PURPOSE
Tamoxifen prevents breast cancer in high-risk women and reduces mortality in the adjuvant setting. Mammographic density change is a proxy for tamoxifen therapy response. We tested whether lower doses of tamoxifen were noninferior to reduce mammographic density and associated with fewer symptoms.
PATIENTS AND METHODS
Women, 40-74 years of age, participating in the Swedish mammography screening program were invited to the 6-month double-blind six-arm randomized placebo-controlled noninferiority dose-determination KARISMA phase II trial stratified by menopausal status (EudraCT 2016-000882-22). In all, 1,439 women were accrued with 1,230 participants accessible for intention-to-treat analysis. The primary outcome was proportion of women treated with placebo, 1, 2.5, 5, and 10 mg whose mammographic density decreased at least as much as the median reduction in the 20 mg arm. The noninferior margin was 17%. Secondary outcome was reduction of symptoms. Post hoc analyses were performed by menopausal status. Per-protocol population and full population were analyzed in sensitivity analysis.
RESULTS
The 1,439 participants, 566 and 873 pre- and postmenopausal women, respectively, were recruited between October 1, 2016, and September 30, 2019. The participants had noninferior mammographic density reduction following 2.5, 5, and 10 mg tamoxifen compared with the median 10.1% decrease observed in the 20 mg group, a reduction confined to premenopausal women. Severe vasomotor symptoms (hot flashes, cold sweats, and night sweats) were reduced by approximately 50% in the 2.5, 5, and 10 mg groups compared with the 20 mg group.
CONCLUSION
Premenopausal women showed noninferior magnitude of breast density decrease at 2.5 mg of tamoxifen, but fewer side effects compared with the standard dose of 20 mg. Future studies should test whether 2.5 mg of tamoxifen reduces the risk of primary breast cancer.
INTRODUCTION
Approximately 490,000 women in Europe are estimated to be diagnosed with breast cancer in 2020.1 This means that nearly one woman a minute will be diagnosed with breast cancer. Despite the high incidence, few preventive programs have been initiated.
CONTEXT
Key Objective
To measure the effect of different doses of tamoxifen on mammographic density and side effects and compare lower doses to the established 20 mg dose.
Knowledge Generated
Lower doses of tamoxifen reduced mammographic density, a proxy for therapy response, noninferior to the full dose of tamoxifen, but with less severe vasomotor symptoms.
Relevance
Tamoxifen prevents breast cancer in high-risk women and reduces mortality in the adjuvant setting but uptake is low because of severe side effects. Adherence may be improved by using low-dose tamoxifen because of less severe vasomotor side effects.
Although tamoxifen was first developed as a contraceptive,2 it was proven to be highly effective as an adjuvant therapy for breast cancer. The impact was found specifically for hormone-positive breast cancer, and because it reduced the risk of distant recurrence, local recurrence, and contralateral breast cancer a series of prevention trials were conducted around the world. Several landmark studies led to (US) Food and Drug Administration approval for breast cancer prevention.3-5 A recent Cochrane review including 28,832 women concluded that 20 mg of tamoxifen, compared with placebo, reduced the risk of breast cancer with approximately 30% in women with above-average risk of developing breast cancer.6
Despite the beneficial effect of tamoxifen, uptake and adherence are low, partly because of symptoms.7,8 Few trials have tested lower doses of tamoxifen as a means to reduce symptoms. A recent study showed that tamoxifen at 5 mg/d for 3 years reduced the recurrence of breast intraepithelial neoplasia by 50% and contralateral breast cancer by 75% with a symptom profile similar to placebo.9
It is well known that mammographic density is associated with increased risk of breast cancer10 and that it decreases the sensitivity of both conventional 2D mammography11 and 3D tomosynthesis.12 Tamoxifen reduces mammographic density, and a change in mammographic density during tamoxifen therapy has been found to be a proxy end point for tamoxifen therapy response, both in the preventive and adjuvant settings.13,14
We performed the double-blind placebo controlled randomized KARISMA trial to test whether lower doses of tamoxifen could reduce mammographic density noninferior to the conventional 20 mg dose and whether a reduction in dose results in fewer symptoms. The primary aim was to identify the lowest dose noninferior in ability to reduce mammographic density compared with the 20 mg dose. The secondary aim was to compare dose-dependent self-reported symptoms.
If a low dose of tamoxifen is as effective as the standard 20 mg and causes fewer symptoms, it has the potential to improve prevention, increase adherence in the adjuvant setting, and increase the sensitivity of a 2D and 3D mammogram.
PATIENTS AND METHODS
Study Design
KARISMA phase II is a double-blind placebo-controlled randomized 6-month and six-arm noninferiority dose-determination trial conducted in Sweden between October 1, 2016, and September 30, 2019 (EudraCT 2016-000882-22). The primary end point was noninferior mammographic density decrease at 6 months. A density responder was defined as a woman with a density decrease as large or larger than the median decrease observed in the 20 mg arm. The primary aim of KARISMA was to demonstrate noninferiority of the proportion density responders in the placebo, 1, 2.5, 5, and 10 mg arms compared with the 20 mg arm.
