Abstract
Epithelial-to-mesenchymal transitions (EMTs) are complex cellular processes where cells undergo dramatic changes in signaling, transcriptional programming, and cell shape, while directing the exit of cells from the epithelium and promoting migratory properties of the resulting mesenchyme. EMTs are essential for morphogenesis during development and are also a critical step in cancer progression and metastasis formation. Here we provide an overview of the molecular regulation of the EMT process during embryo development, focusing on chick and mouse gastrulation and neural crest development. We go on to describe how EMT regulators participate in the progression of pancreatic and breast cancer in mouse models, and discuss the parallels with developmental EMTs and how these help to understand cancer EMTs. We also highlight the differences between EMTs in tumor and in development to arrive at a broader view of cancer EMT. We conclude by discussing how further advances in the field will rely on in vivo dynamic imaging of the cellular events of EMT.
Keywords: Epithelial-to-Mesenchymal Transition, gastrulation, pancreatic ductal adenocarcinoma, breast cancer, cancer progression, intravital imaging
THE EPITHELIAL-TO-MESENCHYMAL TRANSITION
During development tissues are classified as either epithelial or mesenchymal. Epithelial tissues are typically stable structures linked by strong intercellular junctions and often form an impermeable barrier. Mesenchymal tissues comprise loosely packed cells that lack an obvious fixed organization and show greater migratory capability. Transitions between epithelial and mesenchymal states are essential to generate the three-dimensional (3D) organization of many animal tissues; both epithelial-to-mesenchymal transitions (EMTs) and mesenchymal-to-epithelial transitions (METs) are critical. These transformations in tissue organization were first described by embryologists and anatomists in the chick embryo at the beginning of the twentieth century (Lillie 1908; Trelstad et al. 1966, 1967). Elizabeth Hay was the first to formally describe the EMT by observing that cultured epithelia could give rise to migratory mesenchymal cells (Greenburg & Hay 1982). In the adult, the organization of epithelia and the mesenchyme is generally stable, but EMTs occur normally during stem cell differentiation and regeneration in adults.
A 2004 paper from the Weinberg lab made the first link between developmental EMTs and tumor progression when it demonstrated that Twist, a transcription factor discovered for its role in a developmental EMT in Drosophila, promotes breast tumor metastasis (Yang et al. 2004). The majority (80–90%) of human tumors originate in epithelia (carcinomas) (https://training.seer.cancer.gov/disease/categories/classification.html) and lose some epithelial characteristics during tumor progression. Tumor cells can acquire migratory abilities that are characteristic of embryonic mesenchymal cells, which is a critical step in metastasis. Thus, tumor progression very commonly involves an EMT, and some of the same cellular processes and molecular regulators used during development are activated during tumor progression. However, the degree to which the same cellular events and regulatory mechanisms act during tumor progression and in developmental EMTs is not clear and has been a topic of lively debate.
In this review, we focus on the events and molecular regulation of EMTs in vivo, both in developing embryos and in mouse models of tumor progression. We first describe the cellular and molecular events that define developmental EMTs, focusing on gastrulation and neural crest migration in chick and mouse embryos. These studies provide the best mechanistic insight into the regulation and cellular events of EMTs, which can be applied to other developmental and tumor EMTs. We then review the challenges and recent progress toward dissecting the cellular basis and genetic regulation of EMT-like events during tumor progression in vivo. Finally, we describe the progress toward the dynamic imaging of EMTs in vivo during development and cancer, which will be crucial for understanding how EMTs initiate and unfold.
SWITCHES BETWEEN EPITHELIAL AND MESENCHYMAL STATES: KEY REGULATORS
A defined set of molecules that can control and implement EMT have been identified in a variety of studies (Figure 1). Signaling pathways, including the Fgf, TGF-β, and Wnt pathways, can initiate EMT (Shook & Keller 2003, Thiery et al. 2009). Snail1 and Snail2 (Slug) are two transcription factors that frequently promote EMT during development. Both primarily act to repress epithelial genes such E-cadherin and can also activate mesenchymal genes; both have been implicated in cancer EMTs. Twist, Zeb1, and Zeb2 are transcription factors that promote EMT and have different target genes in different contexts (Shook & Keller 2003, Thiery et al. 2009). Together, these transcription factors control the exit of cells from the epithelium and the acquisition of migratory properties that constitute the EMT.
Figure 1:
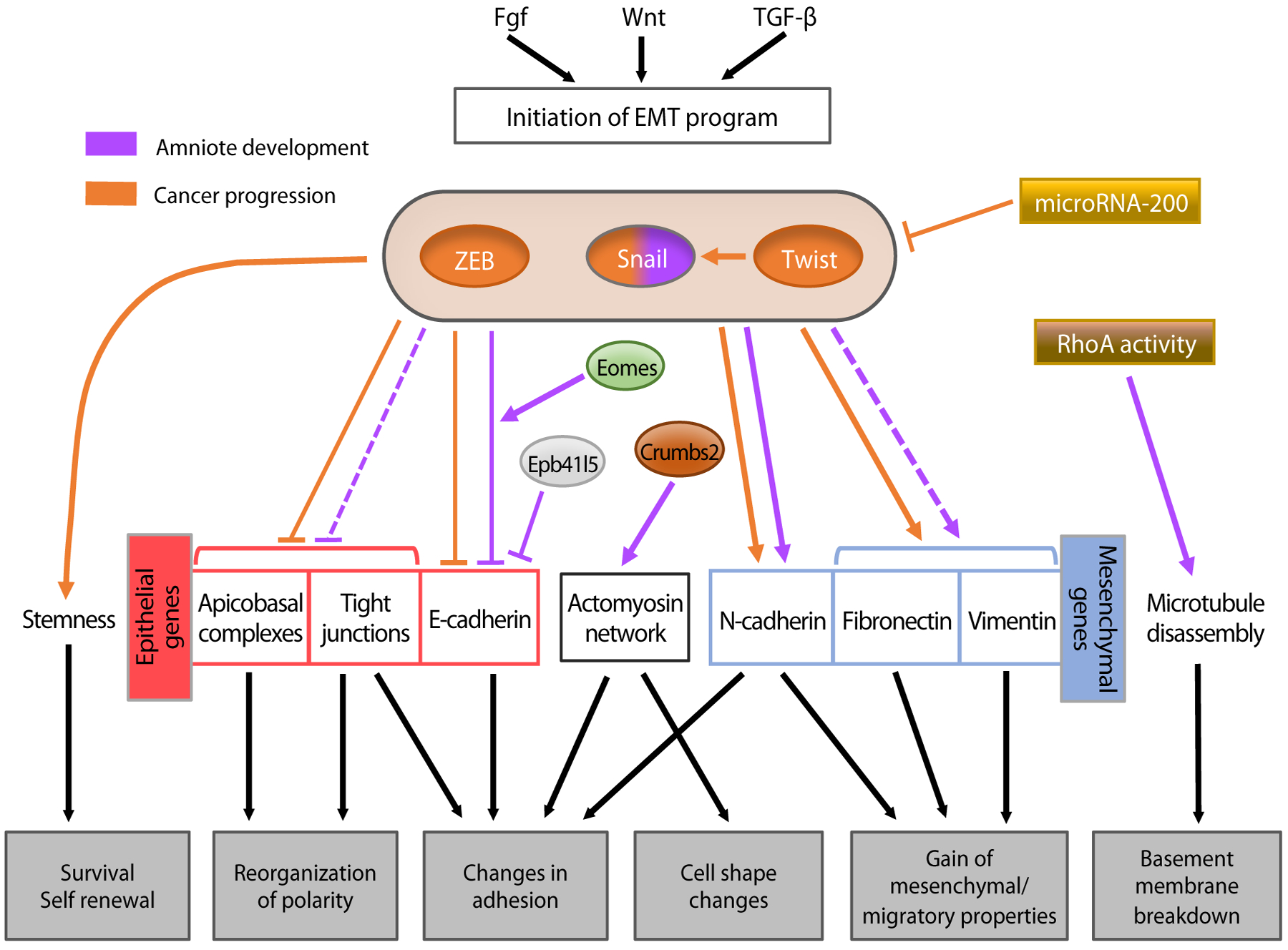
Regulatory networks controlling epithelial-to-mesenchymal transition (EMT) during amniote development and cancer progression. Upstream signaling initiates the EMT program. Three major transcription factor families regulate the process: Snail (zinc finger proteins), Twist (a basic helix-loop-helix protein), and Zeb (zinc finger proteins that, like Twist, bind to E-box motifs). Each of these transcription factors has been implicated in cancer progression, but only Snail has been shown to play a role in developmental EMT in the mouse. The transcription factors act on downstream effectors, mainly by repressing epithelial genes and activating mesenchymal genes. Snail and Zeb mainly act as repressors and Twist acts as an activator.
DEVELOPMENT: CELL BIOLOGICAL MECHANISMS AND MASTER REGULATORS OF EMTS
EMT occurs repeatedly during animal development, with both common and distinct features that depend on the animal, the tissue, and the context (Nakaya & Sheng 2013). All animals share an early developmental EMT during gastrulation, the developmental process that generates endoderm or mesoderm cells from an epithelial layer (Nakaya & Sheng 2008, Nowotschin & Hadjantonakis 2010).
The genetic and cell biological regulation of Drosophila gastrulation is the paradigm for the dissection of EMTs, with extracellular signals controlling expression of master transcription factors that, in turn, regulate specific cellular behaviors. Drosophila mesoderm forms by invagination of the ventral furrow epithelium, followed by a simultaneous transition to mesenchyme of all invaginated cells (Figure 2) (Leptin 1999, Nakaya & Sheng 2008). The Twist and Snail transcription factors were discovered in Drosophila genetic screens because they are required for mesoderm formation, where they controls the EMT through cellular processes of apical constriction and invagination (Figure 2) (Leptin 1991, 1999; Leptin & Grunewald 1990; Seher et al. 2007).
