Abstract
Social interaction promotes survival by helping animals to form stable and supportive groups. Additionally, maladaptive social behavior is a hallmark of disorders such as autism and schizophrenia. In many different animal species, including humans, social interaction can be inherently rewarding. Lately there has been growing interest in studying the neurobiological underpinnings of social interaction and learned social behavior in rodent behavioral models. One common procedure is conditioned place preference (CPP) to measure the rewarding effects of social interaction and social reward learning. Social CPP was originally used in rats but has been adapted recently for use in mice, enabling use of the vast array of genetic tools available in mice. Here we studied the role of age, sex, bedding cues, and prior social isolation on the expression of social CPP in male and female mice. We found that without social deprivation male but not female mice display moderate but temporary social CPP during early adolescence but not adulthood. Early life social isolation increased social CPP in female but not male mice. In contrast, cocaine CPP was robust and long-lasting in male and female mice. Our results demonstrate that social CPP in mice is variable, occurring only under specific conditions, and that social isolation promotes social reward in female but not male mice. We discuss potential methodological and interpretive issues of the mouse social CPP model.
Keywords: Social behavior, conditioned place preference, social reward, social learning
Introduction
Social interaction promotes survival by aiding animals in forming and sustaining stable social groups, and it can be inherently rewarding (Church, 1959; de Waal & Preston, 2017; Rice & Gainer, 1962). Maladaptive social behavior is a hallmark of many neuropsychiatric disorders including, but not limited to, autism, schizophrenia and drug addiction (Fett et al., 2011; McPartland & Volkmar, 2012). In this regard, it has been suggested that the incorporation of social factors into animal models of addiction is critical to finding more successful treatment strategies (Heilig, Epstein, Nader, & Shaham, 2016). As a result, there has been growing interest in using preclinical models to study the interplay between both positive and negative social factors and addiction. An obvious benefit of developing reliable mouse models of innate and learned social behaviors, for example social preference, is the availability of a wide range of genetic tools useful for identifying, characterizing, and manipulating specific cell types and circuits involved in various types of behavior.
Conditioned place preference (CPP) is a Pavlovian procedure where during the training phase one context is paired with drug injections (or a nondrug reward), while another context is paired with vehicle injections (or no reward). During subsequent drug-free CPP tests, laboratory animals choose between the drug- and vehicle-paired contexts. Increased preference for the drug context serves as measures of the drug’s (or non-drug’s) rewarding effects (Van der Kooy, 1987) and incentive motivational effects of drug (or non-drug) contextual cues (Mueller & Stewart, 2000). Both rats and mice demonstrate CPP for addictive drugs, including cocaine, amphetamine, ethanol, heroin, and morphine (Cunningham, 1995; Cunningham, Gremel, & Groblewski, 2006; Schenk, Hunt, Colle, & Amit, 1983; Schenk et al., 1986; Tzschentke, 2007), as well as natural rewards such as food or access to a social partner (Guyon, Assouly-Besse, Biala, Puech, & Thiebot, 1993; Panksepp & Lahvis, 2007; Trezza, Campolongo, & Vanderschuren, 2011). CPP has been demonstrated following a single conditioning trial with amphetamine in rats that were isolated during the adolescent period (Whitaker, Degoulet, & Morikawa, 2013), but more typically conditioning happens in 15–60 minute sessions that are performed over the course of 3–10 days. CPP has been performed primarily in rodent models, although recently a CPP-like phenomenon has been demonstrated in humans (Childs & de Wit, 2009).
The majority of studies using CPP have focused on pharmacological manipulations. More recently, studies have demonstrated social CPP in which animals form an association between an environmental context or cue and the rewarding effects of social interaction. Several studies have demonstrated robust social CPP in rats (Calcagnetti & Schechter, 1992; Crowder & Hutto, 1992; Douglas, Varlinskaya, & Spear, 2004), and in one study, rats showed preference for a social-paired context over a cocaine-paired context in a mutually exclusive choice test (Zernig, Kummer, & Prast, 2013). Interaction with a conspecific can be rewarding (Panksepp & Lahvis, 2007; Vanderschuren, Niesink, & Van Ree, 1997), and has been shown to induce place preference in both rats and mice (Calcagnetti & Schechter, 1992; Trezza et al., 2011); however, successful implementation of social CPP in mouse models is much more sensitive to specific parameters including age, strain, context, and experience. Of the strains examined, C57BL/6J mice are the most social, and consequently display the greatest propensity to develop social CPP (Panksepp et al., 2007; Panksepp & Lahvis, 2007). One recent study found that C57BL/6J male and female mice express social CPP exclusively during the adolescent period (Nardou et al., 2019). Another study found that social CPP in male mice can be expressed in mice during adulthood, but the effect was dependent on the social partner being considerably younger than the experimental mouse (8–9 weeks old for the experimental mouse, 3–4 weeks old for the social partner) (Bariselli, Contestabile, Tzanoulinou, Musardo, & Bellone, 2018).
This state of affairs has led to questions about the strength and reliablity of social interaction as a reward in mice. Here, we sought to determine the conditions under which C57BL/6J mice will express social CPP. We followed a published social CPP procedure in mice (Dolen, Darvishzadeh, Huang, & Malenka, 2013; Nardou et al., 2019). Mice were housed in groups of 4, or isolated for 5 days prior to the beginning of conditioning. We gave mice a single 24-h social conditioning session when social interaction was paired with a distinct type of bedding in the social cage, followed by a 24-h isolated conditioning session in which mice were alone and placed on a separate kind of bedding. During testing, performed immediately after the 24-h isolation session, beddings were placed on either side of the test chamber and mice freely explored. We measured social CPP by assessing how much time was spent in the chamber that contained the social-paired bedding. Our data showed that social CPP in C57BL/6J mice only occured when male but not female mice were tested during adolescence, and that early life social isolation facilitates CPP in female but not male mice. Regardless of prior experience, social CPP in mice is highly variable and transiently expressed with mice no longer expressing a preference for social contexts or cues 4 days after social CPP.