The trial was approved by the ethics review board at Karolinska Institutet, Stockholm, Sweden (2016/65-31/2), and Swedish Medical Products Agency (5.1-2016-41112). The study Protocol is available online only.
Participants
Pre- and postmenopausal women, 40-74 of age, attending the population based national mammography screening program were invited. The main exclusion criteria were cardiovascular disorder and low mammographic density (BI-RADS A).15 All participants signed informed written consent and were informed that they were not invited based on individual risk but based on breast density.
Random Assignment and Masking
Participants were randomly assigned based on a computer procedure in a 1:1 ratio to receive placebo, 1, 2.5, 5, 10, or 20 mg of tamoxifen in blocks of 60 women to maintain balanced random assignment over shorter periods. In all, 1,440 women were randomly assigned, stratified by menopausal status. One randomly assigned premenopausal participant was found to have no measurable density and was therefore excluded, leaving 1,439 women, 566 premenopausal women and 873 postmenopausal women, in the study. The allocation was 242, 239, 235, 240, 242, and 241 women for placebo, 1, 2.5, 5, 10, and 20 mg, respectively. In the premenopausal group, the corresponding allocation was 89, 100, 91, 94, 95, and 97 women, and in the postmenopausal group, it corresponded to 153, 139, 144, 146, 147, and 144 women (Fig 1). The participants received tablet boxes with identical appearance marked with a personal participant random assignment ID. A computer software program (SAS Institute Inc) generated the block random assignment sequence, and P.H., M.B., S.B., S.M., and L.T. enrolled the participants. All study participants, personnel, and clinicians were blinded to treatment allocation. The database was unblinded after the database lock following termination of the last study participant finalizing the 6-month therapy period.
FIG 1.
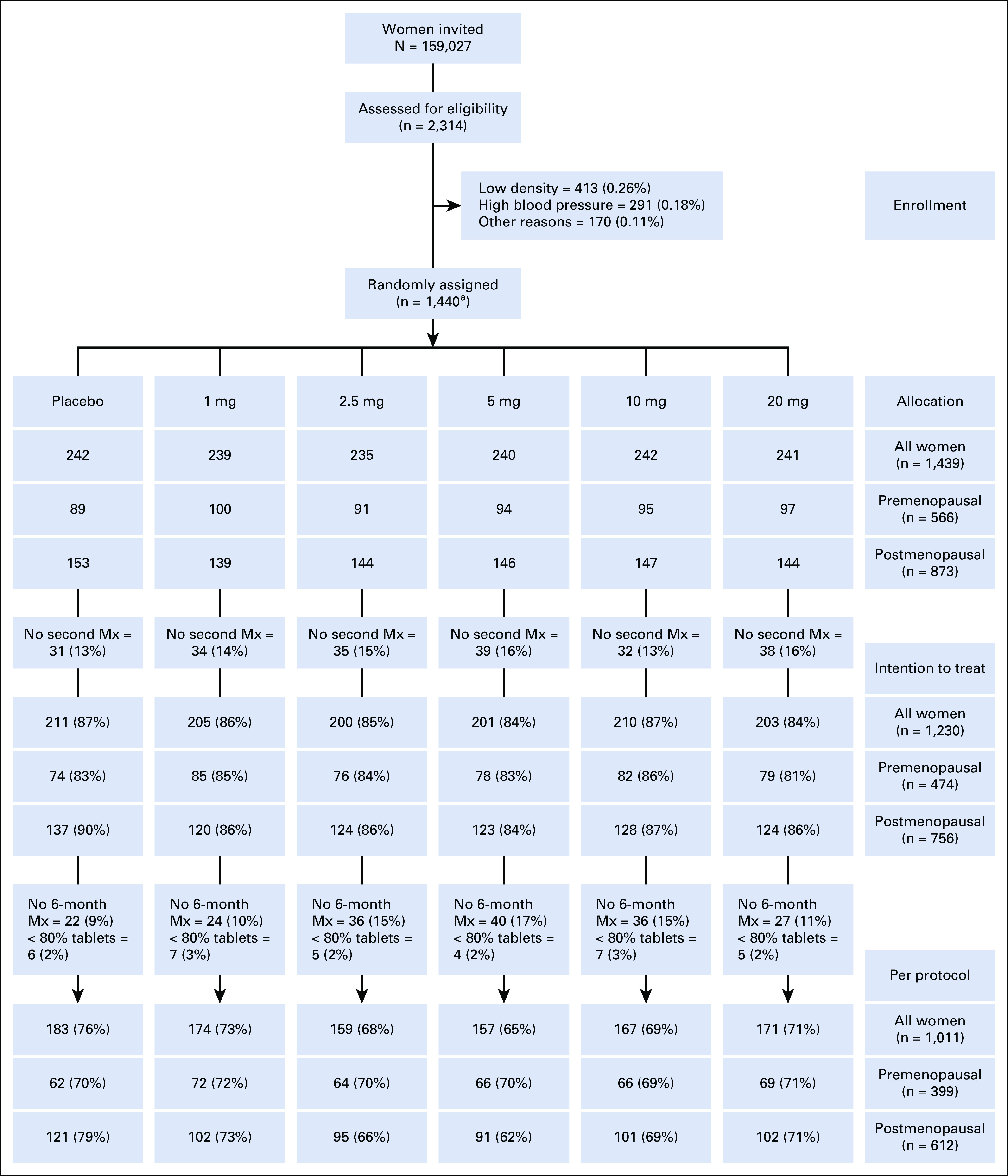
CONSORT diagram for the KARISMA phase II trial. In total, 159,027 women were invited to participate, and 2,314 (1.4%) women were investigated for inclusion to the study. In all, 874 (0.55%) women were excluded because of too low mammographic density (0.26%), hypertension (0.18%), and other reasons (0.11%). The remaining 1,440 (0.9%) women were randomly assigned into placebo, 1, 2.5, 5, 10, or 20 mg of tamoxifen, 240 women in each arm. One randomly assigned participant was found to have no measurable density and was therefore excluded, leaving 1,439 women in the study. A total of 209 participants (14.5%) did not perform a second mammogram and density change could thus not be measured, leaving 1,230 participants in the intention to treat population. Of these women, 185 (12.9%) participants did not complete the full 6-month trial period (but performed an exit mammogram when leaving the trial) or did not take at least 80% of the tablets, 34 (2.4%), leaving 1,011 (70.2%) in the per-protocol population. aOne premenopausal woman was later found to have to measurable density and was excluded, leaving 1,439 in the study. Mx, mammogram.
Procedures
Women participating in the national mammography screening program at Södersjukhuset in Sweden were invited to participate in the KARISMA study. Volunteering women were screened for eligibility. A detailed description of inclusion routines, eligibility criteria, random assignment procedure, and baseline characteristics of the participants are available in the Data Supplement (online only). Eligible participants performed a baseline mammogram and answered questions on background factors and symptoms possibly related to tamoxifen symptoms. The participants received 6 months of medication supply at study entry, and adherence to medication was based on the difference between the tablets received and returned by the study participant.
