PURPOSE
The rho-associated coiled-coil–containing protein kinase-2 (ROCK2) signaling pathway regulates the Th17/regulatory T cells balance and controls profibrotic pathways. Selective ROCK2 inhibition with belumosudil (KD025) may offer a novel approach to the management of chronic graft-versus-host disease (cGVHD).
PATIENTS AND METHODS
A phase IIa, open-label, dose-finding study of belumosudil enrolled 54 patients with cGVHD who had received one to three prior lines of therapy (LOTs). The primary end point was overall response rate (ORR).
RESULTS
The median time from cGVHD diagnosis to enrollment was 20 months. Seventy-eight percent of patients had severe cGVHD, 50% had ≥ 4 organs involved, 73% had cGVHD refractory to their last LOT, and 50% had received ≥ 3 prior LOTs. With an overall median follow-up of 29 months, the ORR (95% CI) with belumosudil 200 mg once daily, 200 mg twice daily, and 400 mg once daily was 65% (38% to 86%), 69% (41% to 89%), and 62% (38% to 82%), respectively. Responses were clinically meaningful, with a median duration of response of 35 weeks, and were associated with quality-of-life improvements and corticosteroid (CS) dose reductions. CS treatment was discontinued in 19% of patients. The failure-free survival rate was 76% (62% to 85%) and 47% (33% to 60%) at 6 and 12 months, respectively. The 2-year overall survival rate was 82% (69% to 90%). Belumosudil was well-tolerated, with low rates of cytopenia. There were no unexpected adverse events and no apparent increased risk of infection, including cytomegalovirus infection and reactivation.
CONCLUSION
Belumosudil treatment resulted in a high ORR and overall survival rate and demonstrated quality-of-life improvements, CS dose reductions, and limited toxicity. Data from the study indicated that belumosudil may prove to be an effective therapy for patients with treatment-refractory cGVHD.
INTRODUCTION
Chronic graft-versus-host disease (cGVHD) is an immune-mediated inflammatory and fibrotic disorder.1 It is a leading cause of morbidity,2 mortality,2,3 and impaired quality of life (QOL)4 after an allogeneic hematopoietic cell transplant (alloHCT).2-4 cGVHD affects up to 70% of all alloHCT recipients,2,5-10 with an incidence of 20%-50% in children,9 who survive more than 100 days after alloHCT.10 It is the leading cause of nonrelapse mortality beyond 2 years after alloHCT.10
The estimated prevalence of cGVHD is 14,000 patients in the United States (as of 2016).11 Of those patients, approximately 40% have severe disease.12,13 In addition, 42% of patients have ≥ 4 organs involved at the time of diagnosis.14
Patients with cGVHD have substantial impairment in QOL,4,15,16 as assessed by the Lee Symptom Scale (LSS), which measures the effect of cGVHD on patients' functioning and well-being.16 Only one third of patients who have cGVHD and start systemic treatment will be alive, in remission and off immunosuppressive therapy by 5 years.12
The pathophysiology of cGVHD can be separated into three phases: early inflammation because of tissue injury, a dysregulated adaptive immune system, and chronic inflammation and aberrant tissue repair with fibrosis.8
First-line therapy for National Institutes of Health (NIH)–defined moderate to severe cGVHD is corticosteroids (CSs) alone or in combination with sirolimus or a calcineurin inhibitor.17 However, up to 70% of patients require additional lines of therapy (LOTs).11,12,18 Furthermore, the long-term use of CS is associated with significant side effects.18,19
Management of cGVHD continues to evolve with the advent of targeted therapies. In 2017, the US Food and Drug Administration approved ibrutinib, a Bruton's Tyr kinase inhibitor, for the treatment of adults with cGVHD after failure of ≥ 1 systemic LOTs.20 In patients with cGVHD who were required to have either > 25% body surface area erythematous rash or an NIH mouth score of > 4,21 a study with ibrutinib reported an overall response rate (ORR) of 67% and a discontinuation rate because of treatment-emergent adverse events (TEAEs) of 43%.22 There remains an opportunity to study other treatment options for patients who have failed ≥ 1 LOTs.
Belumosudil is an oral selective rho-associated coiled-coil–containing protein kinase-2 (ROCK2) inhibitor. ROCK2 has been shown to be activated in a Th17-skewed milieu, leading to the upregulation of signal transducer and activator of transcription 3 (STAT3) phosphorylation and the consequent increased expression of Th17-specific transcription factors, such as RAR-related orphan receptor and interferon regulatory factor 4.23 Moreover, selective ROCK2 inhibition restores immune homeostasis and shifts the Th17/regulatory T cells (Tregs) balance toward Tregs via an STAT5-dependent mechanism.23,24 Belumosudil demonstrated a significant reduction of lung and skin fibrosis in animal models of bronchiolitis obliterans syndrome and sclerodermatous cGVHD, respectively,24 which was consistent with the central role of ROCK in facilitating multiple fibrotic pathways.25
Based on the biologic rationale and compelling preclinical data, a phase IIa study was conducted to evaluate the safety and efficacy of belumosudil in patients with cGVHD.
PATIENTS AND METHODS
Patient Eligibility
This phase IIa, dose-finding, open-label study was conducted at seven centers in the United States. Eligible patients were allogeneic bone marrow transplant or alloHCT recipients of age ≥ 18 years with persistent cGVHD manifestations after having received one to three prior systemic LOTs and who were receiving CS treatment with or without a calcineurin inhibitor and/or concurrent extracorporeal photopheresis. Belumosudil was continued until cGVHD progression or unacceptable toxicity.
Study Design and Treatment
Patients were enrolled into three sequential cohorts: cohort one received belumosudil 200 mg once daily, cohort two received belumosudil 200 mg twice daily (twice a day), and cohort three received belumosudil 400 mg once daily. Sixteen patients were planned for each of the cohorts. Before enrollment of the subsequent cohort, safety data in each previous cohort were analyzed after eight patients reached 2 months of treatment to assure that there was no safety signal. The 2-month timeframe was selected because all clinically significant belumosudil-related adverse events (AEs) to date had occurred in ≤ 36 days of starting belumosudil. No safety concerns were identified, allowing for planned dose escalation.
Belumosudil was administered orally in 28-day cycles until disease progression or unacceptable toxicity. Progression was defined per the 2014 NIH cGVHD Consensus Criteria.26 Long-term follow-up was conducted via telephone every 8 weeks until study closeout. After 4 weeks of belumosudil therapy, CS could be tapered at the investigators' discretion.
Screening was conducted within 28 days of the first study dose. Response was initially assessed after two cycles; however, this was amended to evaluate response on day 1 of each cycle, starting at cycle 2 day 1.
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The institutional review board/independent ethics committee at each center approved the study. All patients provided written informed consent.
The study was registered with ClinicalTrials.gov identifier (NCT02841995) and sponsored by Kadmon Corporation, LLC. All authors reviewed and approved the submitted manuscript.
Study End Points
The primary efficacy end point was ORR, defined as the proportion of patients who achieved either a complete response (CR) or partial response (PR), per the 2014 NIH cGVHD Consensus Criteria, at any time point.26 Only response assessments before the next LOT after belumosudil were counted toward ORR. All responses were assessed by the investigators.
Secondary end points included the number and the percentage of patients with steroid-dependent cGVHD who had a best response of PR or CR, duration of response (DOR), response rate by organ system, LSS score, CS dose reductions, time to next treatment (TTNT), failure-free survival (FFS), and overall survival (OS). The safety and tolerability of belumosudil were evaluated via AE assessments, physical examinations, vital sign measurements, laboratory tests, and electrocardiograms throughout the study. Predose samples were collected for pharmacodynamic (PD) evaluation, which included the assessment of immune cell subtypes in peripheral blood.
