Abstract
PURPOSE:
We evaluated proof of principle for resource-efficient, risk-based screening through reanalysis of the Kerala Oral Cancer Screening Trial.
METHODS:
The cluster-randomized trial included three triennial rounds of visual inspection (seven clusters, n = 96,516) versus standard of care (six clusters, n = 95,354) and up to 9 years of follow-up. We developed a Cox regression–based risk prediction model for oral cancer incidence. Using this risk prediction model to adjust for the oral cancer risk imbalance between arms, through intention-to-treat (ITT) analyses that accounted for cluster randomization, we calculated the relative (hazard ratios [HRs]) and absolute (rate differences [RDs]) screening efficacy on oral cancer mortality and compared screening efficiency across risk thresholds.
RESULTS:
Oral cancer mortality was reduced by 27% in the screening versus control arms (HR = 0.73; 95% CI, 0.54 to 0.98), including a 29% reduction in ever-tobacco and/or ever-alcohol users (HR = 0.71; 95% CI, 0.51 to 0.99). This relative efficacy was similar across oral cancer risk quartiles (P interaction = .59); consequently, the absolute efficacy increased with increasing model-predicted risk—overall trial: RD in the lowest risk quartile (Q1) = 0.5/100,000 versus 13.4/100,000 in the highest quartile (Q4), P trend = .059 and ever-tobacco and/or ever-alcohol users: Q1 RD = 1.0/100,000 versus Q4 = 22.5/100,000; P trend = .026. In a population akin to the Kerala trial, screening of 100% of individuals would provide 27.1% oral cancer mortality reduction at number needed to screen (NNS) = 2,043. Restriction of screening to ever-tobacco and/or ever-alcohol users with no additional risk stratification would substantially enhance efficiency (43.4% screened for 23.3% oral cancer mortality reduction at NNS = 1,029), whereas risk prediction model–based screening of 50% of ever-tobacco and/or ever-alcohol users at highest risk would further enhance efficiency with little loss in program sensitivity (21.7% screened for 19.7% oral cancer mortality reduction at NNS = 610).
CONCLUSION:
In the Kerala trial, the efficacy of oral cancer screening was greatest in individuals at highest oral cancer risk. These results provide proof of principle that risk-based oral cancer screening could substantially enhance the efficiency of screening programs.
BACKGROUND
Cancers of the oral cavity cause substantial morbidity and mortality worldwide, with approximately 300,000 incident cases and 145,000 deaths annually.1 Tobacco smoking, chewing of betel-quid or betel-nut with or without tobacco, and alcohol use cause the majority of oral cancers.1,2 Oral cancer incidence is particularly high in the Indian subcontinent and other parts of South and East Asia, owing to the high prevalence of betel-quid or tobacco chewing.1,2
CONTEXT
Key Objectives
We evaluate proof of principle for resource-efficient, risk-based oral cancer screening through a risk-based reanalysis of the Kerala Oral Cancer Screening Trial.
Knowledge Generated
We provide the first proof of principle for the utility of risk-based oral cancer screening. Specifically, the study presents a validated oral cancer risk prediction model for the identification of high-risk individuals for screening. Through the use of this model, the study demonstrates that (1) in the Kerala trial, the efficacy of screening increased with increasing model-predicted prescreening oral cancer risk and (2) even among high-risk ever-tobacco and/or ever-alcohol users, individuals at highest oral cancer risk experienced the greatest oral cancer mortality reduction from screening.
Relevance
Oral cancer screening through visual inspection by trained health workers is efficacious in reducing oral cancer mortality. Risk-based oral cancer screening strategies could substantially enhance the efficacy of screening programs while maintaining high program sensitivity.
Only one randomized trial has investigated the effect of screening on oral cancer mortality—the Kerala Oral Cancer Screening Trial.3 This trial found that screening through visual inspection by trained health workers (three triennial rounds) resulted in a 34% reduction in oral cancer mortality among ever-tobacco and/or ever-alcohol users in the intervention group but no benefit in never-users of tobacco and/or alcohol.3 Such restriction of the screening benefit to ever-tobacco and/or ever-alcohol users highlights the potential for risk-based screening strategies—that is, selective screening of high-risk individuals most likely to experience oral cancer mortality reductions from screening.
A risk-based oral cancer screening strategy could have substantial logistical and resource implications, particularly in resource-constrained settings. However, neither validated oral cancer risk prediction models nor empirical evidence for the efficacy and efficiency of risk-based oral cancer screening exists in the literature.
