PURPOSE:
Given the widespread introduction of tyrosine kinase inhibitors (TKIs), we evaluated the cost associated with chronic myelogenous leukemia (CML) care compared with the cost of care for patients with hematologic malignancies (HEM) and for patients without cancer (GEN), to aid with resource allocation and clinical decision making.
METHODS:
A retrospective cohort was constructed from the OptumLabs Data Warehouse using claims from 2000 to 2016. Eligible patients had ≥ 2 CML claims and were enrolled continuously for ≥ 6 months before diagnosis and ≥ 1 year afterward (n = 1,909). Patients with CML were frequency matched 4:1 with HEM and GEN cohorts and were observed through October 2017. We used generalized linear models to assess the variation in total mean annualized health care costs in the 3 cohorts and to examine the influence of factors associated with costs.
RESULTS:
Mean annualized costs for CML were $82,054 (ie, $25,471 [95% CI, $20,808 to $30,133] more than those for HEM and $74,993 [95% CI, $70,818 to $79,167] more than those for GEN); these differences were driven by pharmacy costs in the CML group. The cost of CML care exceeded that for HEM and GEN for all index years in this study and increased over each diagnostic interval until 2015, peaking at $91,990. The mean annual cost of all TKIs increased. Imatinib’s mean annualized cost was $41,546 in the period 2000-2004 but increased to $105,069 in the period 2015-2017. In multivariable analysis, percent days on TKIs had the greatest influence on cost: ≥ 75% of the time versus none showed a difference in cost of $108,716 (95% CI, $99,193 to $118,239).
CONCLUSION:
Contemporary CML costs exceeded the cost of treatment of other hematologic malignancies. Cost was primarily driven by TKIs, whose cost continued to increase over time.
INTRODUCTION
Chronic myelogenous leukemia (CML) comprises 15% of leukemia diagnoses in the United States. An estimated 8,990 new patients will be diagnosed with CML in 2019.1 The initial IRIS study of imatinib showed that, for those patients randomly assigned to imatinib, the estimated overall survival at 10 years was 83.3%.2 In real-world practice, with the approval of imatinib as a first-line therapy for CML in 2001 and the introduction of second- and third-generation tyrosine kinase inhibitors (TKIs) into the market, annual mortality from CML decreased from 0.9 deaths per 100,000 in 1998 to 0.3 deaths per 100,000 persons in 2016.3 As a result, the prevalence of patients with CML has greatly increased, with CML now being treated more as a “chronic, indolent” disease. There were an estimated 54,226 people living with CML in the United States in 2016 and estimates of 10-12/100,000 people affected.1 At this time, 5 TKIs (bosutinib, dasatinib, imatinib, nilotinib, and ponatinib) have been US Food and Drug Administration (FDA) approved for the treatment of CML
As the prevalence of people living with CML increases, understanding the cost of ongoing care in this patient population both at diagnosis and in subsequent years becomes more important. The economic burden of care on patients and payers, and the drivers of these costs, become increasingly important as longevity increases. Benchmarking the cost of care for those with other hematologic malignancies and the general commercial insurance population provides perspective as to the relative financial impact of CML therapy. We hypothesized that the cost of CML care would exceed that of hematologic malignancies once the more intense inpatient phases of care have passed after the first 6 months, and cost differences would be primarily associated with the cost of TKI prescriptions. In 2013, a group of more than 100 experts in the CML field highlighted concerns regarding the cost of oral TKI therapy in the United States.4 In this statement paper, this international group compiled the price in thousands of US dollars of annual treatment of CML: imatinib at $92,000, nilotinib at $115,500, and dasatinib at $123,500.4 Although other groups have demonstrated that in the Medicare population, CML care exceeds the cost of care of those without a cancer diagnosis, to our knowledge, the comparison with those affected by other hematologic malignancies has not been established.5
METHODS
Study Population