Figure 2:
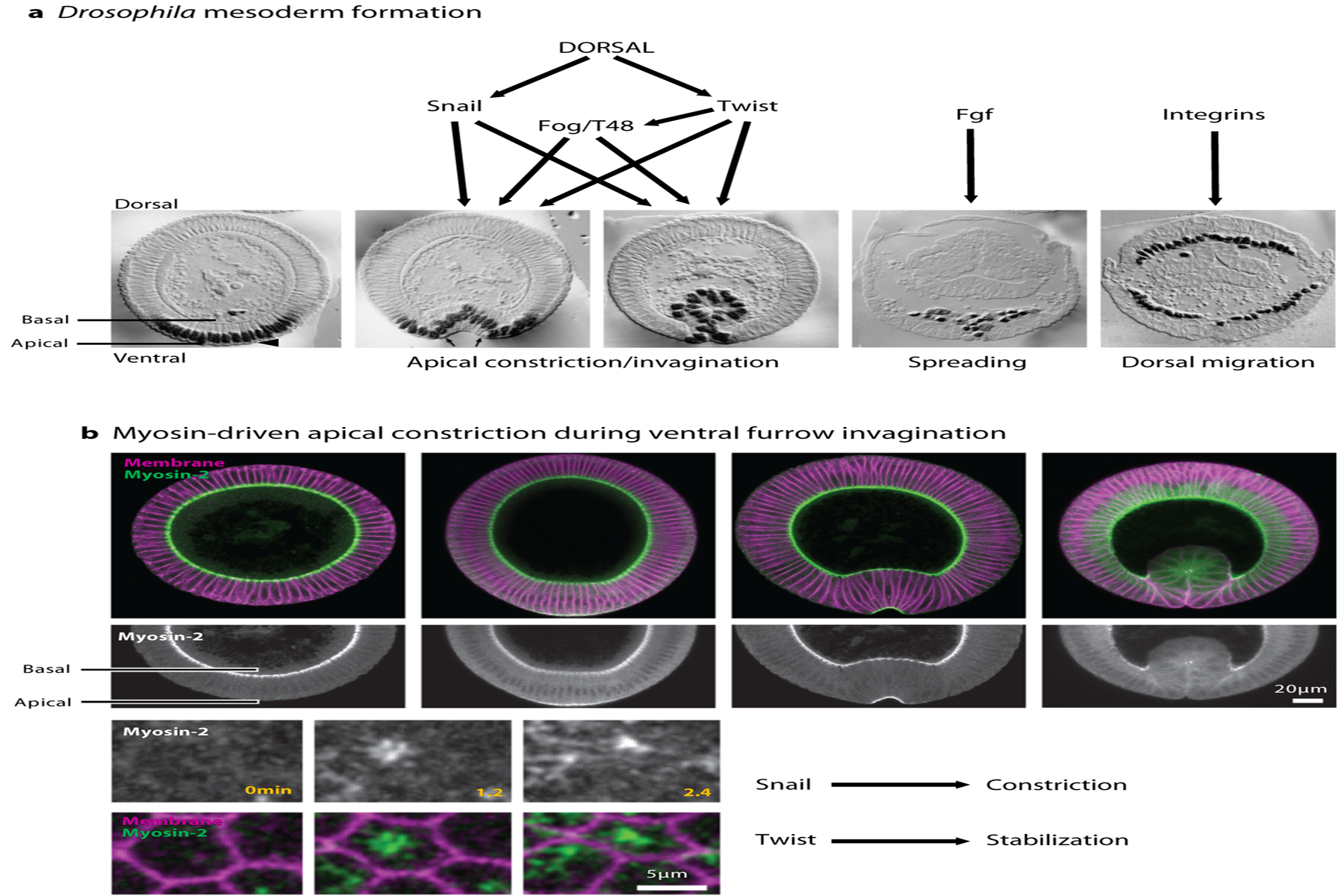
Drosophila mesoderm formation during gastrulation. (a) Drosophila embryo sections at different stages during gastrulation, showing apical constriction and invagination of the ventral cells that will make the mesoderm, as well as spreading and dorsal migration of mesodermal cells after EMT. Black nuclei represent Twist immunostaining. Panel adapted with permission from Leptin & Grunewald (1990). (b) Myosin-2 accumulates on the apical side of ventral furrow cells and triggers apical constriction. Apico-medial accumulation of Myosin regulates pulsed apical constriction that leads to invagination. Snail controls the constriction phase and Twist stabilizes the apical surface. Figure adapted with permission from Vasquez et al. (2014).
The molecular control of apical constriction of invaginating Drosophila mesoderm cells has been extensively studied (Martin & Goldstein 2014, Sawyer et al. 2010). Apical secretion and autocrine action of the protein Fog control apical actomyosin activity (Figure 2) (Lim et al. 2017, Mitrossilis et al. 2017). Apical constriction of invaginating cells is driven by a pulsatile ratchet-like actomyosin network (Figure 2b) (Martin et al. 2009, Mason et al. 2013, Roh-Johnson et al. 2012), and tissue invagination appears to be a passive mechanical response to cell shape changes (Polyakov et al. 2014). Loss of basal Myosin and basal membrane relaxation have also been implicated in ventral furrow invagination (Krueger et al. 2018). After invagination, Fgf signaling is required for spreading of mesoderm cells (Figure 2) (McMahon et al. 2010, Wilson et al. 2005), regulating adherens junctions remodeling and cell divisions (Sun & Stathopoulos 2018). Integrins also play a role in dorsal movement of the mesoderm (McMahon et al. 2010). As in other EMTs, a transcriptional switch from E-cadherin to N-cadherin occurs during mesoderm formation, where E-cadherin is repressed by Snail and N-cadherin is activated by Twist, although the role of E-cadherin downregulation in the mesoderm may be complex (Oda et al. 1998, Schafer et al. 2014).
The Gastrulation EMT in Chicks and Mice
In the development of amniotes (reptiles, birds, and mammals), the EMT that produces the mesoderm layer occurs at the primitive streak, a spatially defined but dynamic structure in the epiblast tissue (Figure 3a,b). Epiblast cells are pluripotent and give rise to the entire proper embryo, and the morphogenetic events of EMT are coupled to loss of pluripotency, as pluripotency genes are downregulated and differentiation of germ layers are activated during the EMT (Nakaya & Sheng 2008, Nowotschin & Hadjantonakis 2010).
Figure 3:
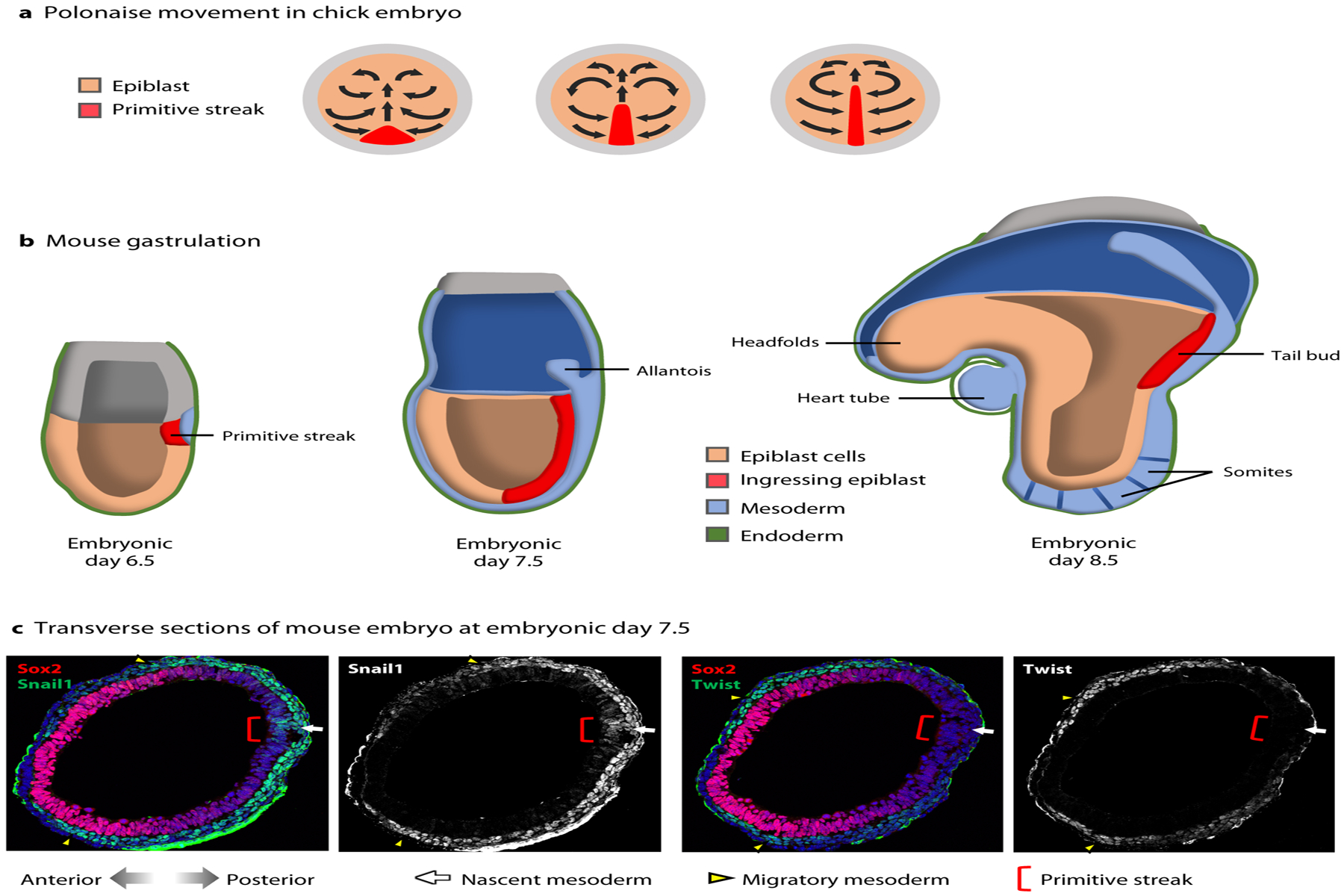
Cell movements and gene expression during chick and mouse gastrulation. (a) Polonaise movement of epiblast cells associated with primitive streak elongation in the developing chick embryo, viewed from above the dorsal side. (b) Mouse gastrulation at E6.5–E8.5 from primitive streak formation, elongation, and tail bud stage, viewed from a lateral side (c) Transverse sections of E7.5 mouse embryos. Snail1 is expressed in a small population of cells in the epiblast at the primitive streak (red bracket), in the nascent mesoderm (white arrow), and in the migratory mesoderm (yellow arrowheads). Twist is not expressed at the primitive streak or in the nascent mesoderm but is turned on later in the migratory mesoderm. Images courtesy of Nitya Ramkumar.
Cell Ingression
In the mouse embryo, gastrulation begins with formation of the primitive streak at the most proximal posterior region of the embryonic epiblast (Figure 3b) (Nowotschin & Hadjantonakis 2010, Williams et al. 2012). A network of signaling pathways, Wnt, Nodal, BMP, and Fgf, initiates the gastrulation EMT (Ramkumar & Anderson 2011). In the mouse, cells continue to undergo EMT at the streak as it elongates to the distal tip of the embryo. This EMT, unlike in Drosophila, does not involve tissue folding; instead, single cells ingress and exit the epithelium in an apparently stochastic manner in the primitive streak and then in its progeny, the tail bud (Figure 3b) (Ramkumar et al. 2016, Williams et al. 2012). As cells ingress at the streak, cells from more lateral epiblast converge toward the streak and ingress at later times (Williams et al. 2012).
In the early chick embryo, the primitive streak initiates on the most posterior region of the flat epiblast disc. Elongation of the streak occurs by the combination of convergence and extension of streak cells, the site ingression, and so-called polonaise movements that allow epiblast cells to converge and integrate the streak (Figure 3a) (Voiculescu et al. 2007, 2014).