Here, we evaluated proof of principle for resource-efficient risk-based oral cancer screening through reanalysis of the Kerala trial.3
METHODS
Study Design and Subjects
The Kerala trial was a cluster-randomized study initiated in 1996 in the district of Trivandrum, India.3 The study design has been described in prior publications.3-6 Briefly, 13 geographic clusters were randomized through restricted block randomization to either the screening or intervention arm (seven clusters, n = 96,516 participants) or the standard-of-care or control arm (six clusters, n = 95,354 participants; Fig 1).3-6 Eligible participants (those of age 35 years or older without a prior history of oral cancer) were identified through a household survey, which encompassed enumeration of households and eligible individuals as well as collection of data on demographics, socioeconomic status, risk behaviors, diet, and medical history.3-6
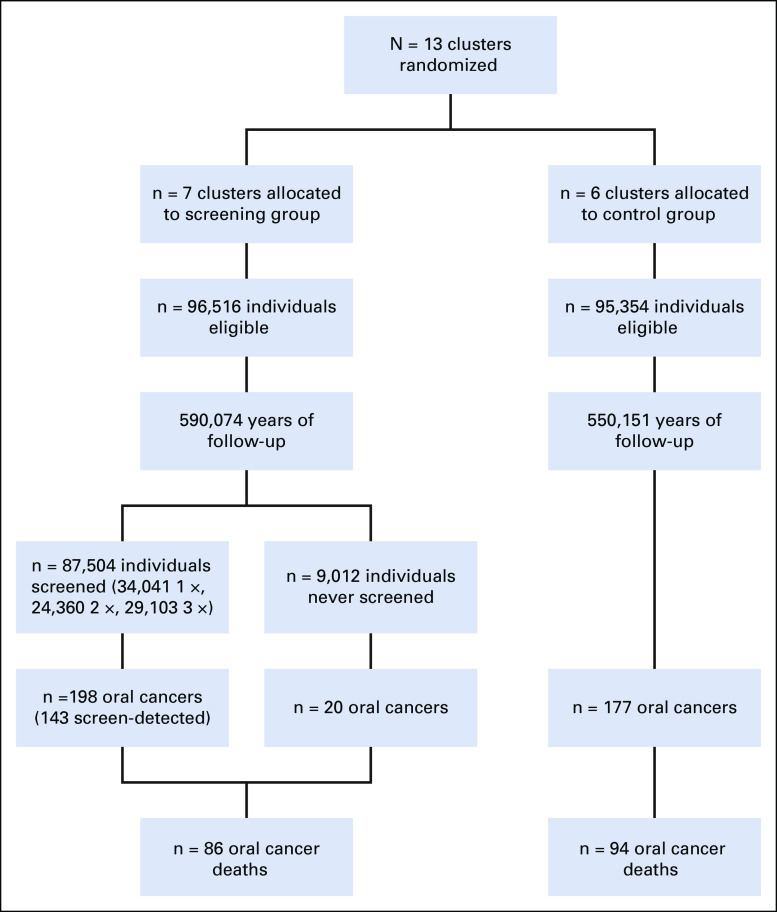
FIG 1.
CONSORT diagram. The figure depicts the number of clusters, individuals, years of follow-up, screening visits (for the screening group), oral cancers diagnosed, and oral cancer deaths in the screening and control arms of the Kerala trial. The number of screen-detected cancers reported is the number with positive screening results. Of note, the number of participants (n = 3) and number of oral cancer events (n = 32 oral cancers and n = 16 oral cancer deaths) differ between our analysis and the 2005 report by Sankaranarayanan et al. These differences arise from the exclusion of duplicate participants (n = 3) and data updates available from additional follow-up.
All participants provided written informed consent. The study was approved by the institutional ethics review boards of the International Agency for Research on Cancer (IARC) and the Regional Cancer Centre (RCC), Trivandrum. Given the conduct of the study prior to 2005, the trial was retrospectively registered at Clinicaltrials.gov identifier: NCT04494620.
Screening and Referral Procedures
In the intervention group, during the household visit, trained health workers conducted oral cancer screening under conventional lighting (daylight, aided by white flashlight).3-6 Prior validation studies in the trial show high validity (sensitivity = 94.3% and specificity = 98.3%) for the diagnosis of precancer or cancer by health workers versus clinicians as the gold standard.7 Screening was conducted in three waves (1996-1998, 1999-2001, and 2002-2004), with a 3-year interval between screens for each participant. Of 96,516 participants in the intervention group, 9,012 were never screened and 87,504 (90.7%) were screened at least once (34,041 screened once, 24,360 screened twice, and 29,103 screened three times) (Fig 1).3 The control group received standard of care (no screening). Both arms received counseling for cessation of tobacco and alcohol use.
Individuals with precancerous lesions (eg, leukoplakia, erythroplakia, and oral submucous fibrosis) or lesions suspicious for cancer (eg, abnormal growths or ulcers) were referred to a dentist or an oncologist for a detailed examination, biopsy, excision, or cancer treatment, as appropriate.3,4,6 Screening resulted in 5,145 screen-positive individuals, 3,218 (62.5%) of whom complied with referral, and 2,383 were diagnosed with oral precancers or oral cancers (positive predictive value [PPV] = 74%). Biopsies were conducted on 708 lesions with clinical suspicions of cancer, resulting in 201 dysplasias and 131 cancers.3
Follow-Up and Outcome Ascertainment
Data on oral cancer incidence and mortality were collected from multiple sources, including the population-based cancer registry of Trivandrum, hospital-based cancer registry of the RCC, local hospitals, municipal death registers, and household visits.3,4,6 Deaths occurring in individuals with a diagnosis of oral cancer and/or advanced oral cancer (regional or distant spread) were considered as oral cancer deaths.3,4,6
Statistical Analyses
Follow-up for oral cancer incidence began at the date of first household interview and ended at the earliest of date of oral cancer incidence (for incidence analyses), oral cancer death (for mortality analyses), death, or end of the study (December 31, 2004), as was done in the study by Sankaranarayanan et al.3 Of note, the number of participants and oral cancer events differ from the report by Sankaranarayanan et al because of data updates during additional follow-up (Data Supplement, online only).
Analyses were intention-to-treat (ITT) without any exclusion of nonparticipants. We used multiple imputation for missing data on education, body mass index (BMI), chewing, smoking, and alcohol use (Data Supplement).