This retrospective study used de-identified data from the OptumLabs Data Warehouse (OLDW), which includes claims data for commercially insured and Medicare Advantage enrollees, representing a diverse mixture of ages, ethnicities, and geographic regions across the United States.6 Three mutually exclusive matched cohorts were defined for this analysis: (1) patients with CML, (2) patients with non-CML hematologic malignancy (HEM), and (3) the general population (GEN). Eligible patients with CML were those who had a nondiagnostic medical claim for CML (International Classification of Diseases, 9th revision [ICD9], code 205.1 or International Classification of Diseases, 10th revision [ICD10] code C92.1) beginning January 1, 2000, through October 31, 2016. We defined the initial CML claim date as our “index” date. To reduce the possibility of misclassification, eligible patients with CML were required to have at least 1 additional CML code within 30 to 365 days of the initial claim. The HEM group was defined similarly on the basis of ICD9/10 codes for lymphoid leukemias, lymphomas, and myeloma. We excluded patients with acute myeloid leukemia codes, given the possibility of misclassification with CML. The GEN group included enrollees without any cancer diagnoses over their enrollment period. Because patients in the GEN group did not have a natural diagnosis date, a proxy index date was chosen for these individuals, defined as their health plan enrollment date plus a randomly generated number of days, on the basis of a gamma distribution. A gamma distribution was chosen because it best represented the distribution of days between the enrollment date and the diagnosis date in the CML group. Individuals chosen for all 3 cohorts were required to be continuously enrolled in the OLDW for at least 180 days before the index date and for at least 365 days after the index date. For the CML and HEM groups, we excluded individuals who had any solid tumor cancer diagnosis codes during the 180 days before the index date or the 365 days after the index date. Because the CML group was the primary group of interest, the HEM and GEN groups were each frequency matched on an approximate 4:1 ratio to the CML group on the basis of age (10-year increments), sex, index year (3-year increments), geographic region (10 US census divisions), and insurance (commercial v Medicare). Overall, follow-up data were available for this analysis through October 31, 2017.
Outcomes
The OLDW contains claims information for all prescription medications and medical services submitted to a health plan for payment. For our primary outcome, we focused on mean annualized total health care costs, which included both health plan liability and patient liability (ie, out-of-pocket copayments). The components of total cost consisted of all covered prescription medications and medical services received. In subanalyses, we also examined mean pharmacy (prescription medications) costs, including those directly attributed to TKIs used for CML care (ie, imatinib, dasatinib, nilotinib, ponatinib, and bosutinib) and patient out-of-pocket costs. All costs were converted to 2017 dollars using the medical component of the Consumer Price Index.
Explanatory Variables
In addition to the demographic characteristics mentioned earlier in the text, the OLDW provides information on each individual’s race/ethnicity, household income, and baseline Charlson comorbidity index (CCI). Ethnicity, household income, and educational level are sourced from a national supplier of consumer marketing data. Household income is imputed on the basis of a model using both public and private consumer data. The CCI was defined using claims data from the initial 120 days before the index date (ie, we excluded the 2-month period immediately before the index date, given concern that the presentation and diagnosis of cancer may inflate one’s baseline’s CCI) and excluded cancer-related items. From the OLDW, we also determined the following annualized variables for health care use: number of ambulatory visits (< 5, 5-9, 10-19, and ≥ 20); emergency room visits (0, 1, ≥ 2); and inpatient days (0, > 0 to < 2, 2-6, ≥ 7); days with TKI prescription coverage (0, 1% to 49%, 50% to 74%, and ≥ 75%); and any receipt of a hematopoietic-cell transplantation (HCT; ICD9 codes 279.5, 996.85, and V42.81, V42.82; ICD10 codes T86.0 and Z94.8).
Statistical Analyses
Annualized costs (total and out of pocket) were calculated by summing costs from all medical and pharmacy claims between the index date and the end of continuous enrollment or the end of the study period (whichever occurred first) and dividing by the accrued time in that period (in years). We compared costs across the 3 cohorts using 1-way analysis of variance (ANOVA) and did pairwise comparisons using t tests. We used generalized linear models (GLM) to assess the variation in total costs in the 3 cohorts, using a gamma family and a log link. Effects are expressed as the exponentiated beta coefficient (with 95% CIs), which can be interpreted as the relative cost in the indicated group, and also as absolute differences (with 95% CIs) using predictive margins. Models were adjusted for the frequency-matched factors (sex, age at index date, index year, geographic region, and insurance). Within the CML cohort, GLM was also used to examine the influence of various factors hypothesized to be associated with costs: sex, age at index date, index year, race/ethnicity, insurance, CCI, TKI prescription coverage, HCT, and annualized health care use. Analyses were completed using Stata/SE 15 (College Station, TX) and R versions 3.5.1 and 3.6.1 software.