Full-field digital mammograms of the mediolateral oblique view, from the left and right breasts, were collected at baseline and the end of study participation. Mammographic density was assessed as area density (squared centimeter) using the fully automated STRATUS method.16 The average dense area (squared centimeter) of left and right breasts at baseline was calculated and compared with average dense area at the end of the trial period, and density change was defined as the relative difference between these two measures. Before measurements and comparisons were done, images of the same breast were aligned to reduce technical differences between images.16
Each participant answered questions on symptoms at baseline and after 1, 3, and 6 months. We created a questionnaire based on an established tool to assess symptoms from hormonal treatments of breast cancer.17-19 Questions addressing vasomotor, gynecologic, sexual functioning, and musculoskeletal symptoms were answered on each occasion (Data Supplement). Each symptom was assessed on a five-grade symptom severity Likert scale (not at all, a little bit, somewhat, quite a bit, and very much). A severe symptom was defined as a symptom with severity quite a bit or very much.
Outcomes
The primary objective was to demonstrate noninferior reduction of mammographic density, with the proportion of density responders as the end point, in lower doses compared with standard-dose 20 mg. The secondary outcomes were symptom severity and adherence in the lower doses compared with the standard dose. Safety and adverse events were assessed by study personnel available through study center phone, a phone app for spontaneous reports, and through scheduled questionnaires at months 1, 3, and 6. The study participants were invited for personal meetings with a medical doctor at the end of study participation.
Statistical Analysis
KARISMA's sample size was determined to achieve 80% power to demonstrate noninferiority with respect to the proportion of responders in the placebo, 1, 2.5, 5, and 10 mg arms compared with the 20 mg arm. The study sample size accounted for a dropout rate up to 30% and for correction for multiple testing. The significance level (alpha) was set to .025.
The noninferiority margin was based on the proportion of responders and was set to 17%. That is, we defined noninferiority to mean that the proportion of responders in the nonreferent study arms is not less than one third of the responders in the 20 mg group (50% minus 33% = 17%) (Data Supplement).
We performed analyses on the intention-to-treat population (ITT; all participants with a measure of mammographic density at baseline and when ending the trial period) as detailed in the Data Supplement. In a prespecified sensitivity analysis, we used multiple imputation to calculate the relative change in density after 6 months for all women who did not complete 6 months of treatment, including those who did not have any measure of mammographic density postbaseline (full population; Data Supplement). As a sensitivity analysis, we also performed analyses on the per-protocol population (PP; women completing the 6 months of tamoxifen and took at least 80% of the pills).
