PURPOSE:
As immune checkpoint inhibitors (ICIs) have transformed the care of patients with cancer, it is unclear whether treatment at the end of life (EOL) has changed. Because aggressive therapy at the EOL is associated with increased costs and patient distress, we explored the association between the Food and Drug Administration (FDA) approvals of ICIs and treatment patterns at the EOL.
METHODS:
We conducted a retrospective, observational study using patient-level data from a nationwide electronic health record–derived database. Patients had advanced melanoma, non–small-cell lung cancer (NSCLC; cancer types with an ICI indication), or microsatellite stable (MSS) colon cancer (a cancer type without an ICI indication) and died between 2013 and 2017. We calculated annual proportions of decedents who received systemic cancer therapy in the final 30 days of life, using logistic regression to model the association between the post-ICI FDA approval time and use of systemic therapy at the EOL, adjusting for patient characteristics. We assessed the use of chemotherapy or targeted/biologic therapies at the EOL, before and after FDA approval of ICIs using Pearson chi-square test.
RESULTS:
There was an increase in use of EOL systemic cancer therapy in the post-ICI approval period for both melanoma (33.9% to 43.2%; P < .001) and NSCLC (37.4% to 40.3%; P < .001), with no significant change in use of systemic therapy in MSS colon cancer. After FDA approval of ICIs, patients with NSCLC and melanoma had a decrease in the use of chemotherapy, with a concomitant increase in use of ICIs at the EOL.
CONCLUSION:
The adoption of ICIs was associated with a substantive increase in the use of systemic therapy at the EOL in melanoma and a smaller yet significant increase in NSCLC.
INTRODUCTION
Improving end-of-life (EOL) care is critical for patients with cancer.1 It is well established that aggressive cancer treatment at the EOL neither improves quality of life nor prolongs survival.1-3 Patients who receive systemic cancer treatment at the EOL may experience less patient-centered care, because they are less likely to receive hospice services and are exposed to a higher risk of acute care use, such as emergency department visits, admissions to the intensive care unit, and death in the hospital.3-11 Clinical practice guidelines recommend against the use of cytotoxic chemotherapy in patients with solid tumor malignancies at the EOL.12,13 Despite these recommendations, the literature has demonstrated varying results with regard to the use of cytotoxic chemotherapy at the EOL. Although several studies have shown a decline in chemotherapy use at the EOL, others have demonstrated no significant change in cytotoxic chemotherapy use,14-18 with one study showing a stable to marginal increase in the use of targeted therapies at the EOL.18
As immune checkpoint inhibitors (ICIs) have become part of standard therapy in the treatment of advanced non–small-cell lung cancer (NSCLC) and melanoma, concerns about systemic therapy at the EOL must be reframed in the context of this rapidly changing treatment paradigm.15 In both NSCLC and melanoma, ICIs represent a well-tolerated option relative to cytotoxic chemotherapy, contributing to widespread adoption of these agents into clinical practice,14,16 while also leading to increasing concerns regarding the potential for inappropriate use near the EOL.11,19 In fact, the clinical decision to use ICIs toward the EOL among patients with advanced cancer is fraught with uncertainty for both patients and providers. Although ICIs are tolerated by most patients, immune-mediated toxicity is not uncommon, and life-threatening adverse events have been reported.19-25 Additionally, responses are difficult to predict in the absence of a reliable biomarker. Because median time to response ranges from 2-6 months in NSCLC and nearly 3 months in melanoma,26,27 even responders may not live long enough to derive benefit from these agents. Finally, Glisch et al11 showed that the use of ICIs at the EOL was associated with lower hospice enrollment and higher rates of in-hospital death.
Given the prior evidence concerning lack of benefit for systemic therapy at the EOL, it is important to understand whether the availability and adoption of ICIs has altered patterns of systemic therapy use during this period. To begin to address this gap in knowledge, we undertook a study to examine whether and how availability of ICIs altered use of systemic therapy at the EOL. Specifically, we determined (1) changes in the use of overall systemic therapy at the EOL in patients with 2 cancer types for which ICIs are approved by the Food and Drug Administration (FDA; melanoma and NSCLC), as well as for patients with a cancer type for which ICIs are not FDA approved or used in clinical practice (microsatellite stable [MSS] colon cancer), and (2) the association between adoption of ICIs into clinical practice and use of other systemic cancer therapies, such as cytotoxic chemotherapy and biologics/targeted therapies at the EOL. We hypothesized that the availability and adoption of ICIs has led to increased use of systemic therapy at the EOL in melanoma and NSCLC and that ICIs have replaced the use of other types of therapy (ie, cytotoxic chemotherapy and/or targeted/biologic therapies) at the EOL for these cancer types. We compared these patterns with those of MSS colon cancer, a cancer type for which there is no FDA approval for ICIs. In MSS colon cancer, prior literature has described no change in use of cytotoxic chemotherapy at the EOL.28,29 Therefore, we hypothesized that in contrast to cancer types for which ICIs have been FDA approved, there has been no significant change in use of systemic therapy in MSS colon cancer at the EOL during the study time period.
METHODS
Study Design
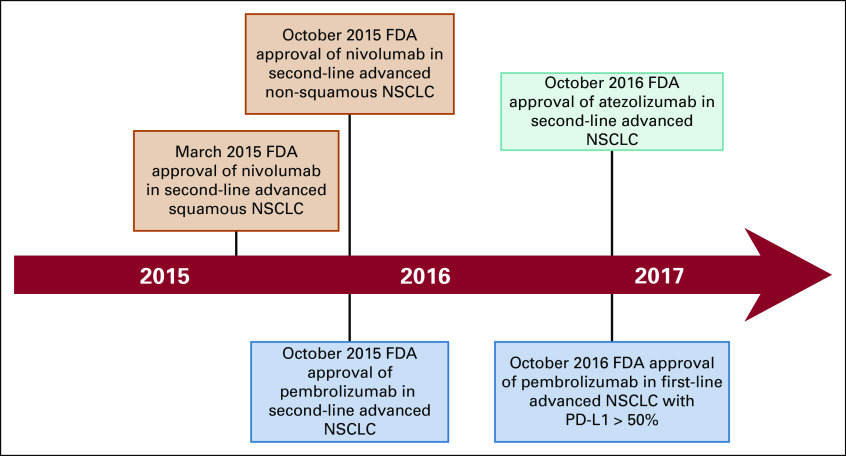
We conducted a retrospective cohort study using a large, national database to determine the association between the FDA approval of ICIs in melanoma and NSCLC and patterns of systemic cancer treatment at the EOL, using MSS colon cancer as a comparator. The pre-ICI and post-ICI treatment periods were centered around the FDA approval dates of the programmed death-1 (PD-1) inhibitors, pembrolizumab on September 4, 2014, and nivolumab on March 4, 2015, for melanoma and NSCLC, respectively (Appendix Figures A1 and A2; online only).30 We examined the relation between the FDA approval of ICIs and systemic therapy use at the EOL among patients with each cancer type using logistic regression analysis. We assessed the use of ICIs, cytotoxic chemotherapy, and/or targeted/biologic therapies at the EOL before and after the FDA approval of ICIs, using Pearson chi-square test.
