Abstract
High blood pressure (BP) negatively affects brain structure and function. Hypertension is associated with white matter hyperintensities (WMH), cognitive and mobility impairment in late-life. However, the impact of BP exposure from young adulthood on brain structure and function in mid-life is unclear. Identifying early brain structural changes associated with BP exposure, before clinical onset of cognitive dysfunction and mobility impairment, is essential for understanding mechanisms and developing interventions. We examined the effect of cumulative BP exposure from young adulthood on brain structure in a substudy of 144 (61 female) individuals from the Coronary Artery Risk Development in Young Adults (CARDIA) study. At year 30 (Y30, 9th visit), participants (56±4 years old) completed brain MRI and gait measures (pace, rhythm, and postural control). Cumulative systolic and diastolic BP (cSBP, cDBP) over 9 visits were calculated, multiplying mean values between two consecutive visits by years between visits. Surface-based analysis of basal ganglia and thalamus was achieved using FreeSurfer-initiated Large Deformation Diffeomorphic Metric Mapping. Morphometric changes were regressed onto cumulative BP to localize regions of shape variation. Y30 WMH volumes were small, and positively correlated with cumulative BP, but not gait. Negative morphometric associations with cSBP were seen in the caudate, putamen, nucleus accumbens, pallidum and thalamus. A concave right medial putamen shape mediated the relationship between cSBP and stride width. Basal ganglia and thalamic morphometric changes, rather than volumes, may be earlier manifestation of grey matter structural signatures of BP exposure that impact midlife gait.
Keywords: Blood pressure, Basal ganglia, Thalamus, Middle Aged, Magnetic Resonance Imaging, Gait
Graphical Abstract
The deleterious impact of high blood pressure (BP) on cognition and dementia is well established1-4. However, high BP is also associated with mobility impairment5. More importantly, gait slowing often precedes cognitive decline6-8, suggesting that mobility changes may be an earlier marker of neurodegeneration than cognitive decline. High BP also negatively affects brain structure, and is strongly associated with white matter hyperintensities (WMH)9, basal ganglia injury10, as well as atrophy of subcortical structures11. While studies have examined the relationship between BP in midlife, brain structure, and cognition4, 12, 13, data on the relationship between BP exposure and midlife brain structure and gait is lacking. A better understanding of preclinical structure and function changes associated with BP exposure will help elucidate underpinning mechanisms linking high BP to brain injury, and facilitate targeted therapies to preserve brain structure and prevent mobility and cognitive decline in late life.
Age-related mobility impairments have been associated with WMH14, thalamic lacunar infarcts14 basal ganglia volume15, and morphology16. WMH, being easy to identify and grade in neuroimaging studies, have been studied widely in age-related gait disorders. While the importance of WMH14 can not be underestimated, age-related motor impairment cannot be entirely explained by WMH burden17, and in fact, gray matter disturbances may have a stronger influence18. Disorders affecting the basal ganglia including Parkinson’s and Huntington’s disease are characterized by gait impairments. However, understanding the relationship between BP, WMH, basal ganglia and mobility impairment in an aged population with endstage disease has been challenging due to multiple comorbidities, accumulation of risk factors and complexity of aging. As such, we sought to explore these relationships in midlife, and to determine the early brain structural changes associated with cumulative BP exposure measured from young adulthood.
To achieve this aim, we leveraged the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) cohort. This cohort provides a unique opportunity to investigate cumulative BP exposure from young adulthood as it relates to WMH and subcortical gray matter structure in midlife. We hypothesized that BP assessed longitudinally from young adulthood would be positively associated with volumes of WMH and negatively with volumes of basal ganglia and thalamus in midlife. In exploratory analyses, we further examined whether these changes would synergistically contribute to gait changes in midlife.
Methods
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). The National Heart, Lung, and Blood Institute policies governing the data and describing it’s access is online (https://www.cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
Participants
The CARDIA study is a longitudinal study of cardiovascular risk factors that began in 1985 and recruited 5115 individuals aged 18-30 years. Follow-up examinations were conducted 2 (Y2), 5 (Y5), 7 (Y7), 10 (Y10), 15 (Y15), 20 (Y20), 25 (Y25), and 30 (Y30) years after baseline (Y0). Chicago is one of the 4 CARDIA field centers and all active participants from Chicago were invited to participate in this substudy (N=513). Our response rate was 71% (N=362) and 154 were willing and eligible for the MRI study. Exclusion criteria were MRI contraindications, body habitus, and suspected pregnancy. All participants provided written consent, approved by the Northwestern University IRB.
Of the 154 participants, two were excluded due to incidental stroke findings, one did not complete the T2 weighted FLAIR sequence thus did not have WMH data, one had an extreme score for WMH volume, one did not have gait assessment, one only had BP from 4 visits and four individuals were excluded due to poor segmentation of the T1 weighted image (described below), resulting in a final sample of 144 for this analysis (Table 1).
Table 1.