Statistical Analysis
With a sample size of 16 patients per cohort, the study had a > 90% probability of ≥ 1 study participants experiencing an AE with an underlying rate of ≥ 14%, which was derived from the probability calculations of the assumed sample size. Assuming a best ORR of 25%, which was determined to be clinically meaningful, the study was expected to have approximately 90% probability to show a response in ≥ 2 patients per cohort. This study was not powered to show significant differences between cohorts with respect to efficacy, AEs, or PD analyses. The primary analysis was conducted using the safety population, defined as enrolled patients who received ≥ 1 dose of study medication. The Clopper-Pearson (exact) method was used to construct the two-sided 95% CI for ORR. The Kaplan-Meier (K-M) method was used to calculate estimates of FFS and OS.
RESULTS
Patients
Fifty-four patients in total were enrolled in sequential cohorts between September 2016 and March 2018: 17 patients in cohort 1, 16 patients in cohort 2, and 21 patients in cohort 3. As of the data cutoff for this analysis, February 19, 2020, the median duration of follow-up was 36 months in cohort 1, 32 months in cohort 2, and 24 months in cohort 3. The overall median duration of follow-up was 29 months (range, 1-39 months).
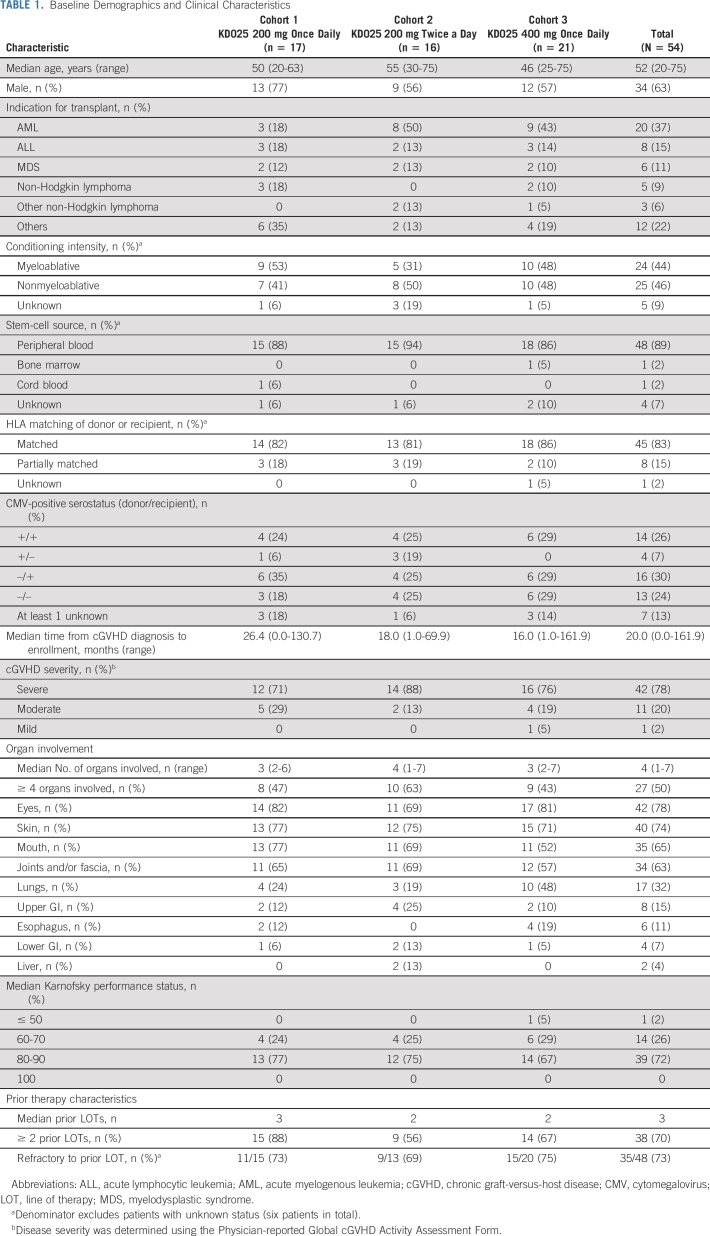
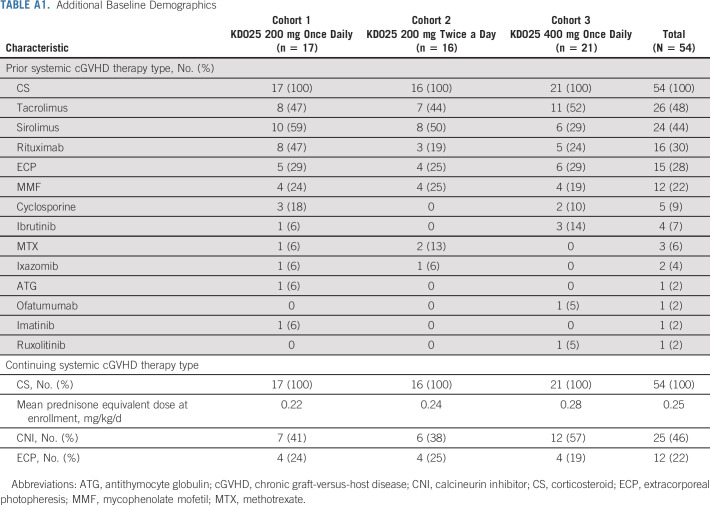
Demographics and baseline characteristics were overall comparable across cohorts (Table 1, Appendix Table A1, online only). The median age at baseline was 52 years (range, 20-75 years). The median time from cGVHD diagnosis to treatment was longest in cohort 1 at 26 months (compared with 18 months and 16 months in cohorts 2 and 3, respectively). Seventy-eight percent of patients had severe cGVHD per investigator assessment. Half of the patients had involvement of ≥ 4 organs, and more patients in cohort 3 had lung involvement (48%) compared with those in cohorts 1 (24%) and 2 (19%). The baseline median CS dose (mg/kg/d prednisone equivalent) was 0.22, 0.19, and 0.17 across cohorts, respectively. Patients in cohort 1 had received a median of three prior LOTs, whereas patients in cohorts 2 and 3 had received a median of two prior LOTs. Seventy-three percent (35 of 48, data not available for six patients) of patients were refractory to their last LOT before study enrollment.
TABLE 1.
Baseline Demographics and Clinical Characteristics
The CONSORT diagram (Fig 1) shows patient disposition. The median duration of treatment was 8.5 months (range, 2-39 months) in cohort 1, 7.5 months (range, 1-35 months) in cohort 2, and 9 months (range, 1-29 months) in cohort 3. Twenty-eight percent of patients have received > 18 months of belumosudil.
FIG 1.
