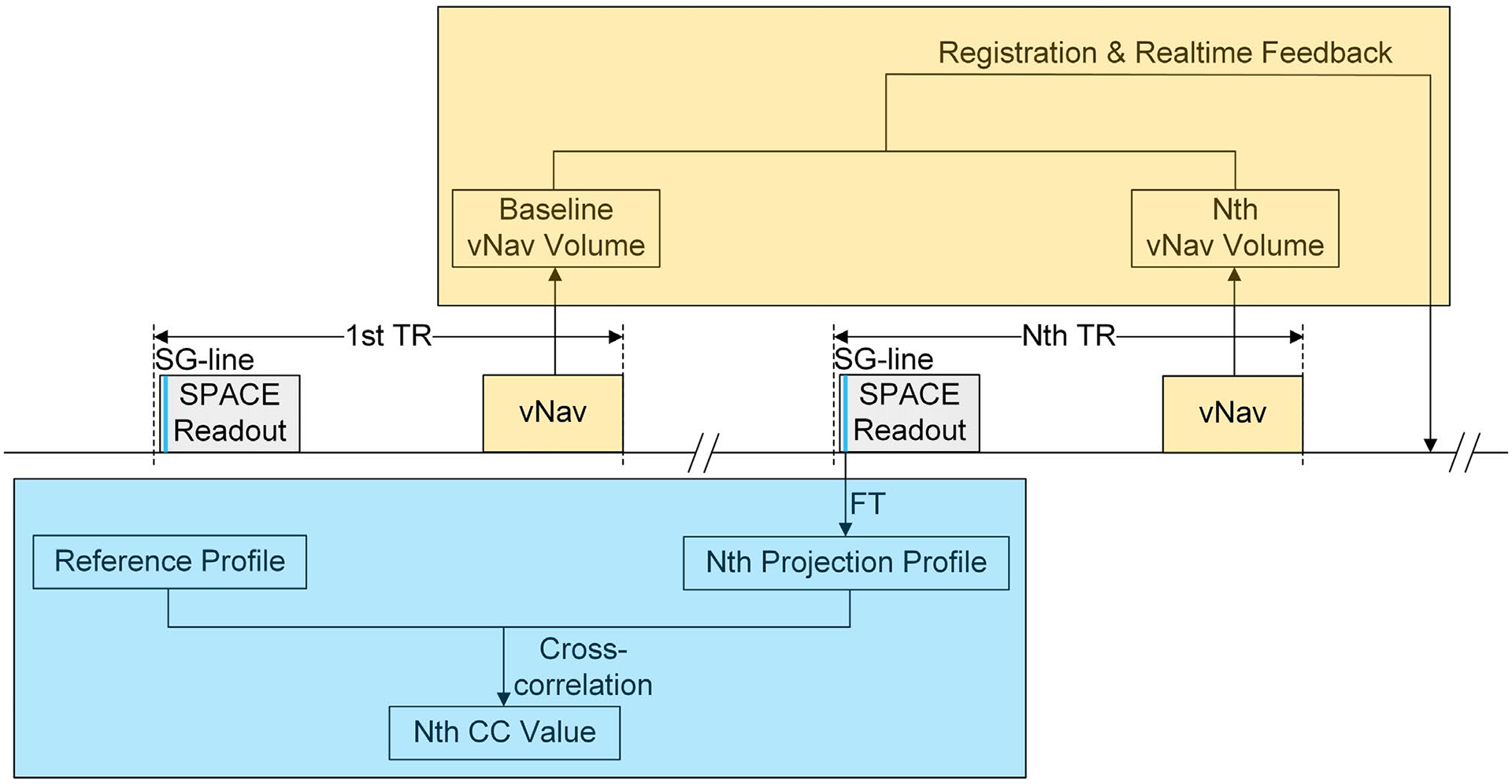
Volumetric navigators (vNav)– self-gating (SG) SPACE sequence diagram. For each TR, an SG line is first acquired from the first echo of the SPACE readout and is used to derive the projection profile of the entire imaging volume by Fourier transform (FT). The projection profiles acquired in later TRs are cross-correlated (CC) to the reference projection collected at the beginning of the scan, which is automatically reestablished when head position changes or signal drift occurs. All CC values are prioritized, and the most motion-affected TRs are reacquired at the end of the scan. The vNavs are implemented as a 3D-EPI module, consisting of acquisition, registration and communication, and are inserted at the end of each TR. The subsequent vNavs acquired in later TRs are registered back to the first navigator to realign the imaging coordinates
The vNav is implemented as a 3D-EPI module with 8-mm resolution and 256-mm FOV in all three directions.20 We insert one such 3D navigator at the end of each TR to ensure that motion estimation is as close as possible to the following SPACE readout train. Each subsequent vNav acquired in a later TR is registered back to the navigator acquired in the first TR, to realign the imaging coordinates. Registration of the volumetric navigators is performed by the optimized prospective acquisition correction algorithm.14 Low-flip-angle (2°) excitation is adopted to minimize the effect of the vNavs on the final image contrast. The entire vNav module consumes approximately 355 ms, thus easily fitting in the TR gap.
The SG method used to compensate for localized movement is a projection-based motion-gating strategy.22 A center k-space line along the superior– inferior direction, denoted as an “SG line,” is acquired from the first echo of the SPACE echo train within each TR. The projection of the entire imaging volume at any TR is then derived by the Fourier transform of the corresponding SG line. The projection profiles acquired in subsequent TRs are cross-correlated (CC) to the reference projection collected at the beginning of the scan. Based on their CC values, which is an inverse surrogate for the severity of motion contamination, all acquired TRs are prioritized, and the most motion-affected TRs are reacquired at the end of the scan. The maximum number of reacquisitions is set on the user interface. The reference projection profile is automatically reestablished if the head position changes or signal drift occurs, using a method described previously.10
Real-time feedback and online reconstruction are accomplished with the vNav-SG SPACE sequence. In each TR, vNav and SG signals are acquired in the data-acquisition module and transferred to the image calculation module to be processed. The vNav registration and communication steps are executed once per TR for image coordinate correction, and the TR priority queue based on the CC values is delivered back to the data-acquisition module near the end of the scan for reacquisition. The sequence binaries for the proposed technique are available upon request.
