Abstract
Introduction
Deficits in goal-directed behavior are common in individuals with Parkinson's disease (PD) and have been ascribed to apathy. In addition to apathy, individuals' beliefs in their competence (self-efficacy) and capacity to regulate emotions, thoughts, and actions (self-regulation) are critical skills for goal-directed behavior. We investigated these skills and their relationship to motor and non-motor symptoms in individuals with PD. We also examined the neural correlates of these skills using functional magnetic resonance imaging (fMRI).
Methods
We enrolled 35 subjects with mild PD (Hoehn and Yahr stage ≤2.5) and used the new general self-efficacy (NGSES) and self-regulation scales (SRS). We correlated the scores on these scales with measures of cognition, anxiety, depression, apathy, fatigue, quality of life, and disease burden using stepwise regression analyses. We collected resting-state fMRI data in a 3-Tesla scanner and computed the pairwise functional connectivity among nodes of major networks. We correlated the connectivity maps with the NGSES and SRS scores.
Results
Our PD cohort demonstrated intact NGSES and SRS scores compared with respective population data. These scores showed significant negative correlation with apathy and disease burden. Stronger connectivity in the salience network and decoupling from the default mode network supported self-efficacy and self-regulation.
Conclusions
Self-efficacy and self-regulation capacity seems preserved, but vulnerable to disease-related factors in individuals with mild PD. Educational programs cultivating this capacity could improve the coping skills of these individuals. Functional connectivity changes in salience and default mode networks may serve as neurobiological markers to demonstrate the effectiveness of such interventions.
Keywords: Parkinson's disease, Functional MRI, Neural networks, Apathy
Highlights
-
•
Self-efficacy and self-regulation skills are critical for goal-directed behavior.
-
•
These skills and their neural bases were tested in mild Parkinson's disease.
-
•
These skills are intact and correlate negatively with apathy and disease burden.
-
•
Functional connectivity among nodes of major neural networks supports these skills.
-
•
These skills can be cultivated in educational programs for better health outcomes.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by motor and non-motor (e.g., anxiety, depression, fatigue) symptoms. Like many chronic and progressive neurological conditions, PD has a negative impact not only on the physical, but also on the psychological and social wellbeing of individuals. A distinct feature of PD is its propensity to impair goal-directed behavior, also known as apathy [1]. Apathy is a syndrome with subdomains including lack of motivation, emotional distress, executive dysfunction, and auto-activation deficits (i.e., inability to self-activate mental processes) [1,2]. Each subdomain is associated with deficits in specific limbic, motor, and cognitive frontostriatal circuits and neurotransmitter systems, all of which can be affected in PD [2,3].
While this multi-domain approach to apathy in PD provides a useful psycho-neurobiological framework, goal-directed behavior is complex and cannot be reduced to emotional/motivational or cognitive factors. It also requires a metacognitive capacity to reflect on one's capabilities, thoughts, and actions; and to regulate oneself in the face of challenges. This capacity has been conceptualized under two related psychological constructs, namely, perceived self-efficacy (from here on, self-efficacy) and self-regulation. In this study, we aimed to expand the scope of investigation of deficits in goal-directed behavior in individuals with PD beyond apathy and evaluated their capacity for self-efficacy and self-regulation.
Self-efficacy is a concept rooted in social cognitive theory. According to this theory, human beings are not just reactive organisms shaped by external events, but active agents who can intentionally shape events and courses their lives take [4]. Self-efficacy is defined as one's perceived capacity to mobilize the motivation and cognitive resources to exert control over events [5,6]. People of high self-efficacy set motivating goals for themselves and figure out ways to overcome obstacles. Self-efficacy is also a major determinant of health outcomes. For example, in chronic neurological conditions including PD, specific self-efficacy measures (e.g., managing daily activities) were found to be better predictors of global health and quality of life than disease severity or diagnosis [7].
Self-regulation refers to processes that enable people to guide their goal-directed activities over time and across changing circumstances [8]. Successful self-regulation requires motivation to achieve the goal, management of stress and somatic factors (e.g., fatigue), and intact executive functioning to maintain goal direction [9]. A specific form of self-regulation is attention control, which is the ability to focus attention on a task and control external and internal distractions [10].
In the context of goal-directed behavior, self-efficacy would facilitate goal selection and commitment, and the belief that one has the capacity to achieve the goal. Subsequently, self-regulatory processes would help to direct motivational and cognitive resources toward the goal; control distractions, and maintain goal-directedness and adaptability in challenging situations.