For the primary end point (proportion of responders), we calculated one-sided P values for noninferiority using Wald tests. The standard errors were estimated by bootstrapping to take into account the variability of the estimate for the median relative dense area change in the 20 mg arm. P values were corrected for multiple comparisons using the Holm-Bonferroni method (Data Supplement).20-22 Noninferiority was declared if the corrected P values were less than α = .025. We reported one-sided 97.5% normal-based CIs.
For the secondary end point, we compared the proportions of severe symptoms and the proportion of women who dropped out in lower doses with the 20 mg dose including 95% CIs. Prevalence ratios, stratified by menopausal status and tamoxifen dose, were estimated using a log-binomial model with Wald 95% CIs.23
We performed additional post hoc analyses. We estimated the mean relative mammographic density change by arm as the differences between means by arm, and 95% CIs, using analysis of variance. We also performed subgroup analyses of primary and secondary end points in pre- and postmenopausal women. The heterogeneity of the treatment effect in terms of mean relative mammographic density change was assessed by jointly testing the interaction terms between study arm and menopausal status in a two-way analysis of variance model.
All analyses were done in SAS 9.4 and R 3.6. Data monitoring of the study was performed as described in the study Protocol. The trial was registered at clinicaltrialsregister.eu EudraCT number 2016-000882-22.
RESULTS
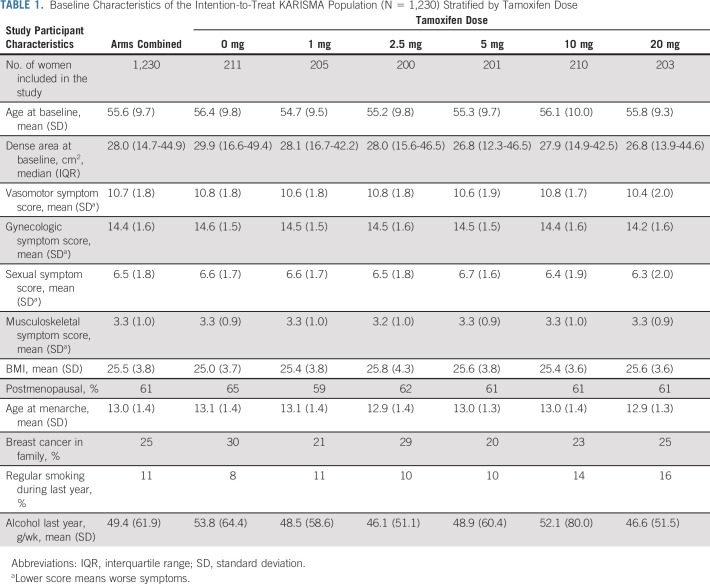
In total, 2,314 women were screened for eligibility between October 1, 2016, and September 30, 2019, and 1,439 women were randomly assigned in six arms, of whom 566 were premenopausal and 873 postmenopausal (Fig 1). The participants were evenly distributed across the six dose arms. A total of 209 participants (14.5%) did not perform a second mammogram, leaving 1,230 participants in the ITT population. Of these women, 185 (12.9%) participants did not complete the full 6-month trial period (but performed an exit mammogram when leaving the trial) or did not take at least 80% of the tablets, 34 (2.4%), leaving 1,011 (70.2%) in the PP (Fig 1). There was no major difference in baseline characteristics except for tobacco use being more prevalent in the 20 mg arm (16%) than in the placebo arm (8%; Table 1). We present the results of the ITT population in the main manuscript and results of the PP and multiple imputation full population in the Data Supplement.
TABLE 1.
Baseline Characteristics of the Intention-to-Treat KARISMA Population (N = 1,230) Stratified by Tamoxifen Dose
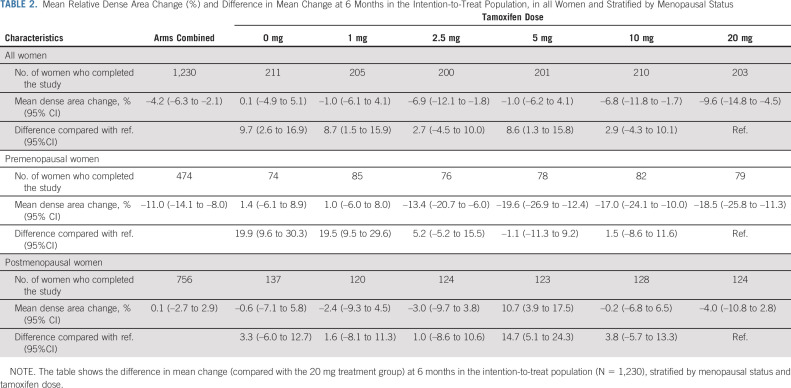
The mean overall area density decrease in the 20 mg group was 9.6% (Table 2; Data Supplement) and similar decreases were seen in the 2.5 and 10 mg of tamoxifen groups but not in the placebo and 1 mg arms. The density change was, however, driven by the decrease seen among premenopausal women where the 20 mg mean decrease was 18.5% (P < .001 for interaction with menopausal status) with small differences compared with the 2.5, 5, and 10 mg treatment arms (Table 2; Data Supplement). Postmenopausal participants randomly assigned to 20 mg had a density change of –4.0%, not substantially different to the placebo, 1, 2.5, and 10 mg treatment arms. Similar results were seen in the full population and PP populations (Data Supplement). The difference in density decrease between pre- and postmenopausal women was not dependent on the difference in baseline mammographic density between the two groups (Data Supplement).
