PURPOSE
To evaluate the addition of the humanized monoclonal antiprogrammed death ligand-1 (PD-L1) antibody, atezolizumab, to platinum-based chemotherapy and bevacizumab in newly diagnosed stage III or IV ovarian cancer (OC).
METHODS
This multicenter placebo-controlled double-blind randomized phase III trial (ClinicalTrials.gov identifier: NCT03038100) enrolled patients with newly diagnosed untreated International Federation of Gynecology and Obstetrics (FIGO) stage III or IV OC who either had undergone primary cytoreductive surgery with macroscopic residual disease or were planned to receive neoadjuvant chemotherapy and interval surgery. Patients were stratified by FIGO stage, Eastern Cooperative Oncology Group performance status, tumor immune cell PD-L1 staining, and treatment strategy and randomly assigned 1:1 to receive 3-weekly cycles of atezolizumab 1,200 mg or placebo (day 1, cycles 1-22), with paclitaxel plus carboplatin (day 1, cycles 1-6) plus bevacizumab 15 mg/kg (day 1, cycles 2-22), omitting perioperative bevacizumab in neoadjuvant patients. The co-primary end points were investigator-assessed progression-free survival and overall survival in the intention-to-treat and PD-L1–positive populations.
RESULTS
Between March 8, 2017, and March 26, 2019, 1,301 patients were enrolled. The median progression-free survival was 19.5 versus 18.4 months with atezolizumab versus placebo, respectively (hazard ratio, 0.92; 95% CI, 0.79 to 1.07; stratified log-rank P = .28), in the intention-to-treat population and 20.8 versus 18.5 months, respectively (hazard ratio, 0.80; 95% CI, 0.65 to 0.99; P = .038), in the PD-L1–positive population. The interim (immature) overall survival results showed no significant benefit from atezolizumab. The most common grade 3 or 4 adverse events were neutropenia (21% with atezolizumab v 21% with placebo), hypertension (18% v 20%, respectively), and anemia (12% v 12%).
CONCLUSION
Current evidence does not support the use of immune checkpoint inhibitors in newly diagnosed OC. Insight from this trial should inform further evaluation of immunotherapy in OC.
INTRODUCTION
Epithelial ovarian cancer (OC) is a leading cause of cancer-related mortality among women worldwide: it is estimated that in 2018, there were almost 185,000 deaths from OC globally.1 Standard-of-care therapy at initial diagnosis includes a combination of cytoreductive surgery and platinum–taxane chemotherapy. Adding the antiangiogenic agent bevacizumab to chemotherapy followed by maintenance bevacizumab significantly improves progression-free survival (PFS) for patients with advanced-stage OC and is a front-line therapy option in many countries, based on the results from the GOG-0218 and ICON7 phase III trials.2,3 More recently, benefit from poly(ADP-ribose) polymerase (PARP) inhibitors, particularly in patients with BRCA-mutant or homologous recombination-deficient (HRD) tumors, has been demonstrated in the SOLO-1,4 PAOLA-1,5 PRIMA,6 and VELIA7 phase III trials. Nevertheless, there remains room for improvement, particularly in women whose disease is unresponsive to chemotherapy alone or in whom maintenance PARP inhibition has limited benefit.
CONTEXT
Key Objective
Does adding the antiprogrammed death ligand-1 (PD-L1) antibody atezolizumab to a standard platinum-based chemotherapy regimen plus bevacizumab improve efficacy in patients with newly diagnosed stage III or IV ovarian cancer?
Knowledge Generated
In this placebo-controlled double-blind randomized phase III trial, atezolizumab did not significantly improve progression-free survival in either the intention-to-treat or the PD-L1–positive population. Post hoc exploratory analyses suggested an effect in patients with high PD-L1 expression; further exploration of this observation is warranted.
Relevance
Current evidence does not support adding PD-L1–targeted immune checkpoint inhibitors to the standard-of-care regimen for patients with newly diagnosed ovarian cancer.
Atezolizumab, a humanized monoclonal antibody targeting programmed death ligand-1 (PD-L1), has demonstrated significantly improved PFS and overall survival (OS) when combined with first-line bevacizumab-containing therapy for non–small-cell lung cancer8 and with bevacizumab versus single-agent sorafenib in hepatocellular carcinoma.9 Single-agent atezolizumab demonstrated encouraging activity and tolerability in the PCD4989g study, with sustained responses in OC.10
In tumors associated with increased vascular endothelial growth factor production, such as OC, vascular endothelial growth factor blockade may promote T-cell infiltration into the tumor bed and reduce immunosuppression within the tumor microenvironment, providing the rationale to combine immunotherapeutic and antiangiogenic strategies. The atezolizumab and bevacizumab combination demonstrated durable responses and a safety profile consistent with the known effects of the individual agents in a single-arm study (GP28328) in platinum-resistant OC.11
IMagyn050 is the first randomized trial to provide efficacy and safety results for the addition of an immune checkpoint inhibitor to standard-of-care bevacizumab-containing therapy in epithelial ovarian, fallopian tube, or primary peritoneal cancer. In the PCD4989g study, responses to single-agent atezolizumab were limited to patients whose tumors showed high PD-L1 expression,10 whereas responses to atezolizumab plus bevacizumab were seen irrespective of PD-L1 expression in the small OC cohort of the GP28328 study.11 This provided the rationale to evaluate outcomes in both PD-L1–positive and all-comer populations in IMagyn050. Here, we report the primary results from the IMagyn050 trial.
METHODS
Study Design
This global randomized double-blind placebo-controlled two-arm phase III trial (ClinicalTrials.gov identifier: NCT03038100) was conducted in North and South America, Europe, Asia, and Australia according to the guidelines of Good Clinical Practice and the principles of the Declaration of Helsinki. The Protocol (online only) was approved by institutional review boards or ethics committees at each site.
Patients
Eligible patients had newly diagnosed untreated International Federation of Gynecology and Obstetrics (FIGO) stage III or IV epithelial ovarian, fallopian tube, or primary peritoneal cancer and either had undergone primary cytoreductive surgery resulting in gross (macroscopic or palpable) residual disease or were planned to receive neoadjuvant therapy followed by interval surgery. Additional eligibility criteria included the following: age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; adequate hematologic, renal, and hepatic function; and availability of a representative formalin-fixed paraffin-embedded tumor specimen for evaluation of PD-L1 status before random assignment. Patients with borderline epithelial ovarian tumors, nonepithelial ovarian tumors, or recurrent OC treated with surgery alone were ineligible, as were patients with contraindications for bevacizumab and atezolizumab. All patients provided written informed consent before any trial-specific procedures or treatment.
Procedures
Eligible patients were randomly assigned 1:1, stratified by FIGO stage (III v IV), ECOG performance status (0 v 1/2), PD-L1 status (PD-L1–expressing immune cells [ICs] as percentage of tumor in < 1% v ≥ 1% [PD-L1–positive], assessed using VENTANA SP142 PD-L1 immunohistochemistry assay [VENTANA Medical Systems, Tucson, AZ]), and treatment strategy (primary cytoreductive surgery v neoadjuvant).
