PURPOSE
There remains a lack of clarity regarding the influence of sequencing of androgen deprivation therapy (ADT) and radiotherapy (RT) on outcomes in prostate cancer (PCa). Herein, we evaluate the optimal sequencing of ADT with prostate-directed RT in localized PCa.
METHODS
MEDLINE (1966-2018), Embase (1982-2018), ClinicalTrials.gov, and conference proceedings (1990-2018) were searched to identify randomized trials evaluating the sequencing, but not duration, of ADT with RT. Two randomized phase III trials were identified, and individual patient data were obtained: Ottawa 0101 and NRG Oncology's Radiation Therapy Oncology Group 9413. Ottawa 0101 randomly assigned patients to neoadjuvant or concurrent versus concurrent or adjuvant short-term ADT. Radiation Therapy Oncology Group 9413, a 2 × 2 factorial trial, included a random assignment of neoadjuvant or concurrent versus adjuvant short-term ADT. The neoadjuvant or concurrent ADT arms of both trials were combined into the neoadjuvant group, and the arms receiving adjuvant ADT were combined into the adjuvant group. The primary end point of this meta-analysis was progression-free survival (PFS).
RESULTS
The median follow-up was 14.9 years. Overall, 1,065 patients were included (531 neoadjuvant and 534 adjuvant). PFS was significantly improved in the adjuvant group (15-year PFS, 29% v 36%, hazard ratio [HR], 1.25 [95% CI, 1.07 to 1.47], P = .01). Biochemical failure (subdistribution HR [sHR], 1.37 [95% CI, 1.12 to 1.68], P = .002), distant metastasis (sHR, 1.40 [95% CI, 1.00 to 1.95], P = .04), and metastasis-free survival (HR, 1.17 [95% CI, 1.00 to 1.37], P = .050) were all significantly improved in the adjuvant group. There were no differences in late grade ≥ 3 gastrointestinal (2% v 3%, P = .33) or genitourinary toxicity (5% v 5%, P = .76) between groups.
CONCLUSION
The sequencing of ADT with prostate-directed RT has significant association with long-term PFS and MFS in localized PCa. Our findings favor use of an adjuvant over a neoadjuvant approach, without any increase in long-term toxicity.
In most cancer types, there is level 1 evidence for the benefit of the addition of systemic therapy to radiotherapy (RT).1-5 Furthermore, randomized trials have demonstrated that the sequencing of systemic therapy and RT is of importance to optimize survival outcomes.1,6 In localized and recurrent prostate cancer (PCa), over 20 randomized trials have established the important role of combining various durations of androgen deprivation therapy (ADT) with RT. However, these trials have almost exclusively focused on the use and duration of ADT rather than the sequencing of ADT with RT.7-19
CONTEXT
Key Objective
To compare the long-term outcomes of men with localized prostate cancer receiving radiotherapy (RT) and neoadjuvant versus adjuvant short-term hormone therapy.
Knowledge Generated
After a median follow-up of approximately 15 years, we demonstrate using individual patient data from two randomized trials that men treated with adjuvant hormone therapy with prostate-directed RT had improved biochemical control and metastasis-free survival compared to a neoadjuvant or concurrent approach of equal duration. There were very low rates of late gastrointestinal or genitourinary grade 3 or higher toxicity, and no differences were found in toxicity based on hormone therapy timing.
Relevance
Delaying the start of RT by use of a neoadjuvant component of hormone therapy did not provide an advantage in tumor control or late toxicity based on our pooled randomized analysis but may have inferior clinically meaningful outcomes than use of an adjuvant sequence with prostate-directed RT.
The sequencing strategy of ADT and RT varies internationally and among the research cooperative groups. For example, NRG Oncology’s Radiation Therapy Oncology Group (RTOG) trials often used a 2-month neoadjuvant component while the Canadian and Australian trialist groups commonly used longer durations of neoadjuvant ADT. By contrast, the European Organization for Research and Treatment of Cancer (EORTC) group often started ADT concomitantly with RT without a neoadjuvant component. Although these studies provide us clarity on the optimal duration of ADT, optimal sequencing approach of ADT with RT remains unclear.
Early preclinical studies by Zietman et al20 used a mouse-derived breast tumor model and demonstrated superior results with neoadjuvant compared with adjuvant surgical castration. However, the neoadjuvant group was functionally neoadjuvant, concurrent, and adjuvant, given this was surgical castration. A single-institution retrospective analysis (N = 515 patients) was unable to identify a difference in any oncologic outcome between the sequencing of neoadjuvant versus adjuvant ADT with RT.21 Only two randomized trials have investigated the impact of sequencing of ADT with RT without altering the total duration of ADT: the Ottawa 0101 randomized phase III trial, and the NRG Oncology’s RTOG 9413 trial (Protocol, online only), a 2 × 2 factorial randomized phase III trial.22,23 Each of these two trials have individually demonstrated the possibility of a superior outcome with an adjuvant versus a neoadjuvant component of ADT when combined with prostate-directed RT, but neither was conclusive. Therefore, we performed the first individual patient-level meta-analysis of these two phase III randomized controlled studies that investigated the optimal sequencing of ADT with prostate-directed RT.