TABLE 2.
Mean Relative Dense Area Change (%) and Difference in Mean Change at 6 Months in the Intention-to-Treat Population, in all Women and Stratified by Menopausal Status
The cut-point for a responder, defined as the median relative dense area decrease in the 20 mg group, was –10.1%. More than 50% of the women receiving tamoxifen 2.5, 5, and 10 mg were responders (Fig 2). However, the findings were confined to the premenopausal women, where the proportions of responders in the 2.5, 5, 10, and 20 mg treatment arms ranged from 69.7% to 74.4%. For postmenopausal women, small differences in the proportions of responders were seen between treatment groups (Fig 2). Similar results were seen in the PP population (Data Supplement).
FIG 2.
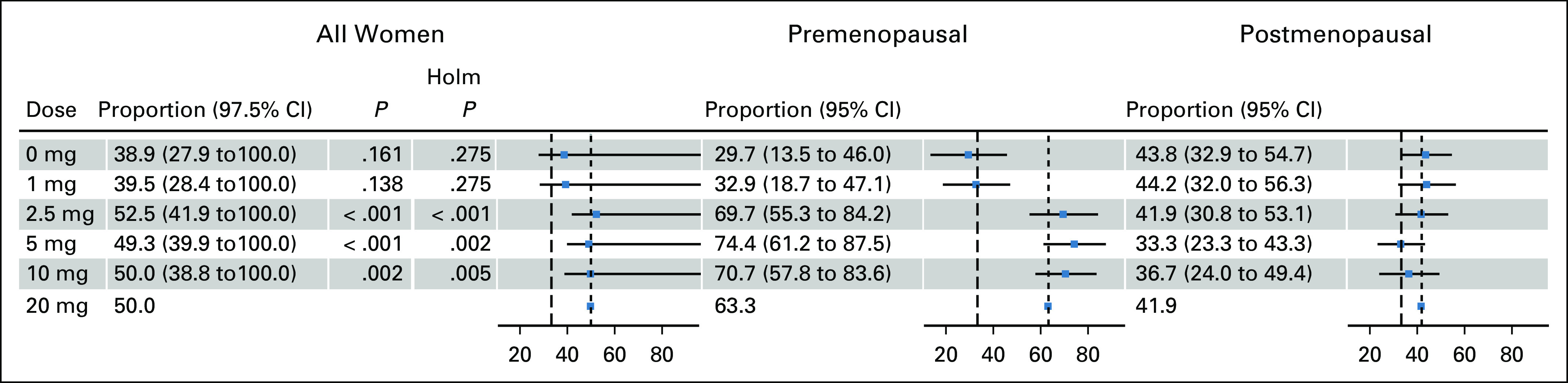
Noninferiority analysis of proportion of responders for the intention-to-treat (ITT) population in all women and stratified by menopausal status. In the ITT population (N = 1,230), the figure shows the proportions of women who had a larger decrease than median relative density decrease in the 20 mg arm (–10.1%) for all women at study exit, stratified by menopausal status and tamoxifen dose. The proportions of women in the 20 mg arms who had a larger decrease than median decrease are denoted with short-dashed lines, and the noninferiority margins of 33% are denoted with long-dashed lines. The Holm P values show Bonferroni-Holm–corrected one-sided tests with rejected null hypotheses for noninferiority of all women.