Materials and Methods
Subjects
We used 88 male and 24 female C57BL/6J mice (purchased and shipped from Jackson Laboratories), post-natal day 25 to 56 when testing began. Sixty-two male mice underwent social CPP as test mice. Sixteen male mice underwent cocaine CPP. Eight additional mice were used as partners for Experiment 5. Two mice were excluded from Experiment 1 due to technical problems with our tracking software. Mice were maintained on a 12-h reversed light-dark cycle with food and water available ad libitum. All experiments were conducted in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. This study was approved by the NIDA IRP Animal Care and Use Committee.
Conditioned Place Preference Apparatus
We used a three-chambered apparatus consisting of two equal-sized chambers separated by a smaller middle chamber (59.6 × 17.8 × 15.2 cm). The compartments have both visual and tactile cues to differentiate the chambers. One compartment contains black and white striped walls with paper bedding while the other compartment has solid gray walls with granule bedding. Beddings used for conditioning are different from home cage bedding.
24 hour Social Conditioned Place Preference Procedure: social interaction
The following procedure was used for all social CPP experiments with the exception of the experiment presented in Figure 3C. We tested male mice for social CPP using a procedure in which mice were conditioned using two separate types of bedding, one paired with social interaction and the other paired with isolation (Dolen et al., 2013; Panksepp & Lahvis, 2007). Prior to testing, male mice were housed in groups of 4 conspecifics. Mice were habituated to the experimenter and weighed two days prior to the pretest. Experiments were performed 3 h before the beginning of the next dark cycle. Thirty minutes prior to the pretest, mice were transported to the behavior room. During the pretest, mice were exposed to the three-chamber place preference apparatus with two types of novel, distinct bedding (paper and granule), on either side of the chamber for 30 min. Beddings were different from the one used in the home cage condition. Immediately following the pretest, mice were transported to a different room and placed in a separate cage containing 3 familiar mice (their cagemates) with one of the beddings used during the pretest for 24-h (e.g. granule). Next, the mice were individually housed in a cage containing the other bedding type (e.g. paper) for 24-h. Mice were removed and taken back to the testing room and given a 30 minute test session immediately following the isolated conditioning session (Dolen et al., 2013; Nardou et al., 2019). During the test, mice are allowed free access to the three-chamber apparatus. The social or isolation-paired bedding were present on either side of the test chamber, and mice were in physical contact with the beddings. The pretest measures baseline side/bedding preference. For experiments shown in Figure 1A and 1B SoPhresh paper bedding and Kaytee granule bedding was used for social conditioning and test sessions. For experiments shown in Figure 2B (right), Carefresh paper bedding and Kaytee granule beddings were used for conditioning and test sessions. For the remaining experiments using this social conditioning procedure, Alpha-Dri Cotton bedding and Kaytee granule beddings were used for conditioning and test sessions. In between sessions, chambers were cleaned with ethanol or Nature’s Miracle de-odorizing spray (Nature’s Miracle Cage Cleaner for Small Animals, Petco). Cohorts of mice that displayed social place preference were retested either 4 or 9 days after the initial test session.
Figure 3.
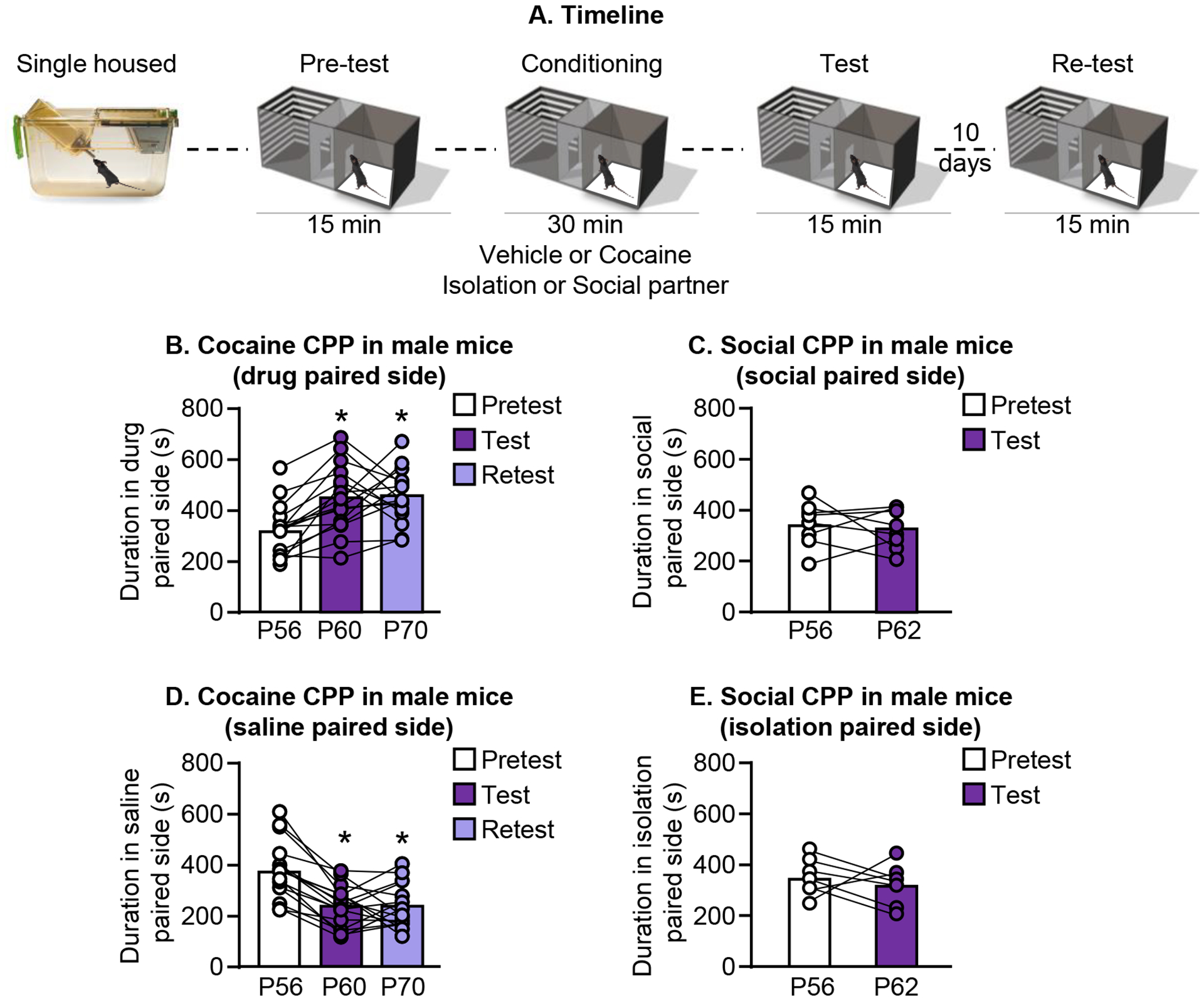
Early adolescent male mice demonstrate cocaine CPP that is stable ten days after the initial test. A. Experimental timeline of cocaine or social CPP. B/D. Cocaine CPP measured as the duration spent in the drug-paired chamber during test; retest was conducted ten days later (P56 at pretest). Time spent in the saline-paired chamber is shown in D. C/E. Using a procedure identical to the cocaine CPP procedure and substituting social interaction for drug reward does not change the time spent in the social-paired chamber (C) or the time spent in the isolation-paired chamber (E). produce place preference (P56 at pretest).
