Dear Editor,
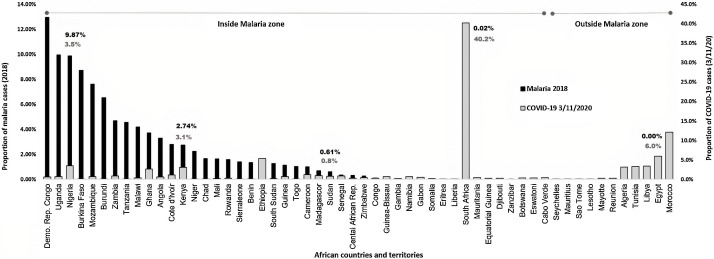
The highly contagious severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) which causes COVID-19 started at the end of 2019 in Wuhan city in China from where it stretched to the entire globe affecting different nations in different manners. Though this virus showed high potential to spread more quickly in certain territories within a short period of a few months. African countries with the most fragile health and environmental systems that render them vulnerable to the rapid spread of infectious diseases, continue to surprisingly report the lowest number of COVID-19 cases and mortality rates as of November 2020 [[1], [2], [3]]. Several hypotheses have been put forward to justify this open query; factors such as climate conditions [4], young African population’s age (median 19.5 years) [1], poor diagnostic tests and medical services [5], immunity due to back malaria infection [6] and the use of antimalarial drugs [7,8] have been reported. Statistical studies have pointed out that hot weather may relatively lower the rapidness of the virus dissemination but doesn’t cease it [9]. The central and western parts of the continent, which extend from Sudan to Mauritania at the extreme western border of Africa, are least affected by the devastating pandemic (Fig. 1 ). Meanwhile, this belt is known for being heavily struck by malaria, a disease of the high burden caused by four Plasmodium species i.e., vivax, malariae, ovale, and lastly falciparum the main culprit of the majority of the death cases in sub-Saharan Africa [10]. The possible COVID-19 immuno-protective effect in response to past malaria infection has been repeatedly discussed in recent literature. This relation has been supported by several observations, firstly both could be cured by antimalarial drugs [11,12], malaria becomes severe in patients with “A” blood group, while “O” blood carriers are protected, the same ABO blood group system’s susceptibility was found to apply to COVID-19 patients [13,14], at severe stages, both are associated with coagulopathy [15,16], and lastly the sharing of certain immunodominant regions between the two microbes [17]. Though antimalarial drugs like chloroquine, hydroxychloroquine, and artemisinin are used by some countries’ health authorities for the treatment of COVID-19; chloroquine and hydroxychloroquine have been removed a few years ago from the malaria protocol due to detected Plasmodium resistance to the drugs [18]. Many West African countries adopted chloroquine for many years as one of the most common drugs for the treatment of malaria, among which Uganda, Burkina Faso, Mali and Guinea-Bissau. Interestingly, these countries remained to report the least number of COVID-19 incidences (Fig. 1). The malaria data presented in this figure were attained from WHO 2018 malaria country report. To discuss malaria endemicity and COVID-19 in Africa in a more comprehensive way, It would have been ideal to incorporate malaria data for 2019, however, unfortunately, these data have not been made publicly available. The mechanism implicated in SARS-COV2–host cell infection is mediated through interactions of the virus surface spike (S) glycoprotein with the angiotensin-COnverting enzyme-2 (ACE2) receptor, a receptor that expressed widely in most of the human organ's cells [19] but lacked in mature erythrocytes [20]. CD147 a member of the human immunoglobulin superfamily which is also termed basigin, a transmembrane glycoprotein receptor is expressed in the erythrocytes. This receptor is noted to mediate the blood stage of P. falciparum infection, blockage of this receptor by a humanized antibody inhibits the parasite infection [21]. Under in vivo conditions, a nano-lipid formulation Metadichol® was found to inhibit the host cell-SARS-CoV-2 interactions and consequently the virus entry [22]. Concurrently and strikingly, the same chemical could successfully inhibit the P. falciparum logically by obstructing the CD147 receptor [23]. Though the fact of the use of the CD147 (basigin) receptor by SARS-CoV2 as an alternative entry route remained controversial, some in-vitro proofs have recently arisen in support of this allegation. Wang and his colleagues have proven distinct strong interactions between CD147 and SARS-CoV2 spike glycoproteins, these interactions were obstructed by using Meplazumab a humanized anti-CD147 immunoglobulin-2 antibody which consequently resulted in viral replication inhibition. Moreover, using the CD147 knockout stable cells Vero E6, led to a sharp decline of the viral load. These developments bolstered the authors’ suggestion of the possibility of the existence of an alternative SARS-CoV-2 infection route via CD147 [24]. A Chinese controversial in-silico study demonstrated that the viral non-structural proteins Orf3a and Orf1ab assist in viral haemoglobin attacks, by dissociating the iron from heme [25]. Major of the criticism faced by these authors was based on the fact that erythrocytes do not possess replication machinery (no nucleus) that would sustain the virus’s life cycle. However, other suggestion in support of the Liu and Li hypothesis is already proclaimed, in which the attack of SARS-CoV-2 to the marrow’s immature erythrocytes (contain a nucleus) is hypothesized [26]. This data has been recently substantiated