Oral cancer risk prediction model development and validation.
We developed a Cox proportional hazards risk prediction model for 7-year oral cancer incidence, with follow-up time as the time scale and covariates chosen a priori—age, sex, education, BMI, tobacco chewing (duration, intensity, and former chewing status), smoking (duration and intensity), alcohol use (duration and intensity), a multiplicative interaction between chewing duration and smoking intensity, and study group or arm (intervention or control). The baseline hazard was stratified by tobacco chewing status (ever or never) because of nonproportionality. The best parameterizations for continuous covariates (across linear, log, square-root, and squared transformations) were selected on the basis of the Akaike Information Criterion. The oral cancer risk prediction model was validated through five-fold cross-validation, evaluated on calibration (ratio of observed/expected [O/E] cases) and discrimination (C-index), accounting for right-censoring.8,9
Estimation of the efficacy of screening.
We estimated the relative and absolute efficacy of screening on oral cancer mortality. These analyses were conducted in the overall trial, among ever-tobacco and/or ever-alcohol users, and across quartiles of 7-year predicted oral cancer risk (cut points defined on the basis of the respective risk distribution in the control group).
We calculated the relative efficacy of screening on oral cancer mortality through 9 years of follow-up using Cox regression models, with follow-up time as the time scale and study group (intervention or control) and (log) 7-year predicted risk of oral cancer incidence as covariates. Multiplicative statistical interactions between study group and risk of oral cancer incidence (continuous or quartiles) were evaluated through product terms. Taylor series linearization was used to calculate 95% CIs around the hazard ratios (HRs).
The absolute efficacy of screening on oral cancer mortality through 9 years of follow-up was estimated through mortality rate differences (RDs). Briefly, model-based mortality RDs were estimated through adjusted population-attributable fractions (PAF, adjusted for the 7-year predicted oral cancer risk) using the methods described by Flegal et al10 (Data Supplement). Jackknife variances were used to compute 95% CIs around RDs.11 Trend tests for RDs across quartiles of oral cancer incidence risk were evaluated using inverse-variance–weighted linear regression.
Estimated efficiency across different scenarios for the selection of individuals for screening.
For each individual in the trial (screening plus control arms combined), using the Cox regression model (coefficients for arm and 7-year predicted risk of oral cancer incidence), we calculated the counterfactual hazard of oral cancer mortality in the absence of screening and the presence of screening (mortality deficit commensurate with the overall HR for screening v control arms). We then compared the estimated screening efficiency across two hypothetical strategies for the selection of individuals for oral cancer screening: (1) a risk-based strategy (incremental selection, highest to lowest model-predicted 7-year risk of oral cancer); and (2) an age-based strategy (incremental selection, oldest to youngest).
