Background:
Between April and June 2016, an outbreak of rhombencephalitis (RE) caused by enterovirus (EV) A71 was detected in Catalonia, Spain—the first documented in Western Europe. The clinical characteristics and outcome of patients with this condition differed from those reported in outbreaks occurring in Southeast Asia.
Methods:
Observational, multicenter study analyzing characteristics, treatment and outcome of patients with EV-A71 rhombencephalitis diagnosed in 6 publicly funded hospitals within the Catalonian Health Institute. A review of clinical characteristics, diagnosis, treatment and outcome of these patients was conducted.
Results:
Sixty-four patients met the clinical and virologic criteria for rhombencephalitis caused by EV-A71. All patients had symptoms suggesting viral disease, mainly fever, lethargy, ataxia and tremor, with 30% of hand-foot-mouth disease. Intravenous immunoglobulin therapy was given to 44/64 (69%) patients and methylprednisolone to 27/64 (42%). Six patients (9%) required pediatric intensive care unit admission. Three patients had acute flaccid paralysis of 1 limb, and another had autonomic nervous system (ANS) dysfunction with cardiorespiratory arrest. Outcome in all patients (except the patient with hypoxic-ischemic encephalopathy) was good, with complete resolution of the symptoms.
Conclusions:
During the 2016 outbreak, rhombencephalitis without ANS symptoms was the predominant form of presentation and most patients showed no hand-foot-mouth disease. These findings contrast with those of other patient series reporting associated ANS dysfunction (10%–15%) and hand-foot-mouth disease (60%–80%). Complete recovery occurred in almost all cases. In light of the favorable outcome in untreated mild cases, therapies for this condition should be reserved for patients with moderate-severe infection. The main relevance of this study is to provide useful information for setting priorities, management approaches and adequate use of resources in future EV-A71 associated rhombencephalitis outbreaks.
Keywords: Enterovirus rhonbencephalitis
Enteroviruses (EV) are a group of positive-sense, single-stranded RNA viruses lacking a lipid envelope. They belong to the genus Enterovirus within the Picornaviridae family and are classified into 4 species (EV-A to EV-D) according to their genotypic and phenotypic characteristics. EV-A71, a well-recognized human pathogen, has been divided into several genogroups (A to F) as well as subgenogroups (in Europe, mainly C1 and C2 are found).1,2
Although EV-A71 infection is usually asymptomatic, this virus is a known etiologic agent of hand, foot and mouth disease (HFMD) epidemics, with neurologic involvement in a small percentage of cases, including aseptic meningitis, rhombencephalitis (RE), acute flaccid paralysis (AFP) and neurogenic pulmonary edema (mainly in children younger than 5 years).1,3–5 The largest outbreaks have been described in Asia and the Pacific area (Australia, China and Taiwan), where a surveillance program has been set up, coordinated by the World Health Organization (WHO).1,4
In the northern hemisphere, EV circulation follows a seasonal pattern, with the highest incidence in summer and autumn, whereas in tropical regions, no clear seasonal trends are evident. Since 2000, sporadic cases of EV-A71 infection with neurologic involvement other than aseptic meningitis have been documented in the United Kingdom, The Netherlands, Germany, Denmark and France.6
Between April and July in 2016, several cases of EV infection with unusual clinical characteristics were detected in Catalonia (Spain). A large number of patients showed neurologic symptoms of severity, including AFP, RE with cranial nerve involvement and bulbar symptoms (eg, dysarthria and difficulty swallowing), as well as other less severe manifestations, such as lethargy, tremor and ataxia. As there was a progressive increase in these cases in the first few weeks, the Catalan health authorities decided to establish consensus guidelines for the care of these patients, to unify the diagnosis and therapeutic management.
The aim of this study is to summarize the clinical and virologic characteristics of EV-A71-associated neurologic disease in patients admitted to the pediatric departments of hospitals within the Catalan Health Institute (ICS, Institut Català de la Salut) between April and July 2016, as well as to describe the diagnostic and therapeutic strategies implemented in these patients.