In the primary cytoreductive surgery cohort, eligible patients were randomly assigned within 42 days after primary surgery to receive either atezolizumab 1,200 mg or placebo on day 1 of cycles 1-22, combined with paclitaxel 175 mg/m2 and carboplatin area under the curve 6 on day 1 during cycles 1-6, and bevacizumab 15 mg/kg on day 1 during cycles 2-22. In the neoadjuvant cohort, eligible patients were randomly assigned before starting study therapy to receive either atezolizumab 1,200 mg or placebo on day 1 of cycles 1-22, both combined with paclitaxel and carboplatin during cycles 1-6 as above. Patients who underwent interval surgery (planned to occur between cycles 3 and 4) omitted both perioperative cycles of bevacizumab. In both cohorts, cycles were repeated every 3 weeks. Treatment was discontinued in the event of disease progression, unacceptable toxicity, or patient or physician decision to discontinue.
PD-L1 expression was determined in the baseline tumor tissue sample collected during primary cytoreductive surgery in the primary surgery cohort and from pretreatment tumor tissue samples in the neoadjuvant cohort. Additional tissue samples were collected at the time of interval surgery in the neoadjuvant cohort. In post hoc exploratory analyses, tumors with ≥ 5% PD-L1 IC expression were categorized as PD-L1–positive high. Samples were also evaluated for tumor cell (TC) staining, with < 1% TC staining considered to be PD-L1 TC–negative and ≥ 1% TC considered to be PD-L1 TC–positive.
In the primary cytoreductive surgery group, tumors were assessed by computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis within 28 days before random assignment, then every 9 weeks during the concurrent treatment phase, every 12 weeks in the maintenance phase, every 3 months for the first 2 years after completing treatment, and every 6 months for the next 3 years. Thereafter, patients were followed as clinically indicated. Patients in the neoadjuvant cohort followed a similar tumor assessment schedule; however, an additional tumor assessment was performed after interval surgery to determine a new baseline tumor status. The next scan was to be done 9 weeks later. Thereafter, the tumor assessment schedule matched that described for the primary cytoreductive surgery group.
Adverse events (AEs) were recorded at every cycle and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Outcomes
The co-primary end points were investigator-assessed PFS (according to RECIST v1.1) and OS in the intention-to-treat (ITT) population and in the population of patients with PD-L1–positive tumors.
Secondary end points included objective response rate (confirmed complete or partial response according to RECIST v1.1 in patients with measurable residual disease after primary surgery), duration of response in these patients, patient-reported outcomes, and the occurrence and severity of AEs.
Statistical Analysis
The planned sample size was 1,300 patients, calculated based on the number of deaths required to demonstrate improved OS in the PD-L1–positive and ITT populations.
PFS was tested in parallel in the PD-L1–positive and ITT populations (two-sided P = .002); OS was tested hierarchically (with the actual alpha spent dependent on the PFS results) first in the PD-L1–positive population; if statistical significance was reached, OS was tested further in the ITT population.12
The primary PFS analysis was prespecified to occur after approximately 601 PFS events in the ITT population and 347 PFS events in the PD-L1–positive subgroup. This provides 90% power to detect a PFS improvement with a hazard ratio (HR) of 0.70 in the ITT population and 91% power to detect an HR of 0.62 in the PD-L1–positive population, both with a two-sided significance level of 0.002. The first interim analysis of OS was prespecified to occur at the time of the primary PFS analysis.
PFS and OS were compared between treatment groups using stratified log-rank testing; HRs were estimated using a stratified Cox proportional hazards model and reported with associated 95% CIs. Kaplan-Meier methodology was used to estimate medians, and associated 95% CIs were calculated using Brookmeyer-Crowley methodology.
Efficacy was analyzed in all randomly assigned patients in the relevant populations (ITT and PD-L1–positive). Safety was analyzed in the safety-evaluable population, defined as all randomly assigned patients who received at least one dose of study drug, with patients analyzed according to the treatment actually received.
RESULTS
Between March 8, 2017, and March 26, 2019, 1,301 patients were enrolled and randomly assigned: 651 to atezolizumab plus bevacizumab plus chemotherapy and 650 to placebo plus bevacizumab plus chemotherapy; of these, 784 (60%) had PD-L1–positive tumors. Overall, 1,286 patients received at least one dose of study treatment (Fig 1).
FIG 1.
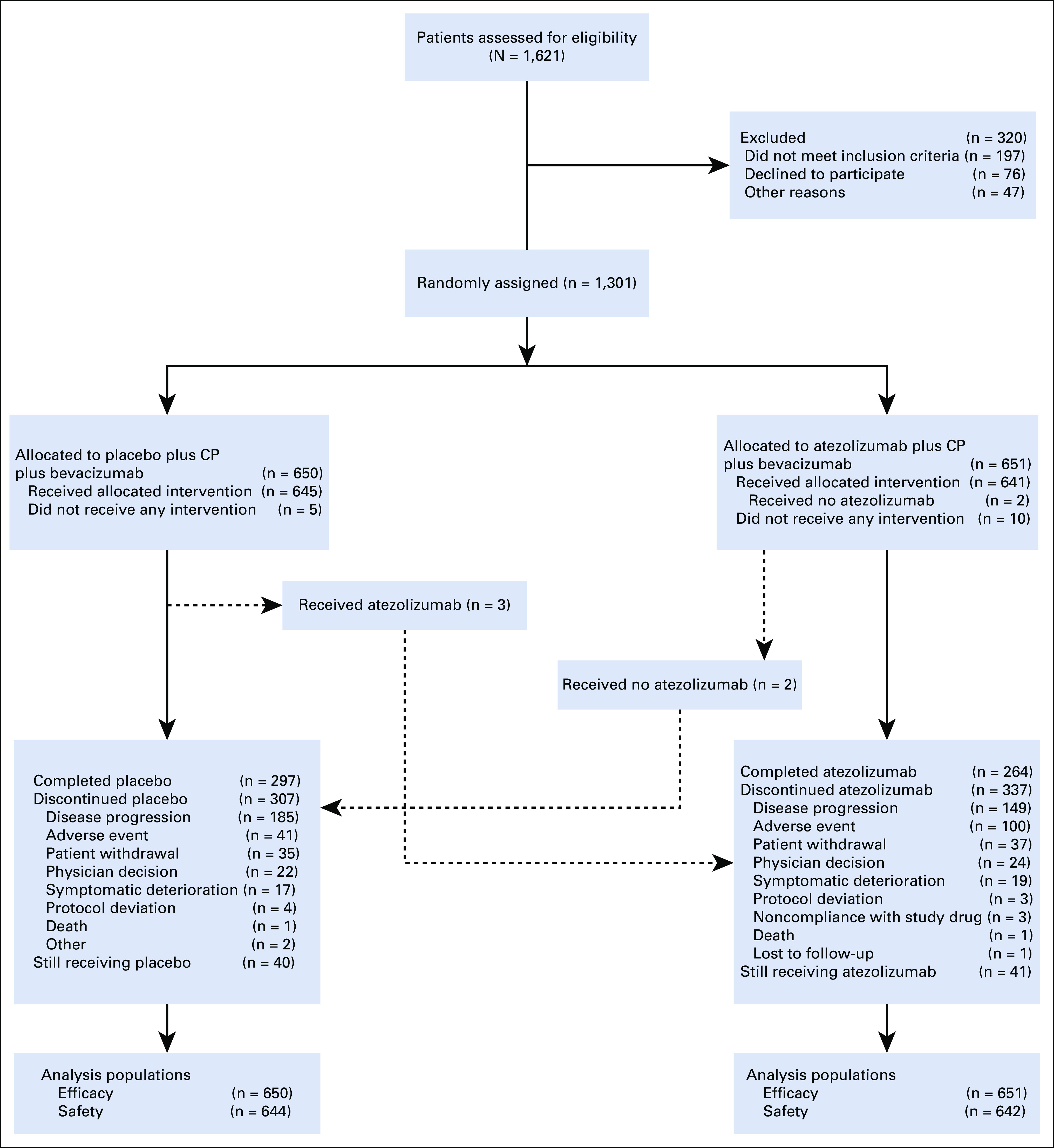
Trial profile. CP, carboplatin plus paclitaxel.