RESULTS
Patient Demographics and Clinical Characteristics
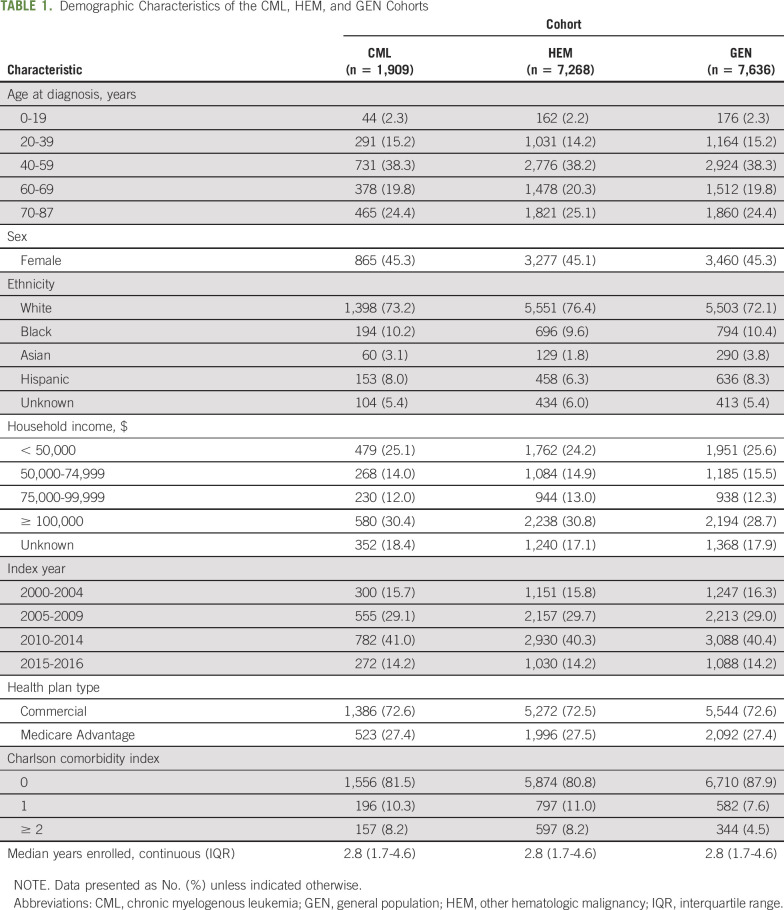
A total of 1,909 enrollees with CML between 2000 and 2016 who met the inclusion criteria were identified. These enrollees were matched with 7,268 HEM and 7,636 GEN enrollees over the observation period. The HEM group consisted of 32.7% lymphoid leukemia, 25.7% non-Hodgkin lymphoma, 22.3% Hodgkin lymphoma, 18.2% myeloma, and 1% other. Demographic characteristics of these cohorts were similar by design (Table 1). Median ages were as follows: CML, 57 years (interquartile range [IQR], 45-69 years); HEM, 58 years (IQR, 46-70 years); and GEN, 56 years (IQR, 44-69); the median follow-up time for all 3 groups was 2.8 years (IQR, 1.7-4.6 years in all groups).
TABLE 1.
Demographic Characteristics of the CML, HEM, and GEN Cohorts
Cost Comparisons
Total mean annualized costs in the CML cohort were greater than the costs in the HEM and GEN cohorts ($82,054 v $56,886 and $7,139, respectively; P < .01 for ANOVA and all pairwise comparisons; Fig 1 and Table 2). There was no statistical difference in mean inpatient costs between the CML and the HEM cohort, with annualized costs for all years for CML care of $15,105 and HEM care of $16,184 (P = .44). However, total pharmacy costs were significantly greater in the CML group than in the HEM and GEN groups ($48,233 v $7,607 and $1,833, respectively, P < .01), which was driven by the cost of TKI prescriptions ($44,035 out of $48,233). When differences in total annualized costs were adjusted for frequency-matched variables, results were essentially unchanged (CML, $25,471 [95% CI, $20,808 to $30,133] more v HEM and $74,993 [95% CI, $70,818 to $79,167] more v GEN).
FIG 1.