Transcription of Snail1 is activated as cells ingress to form the mesoderm (Figures 3c and 4a,b) (Carver et al. 2001, Nieto et al. 1994, Ramkumar et al. 2016), which downregulates E-cadherin transcription. Like in Drosophila, the role of mouse Snail1 in the ingression itself is unclear: Snail1-mutant embryos die during gastrulation but several cells ingress and form a mesoderm population that retains E-cadherin expression (Carver et al. 2001). Embryos that lack Snail1 only in derivatives of the epiblast die later by E9.5 (embryonic day 9.5) due to vascular defects (Lomeli et al. 2009). Thus, Snail1 regulates mesoderm migration rather than cell ingression; it has also been implicated in left-right asymmetry and cardiac looping (Murray & Gridley 2006, Ocana et al. 2017). The role of Snail2 in the chick gastrulation EMT is also not clear, as it is sufficient to induce EMT in some studies (Acloque et al. 2011), whereas others show that it is not able to induce cell ingression (Hardy et al. 2011, Moly et al. 2016). Twist1 appears not to be a part of the EMT program in mouse, although it is expressed in mesoderm cells shortly after they initiate migration (Figure 3) (Fuchtbauer 1995, Gitelman 1997). Twist1 mutants survive gastrulation and die from cranial defects (Chen & Behringer 1995). The EMT transcription factors Zeb1 and Zeb2 are expressed in the mouse embryo at E7.5 (Miyoshi et al. 2006, Takagi et al. 1998, Van de Putte et al. 2003), but Zeb1/2 double compound mutants die after gastrulation, with defects like neural tube closure and maxillonasal morphogenesis (Miyoshi et al. 2006). In the chick embryo, both Zeb2 and Snail2 control P-cadherin transcription in the epiblast (Acloque et al. 2017).
Figure 4:
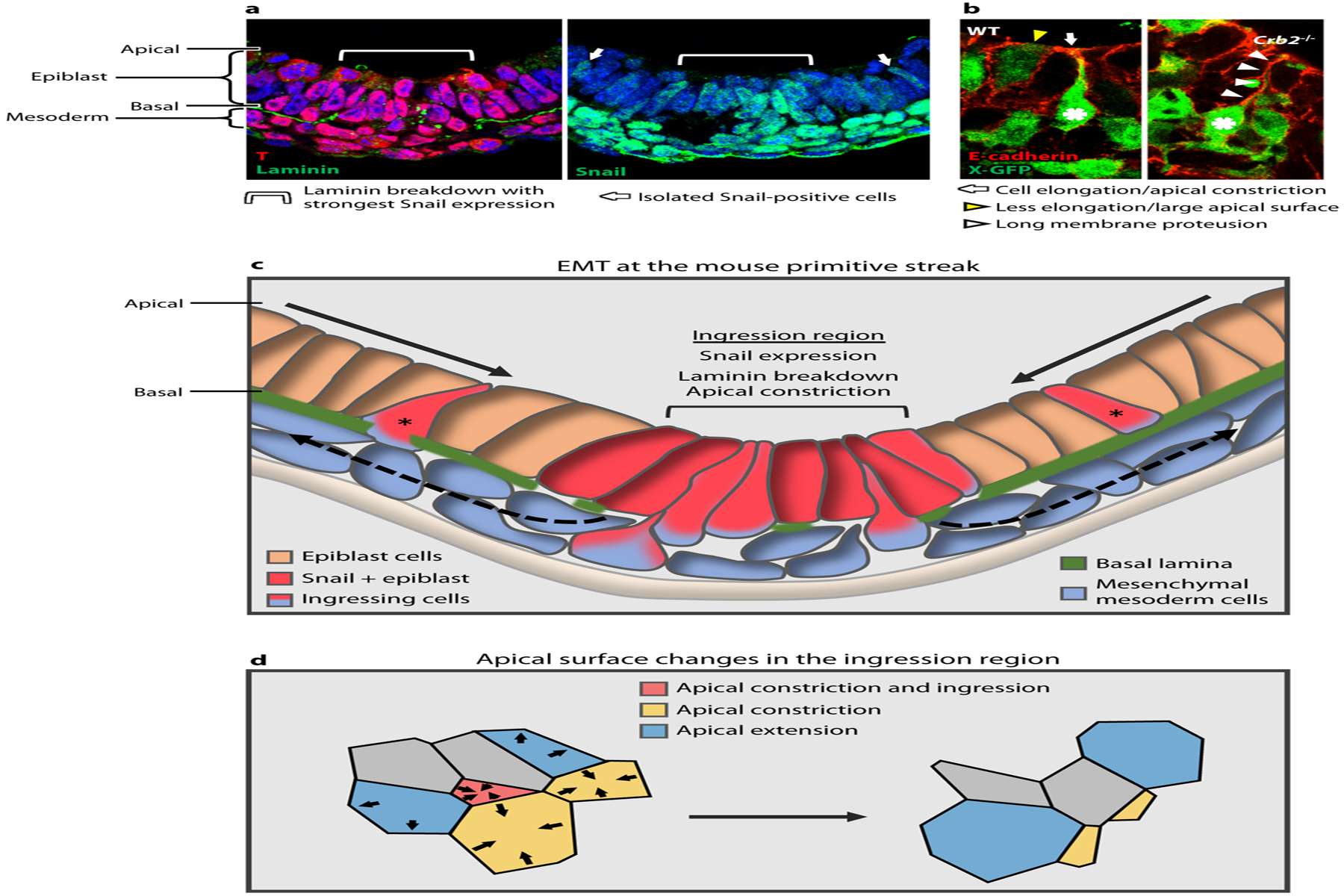
Mouse primitive streak and gastrulation EMT (epithelial-to-mesenchymal transition). (a) Cross-section of the posterior side of a mouse embryo showing T staining at the primitive streak in the epiblast and mesoderm; apical epiblast is up and basal is down. Laminin breakdown occurs beneath a group 6–8 cells wide, corresponding to the strongest Snail expression in the epiblast (light blue brackets). This also corresponds to the region where 80–90% of the ingression events occur, as determined by analyzing whole-embryo time-lapse imaging (data not shown). Isolated Snail positive cells are also observed (white arrows). (b) E-cadherin staining and X-GFP at the primitive streak, show a WT embryo with an ingressing cell (asterisk) elongating and constricting its apical surface (white arrow) and neighboring cell in a different step of the process, which is less elongated and with a larger apical surface (yellow arrowhead). Crb2−/− cell moves further basally (asterisk) and retains a long membrane protrusion attached apically (white arrowheads). Panel adapted with permission from Ramkumar et al. (2016). (c) Schematic of the EMT at the primitive streak during mouse gastrulation. Epiblast cells converge toward the ingression region, which is characterized by expression of Snail, breakdown of the basal lamina, and apical constriction of individual cells. Ingression of a subset of cells at that position appears to be stochastic. Some isolated ingressions are observed outside of this region (asterisks), which may correspond to isolated Snail-positive cells. (d) In the ingression region, cells constrict their apical surfaces in an asynchronous and apparently stochastic manner.
Genetic experiments have identified other proteins required for gastrulation EMT (Figure 1). Fgf signaling is necessary for EMT and transcriptional downregulation of E-cadherin (Ciruna & Rossant 2001). The T-box transcription factor Eomesodermin (Eomes) is required for mesoderm formation in mice and appears to regulate E-cadherin repression at a posttranscriptional level (Arnold et al. 2008). The FERM protein Epb41l5, an actin-binding protein, is also required for gastrulation EMT and participates in E-cadherin downregulation (Hirano et al. 2008, Lee et al. 2007). The apical protein Crumbs2 is required for the EMT process, and null mutants have strong ingression defects, without disruption of apicobasal polarity (Ramkumar et al. 2016).
Basement Membrane Breakdown
In mouse and chick embryos, a basement membrane composed of laminin, fibronectin, and collagen forms on the basal side of the epiblast, and its degradation at the primitive streak is the first step of the EMT process, allowing the cells to move out of the epithelium and ingress to contribute to the mesoderm layer (Figure 4a,c) (Nakaya & Sheng 2008, Nakaya et al. 2008, Shook & Keller 2003, Williams et al. 2012). Basement membrane breakdown is, however, not sufficient for cell ingression. In several mutants that block gastrulation, such as Epb41l5 and Crumbs2, the basement membrane breaks down but most cells fail to ingress (Lee et al. 2007, Ramkumar et al. 2016), and cells at the wild-type primitive streak that lack a basement membrane do not ingress immediately.
The mechanisms that regulate basement membrane breakdown are unclear. No mouse mutants have been described that specifically affect this process. In the chick, it has been proposed that basal loss of RhoA activity triggers the disassembly of microtubules that form focal adhesions with the basement membrane, leading to its breakdown (Nakaya et al. 2008, 2013).
Polarity Changes and Cadherin Switching
During the EMT, cells need to dissolve their junctions, change their adhesion, and break their apicobasal polarity. One of the most studied changes in adhesion in mouse and chick gastrulation is cadherin switching. Downregulation of E-cadherin is a hallmark of EMT in development and metastasis, but the necessity of downregulation is not clear. Ingressing cells in the mouse embryo transiently express E- and N-cadherin (Nakaya et al. 2008, Ramkumar et al. 2015) and progressively switch from one to the other. Snail1 and Snail2 have been implicated in E-cadherin downregulation at the transcriptional level in vivo and in cultured cells (Bolos et al. 2003, Cano et al. 2000). In Snail1-mutant mouse embryos, cells fail to downregulate E-cadherin expression but many cells ingress (Carver et al. 2001). In the chick embryo, Snail2 represses P-cadherin transcription early in gastrulation and later at the level of the streak (Acloque et al. 2017). Snail has also been reported to downregulate tight junction and apicobasal polarity components like Crumbs3 in cultured cells but not in vivo (Ohkubo & Ozawa 2004, Whiteman et al. 2008). In both chick and mouse, Snail and Sox3 transcription factors show complementary expression pattern in the epiblast, enforced by a reciprocal transcriptional repression (Acloque et al. 2011).
The protein Epb41l5 participates in E-cadherin downregulation at a posttranscriptional level (Hirano et al. 2008). In Epb41l5-mutant embryos, the mesoderm cells that are formed retain E-cadherin expression and interestingly express N-cadherin as well. Like Epb41l5, p38 mitogen-activated protein kinase–mutant embryos also downregulate E-cadherin expression at a posttranscriptional level (Arnold et al. 2008, Ciruna & Rossant 2001, Zohn et al. 2006).
Cell Shape Changes and Cytoskeletal Rearrangements
Cells undergo dramatic cell shape changes during gastrulation EMT (Shook & Keller 2003). Cells constrict their apical surface in order to detach from the epiblast epithelium. Myosin motor and actomyosin networks are implicated in membrane rearrangements and apical constriction in multiple tissues and species. A study in chick embryos showed that apical junctional contraction and cell intercalation during primitive streak formation is dependent on Myosin-2 activity (Rozbicki et al. 2015). However, it is not clear how Myosin-2 is directly implicated or how it regulates apical constriction associated with ingression itself, as intercalation is happening at the same time. Apical localization of the actomyosin network is likely to be important for cell ingression in mouse embryos as well (Lee et al. 2007, Ramkumar et al. 2016). As cells constrict their apical surface, they elongate in the apico-basal axis to adopt a bottle shape, which is probably the first step in apical constriction and delamination out of the layer (Figure 4b–d) (Ramkumar et al. 2016, Williams et al. 2012).