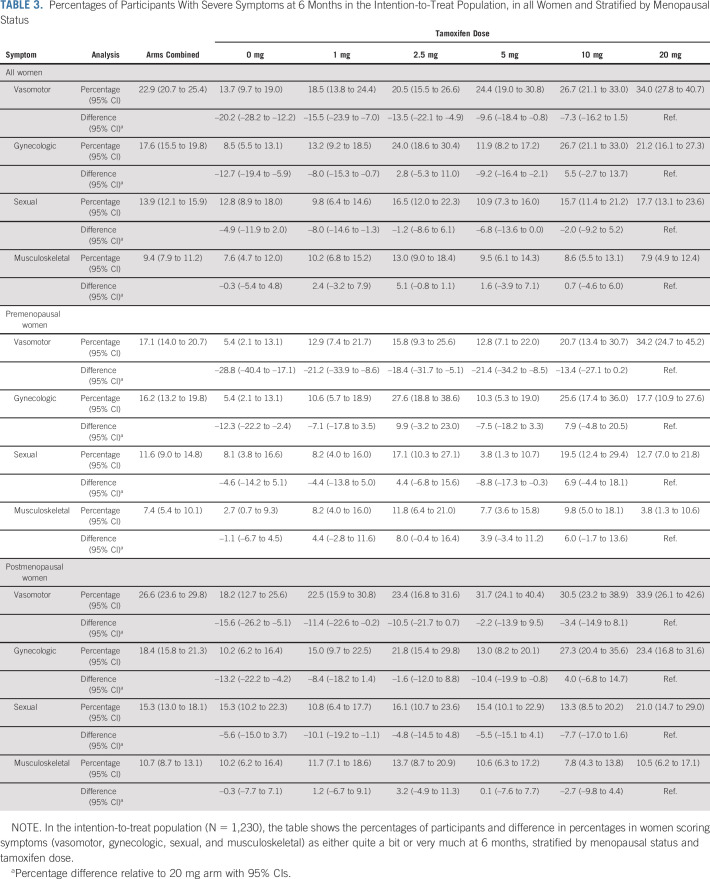
A total of 34.0% of the women in the 20 mg arm experienced severe vasomotor symptoms (Table 3). Significantly lower symptom burdens were seen for 0, 1, 2.5, and 5 mg arms of tamoxifen. The effect of tamoxifen on vasomotor symptoms was similar for pre- and post-menopausal women. No apparent trend was seen for gynecologic symptoms (Table 3). The sexual and musculoskeletal symptoms were similar in placebo as in the tamoxifen arms (Table 3). Similar results were seen in the PP population (Data Supplement). The symptom findings were mirrored in the prevalence ratios in the ITT and PP populations (Data Supplement). No apparent trend was seen for discontinuation across the arms (Data Supplement).
TABLE 3.
Percentages of Participants With Severe Symptoms at 6 Months in the Intention-to-Treat Population, in all Women and Stratified by Menopausal Status
DISCUSSION
We show that a tamoxifen dose of 2.5 mg was the minimal dose that reduces mammographic density noninferior to the standard dose of 20 mg. The reduction was, however, confined to premenopausal women, and no density reduction was seen for postmenopausal women. Vasomotor problems were the most reported symptoms in both pre- and postmenopausal women, and a more than 50% symptom reduction was seen in women receiving placebo, 1, 2.5, and 5 mg tamoxifen compared with 20 mg.
The reduction of mammographic density in pre-menopausal women, but not in postmenopausal women, has been observed previously10 but is in striking contrast to the effect of adjuvant tamoxifen therapy for postmenopausal women,24 and the preventive effect seen in several large randomized controlled trials.6
Tamoxifen blocks the effect of circulating estrogen, reduces insulin growth factor-1 levels,25 alveolar development, epithelial cell proliferation, and extracellular matrix turnover,26 effects that might influence the premenopausal breast tissue to a larger extent than the postmenopausal breast tissue. It could also be that the postmenopausal tissue needs a longer exposure for an effect to be seen.27 In our previous study, only including postmenopausal women, the mean time difference between baseline and follow-up mammogram was 1.4 years.14 However, an explanation to our findings of a menopause status dependent difference in density change must await a better understanding of the biological basis of breast density.
For premenopausal women, there did not seem to be a dose-response relationship on density, rather a threshold at 2.5 mg. Below this dose, there did not seem to be an effect of tamoxifen on mammographic density and at higher doses, no additional influence on decrease was seen. Sex steroid hormone receptors, such as the estrogen receptor, are transcription factors mediating the biological effects of estrogen by regulating gene expression. Tamoxifen results in a downregulation of target genes. The effect of tamoxifen is mediated through metabolites with a higher affinity to the estrogen receptor than the prodrug tamoxifen.28 It could be that the mechanism behind the apparent saturation effect in density reduction is simply a reflection of the necessary metabolite levels achieved in the different dosage groups.
In our study on mammographic density reduction, adherence to therapy was not significantly influenced by tamoxifen dose. Previous tamoxifen-prevention studies have shown similar results.29,30 The high proportion of participants with family history of breast cancer indicate that adherence could be influenced by a dedication to support breast cancer research.
Approximately one third of pre- and postmenopausal women on tamoxifen 20 mg scored vasomotor symptoms (hot flashes, cold sweats, and night sweats) as quite a bit or very much. In comparison to placebo, the severe vasomotor symptoms in the 20 mg arm were 15% more frequent in postmenopausal women and 30% more frequent in premenopausal women. The fact that vasomotor symptoms, in contrast to gynecologic, sexual, and musculoskeletal symptoms, seemed to be associated with tamoxifen is in line with previous findings.6
The weakness and strength of the study is the trial design. The blinded randomized controlled design reduces bias in baseline characteristics, treatment assignment, and follow-up. The few invited participants finally agreeing to participate in the trial could cause a selection bias, but such a bias is of lesser relevance in a dose-determination study.