For each selection strategy, measures of screening efficiency for three triennial screens and up to 9 years of follow-up were as follows: percentage of the population screened, sensitivity for oral cancer mortality in the absence of screening (ie, percentage of all oral cancer deaths in the population covered), effective reduction in oral cancer mortality from screening (sensitivity multiplied by the HR for screening), PPV, complement of the negative predictive value (cNPV = 1 − NPV), oral cancer deaths per million in the absence or presence of screening, oral cancer deaths averted per million screened, and the number needed to screen (NNS) to prevent one oral cancer death.
All analyses accounted for the cluster-sampling design and multiple imputation.9,11 Two-sided P values < 0.05 were considered statistically significant.
RESULTS
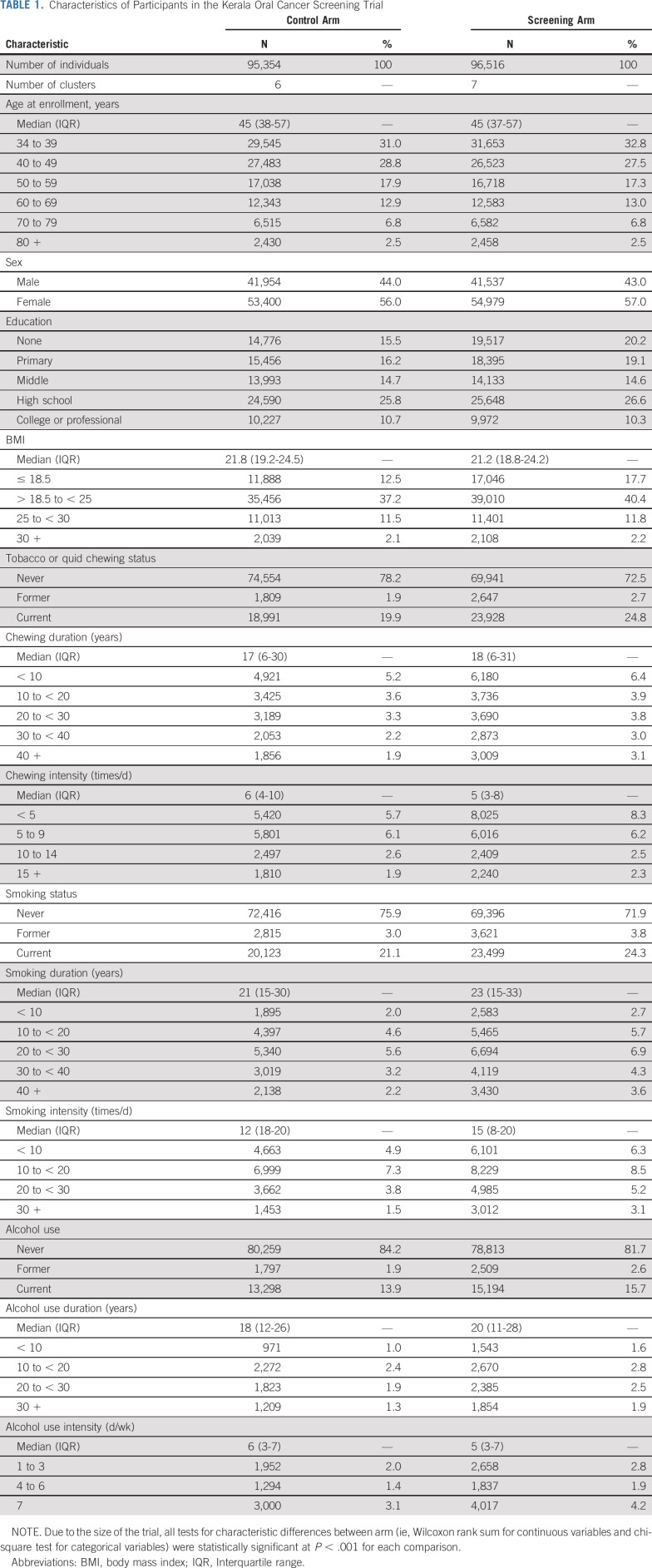
Table 1 shows the characteristics of participants in the control arm (n = 95,354 participants) and the screening arm (n = 96,516 participants). Both arms were balanced on demographic, socioeconomic, and anthropometric characteristics. However, screening arm participants were more likely to be ever chewers, be users of pan with tobacco, have greater duration and intensity of chewing, be current smokers, and have greater duration and intensity of smoking (P < .001 for all comparisons).
TABLE 1.
Characteristics of Participants in the Kerala Oral Cancer Screening Trial
In the control arm (Fig 1), more than 550,151 person-years of follow-up (median = 7.0 years; interquartile range [IQR] = 3.2 to 8.0), 177 incident oral cancers and 94 oral cancer deaths occurred. In the screening arm (Fig 1), more than 590,074 person-years of follow-up (median = 7.2 years; IQR = 3.7 to 8.2), 218 incident oral cancers and 86 oral cancer deaths occurred.
Risk Prediction Model for Oral Cancer Incidence
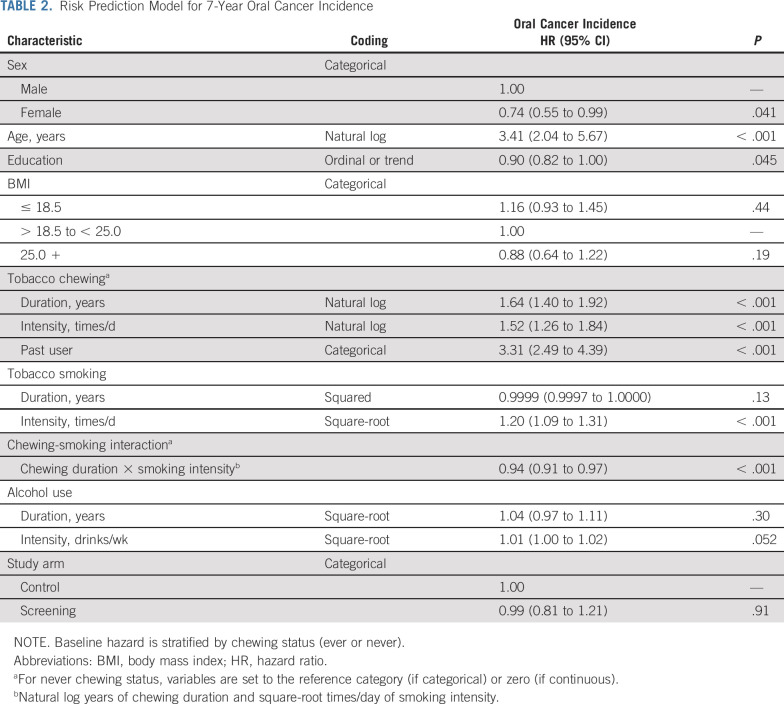
Predictors of increased 7-year risk of oral cancer were older age, male gender, lower education, former chewer status, increased duration and frequency of chewing, increased duration of smoking, and increased frequency of alcohol consumption (Table 2). There was a significant negative correlation between chewing duration and smoking frequency, which manifested as a multiplicative interaction.
TABLE 2.
Risk Prediction Model for 7-Year Oral Cancer Incidence
Screening arm participants had higher predicted 7-year risk of oral cancer incidence when compared with control arm participants (median risk = 0.085% v 0.074%; P < .001). Importantly, after adjustment for this imbalance, oral cancer incidence was similar in the screening and control arms (HR = 0.99; 95% CI, 0.81 to 1.21; Table 2).
The risk- prediction model had good calibration and good discrimination in the overall trial population (O/E = 1.08; 95% CI, 0.81 to 1.44; C-index = 0.84; 95% CI, 0.77 to 0.90) as well as in ever-tobacco and/or ever-alcohol users (O/E = 1.07; 95% CI, 0.77 to 1.43; C-index = 0.75; 95% CI, 0.67 to 0.83), including within each risk quartile (Data Supplement).