The apical protein Crumbs2 has recently been implicated in the ingression process during mouse gastrulation (Ramkumar et al. 2015, Ramkumar et al. 2016). Cells at the primitive streak of Crumbs2-mutant embryos have strong morphological defects: They have a very constricted apical surface, are extensively elongated apicobasally, and stay attached to the apical surface and packed at the streak (Figure 4b) (Ramkumar et al. 2016). It is unclear whether Crumbs2 acts directly in these cell shape changes or if the defects are a passive mechanical constraint due to cell crowding. In the Epb41l5-mutant embryos, proteins that have a known role in apical constriction in cell culture and in Xenopus neural tubes (Chu et al. 2013, Nakajima & Tanoue 2010), cells at the streak also fail to undergo correct ingression, accumulating in the epithelium (Hirano et al. 2008, Lee et al. 2007). Cell shape has not been analyzed in this mutant, but the F-actin network seems clearly perturbed in cells at the streak (Lee et al. 2007).
Mesoderm migration is the final step of gastrulation happening after EMT and required actin cytoskeleton reorganization, dependent on the WAVE complex, its regulator Nap1, the small GTPase Rac1, and the Strip1 protein (Bazzi et al. 2017, Migeotte et al. 2011, Rakeman & Anderson 2006). Mesoderm migration is collective, with adjacent migrating cells making many contacts through filopodia (T. Omelchenko, unpublished manuscript). In this respect, the gastrulation EMT may resemble the so-called partial EMT seen in some tumors (see below).
Diversity of EMTs During Amniote Development
EMT events occur repeatedly during development: in the developing somites, during formation of the cardiac cushions, during craniofacial development, and in neural crest cells, with the neural crest EMT being the best understood (Mayor & Theveneau 2013). Many of the signaling pathways and transcriptions factors that are active during the neural crest EMT are common with gastrulation.
Neural crest cell precursors are located in the most dorsal part of the neural tube epithelium and undergo EMT to exit the neural tube before migrating ventrally (Figure 5). Although Snail transcription factors are expressed in neural crest cells, cells can still delaminate in Snail1-mutant mice (Murray & Gridley 2006). Twist appears to regulate neural crest cells’ migration and differentiation and is required for cranial morphogenesis (Chen & Behringer 1995, Soo et al. 2002). Zeb2 is expressed in premigratory and migratory neural crest cells, but it is not implicated in EMT even though it is required for proper neural crest cell migration (Van de Putte et al. 2003).
Figure 5:
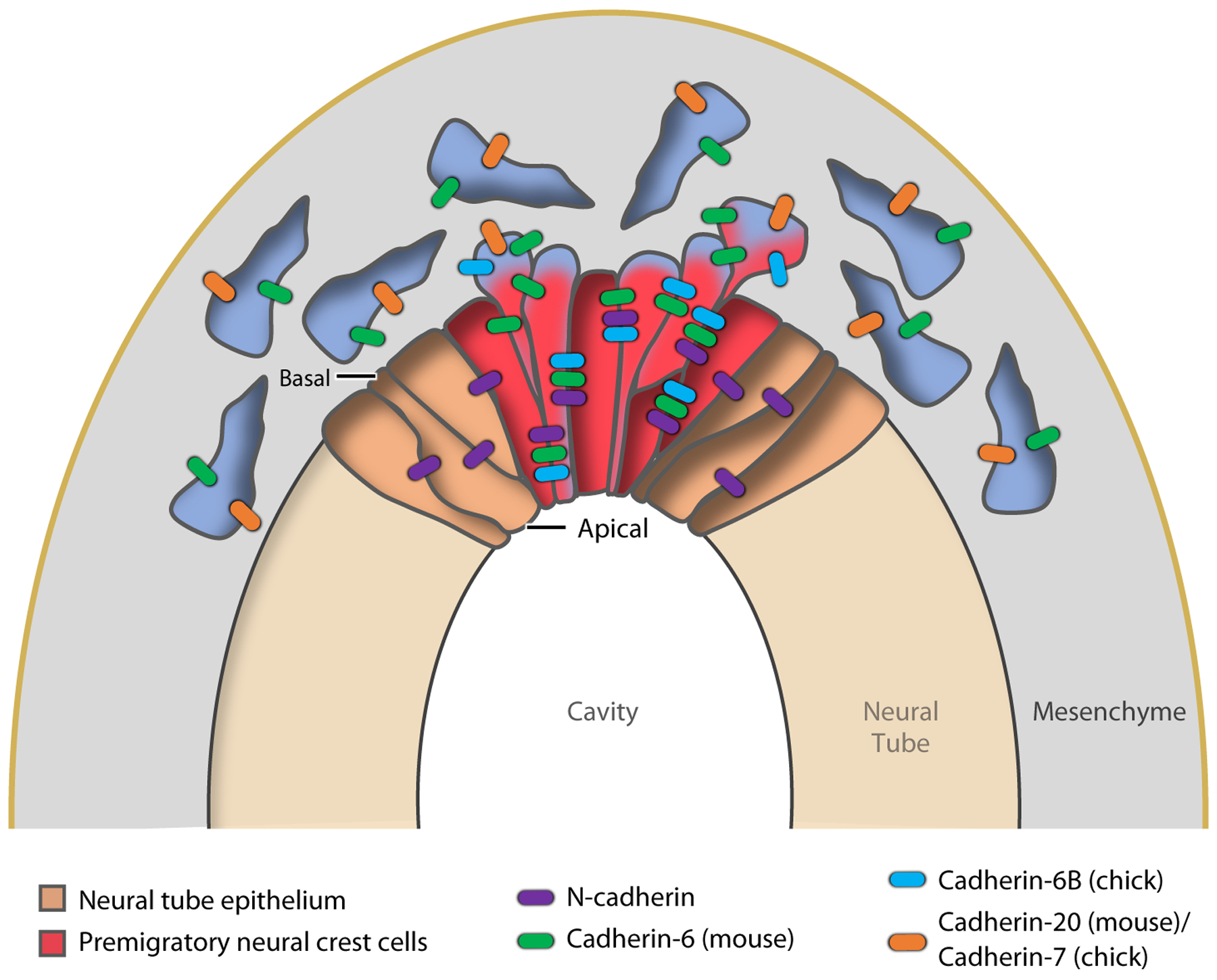
EMT (epithelial-to-mesenchymal transition) and cadherin switching in neural crest cells. Premigratory neural crest cells (red) in the dorsal region of the neural tube epithelium (brown) undergo EMT, delaminate, and then migrate ventrally, switching cadherin expression.
Different cadherins are expressed in neural crest cells than in the primitive streak. In the mouse, cells express N-cadherin and cadherin-6 in the neural tube, and then downregulate N-cadherin and switch to cadherin-20 and −11 during ingression (Figure 5) (Gheldof & Berx 2013). In chick, crest cells express N-cadherin and cadherin-6B and switch to cadherin-7 (Figure 5) (Nakagawa & Takeichi 1998), and the switch has complex kinetics along the anterior-posterior axis (Dady et al. 2012). The FoxD3 transcription factor regulates adhesion and cadherin expression during the neural crest EMT (Cheung et al. 2005, Ray & Niswander 2016). As in the mesoderm and in some tumors, migration of the neural crest is collective, and expression of cadherins in migrating neural crest cells is essential for their normal migration (Barriga & Mayor 2015). Balancing the activity of the pro-EMT factors, the Grhl2 transcription factor controls epithelial integrity and suppresses EMT in non-neural ectoderm (Ray & Niswander 2016).
EMT IN TUMOR PROGRESSION AND METASTASIS
Since the landmark paper from the Weinberg lab on Twist in breast tumor metastasis (Yang et al. 2004), more than 10,000 publications have investigated the relation between EMT and cancer progression. Despite this intense interest, the mechanisms of EMT in tumor progression and metastasis remain a subject of debate. As we have described, different developmental EMTs are regulated by distinct factors and can have different outcomes, so it is to be expected that tumor-related EMTs will vary between tissues and tumor types, and that EMT may be relevant for the progression of some tumors but not others.
Many studies have used cancer cells in culture to understand the regulation of EMT related to cancer progression. Although cell culture models are easy to manipulate and image, conflicting outcomes suggest that the findings may not reflect the in vivo situation. Here we focus on two examples of genetic models of mouse tumor progression, for pancreatic and breast cancer, that have provided useful perspectives on the molecular regulation and biological roles of EMT in tumors.
EMT in Pancreatic Cancer Progression
Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer, and it is also one of the most aggressive and difficult to treat. PDAC progression leads to dissemination of cancer cells and formation of metastatic secondary tumors at a distance from the primary site (Figure 6). Several studies have implicated EMT in PDAC progression and dissemination, but this topic has been hotly debated and provides a good example of the controversies and challenges of the field.
Figure 6:
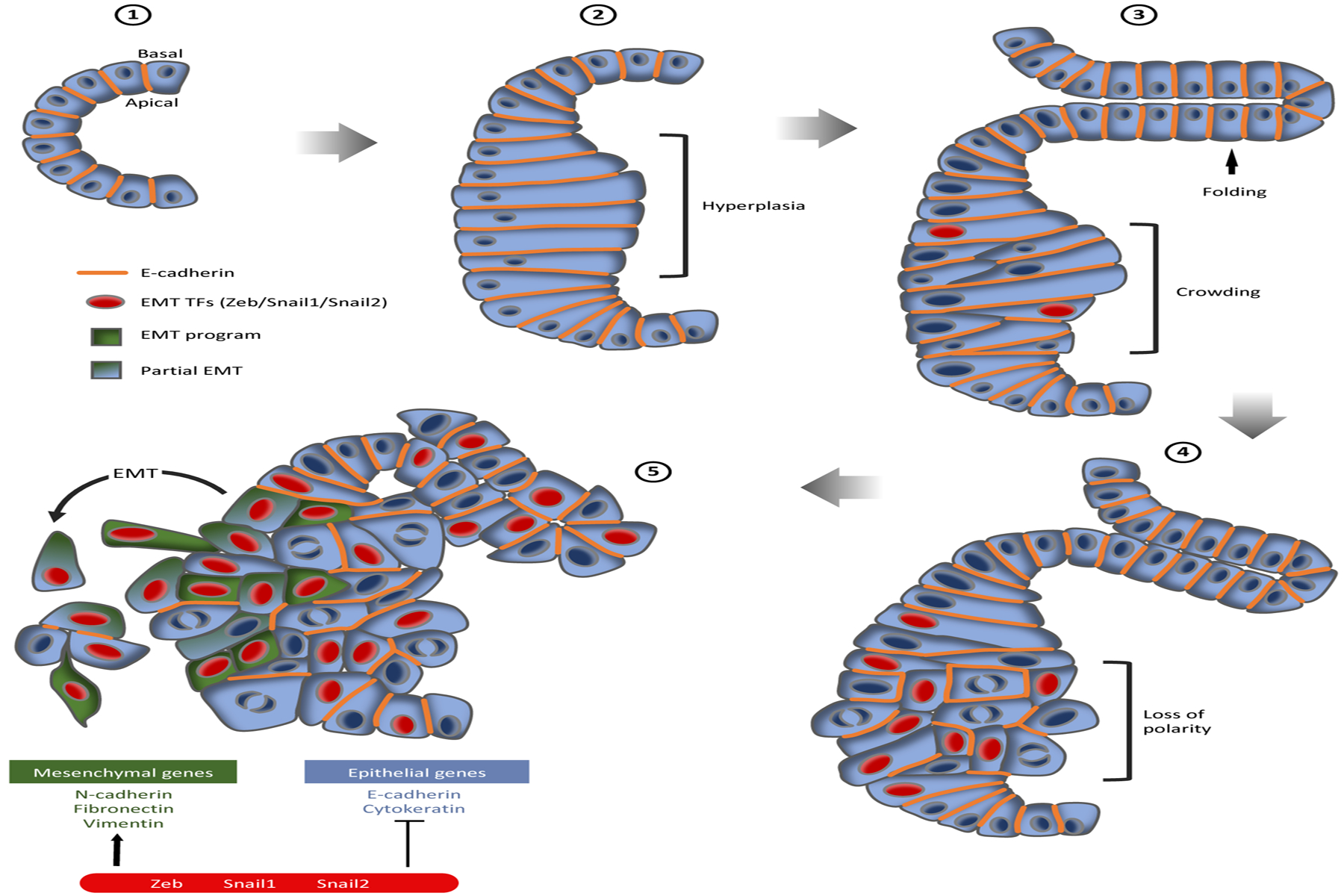
The steps of pancreatic cancer progression from a normal pancreatic duct through the stages of pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC). (❶) A normal pancreatic duct is a simple cuboidal epithelium. (❷) PanIN-1 lesions are associated with cell and tissue hyperplasia (bracket). (❸) PanIN-2 shows some nuclear enlargement, crowding (bracket), and folding of the epithelium (arrow). (❹) PanIN-3 is characterized by nuclear enlargement, loss of polarity (bracket), and the presence of frequent mitotic figures. (❺) PanIN-3 can progress into PDAC, showing loss of polarity and organization of the epithelium and partial loss of E-cadherin. Epithelial-to-mesenchymal (EMT) transcription factor (TFs) expression is observed early in PanIN-2. Cells can activate an EMT-like program or a partial-EMT program and invade surrounding tissue as isolated cells or as a cluster.