2.2 |. In vivo study
The in vivo study was approved by the local institutional review board, and all subjects provided written, informed consent before participation. All scans were performed on a 3T MR system (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany) equipped with a 20-channel head-neck coil. The coil element closest to nasal and oral cavities was used to derive SG signals. A sagittally oriented imaging volume was prescribed to cover the head and part of the neck.8 Three 35–40-mm spatial presaturation bands placed left (L), right (R), and anterior (A) to the head were applied to suppress the signals from the out-of-volume nose and ears.
2.2.1 |. Study I: To investigate the degradation effect of localized movement on intracranial VWI and the effectiveness of SG in artifact reduction
Eight healthy volunteers were recruited. All subjects underwent two vessel wall scans using the vNav-SG SPACE with the following imaging conditions:
Subject asked to remain still; imaging without vNav or SG (denoted as “No motion”); and
Subject asked to conduct predesigned localized movement; imaging with SG only (“With SG”).
Subjects were instructed over the intercom to cough twice at the 50th, 150th, 250th, 350th, and 450th TR of the scan, and to hold their heads and necks still for the rest of the scan. In addition to the online-reconstructed images from the “With SG” scan, raw data were used to reconstruct the corresponding motion-contaminated images (denoted as “Without SG”) by retrospectively retrieving the data acquired in motion-corrupted TRs.
An 8-minute-long sequence previously set up for intracranial VWI was used,23 including FOV = 170 × 170 × 136 mm3, matrix size = 320 × 320 × 256 with 6.7% slice oversampling, spatial resolution = 0.53 mm isotropic, TR/TE = 900/16 ms, 6/8 partial Fourier in the partition-encoding direction, parallel imaging (GRAPPA) acceleration rate = 2 in the phase-encoding direction, echo train length = 52, number of reacquisitions = 30, and 8.1-minute acquisition time (a total of 542 TRs) without motion or vNav-SG.
2.2.2 |. Study II: To demonstrate the robustness of vNav-SG SPACE for intracranial VWI in the presence of both bulk head motion and localized movement
Seven healthy volunteers and 3 acute ischemic stroke patients were recruited. A 0.3-second test shot was first run to allow subject-specific changes to the navigator protocol. The protocol was saved and used as the basis for the vNav modules in our proposed sequence. Following the test shot, all healthy subjects underwent the “directed-motion” study using vNav-SG SPACE. The study involved five separate scans:
Subject asked to remain still; imaging without vNav or SG (denoted as “Without motion, without vNav-SG”);
Subject asked to conduct motion; imaging without vNav or SG (“With motion, without vNav-SG”);
Subject asked to conduct motion; imaging with vNav-only (“With motion, with vNav”);
Subject asked to conduct motion; imaging with SG only (“With motion, with SG”); and
Subject asked to conduct motion; imaging with vNav and SG (“With motion, with vNav-SG”).
Motion instructions were given through the intercom at eight preset stages. Subjects were asked to cough twice at four (100th, 170th, 230th, and 300th TR) of the eight stages and change their head positions at the other four stages (130th, 200th, 270th, and 330th TR). When changing head positions, subjects were asked to rotate their heads less than 8° in one of four directions (left, right, up, and down), hold this position for 10 seconds, and then return to the original position. During the rest of the imaging period, subjects were asked to hold their heads and necks still.
Expedited SPACE imaging-parameter settings were used to reduce the total study time, including FOV = 155 × 155 × 144 mm3, matrix size = 256 × 256 × 224 with 7.1% slice oversampling, spatial resolution = 0.6 mm isotropic, TR/TE = 900/16 ms, 6/8 partial Fourier in the partition-encoding direction, parallel imaging (GRAPPA) acceleration rate = 2 in the phase-encoding direction, echo train length = 52, number of reacquisitions = 30, and 6-minute acquisition time (a total of 400 TRs) without motion or vNav-SG.
Three ischemic stroke patients underwent two consecutive SPACE scans, with and without vNav-SG, respectively, in random order. The comparison was conducted after contrast injection and toward the end of the brain MRI/MRA examination when intrascan motion often occurs because of patient intolerance. The same sequence setup as in study I was used.
2.3 |. Image analysis
All 3D image sets were loaded to a workstation (LEONARDO; Siemens Healthcare, Erlangen, Germany) for processing and viewing. For study I, vessel wall sharpness was measured at the inner and outer boundaries of four major vessel segments, including middle cerebral artery (MCA), internal carotid artery (ICA), basilar artery (BA) and vertebral artery (VA). For this purpose, three contiguous 2D cross-sectional slices of 0.53-mm thickness were reconstructed with multiplanar reformation for each vessel segment. Sharpness was measured from three evenly distributed locations in each slice, with the first location selected at the most blurred position of the vessel wall boundary. The sharpness of each segment was then estimated by averaging across the nine selected locations. Measurement of vessel wall sharpness was based on a previously developed method10,24 using an in-lab MATLAB (R2018a; MathWorks, Natick, MA) program.
For study II in healthy volunteers, all 35 image sets (5 imaging conditions × 7 subjects) were randomized and shown to a blinded neuroradiologist with 6 years of experience in intracranial VWI. The overall image quality for each image set was graded based on a 5-point scale: 0-poor, 1-fair, 2-moderate, 3-good, and 4-excellent. Vessel wall sharpness was measured using the aforementioned methods, at the inner and outer boundaries of two major intracranial vessel segments (BA and MCA). For the patient study, the 3D-SPACE images with and without vNav-SG underwent diagnostic evaluation by the same neuroradiologist, blinded to patient and sequence information.
All image sets from study II were selected to assess the interreader reliability. An MR physicist with 3 years of experience in MR VWI regraded the overall image quality scores and conducted sharpness measurement at the same vessel segments independently, following the same criteria and methods.