Baseline characteristics were well-balanced between treatment groups in the ITT and PD-L1–positive populations (Table 1).
TABLE 1.
Baseline Characteristics (ITT Population)
At the data cutoff for the primary analysis (March 30, 2020), the median duration of follow-up was 19.9 months (interquartile range [IQR], 15.1-23.6 months) in the atezolizumab group and 19.8 months (IQR, 15.4-23.5 months) in the placebo group. In the PD-L1–positive population, the median duration of follow-up was 19.6 months (IQR, 15.1-23.2 months) versus 19.4 months (IQR, 15.4-23.4 months), respectively.
The primary PFS analysis was performed after 664 PFS events had been recorded in the ITT population (323 [50%] atezolizumab-treated patients and 341 [52%] placebo-treated patients). The median PFS was 19.5 months (95% CI, 18.1 to 20.8) with atezolizumab versus 18.4 months (95% CI, 17.2 to 19.8) with placebo. The HR for PFS in the ITT population was 0.92 (95% CI, 0.79 to 1.07, stratified log-rank P = .28), which did not reach statistical significance (Fig 2A).
FIG 2.
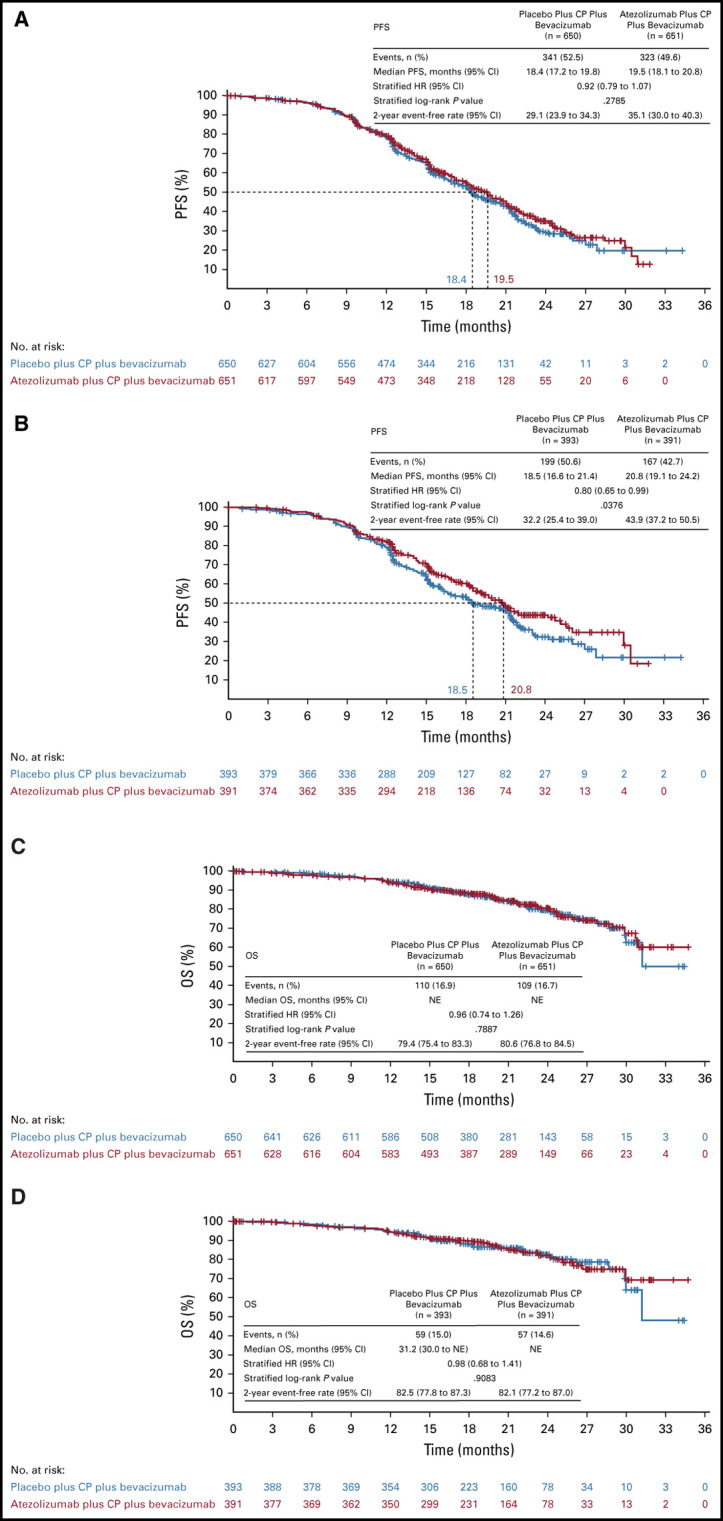
Efficacy: PFS in (A) ITT population and (B) PD-L1+ population; OS in (C) ITT population and (D) PD-L1+ population. CP, carboplatin plus paclitaxel; HR, hazard ratio; ITT, intention-to-treat; NE, not evaluable; OS, overall survival; PD-L1, programmed death ligand-1; PFS, progression-free survival.
In the PD-L1–positive population, a total of 366 patients had PFS events (167 [43%] atezolizumab-treated and 199 [51%] placebo-treated patients). The median PFS was 20.8 months (95% CI, 19.1 to 24.2) with atezolizumab versus 18.5 months (95% CI, 16.6 to 21.4) with placebo. The HR for PFS in the PD-L1–positive population was 0.80 (95% CI, 0.65 to 0.99; stratified log-rank P = .038), which did not reach statistical significance (Fig 2B).
The OS results were immature at the data cutoff for the primary PFS analysis. Deaths had been recorded in 219 patients (17%) in the ITT population and 116 (15%) in the PD-L1–positive population (Figs 2C and 2D). Two-year OS rates were 81% (95% CI, 77 to 84) in atezolizumab-treated patients and 79% (95% CI, 75 to 83) in placebo-treated patients in the ITT population. In the PD-L1–positive population, 2-year OS rates were 82% (95% CI, 77 to 87) with atezolizumab versus 83% (95% CI, 78 to 87) with placebo.
In the ITT population, objective responses were achieved in 233 of 251 response-evaluable patients in the atezolizumab group (93%; 95% CI, 89 to 96) versus 212 of 239 in the placebo group (89%; 95% CI, 84 to 92). In the PD-L1–positive population, objective responses were achieved in 156 of 169 (92%; 95% CI, 87 to 96) and 142 of 158 (90%; 95% CI, 84 to 94) patients, respectively.
Exploratory subgroup analyses of PFS showed generally consistent effects irrespective of baseline characteristics, with the possible exception of FIGO stage (HR, 0.80 [95% CI, 0.67 to 0.97] in patients with stage III disease and 1.24 [95% CI, 0.95 to 1.63] in those with stage IV disease) (Fig 3). In post hoc subgroup analyses according to histologic subtype, the PFS HR was 1.01 (95% CI, 0.84 to 1.20) in patients with high-grade serous histology, representing 76% (993 of 1,301) of the study population. In other histologic subtypes (high-grade nonserous including clear cell, and low-grade serous), PFS was more favorable with atezolizumab, but the numbers of patients in these subgroups were small and 95% CIs for the HRs crossed 1. There was no apparent enrichment of any particular histologic subtype in the PD-L1–positive population (Table 1).