Data Source
We used the Flatiron Health Database31 which contains longitudinal, de-identified patient-level electronic health record (EHR) data from a nationwide, geographically and demographically diverse population. At the time of this study, it included data from 265 cancer clinics at approximately 800 sites of care, with more than 2 million patients with cancer in the United States available for analysis. Flatiron Health captures EHR data from both structured and unstructured sources, using technology-enhanced abstraction techniques.31 Abstracted diagnosis dates, stage at diagnosis, oral anticancer medications, and line of therapy were used in this study. Flatiron Health holds an institutional review board approval that was obtained before study conduct with a waiver of informed consent. The data provided to Yale were de-identified, with provisions to prevent reidentification to protect patients' confidentiality. The Yale Human Investigations Committee deemed this study nonhuman subjects research.
Cohort Selection
The study sample included decedents with a diagnosis of advanced melanoma, NSCLC, or MSS colon cancer who died between January 1, 2013, and April 30, 2017. Advanced disease was defined as the presence of stage IIIB/IV disease at the time of diagnosis or the development of metastasis after initial diagnosis in patients with melanoma, NSCLC, or MSS colon cancer by the American Joint Committee on Cancer staging, 7th edition, at the time of diagnosis.32 Patients were excluded if there was no evidence that the patient was actively seeking care in the health care system through presence of recorded vitals, clinic visits, medication administration/medication orders, which also included supportive care, such as administration of intravenous fluids. Patients receiving treatment in a clinical trial at the EOL were also excluded because the details regarding which drugs were administered in the clinical trial were not available.
Construction of Variables
The primary dependent variable was systemic therapy in the final 30 days of life, where systemic therapy was defined as any cytotoxic chemotherapy, ICI, or targeted/biologic therapy administered to the patient. Hormone therapies were not included as systemic therapy for the specific cancer types included in this study. Details of systemic therapy use was derived from medication orders and administration data. In addition to PD-1 inhibitors, cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) inhibitors first approved in 2011 and programmed death-ligand 1 (PD-L1) inhibitors first approved in 2016 were also categorized as ICIs. Patient characteristics included age at death, sex, race, year of diagnosis, number of comorbidities, stage at initial diagnosis, and date of death. Comorbidities were assessed by using the categories outlined by Elixhauser et al33 by International Classification of Diseases (9th revision; ICD9)/ICD10 diagnosis codes. The comorbidity variable was defined as a sum of the number comorbidities. Race was included as a covariate because racial disparities exist in the treatment of cancer.34,35 There have been substantial disparities in receipt of recommended treatments between Black patients and White patients, and these disparities have remained unchanged over time.35 Date of death details were provided at the level of month and year of death for each patient to guard against identification of protected health information. To ascertain systemic therapy use in the final 30 days of life, we assigned death date as the 15th day of the month. To account for this imprecision, this was followed by a sensitivity analysis, with date of death assigned alternatively to the first or last days of the month. Notably, Flatiron Health mortality information is derived from EHR data, supplemented with Flatiron’s commercial mortality source and the US Social Security Death Index. The data are highly reliable, with sensitivity between 85% and 91%, and a specificity > 97%, relative to the National Death Index.36
Statistical Analysis
We used descriptive statistics to define the demographic, clinical, and treatment characteristics of the study samples. We assessed the relation between demographic and clinical characteristics and receipt of cancer therapy at the EOL by using the Pearson’s chi-square test.
Change in use of any systemic therapy.
We used logistic regression to model the association between the post-ICI approval time period (the independent variable) and the use of systemic therapy in the final 30 days of life (the dependent variable), adjusting for sex, age at diagnosis, race, comorbidities, and stage at diagnosis. Pre- and post-ICI time periods were set by the respective ICI approval dates for NSCLC and melanoma. For each of these 2 cancer types, we also constructed logistic regression models with colorectal cancer as the control, using these ICI approval dates as the before and after time periods to create comparison groups.
Change in use by therapeutic classes.
To understand the association between ICI approval and the use of other systemic cancer treatments at the EOL, such as cytotoxic chemotherapy and biologic/targeted therapies, we determined the proportion of each type of systemic therapy at the EOL in the pre- and post-ICI approval time periods. Pearson chi-square test was used to assess change of systemic therapy type at the EOL on the FDA approval of ICI across cancer types. We used SAS 9.4 (SAS Institute, Cary, NC) to perform all statistical analyses and used a 2-sided P of < .05 for statistical significance.