2.4 |. Statistical analysis
Statistical analyses were performed using SPSS (version 24; IBM, Armonk, NY). To compare vessel wall sharpness under different imaging conditions, a paired two-tailed Student’s t-test was used for study I. For study II, interreader reliabilities of image-quality scores and vessel wall sharpness were assessed by weighted Cohen’s kappa (κ) and intraclass correlation coefficients (ICCs), respectively. A paired two-tailed Wilcoxon signed-rank test was used for the comparison of image-quality scores between the “With motion, with vNav-SG” scan and each of the other imaging conditions, and a paired two-tailed Student’s t-test was used for the comparison of vessel wall sharpness. A p-value < .05 was considered to indicate statistical significance.
3 |. RESULTS
The vNav-SG SPACE imaging was performed successfully on all subjects. For the scans including SG functionality, the number of motion-contaminated TRs detected by SG was greater than 30; thus, 30 TRs were reacquired.
3.1 |. Study I
In general, the internal localized movement caused by coughing resulted in noticeable blurring and noise at the vessels close to the nasal and oral cavities. These artifacts were well suppressed by reacquiring motion-corrupted k-space lines detected by SG signals (Figure 2A–F). Representative time courses of projection profiles and CC values were retrospectively derived and are shown in Figure 2G,H, respectively. The projection profile during coughing deviated substantially in magnitude, leading to drops in CC values.
FIGURE 2.
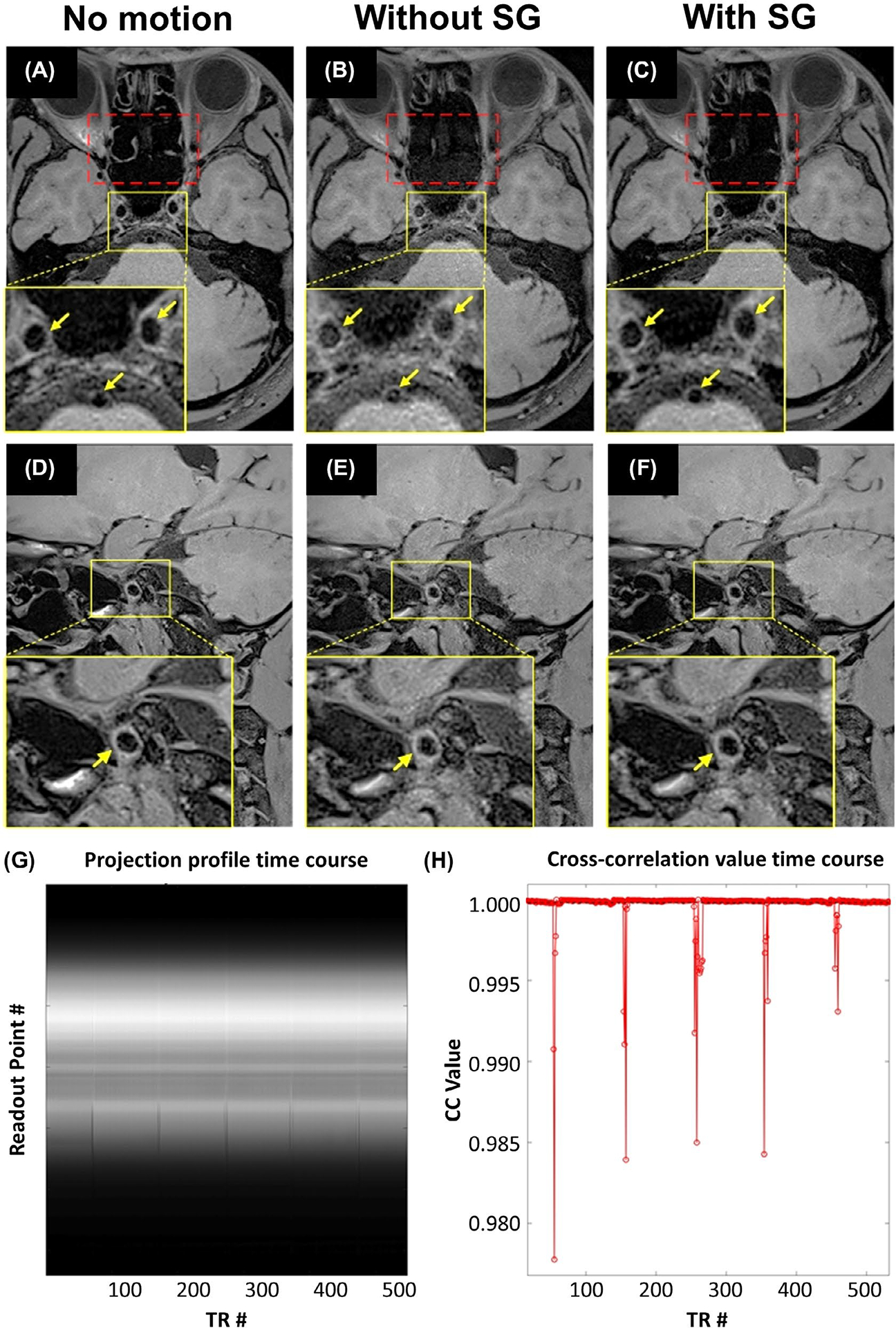
A-F, Representative images demonstrating the effect of localized motion, caused by coughing, on intracranial vessel wall imaging, and the effectiveness of the SG motion-gating technique in mitigating motion artifacts in a representative healthy control. Coughing introduced noise (red dotted box) and substantially obscured vessel wall boundaries (yellow arrows), particularly at the internal carotid artery and basilar artery (A-C) and middle cerebral artery (D-F). The proposed SG technique dramatically mitigated artifacts and provided comparable vessel-wall delineation quality to the scan without motion (“No motion”). G,H, Retrospectively derived representative time courses of projection profiles (G) and CC values (H). In contrast to the projection profiles devoid of motion, motion-contaminated projection profiles deviated substantially in magnitude and were always detected by the SG signal
Localized movements significantly reduced vessel wall sharpness at either the outer or inner vessel wall boundary compared with that obtained in “With SG” scans, as shown in Table 1 (BA outer: 0.68 ± 0.27 vs 0.86 ± 0.17; P = .037; VA outer: 0.43 ± 0.29 vs 0.93 ± 0.29; P = .003; VA inner: 0.37 ± 0.23 vs 0.99 ± 0.32; P = .001; ICA outer: 1.35 ± 0.24 vs 1.61 ± 0.19; P = .034; ICA inner: 0.86 ± 0.30 vs 1.34 ± 0.20; P < .001). The sharpness of wall boundaries was preserved by exploiting SG when imaging subjects with localized motion, comparable to that obtained in the “No motion” group except for the MCA outer (0.77 ± 0.30 vs 0.97 ± 0.24; P = .047) and ICA inner (1.34 ± 0.20 vs 1.66 ± 0.15; P = .029) boundary.