FIG 3.
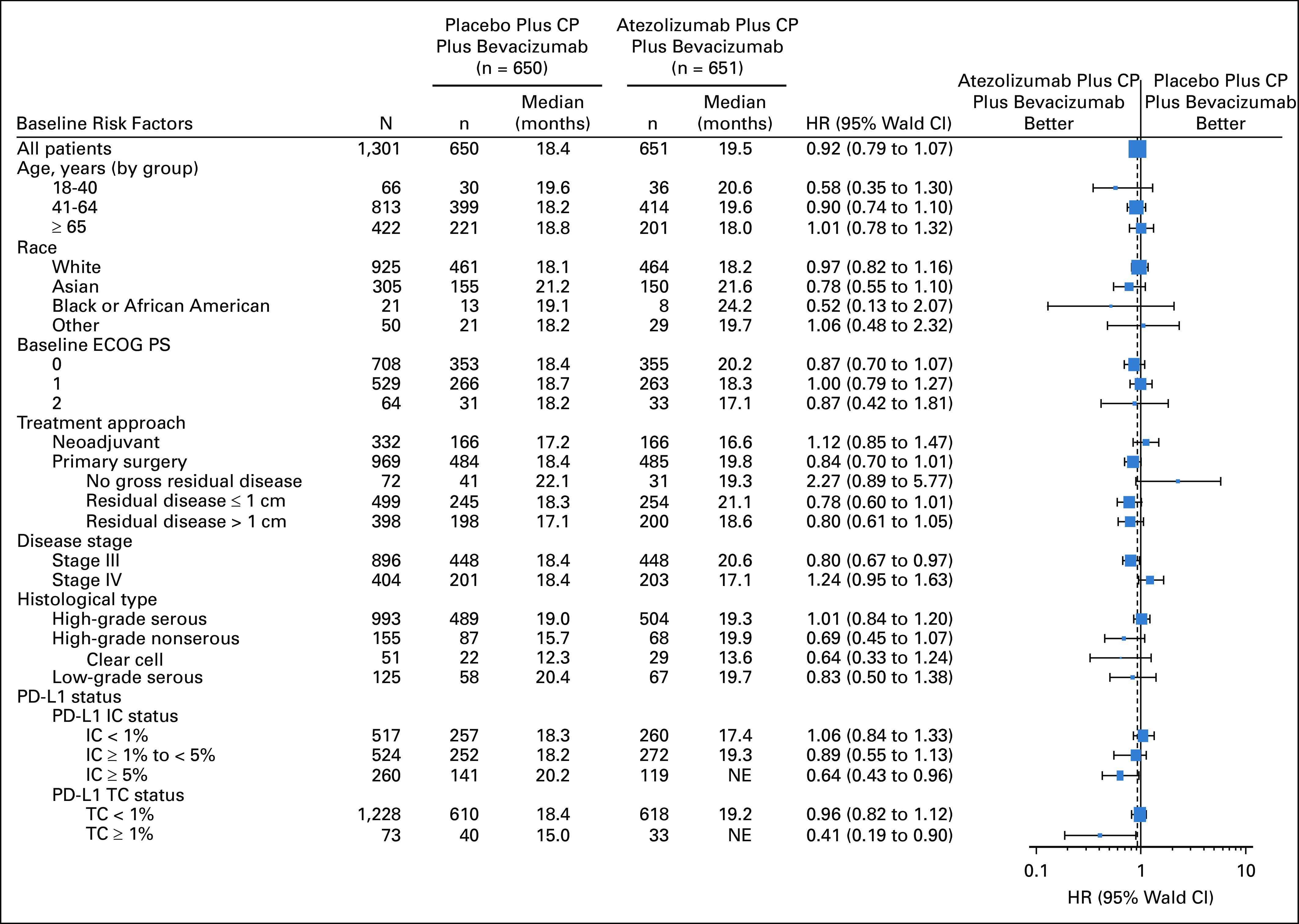
PFS by subgroup. CP, carboplatin plus paclitaxel; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IC, immune cell; NE, not evaluable; PD-L1, programmed death ligand-1; PFS, progression-free survival; TC, tumor cell.
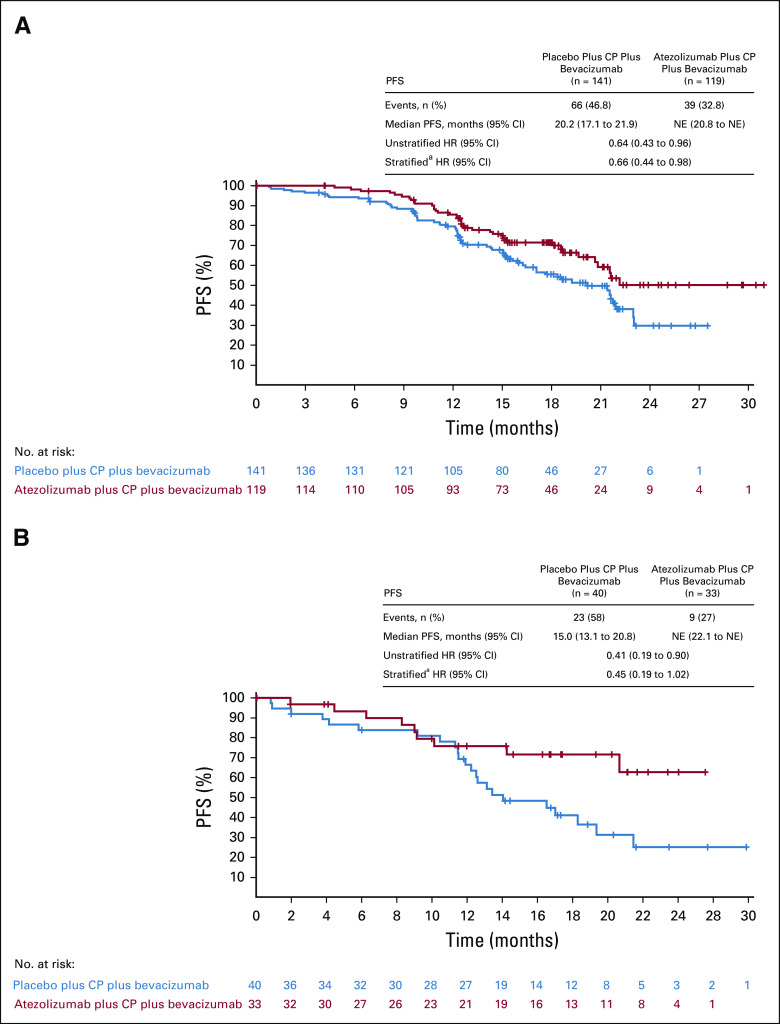
Additional prespecified exploratory analyses exploring the effect of atezolizumab on PFS in 260 patients (20%) with PD-L1 expression on ≥ 5% of ICs suggested a potential benefit from atezolizumab in this subgroup. The median PFS was not reached in the atezolizumab group after events in 39 of 119 patients (33%) and was 20.2 months (95% CI, 17.1 to 21.9) after events in 66 of 141 patients (47%) in the placebo group (Fig 4). The PFS HR in this subgroup was 0.64 (95% CI, 0.43 to 0.96). A small subgroup of patients displayed PD-L1 expression on ≥ 1% of TCs, representing 6% of the ITT population. The median PFS in the PD-L1 TC ≥ 1% subgroup was not reached in atezolizumab-treated patients and was 15.0 months (95% CI, 13.1 to 20.8 months) in placebo-treated patients (HR, 0.41 [95% CI, 0.19 to 0.90]; Fig 4).