The expression level of transcriptions factors and molecules potentially implicated in pancreatic cancer EMT have been analyzed in a multitude of cell lines and human tissue samples, in an effort to identify the molecular signature of EMT in PDAC. Several molecules, some known to play a role in developmental EMTs, have been implicated as EMT inducers, including Snail1/2, Twist, VEGF, TGF-β, BMP4, and Zeb1/2, as well as E-cadherin repression and N-cadherin and Vimentin upregulation (Burk et al. 2008, Ellenrieder et al. 2001, Friess et al. 1993, Hamada et al. 2007, Imamichi et al. 2007, Nishioka et al. 2010, Peinado et al. 2004, Yang et al. 2006). These studies have had differing results: Some claim that increased expression of N-cadherin, Snail1, and Snail2 is a signature of EMT in pancreatic cancer, whereas others found that decreased expression of Twist is a mark of EMT but that Snail and N-cadherin levels are not necessarily modified (Cates et al. 2009, Hotz et al. 2007, Nakajima et al. 2004).
Other interesting developmental genes have been implicated in the PDAC EMT. The EMT suppressor Grainyhead-like 2 (GRHL2) is highly correlated with E-cadherin expression in human samples, and it regulates epithelial morphology and cell proliferation in vitro (Nishino et al. 2017, Ray & Niswander 2016). Aurora A kinase has been implicated in pancreatic tumor progression through increased Twist1 activity (Wang et al. 2017). The developmental transcription factor SOX6 is downregulated in patients with pancreatic cancer metastasis and inhibits EMT of cancer cells in vitro and in vivo in mice by modulating Twist1 activity (Jiang et al. 2018).
E-cadherin has also been implicated in pancreatic cancer progression in vivo. Pancreatic xenografts formed by injection of highly metastatic pancreatic cancer cells are characterized by an EMT-like phenotype, reduction of E-cadherin expression, and SNAIL nuclear localization (von Burstin et al. 2009). Knockdown of E-cadherin in these cells significantly increased their dissemination and metastasis potential. As in development, Snail transcriptionally represses E-cadherin, although the necessity of E-cadherin downregulation to trigger EMT in PDAC has not been tested.
In addition to these cell-based studies, the KPC (KrasLSL-G12D/+;Trp53LSL-R172H/+;Pdx1-Cre) mouse is a well-established and relevant genetic model of PDAC (Hingorani et al. 2005). In these mice, tumorigenesis is driven by inducible activation of the KrasG12D allele (Jackson et al. 2001, Johnson et al. 2001) together with deletion of one allele of the tumor suppressor Trp53R172H, specifically in the pancreatic epithelium. Inducible activation of a YFP (yellow fluorescent protein) in the pancreatic epithelium using Pdx1-Cre (KPCY mouse model) for lineage tracing showed that pancreatic tumor epithelial cells undergo EMT, invade surrounding tissue, and can enter the bloodstream (Rhim et al. 2012). The circulating cells exhibit mesenchymal characteristics and stem cell properties, and can integrate and seed in the liver.
In 2015, two in vivo studies challenged the classic models of EMT in the progression and metastasis formation of pancreatic and breast cancer and disputed the role of Snail1 and Twist1 transcription factors in the progression of these cancers (Fischer et al. 2015, Zheng et al. 2015). Zheng et al. used the KPCY mouse model to induce pancreatic tumor formation, while simultaneously deleting Snail or Twist genes. They found that deletion of neither Snail nor Twist reduced primary tumor initiation and metastasis. Using lineage tracing, staining for selected EMT markers, and microarray analysis, the authors concluded that pancreatic epithelial tumor cells do not undergo EMT, and that EMT is not necessary for metastasis.
Two years after these controversial studies, another group used the KPC pancreatic cancer model, but deleted the Zeb1 transcription factor instead of Snail or Twist (Krebs et al. 2017). Zeb1 depletion in the pancreatic epithelium reduced invasion of cancer cells and distant metastasis formation, and appeared to be important for stemness and plasticity of cancer cells. The authors concluded that EMT is necessary for pancreatic cancer progression and metastasis, and that Zeb1 is a key factor. Others pointed out that the original Zheng et al. studies used α-SMA as a marker of EMT on their lineage tracing in the pancreatic epithelium, that α-SMA is not necessarily activated during EMT, and that other EMT markers were stained without the lineage tracing (Aiello et al. 2017, Ye et al. 2017). These different contradictory views show the difficulties of identifying the EMT process in tumors in vivo. More recently, lineage tracing using the KPCY model showed that several tumors can undergo an alternative EMT program involving protein internalization rather than transcriptional repression. This causes a partial EMT, in which cells retain some epithelial characteristics like E-cadherin expression and invade and migrate as clusters, similar to what is observed in breast metastasis (see the next section) (Aiello et al. 2018). Mesenchymal genes and markers are not universal and are not necessarily always turned on during EMT. Cells can undergo partial EMT and by consequence express different levels of markers or retain epithelial proteins. The different known transcription factors regulating EMT are probably context dependent and differentially regulate the process in different tissues. Moreover, EMT is a complex process that simultaneously needs several factors and signaling, and depletion of one transcription factor is maybe not sufficient to inhibit EMT depending on the tissue.
EMT in Breast Tumor Progression
Twist was the first EMT inducer implicated in metastasis in spontaneous breast tumors in the mouse (Yang et al. 2004). In this unbiased screen, high levels of Twist were correlated with the likelihood of metastasis, and knockdown of Twist decreased the number of metastases. Snail1 and Snail2 transcription factors have also been implicated in breast cancer progression (Casas et al. 2011).
Using fluorescent reporters in an MMTV (mouse mammary tumor virus)-PyMT (polyoma virus middle T) breast tumor mouse model (where breast carcinogenesis in induced by expression of the oncogene PyMT under the control of the MMTV promoter), Snail 1 and 2 are expressed in different cell types in mammary gland tissue, and they control different target genes and activate different EMT programs (Ye et al. 2015). Snail2 and Sox9 transcription factors cooperate to define the normal mammary stem cell state, and when both co-overexpressed they promote the metastasis abilities of implanted breast cancer cells (Guo et al. 2012). FOXC2 is associated with the metastatic capabilities of implanted mammary cancer cells; its expression is triggered by TGF-β1, Snail, and Twist, and its overexpression enhances metastasis (Mani et al. 2007).
Carcinoma-associated fibroblasts (CAFs) constitute the majority of the stroma and seem to play an important role in tumor cell dissemination. In vitro coculture with CAFs significantly increases the invasion ability of cancer cells (Dang et al. 2011). The YAP transcription factor is activated in breast CAFs, where it modulates the expression of several cytoskeletal regulators and is required for ECM remodeling. Actomyosin cytoskeleton contractility is required for YAP activation in CAFs, creating an activation feedback loop and promoting cancer cell invasion (Calvo et al. 2013).
Although breast tumor cells can activate an EMT program during metastasis, several studies have shown that cells can go through a so-called partial EMT program and retain some epithelial properties. Breast cancer cells invade collectively as clusters, and ex vivo 3D culture assay has shown that clusters of cells leaving the primary tumor are headed by a group of cancer cells that express epithelial proteins, including Cytokeratin-14, E-cadherin, and P-cadherin, and do not express EMT markers like Twist, Snail2, and Vimentin (Cheung et al. 2013). Using a multicolor lineage tracing strategy in MMTV-PyMT-implanted organoids, breast carcinoma metastases in the lung have also been shown to arise from a polyclonal tumor cell cluster enriched in Cytokeratin-14, Desmosome, and E-cadherin, as required for metastasis formation (Cheung et al. 2016). In a primary mammary epithelium organoid assay, Twist1 promoted the dissemination of cells, while E-cadherin downregulation did not, and dissemination can occur with retention of epithelial identity. Twist1 expression modifies ECM and cell-matrix adhesion genes (Shamir et al. 2014). Different ECM composition impacts the ability of mammary cancer cells to migrate collectively (Nguyen-Ngoc et al. 2012).
These studies showed that to collectively invade surrounding tissue, metastatic breast carcinoma cells need to retain epithelial characteristics while simultaneously gaining migratory properties. In this collective invasion process, nonmalignant mesenchymal stem cells in the stroma surrounding the tumor participate in metastasis, enhancing cancer cells’ motility and invasion by secreting chemokines (Karnoub et al. 2007). While a lineage tracing study using a MMTV-PyMT model claimed that EMT is not required and that metastases are mainly composed of non-EMT cancer cells (Fischer et al. 2015), a more recent analysis of cell surface markers using a MMTV-PyMT model identified the existence of multiple mammary tumor subpopulations localized in different niches, associated with different EMT stages, from epithelial to completely mesenchymal and with intermediate hybrid states (Pastushenko et al. 2018).
These in vivo studies of pancreatic and breast cancer progression highlight the complexity of the EMT process and show that lineage tracing and marker gene expression, even at cellular resolution, are not sufficiently clear to define EMT in vivo.