All of these regulatory processes involved in goal-directed behavior clearly require intact emotional and interoceptive processes, and also cognitive resources and top-down control, which are closely linked to fundamental executive functions subserved by the frontal executive brain networks [9].
We aimed to explore the capacity of individuals with PD for self-efficacy and self-regulation, examine the relationship between these skills and emotional, motivational, and cognitive factors as well as disease burden; and finally, to investigate the neural correlates of self-regulation and self-efficacy using resting-state functional MRI (fMRI). We were particularly interested in the interplay between the dorsal attention (goal-oriented behavior), default mode (internally-directed mental processes), and salience (“switch” between the first two) networks. A comprehensive understanding of the role of self-efficacy and self-regulation skills in goal-directed behavior in individuals with PD has clinical implications. These skills can be cultivated in patient-oriented education programs to improve coping mechanisms and health outcomes.
2. Material and methods
2.1. Subjects
All subjects participated in the study after giving written informed consent in accordance with the procedures approved by the Human Research Protection Office of the Yale School of Medicine. Subjects were recruited primarily through the Movement Disorders Clinic at the Yale School of Medicine and the Connecticut Advocates for Parkinson's group. The study was conducted at the Yale Magnetic Resonance Research Center. All subjects underwent an initial screening for medical history and MRI safety.
We included 35 subjects with a diagnosis of idiopathic PD according to the UK PD Brain Bank criteria [11] and on a stable PD medication regimen for at least one month. We excluded subjects with PD who met any of the following criteria: They were not fully independent (n = 1), had a neurological or psychiatric disorder (other than PD and comorbid depression or anxiety), had a medical condition that might affect the central nervous system, had a history of alcohol or illicit drug abuse, had a history of head injury resulting in loss of consciousness (n = 1), had dementia (Montreal Cognitive Assessment (MoCA) score < 21) [12], had contraindications for MRI.
2.2. Clinical data collection and analysis
We assessed disease severity and stage using the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) [13] and the Hoehn and Yahr (H & Y) scale [14]. The cut-off for H & Y for inclusion was ≤2.5 (i.e., mild bilateral disease with some impairment in balance) to ensure that subjects were fully independent and could tolerate being off medication. We examined the subjects in the morning when they were off dopaminergic medications for 12 h and collected the MRI scans after they took the first dose of their dopaminergic medication (3.5 h ± 1.0).
We administered the Spielberger Trait Anxiety Inventory (STAI-T) [15], Beck Depression Inventory-II (BDI-II) [16], Starkstein Apathy Scale [17], Parkinson's Fatigue Scale (PFS-16) [18], and PD Quality of Life Questionnaire (PDQ-39) [19].
We used the Self-Regulation Scale (SRS) [20], which evaluates individuals' overall skills when employing attention control (Supplementary Material). The psychometric properties of the SRS have been validated in young, middle-aged, and older adults [10]. We used the New General Self-Efficacy Scale (NGSES) [6], which is a measure of individuals' belief in their overall competence to perform in a variety of situations (Supplementary Material). The psychometric properties of NGSES have been validated in young adults [6,21].
We assessed the normality of distribution of the scores using the Shapiro-Wilk test. The mean values and standard deviations (SD) of the normally distributed, and median values and median absolute deviations of the non-normally distributed scores were compared with population mean scores of the respective tests, when applicable, using one-sample t-tests (p < 0.05, two-tailed).
We also performed stepwise linear regression analyses to explore the relationship between motor and non-motor features of PD and self-regulation and self-efficacy. We created two separate models in which SRS and NGSES scores were used as independent variables and age, disease duration, MoCA, STAI-T, BDI-II, apathy, PFS-16, PD Summary Index (PDSI) based on the PDQ-39 scores, and MDS-UPDRS total scores were entered as predictor variables in a stepwise manner (p < 0.05, two-tailed).
We used the SPSS 26 software for statistical analyses.
2.3. MRI data collection and analysis
We collected the MRI data in a 3.0 Tesla Siemens Trio TIM human research scanner using a 32-channel head coil.