MATERIALS AND METHODS
This is a prospective, observational, multicenter study focusing on the clinical characteristics, virologic features, treatment and outcome of all cases of AFP and RE (with or without myelitis) in patients admitted to the pediatric departments of the 6 ICS pediatric hospitals (Vall d’Hebron in Barcelona, Joan XXIII in Tarragona, Germans Trias i Pujol in Badalona, Doctor Josep Trueta in Girona, Arnau de Vilanova in Lleida and Verge de la Cinta in Tortosa) during the outbreak of acute neurologic disease occurring between April and July 2016.
The definitions of RE case used in the study are shown in Table 1.
TABLE 1.
Definitions of Rhombencephalitis Case
| Clinical Manifestation | Laboratory Confirmation | MRI | |
|---|---|---|---|
| Clinically suspected case | Tremor, myoclonus, lethargy, bulbar symptoms and myelitis with no other known cause | ||
| Clinical case | Tremor, myoclonus, lethargy, bulbar symptoms and myelitis with no other known cause | EV-A71 laboratory confirmation | |
| Confirmed case | Tremor, myoclonus, lethargy, bulbar symptoms and myelitis with no other known cause | EV-A71 laboratory confirmation | Abnormalities |
Detection of EV and other respiratory viruses in nasopharyngeal aspirates was carried out using a real-time multiplex RT-PCR assay (Allplex Respiratory Panel Assay, Seegene, Korea). EV detection in pharyngeal swabs, whole blood, stool samples and cerebrospinal fluid (CSF) was performed with an EV-specific real-time RT-PCR (RealCycler Enterovirus+Parechovirus kit, Progenie Molecular, Spain). In addition, the GeneXpert EV test (Cepheid, United States) was performed in some cases for rapid EV detection in CSF or nasopharyngeal aspirates, following manufacturer’s protocol with minor modifications. Before PCR-based assays, total nucleic acids were extracted using NucliSense easyMAG (bioMérieux, Marcy l’Etoile, France) according to the manufacturer’s instructions, and kept frozen (−20°C) until use. In addition, a complete blood count and general biochemistry tests were performed in all patients.
Phylogenetic analysis of the partial VP1 coding-region sequence of the detected EV was carried out for molecular characterization. Partial VP1 amplification and sequencing were based on the protocol recommended by the WHO,7 with minor modifications. Nucleotide sequences were edited and assembled using MEGA v5.2,8 together with reference sequences of EV types belonging to the 4 species (EV-A to EV-D) downloaded from NCBI GenBank. Before phylogenetic analysis, the molecular evolutionary models of nucleotide substitutions were fitted to the multiple sequence alignments using evolutionary analysis, conducted in MEGA v5.2.8 Phylogenetic trees were constructed using a neighbor-joining distance method, as implemented in MEGA v5.2, with the evolutionary model with the lowest Bayesian information criterion score.8 Topologic accuracy of the internal branch was evaluated using the bootstrap method (1000 replicates).
Emergent MRI of the head and spine was performed in all patients with AFP and moderate/severe forms of RE (according to the consensus protocol), whereas MRI was carried out at the discretion of the attending physician in patients with mild RE. The head imaging protocol included axial T1, T2, FLAIR and diffusion sequences, as well as coronal FLAIR sequences with an optional postcontrast T1 sequence, followed by sagittal T2 sequences with an optional diffusion study of the entire spinal cord. A follow-up MRI (1–3 months after discharge, based on clinical criteria) was carried out only in patients whose first MRI study showed significant abnormal findings and was limited to the affected anatomical region.
In cases of acute moderate/severe neurologic dysfunction and high suspicion of EV infection, hospital admittance was indicated for observation. Patients were monitored for signs of bulbar motor neuron involvement (dysarthria, dysphagia, drooling, absent gag reflex, apneas, or respiratory abnormalities), spinal cord involvement (paresis or flaccid paralysis), myocardial involvement, neurogenic pulmonary edema, lethargy, or convulsions.
The treatment protocol according to the patients’ clinical involvement is shown in Table 2.
TABLE 2.