Figure 1.
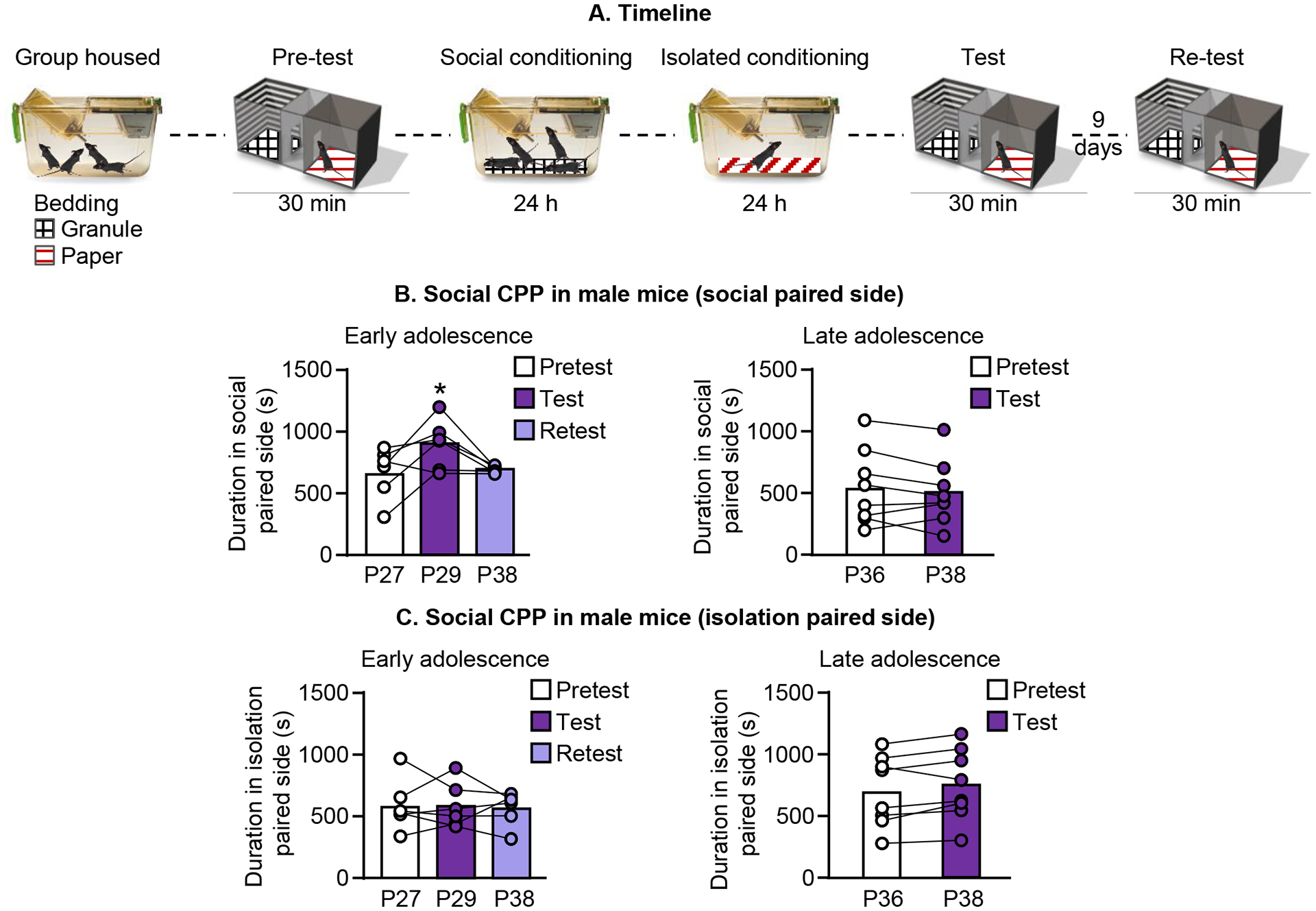
Male mice tested during the early adolescent period (P27 at pretest) demonstrate transient preference for social-paired cues. A. Experimental timeline of social CPP. B. Summary of social CPP as measured by the time spent in the side of the apparatus containing social-paired bedding during early (left) and late (right) adolescence (P36 at pretest). C. Time spent in the side of the apparatus paired with isolation following social CPP in early (left) and late (right) adolescence.
Figure 2.