We collected high-resolution T1-weighted MPRAGE anatomical images (176 slices, slice thickness: 1 mm, in-plane resolution: 1 × 1 mm, FoV: 250 mm, Matrix: 256 × 256, TR: 1900 ms, TE: 2.52 ms, TI: 900 ms, flip angle: 9 degrees) for an accurate localization of the fMRI data in the beginning of each scan session. Then, we obtained axial T2-weighted, echo planar functional images at rest for 10 min and 8 s (36 slices, slice thickness: 4 mm, no spacing; in-plane resolution: 3.5 × 3.5 mm, FoV: 224 mm, Matrix: 64 × 64, TR: 2000 ms, TE: 25 ms, flip angle: 90 degrees, number of acquisitions: 304). We instructed the subjects to keep their eyes closed, avoid any voluntary movement, and let their mind wander. We assessed wakefulness at the end of the scan by subjects' report.
We used the Connectivity toolbox for the resting-state data analysis [22]. Preprocessing steps included the removal of the first four scans to reach magnetization steady state, motion correction, outlier detection, coregistration of functional scans with the anatomical scan, normalization to the standard MNI template, and smoothing with an 8-mm kernel to account for inter-individual anatomical variability. De-noising steps included the elimination of signal originating from the white matter and cerebrospinal fluid, regression of motion artifacts and outliers from the time series, scrubbing, and quadratic detrending. Finally, we bandpass-filtered (0.008 < f < 0.1 Hz) the data to capture the fluctuations of the blood oxygenation level-dependent (BOLD) signal that typically occur within this frequency range at rest.
We used the functionally defined nodes of major networks (default mode, dorsal attention, salience, sensorimotor, visual, language, and cerebellar) in the Connectivity toolbox for the functional connectivity analyses [22]. For each subject, we extracted the BOLD signal time courses from these nodes and correlated them with each other using Pearson correlations. The “r” values corresponded to the functional connectivity strength between node pairs. We Fisher z-transformed the “r” values and obtained group-level functional connectivity maps for statistical analyses. Finally, we used the SRS and NGSES scores as the covariates of interest and age as a covariate of no interest in separate models to be correlated with these maps. We used the false discovery rate (FDR) method for correction for multiple comparisons (p < 0.05). In an exploratory analysis, we used the apathy scores alone as the covariate of interest for correlation with these maps.
3. Results
3.1. Demographic and clinical
Table 1 summarizes the data. The detailed statistical results are shown in the Supplementary Table 1. Briefly, compared to the respective population means, our PD cohort did not have significant apathy, depression, anxiety, or fatigue, and reported good quality of life. The SRS and NGSES scores were also not significantly different from those of the respective population mean scores [6,10]. The SRS and NGSES scores showed significant positive correlation (Spearman's rho = 0.62, p = 0.000). In the stepwise linear regression analyses, only the MDS-UPDRS total and apathy scores showed significant negative correlations with the SRS and NGSES scores (Table 2). The total MDS-UPDRS and apathy scores together accounted for 65.6% and 60.3% of variance in the NGSES and SRS scores, respectively (NGSES: F (2, 32) = 30.45, p = 0.000 and SRS: F (2, 32) = 24.34, p = 0.000).
Table 1.
Demographic and clinical data (N = 35).
| Age (yr) | 64.3 ± 8.7 |
| Gender | 10 F, 25 M |
| Handedness | 33 R, 2 L |
| Onset side | 22 R, 13 L |
| Disease duration (yr) | 6.2 ± 3.8 |
| H & Y | 2.0 ± 0.0 |
| MDS-UPDRS | |
| Part I | 8.5 ± 4.1 |
| Part II | 11.2 ± 5.4 |
| Part III | 27.7 ± 7.3 |
| Part IV | 1.9 ± 2.6 |
| Total | 49.4 ± 14.3 |
| LEDD (mg) | 523.8 ± 335.3 |
| MoCA | 27.9 ± 1.9 |
| STAI-T | 34.5 ± 10.5 |
| BDI-II | 7.6 ± 7.2 |
| Apathy | 8.7 ± 5.2 |
| PDSI | 17.5 ± 11.7 |
| PFS | 2.3 ± 0.9 |
| SRS | 30.8 ± 4.6 |
| NGSES | 4.1 ± 0.6 |
The age range was 45–79 years. Disease duration was defined as the time since the first motor symptom was noticeable to the subject. BDI-II: Beck Depression Inventory-II, H & Y: Hoehn & Yahr, LEDD: Levodopa Equivalent Daily Dose, MDS-UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale, MoCA: Montreal Cognitive Assessment battery, NGSES: New General Self-Efficacy Scale, PDSI: Parkinson's Disease Summary Index based on the PD Quality of Life-39 questionnaire, PFS: Parkinson's Fatigue Scale mean score, SRS: Self-Regulation Scale, STAI-T: Spielberger Trait Anxiety Inventory, F/M: Female/Male, L/R: Left/Right.