Treatment Indication According to the Patient’s Clinical Signs and Symptoms
| Clinical Severity | Signs and Symptoms | Treatment |
|---|---|---|
| Mild | Tremor | No treatment |
| Myoclonus | ||
| Mild lethargy | ||
| Mild ataxia | ||
| Moderate | Significant lethargy | IVIg: 1 g/kg/24 h during 2 d |
| Incipient bulbar symptoms | ||
| Severe | Established bulbar symptoms | IVIg: 1 g/kg/24 h during 2 d |
| Spinal cord symptoms (paresis or flaccid paralysis) | Consider: IV methylprednisolone 30 mg/kg/24 h (maximum dose, 1 g) during 3 d | |
| MR showing pattern of restricted diffusion |
The study was approved by the Vall d’Hebron Hospital Research Ethics Committee.
RESULTS
From April to July 2016, 123 patients meeting the definition of a clinically suspected case were hospitalized. EV-A71 was detected in at least one of the tested samples from 64/123 (52%) patients of which 54/64 were confirmed cases and 10/64 were clinical cases (see Table 1 for definitions). In the remaining 59/123 (48%) patients, other EVs (1 EV-E30 and 3 EV-C109) were found, or no virus was detected. Based on phylogenetic analysis of partial VP1 sequences from detected EV-A71 viruses, 61/64 (95%) strains belonged to subgenogroup C1, whereas the remaining 3/64 (5%) did to subgenogroup C2.
Mean age of cases was 21 months (IQR 12–39 months) and 58% (37/64) were males. Before hospital admission, all patients had experienced signs and symptoms suggesting a viral infection for a mean of 3 days (range 0–9 days). Fever, lethargy, ataxia and tremor predominated among the symptoms recorded in the emergency room (Fig. 1). The findings from complementary examinations are summarized in Table 3.
FIGURE 1.
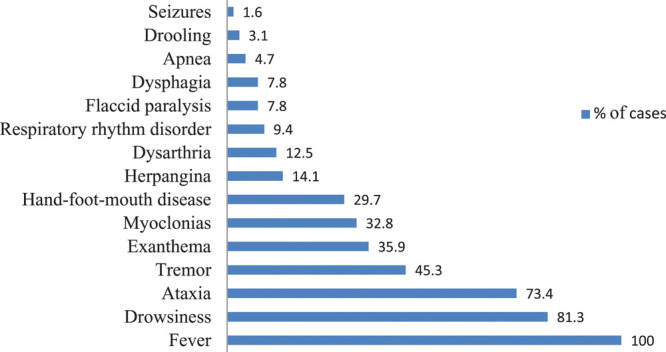
Most frequent signs and symptoms at hospital admission in patients with EV-A71S rhombencephalitis.
TABLE 3.
Findings from Complementary Examinations in Patients With a Microbiologic Diagnosis of EV-A71 Infection
| Number of Cases | ||
|---|---|---|
| Blood analysis | 48/49* | Mean |
| Leukocytes | 12,000 leukocytes/μL | |
| Neutrophils | 6700 neutrophils/μL | |
| C-reactive protein | 0.9 mg/dL | |
| Procalcitonin | 0.4 ng/mL | |
| Lumbar puncture | 40/49* | Mean: |
| Leukocytes | 145 leukocytes/μL | |
| Lymphocytes | 80% | |
| Proteins | 40 mg/dL | |
| Glucose | 64 mg/dL | |
| Bacterial culture | 100% negative | |
| Bacterial PCR | 100% negative | |
| Viral PCR | 2 positives cases: EV-A71 and HHV6 | |
| EV isolated | 64/64 | |
| NPA | 86% (55/64) | |
| Stools | 89% (57/64) | |
| Viral coinfections | 15/49* | |
| Rhinovirus | 60% | |
| Adenovirus | 26% | |
| Bocavirus | 7% | |
| Rotavirus | 7% | |
| Brain and spinal cord MRI | 60/64 | |
| No evident abnormalities | 9.3% (6/64) | |
| Sign of rhombencephalitis† | 84.4% (54/64) | |
| Signs of myelitis | 67.2% (43/64) | |
| Restricted diffusion | 5/60 | 8.3% |
*Data are available only for the 49 patients hospitalized in Hospital Universitario Vall d’Hebron
†Signs of rhombencephalitis: T2 and FLAIR hyperintense lesions in the brainstem particularly in pontine tegmentum (posterior aspect of the pons) or medulla, and or dentate nuclei area. Signs of myelitis: Lineal hyperintensity within the upper aspect cervical cord with prediction to the anterior horns. Restricted diffusion: Additional restricted diffusion (B:1000 hyperintensity, low ADC values) within focal T2 lesions at the posterior aspect of the pons or medulla (poor prognosis).