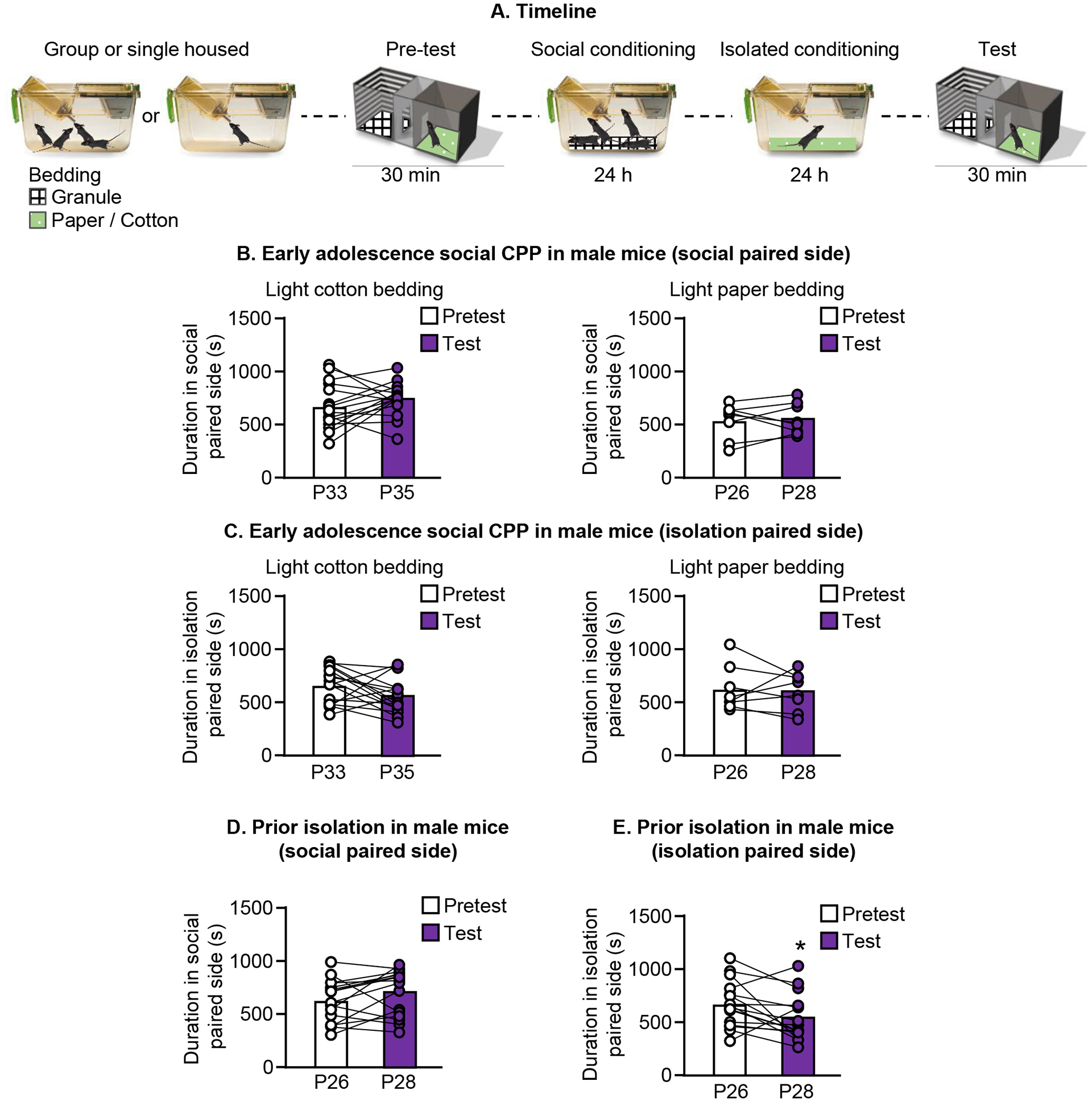
Social CPP is sensitive to tactile cues and early life experience. A. Experimental timeline of social CPP. B/C. Social CPP using two different bedding cues (P33 at pretest on left, P26 at pretest on right). Duration spent in the side of the apparatus paired with social bedding is shown in B, duration spent in the unpaired side of the apparatus is shown in C. D/E. Male mice that were isolated prior to testing do not show social CPP (P26 at pretest). D. Duration spent in the side of the apparatus paired with social bedding; E. Duration spent in the side of the apparatus paired with isolation.
Daily Conditioned Place Preference Procedure: cocaine and social interaction
Mice were habituated to saline injections 2 days prior to Day 1. Experiments were performed 3 h after the end of the dark cycle. During the 15 min pretest, mice were allowed to explore all three chambers of the apparatus to measure baseline side preference. During conditioning sessions, mice received a saline injection in one chamber and an injection of cocaine HCl (10 mg/kg, i.p.; NIDA pharmacy) in the other chamber. Injection context and order of injection were counterbalanced between groups. We conditioned mice for 3 days, with one 30 min session in the morning and one 30 min session in the afternoon separated by a 5–6 hour period. Mice were allowed to explore the three chambers during the 15 min post-test. Mice were retested ten days later. For data presented in Figure 3C, an identical procedure was used to this one described for cocaine, except pairings were done with a social partner instead of an injection of cocaine. Standard bedding used in the home cage was present underneath the flooring for this procedure, but bedding cues were not used for either daily CPP procedure. Tactile cues were present in the form of different floorings in each chamber (grid floor on one side, steel rod floor on the other side). See timeline in Figure 3 for additional experimental details. Mice were considered to show place preference if they significantly increased time spent in the reward-paired (social interaction or cocaine) side of the apparatus.
Specific experiments
Experiment 1: Social CPP in early adolescent and late adolescent mice.
Early adolescent male mice (n=6, PD 27, Figure 1B, left panel), and late adolescent male mice, (n=8, PD 38, Figure 1B, right panel) underwent social CPP according to the above protocol. Half of the mice underwent social conditioning on paper bedding and received granule bedding during isolated conditioning. The other half received granule then paper bedding. Mice were group housed prior to pretest.
Experiment 2: The effect of different bedding materials on social CPP in early adolescent male mice.
We sought to identify additional factors that influence the expression of social CPP by using different types of paper bedding to determine if social CPP is sensitive to cues used during the conditioning session. We followed the same social CPP procedure described in Experiment 1. We performed social conditioning in male mice (n=16, PND 30, Figure 2B, left panel) using cotton bedding or paper bedding (n=8, PD 26, Figure 2B, right panel). Mice were group housed prior to pretest.
Experiment 3: The effect of prior social isolation on social CPP in early adolescent male mice.
Previous studies have shown that early life experience is an important modulator of subsequent reward learning (Bardo & Bevins, 2000; Solinas, Chauvet, Thiriet, El Rawas, & Jaber, 2008; Stelly, Pomrenze, Cook, & Morikawa, 2016; Whitaker et al., 2013) For this reason, we tested the effect of early life social isolation on social CPP in adolescent male mice. We followed the social CPP procedure described in Experiment 1. Male mice (n=16, PD 26, Figure 2C, left panel, same mice were used for right panel but 6 were excluded due to high baseline side preference) were isolated for 5 days prior to the pretest.