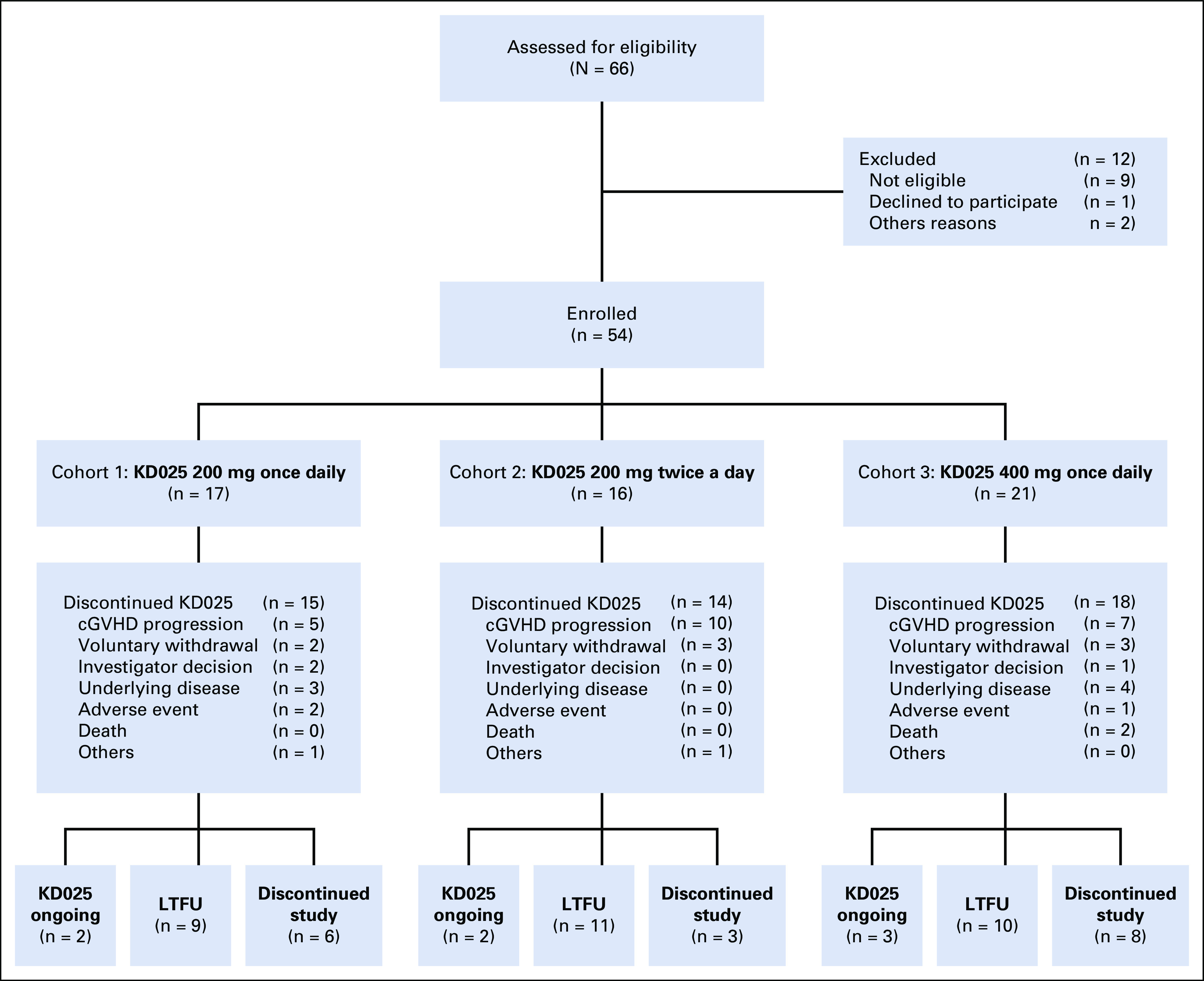
CONSORT diagram. cGVHD, chronic graft-versus-host disease; LTFU, long-term follow-up.
Seven patients (13%) remained on belumosudil at the time of this analysis. Reasons for discontinuing belumosudil included cGVHD progression (n = 22), voluntary withdrawal by patients (n = 8), relapse of underlying disease (n = 7), investigator decision (n = 3), AEs considered to be possibly treatment related (n = 3), and death (n = 2).
Efficacy
Overall response rate
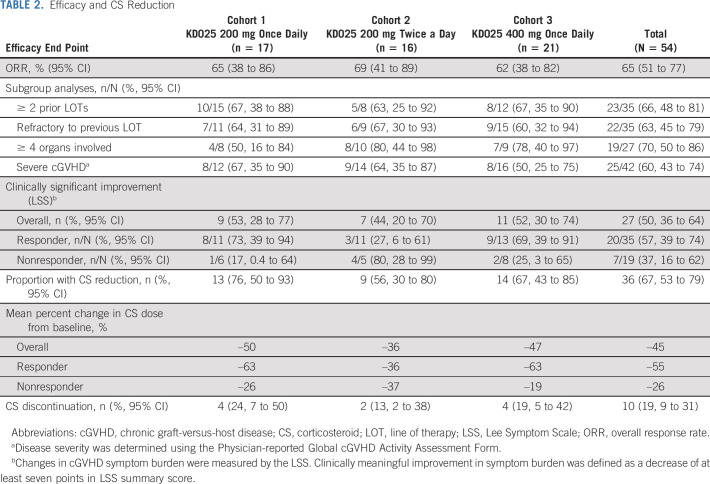
In the safety population (N = 54), the ORR (95% CI) was 65% (51% to 77%). The ORR (95% CI) was similar across cohorts: 65% (38% to 86%) in cohort 1, 69% (41% to 89%) in cohort 2, and 62% (38% to 82%) in cohort 3 (Table 2). Efficacy data for subgroups and secondary end points are presented as pooled data across cohorts.
TABLE 2.
Efficacy and CS Reduction
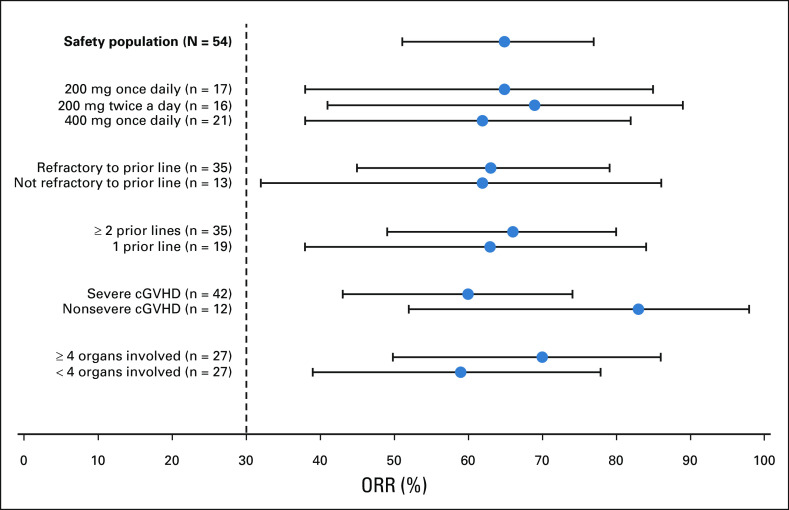
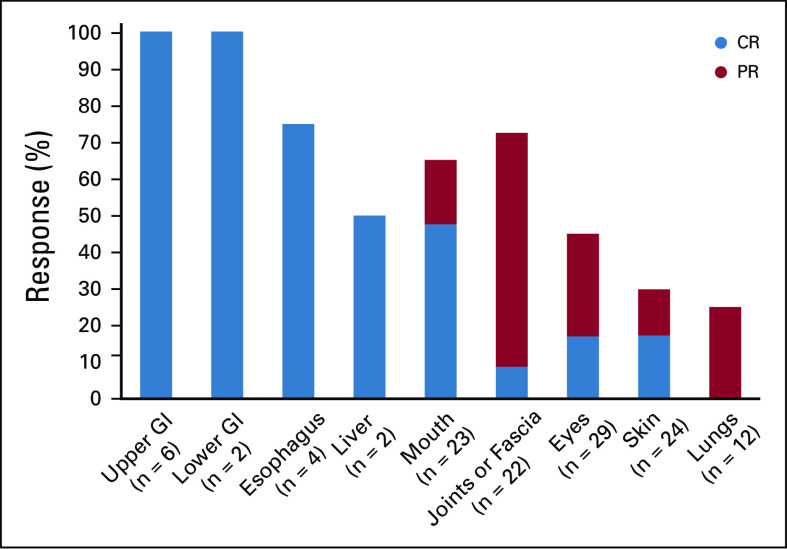
Responses were achieved across key subgroups, with ORRs of 60% (25 of 42) in patients with severe cGVHD, 66% (23 of 35) in patients who had received ≥ 2 prior systemic LOTs, 63% (22 of 35) in patients who were refractory to their last LOT before enrollment, and 70% (19 of 27) in patients with ≥ 4 organs involved (Fig 2). All responses at the patient level were PR; however, organ-specific analyses showed that CR was achieved across all affected organs, with the exception of the lungs, where PR was the best response achieved (Appendix Figs A1 and A2, online only).