METHODS
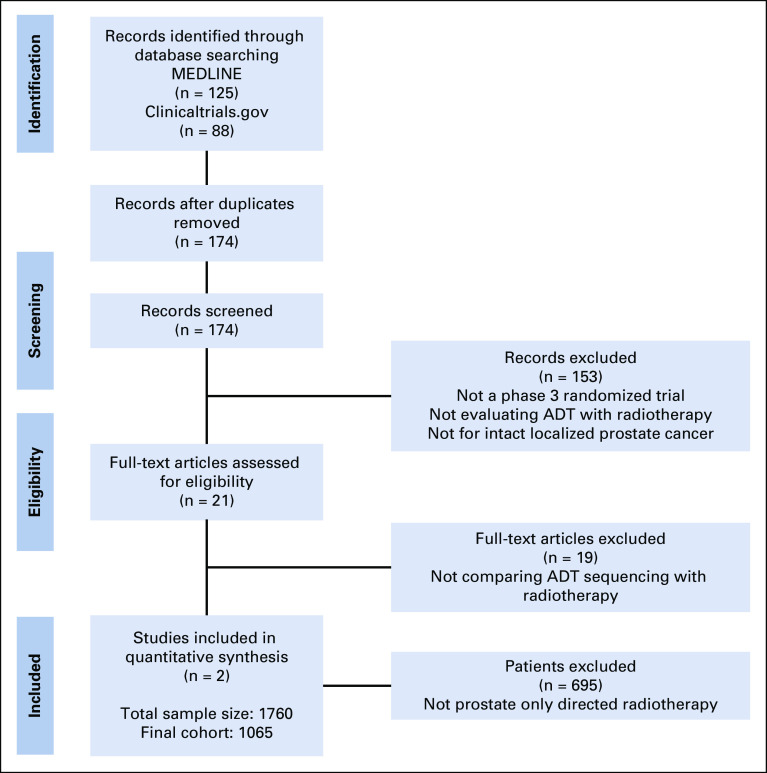
A systematic literature search was performed using MEDLINE (1966-2018), Embase (1982-2018), trial registries (Cochrane Central Register of Controlled Trials and ClinicalTrials.gov), and major urology and oncology conference proceedings (1990-2018) to retrieve studies that evaluated the optimal sequencing of definitive RT with ADT in men with localized PCa. Controlled vocabulary was leveraged for studies involving humans using the following terms: randomized AND prostate AND (androgen deprivation OR hormone therapy) AND (radiotherapy OR radiation) AND (neoadjuvant OR adjuvant) NOT prostatectomy. The search was conducted on January 2, 2020. Two randomized trials were identified and were included in this meta-analysis (Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data can be found in the Appendix Fig A1 [online only]). After institutional review board and data-sharing agreements were approved, individual patient data were collected from the NRG Oncology's RTOG 9413 trial23 (Protocol) and Ottawa 0101 randomized phase III trial.22
Study Cohorts
NRG Oncology's RTOG 9413
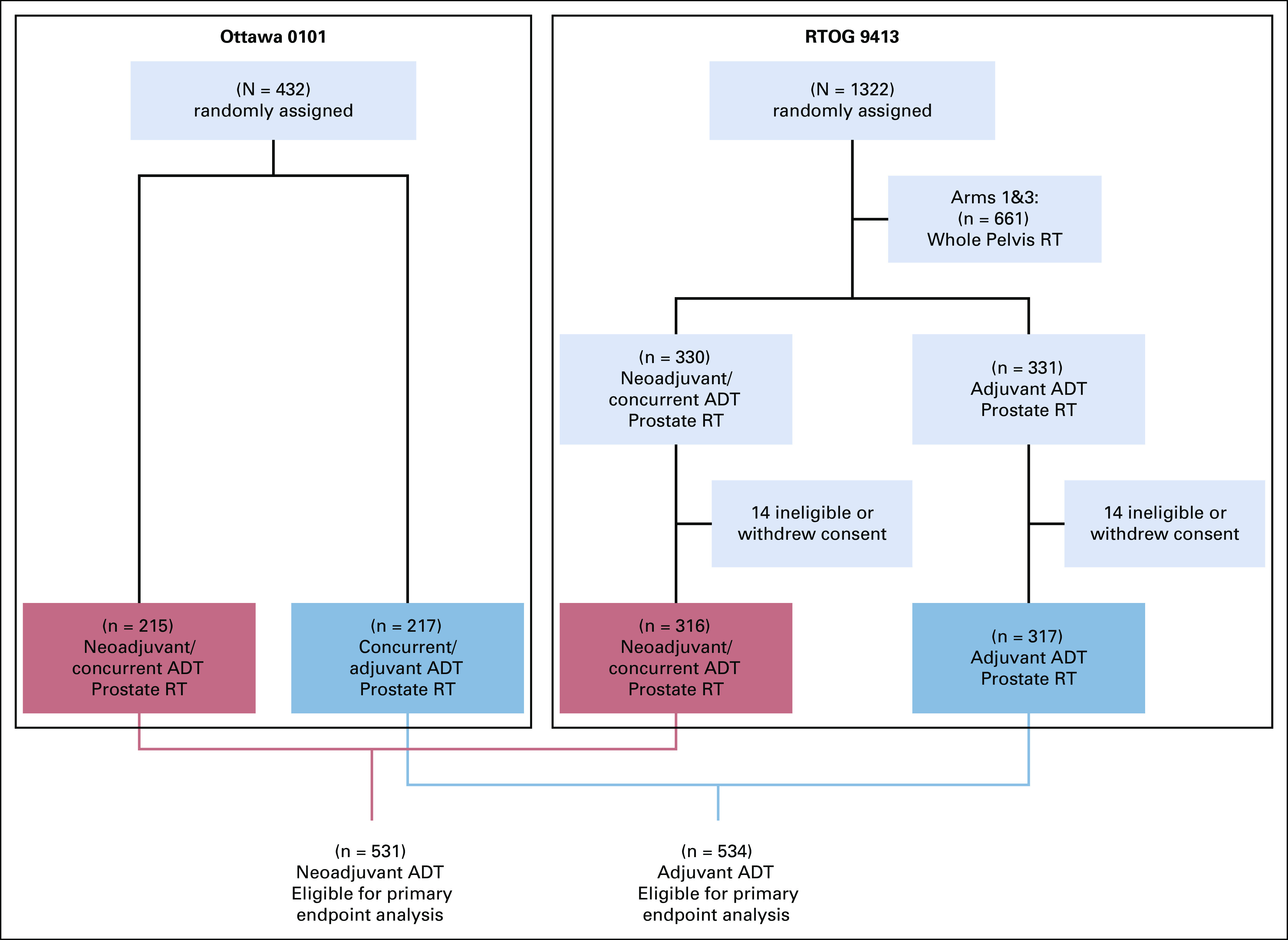
The NRG Oncology's RTOG 9413 study (Protocol) was designed as a 2 × 2 factorial phase III randomized study with hormonal sequencing as one stratification factor and RT field size as the other factor. The primary end point related to ADT sequencing was whether neoadjuvant ADT improved progression-free survival (PFS) compared to adjuvant ADT. Eligible patients had histologically confirmed, clinically localized prostate adenocarcinoma with an estimated risk of lymph node involvement > 15% and a Karnofsky performance status > 70. Patients were randomly assigned (1:1:1:1) by permuted block random assignment to receive either neoadjuvant and concurrent ADT 2 months before and 2 months during prostate-directed RT versus prostate-directed RT followed by 4 months of adjuvant ADT. This was done similarly for the whole pelvis RT arms. Random assignment was stratified by clinical tumor (T) stage, Gleason score, and prostate-specific antigen (PSA) concentration. ADT was combined androgen suppression, consisting of goserelin acetate 3.6 mg once a month subcutaneously or leuprolide acetate 7.5 mg once a month intramuscularly, and flutamide 250 mg orally three times a daily for 4 months. The primary end point of PFS was defined as the time from random assignment to the first occurrence of local progression, regional or nodal failure, distant metastasis (DM), biochemical failure (BF) (Phoenix definition), or death from any cause.