RESULTS
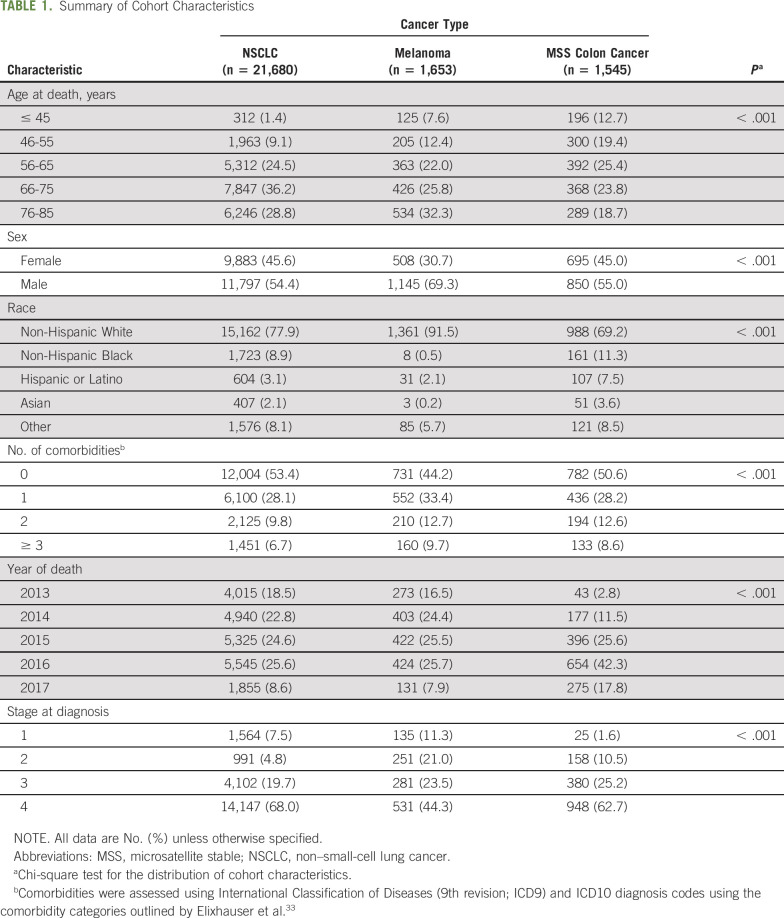
Patient cohorts consisted of 21,680 patients with NSCLC, 1,653 with melanoma, and 1,545 with MSS colon cancer. The majority (70.4%) were non-Hispanic White patients. The melanoma cohort consisted primarily of men (69%), whereas both NSCLC and MSS colon cancer had a relatively even distribution between male and female decedents. The majority of decedents in each cohort had stage IV disease at the time of treatment initiation (Table 1).
TABLE 1.
Summary of Cohort Characteristics
Change in Use of Any Systemic Therapy
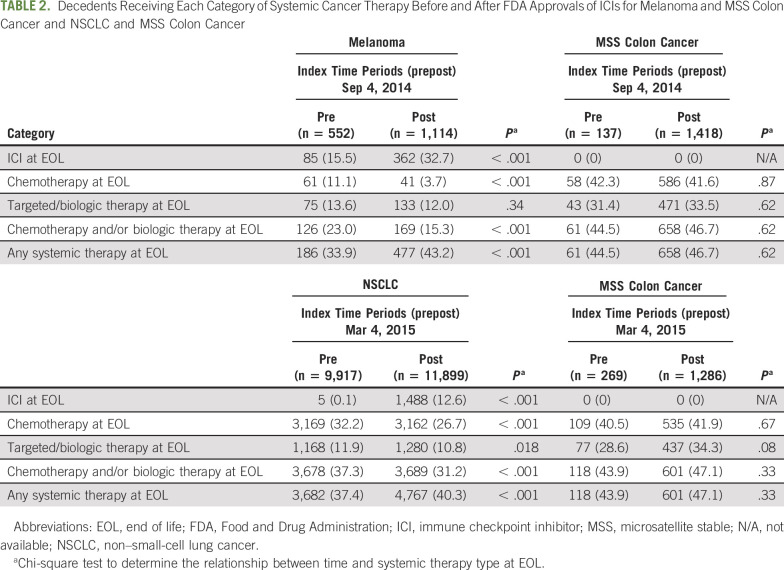
In the pre-ICI study time period, 33.9% of patients with melanoma received systemic therapy at the EOL, which increased to 43.2% in the post-ICI time period (P < .001; Table 2; Appendix Table A1, online only). In patients with advanced NSCLC, the use of systemic therapy at the EOL increased from 37.4% in the pre-ICI time period to 40.3% in the post-ICI time period (P < .001; Table 2; Appendix Table A2, online only). In contrast, the control group of decedents with MSS colon cancer demonstrated no significant increase in use of systemic therapy at the EOL (P = .62 and .37 for each FDA approval date of ICIs in melanoma and NSCLC, respectively; Tables 2 and 3).
TABLE 2.
Decedents Receiving Each Category of Systemic Cancer Therapy Before and After FDA Approvals of ICIs for Melanoma and MSS Colon Cancer and NSCLC and MSS Colon Cancer
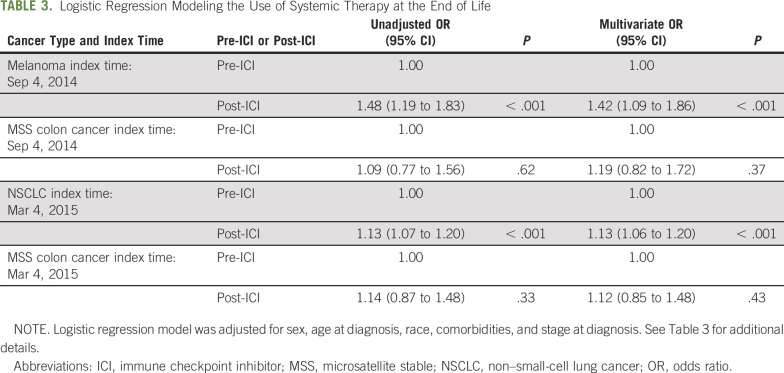
TABLE 3.
Logistic Regression Modeling the Use of Systemic Therapy at the End of Life
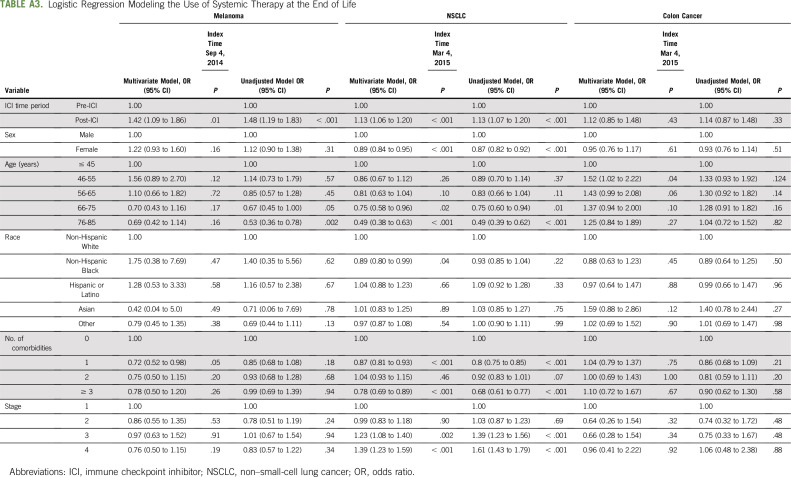
After controlling for patient characteristics, there were significantly higher odds of receiving systemic treatment at the EOL in the post-ICI time period compared with the pre-ICI time period in melanoma (odds ratio [OR], 1.42; 95% CI, 1.09 to 1.86; P < .001) and NSCLC (OR, 1.13; 95% CI, 1.06 to 1.20; P < .001; Table 3; Appendix Table A3, online only). For patients with MSS colon cancer, there was no significant difference in receipt of systemic therapy in the pre- and post-melanoma ICI approval time period (OR, 1.18; 95% CI, 0.81 to 1.71; P = .38; Appendix Table A3), as well as in the pre- and post-NSCLC ICI approval time period (OR, 1.11; 95% CI, 0.84 to 1.48; P = .45; Table 3; Appendix Table A3).