Relative and Absolute Efficacy of Screening
With up to three rounds of triennial screening with visual inspection (average of 1.76 screens received by screening arm participants) and up to 9 years of total follow-up, after adjustment for model-predicted 7-year risk of oral cancer incidence, there was a 27% reduction in oral cancer mortality in the screening arm versus control arm (HR = 0.73; 95% CI, 0.54 to 0.98; P = .035). The effect of screening was stronger in ever-tobacco and/or ever-alcohol users (HR = 0.71; 95% CI, 0.506 to 0.995, P = .047) versus nonusers (HR = 0.89; 95% CI, 0.38 to 2.09; P = .79), although this difference was not statistically significant (P heterogeneity = .64).
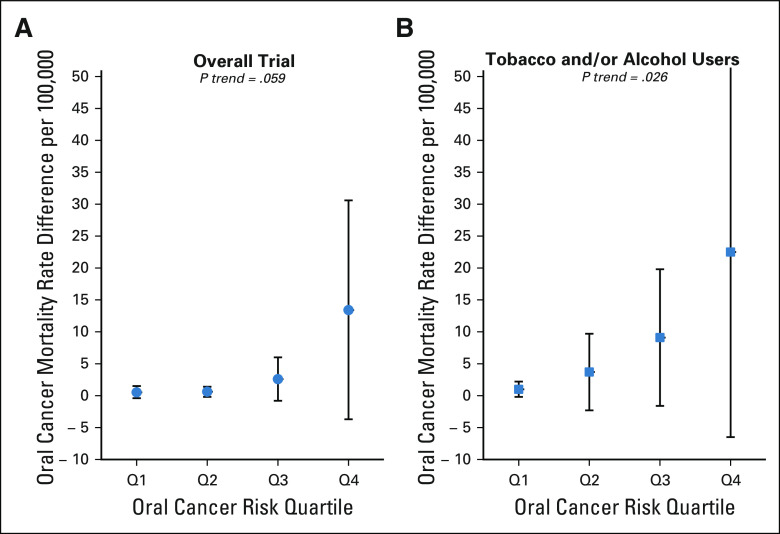
The relative efficacy of screening did not differ by predicted 7-year risk of oral cancer incidence (P interaction of arm × risk quartile = 0.59). Consequently, the absolute efficacy of screening (ie, adjusted oral cancer mortality RDs) increased significantly with increasing oral cancer risk and was greatest in individuals with the highest risk of oral cancer incidence (Figs 2A and 2B). For example, in the overall trial population (Fig 2A), the adjusted oral cancer mortality RD between screening versus control arms increased from 0.5 per 100,000 for individuals in the lowest quartile (Q1) of oral cancer risk to 13.4 per 100,000 in the highest quartile (Q4) of oral cancer risk (P trend = .059). Likewise, among ever-tobacco and/or ever-alcohol users (Fig 2B), the oral cancer mortality RD between the screening versus control arms increased from 1.0 per 100,000 for individuals in Q1 of oral cancer risk to 22.5 per 100,000 in Q4 (P trend = .026).
FIG 2.
Oral cancer mortality RD (screening v control arms) across oral cancer risk in the Kerala Oral Cancer Screening Trial. Shown are adjusted oral cancer mortality RDs between the screening and control arm participants in the overall trial participants (A) and among ever-tobacco and/or ever-alcohol users (B). Estimates (circles and squares) and 95% jackknife CIs (error bars) are shown across oral cancer risk prediction model–based quartiles (defined on the basis of the control population). P values shown are for trend across quartiles. See statistical methods for additional details. RD, rate difference.
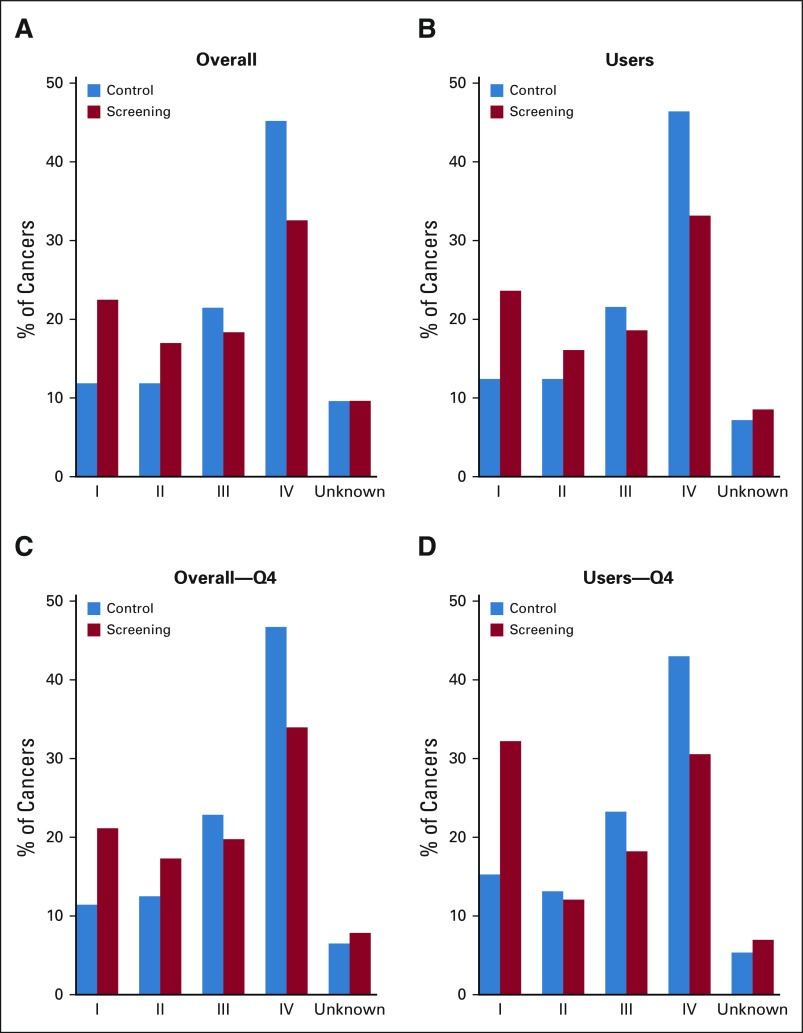
The observed oral cancer mortality reduction from screening was consistent with diagnosis of oral cancers at early stages (I/II) versus late stages (III/IV) in the screening arm versus control arm, both in the overall trial population and ever-tobacco and/or ever-alcohol users (Figs 3A-3D, Data Supplement).