Only the prostate-directed RT randomized arms were used in this study to avoid potential interactions of field size and ADT sequencing, and to harmonize with the Ottawa 0101 trial, which used prostate-directed RT. Between April 1, 1995, and June 1, 1999, a total of 1,322 patients were enrolled from 53 centers and randomly assigned to one of the four treatment groups.
Ottawa 0101
The Ottawa 0101 was an open-label, parallel, two-arm phase III randomized trial. The primary end point was biochemical relapse-free survival (defined as time since random assignment to either biochemical recurrence as per Phoenix criteria, commencement of salvage ADT even in the absence of per-protocol progression, or clinical progression). All patients received a total 6 months of ADT. Patients in arm 1 received 4 months of neoadjuvant ADT followed by RT concurrently with 2 months of ADT. In arm 2, patients received ADT concomitantly with RT for the first 2 months followed by 4 months of adjuvant ADT. Eligible patients had histologically confirmed prostate adenocarcinoma with a Gleason score ≤ 7, clinical stage T1b to T3a, and PSA < 30 ng/mL. Patients with low-risk PCa were excluded. ADT comprised bicalutamide 50 mg orally once a day plus 10.8 mg goserelin subcutaneously once starting 7 days after bicalutamide, and again with a second injection administered 3 months later. Between July 12, 2002, and March 28, 2012, a total of 438 patients were enrolled from two centers and randomly assigned to one of the two treatment groups.
In this meta-analysis, the neoadjuvant and concurrent arms of both trials were combined into the neoadjuvant group, and the concurrent and adjuvant (Ottawa 0101) and adjuvant arm (RTOG 9413; Protocol) were combined into the adjuvant group.
End Points
The primary end point of this analysis was PFS, defined as the time from random assignment to the first occurrence of local, regional, or nodal failure, DM, BF (Phoenix definition), or death from any cause. Secondary end points included metastasis-free survival (MFS) defined as time since random assignment until metastasis or death, overall survival (OS), and the cumulative incidence of BF, DM, and PCa-specific mortality (PCSM; death from PCa as the event). Patients who were event-free were censored on the last date of follow-up or on the date of last follow-up with known biochemical or clinical status. Late toxicity was analyzed as reported by each study and was categorized into genitourinary (bladder) and gastrointestinal (bowel) toxicity. Rates of severe toxicity in each category, defined as grade 3-5, were estimated for the two treatment groups.
Statistical Analysis
An a priori statistical plan was created prior to data pooling and analysis. Baseline characteristics were compared by treatment group using the Mann-Whitney-Wilcoxon test for age and the χ2 test for all other baseline characteristics. Treatment effects on PFS, MFS, and OS were reported using the hazard ratio (HR) with 95% CIs from Cox regression models along with absolute differences in Kaplan-Meier event probability estimates.24,25 The Grambsch-Therneau test was applied to identify violation of proportionality assumption of the Cox regression models. For cumulative incidence of BF, DM, and PCSM, the subdistribution HRs (sHR) with 95% CI were reported from Fine-Gray's competing risk regression models along with cumulative incidence of events. Death from any cause served as competing events for BF and DM, and deaths not resulting from PCa were considered competing events for PCSM.26-29 HRs were defined such that HR > 1 favored the adjuvant group compared with the neoadjuvant group. Formal interaction tests between treatment and clinicopathologic covariables and the National Comprehensive Cancer Network risk group were performed.
For each treatment group, we also estimated 15-year restricted mean survival time (RMST) for OS, MFS, and PFS, and 15-year restricted mean time lost (RMTL) for DM, BF, and PCSM to account for competing risk of death. RMST was determined from the Kaplan-Meier estimate of the survival function, whereas RMTL was determined from the estimated cumulative incidence function. The treatment effect on each end point was assessed by taking the difference in RMST estimates between adjuvant and neoadjuvant groups; however, for RMTL, the direction was reversed to keep the interpretation consistent. Analyses were repeated after adjustment for trial enrollment (RTOG 9413 [Protocol] v Ottawa 0101) to account for any potential systematic differences between trials. Grade 3-5 late toxicity events were summarized for both treatment groups. The effect of treatment arms was assessed using odds ratios, and the 95% CIs and P values were obtained using the exact test with mid-P adjustment. Analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P < .05 was deemed statistically significant.
RESULTS
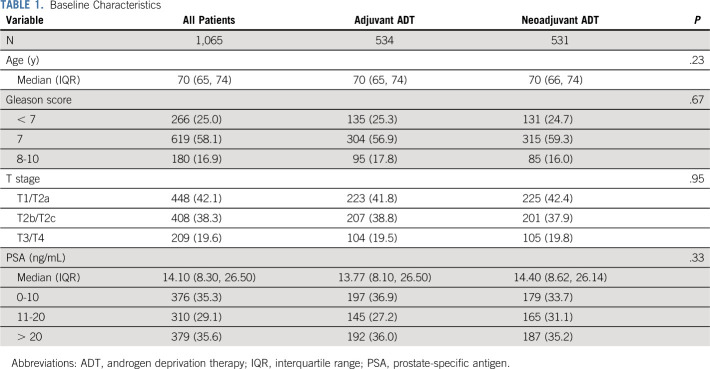
The median follow-up was 14.9 years, and a total of 1,065 patients were included (531 in the neoadjuvant ADT group and 534 in the adjuvant ADT group; Fig 1). All baseline characteristics were well balanced (Table 1). The median age was 70 years (interquartile range [IQR], 65-74); 58.1% had a Gleason score of 7 and 16.9% had a Gleason score of 8-10. Overall, 19.6% had clinical T3 and T4 disease, and the median PSA was 14.1 ng/mL (IQR, 8.30-26.50 ng/mL).
FIG 1.
Consolidated Standards of Reporting Trials (CONSORT) Diagram. ADT, androgen deprivation therapy; RT, radiotherapy.
TABLE 1.