TABLE 1.
Vessel wall sharpness–measurement results at the outer and inner boundary of four major intracranial vessel segments, MCA, BA, VA, and internal carotid artery (ICA), within the imaging conditions in study I
| Sharpness of wall boundary (mm−1) | |||||
|---|---|---|---|---|---|
| Imaging conditions | Vessel segments | Outer boundary | p-Value | Inner boundary | p-Value |
| No motion | MCA | 0.97 ± 0.24 | .047 | 0.78 ± 0.14 | .362 |
| BA | 1.03 ± 0.28 | .160 | 0.68 ± 0.25 | .689 | |
| VA | 1.02 ± 0.34 | .147 | 1.20 ± 0.30 | .275 | |
| ICA | 1.89 ± 0.26 | .058 | 1.66 ± 0.15 | .029 | |
| With SG | MCA | 0.77 ± 0.30 | NA | 0.70 ± 0.22 | NA |
| BA | 0.86 ± 0.17 | NA | 0.66 ± 0.15 | NA | |
| VA | 0.93 ± 0.29 | NA | 0.99 ± 0.32 | NA | |
| ICA | 1.61 ± 0.19 | NA | 1.34 ± 0.20 | NA | |
| Without SG | MCA | 0.65 ± 0.37 | .243 | 0.62 ± 0.31 | .435 |
| BA | 0.68 ± 0.27 | .037 | 0.60 ± 0.31 | .385 | |
| VA | 0.43 ± 0.29 | .003 | 0.37 ± 0.23 | .001 | |
| ICA | 1.35 ± 0.24 | .034 | 0.86 ± 0.30 | < .001 | |
Bold values demonstrate significant difference (p < .05).
Abbreviation: NA, not available.
3.2 |. Study II
Compared with regular SPACE imaging in the absence of motion (Figure 3A), motion artifacts led to severe blurring of vessel wall boundaries, impaired wall continuity, and reduced wall-tissue contrast (Figure 3B), which were suppressed by either vNav (Figure 3C) or SG (Figure 3D). Note that, compared with vNav-only, the addition of the SG approach to vNav further improves delineation of the small vessels close to nasal and oral cavities (arrowheads in Figure 3C,E). Overall image quality was restored by the combined vNav-SG strategy in the presence of motion (Figure 3E).
FIGURE 3.
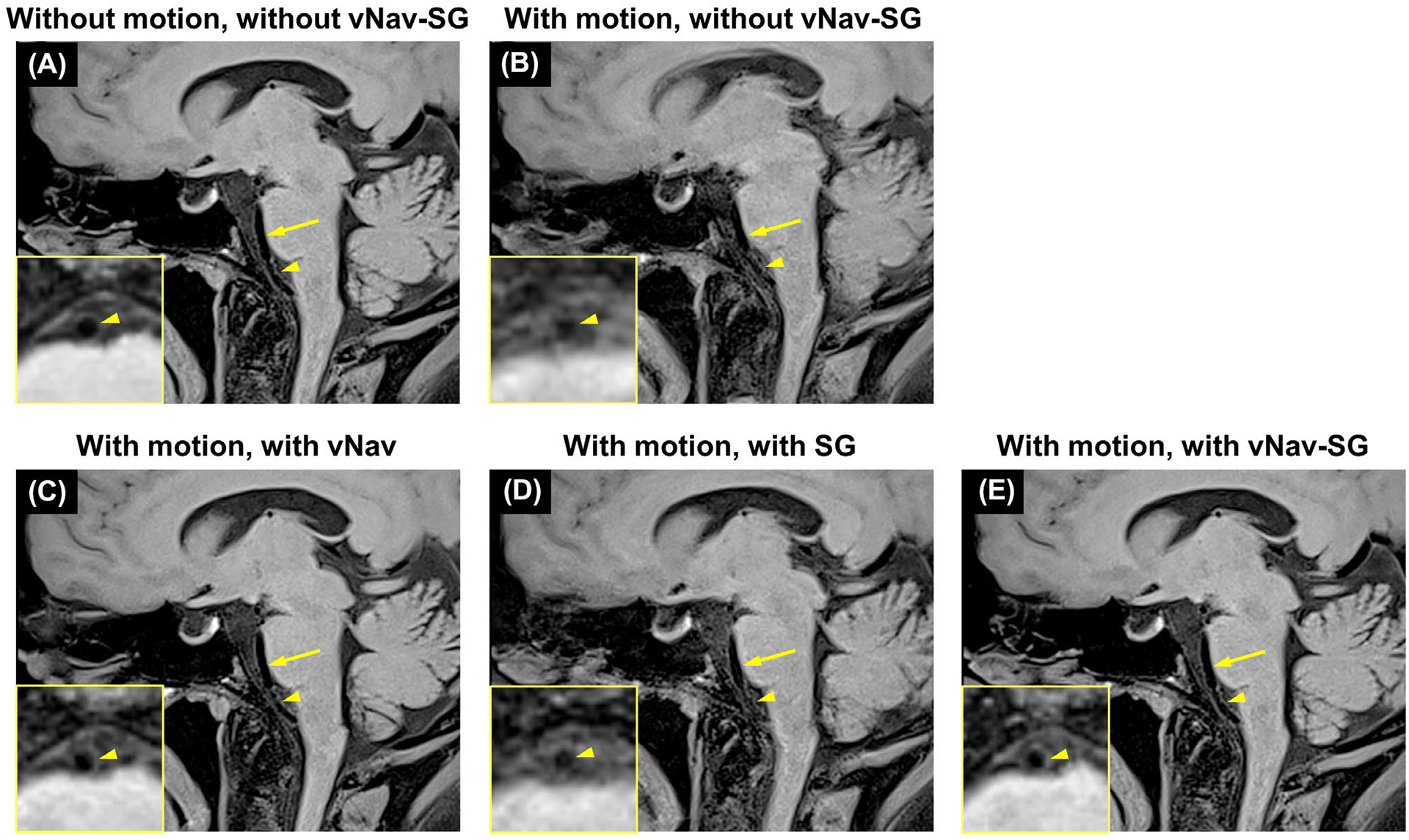
Imaging results of the five imaging conditions in 1 representative healthy subject in study II. Compared with conventional SPACE imaging without subject motion (A), combined bulk head motion and internal localized movement severely degraded image quality as well as vessel wall delineation (yellow arrows and arrowheads) (B). C,D, Motion artifacts were suppressed if either vNavs or the SG approach was used, respectively. Compared with vNav-only, the addition of the SG strategy further improves delineation of the small vessel close to nasal and oral cavities (yellow arrowheads). E, Overall image quality was significantly improved by adopting a combined vNav-SG technique