Demographic variables and cardiovascular risk factors at Y30
| Variables | Mean (SD) or n |
|---|---|
| Sex | 83 Male, 61 Female |
| Race | 88 White, 56 Black |
| Age (years) | 56 (4) [range=48-63] |
| Education (years) | 15 (3) |
| Handedness | 123 Right, 21 Left |
| Intracranial volume (mm3) | 1,520,406 (146,767) |
| Cumulative SBP (mmHg in 30 yrs) | 3,284 (256) |
| Cumulative DBP (mmHg in 30 yrs) | 2,064 (206) |
| Y0 SBP (mmHg) | 107 (10) |
| Y0 DBP (mmHg) | 65 (10) |
| Y30 SBP (mmHg) | 118 (14) |
| Y30 DBP (mmHg) | 72 (10) |
| Body Mass Index | 28 (4) |
| Self-reported hypertension | 104 No, 40 Yes |
| Self-reported diabetes | 131 No, 13 Yes |
| Glucose Y30 (mg/dL) | 99 (18) |
| Self-reported high cholesterol | 94 No, 50 Yes |
| Cholesterol Y30 (mm/dL) | 196 (37) |
| Self-reported smoker | 123 No, 21 Yes |
| Taking diabetes medication Y30 | 132 No, 12 Yes |
| Taking BP medication Y30 | 114 No, 30 Yes |
| Taking cholesterol medication Y30 | 119 No, 25 Yes |
Measures
Blood Pressure
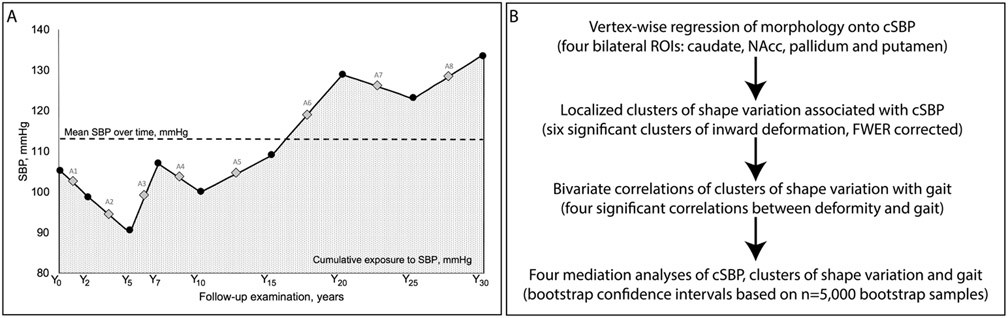
Measurement of BP has been described previously19, 20. Trained research staff measured BP from the right arm brachial artery while the participant was in a seated position. For Y0 to Y15 a Hawksley random-zero sphygmomanometer was used, and for subsequent visits, an automated oscillometric BP monitor (Omron HEM-907XL) was used. A calibration study standardized values to the sphygmomanometer to eliminate machine bias (see 19). Cumulative systolic blood pressure (cSBP) was calculated per previous CARDIA studies (Yano et al., 2014, 2017), detailed in Figure 1A. Mean SBP (and mean DBP) was calculated between two consecutive visits, multipled by the number of years between those two visits, then summed to determine cSBP (and cDBP) over 30 years. cSBP and cDBP were normally distributed. All 144 participants had BP measures for Y0 and Y30, and all had BP measures for at least 5 separate visits. For Y2, Y5, Y7, Y10, Y15, Y20, and Y25, BP data were unavailable for 3, 4, 10, 7, 2, 3 and 2 participants, respectively.
Figure 1.
A) (Adapted from Yano et al., 2017) cSBP calculation across 9 visits in one participant. Average SBP between consecutive visits is shown as A1-A8. cSBP was calculated as (A1 x 2 years + A2 x 3 years + A3 x 2 years + A4 x 3 years + A5 x 5 years + A6 x 5 years + A7 x 5 years + A8 x 5 years), shown by the dotted area, mm Hg x years. B) Statistical analysis for the study.
Gait
Gait was measured during a 40-foot walk on a 20 × 2 foot Zeno walkway. Data were captured with Prokinetics Movement Analysis Software, which has established reliability and validity21. Participants walked at their normal pace. The most frequently reported gait variables in the related to the gait domains of pace, rhythm and postural control were measured22, 23. Velocity (pace domain) is reported as centimeters per second. Cadence (rhythm domain) is reported as steps per minute. Stride width (postural control domain) is defined as the perpendicular distance of a line connecting two heels from the same foot and the heel of the contralateral foot. This value can be negative in out-toe walking individuals whos heels overlap when they land.
Image acquisition and surface mapping
A 3T Siemens Prisma MRI scanner was used to collect T1-weighted Magnetization prepared rapid gradient echo (MPRAGE) images in the sagittal plane. Voxels resolution was 0.98 × 0.98 × 1.0, slices=176, FOV=256 × 256, TR=1900, TE=2.93, TI=900, flip=9. T2-weighted FLAIR images were also collected in the sagittal plane with the same voxel resolution, slices and FOV, with a TR=1900, TE=2.93 and TI=900. WMH volume was calculated by manually delineating hyperintense voxels on FLAIR weighted images using Freeview by research assistants (S.K. and J.H) blinded to the other study data and under the supervision of T.P. Cases where there were any uncertainties were reviewed by a neuroradiologist (A.N.). WMH values were normalized by each participant’s intracranial volume (ICV) and multiplied by 1000. A log transformation was applied to achieve normality, and correlations with both the normalized and log transformed normalized results are reported.