EV indicates enterovirus; MRI, magnetic resonance imaging; NPA, nasopharyngeal aspirate; HHV6, Human herpesvirus 6.
Cranial computed tomography was performed in 5 patients, with normal findings in each test. Although this imaging technique can be useful to rule out other conditions, it is not very useful for identifying EV-A71 related hindbrain lesions.3 Imaging test of choice is head and spine MRI, which shows hyperintensities on T2 and FLAIR imaging and hypointensities on T1, with or without diffusion restriction in the dorsal part of the hindbrain, the dentate nuclei of the cerebellum and the ventral portion of the spinal cord4 (Fig. 2).
FIGURE 2.
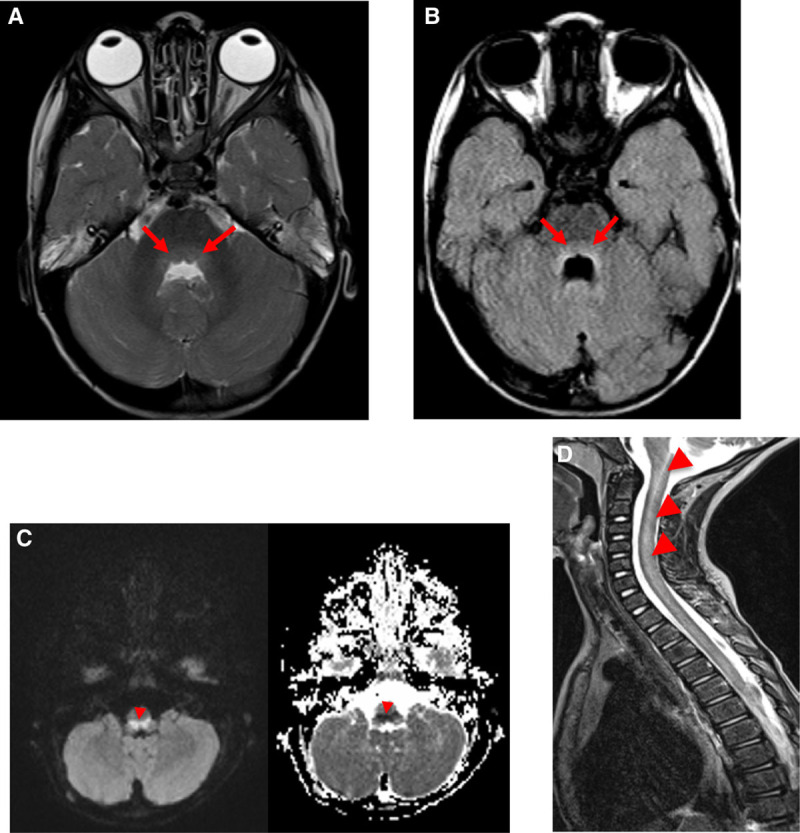
A, hyperintensity on T2 and (B) hypointensity on T1-weighted images in the dorsal hindbrain region. C, Image of restricted diffusion (B1000 hyperintensity, ADC low values) in the dorsal aspect of the medulla caused by cytotoxic edema. D, Lineal hyperintensity within the upper anterior aspect of cervical cord.
Intravenous immunoglobulins (IVIg) were given to 44/64 (69%) patients and methylprednisolone to 27/64 (42%), as soon as the indication was determined according to Table 2, with no significant adverse effects. Six patients (9%) were admitted to the pediatric intensive care unit (PICU), and 5 required respiratory support (2 of them needed invasive mechanical ventilation, 2 noninvasive mechanical ventilation and 1 high-flow nasal cannulas). Three of these 5 patients had AFP of 1 limb, and another showed autonomic dysfunction with acute neurogenic, noncardiogenic pulmonary edema. While in the PICU, this last patient experienced cardiorespiratory arrest, with severe neurologic sequelae (hypoxic-ischemic encephalopathy). The patient who did not require respiratory support was admitted to the PICU for suspected myocardial dysfunction, which was not confirmed later.