Interreader agreement was excellent for overall image quality analysis (Cohen’s κ = 0.883, 95% confidence interval [CI] 0.802–0.963). Interreader agreements for both outer and inner boundary at BA and MCA were all moderate to high (BA outer: ICC 0.768, 95% CI 0.570–0.921; BA inner: ICC 0.744, 95% CI 0.658–0.833; MCA outer: ICC 0.875, 95% CI 0.607–0.961; and MCA inner: ICC 0.805, 95% CI 0.547–0.903).
Because of the moderate to high interreader agreement, the following analyses were based on the evaluation of the more experienced neuroradiologist. The motion-compensation effects of vNav-SG were qualitatively validated by image-quality scores (Table 2). Specifically, the average image-quality score for “With motion, with vNav-SG” scans was significantly higher than that obtained in “With motion, without vNav-SG” scans (P = .026), and comparable to that obtained in “Without motion, without vNav-SG” scans (P = .317). Similar scores were acquired in “With motion, with vNav,” “With motion, with SG,” and “With motion, with vNav-SG” scans (P = .317 and P = .059, respectively), among which “With motion, with vNav-SG” provided the highest score.
TABLE 2.
Image-quality scores (5-point scale: 0-poor, 1-fair, 2-moderate, 3-good, 4-excellent) of the imaging conditions in study II, given by the experienced neuroradiologist
| Without motion without vNav-SG | With motion without vNav-SG | With motion with vNav | With motion with SG | With motion with vNav-SG | |
|---|---|---|---|---|---|
| Subject 1 | 4 | 3 | 4 | 4 | 4 |
| Subject 2 | 4 | 1 | 3 | 3 | 4 |
| Subject 3 | 4 | 1 | 4 | 1 | 4 |
| Subject 4 | 4 | 2 | 3 | 3 | 3 |
| Subject 5 | 4 | 0 | 4 | 3 | 4 |
| Subject 6 | 4 | 4 | 4 | 4 | 4 |
| Subject 7 | 4 | 1 | 4 | 3 | 4 |
| Mean ± SD | 4.00 ± 0.00 | 1.71 ± 1.38 | 3.71 ± 0.49 | 3.00 ± 1.00 | 3.86 ± 0.38 |
| p-Value | .317 | .026 | .317 | .059 | NA |
Bold values demonstrate significant difference (p < .05).
Abbreviation: NA, not available.
Vessel wall sharpness deteriorated significantly when imaging subjects with motion but without any motion-compensation strategy, as compared with that obtained in “With motion, with vNav-SG” scans (BA outer: 0.73 ± 0.24 vs 0.94 ± 0.24, P = .033; BA inner: 0.49 ± 0.08 vs 0.59 ± 0.20, P = .026; MCA outer: 0.85 ± 0.17 vs 1.19 ± 0.84, P = .027; MCA inner: 0.48 ± 0.11 vs 0.57 ± 0.14, P < .001) (Figure 4). The measurements in “With motion, with vNav-SG” scans were higher, although not significantly, than those in other motion-correction scans with either vNav or SG, and were the closest to those obtained in “Without motion, without vNav-SG” scans (BA outer: 0.94 ± 0.24 vs 0.96 ± 0.31, P = .815; BA inner: 0.59 ± 0.20 vs 0.62 ± 0.22, P = .481; MCA outer: 1.19 ± 0.84 vs 1.33 ± 0.56, P = .367; MCA inner: 0.57 ± 0.14 vs 0.65 ± 0.27, P = .275).
FIGURE 4.