FIG 2.
Forest plot for subgroup analyses of ORR in the safety population. Note that subgroups were defined based on baseline assessment. cGVHD, chronic graft-versus-host disease; ORR, overall response rate.
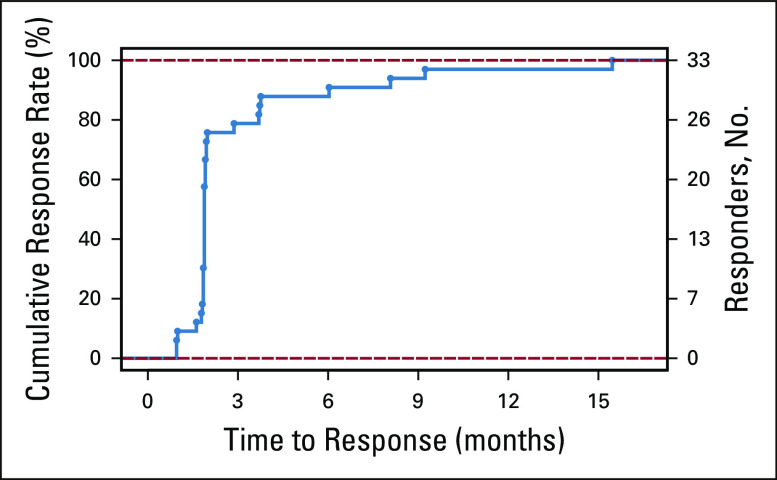
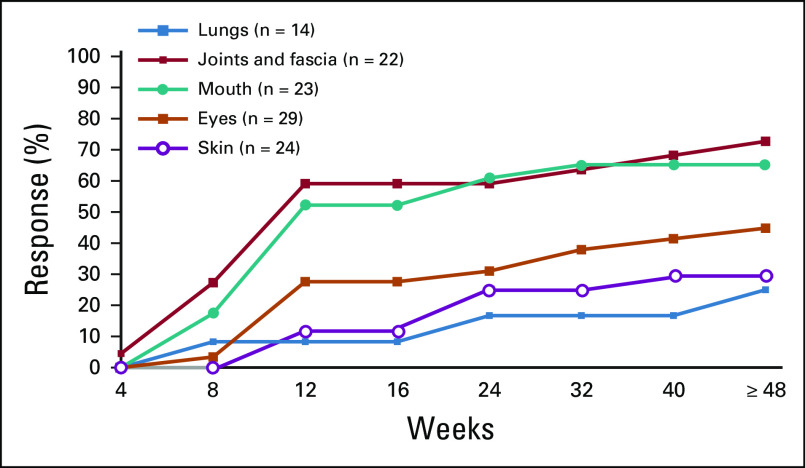
Responses were generally rapid, with > 75% of all responses achieved by the first response assessment at week 8 (Appendix Fig A3, online only). Four of 35 responses occurred after 24 weeks of belumosudil treatment, with late organ responses observed in the lungs, joints and/or fascia, and eyes (Appendix Fig A4, online only). Among responders, the K-M median DOR across cohorts was 35 weeks (Fig 3A). The K-M median DOR was 38 weeks for patients who had received ≥ 2 prior systemic LOTs.
FIG 3.
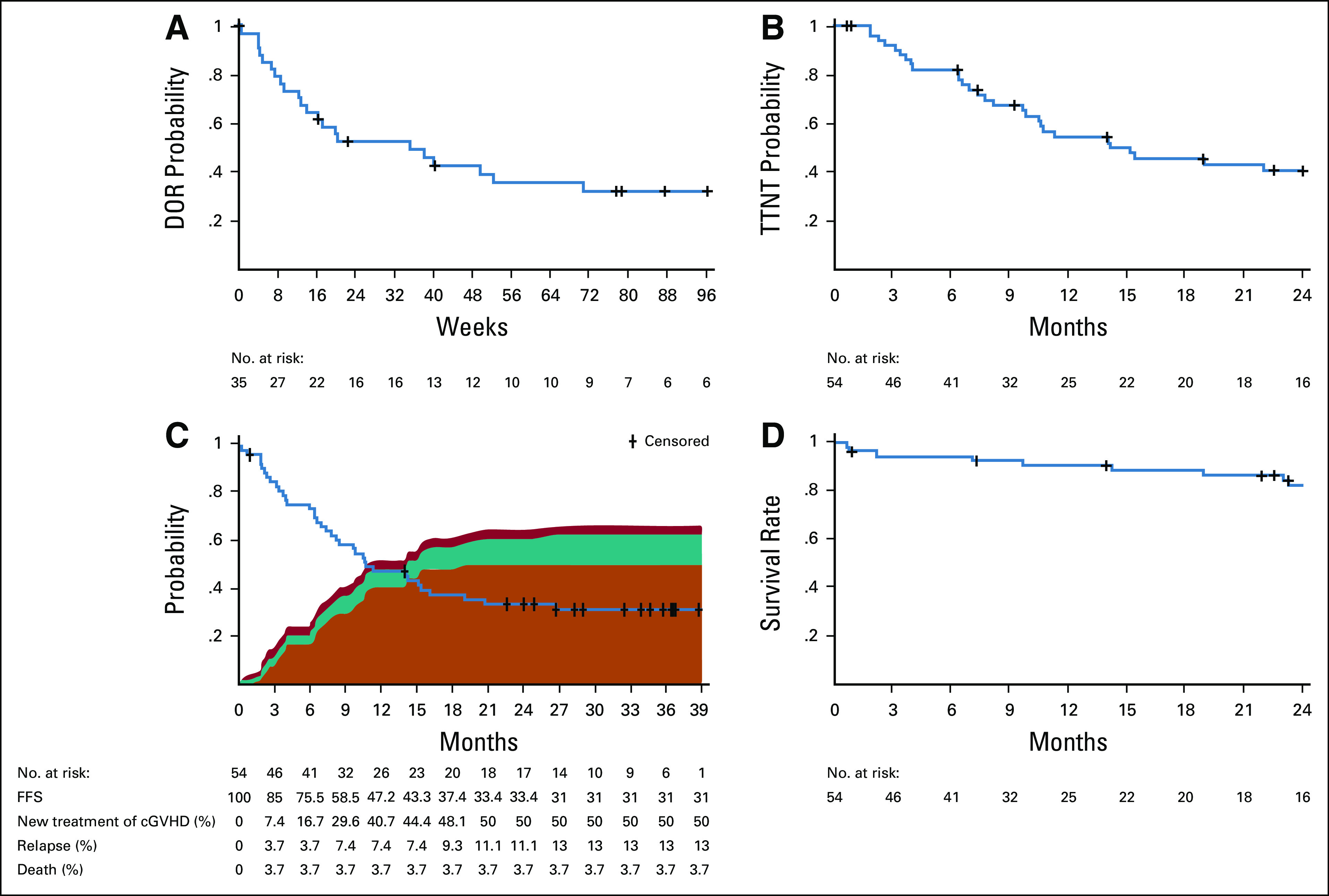
Durability of response to belumosudil in patients with cGVHD. Kaplan-Meier curves of estimated (A) DOR for responders, (B) time to next cGVHD systemic treatment (TTNT) in the safety population, (C) FFS in the safety population (including reasons for failure), and (D) overall survival in the safety population. DOR was defined as the time from response until documented progression. FFS was defined as the time from first dose of study drug to the start of another cGVHD systemic treatment, relapse of the underlying disease, or death.28,29 cGVHD, chronic graft-versus-host disease; DOR, duration of response; FFS, failure-free survival; TTNT, time to next treatment.