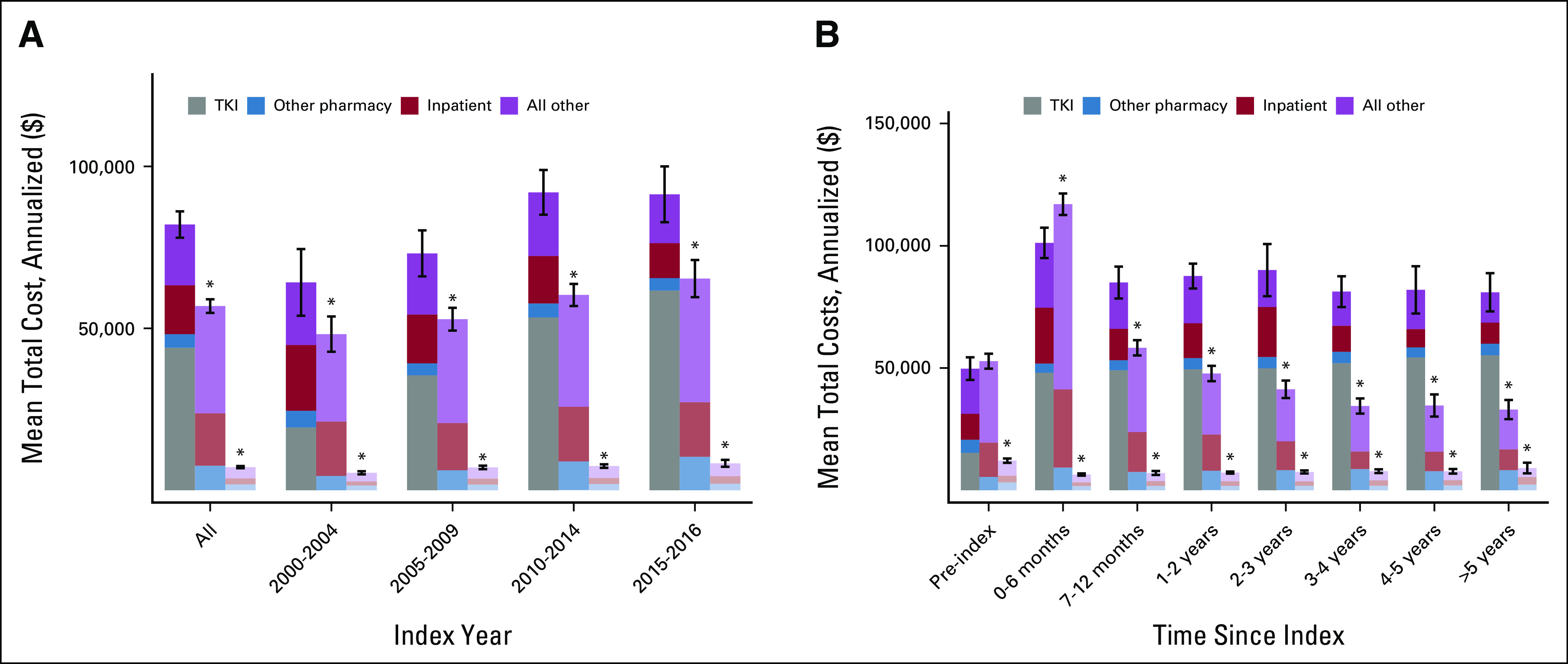
Mean total costs by (A) index year and (B) time since index. TKI, tyrosine kinase inhibitor. (*) Significantly different from the CML cohort (P < 0.05).
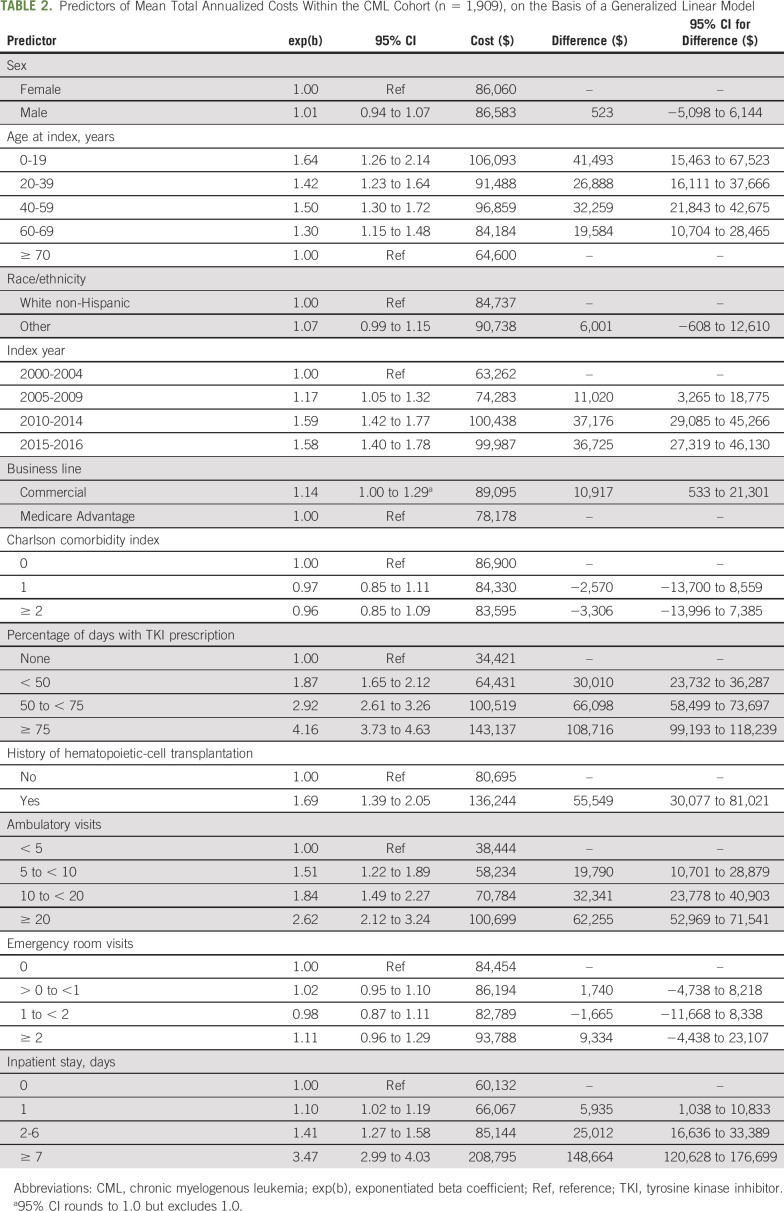
TABLE 2.
Predictors of Mean Total Annualized Costs Within the CML Cohort (n = 1,909), on the Basis of a Generalized Linear Model
In subanalyses, the cost of care for the CML cohort was greater than that for the HEM and GEN cohorts over each diagnostic epoch (P < .01 for ANOVA and all pairwise comparisons; Fig 1). Costs for CML care also increased over each 5-year diagnostic interval until 2015 ($64,188 in 2000-2004, peaking at $91,990 in 2010-2014). To better understand the contribution of time from diagnosis on the cost of care, we also evaluated the costs since index date. The cost of HEM care exceeded the cost of CML care only for the first 6 months after diagnosis (CML, annualized $101,190 v HEM, $116,991). Subsequently, the cost of care for CML exceeded costs for the 2 comparison cohorts after the first 6 months after the index date (P < .01 for 3-way comparison and for each pairwise comparison).
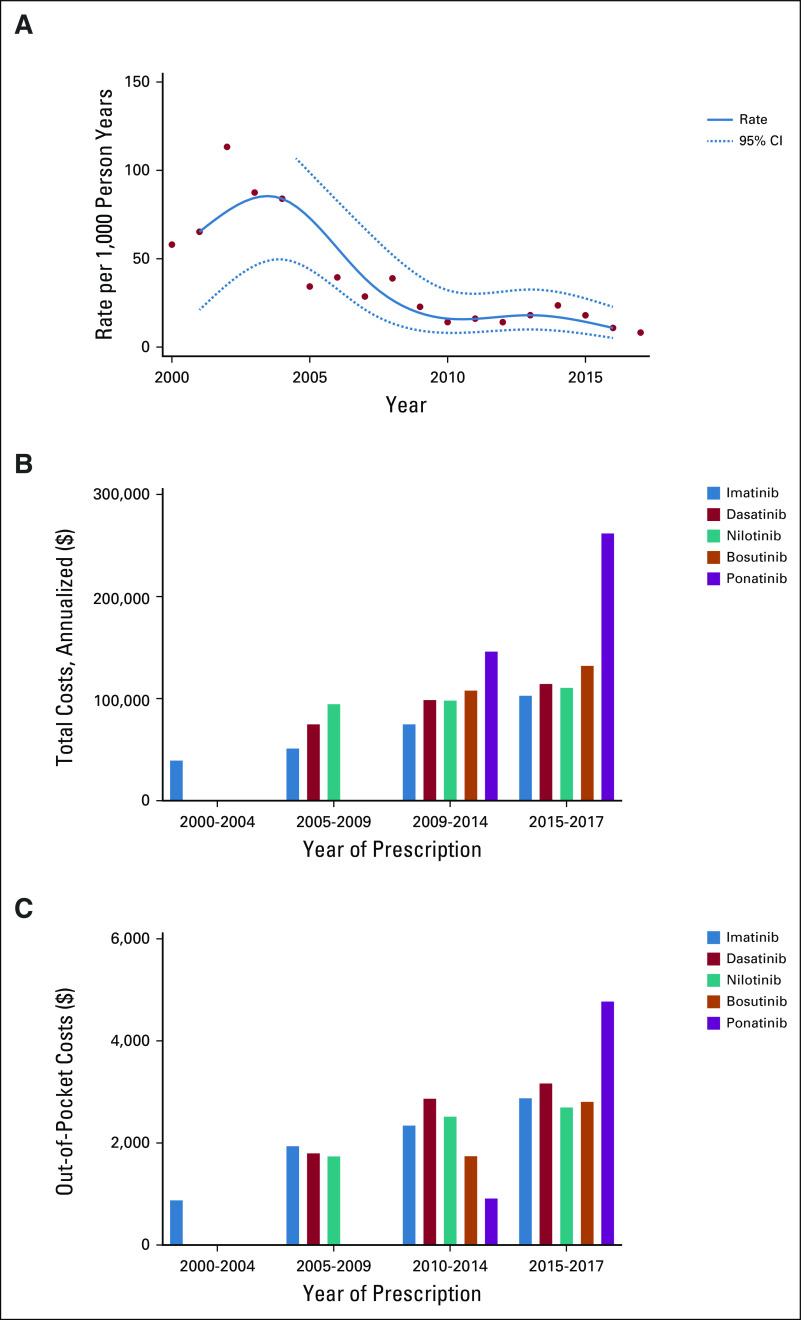