Baseline Characteristics
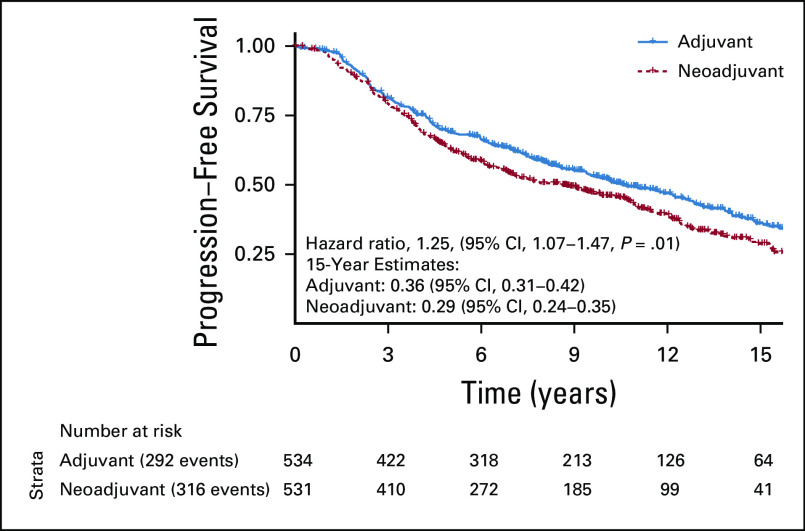
In the pooled cohort, the primary end point of PFS was significantly improved in the adjuvant group compared with the neoadjuvant group. Up to a 15-year truncation point, RMST for progression was improved with adjuvant ADT by 10.8 months (95% CI, 2.7 to 18.8) (Fig 2). Fifteen-year PFS rates for neoadjuvant and adjuvant groups were 29% and 36%, respectively (Fig 3). On the Cox regression, the HR for the neoadjuvant group was 1.25 (95% CI, 1.07 to 1.47, P = .01). Similarly, the cumulative incidences of BF (15-year incidence rates, 43% v 33%, sHR for neoadjuvant group, 1.37 (95% CI, 1.12 to 1.68, P = .002); Fig 4A) and DM (15-year incidence rates, 18% v 12%, sHR for neoadjuvant group, 1.40 [95% CI, 1.00 to 1.95], P = .04; Fig 4B) were significantly lower in the adjuvant group compared with the neoadjuvant group. The adjuvant group was associated with significantly superior MFS relative to the neoadjuvant group (HR for neoadjuvant group, 1.17 [95% CI, 1.00 to 1.37], P = .050).
FIG 2.
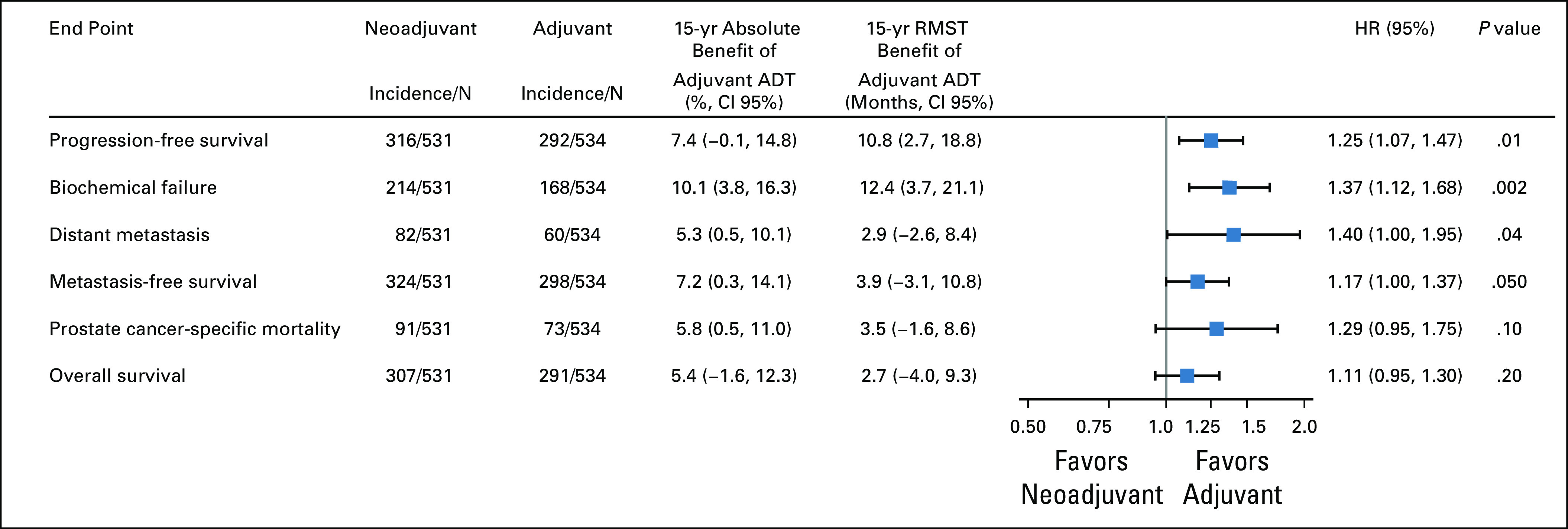
Forest plot of oncologic outcomes comparing neoadjuvant versus adjuvant ADT. ADT, androgen deprivation therapy; HR, hazard ratio; RMST, restricted mean survival time.
FIG 3.
Kaplan-Meier of the primary end point of progression-free survival comparing adjuvant versus neoadjuvant ADT with RT. ADT, androgen deprivation therapy; HR, hazard ratio; RT, radiotherapy.
FIG 4.