FIG 3.
Stage distribution of oral cancers detected in the screening and control arms. The figure depicts the percentage of oral cancers detected at stages I, II, III, and IV and with unknown staging in the screening and control arms in the overall trial population (A) and restricted to ever-tobacco and/or ever-alcohol users (B), the highest risk quartile in the overall trial (C), and the highest risk quartile among ever-tobacco and/or ever-alcohol users (D). Chi-square test P = .0059, P = .013, P = .019, and P = .022 for A, B, C, and D, respectively.
Efficiency Across Selection Strategies for Screening
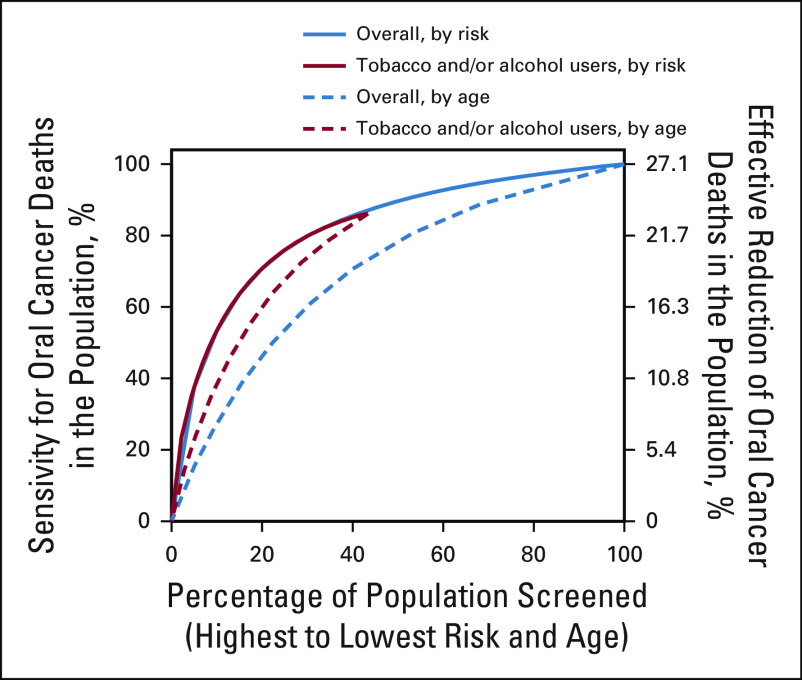
We evaluated the efficiency of two counterfactual screening strategies in the Kerala trial population (screening plus control arms combined)—risk-based and age-based. These analyses provide two broad observations (Fig 4). First, risk-based selection of individuals for screening would outperform age-based selection and provide greater sensitivity for oral cancer mortality (ie, percentage of all oral cancer deaths in the population covered by a specific selection strategy). Thus, for a fixed size of the population selected for screening, a risk-based strategy would avert a higher number of oral cancer deaths than age-based selection (Fig 4). Second, the similarity in performance of risk-based selection in the overall trial population and in ever-tobacco and/or ever-alcohol users indicates that almost all individuals at highest risk in the trial were tobacco and/or alcohol users (Fig 4).
FIG 4.
Performance of risk-based and age-based strategies for selection of individuals for oral cancer screening in the Kerala Oral Cancer Screening Trial. The figure depicts the percentage of all oral cancer deaths in the Kerala trial population targeted at varying levels of risk-based and age-based thresholds for the counterfactual selection of individuals for oral cancer screening. Results are shown for each of the selection strategies in the overall trial population and in ever-tobacco and/or ever-alcohol users. See statistical methods for additional details.