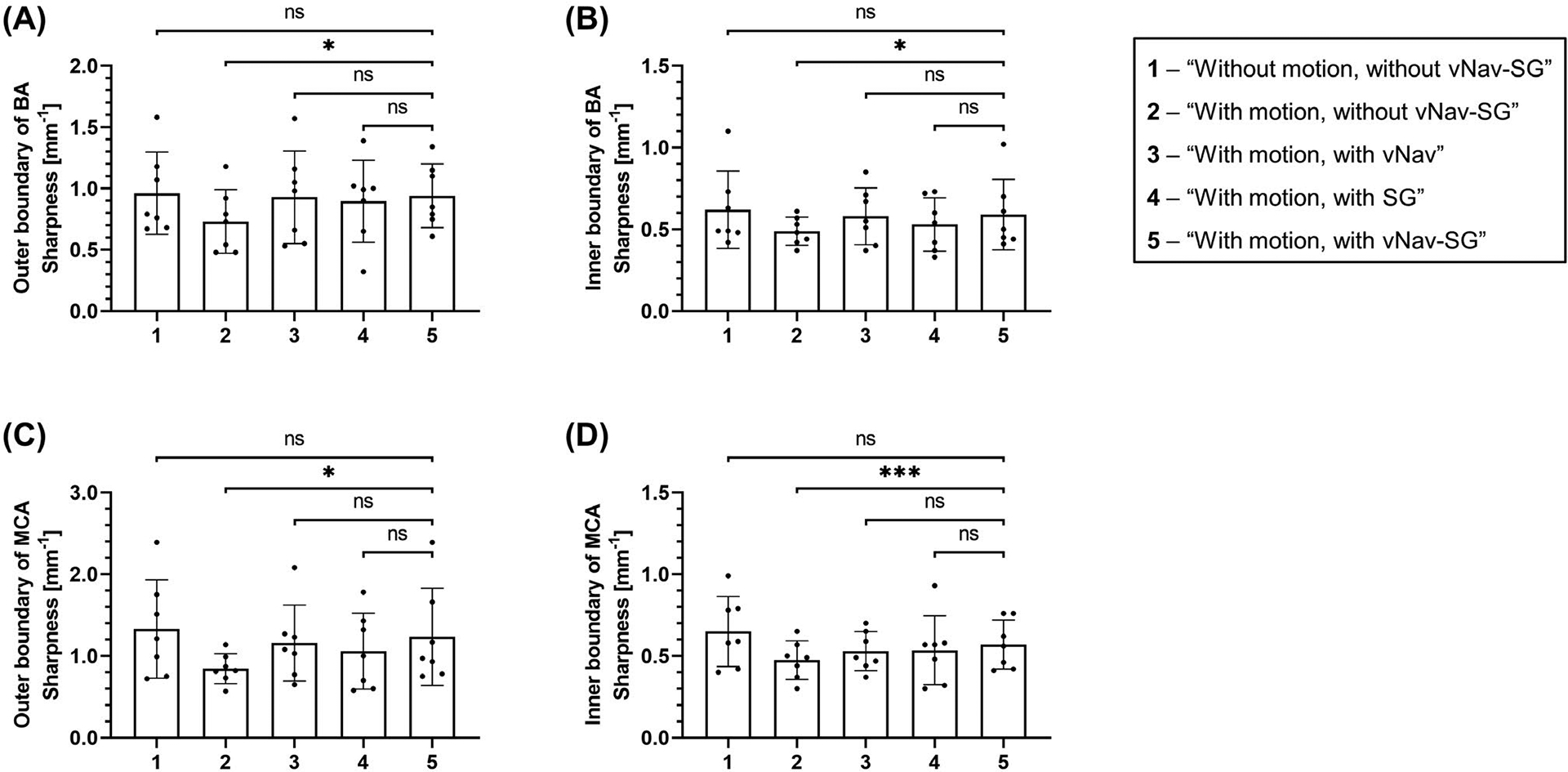
Bar graphs showing the mean values and SDs of the outer and inner vessel wall boundary of the basilar artery (BA) (A,B) and middle cerebral artery (MCA) (C,D), respectively, with regard to the five imaging conditions. The sharpness measurement results obtained from the “With motion, with vNav-SG” scan were compared to those obtained from the other four imaging conditions. The p-values were calculated based on a paired two-tailed Student’s t-test and are marked on the top of each bar graph. Comparable vessel-wall sharpness results were obtained in the “Without motion, without vNav-SG” group and those scans with either the vNav or SG approach or both. Adoption of the proposed vNav-SG strategy (“With motion, with vNav-SG”) led to the best vessel wall sharpness compared to the other two scenarios (“With motion, with vNav” and “With motion, with SG”). The significant difference between “With motion, without vNav-SG” and “With motion, with vNav-SG” scans illustrate the effectiveness of vNav-SG approach in mitigating motion artifacts when imaging subjects with motion. Abbreviation: ns, not significant
Figure 5 shows the imaging results from a representative patient with ischemic stroke (Figure 5A) caused by intra-aneurysmal thrombosis at the left MCA (arrow in Figure 5B). A mild stenosis caused by atherosclerotic plaque was found in the left VA (arrowhead in Figure 5B). During the intracranial VWI session, the patient could not hold his head and neck still for the entire 8 minutes. Therefore, the conventional SPACE images suffered from severe motion artifacts (ie, blurry aneurysm) (Figure 5C) and nondiagnostic vessel wall boundary (Figure 5D). In contrast, the proposed vNav-SG technique dramatically improved image quality and the lesions were much better depicted. Notice that in Figure 5E, the aneurysm was better delineated, and wall thickening as well as contrast enhancement were detected in Figure 5F.
FIGURE 5.