Time to next treatment
The K-M median TTNT was 14 months (Fig 3B). Subsequent systemic cGVHD therapies included tacrolimus, sirolimus, ibrutinib, ruxolitinib, extracorporeal photopheresis, and mycophenolate mofetil.
FFS and OS
The FFS rate (95% CI) was 76% (62% to 85%), 47% (33% to 60%), and 33% (21% to 46%) at 6, 12, and 24 months, respectively (Fig 3C). FFS was defined as the time from the first dose of belumosudil to a failure event.27 Reasons for failure included initiation of a new systemic therapy (n = 27), relapse of the underlying disease (n = 7), and death (n = 2). An important end point is the percentage of patients achieving FFS with response (CR/PR) at 12 months,28 which was 24% in this study. The OS rate (95% CI) was 91% (79% to 96%) and 82% (69% to 90%) at 12 and 24 months, respectively (Fig 3D).
QOL assessment
Clinically meaningful improvement in LSS score, defined as a decrease of ≥ 7 points in the LSS summary score,16 during belumosudil treatment was observed in 50% of patients. Thirty-five percent of all patients (37% of responders and 32% of nonresponders) reported a clinically meaningful improvement in LSS score on consecutive assessments.
CS sparing
During belumosudil treatment, 67% of patients reduced CS dose and 19% completely discontinued CS. The mean CS dose was reduced by 45%. The median time to CS discontinuation was 29 weeks (range, 8-77 weeks). The mean CS dose reduction was 55% in responders and 26% in nonresponders (Table 2).
Safety
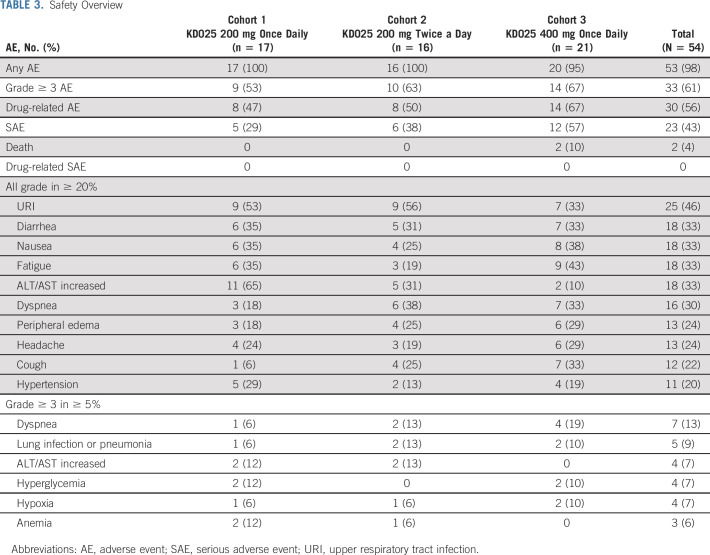
Belumosudil was well-tolerated, with > 56 patient-years of belumosudil exposure. The median relative dose intensity was 98% overall. The percentage of patients with a relative dose intensity > 95% was 77%, 63%, and 71% across cohorts, respectively. Dose reductions occurred in 9% of patients, and the median duration of reduction was 97 days (range, 21-859 days). Dose interruptions occurred in 41% of patients, and the median duration of interruption was 10 days (range, 2-39 days).
AEs were consistent with those expected in a population of patients with advanced cGVHD receiving CS. AEs reported in ≥ 20% of patients were upper respiratory infection (46%), diarrhea (33%), fatigue (33%), nausea (33%), increased liver function tests (33%), dyspnea (30%), headache (24%), peripheral edema (24%), cough (22%), and hypertension (20%) (Table 3). Serious AEs were reported in 43% of patients, and serious AEs reported in > 1 patient were dyspnea (7%), lung infection (6%), hypoxia (4%), and influenza-like illness (4%). Sixty-one percent of patients had a grade ≥ 3 AE, with the most common being dyspnea (13%), increased liver function tests (7%), hyperglycemia (7%), and hypoxia (7%) (Table 3).
TABLE 3.
Safety Overview
Grade ≥ 3 cytopenias were reported in two patients (4%). These occurred at relapse of underlying malignancy in patients who had otherwise maintained normal blood counts during their belumosudil treatment.
No cases of cytomegalovirus (CMV) infection or reactivation were reported with belumosudil.
Three patients discontinued belumosudil because of potentially drug-related AEs (cohort 1: diarrhea and headache; cohort 3: fatigue). Four patients, all in cohort 3, died during the study—secondary to relapse of leukemia, pneumonia (unknown pathogen), cardiac arrest, and cGVHD progression—with none of the deaths attributed to belumosudil. There was no dose response with respect to the observed AEs.
PD Analyses
In exploratory PD analyses of peripheral blood mononuclear cells across cohorts, the percentage of CD4+ Tregs demonstrated an increasing trend early on by cycle 2 day 1 of belumosudil treatment. A simultaneous decrease in Th17 cells was also observed. The Th17 cells continued to decrease through C4D1 and C6D25. The percentage of CD4+ Tregs continued to increase through C4D1 and C7D1, as shown in Figure 4. Because of the small sample size, correlative data with steroid dose were limited for any statistical analysis.
FIG 4.
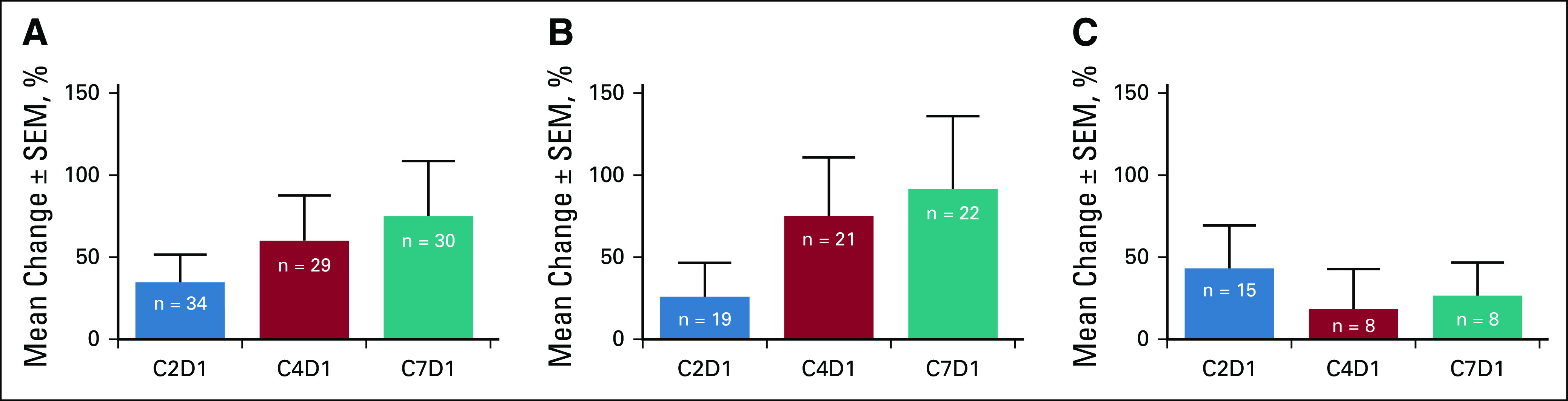
Changes in percentage of CD4+ Tregs following treatment with belumosudil compared with baseline. (A) Tregs (all), (B) Tregs (responders), and (C) Tregs (nonresponders). Predose peripheral blood samples were collected on C1D1, C2D1, C4D1, C7D1, and end-of-treatment visits. C1D1, cycle 1 day 1; C2D1, cycle 2 day 1; C4D1, cycle 4 day 1; C7D1, cycle 7 day 1; Tregs, regulatory T cells.