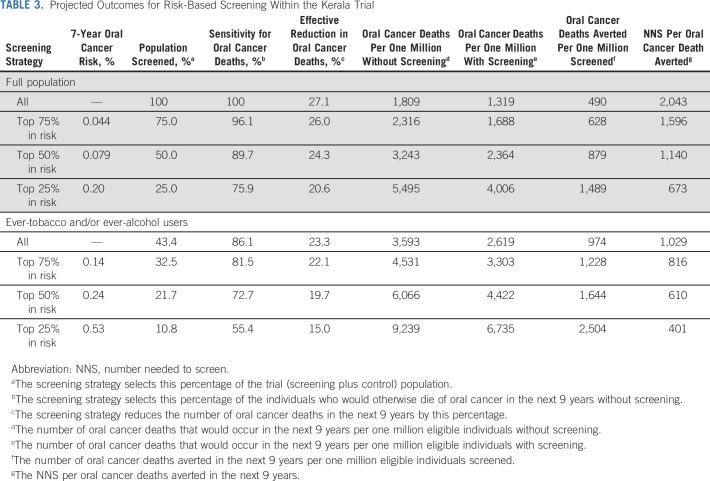
As shown in Table 3, screening of 100% of individuals in the Kerala trial would provide a 27.1% effective reduction in oral cancer mortality over 9 years (490 averted oral cancer deaths per one million screened at NNS to prevent one oral cancer death of 2,043). Restriction of screening to all ever-tobacco and/or ever-alcohol users (43.4% of the population) without any additional oral cancer risk assessment would provide 23.3% effective reduction in oral cancer mortality (974 averted oral cancer deaths per one million tobacco and/or alcohol users screened at NNS of 1,029). Restriction of risk assessment to ever-tobacco and/or ever-alcohol users and selection of 50% of ever-tobacco and/or ever-alcohol users at highest risk (21.7% of the population) would provide approximately 20% effective reduction in oral cancer mortality at substantially better efficiency (1,644 averted oral cancer deaths per one million high-risk tobacco and/or alcohol users screened at NNS of 610).
TABLE 3.
Projected Outcomes for Risk-Based Screening Within the Kerala Trial
DISCUSSION
In our risk-based reanalysis of the Kerala Oral Cancer Screening Trial, we present four key results. First, we provide a risk prediction model for oral cancer incidence to enable the identification of high-risk individuals. Second, in an ITT analysis, we show that screening with visual inspection by trained health workers resulted in a significant 27% relative reduction in oral cancer mortality. Third, we show that the absolute benefit of screening (as measured by the oral cancer mortality RD in the screening v control arms) increased significantly with increasing model-predicted risk of oral cancer, both overall and in high-risk ever-tobacco and/or ever-alcohol users. Fourth, oral cancer incidence was similar between the screening and control arms, underscoring no cancer overdiagnosis from screening. Collectively, these results provide proof of principle for risk-based oral cancer screening.
The key 2005 report from the Kerala trial by Sankaranarayanan and colleagues showed a nonsignificant 21% reduction (screening v control arms) in oral cancer mortality in the overall trial population and a significant 34% reduction among ever-tobacco and/or ever-alcohol users.3 Despite such efficacy, citing methodologic limitations, most guidelines committees have failed to adopt or recommend oral cancer screening with visual inspection.12-16 These methodologic criticisms include the small number of clusters and the resultant risk imbalance between study arms, lack of analytic accounting for the cluster-sampling design in a few interim reports,4,6 the post hoc subgroup nature of the observation of significant screening efficacy in ever-tobacco and/or ever-alcohol users, and moderate compliance with screening and referral procedures.12-16
We quantitatively addressed the prior criticisms of the Kerala trial to provide empirical evidence that screening with visual inspection was efficacious in reducing oral cancer mortality. We conducted an ITT analysis to conservatively address low compliance, used survey analysis methods to account for the cluster-sampling design, and implemented multiple imputation and risk prediction model–based adjustment to address the risk imbalance between study arms. Even after such accounting for the prior criticisms in the literature, we demonstrate a 27% statistically significant reduction in oral cancer mortality in the screening arm participants, including a 29% statistically significant reduction among ever-tobacco and/or ever-alcohol users.
Our study also extends the Kerala trial results3 to show that the relative efficacy of screening was homogeneous across risk subgroups. Consequently, as a result of the expected increase in baseline risk across risk prediction model quartiles, the 27% relative efficacy translated to greater absolute efficacy in high-risk individuals. Thus, the 25% of individuals at greatest model-predicted oral cancer risk experienced 76% of the mortality reduction from screening.
The efficacy and efficiency results presented herein need to be interpreted within the context of some key limitations. First, our estimates are conservative, given the moderate compliance with screening (three screens planned by design v 1.76 screens received) and with referral procedures (67% compliance with referral to experts).3 Thus, our estimated efficacy and efficiency likely reflect real-life effectiveness of oral cancer screening in India. Second, the generalizability of the Kerala trial results to other parts of the world has previously been questioned.12 We submit that the trial setting as well as the results are broadly generalizable to the Indian Subcontinent, in view of the similar exposure experience, particularly chewing tobacco. Third, the similar exposure experience notwithstanding, the transportability of our risk prediction model to other geographic regions in India and neighboring countries needs to be determined. Last, there was no formal evaluation in the trial of the potential harms of screening, such as overdiagnosis of oral precancer, patient anxiety, and unnecessary biopsies and treatments.
Our results have public health relevance for oral cancer screening in resource-limited developing countries, such as India, which are also the global hot spots for oral cancer burden.1,2 Indeed, oral cancer is the most common cancer in Indian men and third most common in Indian women. Given this, the Government of India has recently formulated guidelines for oral cancer screening as part of a larger screening effort for five chronic diseases (diabetes; hypertension; and oral, cervical, and breast cancers).17,18 These guidelines recommend oral cancer screening with visual inspection by trained health workers (akin to the Kerala trial) once every five years in all adults of age 30 + years, an endeavor with substantial human and economic resource implications.