Change in Use by Therapeutic Classes
The approval of ICIs in melanoma and NSCLC was associated with a change in treatment patterns of cytotoxic chemotherapy and targeted/biologic therapy use at the EOL. After the approval of ICIs in melanoma, the use of cytotoxic chemotherapy decreased from 11.1% to 3.7% (P < .001; Table 2), and the use of biologic/targeted therapies remained stable (13.6%-12.0%; P .34; Table 2) . Conversely, the use of ICIs at the EOL increased from 15.5% to 32.7% (P < .001; Table 2; Fig 1). Notably, ICI use in patients with melanoma before FDA approval of pembrolizumab in 2014 represented treatment with the CTLA-4 inhibitor, ipilimumab, rather than the PD-1 or PD-L1 inhibitors, which were the main focus of this study.
Fig. 1.
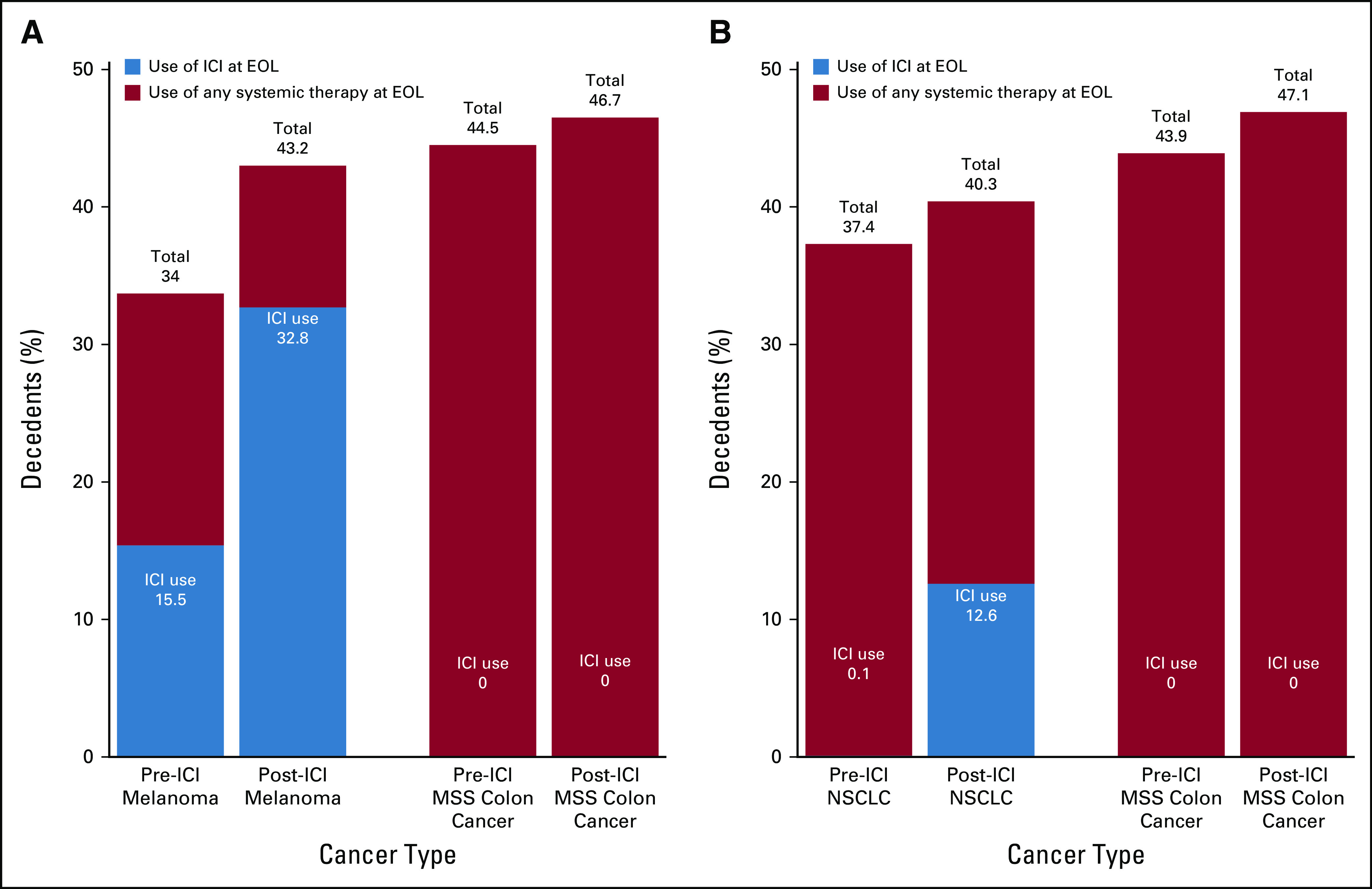
Change in use of immune checkpoint inhibitors (ICIs) and any systemic therapy across pre– and post–Food and Drug Administration (FDA) approval time periods for each cancer type. (A) Depicts the use of ICIs and any systemic therapy at the end of life (EOL) for melanoma and microsatellite stable (MSS) colon cancer before and after the FDA approval of ICIs in melanoma on September 4, 2014. (B) Depicts the use of ICIs and any systemic therapy at EOL for non–small-cell lung cancer (NSCLC) and MSS colon cancer before and after the FDA approval of ICIs for NSCLC on March 4, 2015.