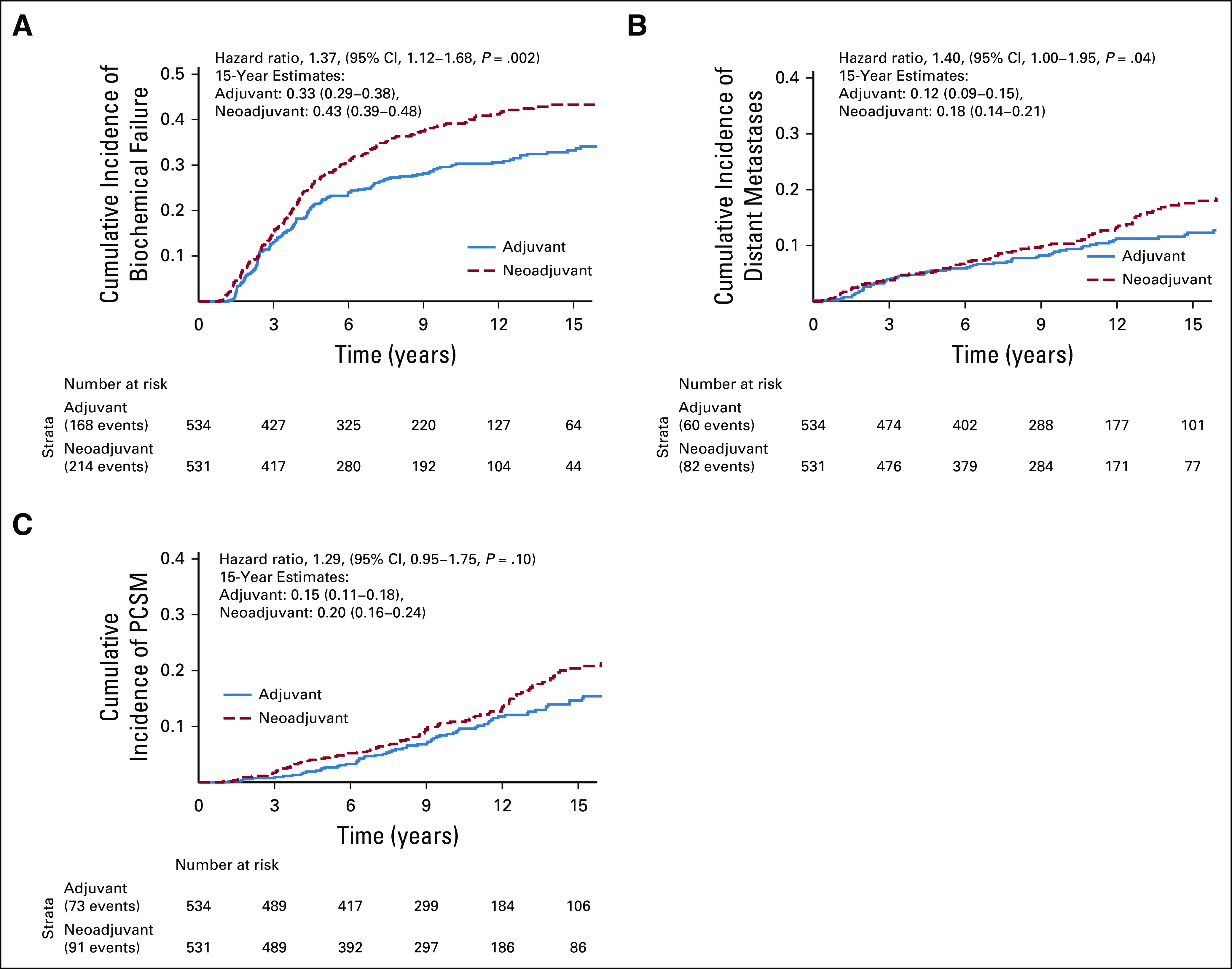
Comparison of adjuvant versus neoadjuvant ADT with RT. (A) Cumulative incidence of biochemical failure, (B) distant metastasis, and (C) prostate cancer–specific mortality. ADT, androgen deprivation therapy; RT, radiotherapy.
Adjuvant ADT yielded lower PCSM (15-year incidence rates, 20% v 15%, sHR for neoadjuvant group, 1.29 (95% CI, 0.95 to 1.75, P = .10; Fig 4C), but this did not reach the level of statistical significance. In men with high-risk PCa, the effect size of benefit from adjuvant ADT was larger for PCSM (sHR, 1.39 [95% CI, 1.00 to 1.93, P = .053; Appendix Fig A2), but remained statistically nonsignificant. There was no statistical evidence of a treatment interaction by the National Comprehensive Cancer Network risk group (P = .32). There was also no significant difference in OS between the groups (15-year rates, 34% v 39% for the neoadjuvant and adjuvant groups, respectively; HR for the neoadjuvant group, 1.11 [95% CI, 0.95 to 1.30], P = .20).
Given trial-level differences (eg, years of enrollment, ADT duration, country of primary enrollment, etc), analyses for all oncologic outcomes were repeated after adjustment for clinical trial. As shown in the Appendix Fig A3, the outcomes remained effectively unchanged, with adjuvant ADT having superior outcomes for all end points.
There were no significant differences in either late grade 3-5 gastrointestinal (2% v 3%, P = .33) or genitourinary toxicity (5% v 5%, P = .76) between the neoadjuvant versus adjuvant groups, respectively.
DISCUSSION
The current individual patient-level meta-analysis of two randomized phase III trials with long-term follow-up demonstrated significant improvements in several clinically meaningful oncologic outcomes with short-term adjuvant ADT relative to neoadjuvant ADT when used in conjunction with prostate-directed RT. Additionally, overall rates of late grade 3-5 toxicities were low, and we did not find any significant difference in the incidence of severe gastrointestinal or genitourinary toxicities between the two groups. When the trials are viewed individually, there were trends noted for improved BF and PFS in the RTOG 9413 (Protocol) and Ottawa 0101 trials. This meta-analysis with a larger patient population consolidates the findings of the above trials and demonstrates a significant reduction in the relative incidence of DM with improved MFS, which is a unique and important observation.
Several randomized trials have investigated the utility of prolongation of the neoadjuvant component. The Quebec L-101 (N = 120),12 RTOG 9910 (N = 1,579),19 Canadian Multicenter Trial (N = 378),10 and Irish Clinical Oncology Research Group 97-01 (N = 261)18 randomized trials failed to demonstrate any improvement in oncologic outcomes from the prolongation of the course of neoadjuvant ADT. By contrast, the use and increased duration of adjuvant ADT have consistently resulted in meaningful improvements in MFS, PCSM, and/or OS (ie, TROG RADAR and DART trials 01/05, RTOG 9202, and EORTC 22961).8,9,14,30 These results reflect that an increased duration of ADT might not be the driver of improved oncologic outcomes, and that the timing of ADT in relation to RT is important to optimize outcome in localized PCa.
In this context, the interpretation of a prior pooled analysis of three randomized trials merits reconsideration.11 This analysis, by D'Amico et al,11 included two trials of predominately neoadjuvant ADT with 1 month of concurrent ADT (a total duration of 3-4 months), and compared this group with patients who received 4 months of neoadjuvant and concurrent ADT plus 2 months of adjuvant ADT (a total of 6 months). They demonstrated that 6 months of ADT conferred a large improvement in PCSM (HR, 0.55; 95% CI, 0.36 to 0.82) compared with the shorter duration of ADT. The authors attributed the improvement to increased duration of ADT rather than the difference in sequencing. However, as previously noted, multiple trials that compared 3-4 months of ADT with an increased duration of neoadjuvant ADT (a total duration > 6 months) failed to demonstrate any difference in oncologic outcomes.10,19 Thus, perhaps the sequencing of ADT was the true reason for the improvement of outcomes.
Neoadjuvant and concurrent ADT effectively provide a short period of adjuvant ADT due to prolonged testosterone suppression. This begs the question if trials like RTOG 9408, which prescribed neoadjuvant and concurrent ADT, provided most of the benefit from the short duration of residual adjuvant testosterone suppression rather than the neoadjuvant and concurrent active drug delivery. Similarly, the TROG 96.01 trial of 0 versus 3 months versus 6 months ADT (no adjuvant ADT given) demonstrated that the primary end point of PCSM was only significantly improved once 6 months of ADT was given, potentially due to prolonged adjuvant testosterone suppression. This theory was effectively examined in the Princess Margaret Hospital 9907 (N = 252) randomized trial of men with intermediate-risk disease, in which men were randomly assigned positive or negative antiandrogen therapy rather than ADT. Thus, there was no adjuvant testosterone suppression.31 This trial gave 150 mg of bicalutamide once a day for 5 months in the neoadjuvant and concurrent phase with RT, and showed no improvement in any oncologic end point, or even in biochemical control, from neoadjuvant and concurrent antiandrogen therapy. All these findings taken together point toward the importance of adjuvant ADT when used in conjunction with RT in localized PCa.