by investigating COVID-19 patients’ RBCs in which deformed erythrocytes with sickled shape and vacuolization of the cytoplasm that customarily induced by viruses attack were spotted [27]. Furthermore, a very recent study put forward by an English research group has proven a direct infection of erythroid progenitors by SARS-CoV-2 at severe stages of the disease [28]. The rosette formation phenomenon is a situation associated with severe cases of malaria in which P. falciparum-infected RBCs become stickier and subsequently facilitate the adherence of the healthier ones to them, which would culminate in clots formation [29]. Also, Coagulopathy or thrombosis in COVID-19 acute cases is well documented, and high morbidity rates were attributed, on several occasions, to clot formation that would finally lead to thromboembolism and death [30,31]. These manifestations are usually accompanied by elevated laboratory markers such as D-dimer, fibrinogen, and low lymphocyte numbers [[32], [33], [34]]. A similar outcome is also noticeable in some malaria patients [35,36]. In our research group’s recent findings, we have demonstrated possible shared targets for immune response between SARS-CoV-2 and P. falciparum by immunoinformatic approach. P. falciparum-thrombospondin related adhesive protein (PfTRAP), a protein family that aids with the erythrocyte invasion by the parasite is found to share five immunodominant regions located at three SARS-CoV-2 proteins (i.e., nucleocapsid, Orf1ab, and Orf3a proteins) [17]. Malaria is often a disease of children from the first few months of life to the age of about 5 years, becoming less common in older children and adults as specific acquired immunity gives increasing (although always incomplete) protection [37]. This acquired immunity might likely reduce the susceptibility to possible SARS-CoV-2 infection. Moreover, SARS-CoV-2 detection in peripheral blood specimens occurs merely in hospitalized patients and severe cases, with a detection rate between 9 to 40% [38]. Hence it is unlikely that all Africans who are susceptible to malaria be also for COVID-19.
Fig. 1.
The proportion of confirmed cases of malaria in 2018 in Africa (according to WHO countries profile reports from health facilities) and the proportion of accumulative confirmed cases of COVID-19 reported on Nov 3, 2020 (WHO situation report).
The common pathological manifestations of COVID-19 and malaria in terms of (1) the blood group susceptibility, (2) the coagulopathy associated with severe stages of the two diseases, (3) the elevation or decrease of certain serum biomarkers, (4) the shared immunodominant epitopes regions between SARS-CoV-2 and P. falciparum and lastly (5) the low incidence rate of COVID-19 in the malaria-endemic zones, all together may permit us to hypothesize a conceivable role for the erythrocytes in COVID-19 pathogenicity. The erythrocyte’s SARS-CoV-2 invasion is presumably taking place through RBC’s CD147 receptor. Furthermore, these striking pathological and immunological resemblances between SARS-CoV-2 and P. falciparum may likely pave the way in the chase for efficient vaccine manufacturing for both diseases.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Chitungo I., Dzobo M., Hlongwa M., Dzinamarira T. COVID-19: unpacking the low number of cases in Africa. J Public Health Manag Pract. 2020;1:100038. doi: 10.1016/j.puhip.2020.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawal Y. Africa’s low COVID-19 mortality rate: a paradox? Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.10.038. (2020/10/16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2020. WHO coronavirus disease (COVID-19) DashboardNov. [Google Scholar]
- 4.Briz-Redón Á., Serrano-Aroca Á. The effect of climate on the spread of the COVID-19 pandemic: a review of findings, and statistical and modelling techniques. Prog Phys Geogr Earth Environ. 2020;44(5):591–604. [Google Scholar]
- 5.Lone S.A., Ahmad A. COVID-19 pandemic — an African perspective. Emerg Microbes Infect. 2020;9(1):1300–1308. doi: 10.1080/22221751.2020.1775132. (2020/01/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda A.K., Tripathy R., Das B.K. Plasmodium falciparum infection may protect a population from severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2020;222(9):1570–1571. doi: 10.1093/infdis/jiaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parodi A., Cozzani E. Coronavirus disease 2019 (COVID 19) and Malaria: have anti glycoprotein antibodies a role? Med Hypotheses. 2020;143:110036. doi: 10.1016/j.mehy.2020.110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L., Dai L., Zhang X., Zhang Z., Zhang Z. Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch Pharm Res. 2020;43(8):765–772. doi: 10.1007/s12272-020-01258-7. (2020/08/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukhari Q., Massaro J.M., D’Agostino R.B., Sr., Khan S. Effects of weather on coronavirus pandemic. Int J Environ Res Public Health. 2020;17(15):5399. doi: 10.3390/ijerph17155399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2014. WHO malaria. Annual report. 14 January. [Google Scholar]