Our study underscores the potential for a resource-efficient, risk-based oral cancer screening program in India. Assuming generalizability of the Kerala trial results to the Indian population, on the basis of the Global Adult Tobacco Survey (GATS) data,19 the current guidelines to screen all adults of age 30 years or older would target approximately 584.2 million individuals for 100% program sensitivity (ie, target all oral cancer deaths in the population). Instead, mere restriction of screening to ever-tobacco users without any additional risk stratification would target 234.6 million individuals to achieve 86.1% program sensitivity. Further gains in screening program efficiency could be achieved through the use of a risk prediction model—screening of 50% of ever-tobacco users at highest model-predicted risk would target only 117.3 million individuals (20.1% of the eligible population), yet maintain high program sensitivity (72.7%).
The global burden of oral cancers is estimated to increase by 62% by the year 2035, particularly in Asia.1 In addition to primary prevention of tobacco and/or alcohol use, screening and early detection could lead to significant reductions in oral cancer mortality.1,2 To enable such early detection, we provide empirical evidence of the efficacy of screening with visual inspection, a validated oral cancer risk prediction model for the identification of high-risk individuals, and proof of principle that risk-based screening of high-risk individuals could provide substantial gains in the efficiency of oral cancer screening programs.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
EQUAL CONTRIBUTION
K.R. and A.K.C. contributed equally to this work. L.C.C. and A.K.C. are cocorresponding authors.
SUPPORT
The trial was funded by Worldwide Cancer Research and Cancer Research UK and supported by the International Agency for Research on Cancer. This reanalysis was funded in part by the Intramural Research Program of the US National Institutes of Health (NIH)/National Cancer Institute. The study sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. L.C.C. and A.K.C. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Relevant data for research purposes from the Kerala Oral Cancer Trial can be requested from the International Agency for Research on Cancer.
AUTHOR CONTRIBUTIONS
Conception and design: Li C. Cheung, Kunnambath Ramadas, Barry I. Graubard, Rengaswamy Sankaranarayanan, Thara Somanathan, Anil K. Chaturvedi
Administrative support: Kunnambath Ramadas, Partha Basu
Provision of study materials or patients: Kunnambath Ramadas, Gigi Thomas, Rengaswamy Sankaranarayanan
Collection and assembly of data: Kunnambath Ramadas, Richard Muwonge, Gigi Thomas, Rengaswamy Sankaranarayanan
Data analysis and interpretation: Li C. Cheung, Kunnambath Ramadas, Richard Muwonge, Hormuzd A. Katki, Barry I. Graubard, Partha Basu, Rengaswamy Sankaranarayanan, Anil K. Chaturvedi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk-Based Selection of Individuals for Oral Cancer Screening
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Barry I. Graubard
Stock and Other Ownership Interests: Medtronic
Rengaswamy Sankaranarayanan
Employment: Karikinos Healthcare Pvt Ltd.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shield KD, Ferlay J, Jemal A, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64. doi: 10.3322/caac.21384. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ramadas K, Amarasinghe H, et al. Oral cancer: Prevention, early detection, and treatment. In: Gelband H, Jha P, Sankaranarayanan R, et al., editors. Cancer: Disease Control Priorities. ed 3. Volume 3. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2015. [PubMed] [Google Scholar]
- 3.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 4.Ramadas K, Sankaranarayanan R, Jacob BJ, et al. Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncol. 2003;39:580–588. doi: 10.1016/s1368-8375(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R, Ramadas K, Thara S, et al. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013;49:314–321. doi: 10.1016/j.oraloncology.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Mathew B, Jacob BJ, et al. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer. 2000;88:664–673. [PubMed] [Google Scholar]
- 7.Mathew B, Sankaranarayanan R, Sunilkumar KB, et al. Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer. 1997;76:390–394. doi: 10.1038/bjc.1997.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 9.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 11.Korn EL, Graubard BI. Analysis of Health Surveys: Wiley Series in Probability and Statistics. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 12.Brocklehurst P, Kujan O, Glenny AM, et al. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2010;11:CD004150. doi: 10.1002/14651858.CD004150.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Kujan O, Glenny AM, Oliver RJ, et al. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2006;3:CD004150. doi: 10.1002/14651858.CD004150.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Moyer VA, US Preventive Services Task Force Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:55–60. doi: 10.7326/M13-2568. [DOI] [PubMed] [Google Scholar]
- 15.Ramadas K, Arrossi S, Thara S, et al. Keynote comment: Importance of recognising scientific evidence. Lancet Oncol. 2006;7:962–963. doi: 10.1016/S1470-2045(06)70950-0. [DOI] [PubMed] [Google Scholar]
- 16.Mignogna MD, Fedele S. Oral cancer screening: 5 minutes to save a life. Lancet. 2005;365:1905–1906. doi: 10.1016/S0140-6736(05)66635-4. [DOI] [PubMed] [Google Scholar]
- 17.Bagcchi S. India launches plan for national cancer screening programme. BMJ. 2016;355:i5574. doi: 10.1136/bmj.i5574. [DOI] [PubMed] [Google Scholar]
- 18.Rajaraman P, Anderson BO, Basu P, et al. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015;16:e352–361. doi: 10.1016/S1470-2045(15)00078-9. [DOI] [PubMed] [Google Scholar]
- 19.Global Adult Tobacco Survey Datasets for South-East Asian (SEAR) Region, India, India-National. https://nccd.cdc.gov/GTSSDataSurveyResources/Ancillary/DataReports.aspx?Country=180&CAID=2&Survey=4&WHORegion=2&Site=3840002016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data for research purposes from the Kerala Oral Cancer Trial can be requested from the International Agency for Research on Cancer.