The mammographic density surrogate end point was chosen for mainly two reasons. Several studies have shown that density decrease is associated with tamoxifen therapy response.10,13,14,24,31 Cuzick et al.13 showed in the preventive setting that healthy participants in the International Breast Cancer Intervention Study I prevention trial who experienced a density decrease were the only ones who had a protective effect of tamoxifen. Furthermore, a six-armed dose-determination trial with breast cancer incidence as outcome must include well over 100,000 women and follow-up has to exceed 5 years.
Tamoxifen has so far been tested in preventive trials including more than 20,000 women.6,26 Tamoxifen has an established effect, but uptake is low, particularly so in the preventive setting. If low-dose tamoxifen retains its preventive effect with fewer and less severe symptoms, it has the potential to increase the benefit-to-harm ratio. This is particularly relevant for premenopausal women in whom the risk of endometrial cancer and fatal thromboembolism is low.
Our findings are in agreement with the study by DeCensi in which 5 mg of tamoxifen over a 3-year period significantly reduced intraepithelial neoplasia9 and that women using low-dose tamoxifen experience less severe vasomotor side effects. It has been shown that approximately 50% of patients treated with 20 mg of tamoxifen discontinued medication within 5 years.32
The KARISMA trial is a dose-determination study and our results suggest that substantially lower doses of tamoxifen could be as effective as the standard 20 mg dose. We have shown that both 2.5 and 20 mg of tamoxifen reduce density with 15%-20% in premenopausal women and that the lower dose comes with substantially reduced vasomotor symptom. Future studies should test whether 2.5 mg of tamoxifen reduces the risk of primary breast cancer.
ACKNOWLEDGMENT
The authors thank the study participants and study personnel for their devoted support of the study.
Mikael Eriksson
Stock and Other Ownership Interests: Swedish Funds
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Martin Eklund
Stock and Other Ownership Interests: A3P Biomedical
Consulting or Advisory Role: QuantumLeap Health
Patents, Royalties, Other Intellectual Property: I am an inventor of five pending patent applications related to prostate cancer diagnostics. I do not receive any royalties
Signe Borgquist
Honoraria: Pfizer
Travel, Accommodations, Expenses: Roche
Ann Rosendahl
Employment: UCB
Stock and Other Ownership Interests: AstraZeneca, Novo Nordisk, Pfizer
Kristina Lång
Honoraria: AstraZeneca
José Tapia
Travel, Accommodations, Expenses: Rovi
Magnus Bäcklund
Employment: Diagnostiskt Centrum Hud i Sverige AB
Mattias Hammarström
Research Funding: We are conducting an RCT partly financed by Atossa Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Kamila Czene
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Per Hall
Honoraria: iCAD
Research Funding: Atossa Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Travel, Accommodations, Expenses: iCAD
No other potential conflicts of interest were reported.
DISCLAIMER
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
SUPPORT
Per Hall: The Kamprad Foundation—20150052. Per Hall: Swedish Research Council—E0718301. Per Hall: Märit and Hans Rausing's Initiative Against Breast Cancer. Per Hall: Swedish Cancer Society—CAN 2015/649. Per Hall: Stockholm County Council—4-2645/2015. Martin Eklund: Swedish Research Council—2019-01466.
CLINICAL TRIAL INFORMATION
EudraCT number 2016-000882-22
AUTHOR CONTRIBUTIONS
Conception and design: Mikael Eriksson, Martin Eklund, Signe Borgquist, Sara Margolin, Magnus Bäcklund, Marike Gabrielson, Mattias Hammarström, Yvonne Wengström, Kamila Czene, Per Hall
Financial support: Martin Eklund, Kamila Czene, Per Hall
Administrative support: Signe Borgquist
Provision of study materials or patients: Signe Borgquist, Sara Margolin, Linda Thoren, Magnus Bäcklund, Per Hall
Collection and assembly of data: Mikael Eriksson, Martin Eklund, Signe Borgquist, Roxanna Hellgren, Sara Margolin, Kristina Lång, José Tapia, Mattias Hammarström, Kamila Czene, Per Hall
Data analysis and interpretation: Mikael Eriksson, Martin Eklund, Sara Margolin, Linda Thoren, Ann Rosendahl, Kristina Lång, Magnus Bäcklund, Andrea Discacciati, Alessio Crippa, Marike Gabrielson, Kamila Czene, Per Hall
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mikael Eriksson
Stock and Other Ownership Interests: Swedish Funds
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Martin Eklund
Stock and Other Ownership Interests: A3P Biomedical
Consulting or Advisory Role: QuantumLeap Health
Patents, Royalties, Other Intellectual Property: I am an inventor of five pending patent applications related to prostate cancer diagnostics. I do not receive any royalties
Signe Borgquist
Honoraria: Pfizer
Travel, Accommodations, Expenses: Roche
Ann Rosendahl
Employment: UCB
Stock and Other Ownership Interests: AstraZeneca, Novo Nordisk, Pfizer
Kristina Lång
Honoraria: AstraZeneca
José Tapia
Travel, Accommodations, Expenses: Rovi
Magnus Bäcklund
Employment: Diagnostiskt Centrum Hud i Sverige AB
Mattias Hammarström
Research Funding: We are conducting an RCT partly financed by Atossa Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Kamila Czene
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Per Hall
Honoraria: iCAD
Research Funding: Atossa Therapeutics
Patents, Royalties, Other Intellectual Property: Pending patent on compositions and methods for prevention of breast cancer with an option to license to Atossa Therapeutics. Licensed the algorithm for risk prediction based on analyses of mammographic features to iCAD
Travel, Accommodations, Expenses: iCAD