FIG 4.
PFS in the subgroup of patients with PD-L1: (A) IC ≥ 5% (20% of the ITT population) and (B) TC ≥ 1% (6% of the ITT population). Of note, 67 of the 73 patients with TC ≥ 1% were also PD-L1 IC ≥ 1%. Only six patients whose tumors were identified as PD-L1–positive by TC staining were not considered to have PD-L1–positive tumors by IC staining. aUsing the stratification factors, disease stage, ECOG performance status, and treatment approach. CP, carboplatin plus paclitaxel; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IC, immune cell; ITT, intention-to-treat; NE, not evaluable; PD-L1, programmed death ligand-1; PFS, progression-free survival; TC, tumor cell.
The median number of atezolizumab cycles administered was 18 (range, 1-22). In both groups, the median number of cycles administered was 17 (range, 1-21) for bevacizumab and 6 (range, 1-6) for both carboplatin and paclitaxel.
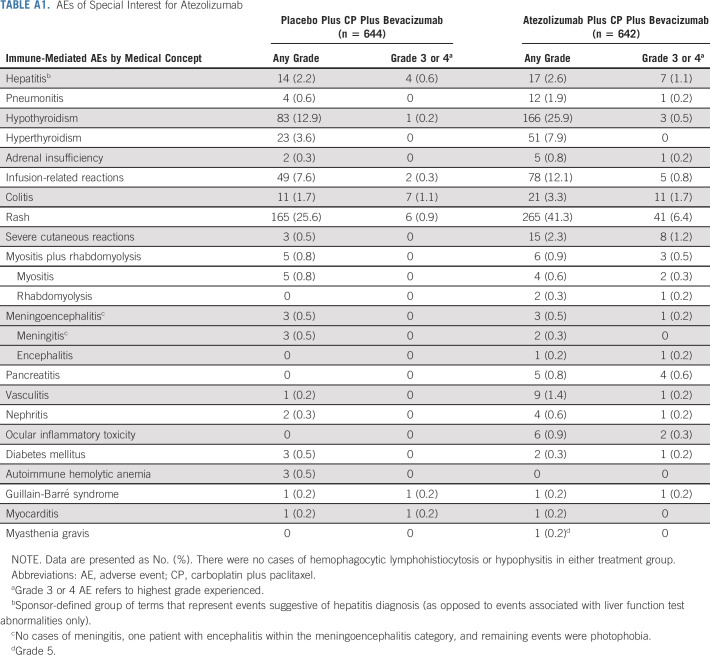
Table 2 summarizes the safety results in the safety-evaluable population, with the most common AEs by treatment group in Table 3. Findings were consistent in the safety-evaluable and PD-L1–positive populations (data not shown). AEs with fatal outcome occurred in 1% of patients in both groups.
TABLE 2.
Overview of Safety (Safety-Evaluable Population)
TABLE 3.
Clinicala AEs (Any Grade in ≥ 25% of Patients in Either Arm and Grade ≥ 3 AEs in > 0.5% of Patients in Either Arm)
The incidence of grade 3 or 4 AEs was numerically higher with atezolizumab than placebo (79% v 73%, respectively). The most common grade 3 or 4 AEs were neutropenia, hypertension, and anemia (Table 3). The only serious AEs (irrespective of investigator-assessed causality) in ≥ 2% of patients in either group were febrile neutropenia (8% v 4% with atezolizumab v placebo, respectively) and pyrexia (4% v 1%, respectively).
AEs of special interest (AESIs) for atezolizumab (Appendix Table A1, online only) were generally manageable and typically grade 1 or 2 (77% of 469 atezolizumab-treated patients with AESIs; 89% of 336 placebo-treated patients). One atezolizumab-treated patient experienced a grade 5 AESI (myasthenia gravis). AESIs (any grade) with a numerical difference between treatment groups were rash (any grade: 41% with atezolizumab v 26% with placebo; grade 3 or 4: 6% v 1%), hypothyroidism (any grade: 26% v 13%; grade 3 or 4: 0.5% v 0.2%), infusion-related reactions (12% v 8%), and hyperthyroidism (grade 1 or 2: 8% v 4%, respectively; no grade ≥ 3). Grade ≥ 3 severe cutaneous reactions occurred in 1% of atezolizumab-treated patients versus none of the placebo group. AESIs for bevacizumab were well-balanced between the two treatment groups (data not shown).
AEs led to discontinuation of any treatment in 26% of atezolizumab-treated patients and 22% of placebo-treated patients. This difference was driven by a higher proportion of patients discontinuing atezolizumab than placebo; the proportion of patients with AEs leading to bevacizumab discontinuation was similar in the two treatment groups.
DISCUSSION
In the IMagyn050 randomized phase III trial in newly diagnosed OC, adding atezolizumab to a chemotherapy plus bevacizumab backbone did not improve PFS compared with chemotherapy plus bevacizumab alone in either the ITT or the PD-L1–positive (IC ≥ 1%) populations. The results are immature for the co-primary end point of OS (deaths in only 17% of patients in the ITT population). OS follow-up continues.
IMagyn050 showed no significant PFS improvement in patients with PD-L1–positive tumors defined as IC ≥ 1%. However, in an exploratory analysis using a threshold of PD-L1 IC ≥ 5% (the cutoff used in urothelial carcinoma, representing 20% of the ITT population in IMagyn050), the PFS HR was 0.64 (95% CI, 0.43 to 0.96). The median PFS was 20.2 months with placebo but was not reached in atezolizumab-treated patients, with an early and sustained separation (Fig 4). The distribution of these biomarkers appeared to be balanced across subgroups. This intriguing signal may warrant further evaluation of atezolizumab in a population with high PD-L1 expression. Additional exploratory analysis in populations defined by PD-L1 expression on TCs was encouraging but difficult to interpret, as this population represents only 6% of the trial population and overlaps largely with PD-L1 IC–positive tumors (IC ≥ 1%). Subgroup analyses according to stage (a stratification factor) suggested an effect in patients with stage III but not stage IV disease. Reasons for such a difference are unclear and require elucidation. All the results from subgroup analyses should be interpreted with caution given their exploratory nature, the small sample sizes of some of the subgroups, and differences in event rates and prognosis between subgroups, biasing toward early events in some groups more than others, and should be considered only as hypothesis generating.
The safety profile of the atezolizumab, bevacizumab, and chemotherapy combination was consistent with previous experience with this regimen.8 Overall, adding atezolizumab to bevacizumab and chemotherapy did not compromise delivery of the backbone therapy. AESIs for bevacizumab were consistent with the known risks, indicating that adding atezolizumab did not worsen the established bevacizumab safety profile. The most common AEs with atezolizumab-containing therapy were typical of chemotherapy and bevacizumab, with the exception of hypothyroidism, hyperthyroidism, and rash, which were more common with atezolizumab.