Median duration of hospitalization was 5 days (range 1–159) and duration of PICU admission was 5 days (IQR 4–7 days). At the time of hospital discharge, 24/64 patients (38%) still showed mild neurologic symptoms: most had ataxia, and 2 patients had weakness of 1 limb. The final outcome of these patients was good, with complete resolution of the symptoms 6 months after discharge from hospital, except for the patient with hypoxic-ischemic encephalopathy.
DISCUSSION
EV infection is common in children younger than 5 years and usually manifests in mild clinical forms, such as HFMD or herpangina. Less commonly, causes aseptic meningitis. The outbreak described here is the most important documented in Western Europe up to 2016 regarding the number of cases compared with another published series, mainly occurred in Southeast Asia and Australia.1,4 In response to this situation, consensus guidelines for the clinical diagnosis and management of this condition were elaborated and adapted according to the accumulated experience during the outbreak. For example, the indication for emergent MRI and the type of treatment were modified along with the outbreak duration. There is a published paper about the same outbreak9 describing cases of EV neurologic infections (none of them included in our study), but our series is exclusively focused on clinical, virologic and radiologic confirmed RE.
The age and symptoms prompting hospitalization in the 64 children with EV-A71-associated RE in our series were similar to those described in previous studies.3,4,10–13 All patients experienced a previous period consistent in viral disease. Ataxia and lethargy are warning signs in the first stages of RE.3,12,14 It is likely that myoclonus during sleep in the first phases of the condition (32.8%, Fig. 1) may have been underestimated in our series, as this variable was not initially included in the clinical data collection.
In patients with an initial suspicion who were included in the study, the most common detected EV was A71. Phylogenetic analysis showed that most of these viruses belonged to subgenogroup C1, and the percentage of C2 was very small, as reported in other European countries in 2016,15,16 and in previous years.15,17 But, in addition to EV-A71, other nonpolio EV were also characterized in several specimens from clinical neurologic cases, as well as no EVs were detected in some cases. Other nonpolio EV viruses can also cause severe neurologic infections, although many cases may go undiagnosed because EV typing data are not currently performed in most European laboratories.18 This outbreak highlighted the lack of information regarding nonpolio EV circulating in the community despite the theoretical neurotropism of all EVs. It supports the idea that virologic surveillance should be strengthened in cases related to mild disease as well as neurologic disease.
The clinical and analytic features of EV-related RE differ from those of EV meningitis. One significant difference is that EV detection in CSF is the rule in meningitis, but rare in RE, even in cases with CSF pleocytosis. In fact, it is likely that in the few cases when EV is identified in CSF, patients first had meningitis clinical features and later RE, as other ones first had HFMD or herpangina. In RE or myelitis with AFP caused by EV, the virus is not expected to be present in CSF,18 but instead in respiratory (nasopharyngeal aspirate, nasopharyngeal smear or pharyngo-tonsillar smear) or stool specimens, and it is currently recommended to establish the microbiologic diagnosis based on results from these samples.18 Because of this, EV-A71 could not be confirmed as the causal agent, although it is possible to establish an association between this virus and the neurologic involvement. Our laboratory findings (Table 3) are in accordance with those described in the literature to date.3,4,10,11
Cranial computed tomography was performed in 5 patients, with normal findings in each test. Although this imaging technique can be useful to rule out other conditions, it is not very useful for identifying EV-A71 related hindbrain lesions.3 Imaging test of choice is head and spine MRI, which shows hyperintensities on T2 and FLAIR imaging and hypointensities on T1, with or without diffusion restriction in the dorsal part of the hindbrain, the dentate nuclei of the cerebellum and the ventral portion of the spinal cord4 (Fig. 2).
Hindbrain involvement on MRI was subtle in many patients, and spinal cord involvement, although it was very evident, does not indicate the same degree of clinical severity in these patients as in myelitis caused by poliovirus or EV-D68. At the beginning of the outbreak, a patient was attended for tetraplegia caused by EV-D68,19 and her MRI findings of RE and myelitis were comparable with those of the EV-A71 infected patients with mild paresis and a favorable outcome. At the start of the outbreak and before the causal agent had been identified, a larger number of neuroimaging tests were performed and treatment was more aggressive. When it was confirmed that these were diseases caused by different etiologic agents, the diagnostic and therapeutic approaches were adapted to the new data.