DYNAMIC IMAGING OF DEVELOPMENTAL EMT IN VIVO
As described above, the EMT is a complex, context-dependent process involving multiple cellular events. Therefore, identifying the occurrence of EMT in a fixed sample with markers is frequently inconclusive. Thus, imaging the dynamic process of EMT in living animals will be crucial to understanding the cellular mechanisms underlying the process. Fortunately, tools for this type of analysis are now becoming available.
Imaging EMT During Embryo Gastrulation
Because of the accessibility of embryos, basic understanding of the cellular processes of gastrulation, markers that make it possible to track single cells, and improved microscopic methods, the cellular dynamics of EMT have been most studied during chick and mouse gastrulation. In the chick, cell electroporation with fluorescent reporters were first used to image streak formation and cell movement in the epiblast, showing cell intercalation and convergence-extension movement at the streak (Voiculescu et al. 2007). Multiphoton microscopy has revealed that a few individual cells scattered in the epiblast undergo EMT before streak formation, and that EMT events accelerate and concentrate around the streak during its formation (Voiculescu et al. 2014). Before ingression, cell bodies of individual ingressing cells move basally several times before finally exiting the epithelium. Light sheet microscopy, combined with a membrane-tagged GFP (green fluorescent protein) transgenic chick line, showed that cells at the streak constrict their apical surface and elongate in the apicobasal axis during ingression (Rozbicki et al. 2015).
In the mouse embryo, imaging of membrane-tagged mouse lines using confocal microscopy showed that epiblast cells converge toward the streak where most of ingression events take place. Cells constrict their apical surface and move their body basally to form bottle-shaped structures (Williams et al. 2012). In the mouse, imaging can be combined with the analysis of mutants in which gastrulation is disrupted. For example, the Crumbs2 apical protein is crucial for the ingression process, as in Crumbs2-mutant embryos, where cells move basally but do not detach from the apical surface and where only a few cells ingress after an extended time (Figure 7a) (Ramkumar et al. 2016). These experiments demonstrate the feasibility of single-cell analysis of EMT. High-resolution time-lapse imaging and fluorescent reporters of proteins implicated in EMT, as well as analysis of mutants, will be essential to understand the complex dynamics of cell ingression during gastrulation.
Figure 7:
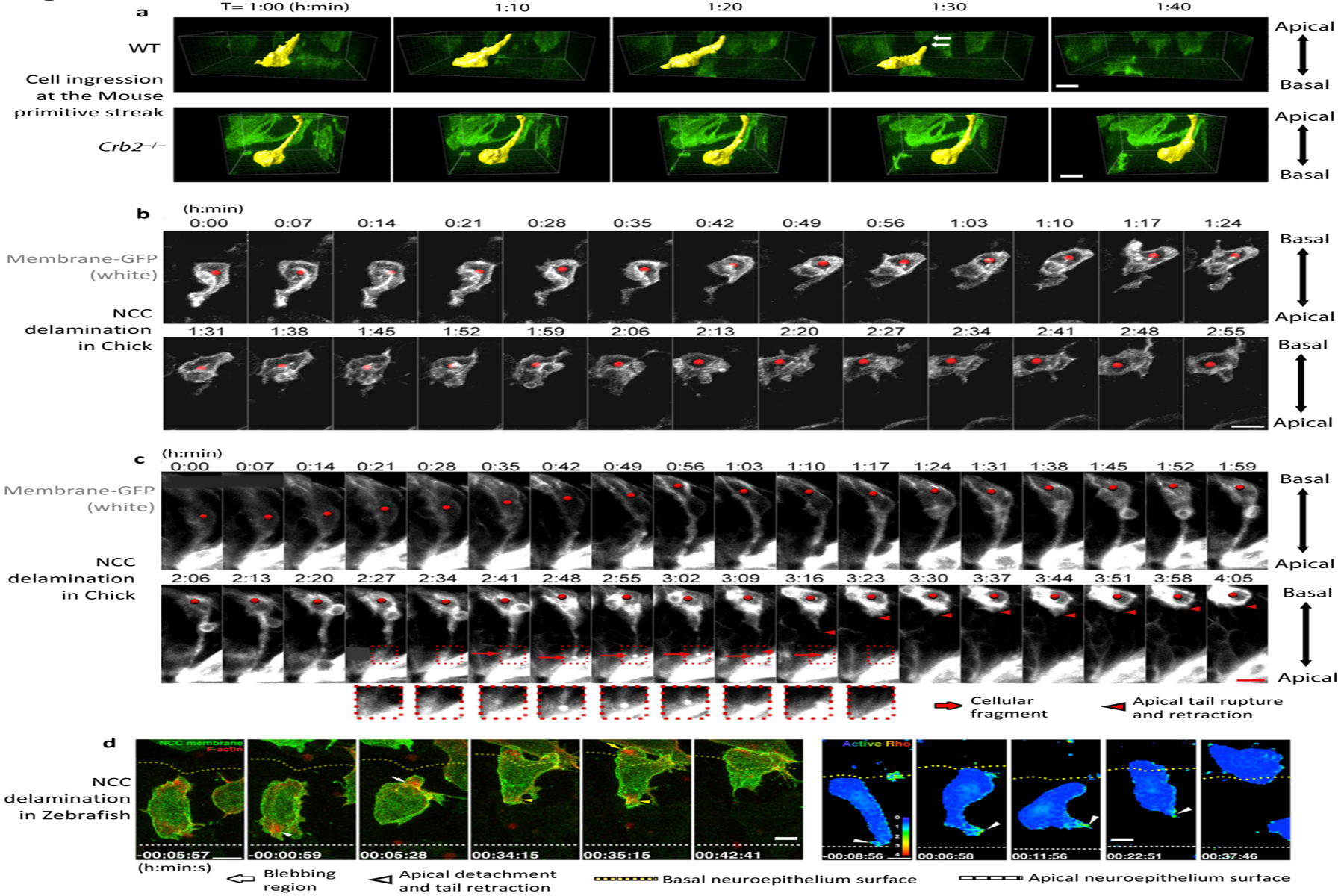
Dynamic imaging of ingression of cells at the primitive streak and neural crest. (a) Three-dimensional surface rendering of individual cells ingressing at the mouse primitive streak. Wild-type (WT) cells elongate in the basal direction and detach from the apical surface, whereas Crb2−/− cells move basally but remain attached to the apical surface of the epiblast. (b) Dynamics of delamination of Neural Crest Cells (NCC)in the chick embryo, where most cells detach from the apical surface, retract their apical tail, and move out of the epithelium. (c) In some cases, cells detach and leave a fragment of membrane on the apical surface. (d) Dynamics of NCC delamination in zebra fish showing F-actin accumulation in the blebbing region and at the apical tail during apical detachment and retraction, as well as Rho activity in the apical tail during detachment and retraction. In d, apical detachment is at 0 minutes. All scale bars represent 10 μm. Panels adapted with permission from (a) Ramkumar et al. (2016), (b,c) Ahlstrom & Erickson (2009), and (d) Clay & Halloran (2013.
Imaging the Neural Crest Cell EMT In Vivo
The ability to electroporate fluorescently tagged reporter genes into chick embryos has facilitated imaging of the dynamics of neural crest cell ingression. Time-lapse imaging of chick embryos has revealed that neural crest cells undergoing EMT, like cells of the primitive streak, constrict apically, detach from the apical surface, and then move the cell body out of the neuroepithelium (Figure 7b) (Ahlstrom & Erickson 2009). In rare cases, the apical region (the apical tail) of the delaminating cell breaks and leaves a fragment of apical membrane at the surface (Figure 7c). Interestingly, some apical junctional component like actin and α-catenin are not always decreased apically before detachment of the apical tail, raising the question of how much apical junction disassembly is required for ingression (Ahlstrom & Erickson 2009). In zebra fish neural crest cells, F-actin accumulates apically before apical detachment and retraction during the EMT process, and Rho-ROCK signaling and cadherin-6 seem to play an important role in apical defacement and cell ingression (Figure 7d) (Clay & Halloran 2013, 2014).
IMAGING TUMOR EMTs IN VIVO
Imaging EMTs in a tumor in vivo is more challenging than during development. Intravital imaging has been used to image the different steps of tumor progression (Fein & Egeblad 2013, Vennin et al. 2016) and to observe and study the interaction between cancer cells and their surrounding environment during invasion (Alexander et al. 2013, Nakasone et al. 2012).
The use of a permanent window implanted in living mice is likely to be a key feature for long-term analysis of tumor progression. Development of mammary imaging windows combined with expression of a switchable fluorescent protein in the tumor made it possible to visualize cell invasion from the primary breast tumor, showing that tumor cell invasion is much higher when the cells are surrounded by blood vessels (Figure 8a) (Kedrin et al. 2008), demonstrating the importance of the surrounding microenvironment. Use of a permanent window on the murine lung made it possible to visualize different stages of metastatic seeding at single-cell resolution, including arrival and extravasation of cells, as well as metastatic tumor cell division (Figure 8b,c) (Entenberg et al. 2018). These kinds of permanent windows have also been used to image deeper organs like pancreatic tumor development and liver metastasis formation (McElroy et al. 2008, Ritsma et al. 2012).