HRD and BRCA mutation status have both shown prognostic value in OC and are associated with sensitivity to platinum-based chemotherapy and PARP inhibitors. BRCA and HRD status were unavailable at the time of random assignment; imbalances between the two treatment arms may exist. Further exploratory analyses according to HRD and BRCA mutation status in IMagyn050 are ongoing. Although the backbone regimen in IMagyn050 represents a standard front-line regimen for OC, the hypothesis that the type of chemotherapy backbone has an impact on outcomes with immunotherapeutic approaches remains unanswered. Exposure to immunogenic chemotherapy agents, such as anthracyclines, may sensitize cells to immune checkpoint inhibitors, converting ‘cold’ tumors to ‘hot’ tumors,13 although the combination of avelumab and pegylated liposomal doxorubicin (PLD) did not significantly improve outcomes in patients with platinum-resistant or platinum-refractory OC versus PLD alone in the JAVELIN-OVARIAN 200 trial.14 Ongoing trials evaluating checkpoint inhibitors with other chemotherapy backbones, including PLD, may help to elucidate this hypothesis and include NRG-GY009 (ClinicalTrials.gov identifier: NCT02839707), ATALANTE (ClinicalTrials.gov identifier: NCT02891824), and AGO-OVAR 2.29/ENGOT-ov34 (ClinicalTrials.gov identifier: NCT03353831).
The lack of PFS benefit from immunotherapy in IMagyn050 is consistent with findings from the JAVELIN-OVARIAN 100 and 200 trials evaluating avelumab in the front-line and recurrent settings, respectively,14,15 albeit the two front-line trials differed with respect to eligibility criteria, backbone regimen (with v without bevacizumab), and the assay used to determine PD-L1 status.
Unlike findings in non–small-cell lung cancer,8 combining atezolizumab with bevacizumab and chemotherapy did not improve efficacy in OC, highlighting intrinsic biologic and molecular differences between the tumor types. Currently, there is no evidence to support using immune checkpoint inhibitors in newly diagnosed OC. Insights from this trial should be considered for further research. Combining observations from this large trial with plausible biologic hypotheses will enable us to embrace specific trial designs in more focused, selected populations and settings.
ACKNOWLEDGMENT
We thank all patients and their families, the study investigators and staff at participating sites (see Appendix 1), the operational staff from the participating study groups (Laura Reese, Katie Campbell, Jennifer Klein, Mary Sharp, and Nicole LaMack [GOG-F]; Friederike Kipkeew and Gabriele Elser [Arbeitsgemeinschaft Gynaekologische Onkologie (AGO)/NOGGO]; Jane Bryce, Piera Gargiulo, and Francesco Perrone [MITO]; Ceylan Aksoy [TRSGO]; Christel Johanneson Bertolt, Joan Løhndorf, and Dorthe Almdal-Schrøder [NSGO]; Elena Biagioli [MaNGO]; Natasha Kitka and Stella Verrarou [HeCOG]; Ana M Levin [GEICO]; Magdalena Studnicka [Polish Gynecologic Oncology Group (PGOG)]; Ivana Nohova [Central and Eastern European Gynecologic Oncology Group (CEEGOG)]; Aurélie Pailhe and Bénédicte Votan [Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens (GINECO)]; Regina Berger [AGO-Austria]; Leora Mccullough [Israeli Society of Gynecologic Oncology (ISGO)]; and Eve Peeraer [Belgium and Luxembourg Gynecological Oncology Group (BGOG)]), members of the Independent Data Monitoring Committee, and F. Hoffmann-La Roche/Genentech. The study was done by GOG-F, AGO Study Group/NOGGO, MITO, TRSGO, NSGO, MaNGO, HeCOG, GEICO, PGOG, CEEGOG, GINECO, AGO-Austria, ISGO, and BGOG. Dr Aghajanian was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. The authors also acknowledge Jennifer Kelly, MA (Medi-Kelsey Ltd, Ashbourne, UK), for medical writing assistance, funded by F. Hoffmann-La Roche Ltd.
APPENDIX 1. Participating Investigators
Y. Cai, D. Cao, W. Cheng, X. Cheng, Y. Gao, L. Li, J. Liu, G. Lou, L. Wang, X. Wu, R. Yin, Q. Zhou, and J. Zhu (China); D. Aoki, T. Enomoto, J. Hamanishi, H. Itamochi, H. Kajiyama, S. Kamiura, H. Kato, H. Kobayashi, E. Kondo, T. Matsumoto, T. Mizushima, S. Nagao, H. Nakamura, K. Oda, M. Okadome, A. Okamoto, K. Takehara, M. Takekuma, H. Tokunaga, K. Ushijima, H. Yahata, and M. Yunokawa (Japan); B.-G. Kim, J.-W. Kim, Y.-M. Kim, and M.C. Lim (Republic of Korea); S. Baron-Hay, M. Grossi, and G. Richardson (Australia); H. Koch, C. Marth, A. Reinthaller, and C. Schauer (AGO-Austria); H. Denys, L. Dirix, E. Van Nieuwenhuysen, and C. Vulsteke (BGOG); D. Cibula, J. Klat, B. Melichar, and J. Prausova (CEEGOG); Z. Alwafai, B. Ataseven, M. Bossart, H. Bronger, A. Burges, P. Buderath, N. De Gregorio, M. Eichbaum, L. Hanker, A. Hasenburg, T.-W. Park-Simon, T. Reimer, B. Schmalfeldt, A. Schneeweiss, J. Sehouli, and P. Wimberger (AGO/NOGGO); M. Anttila, G. Aune, A. Auranen, J. Fernebro, S. Hietanen, G. Lindahl, K. Lindemann, and T.J. Nøttrup (NSGO); J. Alarcon Company, J.A. Arranz Arija, M.J. Bermejo Pérez, S. Hernando Polo, A. Herrero Ibañez, J. Garcia-Donas, Y. Garcia Garcia, A. Martinez Bueno, and A. Santaballa Bertran (GEICO); N. Bonnin, H. Bourgeois, C. Dubot, A. Leary, A. Lortholary, I. Ray-Coquard, and F. Selle (GINECO); G. Aravantinos, A. Bamias, G. Fountzilas, and H. Kalofonos (HeCOG); M. Beiner, I. Bruchim, J. Korach, and O. Rosengarten (ISGO); A. Bologna, S. Cinieri, N. Colombo, P. Conte, U. De Giorgi, S. De Placido, A. Ferrero, A.A. Lissoni, G. Mangili, A.M. Mosconi, S. Pignata, F. Raspagliesi, G. Scambia, S. Siena, S. Tamberi, G. Tognon, C. Zamagni, and P. Zola (MITO/MaNGO); M. Bidzinski, A. Chudecka-Glaz, E. Iwanska, and R. Madry (PGOG); A. Lisyanskaya, R. Safin, A. Smolin, and D. Stroyakovskii (Russian Federation); H. Akbulut, A. Ayhan, T.T. Bese, F. Kose, P. Saip, and C. Taskiran (TRSGO); A.C. De Melo and R. Hegg (Brazil); and C. Aghajanian, H. Ahuja, A. Alvarez-Secord, C. Anderson, J. Barlin, L. Barroilhet, K. Behbakht, M. Bell, D. Bender, S. Blank, C. Boardman, M. Bookman, J. Bottsford-Miller, J. Buscema, M. Callahan, M.E. Carney, C. Castro, P. Celano, J. Chan, L.-M. Chen, N. Cloven, J. Cohen, E. Crane, C. Darus, S. Dewdney, J. Diaz, O. Dorigo, K. Elsahwi, A. Evans, J. Farley, J. Fauci, A. Fields, J. Fiorica, D. Fishman, E. Fleming, H. Frederickson, W. Gao, M. Geller, M. Gold, H. Gray, A. Green, D. Griffin, E. Hernandez, C. Holschneider, W. Houck, M. Indermaur, A. Jackson, V. John, A. Kamat, T.C. Krivak, P. Kumar, C. Landen, J. Lea, C. Lee, S. Lentz, S. Lewin, P. Lim, R. Liu, L. Ma, T. Mahmood, P. Mansky, T. McCarthy, L. McCluskey, M. McCollum, M. Mchale, D. McNamara, M. Messing, M. Miller, K. Moore, T. Moore, M. Morgan, J. Moroney, R. Morris, T. Myers, A. Olawaiye, D. O'Malley, I. Podzielinski, B. Pothuri, T. Reid, T. Rhodes, D. Richards, C. Rivard, K. Robison, K. Rodabaugh, L.-D. Roman, P. Rose, T. Rutledge, A. Santillan-Gomez, R. Schilder, K. Schuler, P. Seago, M. Shahin, S.K. Sharma, D. Slater, N.M. Spirtos, M. Teneriello, M. Tenney, D. Tewari, K. Tewari, P. Thaker, J. Thomes-Pepin, B. Tierney, J. Trinidad, F. Ueland, S.E. Waggoner, D. Warshal, T. Wassenaar, T. Werner, S. Westin, M.Y. Williams-Brown, L. Willmott, A. Wolfson, and K. Yost (GOG-F).