The 5 patients in our series showing a restrictive diffusion pattern on MRI (an indication of neuronal cytotoxic edema) required PICU admission; hence, this finding could be a marker of a poor clinical course. In the 2013 study of patients with acute EV-A71 neurologic disease in Australia, all those with pulmonary edema showed hindbrain diffusion restriction, suggesting acute cytotoxic damage.10 The clinical course of our RE patients clearly differed from that seen in EV-related sepsis or myocarditis. The EV strains implicated in these infections and the clinical course were completely different. The neurogenic pulmonary edema caused by severe autonomic dysfunction occurring in some patients should not be confused with heart failure associated with myocarditis, as this would have clinical and therapeutic repercussions.
IVIg treatment has been used since 200020 in countries with EV-A71-related outbreaks of neurologic disease,11 based on 2 premises: first, that the antibodies in immunoglobulins help to neutralize the virus, and second, that the autonomic nervous system (ANS) dysregulation and pulmonary edema or bleeding associated with EV-A71 disease are related to production of proinflammatory cytokines (interleukin [IL]-6, IL-10 and IL-3) and chemokines (IL-8, IP-10, MCP-1 and possibly Mig).21–23 A retrospective case study in Taiwan (China) suggested that IVIg can have an immunomodulatory effect in children with RE and ANS dysregulation.24
Corticosteroids have also been used since 2000 in some series,10 analogous to the treatment of acute transverse myelitis (an immune-mediated demyelinizing disease),25 although evidence of its true usefulness for treating acute flaccid myelitis is lacking. The reason why we used so many corticosteroids at the beginning of the outbreak was that in February 2016 (2 months before the onset of the EV-A71 rhombencephalitis outbreak), a patient with AFP caused by EV-D68 was admitted to the hospital, which progressed to tetraplegia. For this reason, and given the initial doubt that the new cases of neurologic involvement caused by enterovirus could be due to EV-D68, treatment with corticosteroids was administered to the first patients of the outbreak although the cases were not severe. In 2014, the Center for Disease Control and Prevention (CDC) concluded that corticosteroids are not indicated for the treatment of AFP and that they carry a risk of worsening prognosis because of the immunosuppression. Nonetheless, judicious use of corticosteroids could be helpful for managing severe spinal cord edema, which can worsen cord lesions. Hence, the potential benefits of these drugs for cord edema should be evaluated on an individual basis because of the risk of worsening the viral infection caused by immunosuppression.26
In our patients, the initial approach followed the reported recommendations we considered the most rigorous, and we established a diagnostic and treatment protocol for a potentially severe clinical situation. Because of the effective collaboration of all the ICS pediatric hospitals and by daily review of the situation, we noticed that not all the information in the literature was applicable to our cases and that we could adapt our approach based on our direct data. Thus, we learned that HFMD, herpangina and other signs and symptoms were not as frequent as they were in other outbreaks. We also found that severe forms were already severe at the start of the manifestations of hindbrain involvement. Therefore, when EV infection was clinically suspected, we were able to recommend strict monitoring during the first 24 hours, with lowering of the alert level when clinical symptoms of bulbar involvement did not develop. This led to a reduction in hospital monitoring, IVIg and corticosteroid treatment, and imaging tests. The outbreak had a very acute beginning and end, so we were only able to adjust our approach in a small number of patients, but we believe that our experience may be of value for similar outbreaks in the future.
The outcome was favorable in all our patients with the exception of one who had hypoxic-ischemic sequelae. There were no deaths. In contrast to other studies reporting ANS dysfunction causing cardiac rhythm changes, myocarditis and pulmonary edema in 7%–14% of patients,10,12 only this single patient (2%) had ANS dysfunction.
Our series of EV-A71-related RE cases, collected using well-defined criteria within the protocol developed, indicates that this condition could be a different entity and not a complication of HFMD, as had been considered in Asian outbreaks and in some reports that follow the WHO criteria and recommendations. The clinical course may differ from RE caused by an immune response or by other host genetic factors against infection caused by certain EVs, which remarks the need for the genetic characterization of all EVs detected in the laboratory, especially those associated with severe diseases.