Among patients with NSCLC, although overall EOL therapy use increased, there was a decrease in use of cytotoxic chemotherapy (32.2%-26.7%; P < .001) and biologic/targeted therapies (11.9% to 10.8%; P = .018) from the pre-ICI to post-ICI time periods (Table 2). Hence, the net increase of 2.9% (P < .001) in the use of systemic therapy was associated with the increase in use of ICIs at the EOL (from 0.1% to 12.6%; P < .001; Fig 1). Decedents with MSS colon cancer did not have a statistically significant change in use of cytotoxic chemotherapy or targeted/biologic therapies during the study period.
DISCUSSION
To our knowledge, this is the first study to characterize the relationship between the approval of ICIs and systemic treatment patterns in the final 30 days of life in patients with melanoma and NSCLC. We found that FDA approvals of ICIs for advanced NSCLC and melanoma were associated with a significant increase in the use of systemic cancer therapy at the EOL and that this increase in treatment was associated with the adoption of ICIs into clinical practice. In contrast, there was no significant change in the use of systemic therapy at the EOL for MSS colon cancer, a cancer type for which there is no indication for ICI use.
Prior literature assessing the influence of ICIs on EOL care in patients with urothelial cell carcinoma similarly demonstrated that the proportion of patients who started any systemic therapy at the EOL doubled between 2015 and 2017, and that this change was primarily driven by the adoption of ICIs.37 That study was limited in that it focused on a single tumor type and did not use a comparison group. Furthermore, studies conducted in the pre-ICI era assessing trends in use of cytotoxic chemotherapy at the EOL in multiple tumor types, including colon cancer, demonstrated that there was little to no significant change over time,17,38,39 consistent with our findings of no significant change in use of systemic therapy in decedents with MSS colon cancer. Previous studies reported that 7%-10% of patients with melanoma40 and 43% with NSCLC received chemotherapy at the EOL,41 similar to our findings in the pre-ICI approval time periods.
As newer therapies have become available in NSCLC, more patients have received these new therapies for longer periods of time, suggesting more use at the EOL, as described by Murillo and Koeller.41 On one hand, we have identified a similar trend with the availability of ICIs with an increase in the use of systemic therapy at the EOL coincident with the introduction of this therapeutic option. On the other hand, tumors such as melanoma that are considered to be chemo-insensitive are associated with a lower rate of receiving palliative chemotherapy at any time period compared with chemosensitive tumors.4 However, in the context of the availability of ICIs, to which melanoma is responsive, there may now be a greater willingness to use these drugs at the EOL, leading to an increase in use of systemic cancer therapy as demonstrated by this study.
There are several potential reasons for the adoption of ICIs in the EOL setting. Because patients tend to have a worsening performance status as they near the EOL,42 ICI use may be favored because of the perception that these drugs are better tolerated than cytotoxic chemotherapy. Parikh et al37 found that after the FDA approval of atezolizumab in 2016, there was an increase in the initiation of ICIs in the final 60 days of life among patients with a poor performance status, with no significant change in those with a good performance status. Clinicians may offer, and patients may request, a trial of ICIs without known benefit at the EOL and risk the adverse events.19,40,43
Because ICIs offer the potential for a durable response, oncologists who have limited treatment options to offer may turn to it as a final option. Studies have shown that physicians spend little time discussing prognostic implications of scans, instead focusing on treatment.44,45 Moreover, physicians’ estimation of EOL tends to favor an optimistic prognosis,46,47 which may lead toward the use of systemic therapy near the EOL. Additionally, prolonged physician counseling at the EOL may be limited because of time constraints of busy clinics, which may affect physician willingness and opportunity to address these important topics.4
Prior literature has recognized that limiting chemotherapy at the EOL improves quality of life,48,49 and as a result, chemotherapy use at the EOL is now recognized as a metric of poor quality care.48 Notably, these studies were designed to evaluate the impact of chemotherapy at the EOL and not ICIs, which are associated with a different set of toxicities and potential outcomes. Future studies must explore the impact of EOL ICI use on patient outcomes, including cost of care and quality of life.
Using a study population comprising decedents has advantages and limitations.50-52 One disadvantage of this approach is the potential for introducing biased inferences regarding treatment patterns through the exclusion of patients who may have received treatment near the EOL and subsequently responded to treatment. To mitigate this potential bias, our study evaluated a limited duration of time before death.50,52 A decedent-based analytic approach has the advantage of using a selection of patients that is not dependent on physicians’ clinical prediction of survival, which tends to overestimate survival.53-55 Furthermore, the time relationship to death is known, therefore allowing for the analysis of details regarding systemic therapy use in a well-defined timeframe.50,51
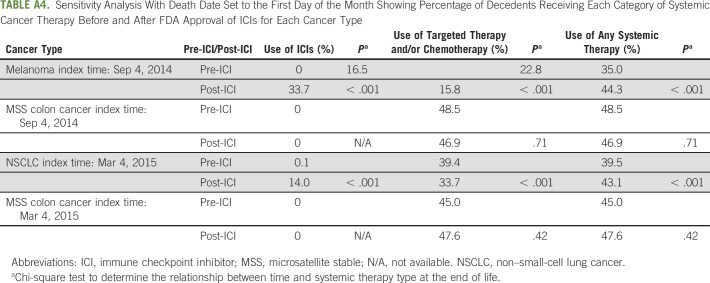
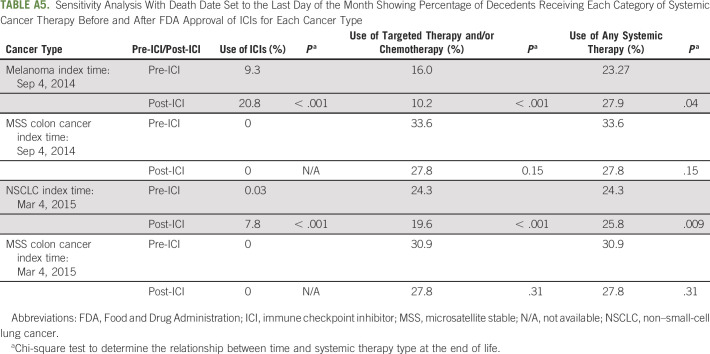
We acknowledge additional limitations to this study. Although geographically and demographically diverse, our population was limited to patients primarily in community oncology practices, limiting our understanding of the academic oncology practice setting. Additionally, we were unable to adjust for line of therapy or performance status, variables that may affect EOL treatment decisions, because of a high degree of missing data for both variables (30% and 51%, respectively). However, we performed a sensitivity analysis incorporating these covariates into the model, with conclusions remaining unchanged. Notably, there was also a restriction on the details of the date of death to month and year, also necessitating a sensitivity analysis with qualitatively unchanged conclusions. By running the sensitivity analysis, we were able to show that despite having death data at the level of month and year, our conclusions did not change based on the specific day of death (Appendix Tables A4 and A5).