Table 2.
Stepwise linear regression results.
| t | p | |
|---|---|---|
| NGSES scores: | ||
| MDS-UPDRS total | −3.75 | 0.001 |
| Apathy | −4.67 | 0.000 |
| SRS scores: | ||
| MDS-UPDRS total | −2.99 | 0.005 |
| Apathy | −4.49 | 0.000 |
Both NGSES and SRS scores showed significant negative correlations with the MDS-UPDRS total (beta = −0.14 and −0.12, respectively) and apathy (beta = −0.49 and −0.49, respectively) scores. The beta coefficients are unstandardized. MDS-UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale, NGSES: New General Self-Efficacy Scale, SRS: Self-Regulation Scale.
3.2. Imaging
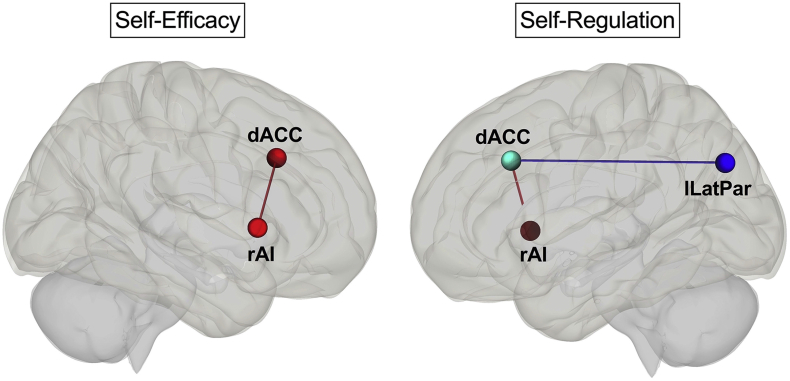
Table 3 shows the node pairs whose functional connectivity showed significant correlations with the SRS and NGSES scores and Fig. 1 shows the nodes. Of note, in the exploratory analysis, the apathy scores did not show a significant correlation with any of the pairwise functional connectivity values.
Table 3.
Correlations of SRS and NGSES scores with nodal pairwise functional connectivity.
| t | p | |
|---|---|---|
| SRS | ||
| Positive correlation: | ||
| dACC (SN) – rAI (SN) | 3.19 | 0.049 |
| Negative correlation: | ||
| dACC (SN) – lLatPar (DMN) | −3.69 | 0.025 |
| NGSES | ||
| Positive correlation: | ||
| dACC (SN) – rAI (SN) | 3.53 | 0.040 |
dACC: Dorsal anterior cingulate cortex, DMN: Default mode network, lLatPar: Left lateral parietal, NGSES: New general self-efficacy scale, rAI: Right anterior insula, SN: Salience network, SRS: Self-regulation scale. p values are FDR-corrected.
Fig. 1.
The pairwise functional connections that showed significant correlations with self-efficacy (NGSES) and self-regulation (SRS) scores (FDR-corrected, p < 0.05). The nodes and connections are displayed on sagittal Montreal Neurological Institute (MNI) brain templates. dACC: Dorsal anterior cingulate cortex, rAI: Right anterior insula, lLatPar: Left lateral parietal. Blue: Negative correlation, red: Positive correlation. The MNI coordinates of the nodes: dACC: x = 0, y = 22, z = 35; lLatPar: x = −39, y = −77, z = 33; rAI: x = 47, y = 14, z = 0. See Table 3 for statistics. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We did not find deficits in self-efficacy or self-regulation in our PD cohort compared with the respective population data [6,10]. The SRS and NGSES scores showed a significant positive correlation with each other suggesting that self-efficacy and self-regulation are related psychological constructs. Considering the characteristics of our PD cohort, we think that our findings are in line with the results of previous studies. In a study assessing self-efficacy for managing daily activities, the responses of a large cohort with chronic medical and neurological conditions were highly skewed to the “very confident” level. A possible explanation for this ceiling effect provided by the authors was that the medical conditions of these individuals might be good enough to live in the community [23]. In another recent study investigating self-efficacy measures in various chronic neurological conditions, lower self-efficacy for managing daily activities and symptoms was found in disorders with progressive symptoms including neuropathy and PD. Overall, low income and greater disease severity were also found to be associated with lower self-efficacy [7]. Our PD cohort comprised participants with mild bilateral disease who were high-functioning and community-dwelling individuals with financial stability and family support. All were followed by movement disorders specialists and were well-informed about PD. Presumably, these factors are associated with their preserved capacity for self-efficacy and self-regulation.