In the treatment of CML, this time period highlighted a paradigm shift. Overall, HCT usage was more common in the early 2000s, peaking around 2004, but it has subsequently declined (Fig 2A). From 2000 to 2004, imatinib was the only TKI being prescribed, with a mean annualized cost of $41,546; 2005-2009 demonstrated increased use of dasatinib and nilotinib, with a mean annualized cost for imatinib of $53,463; dasatinib, $77,142; and nilotinib, $96,912 (Fig 2B). During 2010-2014, the annual mean cost of imatinib ($77,219), dasatinib ($100,983), and nilotinib ($100,385) all increased. Ponatinib and bosutinib were prescribed for few patients (n < 20 claims each year), with total annual mean costs between 2010 and 2014 of $148,304 and $110,131, respectively. From 2015 to 2017, there was continued cost increases for imatinib ($105,069), dasatinib ($116,729), nilotinib ($112,780), and bosutinib ($134,337). During this time, ponatinib had a particularly large cost increase, to a mean annual cost of $264,138 versus $143,304 in prior years. Out-of-pocket costs also increased between 2001 and 2017 (Fig 2C).
FIG 2.
Incidence of hematopoietic-cell transplantation (HCT) in chronic myelogenous leukemia (CML) cohort. (A) Rate per 1,000 person-years. (B) Total cost. (C) Out-of-pocket costs.
In multivariable analysis limited to CML enrollees only, the treatment-level factor that had the largest independent influence on cost was the percentage of days on a TKI (≥ 75% of time v none: $108,716 [95% CI, $99,193 to $118,239]; Table 2). Other factors associated with cost in-cluded a history of HCT, a greater number of inpatient days, and a greater number of ambulatory visits. Patient-level factors influencing costs included younger age but not CCI.
DISCUSSION
Within an insurance database of commercial and Medicare Advantage enrollees, we evaluated the cost of CML therapy in the United States over time from 2000 to 2016 during the time period when TKIs were introduced and became widespread. We sought to compare the cost of care with that of a frequency-matched population of patients with hematologic malignancies and that of patients without a cancer diagnosis. We found that contemporary CML costs greatly exceeded the cost of treatment of other hematologic malignancies after the first 6 months of therapy. Overall, the average annual cost of CML care was more than $25,000 the cost of other hematologic malignancy care and $75,000 more expensive than for an individual from the general population without cancer. This difference was primarily driven by the cost of TKI prescriptions, with patients with CML without a TKI incurring a cost $108,716 less than those who received a TKI more than 75% of the time; this cost difference occurred despite the decrease in use of HCT for CML over time.