Experiment 4: Cocaine CPP in male mice.
Male mice (n=16, PD 56, Figure 3B) underwent three days of conditioning sessions (2 sessions/day). During the 30-minute conditioning sessions, mice received an injection of cocaine and were then confined to one side of the apparatus. They received a saline injection prior to being confined to the other side of the apparatus in the subsequent session. We counterbalanced the order of injection each day. During the 15-min test mice were the allowed to explore the entire apparatus freely. Mice were retested 10 days later. Mice were isolated for 5 days prior to the pretest.
Experiment 5: Social CPP using multiple conditioning sessions without bedding cues in male mice.
We used a procedure identical to that used in Experiment 4 except that instead of receiving an injection male mice (n=8, PD 56, Figure 3C) were placed in one side of the chamber with a social partner mouse during the 30 minute conditioning sessions. Mice were isolated for 5 days prior to the pretest.
Experiment 6: Social CPP in early adolescent female mice.
It has been suggested that female mice may form stronger social bonds than male mice given that male mice are territorial and female mice live in social groups. Based on this idea, we hypothesized that female mice may form social CPP more readily than male mice. Female mice (n=8, PD 25, Figure 4B) underwent the social CPP procedure described in Experiment 1. Mice were group housed prior to pretest.
Figure 4.
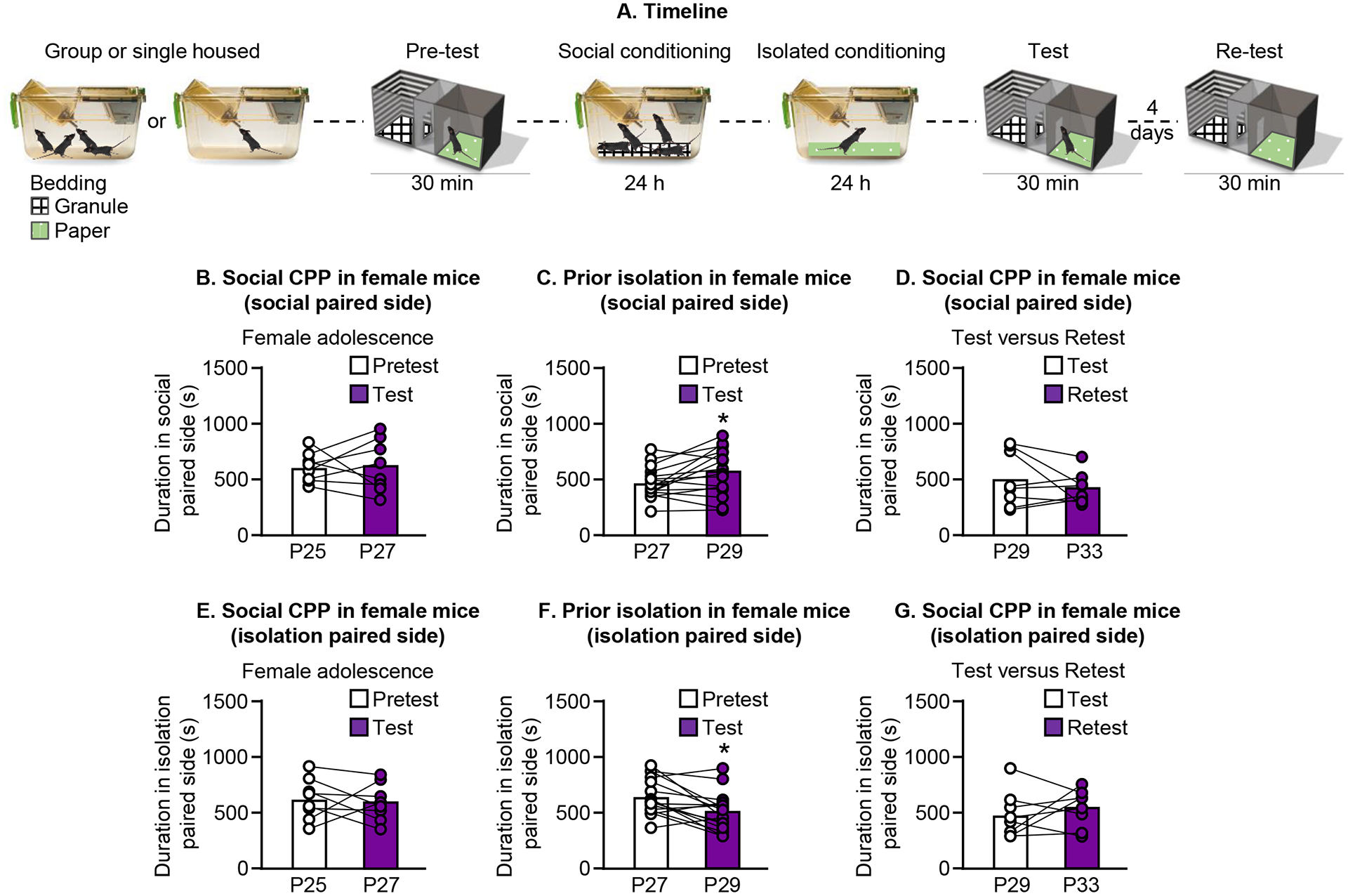
Early adolescent female mice that were isolated prior to testing display social CPP. A. Experimental timeline of social CPP. B/E. Group housed female mice do not change the time spent in the social paired side (B) or the isolation paired side (E) (P25 at pretest) C. Early life social isolation promotes social CPP in female mice (P27 at pretest). Female mice that experienced prior isolation increase time spent in the social paired side (C) and decrease the time spent in the isolation paired side (F). D/G. Social CPP in female mice is not present when mice are retested 4 days later. There is no change in time spent in either the social paired side (D) or the isolation paired side (G).
Experiment 7: The effect of prior social isolation on social CPP in early adolescent female mice.
Female mice (n=16, PD 25, Figure 4C, left panel, same mice were used for right panel but 6 were excluded due to high baseline side preference) underwent the same conditionined procedure described in Experiment 1. Mice were isolated for 5 days prior to the pretest. We retested 8 of these mice 4 days later. We chose 4 days rather than 9–10 days as in the prior experiments to see if preference might still be present when a shorter duration between test and retest is used.