It is not surprising that the apathy and MDS-UPDRS total scores correlated negatively with the NGSES and SRS scores. The motivation and ability to work toward a goal are fundamental for optimal self-efficacy and self-regulation capacity. This finding further indicates that the confidence of our PD cohort in their self-efficacy and self-regulation capacity is grounded in realistic insights regarding their affect and abilities. We did not find a significant correlation between any of the other predictor variables and NGSES and SRS scores. This is partly because our PD cohort reported good quality of life, did not suffer from fatigue, and had no significant depression or anxiety. We did not specifically assess executive functioning, however, the mean MoCA score indicates that overall cognitive functioning was also intact in our PD cohort. Another possible reason is that the scales used here, except trait-anxiety, measure states in a relatively short timeframe (up to one month) whereas the NGSES and SRS are measures of trait-like features that are not specific to any situation or domain of life. These scales conceptualize self-efficacy and self-regulation as relatively stable personality variables [10,21]. A trait-like approach to self-efficacy and self-regulation has received criticism with the argument that the scales should reflect situation-specific, state-like measures; not decontextualized, generalized measures [4]. On the other hand, the trait-like dimension is thought to be more resistant to short-lived influences and more influenced by accumulated life experiences [6,10]. Our results suggest that the NGSES and SRS captured the stable personality traits of our PD cohort and demonstrated their vulnerability to specific factors in the context of a chronic neurological condition.
The NGSES and SRS scores also demonstrated correlations with the functional connectivity between the hubs of major resting-state networks including the salience network (SN) and default mode network (DMN). The DMN is also known as the task-negative network. The traditional DMN functions include self-related, internally oriented mental processes [24]. In contrast, the dorsal attention network (DAN), known as the task-positive network, is involved in goal-oriented tasks [24]. Finally, the SN is involved in detecting salient internal and external events [25] and acts as a “switch” between the DMN and DAN. Its main constituents including the right anterior insula (rAI) and dorsal anterior cingulate cortex (dACC) play a critical role in disengaging from internally-oriented mental focus (mediated by the DMN) and reallocating the attentional resources to goal-directed processes (mediated by the DAN) [26].
The NGSES and SRS scores of our cohort showed significant positive correlations with the functional connectivity between the dACC and rAI. The SRS scores also showed a significant negative correlation with the functional connectivity between the dACC and left lateral parietal cortex, which is a major node of the DMN. These results suggest that attentional self-regulation and self-efficacy require a strong salience-detection mechanism in order to switch efficiently from a self-referential to a goal-oriented mental state. This mechanism seems to be subserved by the coupling among the nodes of the SN and decoupling of the SN from the DMN. Furthermore, the coordinated activity of the rAI and dACC is also crucial for generating appropriate responses to internal and external events based on one's awareness [27]. The successful integration of awareness with response is fundamental for self-regulation and self-efficacy. More specifically, the rAI integrates the physiological, affective, and cognitive states, thereby, plays a critical role in creating body- and self-awareness [27]. This awareness is thought to serve as the basis for the selection of and preparation for context-specific responses facilitated by the dACC [28]. For example, the sense of self-agency during voluntary movements [29] and making intentional action decisions [30] have been found to engage the AI and dACC. In line with these findings, our results suggest that successful self-regulation and self-efficacy are also supported by the strong coupling of the rAI-dACC.
Interestingly, the apathy scores did not show a significant correlation with the functional connectivity between any node pair. Yet, altered metabolism in limbic regions including the ventral striatum, orbitofrontal cortex, and ACC [3]; and reduced resting-state functional connectivity within these limbic circuits have been shown in patients with PD and apathy [31]. The fact that our PD cohort as a group was not apathetic might underlie the lack of a relationship between apathy scores and functional connectivity. More importantly, our findings indicate that self-efficacy and self-regulation are sensitive measures for goal-directed behavior and map onto specific neural correlates even in the absence of apathy.