Other factors, such as other therapies and changes in insurance formularies and reimbursements, could affect use of systemic therapy at the EOL. We mitigated the uncertainty of unmeasured confounders by including colon cancer as a control group, not expecting to have changes in use in response to an FDA approval of an ICI in a different cancer type. Notably, the overall increase in patients with NSCLC and patients with MSS colon cancer was similar (approximately 3% increase); although MSS colon cancer is in many ways an ideal comparator from a clinical perspective, the sample size in the data was relatively small, contributing to the lack of significance in this group. Certainly, MSS colon cancer having had relatively limited FDA drug approvals during the study time period may have affected use of systemic therapy at the EOL in this group. Although ICIs have been approved in many cancer types beyond those studied here, the generalizability of our findings is limited to NSCLC, melanoma, and MSS colon cancer. Furthermore, we were unable to delineate the patients who initiated ICIs near the EOL, which would have allowed for additional insight into treatment patterns at the EOL associated with the adoption of ICIs. However, as more ICIs are developed and approved in new tumor types and in earlier-stage disease, the use of ICIs in patients near the EOL will continue to evolve.
In conclusion, we found that FDA approval of ICIs for metastatic melanoma and NSCLC was associated with an increase in the use of systemic treatment at the EOL attributable to the adoption of ICIs into clinical practice. For patients with melanoma, the adoption of ICIs was associated with a substantive increase in EOL treatment driven by ICIs, whereas in patients with NSCLC, ICIs to large extent were replacing cytotoxic chemotherapy in the EOL setting. Because ICI use at the EOL has been associated with worse patient outcomes,11 the increased use of ICIs at the EOL, as determined by our study, raises concerns of declining value-based care at the EOL.
APPENDIX
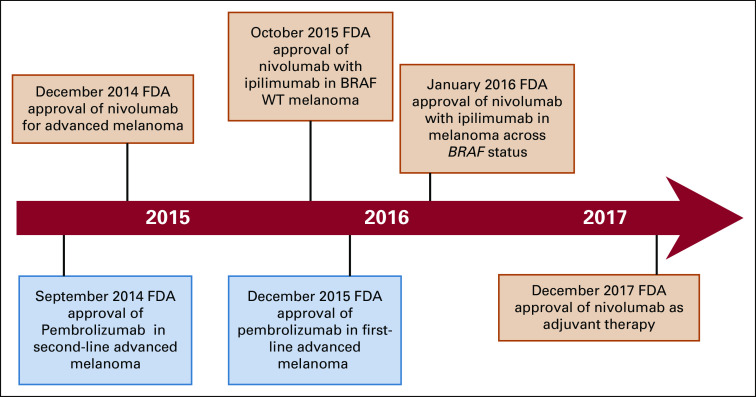
Fig A1.
Timeline showing the US Food and Drug Administration (FDA) approval of immune checkpoint inhibitors in the treatment of melanoma during the study time period. WT, wild type.
Fig A2.
Timeline showing the US Food and Drug Administration (FDA) approval of immune checkpoint inhibitors in the treatment of non–small-cell lung cancer (NSCLC) during the study time period. PD-L1, programmed death-ligand 1.
TABLE A1.
Sample Proportions to Assess the Significance of Change from Pre- to Post-ICI Time Period in NSCLC
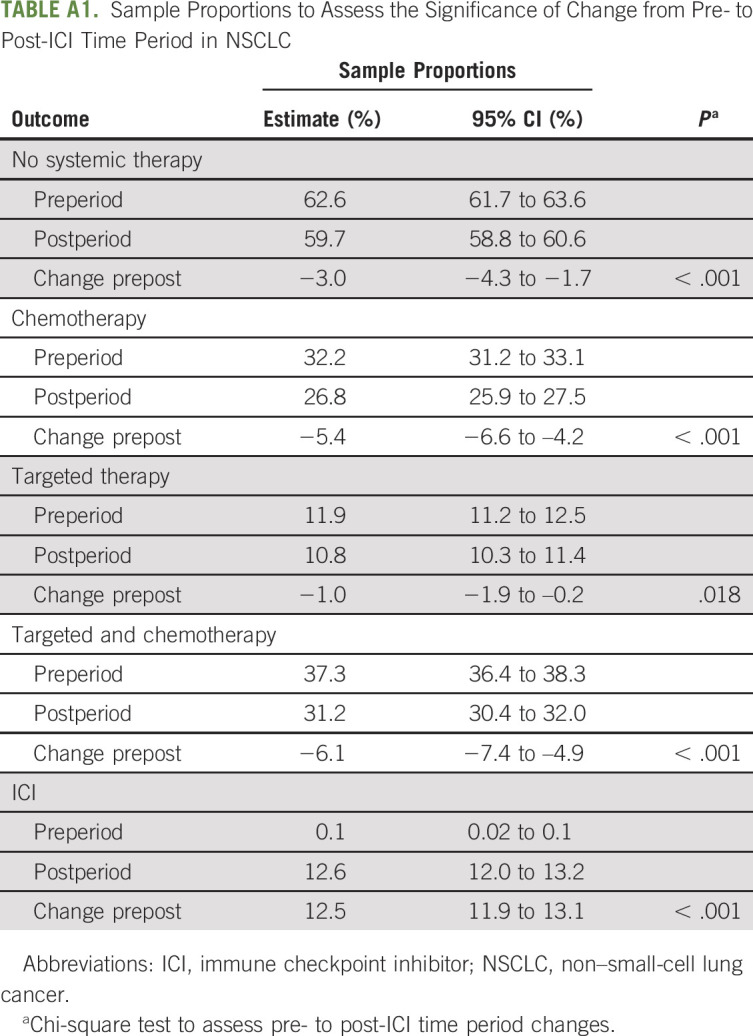
TABLE A2.