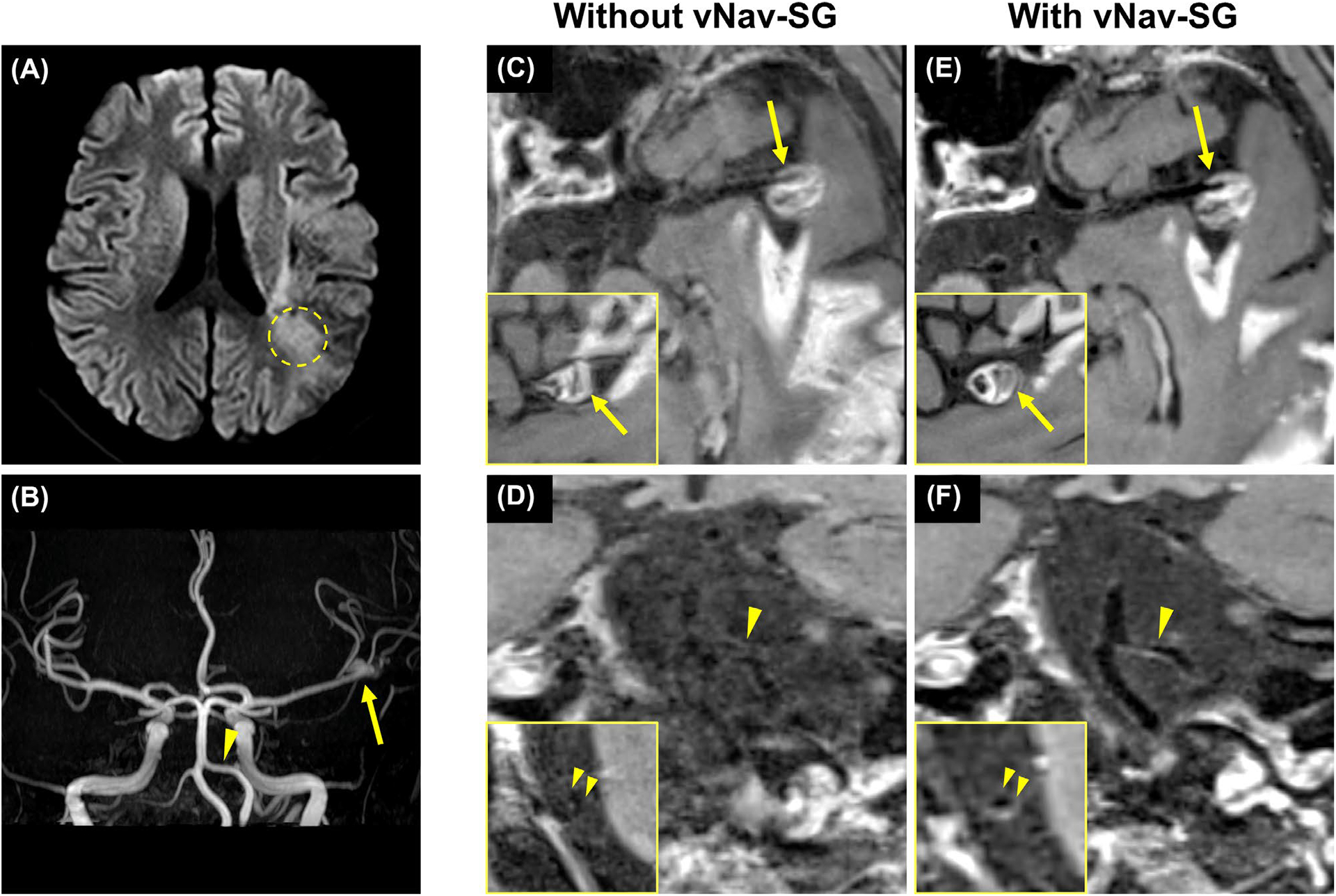
A, In a 62-year-old male patient with ischemic stroke caused by intra-aneurysmal thrombosis, an infarct marked out by the dashed circle was detected on the DWI image during clinical evaluation. B, On time-of-flight MRA, an aneurysm (arrow) and atherosclerotic plaque (arrowhead) were detected on left MCA M1 segment and left vertebral artery (VA) V4 segment, respectively. During the evaluation of intracranial vessel wall imaging, the patient had difficulty holding his head and neck still for the entire 8 minutes. C,D, Therefore, 3D multiplanar reconstructions of postcontrast vessel wall imaging and the cross-sectional view of the left MCA M1 segment and the left VA V4 segment showed that the images were corrupted by severe intrascan motion. E, With the proposed vNav-SG strategy, the aneurysm was better delineated, and the wall thickening as well as contrast enhancement were detected
4 |. DISCUSSION
To our knowledge, this is the first report of technical development work addressing motion coinciding with MR intracranial VWI. Motion is a common reason for image corruption in neuroanatomical MRI. Intracranial 3D VWI is particularly susceptible due to the required high spatial resolutions and relatively long scan times. In a recent healthy volunteer study, motion artifacts were observed in 17.6% of imaging subjects.12 A population-based study reported a failure rate of 9.9% due to motion-related poor image quality.13 Therefore, although underexplored to date, motion susceptibility could critically undermine VWI’s clinical translation.
In intracranial VWI, motion artifacts may arise from either bulk head motion or localized movement of internal anatomic structures.19 Compensation for bulk motion in brain imaging has been investigated extensively through either hardware-based25,26 or software-based approaches.27–29 Hardware-based techniques (ie, camera systems) are compatible with a broad range of sequences and track rigid motions precisely. However, these approaches have been restricted to compensation for rigid bulk motion. Software-based approaches are more cost-effective, as they require only the standard MR system. The vNav approach fits easily in the SPACE readouts and has proved successful in brain imaging.20 However, like hardware-based approaches, it has the drawback that the effects of internal localized movements on image quality in intracranial VWI are not considered. Given the fact that some intracranial vessel segments are located close to the nasal sinuses and oral cavity, the localized motions, caused by swallowing and coughing, may affect the quality of intracranial VWI to some extent. Software approaches have previously been shown to be effective in compensating for these motion sources in extracranial VWI.10,30 We therefore sought to perform a systematic investigation of motion effects and propose a technique capable of simultaneously addressing bulk head motion and localized motion.
We first demonstrated that localized movement can significantly impair vessel wall delineation. The SG technique, when specifically targeted to the nasal sinus and oral cavity, demonstrated promise in detecting motion and significantly mitigating image degradation. By combining vNav and SG strategies, we demonstrated that each component of this combined strategy contributes to restored image quality. Compared with regular SPACE imaging, the proposed vNav-SG SPACE technique provided acceptable overall image quality and vessel wall sharpness in the presence of subject motion.