DISCUSSION
This study was the first to evaluate belumosudil treatment in patients with cGVHD. All phenotypes of cGVHD, without requirements for inflammatory or fibrotic manifestations, were included. Patients with advanced multiorgan cGVHD treated with belumosudil achieved an ORR of 65%, with QOL improvements, CS dose reductions, and limited toxicity. With relatively small sample sizes, there was no difference in the ORR across cohorts.
Belumosudil achieved response rates that were meaningful and consistent across subgroups, including patients with severe cGVHD, patients who had received ≥ 2 prior systemic LOTs, patients who were refractory to their last LOT before enrollment, and patients with ≥ 4 organs involved. The ORR among patients with nonsevere cGVHD was 83%, suggesting that further studies of how belumosudil may benefit patients earlier in their disease are indicated. All responses at the patient level were PR; no CR was achieved. However, given the severity and extent of fibrotic cGVHD manifestations in this patient population, achieving CR in all organs was not expected, as some advanced fibrotic changes in the eyes, mouth, lungs, or joints and/or fascia can be irreversible. CR was observed in all organs except the lungs, where PR was achieved.
Belumosudil response kinetics suggest that most responders achieved responses rapidly within 8 weeks after receiving belumosudil.
Belumosudil was well-tolerated, with a median DOR of 35 weeks across all responders. The ability to stay on therapy is dependent on the safety and long-term tolerability profile of the intervention. The median treatment duration was 8 months (range, 1-39 months). Twenty-eight percent of patients remained on belumosudil for > 18 months, with 11 patients remaining on treatment at the time of this analysis. There was no reported CMV infection or reactivation, despite 57% of patients being CMV seropositive. The incidence of TEAEs and grade ≥ 3 TEAEs was similar across cohorts.
The combination of well-tolerated therapy and efficacy in inducing responses translated into a 2-year OS rate of 82%, a median TTNT of 14 months, and FFS rates of 76% and 47% at 6 and 12 months, respectively.
In a prospective study conducted by the cGVHD Consortium, the 12-month FFS rate with response (CR/PR) after first-line therapy was 12% to 15%.28 In our study (after 1-3 prior LOTs), the 12-month FFS rate with response was 24%.
Belumosudil therapy was associated with a CS-sparing effect. The current treatment paradigm relies on CS as the mainstay of therapy; however, the related long-term toxicities mandate the use of the lowest possible dose or discontinuation whenever possible. The use of CS is tied to QOL, as the side effect profile of CS contributes to patient symptom burden.18,19,30 CS dose reduction was observed across both responders and nonresponders to belumosudil. Approximately 20% of patients were able to discontinue CS during belumosudil treatment. Even in the absence of an NIH-defined response, patients experienced clinical benefit, as evidenced by improvements in LSS score or reductions in CS doses.
The pathophysiology and clinical phenotype of cGVHD reflect both immune and fibrotic components.8 An ideal intervention would target both aspects. Fibrotic cGVHD manifestations, including fasciitis, ocular fibrosis, cutaneous sclerosis, and bronchiolitis obliterans syndrome, are notoriously difficult to treat.18,31-33 As a ROCK2 inhibitor, belumosudil has been shown to decrease inflammation, restore immune homeostasis, and decrease fibrosis.23,24,34 In our study, responses were achieved in patients with fibrotic manifestations in the lungs, joints and/or fascia, and eyes. These responses were observed in some cases after > 24 weeks of treatment,34 further highlighting the need to sustain effective therapy to achieve clinical benefit, particularly in patients with difficult-to-treat disease.
In summary, belumosudil is a selective ROCK2 inhibitor with a novel mechanism of action that targets both inflammation and fibrosis in cGVHD.23 Belumosudil was well-tolerated and achieved clinically meaningful responses in patients with cGVHD across all dose regimens evaluated.35 Responses have been durable and associated with QOL improvements and CS dose reductions or discontinuations. These clinical benefits were also seen in patients who did not achieve NIH-defined responses.35 Data from this study are very encouraging, given the unmet needs of patients with cGVHD. These data have led to belumosudil being granted Breakthrough Therapy Designation by the US Food and Drug Administration. Because the lower belumosudil 200-mg once daily dose was equally safe and effective, it has been further compared in a subsequent randomized registration study (ClinicalTrials.gov identifier: NCT03640481) against the 200-mg twice a day dose for final dose recommendation.
ACKNOWLEDGMENT
The authors would like to thank all the patients, caregivers, health care providers, investigators, and the employees of Kadmon who were involved in the study.
Appendix
FIG A1.
Best individual response by organ system among responders. n = number of responder population for global severity rating and number of specific organs involved at baseline. The percentages are calculated based on the corresponding n. CR, complete response; PR, partial response.
FIG A2.
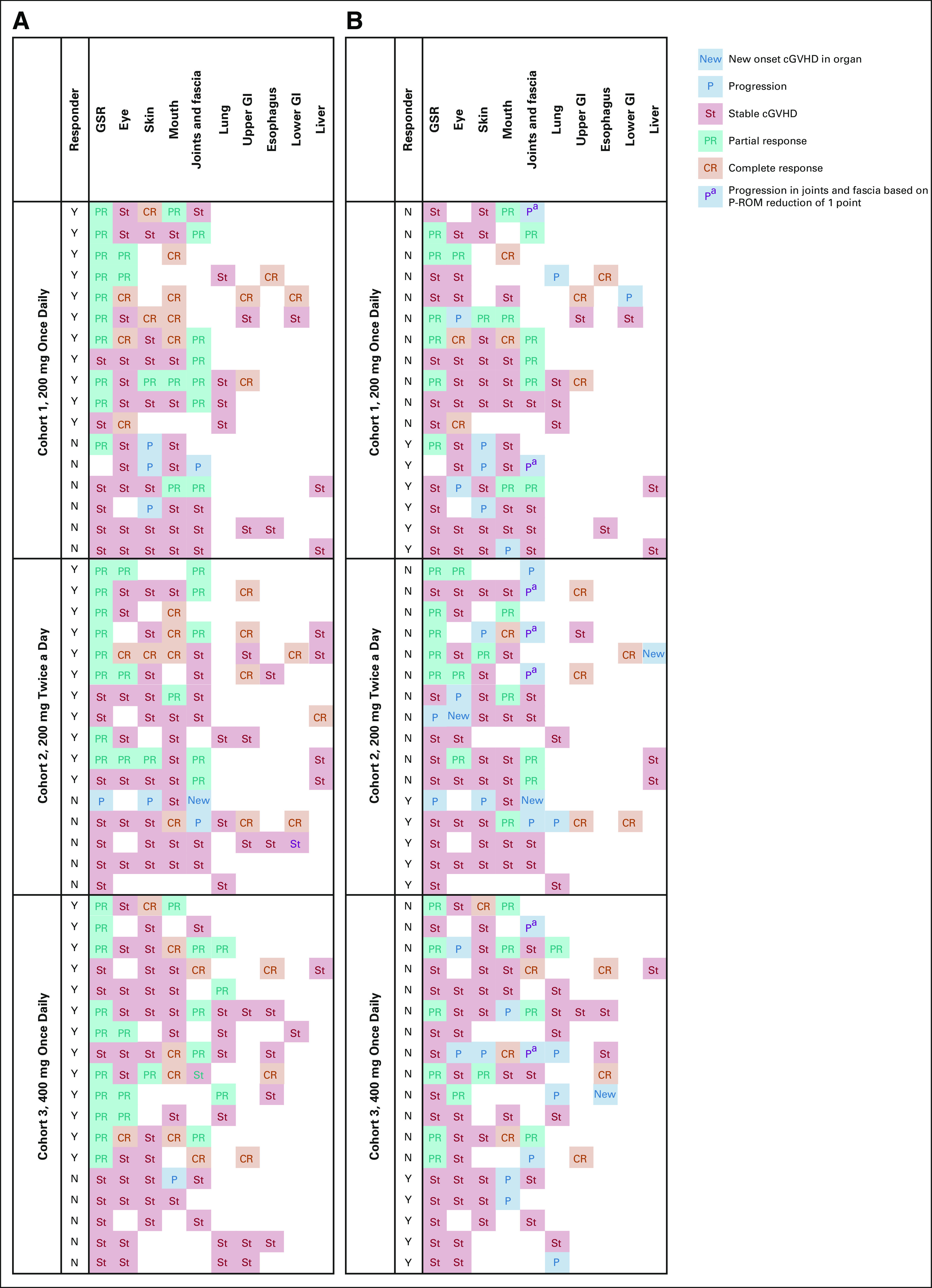
Response and progression heat map for all patients in the safety population. (A) Best response by organ. (B) Organ responses at time of progression or end of study. Of 11 patients with progression in joints, seven had a reduction in P-ROM of just one unit. cGVHD, chronic graft-versus-host disease; CR, complete response; GSR, Global Severity Rating; PR, partial response; P-ROM, photographic range of motion.