This exploratory study has several limitations. The NGSES and SRS are not validated tools for use in clinical populations including PD. Moreover, the validity of generalized trait as opposed to situation-dependent state measurements of self-efficacy and self-regulation is still debated [4,6,10]. We performed our statistical analyses based on the population data from middle-aged and older adults for the SRS, but the population data were obtained from young adults and a small group of older managers for the NGSES. Our cohort size is relatively small and consists of individuals with mild disease limiting the generalizability of our findings. Future studies should investigate self-efficacy and self-regulation in larger cohorts of PD stratified based on disease stage and use both trait and state measurements.
In conclusion, our findings suggest that self-efficacy and self-regulation are valuable psychological constructs that provide a more holistic social-psychological-neurobiological framework in understanding the individuals with PD not only as patients, but also as agents with the capacity to control and change their circumstances despite their disease-specific vulnerabilities. These constructs also contribute to a more comprehensive real-life evaluation of individuals' metacognitive skills beyond the standard neurocognitive tests which are confined to controlled settings [32]. The correlative nature of our study precludes causal inferences, but it is tempting to think that the high-functioning status of our PD cohort might be related to their self-efficacy and self-regulation capacity consistent with previous reports demonstrating that these measures are strong predictors of global health and quality of life [7]. Importantly, this capacity should not be taken as a rigid personality trait, but rather as a plastic moderator facilitating adaptive behavior. Thus, educational programs for individuals with PD to improve self-efficacy and self-regulation skills could be beneficial in coping with various challenges [33]. Our imaging results may serve as neurobiological markers to demonstrate the effectiveness of such interventions.
Acknowledgments
Acknowledgment
This work was supported by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science, a component of the National Institutes of Health (NIH), USA. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Authors' roles
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Declaration of competing interest
Sule Tinaz has received the National Center for Advancing Translational Science Award (CTSA Grant Number UL1 TR001863), USA and the National Institute of Neurological Disorders and Stroke Award (K23 NS099478), USA; she has also received research support from the Connecticut Advocates for Parkinson's group.
Mohamed Elfil reports no disclosures. Serageldin Kamel reports no disclosures. Sai S. Aravala reports no disclosures.
Elan D. Louis has received research support from the National Institute of Neurological Disorders and Stroke, USA; he has also received support from the Claire O'Neil Essential Tremor Research Fund (Yale University); he serves on the Clinical Advisory Board of SAGE Therapeutics, the Clinical Advisory Board for CADENT Therapeutics, and receives publishing royalties from Elsevier for Merritt's Neurology.
Rajita Sinha has received research support from the National Institute on Alcohol Abuse and Alcoholism, USA and National Institute of Diabetes and Digestive and Kidney Diseases, USA; she has also received support from the National Center for Advancing Translational Science, USA; she is on the Scientific Advisory Board for Embera Neurotherapeutics, Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prdoa.2020.100051.
Contributor Information
Sule Tinaz, Email: sule.tinaz@yale.edu.
Mohamed Elfil, Email: mohamed.elfil@yale.edu.
Serageldin Kamel, Email: serageldin.attia@yale.edu.
Sai S. Aravala, Email: sai.aravala@yale.edu.
Elan D. Louis, Email: elan.louis@yale.edu.
Rajita Sinha, Email: rajita.sinha@yale.edu.