Figure 8:
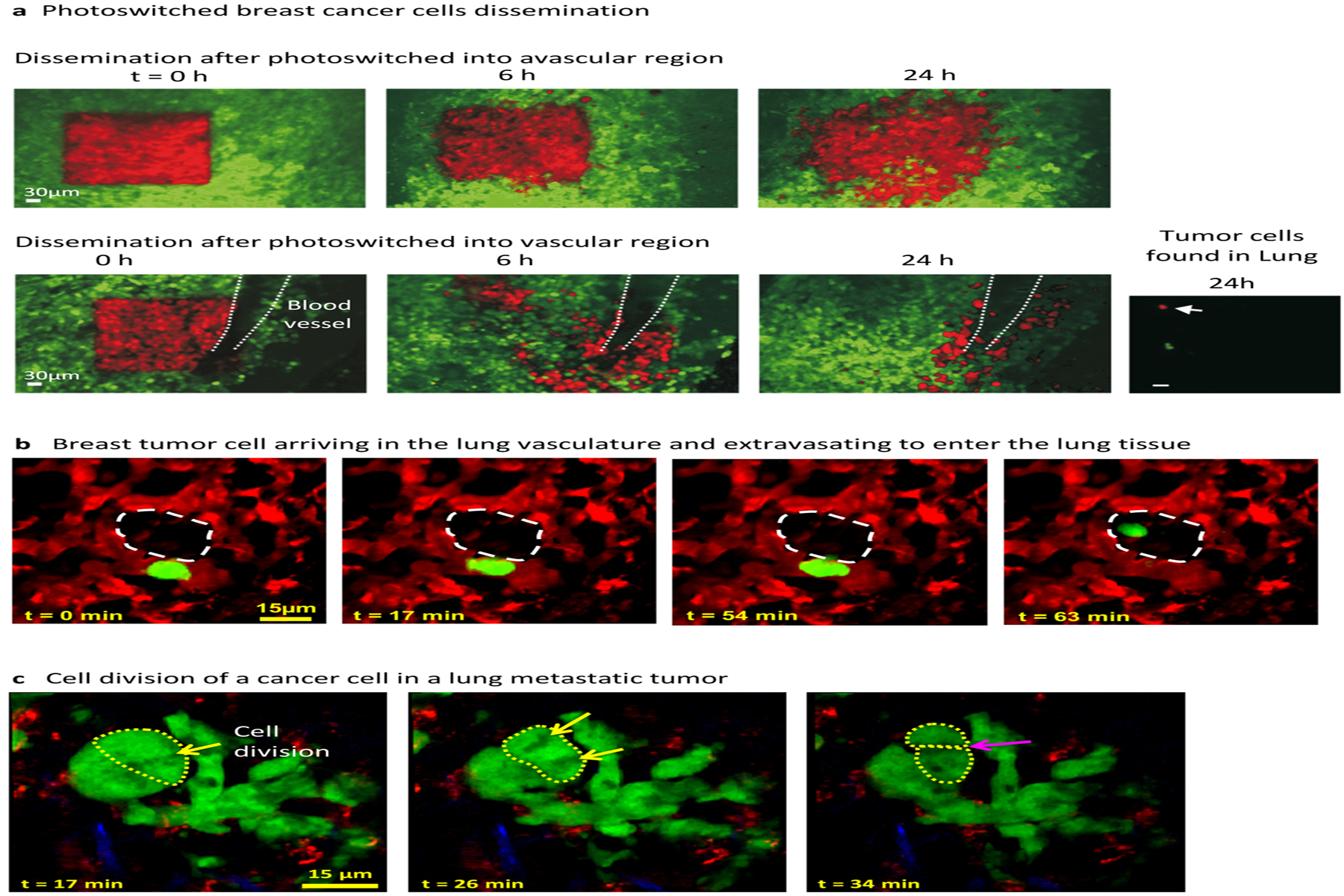
Intravital imaging of breast tumor and lung metastasis. (a) Intravital visualization of an orthotopic breast tumor through a permanent mammary imaging window. Breast cancer cells in the mammary fat pad (green), when photoswitched (red) in an avascular region showed limited invasion (top), whereas when photoswitched into a vascular region, the cells infiltrated a larger area, migrated along the blood vessel, and probably intravasated (bottom); some cells were found in the lung 24 h after photoswitching (right). (b) Spontaneous metastatic cell visualized through a permanent thoracic window. A cell from an orthotopic mammary tumor (green) arriving in the lung vasculature (red), extravasating, and moving into the alveolar space (white dashed region). (c) A cluster of metastatic tumor cells in the lung showing cell division with chromosomal alignment and separation, and cytokinesis (white arrows). Panels adapted with permission from (a) Kedrin et al. (2008) and (b,c) Entenberg et al. (2018).
The development of genetic reporters is also essential for identifying and dynamically visualizing EMT in a tumor, as well as observing protein dynamics during the process. The breast cancer mouse model MMTV-PyMT, Rosa26-RFP-GFP, Fsp1-Cre, where cancer cells switch from expression of RFP (red fluorescent protein) to GFP upon expression of Fsp1 (a marker of EMT initiation), was used in combination with intravital imaging to visualize potential EMT and movement of single cells in the tumor (Zhao et al. 2016). It is however difficult to know when the EMT process itself happens, and it is dependent on the expression of Fsp1 (which is probably not a universal EMT marker depending on the context). Moreover, Cre-based permanent recombination does not permit assessment of the plasticity of cell states during EMT.
A recent reporter system used transgenic expression of GFP and RFP (under the control of Zeb1 and E-cadherin promoters, respectively) to identify epithelial-mesenchymal state changes in vitro, and this system is promising for in vivo studies as well (Toneff et al. 2016). However, once again, the dependence of EMT on changes in Zeb1 and E-cadherin expression may not be the same depending on the tumor context. An E-cadherin-GFP reporter was used with intravital imaging in a pancreatic KPC mouse model and showed, using FRAP (fluorescence recovery after photobleaching), that E-cadherin turnover and mobilization is higher in tumors than in normal tissues (Erami et al. 2016). Fluorescence resonance energy transfer (FRET) biosensor mouse models were also developed to analyze protein activity and provided interesting results: A Rac-FRET transgene used in a KPC tumor model showed that Rac activity is increased in pancreatic tumor tissue compared to normal pancreatic tissue (Johnsson et al. 2014). A RhoA-FRET biosensor was also used to analyze RhoA activity in vivo and showed that RhoA activity decreased during pancreatic tumor progression in the KPC mouse model, with different regions of the tumor itself showing different activity levels (Nobis et al. 2017). These genetic reporters promise to be very helpful for understanding the EMT process in tumors, as E-cadherin, Rac, and RhoA have all been implicated (or are modulated) in certain aspects or steps during EMT in development. The increased number of genetic reporters and the advancement of microscopy technology will allow scientists to obtain more information about the cellular biology of EMT in tumors in vivo.
Cancer EMT—the progression of cells from epithelial to migratory behaviors—is certainly a component of tumor progression. Many of the molecules that regulate this process, including key transcription factors, have roles in both developmental and cancer EMTs. While recent studies have implicated EMT in tumor progression in vivo, they have simultaneously revealed the complexity and variability of EMTs in cancer progression. There is no single cancer EMT program, and the process can involve a complete EMT or the acquisition of migratory behaviors by epithelial-like tumor cells. The EMT program is certainly involved in cancer from initiation to metastasis, with complex and tissue-specific involvement of EMT transcription factors, and a spectrum of partial completion of the process. This complexity means that simple detection methods based on the expression of epithelial and mesenchymal markers or EMT transcription factors are not sufficient to demonstrate the involvement of the EMT program in vivo. Mouse models and tools to follow EMT states and tumor progression in vivo will be crucial to tackle this complex process rigorously, and live imaging of tumor progression in vivo should be a central component of these studies.
ACKNOWLEDGMENTS
We thank the MSKCC (Memorial Sloan Kettering Cancer Center) Molecular Cytology Core Facility for imaging technical support. We thank Nitya Ramkumar for unpublished images. This work was supported by NIH R01/R37 HD03455 to K.V.A., the MSKCC Cancer Center Support Grant (P30 CA008748), and The Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- Acloque H, Ocana OH, Abad D, Stern CD, Nieto MA. 2017. Snail2 and Zeb2 repress P-cadherin to define embryonic territories in the chick embryo. Development 144:649–56 [DOI] [PubMed] [Google Scholar]
- Acloque H, Ocana OH, Matheu A, Rizzoti K, Wise C, et al. 2011. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev. Cell 21:546–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom JD, Erickson CA. 2009. The neural crest epithelial-mesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development 136:1801–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello NM, Brabletz T, Kang Y, Nieto MA, Weinberg RA, Stanger BZ. 2017. Upholding a role for EMT in pancreatic cancer metastasis. Nature 547:E7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, et al. 2018. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45:681–95.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Weigelin B, Winkler F, Friedl P. 2013. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr. Opin. Cell Biol 25:659–71 [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. 2008. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development 135:501–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga EH, Mayor R. 2015. Embryonic cell-cell adhesion: a key player in collective neural crest migration. Curr. Top. Dev. Biol 112:301–23 [DOI] [PubMed] [Google Scholar]
- Bazzi H, Soroka E, Alcorn HL, Anderson KV. 2017. STRIP1, a core component of STRIPAK complexes, is essential for normal mesoderm migration in the mouse embryo. PNAS 114:E10928–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci 116:499–511 [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, et al. 2008. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9:582–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, et al. 2013. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol 15:637–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, et al. 2000. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol 2:76–83 [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. 2001. The mouse Snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol. Cell. Biol 21:8184–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. 2011. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 71:245–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates JM, Byrd RH, Fohn LE, Tatsas AD, Washington MK, Black CC. 2009. Epithelial-mesenchymal transition markers in pancreatic ductal adenocarcinoma. Pancreas 38:e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR. 1995. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 9:686–99 [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. 2013. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155:1639–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, et al. 2016. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. PNAS 113:E854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. 2005. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 8:179–92 [DOI] [PubMed] [Google Scholar]
- Chu CW, Gerstenzang E, Ossipova O, Sokol SY. 2013. Lulu regulates Shroom-induced apical constriction during neural tube closure. PLOS ONE 8:e81854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. 2001. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1:37–49 [DOI] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. 2013. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development 140:3198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. 2014. Cadherin 6 promotes neural crest cell detachment via F-actin regulation and influences active Rho distribution during epithelial-to-mesenchymal transition. Development 141:2506–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dady A, Blavet C, Duband JL. 2012. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev. Dyn 241:1333–49 [DOI] [PubMed] [Google Scholar]
- Dang TT, Prechtl AM, Pearson GW. 2011. Breast cancer subtype-specific interactions with the microenvironment dictate mechanisms of invasion. Cancer Res. 71:6857–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, et al. 2001. Transforming growth factor β1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 61:4222–28 [PubMed] [Google Scholar]
- Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, et al. 2018. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods 15:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erami Z, Herrmann D, Warren SC, Nobis M, McGhee EJ, et al. 2016. Intravital FRAP imaging using an E-cadherin-GFP mouse reveals disease- and drug-dependent dynamic regulation of cell-cell junctions in live tissue. Cell Rep. 14:152–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein MR, Egeblad M. 2013. Caught in the act: revealing the metastatic process by live imaging. Dis. Model. Mech 6:580–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, et al. 2015. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess H, Yamanaka Y, Buchler M, Berger HG, Kobrin MS, et al. 1993. Enhanced expression of the type II transforming growth factor β receptor in human pancreatic cancer cells without alteration of type III receptor expression. Cancer Res. 53:2704–7 [PubMed] [Google Scholar]
- Fuchtbauer EM. 1995. Expression of M-twist during postimplantation development of the mouse. Dev. Dyn 204:316–22 [DOI] [PubMed] [Google Scholar]
- Gheldof A, Berx G. 2013. Cadherins and epithelial-to-mesenchymal transition. Prog. Mol. Biol. Transl. Sci 116:317–36 [DOI] [PubMed] [Google Scholar]
- Gitelman I 1997. Twist protein in mouse embryogenesis. Dev. Biol 189:205–14 [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. 1982. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol 95:333–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, et al. 2012. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148:1015–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Hirota M, Kimura K, Kanno A, et al. 2007. Bone morphogenetic protein 4 induces epithelial-mesenchymal transition through MSX2 induction on pancreatic cancer cell line. J. Cell. Physiol 213:768–74 [DOI] [PubMed] [Google Scholar]
- Hardy KM, Yatskievych TA, Konieczka J, Bobbs AS, Antin PB. 2011. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev. Biol 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, et al. 2005. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–83 [DOI] [PubMed] [Google Scholar]
- Hirano M, Hashimoto S, Yonemura S, Sabe H, Aizawa S. 2008. EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J. Cell Biol 182:1217–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. 2007. Epithelial to mesenchymal transition: expression of the regulators Snail, Slug, and Twist in pancreatic cancer. Clin. Cancer Res 13:4769–76 [DOI] [PubMed] [Google Scholar]
- Imamichi Y, Konig A, Gress T, Menke A. 2007. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene 26:2381–85 [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, et al. 2001. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15:3243–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Yuan Q, Jiang Y, Huang L, Chen C, et al. 2018. Identification of Sox6 as a regulator of pancreatic cancer development. J. Cell. Mol. Med 22:1864–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, et al. 2001. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410:1111–16 [DOI] [PubMed] [Google Scholar]
- Johnsson AE, Dai Y, Nobis M, Baker MJ, McGhee EJ, et al. 2014. The Rac-FRET mouse reveals tight spatiotemporal control of Rac activity in primary cells and tissues. Cell Rep. 6:1153–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, et al. 2007. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–63 [DOI] [PubMed] [Google Scholar]
- Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, et al. 2008. Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5:1019–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, et al. 2017. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol 19:518–29 [DOI] [PubMed] [Google Scholar]