Statistical Analysis
Data were analyzed using GraphPad Prism (version 8). For experiments containing more than two groups, one-way repeated measures ANOVA was used. For experiments comparing the mean of two groups, paired t tests were used. Statistical significance was set at p < 0.05.
Results
Experiment 1: Social CPP in early adolescent and late adolescent mice.
We tested male mice for social conditioned place preference using the 24 hour social conditioned place preference procedure described above. Briefly, mice were exposed to the three-chamber place preference apparatus with two types of bedding on either side of the chamber during pretest. Immediately following the pretest, mice were placed in a separate cage containing 3 familiar mice with one of the beddings used during the pretest for 24 hours. Next, the mice were individually housed in a cage containing the other bedding type for 24 hours. Mice were removed and given free access to the three-chamber apparatus to test for CPP immediately following the isolated conditioning session. The social or isolation-paired bedding were present on either side of the test chamber. See experimental timeline for details (Fig. 1A). Male mice conditioned during the early adolescent period showed preference for the side of the apparatus containing social-paired bedding 1 day after training [One-way repeated measures ANOVA, F(2,5) = 4.9, p=0.03] (Fig. 1B, left panel), but not 9 days after the initial test session. Male mice conditioned later in adolescence (Day 1 = PD 36) did not show preference for the social-paired bedding [Paired t-test, t(7) = 1.2, p=0.3] (Fig. 1B, right panel). There was no significant change in the time spent in the side of the apparatus containing isolation-paired bedding 1 day after training for early adolescent mice [One-way repeated measures ANOVA, F(2,5) = 0.06, p=0.92], (Fig. 1C, left panel) or late adolescent mice [Paired t-test, t(7)=1.9, p=0.10], (Fig. 1C, right panel). The results of Experiment 1 suggeset that social interaction in male mice is rewarding during adolescence but not adulthood.
Experiment 2: The effect of different bedding materials on social CPP in early adolescent male mice.
In Experiment 2 we used different types of paper bedding to determine whether social CPP is sensitive to cues used during the conditioning session. When we substituted a light cotton bedding for the paper bedding we used in our previous experiment, male mice no longer demonstrated social CPP; there was no change in the time spent in either the social-paired side of the chamber [Paired t-test, t(15) = 1.2, p=0.3], (Fig. 2B, left panel) or the isolation-paired side of the chamber [Paired t-test, t(15) = 1.8, p=0.10], (Fig. 2C, left panel). We repeated the experiment using a light-colored paper bedding that was similar to that used previously, and mice did not express social CPP; there was no change in the time spent in the social-paired side of the chamber [Paired t-test, t(7) = 0.4, p=0.7], (Fig. 2B, right panel) or the isolation-paired side of the chamber [Paired t-test, t(7) = 0.27, p=0.80], (Fig. 2C, right panel). The results of Experiment 2 suggest that the type of bedding used for conditioning can influence whether or not adolescent male mice develop social CPP.
Experiment 3: The effect of prior social isolation on social CPP in early adolescent male mice.
We then tested the effect of early life social isolation on social CPP in adolescent male mice. Mice were individually housed for 5 days prior to the pretest, but otherwise the experimental procedure was identical to that used previously. There was no significant increase in the duration of time spent in the social-paired chamber on test day [Paired t-test, t(15) = 1.7, p=0.1] (Fig. 2D). However, mice spent less time in the isolation-paired side of the apparatus [Paired t-test, t(15) = 2.4, p=0.03] (Fig. 2E). The results of Experiment 3 demonstrate that prior isolation does not influence social CPP in adolescent male mice.
Experiment 4: Cocaine CPP in male mice.
Given that social CPP in mice is influenced by a number of factors including age, sex, and prior experience, we decided to test mice for conditioned place preference using a non-social reward to determine if these male mice might have a general deficit in forming CPP. For this reason, we tested mice using a well-established cocaine CPP procedure. Briefly, we gave the mice saline or cocaine injections (i.p.) and placed them in one side of the CPP apparatus for 30 minutes (2 sessions per day for three days total). The male mice showed a robust increase in duration spent in the cocaine-paired side of the apparatus that was stable ten days after the initial test [One-way repeated measures ANOVA, F(2,15) = 11.8, p=0.0006] (Fig. 3B), and a corresponding decrease in time spent in the saline-paired side of the apparatus that was stable ten days after the initial test [One-way repeated measures ANOVA, F(2,15) = 23.97, p<0.0001] (Fig. 3D). This result demonstrates that male mice are capable of forming long-lasting CPP for drug reward using an established CPP procedure.
Experiment 5: Social CPP using multiple conditioning sessions without bedding cues in male mice.
In a separate cohort of mice, we used the same procedure that robustly induces cocaine CPP and gave mice access to a social partner instead of an i.p. injection of cocaine. We did not observe any changes in the time spent in the social-paired chamber [Paired t-test, t(7) = 0.5, p=0.6] (Fig. 3C) or in the isolation-paired chamber [Paired t-test, t(7) = 0.73, p=0.49] (Fig. 3E). This result demonstrates that using a procedure that produces CPP for other types of reward such as cocaine does not produce place preference for social interaction.
Experiment 6: Social CPP in early adolescent female mice.
Next, we tested whether female mice may form social CPP more readily than male mice. We used a procedure similar to what we used in male mice (Fig. 4A) in which group housed female mice were given a single 24-h social conditioning session followed by a single 24-h isolated conditioning session. We then tested their preference for the side of the apparatus containing social-paired bedding. Unlike the results with male mice, female mice did not develop social CPP under these conditions; there was no change in time spent in the social-paired side of the apparatus [Paired t-test, t(7) = 0.1, p=0.9] (Fig. 4B) or the isolation-paired side of the apparatus [Paired t-test, t(7) = 0.39, p=0.71] (Fig. 4E). However, when female mice were isolated for 5 days prior to the start of conditioning, they spent more time in the social-paired chamber during test [Paired t-test, t(15) = 2.5, p=0.03] (Fig. 4C), and less time in the isolation-paired chamber during test [Paired t-test, t(15) =3.29, p=0.005] (Fig. 4F). We retested 8 of the mice that showed social CPP and found that preference was no longer present 4 days later: there was no change in the duration spent in the social-paired side of the apparatus, [Paired t-test, t(7) = 1.1, p=0.3] (Fig. 4D) or the isolation-paired side of the apparatus [Paired t-test, t(7) =0.75, p=0.48] (Fig. 4G).