The developed vNav-SG technique integrated previously published vNav and SG approaches with several technical modifications. First, instead of using the signal from the k-space center point,30 the SG signal in our method came from the center k-space line that was in turn transformed to a projection of the whole 3D volume, which is intuitively more sensitive to translation or distortion. Second, the motion-contaminated TRs detected by SG were reacquired at the end of the scan instead of being reacquired immediately.10 All TRs were ordered in a priority queue based on their CC values. Once all TRs had been executed, the sequence began reacquiring TRs according to their priority. The number of reacquisitions was predefined by the operator at the scanner console, ensuring a fixed total scan time despite potentially compromised image quality in the case of excessive patient movement. Third, in this work, intra-TR motion was monitored by the SG lines, and this replaced the reacquisition by “motion score” used in the original vNav approach.20 Moreover, previous methods compensate for either localized or bulk head motion only, whereas our method allows for simultaneous compensation of bulk and localized movements, which is the major innovation and contribution.
There are some limitations in this work. First, the instructed motion at certain preset intervals is not guaranteed to be identical across all conditions and subjects. Such inconsistency could have biased the comparison of images among different imaging conditions. Second, the reacquisition scheme entails a time penalty, and consequently a trade-off between scan efficiency and overall image quality. A shorter reacquisition period may lead to more motion-contaminated data being retained and contributing to the online reconstruction, thus degrading the final image quality.
5 |. CONCLUSIONS
We have shown that localized movement can induce substantial artifacts in intracranial VWI, and mitigation with the SG approach is feasible. Moreover, the combined vNav-SG strategy has demonstrated the potential to effectively mitigate motion artifacts caused by both bulk head motion and localized movement in healthy subjects and patients. Therefore, the developed vNav-SG SPACE sequence may substantially improve the robustness of intracranial VWI in clinical settings and ensure accurate assessment of vessel wall pathologies.
Funding information
National Institutes of Health; Grant/Award No. R01HL147355
Footnotes
CONFLICT OF INTEREST
Dr. Fei Han and Dr. Xiaoming Bi are employees of Siemens Healthcare.
DATA AVAILABILITY STATEMENT
The sequence binaries for the proposed vNav-SG SPACE technique are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;E139–E596. [DOI] [PubMed] [Google Scholar]
- 2.Bhogal P, Navaei E, Makalanda H, et al. Intracranial vessel wall MRI. Clin Radiol. 2016;71:293–303. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39: 1246. [DOI] [PubMed] [Google Scholar]
- 4.Alexander MD, Yuan C, Rutman A, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry. 2016;87:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2017;38:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30. [DOI] [PubMed] [Google Scholar]
- 8.Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid–attenuated T1-weighted 3 D turbo spin echo. Magn Reson Med. 2017;77:1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang N, Wu J, et al. High resolution three dimensional intracranial arterial wall imaging at 3 T using T1 weighted SPACE. Magn Reson Imaging. 2015;33:1026–1034. [DOI] [PubMed] [Google Scholar]
- 10.Fan Z, Zuehlsdorff S, Liu X, Li D. Prospective self-gating for swallowing motion: a feasibility study in carotid artery wall MRI using three-dimensional variable-flip-angle turbo spin-echo. Magn Reson Med. 2012;67:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu F, Ma Q, Song H, et al. Differential features of culprit intracranial atherosclerotic lesions: a whole-brain vessel wall imaging study in patients with acute ischemic stroke. J Am Heart Assoc. 2018;7:e009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang NA, Zhang F, Deng Z, et al. 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson. 2018;20:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao YE, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology. 2016;280:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. [DOI] [PubMed] [Google Scholar]
- 15.Godenschweger F, Kägebein U, Stucht D, et al. Motion correction in MRI of the brain. Phys Med Biol. 2016;61:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenholz A, van der Kolk AG, Zwanenburg JJ, Hendrikse J. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology. 2018;286:12–28. [DOI] [PubMed] [Google Scholar]
- 17.Pauletto P, Palatini P, Da Ros S, et al. Factors underlying the increase in carotid intima-media thickness in borderline hypertensives. Arterioscler Thromb Vasc Biol. 1999;19:1231–1237. [DOI] [PubMed] [Google Scholar]
- 18.Harteveld AA, Denswil NP, Van Hecke W, et al. Data on vessel wall thickness measurements of intracranial arteries derived from human circle of Willis specimens. Data Brief. 2018;19:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maclaren J, Herbst M, Speck O, Zaitsev M. Prospective motion correction in brain imaging: a review. Magn Reson Med. 2013;69:621–636. [DOI] [PubMed] [Google Scholar]
- 20.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugler JP III. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39:745–767. [DOI] [PubMed] [Google Scholar]
- 22.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: a parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging. 2017;46:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy RM, Shea SM, Deshpande VS, et al. Coronary MR angiography: true FISP imaging improved by prolonging breath holds with preoxygenation in healthy volunteers. Radiology. 2003;227:283–288. [DOI] [PubMed] [Google Scholar]
- 25.Andrews-Shigaki BC, Armstrong BS, Zaitsev M, Ernst T. Prospective motion correction for magnetic resonance spectroscopy using single camera retro-grate reflector optical tracking. J Magn Reson Imaging. 2011;33:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman C, Aksoy M, Hornegger J, Bammer R. Self-encoded marker for optical prospective head motion correction in MRI. Med Image Anal. 2011;15:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Bammer R, Kim DH, Moseley ME. Self-navigated inter-leaved spiral (SNAILS): application to high-resolution diffusion tensor imaging. Magn Reson Med. 2004;52:1388–1396. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Kouwe AJ, Benner T, Dale AM. Real-time rigid body motion correction and shimming using cloverleaf navigators. Magn Reson Med. 2006;56:1019–1032. [DOI] [PubMed] [Google Scholar]
- 29.White N, Roddey C, Shankaranarayanan A, et al. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 2010;63:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyverfeldt P, Deshpande VS, Kober T, Krueger G, Saloner D. Reduction of motion artifacts in carotid MRI using free-induction decay navigators. J Magn Reson Imaging. 2014;40:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]