The increasing virulence and geographic spread of EV-A71 reported in last years has highlighted the need for a safe and effective vaccine to protect against the various genogroups and subgenogroups of EV-A71.27 The developed vaccines have shown cross-protection against some EV-A71 genogroups and subgenogroups, but their protection is not complete and they do not protect against other EV species; hence, their use is limited to controlling EV-A71 outbreaks.28,29
In the absence of available vaccines, prevention and control measures are aimed at interrupting the chain of transmission. Fast detection of outbreaks and implementation of hygiene measures are the fundamental pillars to reduce serious forms of the disease. The virus can be detected in oropharyngeal specimens 2 weeks after the onset of the disease, and viral excretion in stool specimens lasts during 4–8 weeks. Therefore, proper hand hygiene is essential to interrupt transmission during outbreaks and should be stressed during the entire period that EV-A71 is excreted in stool (up to 11 weeks). Transmission by saliva or airborne respiratory droplets, however, cannot be ignored during the acute phase of the infection, when EV-A71 is present in oropharyngeal specimens.3,30
CONCLUSIONS
The EV-A71-associated RE outbreak that occurred in 2016 in Catalonia was the first one with the described characteristics in the European continent. The predominant clinical manifestation was RE. The outcome of the infection was favorable overall, with complete neurologic recovery in all patients except one, who experienced sequelae of hypoxic-ischemic encephalopathy following cardiorespiratory arrest of neurogenic origin. As most patients progressed well, we believe that treatments should be reserved for patients with moderate-severe neurologic involvement. The main value of this article is to transmit information that we lacked at the start of the outbreak, which would have been useful to establish priorities, management criteria and proper use of resources for this infection.
Footnotes
The authors have no conflicts of interest or funding to disclose.
Ethical approval: Institutional Review Board approval (PR(AG) 373/2019) was obtained from the Vall d’Hebron University Hospital (HUVH) Clinical Research Ethics Committee.
N.W. and R.R.-G. have contributed equally to this work.
N.W. and R.R.-G. conceptualized and designed the study, drafted the initial article, and reviewed and revised the article. A.A. and C.A. did virologic studies, analysis and interpretation of microbiologic data. They wrote the virologic part of the article. T.P. did interpretation and of microbiologic data, study supervision. I.D. and E.V. did IMR acquisition, analysis and interpretation. They wrote the radiologic part of the article. E.C., S.G., L.M., M.M., E.S., J.R., A.C., E.L. and M.S. did acquisition, analysis and interpretation of clinical data. They collected the clinical data and described the major clinical findings of the article. M.C. did statistical analysis, analysis and interpretation of epidemiologic data. She wrote the epidemiologic part of the article. C.R. did concept and design of the study, study supervisión and review of the article. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors did critical revision of the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
REFERENCES
- 1.Kok CC. Therapeutic and prevention strategies against human enterovirus 71 infection. World J Virol. 2015; 4:78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lugo D, Krogstad P. Enteroviruses in the early 21st century: new manifestations and challenges. Curr Opin Pediatr. 2016; 28:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ooi MH, Wong SC, Lewthwaite P, et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010; 9:1097–1105. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, Lee MS, Kim DB. Neurologic manifestations of enterovirus 71 infection in Korea. J Korean Med Sci. 2016; 31:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long L, Xu L, Xiao Z, et al. Neurological complications and risk factors of cardiopulmonary failure of EV-A71-related hand, foot and mouth disease. Sci Rep. 2016; 6:23444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Outbreak of Enterovirus A71 with Severe Neurological Symptoms Among Children in Catalonia, Spain 14 June 2016. 2016, ECDC; 1–10. [Google Scholar]