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society : Breast Cancer Facts & Figures 2019-2020. Atlanta, GA, American Cancer Society, 2019 [Google Scholar]
- 2.Viviane M: Quirke; tamoxifen from failed contraceptive pill to best-selling breast cancer medicine: A case-study in pharmaceutical innovation. Front Pharmacol 8:620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzick J Sestak I Cawthorn S, et al. : Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 16:67-75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B Costantino JP Wickerham DL, et al. : Tamoxifen for the prevention of breast cancer: Current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 97:1652-1662, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Powles TJ Ashley S Tidy A, et al. : Twenty year follow-up of the Royal Marsden randomized, double blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst 99:283-290, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Mocellin S, Goodwin A, Pasquali S: Risk-reducing medications for primary breast cancer: A network meta-analysis. Cochrane Database Syst Rev 4:CD012191, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SG Sestak I Forster A, et al. : Factors affecting uptake and adherence to breast cancer chemoprevention: A systematic review and meta-analysis. Ann Oncol 27:575-590, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bober SL Hoke LA Duda RB, et al. : Decision-making about tamoxifen in women at high risk for breast cancer: Clinical and psychological factors. J Clin Oncol 22:4951-4957, 2004 [DOI] [PubMed] [Google Scholar]
- 9.DeCensi A Puntoni M Guerrieri-Gonzaga A, et al. : Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol 37:1629-1637, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzick J Warwick J Pinney E, et al. : Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 96:621-628, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B Scoccianti C Loomis D, et al. : Breast-cancer screening—Viewpoint of the IARC working group. N Engl J Med 372:2353-2358, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Dimitrova N Parkinson ZS Bramesfeld A, et al. : European Guidelines for Breast Cancer Screening and Diagnosis—the European Breast Guidelines. Luxembourg, Publications Office of the European Union, 2016 [Google Scholar]
- 13.Cuzick J Warwick J Pinney E, et al. : Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J Natl Cancer Inst 103:744-752, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Li J Humphreys K Eriksson L, et al. : Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol 31:2249-2256, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J Azziz A Fan B, et al. : Agreement of mammographic measures of volumetric breast density to MRI. PLoS One 8:e81653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson M Li J Leifland K, et al. : A comprehensive tool for measuring mammographic density changes over time. Breast Cancer Res Treat 169:371-379, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallowfield LJ Leaity SK Howell A, et al. : Assessment of quality of life in women undergoing hormonal therapy for breast cancer: Validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189-199, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J Sestak I Forbes JF, et al. : Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 383:1041-1048, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Land SR Walcott FL Liu Q, et al. : Symptoms and QOL as predictors of chemoprevention adherence in NRG oncology/NSABP trial P-1. J Natl Cancer Inst 108:djv365, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonferroni CE: Il calcolo delle assicurazioni su gruppi di teste. Roma, Italy, Tipografia del Senato, 1995 [Google Scholar]
- 21.Holm S: A simple sequentially rejective multiple test procedure. Scand J Stat 6:65-70, 1979 [Google Scholar]
- 22.Romano JP, Shaikh AM, Wolf M: Control of the false discovery rate under dependence using the boot-strap and subsampling. Test 17:417, 2008 [Google Scholar]
- 23.McNutt LA Wu C Xue X, et al. : Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 157:940-943, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C Godwin J Gray R, et al. : Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 378:771-784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decensi A Robertson C Guerrieri-Gonzaga A, et al. : Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol 27:3749-3756, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattar R Maller O McDaniel S, et al. : Tamoxifen induces pleiotrophic changes in mammary stroma resulting in extracellular matrix that suppresses transformed phenotypes. Breast Cancer Res 11:R5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decensi A Gandini S Serrano D, et al. : Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol 25:4201-4209, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Wu X Hawse JR Subramaniam M, et al. : The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells; Cancer Res 69:1722-1727, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Smith SG Sestak I Howell A, et al. : Participant-reported symptoms and their effect on long-term adherence in the International Breast Cancer Intervention Study I (IBIS I). J Clin Oncol 35:2666-2673, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powles T Eeles R Ashley S, et al. : Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial; Lancet 352:98-101, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Nyante SJ Sherman ME Pfeiffer RM, et al. : Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J Natl Cancer Inst 107:dju425, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W Fang F Varnum C, et al. : Predictors of discontinuation of adjuvant hormone therapy in patients with breast cancer. J Clin Oncol 33:2262-2269, 2015 [DOI] [PubMed] [Google Scholar]