Several factors may account for the sequence-dependent interaction between ADT and RT in PCa. These include an inhibitory action of adjuvant ADT on enhanced ligand-dependent activation of the androgen receptors (ARs), which are found to be overexpressed after RT.32 Moreover, due to the prolonged natural history of RT induced cell death in PCa, which occurs over months to years to achieve maximal PSA nadir, continued adjuvant inhibition of AR-regulated DNA repair genes may be critical for optimal outcomes. Additionally, neoadjuvant ADT might have competing benefits from reduction in tumor hypoxia that might enhance sensitivity to RT and a decrease in the proliferation of cancer cells, resulting in reduced radio-sensitivity.33 This latter point formed the basis of the original hypothesis to test ADT sequence in RTOG 9413, as stated in the original RTOG 9413 Protocol.
Our meta-analysis used individual patient data from two trials specifically conceived and conducted to test the hypothesis of our study. Each group in our study included all patients assigned to the relevant groups of the constituent trials to harmonize with consistent prostate-directed target volume for RT delivery. Nonetheless, we acknowledge potential limitations of our meta-analysis. Extensive effort was made to ensure these were the only two phase III randomized trials to have assessed the question of ADT sequencing (with a constant duration of ADT) with RT, but potential limitations exist with any search strategy. Patients allocated to the whole-pelvic RT arm of the RTOG 9413 study (Protocol) were not included in this meta-analysis to harmonize the mode of RT delivery with the Ottawa 0101 trial. There was a hypothesis that generated post hoc, unplanned interaction in RTOG 9413 (Protocol), identified between RT field size and ADT sequencing. However, as stated in the RTOG 9413 Protocol, there was no intention of testing for a treatment interaction given that “these factors are not expected to show any statistical interaction with treatment” (Protocol). Given that the Ottawa trial did not include patients treated with nodal RT, we are unable to validate this potential interaction. There were systematic differences in the two trials in our study (duration of ADT, exact timing of ADT in relation to RT, years of enrollment, formulation of ADT used, etc). However, our findings remained effectively unchanged when adjusting for trial enrollment. Given that the RTOG 9413 trial (Protocol) enrolled patients with more aggressive disease than the Ottawa 0101 trial, our ability to soundly perform subset analyses to compare concurrent and adjuvant versus adjuvant ADT was hindered. Despite these limitations, it is unlikely that a separate suitably powered phase III randomized controlled trial based on the central concept presented herein will ever be launched. Therefore, our findings potentially serve as the highest level of evidence that clearly support a preference for inclusion of adjuvant short-term ADT when administered with prostate-directed RT.
In conclusion, our findings have immediate and significant clinical implications on the management of localized PCa. They emphasize that a delay in initiating RT to allow receipt of neoadjuvant ADT is unnecessary and does not reduce long-term toxicity compared with an adjuvant approach in an unselected group of men treated with prostate-directed RT. Furthermore, adjuvant short-term ADT combined with prostate-directed RT was found to confer superior PFS reduced incidence of biochemical relapse and DM compared with neoadjuvant and concurrent ADT. Ongoing and future clinical research in localized PCa should consider these findings in trial design and conduct.
ACKNOWLEDGMENT
The authors would like to acknowledge NRG Oncology, the National Cancer Institute, and the Ottawa trial groups for their commitment to data sharing. We thank Dr Lipni Eapen for his decades of important contributions to Oncology.
APPENDIX
FIG A1.
PRISMA diagram.
FIG A2.
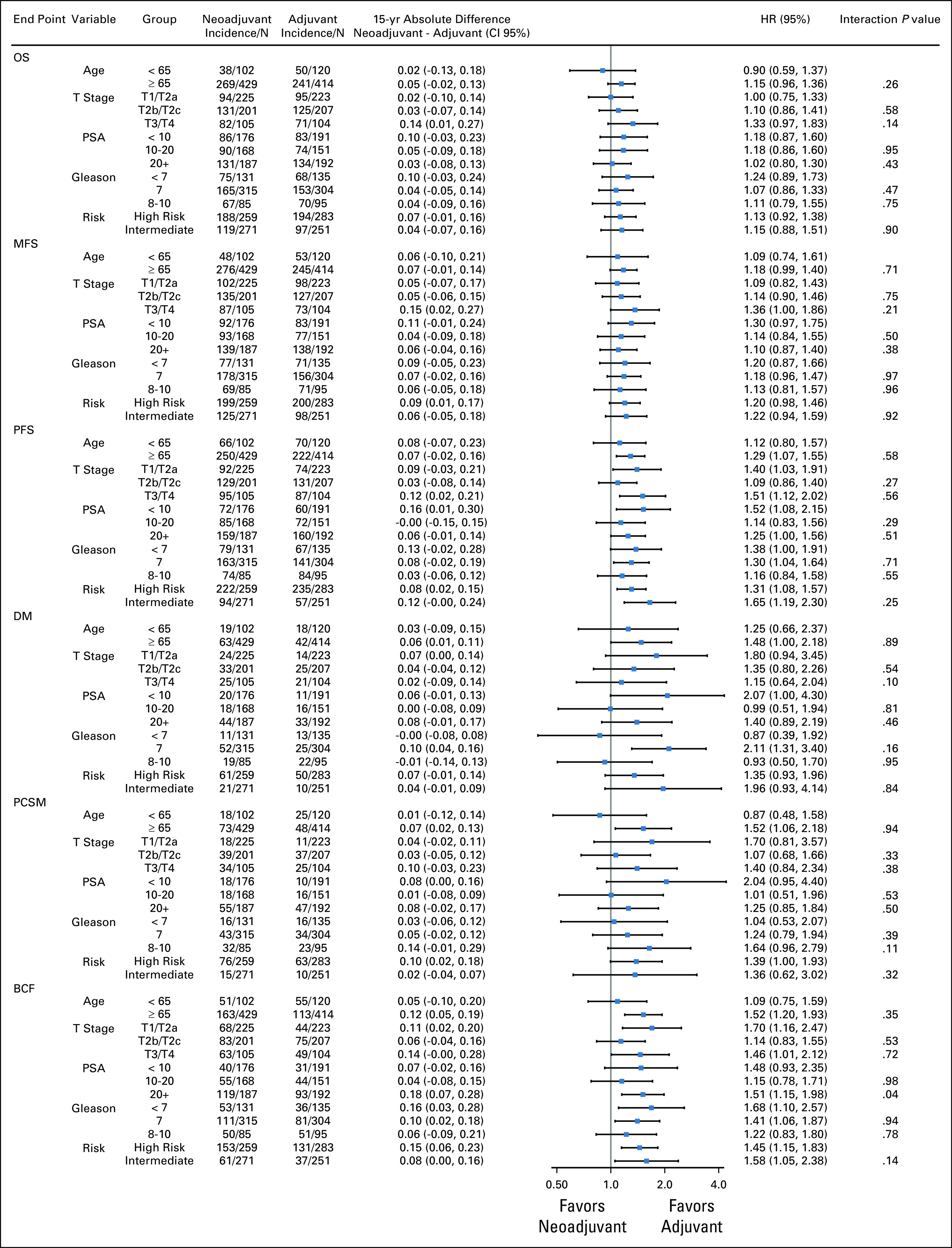
Subgroup analyses by end point.