TABLE A1.
AEs of Special Interest for Atezolizumab
Kathleen N. Moore
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, Tesaro, VBL Therapeutics, Merck, Aravive, Pfizer/EMD Serono, Eisai, AbbVie, Vavotar Life Sciences, Mersana, Myriad Genetics
Research Funding: PTC Therapeutics, Lilly, Merck, Tesaro, Genentech, Clovis Oncology, Lilly Foundation, Regeneron, Advaxis, Bristol-Myers Squibb, Verastem, Novartis Pharmaceuticals UK Ltd, AstraZeneca, Agenus, Takeda, Forty Seven, Stem CentRx, Immunogen, Bayer, Novogen, AbbVie/Stem CentRx
Michael Bookman
Employment: The Permanente Medical Group
Consulting or Advisory Role: AstraZeneca, AbbVie, Immunogen, Genentech/Roche, Seattle Genetics, Aravive, Merck Sharp & Dohme
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche Diagnostics, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis
Research Funding: AstraZeneca, Clovis Oncology, Merck, Bayer, PharmaMar, Pfizer, Tesaro, MSD Oncology, Roche
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, MSD Oncology, Olympus
Austin Miller
Uncompensated Relationships: Genentech
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Johanna Mäenpää
Honoraria: GlaxoSmithKline, AstraZeneca, Roche
Consulting or Advisory Role: GlaxoSmithKline, Eisai Europe, AstraZeneca
Travel, Accommodations, Expenses: GlaxoSmithKline
Lyndsay Willmott
Honoraria: Clovis Oncology, AstraZeneca/Merck, Tesaro/GSK, Eisai, Genentech, GlaxoSmithKline
Consulting or Advisory Role: Tesaro/GSK, AstraZeneca/Merck, Clovis Oncology, Eisai, Genentech
Speakers' Bureau: Tesaro/GSK, AstraZeneca/Merck, Clovis Oncology
Nicoletta Colombo
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, PharmaMar, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Pfizer, Amgen, Immunogen, Novartis, Mersana, Eisai, Merck, Takeda, Biocad
Consulting or Advisory Role: Roche/Genentech, PharmaMar, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Tesaro, BioCad, GlaxoSmithKline, Immunogen, Mersana, Eisai, Merck, Takeda, Biocad
Michalis Liontos
Employment: Pfizer [I]
Stock and Other Ownership Interests: Pfizer [I]
Honoraria: MSD Oncology, Roche, Janssen, Sanofi, Bristol-Myers Squibb/Celgene, AstraZeneca, Ipsen, Astellas Pharma
Consulting or Advisory Role: Amgen, GlaxoSmithKline, AstraZeneca, Janssen
Travel, Accommodations, Expenses: Bayer, Pfizer, Roche, AstraZeneca
Yolanda Garcia
Consulting or Advisory Role: GlaxoSmithKline/Tesaro, Roche, AstraZeneca
Speakers' Bureau: AstraZeneca
Research Funding: Roche, AstraZeneca
Travel, Accommodations, Expenses: GlaxoSmithKline/Tesaro, Roche
Christopher J. Darus
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Aikou Okamoto
Honoraria: AstraZeneca Japan, MSD, Chugai Pharma, Takeda
Consulting or Advisory Role: AstraZeneca Japan, Chugai Pharma
Speakers' Bureau: AstraZeneca Japan
Research Funding: Kissei Pharmaceutical, Meiji Holdings, Pfizer, Fuji Pharma, Taiho Pharmaceutical, Kaken Pharmaceutical, Chugai Pharma, Tsumura & Co, Daiichi Sankyo Co, Ltd, Shinnihonseiyaku, Mochida Pharmaceutical Co, Ltd, ASKA Pharmaceutical Co, Ltd, Takeda, Terumo
Fan Wu
Employment: Roche
Stock and Other Ownership Interests: Roche
Luciana Molinero
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: P35974-US Patent Application Inventorship
Vidya Maiya
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech
Other Relationship: Roche/Genentech
Victor K. Khor
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche, Iovance Biotherapeutics
Yvonne G. Lin
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Consulting or Advisory Role: Venn Biosciences Corporation
Sandro Pignata
Honoraria: AstraZeneca, Roche, PharmaMar, Tesaro, Pfizer, MSD, GlaxoSmithKline, Clovis Oncology
Consulting or Advisory Role: AstraZeneca, Roche, PharmaMar, Pfizer, Tesaro, Clovis Oncology, GlaxoSmithKline
Research Funding: Roche, AstraZeneca, MSD, Pfizer
No other potential conflicts of interest were reported.
See accompanying editorial on page 1833
PRIOR PRESENTATION
Presented at ESMO 2020 Virtual Meeting, September 19–21, 2020 (oral abstract: LBA31).
SUPPORT
Sponsored by F. Hoffmann-La Roche/Genentech. The trial was designed and conducted by F. Hoffmann-La Roche/Genentech in collaboration with The Gynecologic Oncology Group Foundation (GOG-F) and the European Network for Gynaecological Oncological Trial Groups (ENGOT) according to what was subsequently described in 2019 as ENGOT model C.16 The sponsor (F. Hoffmann-La Roche/Genentech) was involved in data collection, analysis, and interpretation. All the authors verify that the trial was conducted according to the protocol and vouch for the accuracy and completeness of the data. All the drafts of the manuscript were prepared by the authors, with medical writing assistance funded by the sponsor.
CLINICAL TRIAL INFORMATION
NCT03038100 (IMagyn050/GOG 3015/ENGOT-OV39)
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the sharing of clinical information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_ commitment_to_data_sharing.htm).