These results from a commercial and Medicare Advantage database reflect similar contributions of TKI use to the cost of care found in the Medicare population alone. A prior study of patients > 65 years of age using a SEER-Medicare linked data set estimated the cost of CML care with TKIs from 2007 to 2012 at $132,607, compared with $41,268 for those with CML not receiving a TKI, adjusted to 2018 dollars.5 In particular, understanding the relative cost of CML care as compared with the care of other hematologic malignancies is helpful to frame the acute need for financial support with the ongoing need for TKI therapy. It also highlights the paradigm shift from earlier HCT to TKI use in CML care. For comparison, a recent study of the economic burden of relapsed and refractory AML (acute myeloid leukemia) active therapy from a commercial database in the United States showed that the mean cost from relapse until death or end of study was $524,595 if transplantation was included, compared with $263,310 without transplantation.7 For patients who had a higher intensity of care, inpatient admissions were the largest component of cost, with prescriptions contributing only $24,640.7 In comparison with the intensity of care delivered in the relapsed/refractory AML setting, the need for inpatient care in CML is smaller when HCT is omitted, and the prescription costs demonstrated in this study are much greater.
The contribution of TKIs to the total direct costs of contemporary CML care is not surprising, given the paradigm shift in CML management after the advent of imatinib. This finding is also similar to the results of a recently published evaluation of patients in 10 integrated health care systems that demonstrated that 81% of their patients with CML received an oral agent between 2000 and 2017.8 Understanding the relative cost of CML care and the impact of TKI use on this cost is of particular importance as the prevalence of patients with CML increases.9 Imatinib was introduced in 2001 at $26,400 per year in 2001 dollars, and by 2016, the price had reached more than $120,000 per year.10 As argued by Chen and Kesselheim,10 the expected decrease in drug pricing with the advent of generic imatinib has been slow to be seen. With the introduction of generic imatinib to the market in the United States in February 2016, there was exclusivity with 1 generic imatinib manufacturer, with an initial pricing of generic imatinib at 30% below the price of the brand name product.10,11 In a study of a tertiary academic center, dasatinib was one of the top medications requiring financial assistance.12 A recently published economic model of cost savings of switching to generic imatinib cited 30-day wholesale acquisition costs in 2018 for dasatinib at $12, 912 and nilotinib at $13,644 versus branded imatinib at $10,122 and generic at $410, whereas other studies estimated generic imatinib at approximately $8,000 in 2017.13,14 We found TKI cost to be increasing over time. Ward et al15 evaluated total costs in patients with CML initiating care with a first-generation versus a second-generation TKI and demonstrated that total costs were 1.3 times higher for those starting with a second-generation TKI because of differences in pharmacy costs. Treatment initiation with second- and third-generation TKIs also seems to be increasingly common, so additional efforts to control the cost of CML care will be needed.14 Cost changes, use of second- and third-generation TKIs, the ongoing impact of cost on medication adherence, and improved understanding of the equivalency of generics will be needed to determine the impact of the introduction of generic TKIs into the market.
Another potential paradigm shift in CML therapy may occur with the publication of de-escalation and discontinuation trials such as STIM, DESTINY, and EURO-SKI, which demonstrated the safety of de-escalation and discontinuation of TKIs in some populations and the safety of restarting if there was a loss of major molecular response.16-18 As a result, discontinuation of TKIs for treatment-free remission is now more likely to move forward as a tenable treatment option for many patients.17,18 Recent modeling of the costs associated with discontinuation indicate that imatinib may still be the most cost-effective TKI, but additional evaluation as generic versions of second- and third-generation TKIs become available will be important.19
There are particular limitations to this study that may affect generalizability. First, because of the design and data source, we cannot address the costs of care of patients who are not insured and cannot account for the indirect costs of care such as lost days of work or transportation costs. Uninsured patients have been shown to have a lower adherence to TKI use and risk additional long-term costs.20 Second, the component of drug costs to the insurer and the patient may vary by insurance type, and our study was limited to patients in the OLDW; however, the costs here reflect paid costs. Third, although the time period we examined extends into late 2017, generic imatinib was not FDA approved until February 1, 2016, and therefore, we may not yet have captured the more recent effects on imatinib and other TKI pricing in response to this market change.
In conclusion, we found that the cost of contemporary CML care exceeded the cost of care for matched patients with hematologic malignancies after the first 6 months of therapy and for the general population. This cost was primarily driven by the cost of the prescription of TKIs as HCT use decreased over time. As therapeutic goals in CML continue to evolve toward treatment-free remission, continuing to monitor the relative cost of treatment will be important to both the health care system and the individual patient. This may also allow for additional advocacy efforts to help curtail costs with the introduction of generics.