Appendix A. Supplementary data
Supplementary Material
References
- 1.Levy R., Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 2.Pagonabarraga J., Kulisevsky J., Strafella A.P., Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- 3.Le Heron C., Apps M.A.J., Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118:54–67. doi: 10.1016/j.neuropsychologia.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandura A. On the functional properties of perceived self-efficacy revisited. J. Manage. 2012;38:9–44. doi: 10.1177/0149206311410606. [DOI] [Google Scholar]
- 5.Bandura A. Toward a psychology of human agency: pathways and reflections. Perspect. Psychol. Sci. 2018;13:130–136. doi: 10.1177/1745691617699280. [DOI] [PubMed] [Google Scholar]
- 6.Chen G., Gully S.M., Eden D. Validation of a new general self-efficacy scale. Organ. Res. Methods. 2001;4:62–83. doi: 10.1177/109442810141004. [DOI] [Google Scholar]
- 7.Shulman L.M., Velozo C., Romero S., Gruber-Baldini A.L. Comparative study of PROMISⓇ self-efficacy for managing chronic conditions across chronic neurologic disorders. Qual. Life Res. 2019;28:1893–1901. doi: 10.1007/s11136-019-02164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karoly P. Mechanisms of self-regulation: a systems view. Annu. Rev. Psychol. 1993;44:23–52. doi: 10.1146/annurev.psych.44.1.23. [DOI] [Google Scholar]
- 9.Hofmann W., Schmeichel B.J., Baddeley A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Diehl M., Semegon A.B., Schwarzer R. Assessing attention control in goal pursuit: a component of dispositional self-regulation. J. Pers. Assess. 2006;86:306–317. doi: 10.1207/s15327752jpa8603_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 13.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N., Agarwal P., Athar S., Bordelan Y., Bronte-Stewart H.M., Camicioli R., Chou K., Cole W., Dalvi A., Delgado H., Diamond A., Dick J.P., Duda J., Elble R.J., Evans C., Evidente V.G., Fernandez H.H., Fox S., Friedman J.H., Fross R.D., Gallagher D., Goetz C.G., Hall D., Hermanowicz N., Hinson V., Horn S., Hurtig H., Kang U.J., Kleiner-Fisman G., Klepitskaya O., Kompoliti K., Lai E.C., Leehey M.L., Leroi I., Lyons K.E., McClain T., Metzer S.W., Miyasaki J., Morgan J.C., Nance M., Nemeth J., Pahwa R., Parashos S.A., Schneider J.S.J.S., Schrag A., Sethi K., Shulman L.M., Siderowf A., Silverdale M., Simuni T., Stacy M., Stern M.B., Stewart R.M., Sullivan K., Swope D.M., Wadia P.M., Walker R.W., Walker R., Weiner W.J., Wiener J., Wilkinson J., Wojcieszek J.M., Wolfrath S., Wooten F., Wu A., Zesiewicz T.A., Zweig R.M. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 14.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 15.Spielberger C.D., Gorsuch R.L., Lushene R.E., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; California: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 16.Beck A.T., Steer R.A., Brown K.G. first ed. Psychological Corporation; Texas: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 17.Starkstein S.E., Mayberg H.S., Preziosi T.J., Andrezejewski P., Leiguarda R., Robinson R.G. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 18.Brown R.G., Dittner A., Findley L., Wessely S.C. The Parkinson fatigue scale. Park. Relat. Disord. 2005;11:49–55. doi: 10.1016/j.parkreldis.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson C., Fitzpatrick R., Peto V., Greenhall R., Hyman N. 1997. The Parkinson’s Disease Questionnaire (PDQ-39): Development and Validation of a Parkinson’s Disease Summary Index Score, Age Ageing. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzer R., Diehl M., Schmitz G.S. Self-Regulation Scale, Retrieved Oct. 1999. http://userpage.fu-berlin.de/~health/selfreg_e.htm
- 21.Scherbaum C.A., Cohen-Charash Y., Kern M.J. Measuring general self-efficacy: a comparison of three measures using item response theory. Educ. Psychol. Meas. 2006;66:1047–1063. doi: 10.1177/0013164406288171. [DOI] [Google Scholar]
- 22.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 23.Hong I., Velozo C.A., Li C.Y., Romero S., Gruber-Baldini A.L., Shulman L.M. Assessment of the psychometrics of a PROMIS item bank: self-efficacy for managing daily activities. Qual. Life Res. 2016;25:2221–2232. doi: 10.1007/s11136-016-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medford N., Critchley H.D. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brass M., Haggard P P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct. Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- 29.Farrer C., Frith C.D. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- 30.Zapparoli L., Seghezzi S., Paulesu E. The what, the when, and the whether of intentional action in the brain: a meta-analytical review. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baggio H.C., Segura B., Garrido-Millan J.L., Marti M.J., Compta Y., Valldeoriola F., Tolosa E., Junque C. Resting-state frontostriatal functional connectivity in Parkinson’s disease-related apathy. Mov. Disord. 2015;30:671–679. doi: 10.1002/mds.26137. [DOI] [PubMed] [Google Scholar]
- 32.Combs H.L., Garcia-Willingham N.E., Berry D.T.R., van Horne C.G., Segerstrom S.C. Psychological functioning in Parkinson’s disease post-deep brain stimulation: self-regulation and executive functioning. J. Psychosom. Res. 2018;111:42–49. doi: 10.1016/j.jpsychores.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Cook D., McRae C., Crist K. 5th World Park. Congr., Kyoto, Japan. 2019. Impact of a self-efficacy enhancing program for recently diagnosed persons with Parkinson’s disease and their care partners. (p. Abstract (P37.03)) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material