- Krueger D, Tardivo P, Nguyen C, De Renzis S. 2018. Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J. 37:e100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Silva-Gagliardi NF, Tepass U, McGlade CJ, Anderson KV. 2007. The FERM protein Epb4.1l5 is required for organization of the neural plate and for the epithelial-mesenchymal transition at the primitive streak of the mouse embryo. Development 134:2007–16 [DOI] [PubMed] [Google Scholar]
- Leptin M 1991. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5:1568–76 [DOI] [PubMed] [Google Scholar]
- Leptin M 1999. Gastrulation in Drosophila: the logic and the cellular mechanisms. EMBO J. 18:3187–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M, Grunewald B. 1990. Cell shape changes during gastrulation in Drosophila. Development 110:73–84 [DOI] [PubMed] [Google Scholar]
- Lillie FR. 1908. The Development of the Chick. New York: Henry Holt & Co. [Google Scholar]
- Lim B, Levine M, Yamazaki Y. 2017. Transcriptional pre-patterning of Drosophila gastrulation. Curr. Biol 27:286–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Starling C, Gridley T. 2009. Epiblast-specific Snai1 deletion results in embryonic lethality due to multiple vascular defects. BMC Res. Notes 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, et al. 2007. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. PNAS 104:10069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Goldstein B. 2014. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141:1987–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457:495–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason FM, Tworoger M, Martin AC. 2013. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol 15:926–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Theveneau E. 2013. The neural crest. Development 140:2247–51 [DOI] [PubMed] [Google Scholar]
- McElroy M, Kaushal S, Bouvet M, Hoffman RM. 2008. Color-coded imaging of splenocyte-pancreatic cancer cell interactions in the tumor microenvironment. Cell Cycle 7:2916–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Reeves GT, Supatto W, Stathopoulos A. 2010. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development 137:2167–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeotte I, Grego-Bessa J, Anderson KV. 2011. Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo. Development 138:3011–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrossilis D, Roper JC, Le Roy D, Driquez B, Michel A, et al. 2017. Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun 8:13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Maruhashi M, Van De Putte T, Kondoh H, Huylebroeck D, Higashi Y. 2006. Complementary expression pattern of Zfhx1 genes Sip1 and δEF1 in the mouse embryo and their genetic interaction revealed by compound mutants. Dev. Dyn 235:1941–52 [DOI] [PubMed] [Google Scholar]
- Moly PK, Cooley JR, Zeltzer SL, Yatskievych TA, Antin PB. 2016. Gastrulation EMT is independent of P-cadherin downregulation. PLOS ONE 11:e0153591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Gridley T. 2006. Snail family genes are required for left–right asymmetry determination, but not neural crest formation, in mice. PNAS 103:10300–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125:2963–71 [DOI] [PubMed] [Google Scholar]
- Nakajima H, Tanoue T. 2010. Epithelial cell shape is regulated by Lulu proteins via myosin-II. J. Cell Sci 123:555–66 [DOI] [PubMed] [Google Scholar]
- Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, et al. 2004. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin. Cancer Res 10:4125–33 [DOI] [PubMed] [Google Scholar]
- Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, et al. 2012. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21:488–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. 2008. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev. Growth Differ 50:755–66 [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. 2013. EMT in developmental morphogenesis. Cancer Lett. 341:9–15 [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Sheng G. 2013. Epiblast integrity requires CLASP and Dystroglycan-mediated microtubule anchoring to the basal cortex. J. Cell Biol 202:637–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. 2008. RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nat. Cell Biol 10:765–75 [DOI] [PubMed] [Google Scholar]
- Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, et al. 2012. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. PNAS 109:E2595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. 1994. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264:835–39 [DOI] [PubMed] [Google Scholar]
- Nishino H, Takano S, Yoshitomi H, Suzuki K, Kagawa S, et al. 2017. Grainyhead-like 2 (GRHL2) regulates epithelial plasticity in pancreatic cancer progression. Cancer Med. 6:2686–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka R, Itoh S, Gui T, Gai Z, Oikawa K, et al. 2010. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo. Exp. Mol. Pathol 89:149–57 [DOI] [PubMed] [Google Scholar]
- Nobis M, Herrmann D, Warren SC, Kadir S, Leung W, et al. 2017. A RhoA-FRET biosensor mouse for intravital imaging in normal tissue homeostasis and disease contexts. Cell Rep. 21:274–88 [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Hadjantonakis AK. 2010. Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr. Opin. Genet. Dev 20:420–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana OH, Coskun H, Minguillon C, Murawala P, Tanaka EM, et al. 2017. A right-handed signalling pathway drives heart looping in vertebrates. Nature 549:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M. 1998. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev. Biol 203:435–50 [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. 2004. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J. Cell Sci 117:1675–85 [DOI] [PubMed] [Google Scholar]
- Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, et al. 2018. Identification of the tumour transition states occurring during EMT. Nature 556:463–68 [DOI] [PubMed] [Google Scholar]
- Peinado H, Marin F, Cubillo E, Stark HJ, Fusenig N, et al. 2004. Snail and E47 repressors of E-cadherin induce distinct invasive and angiogenic properties in vivo. J. Cell Sci 117:2827–39 [DOI] [PubMed] [Google Scholar]
- Polyakov O, He B, Swan M, Shaevitz JW, Kaschube M, Wieschaus E. 2014. Passive mechanical forces control cell-shape change during Drosophila ventral furrow formation. Biophys. J 107:998–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakeman AS, Anderson KV. 2006. Axis specification and morphogenesis in the mouse embryo require Nap1, a regulator of WAVE-mediated actin branching. Development 133:3075–83 [DOI] [PubMed] [Google Scholar]
- Ramkumar N, Anderson KV. 2011. SnapShot: mouse primitive streak. Cell 146:488.e1–2 [DOI] [PubMed] [Google Scholar]
- Ramkumar N, Harvey BM, Lee JD, Alcorn HL, Silva-Gagliardi NF, et al. 2015. Protein O-glucosyltransferase 1 (POGLUT1) promotes mouse gastrulation through modification of the apical polarity protein CRUMBS2. PLOS Genet. 11:e1005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar N, Omelchenko T, Silva-Gagliardi NF, McGlade CJ, Wijnholds J, Anderson KV. 2016. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat. Cell Biol 18:1281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray HJ, Niswander LA. 2016. Grainyhead-like 2 downstream targets act to suppress epithelial-to-mesenchymal transition during neural tube closure. Development 143:1192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, et al. 2012. EMT and dissemination precede pancreatic tumor formation. Cell 148:349–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsma L, Steller EJ, Beerling E, Loomans CJ, Zomer A, et al. 2012. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci. Transl. Med 4:158ra45. [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, et al. 2012. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 335:1232–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozbicki E, Chuai M, Karjalainen AI, Song F, Sang HM, et al. 2015. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol 17:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. 2010. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol 341:5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Narasimha M, Vogelsang E, Leptin M. 2014. Cadherin switching during the formation and differentiation of the Drosophila mesoderm—implications for epithelial-to-mesenchymal transitions. J. Cell Sci 127:1511–22 [DOI] [PubMed] [Google Scholar]
- Seher TC, Narasimha M, Vogelsang E, Leptin M. 2007. Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev 124:167–79 [DOI] [PubMed] [Google Scholar]
- Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, et al. 2014. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol 204:839–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook D, Keller R. 2003. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech. Dev 120:1351–83 [DOI] [PubMed] [Google Scholar]
- Soo K, O’Rourke MP, Khoo PL, Steiner KA, Wong N, et al. 2002. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev. Biol 247:251–70 [DOI] [PubMed] [Google Scholar]
- Sun J, Stathopoulos A. 2018. FGF controls epithelial-mesenchymal transitions during gastrulation by regulating cell division and apicobasal polarity. Development 145:dev161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Moribe H, Kondoh H, Higashi Y. 1998. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 125:21–31 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–90 [DOI] [PubMed] [Google Scholar]
- Toneff MJ, Sreekumar A, Tinnirello A, Hollander PD, Habib S, et al. 2016. The Z-cad dual fluorescent sensor detects dynamic changes between the epithelial and mesenchymal cellular states. BMC Biol. 14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad RL, Hay ED, Revel JD. 1967. Cell contact during early morphogenesis in the chick embryo. Dev. Biol 16:78–106 [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Revel JP, Hay ED. 1966. Tight junctions between cells in the early chick embryo as visualized with the electron microscopy. J. Cell Biol 31:C6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, et al. 2003. Mice lacking Zfhx1b, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am. J. Med. Genet 72:465–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez CG, Tworoger M, Martin AC. 2014. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J. Cell Biol 206:435–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennin C, Herrmann D, Lucas MC, Timpson P. 2016. Intravital imaging reveals new ancillary mechanisms co-opted by cancer cells to drive tumor progression. F1000Research 5:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. 2007. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449:1049–52 [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bodenstein L, Lau IJ, Stern CD. 2014. Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. eLife 3:e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, et al. 2009. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 137:361–71.e5 [DOI] [PubMed] [Google Scholar]
- Wang J, Nikhil K, Viccaro K, Chang L, Jacobsen M, et al. 2017. The Aurora-A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J. Cell Sci 130:1078–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman EL, Liu CJ, Fearon ER, Margolis B. 2008. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27:3875–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Burdsal C, Periasamy A, Lewandoski M, Sutherland A. 2012. Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev. Dyn 241:270–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Vogelsang E, Leptin M. 2005. FGF signalling and the mechanism of mesoderm spreading in Drosophila embryos. Development 132:491–501 [DOI] [PubMed] [Google Scholar]
- Yang AD, Camp ER, Fan F, Shen L, Gray MJ, et al. 2006. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 66:46–51 [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, et al. 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–39 [DOI] [PubMed] [Google Scholar]
- Ye X, Brabletz T, Kang Y, Longmore GD, Nieto MA, et al. 2017. Upholding a role for EMT in breast cancer metastasis. Nature 547:E1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, et al. 2015. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525:256–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhu X, Cui K, Mancuso J, Federley R, et al. 2016. In vivo visualization and characterization of epithelial-mesenchymal transition in breast tumors. Cancer Res. 76:2094–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, et al. 2015. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527:525–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. 2006. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125:957–69 [DOI] [PubMed] [Google Scholar]