FIG A3.
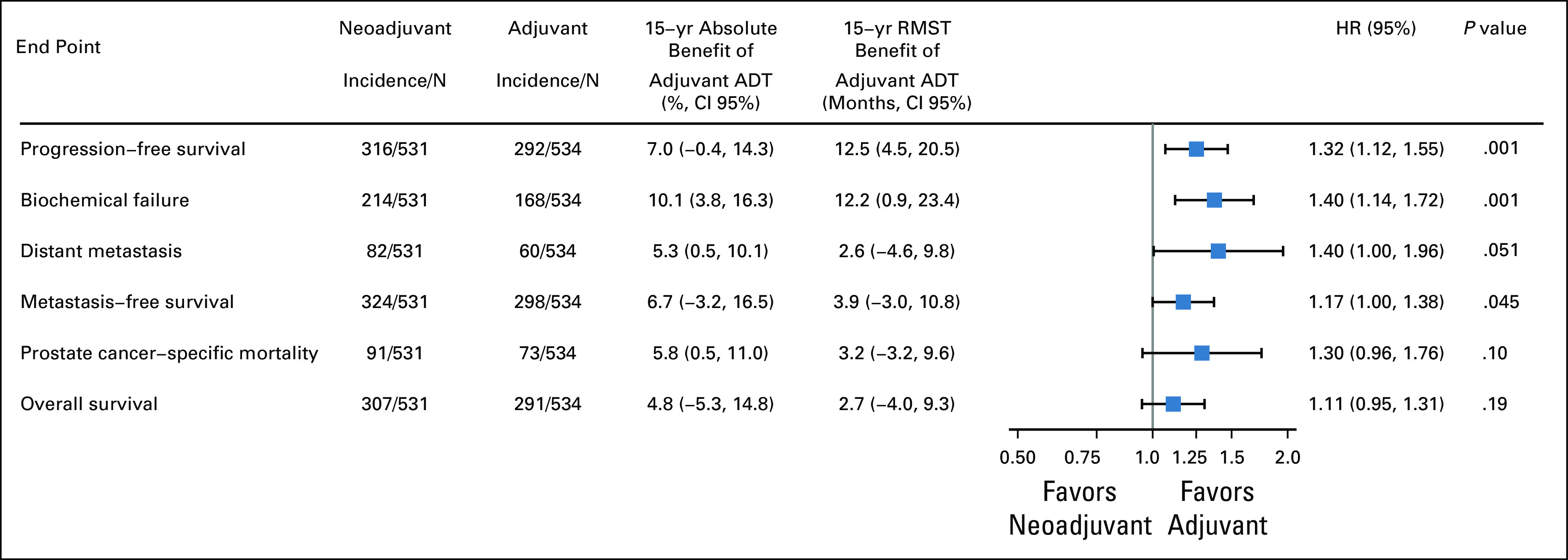
Oncologic outcomes after adjustment for clinical trial. ADT, androgen deprivation therapy; HR, hazard ratio.
PRIOR PRESENTATION
Presented at the ASTRO 2020 Virtual Annual Meeting, October 24-28, 2020.
SUPPORT
Supported by the Prostate Cancer Foundation, Prostate Cancer SPORE, Department of Defense, Rogel Cancer Center, and the National Institutes of Health (D.E.S.), and by AstraZeneca for the Ottawa 0101 Trial (S.W.).
CLINICAL TRIAL INFORMATION
NCT00769548 (RTOG 9413)
EQUAL CONTRIBUTION
D.E.S., S.M., S.R., and C.L. contributed equally to first or senior authorship.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel E. Spratt, Shawn Malone, Libni Eapen, Amar U. Kishan, Mack Roach III, Yilun Sun
Financial support: Daniel E. Spratt
Administrative support: Daniel E. Spratt
Provision of study materials or patients: Daniel E. Spratt, Shawn Malone, Scott C. Morgan, Julia Malone, Jeff Michalski, Thomas M. Pisansky, Mack Roach III, Colleen A. F. Lawton
Collection and assembly of data: Daniel E. Spratt, Shawn Malone, Soumyajit Roy, Scott Grimes, Julia Craig, William Shipley, Howard M. Sandler, Mack Roach III, Yilun Sun
Data analysis and interpretation: Daniel E. Spratt, Shawn Malone, Soumyajit Roy, Libni Eapen, Scott C. Morgan, Julia Malone, Robert T. Dess, Holly E. Hartman, Amar U. Kishan, Rohit Mehra, Todd M. Morgan, Zachery R. Reichert, Joshi J. Alumkal, Jeff Michalski, W. Robert Lee, Thomas M. Pisansky, Felix Y. Feng, William Shipley, Mathew J. Schipper, Mack Roach III, Yilun Sun, Colleen A. F. Lawton
Manuscript writing: All Authors
Final approval of manuscript: All Authors
Accountable for all aspects of the work: All Authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prostate Radiotherapy With Adjuvant Androgen Deprivation Therapy (ADT) Improves Metastasis-Free Survival Compared to Neoadjuvant ADT: An Individual Patient Meta-Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel E. Spratt
Consulting or Advisory Role: Blue Earth Diagnostics, Janssen Oncology, AstraZeneca
Shawn Malone
Honoraria: Astellas Pharma, Janssen, Sanofi, Bayer
Travel, Accommodations, Expenses: Tersera, Sanofi
Scott C. Morgan
Consulting or Advisory Role: Astellas Pharma, Bayer, Janssen, Tersera
Amar U. Kishan
Honoraria: Varian Medical Systems, ViewRay
Consulting or Advisory Role: Janssen
Samuel Kaffenberger
Consulting or Advisory Role: MDxHealth, clovis oncology
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Todd M. Morgan
Consulting or Advisory Role: Myriad Genetics, TerumoBCT
Research Funding: Myriad Genetics, MDxHealth, GenomeDx
Zachery R. Reichert
Consulting or Advisory Role: Dendreon
Research Funding: AstraZeneca
Joshi J. Alumkal
Consulting or Advisory Role: Merck Sharp & Dohme, Dendreon
Research Funding: Aragon Pharmaceuticals, Astellas Pharma, Zenith Epigenetics, Gilead Sciences
Travel, Accommodations, Expenses: Astellas Pharma, Merck Sharp & Dohme
Other Relationship: Astellas Pharma
Jeff Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
(Optional) Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
W. Robert Lee
Stock and Other Ownership Interests: Augmenix
Consulting or Advisory Role: Blue Earth Diagnostics
Patents, Royalties, Other Intellectual Property: UpToDate Editor
Felix Y. Feng
Leadership: PFS Genomics
Stock and Other Ownership Interests: PFS Genomics
Honoraria: Genentech
Consulting or Advisory Role: Blue Earth Diagnostics, Celgene, Janssen Biotech, Genentech, Myovant Sciences, Riovant Sciences, Astellas Pharma, SerImmune
Research Funding: Zenith Epigenetics
Patents, Royalties, Other Intellectual Property: I helped develop a molecular signature to predict radiation resistance in breast cancer, and this signature was patented by the University of Michigan, my employer. It is in the process of being licensed to PFS Genomics, a company that I helped found
Howard M. Sandler
Stock and Other Ownership Interests: Radiogel
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
Mathew J. Schipper
Consulting or Advisory Role: Innovative Analytics
Mack Roach
Honoraria: Bayer, Blue Earth Diagnostics, Ferring, Myriad Genetics, Tolmar
Consulting or Advisory Role: Ferring, Myriad Genetics, Bayer, Blue Earth Diagnostics, Accuray, Tolmar, Noxopharm, Genomic Health, Janssen
Expert Testimony: Ferring
Travel, Accommodations, Expenses: Bayer, Ferring, Myriad Genetics, Blue Earth Diagnostics, Tolmar
Other Relationship: TYME
Colleen Lawton
Honoraria: teledoc
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pignon JP, Le Maitre A, Maillard E, et al. : Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4-14, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Le Pechoux C, Pignon J, et al. : Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol 17:473-483, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Jones C, Pugh S, Sandler H, et al. : Long-term update of NRG Oncology RTOG 94-08. Int J Radiat Oncol Biol Phys 102:S31-S32, 2018 [Google Scholar]
- 4.Stupp R, Mason WP, Van Den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005 [DOI] [PubMed] [Google Scholar]