Discussion
Our results suggest that under certain conditions social CPP in mice can occur during early adolescence but not adulthood. We also report sex differences in the effect of prior social conditions on the expression of social CPP in adolescent mice: in females, social CPP was only observed after social isolation but not group housing, while in males social CPP was only observed after group housing but not isolation. The results of our study also suggest that social CPP in mice is variable, short-lasting, and critically dependent on procedural factors like bedding type. Of note, in adult mice social CPP was not formed under standard CPP experimental conditions under which cocaine CPP was robust and long-lasting.
Other studies have explored factors involved in the expression of social CPP in mice. Panksepp and Lahvis compared social CPP across four different mouse strains using a procedure in which male and female mice were conditioned in a separate cage containing novel and distinct bedding types. In this study, social conditioning was conducted using 24-h sessions over a period of ten days (Panksepp & Lahvis, 2007). Dolen et al utilized a similar but condensed version of this procedure and found that a single 24-h social conditioning session followed by a single 24-h isolated conditioning session was sufficient to produce social CPP in adolescent mice when the CPP test was performed immediately after the isolation session (Dolen et al., 2013; Nardou et al., 2019). We focused our parametric assessment of social CPP in mice on the C57BL/6J strain given that this is a generally social strain (Panksepp & Lahvis, 2007; Pinheiro et al., 2016). Intra-strain differences in social behavior may have interesting implications for the use of C57BL/6J mice in studies of drug reward as well, since prior social experience can alter the subsequent effects of drugs of abuse. Kennedy and colleagues found that adolescent C57BL/6J mice are less susceptible to the rewarding effects of morphine when conditioned in a social group as compared to the relatively less social BALB/CJ mice (Kennedy, Panksepp, Runckel, & Lahvis, 2012).
One potential caveat is that we conditioned mice using partner mice that were age/weight/sex matched. Bariselli et al demonstrated that young adult mice express social CPP when conditioning sessions are done with younger partner mice (Bariselli et al., 2018; Bariselli et al., 2016). It is possible that we might have observed social CPP in a wider range of ages had we employed this method. Additionally, partner mice in the Bariselli study were non-familiar mice, whereas in our study we used familiar mice for social conditioning. Given that social CPP was more robust in this study when non-familiar mice were used for conditioning, it is possible that we would have found stronger social CPP had we used non-familiar mice for conditioning sessions. However, the use of non-familiar mice presents a potential confound in that it becomes difficult to ascertain whether CPP is associated with the rewarding aspects of social interaction or with CPP for a novel experience. Despite these issues, it seems clear that age is an important factor in modulating social reward in mice. Adolescent male and female rats demonstrate social CPP, similar to what we observed in mice. However, unlike mice, both male and female rats will express social CPP in adulthood if they and their partner rats were previously housed under isolated conditions (Douglas et al., 2004).
Another potential issue is that the procedure we and others have used relies on the use of conditioning to a particular type of bedding, and measuring the strength of associations made with a social bedding cue rather than a direct measurement of the association between social interaction and the conditioning context (Dolen et al., 2013; Nardou et al., 2019; Panksepp & Lahvis, 2007). Furthermore, a potential issue in the single conditioning trial social CPP procedure is that mice are tested for the expression of CPP immediately following the termination of the isolated conditioning trial. Thus, the time spent on social bedding may reflect a more immediate aversive reaction to the isolation-paired bedding rather than a positive association with the social-paired bedding. In support of this notion, when we examined the persistence of social CPP compared to cocaine CPP, we found that social CPP was transient, the effect having completely disappeared a few days after the final conditioning session whereas cocaine preference was intact ten days following the final cocaine conditioning session.
The question of whether or not mice engage in social behavior is a critical one to answer when attempting to develop models of social interaction and social learning to facilitate neurobiological investigation. One study investigated the social behavior of juvenile CD-1 mice, demonstrating that mice are social, but that female mice engage in more social behavior than male mice, and that prior isolation facilitates subsequent social behavior (Terranova, Laviola, & Alleva, 1993). The increase in social behavior observed in mice together with the finding that prior isolation selectively facilitates social CPP in female mice suggests that isolation enhances the strength of social reward in adolescent females. Early life experience is a powerful modulator of subsequent learned behavior, including natural and drug-based reward learning. Early life social isolation in rats facilitates CPP for both amphetamine and alcohol (Whitaker et al., 2013), and repeated social stress enhances the expression of cocaine CPP (Stelly et al., 2016). Conversely, exposing mice to an enriched environment that encourages social interaction eliminates cocaine-induced CPP reinstatement (Solinas et al., 2008).
Conclusions
The aim of this study was to identify important factors to consider when developing preclinical mouse CPP models to facilitate neurobiological investigation of social factors in neuropsychiatric disorders. We showed that social CPP is only observed under a specific set of conditions in adolescent mice and that prior isolation increased social CPP selectively in female mice. Social CPP is expressed immediatlely after conditioning but is no longer expressed at the time points tested, either 4 or 9 days later. This limits the ability of researchers to use this model in conjunction with other genetic mouse tools or neurobiological assays. In light of this, and other limitations of the social CPP model such as generally modest effect sizes and high variability, there is a need to move toward operant models of social behavior. Recent studies (Venniro et al., 2020; Venniro, Russell, Zhang, & Shaham, 2019; Venniro & Shaham, 2020; Venniro et al., 2018) demonstrated that rats strongly prefer social interaction to an infusion of drug even in rats showing an addiction-like phenotype (Ahmed & Koob, 1998; Deroche-Gamonet, Belin, & Piazza, 2004). Developing a similar social choice procedure in mice will open new avenues to examine the relative value of social and non-social rewards and will enable us to examine the neurobiological substrates of volitional social interaction and social learning.
Highlights.
Early adolescence is a critical period for social CPP in male mice
Prior isolation enhances social place preference in adolescent female mice
Cocaine CPP is robust and long-lasting in adolescent male mice
Social CPP is a transient phenomenon in adolescent male and female mice
Acknowledgments:
The authors wish to acknowledge Dr. Yavin Shaham for his helpful comments and edits to the manuscript.