- 11.Li X., Wang Y., Agostinis P., Rabson A., Melino G., Carafoli E., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. doi: 10.1038/s41419-020-2721-8. (2020/07/08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gendrot M., Andreani J., Boxberger M., Jardot P., Fonta I., Le Bideau M., et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: an in vitro evaluation. Travel Med Infect Dis. 2020;37(September–October):101873. doi: 10.1016/j.tmaid.2020.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourali F., Afshari M., Alizadeh-Navaei R., Javidnia J., Moosazadeh M., Hessami A. Relationship between blood group and risk of infection and death in COVID-19: a live meta-analysis. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100743. (2020/09/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha S.A.H., Osman M.E.M., Abdoelkarim E.A.A., Holie M.A.I., Elbasheir M.M., Abuzeid N.M.K., et al. Individuals with a Rh-positive but not Rh-negative blood group are more vulnerable to SARS-CoV-2 infection: demographics and trend study on COVID-19 cases in Sudan. New Microbes New Infect. 2020;38 doi: 10.1016/j.nmni.2020.100763. (2020/11/01/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moxon C.A., Heyderman R.S., Wassmer S.C. Dysregulation of coagulation in cerebral malaria. Mol Biochem Parasitol. 2009;166(2):99–108. doi: 10.1016/j.molbiopara.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martín-Rojas R.M., Pérez-Rus G., Delgado-Pinos V.E., Domingo-González A., Regalado-Artamendi I., Alba-Urdiales N., et al. COVID-19 coagulopathy: an in-depth analysis of the coagulation system. Eur J Haematol. 2020;105(6):741–750. doi: 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iesa M.A.M., Osman M.E.M., Hassan M.A., Dirar A.I.A., Abuzeid N., Mancuso J.J., et al. SARS-COV2 and P. falciparum common immunodominant regions may explain low COVID-19 incidence in the malaria-endemic belt. New Microbes New Infect. 2020 doi: 10.1016/j.nmni.2020.100817. (2020/11/19/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frosch A.E.P., Venkatesan M., Laufer M.K. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10(1):116. doi: 10.1186/1475-2875-10-116. (2011/05/09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosic I., Cosic D., Loncarevic I. RRM prediction of erythrocyte Band3 proteinas alternative receptor for SARS-CoV-2 virus. Appl Sci. 2020;10(11):4053. [Google Scholar]
- 21.Zhang M.-Y., Zhang Y., Wu X.-D., Zhang K., Lin P., Bian H.-J., et al. Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium falciparum. Blood. 2018;131(10):1111–1121. doi: 10.1182/blood-2017-08-802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavan P. Metadichol®, a novel nano lipid formulation that inhibits SARS-CoV-2 and a multitude of pathological viruses in vitro. Res Square. 2020 doi: 10.21203/rs.3.rs-34021/v5. PREPRINT (Version 5) (2020/11/05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem cell reviews and reports. 2020;16(June (3)):434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. (2020/12/04) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v11938179. Preprint. [DOI] [Google Scholar]
- 26.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reva Ivan, Yamamoto Tatsuo, Rasskazova Mariya, Lemeshko Tatyana, Usov Victor, Krasnikov Yuriy, et al. Erythrocytes as a target of SARS CoV-2 in pathogenesis of COVID-19. Arch Euromed. 2020;10:3. doi: 10.35630/32199-35885X/32020/35610/35633.35631. [DOI] [Google Scholar]
- 28.Huerga Encabo H., Grey W., Garcia-Albornoz M., Wood H., Ulferts R., Aramburu I.V., et al. Human erythroid progenitors are directly infected by SARS-CoV-2: implications for emerging erythropoiesis in severe COVID-19 patients. Stem Cell Reports. 2021;(February) doi: 10.1016/j.stemcr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho M., Davis T.M., Silamut K., Bunnag D., White N.J. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. 1991;59(6):2135–2139. doi: 10.1128/iai.59.6.2135-2139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polat V., Bostancı G.İ. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J Thromb Thrombolysis. 2020;50(1):239–241. doi: 10.1007/s11239-020-02132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price L.C., McCabe C., Garfield B., Wort S.J. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labò N., Ohnuki H., Tosato G. Vasculopathy and coagulopathy associated with SARS-CoV-2 infection. Cells. 2020;9(7):1583. doi: 10.3390/cells9071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavalcanti D.D., Raz E., Shapiro M., Dehkharghani S., Yaghi S., Lillemoe K., et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41(8):1370–1376. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260:118431. doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta A., Rai S., Das Gupta A. Persistently elevated laboratory markers of thrombosis and fibrinolysis after clinical recovery in malaria points to residual and smouldering cellular damage. Indian J Hematol Blood Transfus. 2012;28(1):29–36. doi: 10.1007/s12288-011-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francischetti I.M.B., Seydel K.B., Monteiro R.Q. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15(2):81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tropical medicine and international health. John Wiley & Sons; 2014. WHO severe malaria. [Google Scholar]
- 38.Azghandi M., Kerachian M.A. Detection of novel coronavirus (SARS-CoV-2) RNA in peripheral blood specimens. J Transl Med. 2020;18(November (1)):412. doi: 10.1186/s12967-020-02589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]