- 5.James ND, Hussain SA, Hall E, et al. : Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366:1477-1488, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Aupérin A, Le Péchoux C, Rolland E, et al. : Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol 28:2181-2190, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Jones CU, Hunt D, McGowan DG, et al. : Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365:107-118, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Bolla M, de Reijke TM, van Tienhoven G, et al. : Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360:2516-2527, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Zapatero A, Guerrero A, Maldonado X, et al. : High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol 16:320-327, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Crook J, Ludgate C, Malone S, et al. : Final report of multicenter Canadian phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 73:327-333, 2009 [DOI] [PubMed] [Google Scholar]
- 11.D'Amico AV, Chen MH, Crook J, et al. : Duration of short-course androgen suppression therapy and the risk of death as a result of prostate cancer. J Clin Oncol 29:4682-4687, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaverdièRe J, Nabid A, De Bedoya LD, et al. : The efficacy and sequencing of a short course of androgen suppression on freedom from biochemical failure when administered with radiation therapy for T2-T3 prostate cancer. J Urol 171:1137-1140, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lawton CAF, Lin X, Hanks GE, et al. : Duration of androgen deprivation in locally advanced prostate cancer: Long-term update of NRG Oncology RTOG 9202. Int J Radiat Oncol Biol Phys 98:296-303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denham JW, Joseph D, Lamb DS, et al. : Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol 20:267-281, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Denham JW, Steigler A, Lamb DS, et al. : Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451-459, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Bolla M, Maingon P, Carrie C, et al. : Short androgen suppression and radiation dose escalation for intermediate-and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol 34:1748-1756, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Bolla M, Van Tienhoven G, Warde P, et al. : External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11:1066-1073, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Armstrong JG, Gillham CM, Dunne MT, et al. : A randomized trial (Irish Clinical Oncology Research Group 97-01) comparing short versus protracted neoadjuvant hormonal therapy before radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 81:35-45, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Pisansky TM, Hunt D, Gomella LG, et al. : Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol 33:332-339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zietman AL, Prince EA, Nakfoor BM, et al. : Androgen deprivation and radiation therapy: Sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys 38:1067-1070, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Weller MA, Kupelian PA, Reddy CA, et al. : Adjuvant versus neoadjuvant androgen deprivation with radiotherapy for prostate cancer: Does sequencing matter? Clin Genitourin Cancer 13:e183-e189, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Malone S, Roy S, Eapen L, et al. : Sequencing of androgen-deprivation therapy with external-beam radiotherapy in localized prostate cancer: A phase III randomized controlled trial. J Clin Oncol 38:593-601, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach M, Moughan J, Lawton CA, et al. : Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol 19:1504-1515, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox DR: Regression models and life‐tables. J R Stat Soc Series B Stat Methodol 34:187-202, 1972 [Google Scholar]
- 25.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 26.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 27.Korn EL, Dorey FJ: Applications of crude incidence curves. Stat Med 11:813-829, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Latouche A, Allignol A, Beyersmann J, et al. : A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 66:648-653, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Dignam JJ, Zhang Q, Kocherginsky M: The use and interpretation of competing risks regression models. Clin Cancer Res 18:2301-2308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawton CA, Lin X, Hanks GE, et al. : Duration of androgen deprivation in locally advanced prostate cancer: Long-term update of NRG oncology RTOG 9202. Int J Radiat Oncol Biol Phys 98:296-303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPartlin AJ, Glicksman R, Pintilie M, et al. : PMH 9907: Long‐term outcomes of a randomized phase 3 study of short‐term bicalutamide hormone therapy and dose‐escalated external‐beam radiation therapy for localized prostate cancer. Cancer 122:2595-2603, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Spratt DE, Evans MJ, Davis BJ, et al. : Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 75:4688-4696, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmut B: Factors implicated in radiation therapy failure and radiosensitization of prostate cancer. Prostate Cancer 2012:593241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]