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen N. Moore, Michael Bookman, Austin Miller, Charles Anderson, Giovanni Scambia, Cagatay Taskiran, Carol Aghajanian, Xiaohua Wu, Fan Wu, Luciana Molinero, Vidya Maiya, Victor K. Khor, Yvonne G. Lin, Sandro Pignata
Provision of study materials or patients: Kathleen N. Moore, Michael Bookman, Jalid Sehouli, Johanna Mäenpää, Jessica Thomes-Pepin, Yolanda Garcia, Sudarshan K. Sharma, Christopher J. Darus, Carol Aghajanian, Xiaohua Wu, Rustem Safin, Fan Wu, Sandro Pignata
Collection and assembly of data: Kathleen N. Moore, Michael Bookman, Jalid Sehouli, Charles Anderson, Giovanni Scambia, Cagatay Taskiran, Katina Robison, Lyndsay Willmott, Nicoletta Colombo, Jessica Thomes-Pepin, Michalis Liontos, Michael A. Gold, Yolanda Garcia, Christopher J. Darus, Aikou Okamoto, Xiaohua Wu, Rustem Safin, Luciana Molinero, Vidya Maiya, Victor K. Khor, Yvonne G. Lin
Data analysis and interpretation: Kathleen N. Moore, Michael Bookman, Jalid Sehouli, Giovanni Scambia, Tashanna Myers, Cagatay Taskiran, Katina Robison, Johanna Mäenpää, Nicoletta Colombo, Michael A. Gold, Yolanda Garcia, Sudarshan K. Sharma, Christopher J. Darus, Fan Wu, Luciana Molinero, Vidya Maiya, Victor K. Khor, Yvonne G. Lin, Sandro Pignata
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathleen N. Moore
Honoraria: Research To Practice, Prime Oncology, Physicans' Education Resource
Consulting or Advisory Role: Genentech/Roche, Immunogen, AstraZeneca, Tesaro, VBL Therapeutics, Merck, Aravive, Pfizer/EMD Serono, Eisai, AbbVie, Vavotar Life Sciences, Mersana, Myriad Genetics
Research Funding: PTC Therapeutics, Lilly, Merck, Tesaro, Genentech, Clovis Oncology, Lilly Foundation, Regeneron, Advaxis, Bristol-Myers Squibb, Verastem, Novartis Pharmaceuticals UK Ltd, AstraZeneca, Agenus, Takeda, Forty Seven, Stem CentRx, Immunogen, Bayer, Novogen, AbbVie/Stem CentRx
Michael Bookman
Employment: The Permanente Medical Group
Consulting or Advisory Role: AstraZeneca, AbbVie, Immunogen, Genentech/Roche, Seattle Genetics, Aravive, Merck Sharp & Dohme
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche Diagnostics, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis
Research Funding: AstraZeneca, Clovis Oncology, Merck, Bayer, PharmaMar, Pfizer, Tesaro, MSD Oncology, Roche
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, MSD Oncology, Olympus
Austin Miller
Uncompensated Relationships: Genentech
Giovanni Scambia
Consulting or Advisory Role: Clovis Oncology, AstraZeneca, PharmaMar, Roche, Tesaro
Speakers' Bureau: Clovis Oncology, MSD
Johanna Mäenpää
Honoraria: GlaxoSmithKline, AstraZeneca, Roche
Consulting or Advisory Role: GlaxoSmithKline, Eisai Europe, AstraZeneca
Travel, Accommodations, Expenses: GlaxoSmithKline
Lyndsay Willmott
Honoraria: Clovis Oncology, AstraZeneca/Merck, Tesaro/GSK, Eisai, Genentech, GlaxoSmithKline
Consulting or Advisory Role: Tesaro/GSK, AstraZeneca/Merck, Clovis Oncology, Eisai, Genentech
Speakers' Bureau: Tesaro/GSK, AstraZeneca/Merck, Clovis Oncology
Nicoletta Colombo
Honoraria: Roche/Genentech, AstraZeneca, Tesaro, PharmaMar, GlaxoSmithKline, MSD Oncology, Clovis Oncology, Pfizer, Amgen, Immunogen, Novartis, Mersana, Eisai, Merck, Takeda, Biocad
Consulting or Advisory Role: Roche/Genentech, PharmaMar, AstraZeneca, Clovis Oncology, Pfizer, MSD Oncology, Tesaro, BioCad, GlaxoSmithKline, Immunogen, Mersana, Eisai, Merck, Takeda, Biocad
Michalis Liontos
Employment: Pfizer [I]
Stock and Other Ownership Interests: Pfizer [I]
Honoraria: MSD Oncology, Roche, Janssen, Sanofi, Bristol-Myers Squibb/Celgene, AstraZeneca, Ipsen, Astellas Pharma
Consulting or Advisory Role: Amgen, GlaxoSmithKline, AstraZeneca, Janssen
Travel, Accommodations, Expenses: Bayer, Pfizer, Roche, AstraZeneca
Yolanda Garcia
Consulting or Advisory Role: GlaxoSmithKline/Tesaro, Roche, AstraZeneca
Speakers' Bureau: AstraZeneca
Research Funding: Roche, AstraZeneca
Travel, Accommodations, Expenses: GlaxoSmithKline/Tesaro, Roche
Christopher J. Darus
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Aikou Okamoto
Honoraria: AstraZeneca Japan, MSD, Chugai Pharma, Takeda
Consulting or Advisory Role: AstraZeneca Japan, Chugai Pharma
Speakers' Bureau: AstraZeneca Japan
Research Funding: Kissei Pharmaceutical, Meiji Holdings, Pfizer, Fuji Pharma, Taiho Pharmaceutical, Kaken Pharmaceutical, Chugai Pharma, Tsumura & Co, Daiichi Sankyo Co, Ltd, Shinnihonseiyaku, Mochida Pharmaceutical Co, Ltd, ASKA Pharmaceutical Co, Ltd, Takeda, Terumo
Fan Wu
Employment: Roche
Stock and Other Ownership Interests: Roche
Luciana Molinero
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: P35974-US Patent Application Inventorship
Vidya Maiya
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech
Other Relationship: Roche/Genentech
Victor K. Khor
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche, Iovance Biotherapeutics
Yvonne G. Lin
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Consulting or Advisory Role: Venn Biosciences Corporation
Sandro Pignata
Honoraria: AstraZeneca, Roche, PharmaMar, Tesaro, Pfizer, MSD, GlaxoSmithKline, Clovis Oncology
Consulting or Advisory Role: AstraZeneca, Roche, PharmaMar, Pfizer, Tesaro, Clovis Oncology, GlaxoSmithKline
Research Funding: Roche, AstraZeneca, MSD, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ferlay J Colombet M Soerjomataram I, et al. : Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941-1953, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Burger RA Brady MF Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Perren TJ Swart AM Pfisterer J, et al. : A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Moore K Colombo N Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Ray-Coquard I Pautier P Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 6.González-Martín A Pothuri B Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Coleman RL Fleming GF Brady MF, et al. : Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381:2403-2415, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socinski MA Jotte RM Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Finn RS Qin S Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Liu JF Gordon M Veneris J, et al. : Safety, clinical activity and biomarker assessments of atezolizumab from a phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol 154:314-322, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Moroney JW Powderly J Lieu CH, et al. : Safety and clinical activity of atezolizumab plus bevacizumab in patients with ovarian cancer: A phase Ib study. Clin Cancer Res 26:5631-5637, 2020 [DOI] [PubMed] [Google Scholar]
- 12.DeMets DL, Lan KK: Interim analysis: The alpha spending function approach. Stat Med 13:1341–1352, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Kepp O, Zitvogel L, Kroemer G: Clinical evidence that immunogenic cell death sensitizes to PD-1/PD-L1 blockade. Oncoimmunology 8:e1637188, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujade-Lauraine E Fujiwara K Ledermann JA, et al. : Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: Primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial. Gynecol Oncol 154, 2019. (suppl 1; abstr LBA1) [Google Scholar]
- 15.Ledermann JA Colombo N Oza AM, et al. : Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: Results from the phase 3 JAVELIN Ovarian 100 trial. Gynecol Oncol 159, 2020. (suppl 1; abstr LBA25) [Google Scholar]
- 16.Vergote I Coleman RL Pignata S, et al. : Joint ENGOT and GOG Foundation requirements for trials with industry partners. Gynecol Oncol 154:255-258, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the sharing of clinical information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_ commitment_to_data_sharing.htm).