FIG A3.
Time to response among belumosudil responders. Percentages are calculated based on the number of responder population.
FIG A4.
Time to response by selected organs among responders. Percentages are calculated based on the number of responder population.
TABLE A1.
Additional Baseline Demographics
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Aleksandr Lazaryan
Stock and Other Ownership Interests: AbbVie, Pfizer
Consulting or Advisory Role: EUSA Pharma
Carlos R. Bachier
Employment: HCA Healthcare
Consulting or Advisory Role: Kadmon, Viracyte, WUGEN, crispr therapeutics, Novartis, Juno/Celgene
Research Funding: Bristol-Myers Squibb
Amandeep Salhotra
Consulting or Advisory Role: Kadmon, Syros Pharmaceuticals
Research Funding: Bristol-Myers Squibb
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
James Essell
Consulting or Advisory Role: AbbVie/Genentech, Celgene/Bristol-Myers Squibb
Speakers' Bureau: Kite/Gilead
Laurie Green
Employment: Kadmon
Olivier Schueller
Employment: Kadmon
Jeegar Patel
Employment: Kadmon Corporation LLC
Stock and Other Ownership Interests: Kadmon Corporation LLC
Travel, Accommodations, Expenses: Kadmon
Alexandra Zanin-Zhorov
Employment: Kadmon Holdings
Stock and Other Ownership Interests: Kadmon Holdings
Jonathan M. Weiss
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
Consulting or Advisory Role: Stellate
Research Funding: Kadmon
Travel, Accommodations, Expenses: Kadmon
Zhongming Yang
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
David Eiznhamer
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
Sanjay K. Aggarwal
Employment: Kadmon, Angiocrine Bioscience
Leadership: Angiocrine Bioscience
Stock and Other Ownership Interests: Kadmon, Angiocrine Bioscience, Amgen, Alnylam
Consulting or Advisory Role: Kadmon
Bruce R. Blazar
Stock and Other Ownership Interests: Five Prime Therapeutics, BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Honoraria: Kadmon, Incyte
Consulting or Advisory Role: Kadmon, BlueRock Therapeutics, Magenta Therapeutics
Research Funding: Fate Therapeutics, BlueRock Therapeutics, Alpine Immune Sciences, AbbVie
Patents, Royalties, Other Intellectual Property: Inducible regulatory T cell generation for hematopoietic cell transplants (UMN Z09026), U.S. 9,228,172, Inducible regulatory T cell generation for hematopoietic cell transplants (UMN Z09026), U.S. 9,228,172, TALEN based gene correction, Patent No. 9,393,257, Generation of natural killer cells and lymphoid tissue inducer-like (LTI-like) NK-22 cells, 9,862,928, Method for correcting a genetic sequence, 10,648,002
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Rheos Medicines
Stephanie J. Lee
Honoraria: Wolters Kluwer
Consulting or Advisory Role: Incyte, Pfizer, EMD Serono, Kadmon, MSD Oncology, Sanofi, Genzyme, Regeneron, 4SC
Research Funding: Kadmon, Takeda, Amgen, Bristol-Myers Squibb, EMD Serono, MSD, Novartis, Incyte, Syndax, Pfizer, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for high affinity T cell receptors that target the Merkel polyomavirus
No other potential conflicts of interest were reported.
See accompanying article on page 1942
PRIOR PRESENTATION
Presented in part at the American Society of Hematology Annual Meetings (Atlanta, GA, December 9-12, 2017; San Diego, CA, December 1-4, 2018; and Orlando, FL, December 7-10, 2019); the Transplantation and Cellular Therapy Meetings (Salt Lake City, UT, February 21-25, 2018; Houston, TX, February 20-24, 2019; and Orlando, FL, February 19-23, 2020); the European Group for Blood and Marrow Transplantation Annual Meetings (Lisbon, Portugal, March 18-21, 2018; Frankfurt, Germany, March 24-27, 2019; and virtual meeting, August 29-September 1, 2020); and the European Hematology Association Congresses (Stockholm, Sweden, June 14-17, 2018; and Amsterdam, the Netherlands, June 13-16, 2019).
SUPPORT
Supported by Kadmon Corporation, LLC, who designed the study with input from the study investigators, provided the study drug, conducted quality assurance, developed the analysis plan, analyzed the results, and funded professional writing assistance provided by RevHealth.
CLINICAL TRIAL INFORMATION
M.J. and A.L. are co-first authors.