Funding:
All work was supported by the National Institute on Drug Abuse Intramural Research Program.
Footnotes
Declarations of interest: none
References
- Ahmed SH, & Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science, 282(5387), 298–300. doi: 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- Bardo MT, & Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl), 153(1), 31–43. doi: 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Bariselli S, Contestabile A, Tzanoulinou S, Musardo S, & Bellone C (2018). SHANK3 Downregulation in the Ventral Tegmental Area Accelerates the Extinction of Contextual Associations Induced by Juvenile Non-familiar Conspecific Interaction. Front Mol Neurosci, 11, 360. doi: 10.3389/fnmol.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Tzanoulinou S, Glangetas C, Prevost-Solie C, Pucci L, Viguie J, … Bellone C (2016). SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci, 19(7), 926–934. doi: 10.1038/nn.4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnetti DJ, & Schechter MD (1992). Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav, 51(4), 667–672. doi: 10.1016/0031-9384(92)90101-7 [DOI] [PubMed] [Google Scholar]
- Childs E, & de Wit H (2009). Amphetamine-induced place preference in humans. Biol Psychiatry, 65(10), 900–904. doi: 10.1016/j.biopsych.2008.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM (1959). Emotional reactions of rats to the pain of others. J Comp Physiol Psychol, 52(2), 132–134. doi: 10.1037/h0043531 [DOI] [PubMed] [Google Scholar]
- Crowder WF, & Hutto CW Jr. (1992). Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav, 41(4), 817–824. doi: 10.1016/0091-3057(92)90233-6 [DOI] [PubMed] [Google Scholar]
- Cunningham CL (1995). Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology (Berl), 120(1), 28–41. doi: 10.1007/bf02246142 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, & Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nat Protoc, 1(4), 1662–1670. doi: 10.1038/nprot.2006.279 [DOI] [PubMed] [Google Scholar]
- de Waal FBM, & Preston SD (2017). Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci, 18(8), 498–509. doi: 10.1038/nrn.2017.72 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, & Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science, 305(5686), 1014–1017. doi: 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, & Spear LP (2004). Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol, 45(3), 153–162. doi: 10.1002/dev.20025 [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, & Krabbendam L (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev, 35(3), 573–588. doi: 10.1016/j.neubiorev.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Guyon A, Assouly-Besse F, Biala G, Puech AJ, & Thiebot MH (1993). Potentiation by low doses of selected neuroleptics of food-induced conditioned place preference in rats. Psychopharmacology (Berl), 110(4), 460–466. doi: 10.1007/bf02244653 [DOI] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA, & Shaham Y (2016). Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci, 17(9), 592–599. doi: 10.1038/nrn.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Runckel PA, & Lahvis GP (2012). Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology (Berl), 219(3), 923–932. doi: 10.1007/s00213-011-2421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J, & Volkmar FR (2012). Autism and related disorders. Handb Clin Neurol, 106, 407–418. doi: 10.1016/B978-0-444-52002-9.00023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, & Stewart J (2000). Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav. Brain Res, 115(1), 39–47. Retrieved from http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=10996406 [DOI] [PubMed] [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, & Dolen G (2019). Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature, 569(7754), 116–120. doi: 10.1038/s41586-019-1075-9 [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, … Lahvis GP (2007). Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One, 2(4), e351. doi: 10.1371/journal.pone.0000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2007). Social reward among juvenile mice. Genes Brain Behav, 6(7), 661–671. doi: 10.1111/j.1601-183X.2006.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro BS, Seidl SS, Habazettl E, Gruber BE, Bregolin T, & Zernig G (2016). Dyadic social interaction of C57BL/6 mice versus interaction with a toy mouse: conditioned place preference/aversion, substrain differences, and no development of a hierarchy. Behav Pharmacol, 27(2–3 Spec Issue), 279–288. doi: 10.1097/FBP.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, & Gainer P (1962). “Altruism” in the albino rat. J Comp Physiol Psychol, 55, 123–125. doi: 10.1037/h0042276 [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Colle L, & Amit Z (1983). Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci, 32(10), 1129–1134. doi: 10.1016/0024-3205(83)90118-2 [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, & Amit Z (1986). Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav, 24(6), 1793–1796. doi: 10.1016/0091-3057(86)90523-x [DOI] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, & Jaber M (2008). Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A, 105(44), 17145–17150. doi: 10.1073/pnas.0806889105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelly CE, Pomrenze MB, Cook JB, & Morikawa H (2016). Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. Elife, 5. doi: 10.7554/eLife.15448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, & Alleva E (1993). Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol, 26(8), 467–481. doi: 10.1002/dev.420260805 [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, & Vanderschuren LJ (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci, 1(4), 444–458. doi: 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2007). Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol, 12(3–4), 227–462. doi: 10.1111/j.1369-1600.2007.00070.x [DOI] [PubMed] [Google Scholar]
- Van der Kooy D (1987). Place conditioning: A simple and effective method for assessing the motivational properties of drugs. In Bozarth MA (Ed.), Methods of assessing the reinforcing properties of abused drugs (pp. 229–240). New York: Springer-Verlag. [Google Scholar]
- Vanderschuren LJ, Niesink RJ, & Van Ree JM (1997). The neurobiology of social play behavior in rats. Neurosci Biobehav Rev, 21(3), 309–326. doi: 10.1016/s0149-7634(96)00020-6 [DOI] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, … Shaham Y (2020). Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc Natl Acad Sci U S A, 117(14), 8126–8134. doi: 10.1073/pnas.2001615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, & Shaham Y (2019). Operant Social Reward Decreases Incubation of Heroin Craving in Male and Female Rats. Biol Psychiatry, 86(11), 848–856. doi: 10.1016/j.biopsych.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, & Shaham Y (2020). An operant social self-administration and choice model in rats. Nat Protoc, 15(4), 1542–1559. doi: 10.1038/s41596-020-0296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, … Shaham Y (2018). Volitional social interaction prevents drug addiction in rat models. Nat Neurosci, 21(11), 1520–1529. doi: 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, & Morikawa H (2013). Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron, 77(2), 335–345. doi: 10.1016/j.neuron.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, & Prast JM (2013). Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry, 4, 100. doi: 10.3389/fpsyt.2013.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]