Presented at the American Society of Hematology 61st Annual Conference, Orlando, FL, December 7-10, 2019.
SUPPORT
Supported by Stand Up 2 Cancer and the US National Institutes of Health, Grant. No. CA015704.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer J. Wilkes, Gary H. Lyman, Lena E. Winestone, Henry J. Henk, Eric J. Chow
Data analysis and interpretation: Jennifer J. Wilkes, Gary H. Lyman, David R. Doody, Shasank Chennupati, Eric J. Chow
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Health Care Cost Associated With Contemporary Chronic Myelogenous Leukemia Therapy Compared With That of Other Hematologic Malignancies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Gary H. Lyman
Consulting or Advisory Role: G1 Therapeutics, Partners Healthcare, Mylan, Spectrum Pharmaceuticals, Invitae, Sandoz-Novartis, Samsung Bioepis, bioTheranostics, BeyondSpring Pharmaceuticals, Daiichi Sankyo
Research Funding: Amgen (Inst)
Travel, Accommodations, Expenses: Bayer
Laura K. Becker
Employment: Optum
Stock and Other Ownership Interests: United Health Group
Pamela E. Morin
Employment: United Healthcare
Stock and Other Ownership Interests: United Healthcare
Research Funding: United Healthcare
Lena E. Winestone
Stock and Other Ownership Interests: Pfizer, Merck
Consulting or Advisory Role: Enlytic (I)
Henry J. Henk
Employment: Optum
Stock and Other Ownership Interests: United Health Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Larson RA, Guilhot F, et al. : Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 376:917-927, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER: SEER*Explorer. https://seer.cancer.gov/explorer/
- 4.Experts in Chronic Myeloid Leukemia : The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood 121:4439-4442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenzik KM, Bhatia R, Williams GR, et al. : Medicare and patient spending among beneficiaries diagnosed with chronic myelogenous leukemia. Cancer 125:2570-2578, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Wallace PJ, Shah ND, Dennin T, et al. : Optum Labs: Building a novel node in the learning health care system. Health Affairs 33:187-94, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Pandya BJ, Chen CC, Medeiros BC, et al. : Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with acute myeloid leukemia (AML) in the USA: A retrospective analysis of a commercial payer database. Adv Ther 36:1922-1935, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banegas MP, Rivera DR, O’Keeffe-Rosetti MC, et al. : Long-term patterns of oral anticancer agent adoption, duration, and switching in patients with CML. J Natl Compr Canc Netw 17:1166-1172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Cortes J, Kantarjian H: Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 118:3123-3127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CT, Kesselheim AS: Journey of generic imatinib: A case study in oncology drug pricing. J Oncol Pract 13:352-355, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Miller J. Sun hopes cut-price generic Gleevec will win third of U.S. market. Reuters, February 1, 2016 [Google Scholar]
- 12.Farano JL, Kandah HM: Targeting financial toxicity in oncology specialty pharmacy at a large tertiary academic medical center. J Manag Care Spec Pharm 25:765-769, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour EJ, Mendiola MF, Lingohr-Smith M, et al. : Economic modeling to evaluate the impact of chronic myeloid leukemia therapy management on the oncology care model in the US. J Med Econ 22:1113-1118, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Cole AL, Dusetzina SB: Generic price competition for specialty drugs: Too little, too late? Health Aff (Millwood) 37:738-742, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Ward MA, Fang G, Richards KL, et al. : Comparative evaluation of patients newly initiating first-generation versus second-generation tyrosine kinase inhibitors for chronic myeloid leukemia and medication adherence, health services utilization, and healthcare costs. Curr Med Res Opin 31:289-297, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Mahon FX, Réa D, Guilhot J, et al. : Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 11:1029-1035, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Clark RE, Polydoros F, Apperley JF, et al. : De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): A non-randomised, phase 2 trial. Lancet Haematol 6:e375-e383, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Saussele S, Richter J, Guilhot J, et al. : Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol 19:747-757, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto C, Nakashima H, Ikeda T, et al. : Analysis of the cost-effectiveness of treatment strategies for CML with incorporation of treatment discontinuation. Blood Adv 3:3266-3277, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry AM, Brunner AM, Zou T, et al. : Association between insurance status at diagnosis and overall survival in chronic myeloid leukemia: A population-based study. Cancer 123:2561-2569, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]