Sample Proportions to Assess the Significance of Change from Pre- to Post-ICI Time Period in Melanoma
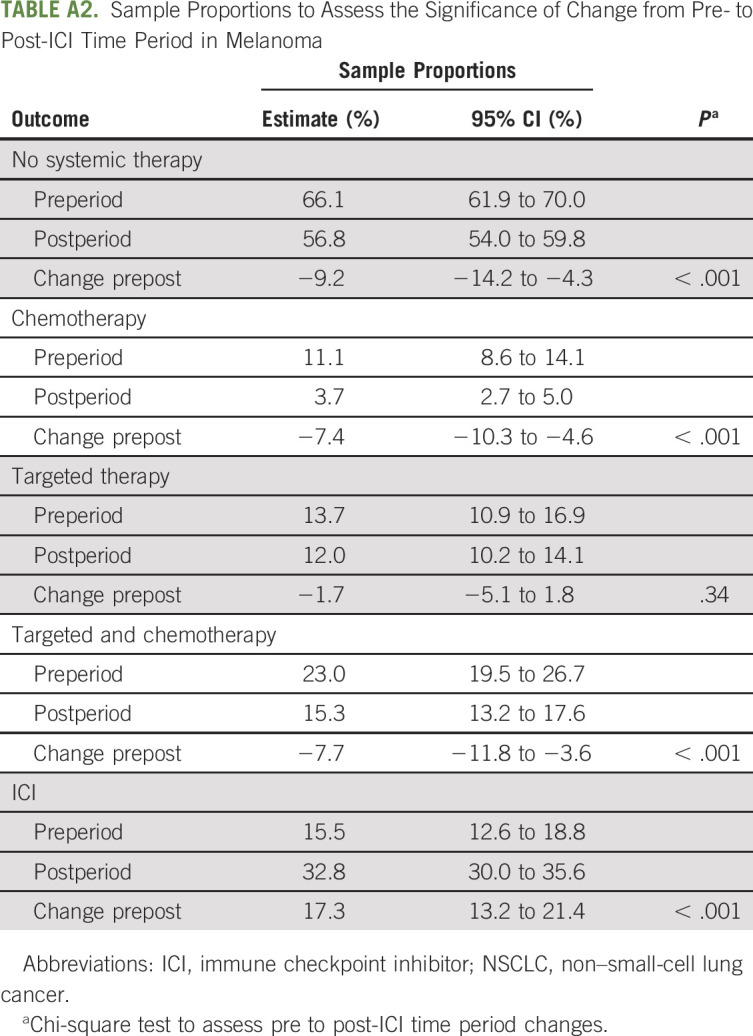
TABLE A3.
Logistic Regression Modeling the Use of Systemic Therapy at the End of Life
TABLE A4.
Sensitivity Analysis With Death Date Set to the First Day of the Month Showing Percentage of Decedents Receiving Each Category of Systemic Cancer Therapy Before and After FDA Approval of ICIs for Each Cancer Type
TABLE A5.
Sensitivity Analysis With Death Date Set to the Last Day of the Month Showing Percentage of Decedents Receiving Each Category of Systemic Cancer Therapy Before and After FDA Approval of ICIs for Each Cancer Type
SUPPORT
Supported by the Yale Cancer Center Support Grant No. P30 CA016359 and the Clinical and Translational Science Awards Grant No. UL1 TR000142 from the National Center for Advancing Translational Sciences.
AUTHOR CONTRIBUTIONS
Conception and design: Fauzia Riaz, Amy J. Davidoff, Kerin B. Adelson, Blythe J. Adamson, Cary P. Gross
Collection and assembly of data: Fauzia Riaz, Blythe J. Adamson, Pooja Shaw, Cary P. Gross
Data analysis and interpretation: Fauzia Riaz, Geliang Gan, Fangyong Li, Amy J. Davidoff, Carolyn J. Presley, Blythe J. Adamson, Ravi B. Parikh, Ronac Mamtani, Cary P. Gross
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adoption of Immune Checkpoint Inhibitors and Patterns of Care at the End of Life
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Geliang Gan
Employment: The Rothberg Institute (I), Thermo Fisher Scientific (I)
Stock and Other Ownership Interests: The Rothberg Institute (I)
Research Funding: The Rothberg Institute (I)
Fangyong Li
Consulting or Advisory Role: IVIVIA
Amy J. Davidoff
Honoraria: Celgene (I), Kyowa Hakko Kirin (I), Jazz Pharmaceuticals (I), Tolero Pharmaceuticals (I)
Consulting or Advisory Role: Celgene (I), AbbVie (I), Amgen (I)
Research Funding: Boehringer Ingelheim (Inst), Celgene (Inst)
Uncompensated Relationships: Flatiron Health
Kerin B. Adelson
Stock and Other Ownership Interests: Lyra Health (I), MindNest Health (I), Carrum Health
Honoraria: Genentech
Consulting or Advisory Role: HERON, Celgene, Roche DIS
Research Funding: Genentech
Patents, Royalties, Other Intellectual Property: Genentech
Travel, Accommodations, Expenses: Genentech, HERON, Celgene, Roche DIS
Other Relationship: Genentech
Carolyn J. Presley
Consulting or Advisory Role: Potentia Metrics
Blythe J. Adamson
Employment: Flatiron Health
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Provisional patent
Pooja Shaw
Employment: Flatiron Health
Stock and Other Ownership Interests: Roche
Ravi B. Parikh
Stock and Other Ownership Interests: Merck, Google, GNS Healthcare
Consulting or Advisory Role: GNS Healthcare, Cancer Study Group
Travel, Accommodations, Expenses: Conquer Cancer Foundation, Flatiron Health
Ronac Mamtani
Honoraria: Flatiron Health
Consulting or Advisory Role: Genentech, Seattle Genetics/Astellas
Cary P. Gross
Research Funding: Johnson & Johnson (Inst), Pfizer (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Flatiron Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wright AA, Keating NL, Ayanian JZ, et al. : Family perspectives on aggressive cancer care near the end of life. JAMA 315:284-292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Gomes B, Higginson IJ: End-of-life care—what do cancer patients want? Nat Rev Clin Oncol 11:100-108, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Prigerson HG, Bao Y, Shah MA, et al. : Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 1:778-784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao S, Shafiq J, Vardy J, et al. : Use of chemotherapy at end of life in oncology patients. Ann Oncol 20:1555-1559, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Schnipper LE, Smith TJ, Raghavan D, et al. : American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol 30:1715-1724, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Wright AA, Zhang B, Keating NL, et al. : Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ 348:g1219, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright AA, Zhang B, Ray A, et al. : Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300:1665-1673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane RL, Wales J, Bernstein L, et al. : A randomised controlled trial of hospice care. Lancet 1:890-894, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Chiang JK, Lee YC, Kao YH: Trend analysis of end-of-life care between hospice and nonhospice groups of cancer patients in Taiwan for 2002-11. Medicine (Baltimore) 96:e7825, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glisch C, Hagiwara Y, Gilbertson-White S, et al. : Immune checkpoint inhibitor use near the end of life is associated with poor performance status, lower hospice enrollment, and dying in the hospital. Am J Hosp Palliat Care 37:179-184, 2020 [DOI] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network: Palliative care. https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf.