AUTHOR CONTRIBUTIONS
Conception and design: Madan Jagasia, Aleksandr Lazaryan, Daniel J. Weisdorf, Jeegar Patel, Sanjay K. Aggarwal, Bruce R. Blazar, Stephanie J. Lee
Administrative support: Sanjay K. Aggarwal
Provision of study materials or patients: Carlos R. Bachier, Amandeep Salhotra, Daniel J. Weisdorf, James Essell, Stephanie J. Lee
Collection and assembly of data: Amandeep Salhotra, James Essell, David Eiznhamer, Sanjay K. Aggarwal
Data analysis and interpretation: Madan Jagasia, Aleksandr Lazaryan, Carlos R. Bachier, Amandeep Salhotra, Behyar Zoghi, James Essell, Laurie Green, Olivier Schueller, Alexandra Zanin-Zhorov, Jonathan M. Weiss, Zhongming Yang, David Eiznhamer, Sanjay K. Aggarwal, Bruce R. Blazar, Stephanie J. Lee
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
ROCK2 Inhibition With Belumosudil (KD025) for the Treatment of Chronic Graft-Versus-Host Disease
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Aleksandr Lazaryan
Stock and Other Ownership Interests: AbbVie, Pfizer
Consulting or Advisory Role: EUSA Pharma
Carlos R. Bachier
Employment: HCA Healthcare
Consulting or Advisory Role: Kadmon, Viracyte, WUGEN, crispr therapeutics, Novartis, Juno/Celgene
Research Funding: Bristol-Myers Squibb
Amandeep Salhotra
Consulting or Advisory Role: Kadmon, Syros Pharmaceuticals
Research Funding: Bristol-Myers Squibb
Daniel J. Weisdorf
Consulting or Advisory Role: Incyte, Fate Therapeutics
Research Funding: Incyte
James Essell
Consulting or Advisory Role: AbbVie/Genentech, Celgene/Bristol-Myers Squibb
Speakers' Bureau: Kite/Gilead
Laurie Green
Employment: Kadmon
Olivier Schueller
Employment: Kadmon
Jeegar Patel
Employment: Kadmon Corporation LLC
Stock and Other Ownership Interests: Kadmon Corporation LLC
Travel, Accommodations, Expenses: Kadmon
Alexandra Zanin-Zhorov
Employment: Kadmon Holdings
Stock and Other Ownership Interests: Kadmon Holdings
Jonathan M. Weiss
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
Consulting or Advisory Role: Stellate
Research Funding: Kadmon
Travel, Accommodations, Expenses: Kadmon
Zhongming Yang
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
David Eiznhamer
Employment: Kadmon
Stock and Other Ownership Interests: Kadmon
Sanjay K. Aggarwal
Employment: Kadmon, Angiocrine Bioscience
Leadership: Angiocrine Bioscience
Stock and Other Ownership Interests: Kadmon, Angiocrine Bioscience, Amgen, Alnylam
Consulting or Advisory Role: Kadmon
Bruce R. Blazar
Stock and Other Ownership Interests: Five Prime Therapeutics, BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Honoraria: Kadmon, Incyte
Consulting or Advisory Role: Kadmon, BlueRock Therapeutics, Magenta Therapeutics
Research Funding: Fate Therapeutics, BlueRock Therapeutics, Alpine Immune Sciences, AbbVie
Patents, Royalties, Other Intellectual Property: Inducible regulatory T cell generation for hematopoietic cell transplants (UMN Z09026), U.S. 9,228,172, Inducible regulatory T cell generation for hematopoietic cell transplants (UMN Z09026), U.S. 9,228,172, TALEN based gene correction, Patent No. 9,393,257, Generation of natural killer cells and lymphoid tissue inducer-like (LTI-like) NK-22 cells, 9,862,928, Method for correcting a genetic sequence, 10,648,002
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Rheos Medicines
Stephanie J. Lee
Honoraria: Wolters Kluwer
Consulting or Advisory Role: Incyte, Pfizer, EMD Serono, Kadmon, MSD Oncology, Sanofi, Genzyme, Regeneron, 4SC
Research Funding: Kadmon, Takeda, Amgen, Bristol-Myers Squibb, EMD Serono, MSD, Novartis, Incyte, Syndax, Pfizer, AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for high affinity T cell receptors that target the Merkel polyomavirus
No other potential conflicts of interest were reported.
REFERENCES
- 1.MacDonald KPA, Hill GR, Blazar BR: Chronic graft-versus-host disease: Biological insights from preclinical and clinical studies. Blood 129:13-21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora M Cutler CS Jagasia MH, et al. : Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl 22:449-455, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard JR Majhail NS Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230-2239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong FL Francisco L Togawa K, et al. : Long-term recovery after hematopoietic cell transplantation: Predictors of quality-of-life concerns. Blood 115:2508-2519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grube M Holler E Weber D, et al. : Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation—Results from a single-center observational study. Biol Blood Marrow Transpl 22:1781-1791, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Sahebi F Garderet L Kanate AS, et al. : Outcomes of haploidentical transplantation in patients with relapsed multiple myeloma: An EBMT/CIBMTR report. Biol Blood Marrow Transpl 25:335-342, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afram G Pérez Simón JA Remberger M, et al. : Reduced intensity conditioning increases risk of severe cGVHD: Identification of risk factors for cGVHD in a multicenter setting. Med Oncol 35:79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiser R, Blazar BR: Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med 377:2565-2579, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Baird K, Cooke K, Schultz KR: Chronic graft versus host disease (GVHD) in children. Pediatr Clin North Am 57:297-322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Vogelsang G, Flowers MED: Chronic graft-versus-host disease. Biol Blood Marrow Transpl 9:215-233, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bachier CR Aggarwal SK Hennegan K, et al. : Epidemiology and real-world treatment of chronic graft-versus-host disease post allogeneic hematopoietic cell transplantation: A US claims analysis. Presented at ASH 2019, Orlando, FL, December 7-10, 2019 [DOI] [PubMed]
- 12.Lee SJ Nguyen TD Onstad L, et al. : Success of immunosuppressive treatments in patients with chronic graft-versus-host disease. Biol Blood Marrow Transpl 24:555-562, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saillard C Crocchiolo R Furst S, et al. : National Institutes of Health classification for chronic graft-versus-host disease predicts outcome of allo-hematopoietic stem cell transplant after fludarabine–busulfan–antithymocyte globulin conditioning regimen. Leuk Lymphoma 55:1106-1112, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Data on file : Trinity Data. New York, NY, Kadmon Holdings Inc, 2019 [Google Scholar]
- 15.Pidala J Kurland B Chai X, et al. : Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: Report on baseline data from the chronic GVHD Consortium. Blood 117:4651-4657, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ Cook EF Soiffer R, et al. : Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transpl 8:444-452, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter PA Logan BR Lee SJ, et al. : A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/sirolimus/calcineurin inhibitor for the treatment of chronic graft-versus-host disease: BMT CTN 0801. Haematologica 103:1915-1924, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flowers MED, Martin PJ: How we treat chronic graft-versus-host disease. Blood 125:606-615, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couriel D Carpenter PA Cutler C, et al. : Ancillary therapy and supportive care of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group report. Biol Blood Marrow Transpl 12:375-396, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Imbruvica. Package Insert. Sunnyvale, CA, Pharmacyclics LLC, 2019 [Google Scholar]
- 21.Miklos D Cutler CS Arora M, et al. : Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 130:2243-2250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waller EK Miklos D Cutler C, et al. : Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-Year update of a phase 1b/2 study. Biol Blood Marrow Transpl 25:2002-2007, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Zanin-Zhorov A Weiss JM Nyuydzefe MS, et al. : Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A 111:16814-16819, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn R Paz K Du J, et al. : Targeted rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood 127:2144-2154, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riches DWH, Backos DS, Redente EF: ROCK and rho: Promising therapeutic targets to ameliorate pulmonary fibrosis. Am J Pathol 185:909-912, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagasia MH Greinix HT Arora M, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl 21:389-401.e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inamoto Y Flowers MED Sandmaier BM, et al. : Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood 124:1363-1371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin PJ Storer BE Inamoto Y, et al. : An endpoint associated with clinical benefit after initial treatment for chronic graft-versus-host disease. Blood 130:360-367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inamoto Y Storer BE Lee SJ, et al. : Failure-free survival after second-line systemic treatment of chronic graft-versus-host disease. Blood 121:2340-2346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dignan FL Amrolia P Clark A, et al. : Diagnosis and management of chronic graft-versus-host disease. Br J Haematol 158:46-61, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ Wolff D Kitko C, et al. : Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transpl 21:984-999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baird K Steinberg SM Grkovic L, et al. : National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: Organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transpl 19:632-639, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imanguli MM Atkinson JC Mitchell SA, et al. : Salivary gland involvement in chronic graft-versus-host disease: Prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transpl 16:1362-1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagasia M Salhotra A Bachier CR, et al. : KD025 for patients with chronic graft-versus-host disease (cGVHD)—Long-term follow-up of a phase 2a study (KD025-208). Presented at ASH 2019, Orlando, FL, December 7-10, 2019
- 35.Jagasia M Salhotra A Bachier CR, et al. : KD025-208: A phase 2a study of KD025 for patients with chronic graft versus host disease (cGVHD)—Pharmacodynamics and updated results. Blood 132:602, 2018. (suppl 1) [Google Scholar]