- 7.World Health Organization Regional Office for Europe. Enterovirus Surveillance Guidelines. 2015, World Health Organization; 1–29. [Google Scholar]
- 8.Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casas-Alba D, de Sevilla MF, Valero-Rello A, et al. Outbreak of brainstem encephalitis associated with enterovirus-A71 in Catalonia, Spain (2016): a clinical observational study in a children’s reference centre in Catalonia. Clin Microbiol Infect. 2017; 23:874–881. [DOI] [PubMed] [Google Scholar]
- 10.Teoh HL, Mohammad SS, Britton PN, et al. Clinical characteristics and functional motor outcomes of enterovirus 71 neurological disease in children. JAMA Neurol. 2016; 73:300–307. [DOI] [PubMed] [Google Scholar]
- 11.Cardosa J, Farrar J, Yeng C. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD). 2011, World Health Organization; 1–71. [Google Scholar]
- 12.Huang CC, Liu CC, Chang YC, et al. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999; 341:936–942. [DOI] [PubMed] [Google Scholar]
- 13.Yang SD, Li PQ, Li YM, et al. Clinical manifestations of severe enterovirus 71 infection and early assessment in a Southern China population. BMC Infect Dis. 2017; 17:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu HK, Lin TY, Hsia SH, et al. Prognostic implications of myoclonic jerk in children with enterovirus infection. J Microbiol Immunol Infect. 2004; 37:82–87. [PubMed] [Google Scholar]
- 15.Midgley SE, Nielsen AG, Trebbien R, et al. Co-circulation of multiple subtypes of enterovirus A71 (EV- A71) genotype C, including novel recombinants characterised by use of whole genome sequencing (WGS), Denmark 2016. Euro Surveill. 2017; 22:30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antona D, Kossorotoff M, Schuffenecker I, et al. Severe paediatric conditions linked with EV-A71 and EV-D68, France, May to October 2016. Euro Surveill. 2016; 21:30402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karrasch M, Fischer E, Scholten M, et al. A severe pediatric infection with a novel enterovirus A71 strain, Thuringia, Germany. J Clin Virol. 2016; 84:90–95. [DOI] [PubMed] [Google Scholar]
- 18.Harvala H, Broberg E, Benschop K, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J Clin Virol. 2018; 101:11–17. [DOI] [PubMed] [Google Scholar]
- 19.Cabrerizo M, García-Iñiguez JP, Munell F, et al. First cases of severe flaccid paralysis associated with enterovirus D68 infection in Spain, 2015-2016. Pediatr Infect Dis J. 2017; 36:1214–1216. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Mao C, Ma S, et al. Generation of neutralizing monoclonal antibodies against enterovirus 71 using synthetic peptides. Biochem Biophys Res Commun. 2009; 390:1126–1128. [DOI] [PubMed] [Google Scholar]
- 21.Wang SM, Lei HY, Yu CK, et al. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J Infect Dis. 2008; 198:1002–1006. [DOI] [PubMed] [Google Scholar]
- 22.Wang SM, Lei HY, Huang KJ, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003; 188:564–570. [DOI] [PubMed] [Google Scholar]
- 23.Wang LC, Yao HW, Chang CF, et al. Suppression of interleukin-6 increases enterovirus A71 lethality in mice. J Biomed Sci. 2017; 24:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SM, Lei HY, Huang MC, et al. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71-associated brainstem encephalitis. J Clin Virol. 2006; 37:47–52. [DOI] [PubMed] [Google Scholar]
- 25.Defresne P, Meyer L, Tardieu M, et al. Efficacy of high dose steroid therapy in children with severe acute transverse myelitis. J Neurol Neurosurg Psychiatry. 2001; 71:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCIRD C. Acute Flaccid Myelitis: Interim Considerations for Clinical Management. Available at: httpwwwcdcgovacute-flaccid-myelitisdownloadsacute-flaccid-myelitispdf. 2014 Nov 7;1–11. Accessed April 22, 2020.
- 27.Lee MS, Tseng FC, Wang JR, et al. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis. 2012; 6:e1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi EJ, Shin YJ, Kim JH, et al. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res. 2017; 6:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed Z, Cardosa MJ. Status of research and development of vaccines for enterovirus 71. Vaccine. 2016; 34:2967–2970. [DOI] [PubMed] [Google Scholar]
- 30.Chung PW, Huang YC, Chang LY, et al. Duration of enterovirus shedding in stool. J Microbiol Immunol Infect. 2001; 34:167–170. [PubMed] [Google Scholar]