- 13.Greer JA, Pirl WF, Jackson VA, et al. : Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 30:394-400, 2012 [DOI] [PubMed] [Google Scholar]
- 14.McDermott D, Lebbé C, Hodi FS, et al. : Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev 40:1056-1064, 2014 [DOI] [PubMed] [Google Scholar]
- 15.O’Connor JM, Fessele KL, Steiner J, et al. : Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol 4:e180798, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Man J, Ritchie G, Links M, et al. : Treatment-related toxicities of immune checkpoint inhibitors in advanced cancers: A meta-analysis. Asia Pac J Clin Oncol 14:141-152, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Kraut J, Mooney K, Sweetenham JW, et al. : Implementing an electronic end-of-life chemotherapy utilization measure. J Oncol Pract 15:220-223, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Fang P, Jagsi R, He W, et al. : Rising and falling trends in the use of chemotherapy and targeted therapy near the end of life in older patients with cancer. J Clin Oncol 37:1721-1731, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fojo T: Desperation oncology. Semin Oncol 45:105-106, 2018 [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1093/annonc/mdv383. Naidoo J, Page DB, Li BT, et al: Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375-2391, 2015 [Erratum: Ann Oncol 27:1362] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolata G: ‘Desperation Oncology’: When patients are dying, some cancer doctors turn to immunotherapy. The New York Times, April 26, 2018.
- 22. Tedeschi B: Doctors want to give their cancer patients every chance. But are they pushing off hard talks too long? https://www.pbs.org/newshour/health/doctors-want-give-cancer-patients-every-chance-pushing-off-hard-talks-long.
- 23.El Osta B, Hu F, Sadek R, et al. : Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 119:1-12, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Villadolid J, Amin A: Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl Lung Cancer Res 4:560-575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishijima TF, Shachar SS, Nyrop KA, et al. : Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: A meta-analysis. Oncologist 22:470-479, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SP: Immune checkpoint blockade for lung cancer: State of the art. Transl Cancer Res 4:415-422, 2015 [Google Scholar]
- 28.Pataky RE, Cheung WY, de Oliveira C, et al. : Population-based trends in systemic therapy use and cost for cancer patients in the last year of life. Curr Oncol 23:S32-S41, 2016. (suppl) 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CEP: National trends in end of life care for veterans with advanced cancer. J Clin Oncol 36:3, 2018. (30; suppl) [DOI] [PubMed] [Google Scholar]
- 30. Raedler LA: Keytruda (pembrolizumab): First PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits 8:96-100, 2015. [PMC free article] [PubMed] [Google Scholar]
- 31. Flatiron: Accelerate research with the most advanced real-world evidence platform in oncology. https://flatiron.com/real-world-evidence/
- 32. Edge SB, Byrd DR, Compton CC, et al: AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010. [Google Scholar]
- 33.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 36:8-27, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Morris AM, Rhoads KF, Stain SC, et al. : Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg 211:105-113, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Hardy D, Liu CC, Xia R, et al. : Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer 115:2199-2211, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Curtis MD, Griffith SD, Tucker M, et al. : Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res 53:4460-4476, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh RB, Galsky MD, Gyawali B, et al. : Trends in checkpoint inhibitor therapy for advanced urothelial cell carcinoma at the end of life: Insights from real-world practice. Oncologist 24:e397-e399, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho TH, Barbera L, Saskin R, et al. : Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 29:1587-1591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SY, Hall J, Pollack CE, et al. : Trends in end-of-life cancer care in the Medicare program. J Geriatr Oncol 7:116-125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. : Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med 138:639-643, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Murillo JR, Koeller J: Chemotherapy given near the end of life by community oncologists for advanced non-small cell lung cancer. Oncologist 11:1095-1099, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Seow H, Barbera L, Sutradhar R, et al. : Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol 29:1151-1158, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Slevin ML, Stubbs L, Plant HJ, et al. : Attitudes to chemotherapy: Comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ 300:1458-1460, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S, Cortez D, Maynard D, et al. : Characterizing the nature of scan results discussions: Insights into why patients misunderstand their prognosis. J Oncol Pract 13:e231-e239, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maynard DW, Cortez D, Campbell TC: ‘End of life’ conversations, appreciation sequences, and the interaction order in cancer clinics. Patient Educ Couns 99:92-100, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang TK, Kim DY, Lee SW, et al. : Trends in treatment during the last stages of life in end-stage gynecologic cancer patients who received active palliative chemotherapy: A comparative analysis of 10-year data in a single institution. BMC Palliat Care 17:99, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lamont EB, Christakis NA: Some elements of prognosis in terminal cancer. Oncology (Williston Park) 13:1165-1170; discussion 1172-1174, 1179-1180. [PubMed] [Google Scholar]
- 48.Lee HS, Chun KH, Moon D, et al. : Trends in receiving chemotherapy for advanced cancer patients at the end of life. BMC Palliat Care 14:4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Barnato AE, Lynn J: Resurrecting treatment histories of dead patients. JAMA 293:1591-1592, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Teno JM, Mor V: Resurrecting treatment histories of dead patients. JAMA 293:1591, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients: A study design that should be laid to rest. JAMA 292:2765-2770, 2004 [DOI] [PubMed] [Google Scholar]
- 53. doi: 10.1016/j.jpainsymman.2015.03.004. Amano K, Maeda I, Shimoyama S, et al: The accuracy of physicians' clinical predictions of survival in patients with advanced cancer. J Pain Symptom Manage 50: 139-146.e1. [DOI] [PubMed] [Google Scholar]
- 54.Hui D, Park M, Liu D, et al. : Clinician prediction of survival versus the Palliative Prognostic Score: Which approach is more accurate? Eur J Cancer 64:89-95, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christakis NA, Lamont EB: Extent and determinants of error in physicians’ prognoses in terminally ill patients: Prospective cohort study. West J Med 172:310-313, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]