Regions of interest (ROIs) were bilateral nucleus accumbens, caudate, putamen, pallidum and thalamus. ROI surfaces were automatically generated for each participant using the FS+LDDMM pipeline24. This combines FreeSurfer’s (FS) probabilistic voxel-based classification and a deformable, high dimensional template-based method of large deformation diffeomorphic metric mapping, (LDDMM)25. FreeSurfer (version 5.3) subcortical labeling for the initial basal ganglia segmentations was followed by image alignment and intensity normalization with LDDMM25, which produces smooth transformations for each ROI.
Surface processing
Each participant’s surface was rigidly registered to atlas space to calculate a population average. For each participant, local shape variation24 was calculated from the population average by quantifying the perpendicular amplitude between surfaces at a vertex-to-vertex level. The quantification of perpendicular change between surfaces was assigned a positive (outward variation from population average) or negative (inward variation from population average) value. For each ROI, shape variation values at each vertex were summed across the whole surface. Overall volume for each participant for each ROI was calculated utilizing the volume enclosed within the surfaces.
Statistical Analysis
Associations between cSBP (or cDBP) and volume of each ROI were examined using partial correlations, controlling for age, sex, race and ICV. We examined associations between cSBP (or cDBP) and local shape variation of each ROI in surface-based analyses using SurfStat26 implemented in MATLAB. To localize regions of local shape variation, multivariate linear regression models evaluated the associations of cSBP with morphometric changes. Separate models were tested for each of the five ROIs, adjusting for age, sex, race and ICV in all models. To account for multiple comparisons within each ROI (p< 0.001), Random Field Theory (RFT; 27) was applied using SurfStat. This approach provided clusters of vertices at the desired family-wise error rate (FWER) of p< 0.05 per ROI. Significance and direction of association were visualized as a color map on the overall average surface.
Exploratory analyses
We were additionally interested in understanding if the multiple comparison corrected, morphological associations with cSBP were also related to gait. Therefore we undertook further exploratory analyses of three measures of gait. To reduce the number of mediation analyses performed, therefore the number of statistical comparisons, we first calculated bivariate correlations (uncorrected) to identify morphometric clusters that were associated with gait (Figure 1). Mediation analyses using ordinary least squares path analysis were conducted using PROCESS28 for SPSS to determine whether deformities negatively associated with cSBP mediated relationships between cSBP and gait. Age, sex, race and ICV covariates were included in the models. Given that our sample did not yet demonstrate substantial WMH burden29, and in line with previous research examining the relationship between basal ganglia and gait15, we chose to covary WMH volume, along with other risk factors of Y30 BMI, Y30 cholesterol (mm/dL), Y30 self-reported diabetes, and Y30 self-reported smoker. Multicollinearity was assessed and found to be absent, with tolerance values ranging from 0.51 to 0.94 and variance inflation factor values ranging from 1.07 to 2.00. 95% confidence intervals were estimated using 5000 bootstraps. We hypothesized that inward morphology of regions associated with cSBP would mediate the relationship between cSBP and gait performance.
Results
Demographic data are summarized in Table 1. Our mid-life sample, without a substantial burden of vascular risk factors, is representative of the CARDIA participants in the Chicago cohort.
BP exposure and Brain Structure in midlife
As expected, we observed positive partial correlations between cSBP and cDBP (0.80, p<0.0001), cSBP and WMH volume (0.21, p= 0.01), and log WHM volume (0.23, p= 0.007), cDBP and WMH volume (0.18, p= 0.04), and log WMH volume (0.20, p= 0.01). Surprisingly, there were no correlations (p> .05) between cSBP or cDBP and any grey matter volumes. WMH volume was negatively correlated with volume of the left pallidum (−0.18, p= 0.04), however not after FWE correction for multiple comparisons (p> .05).
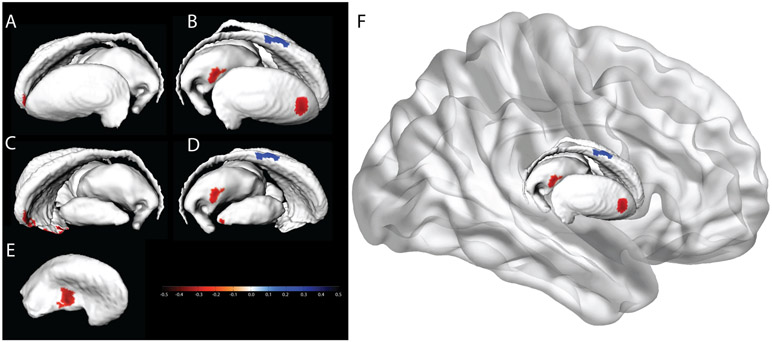
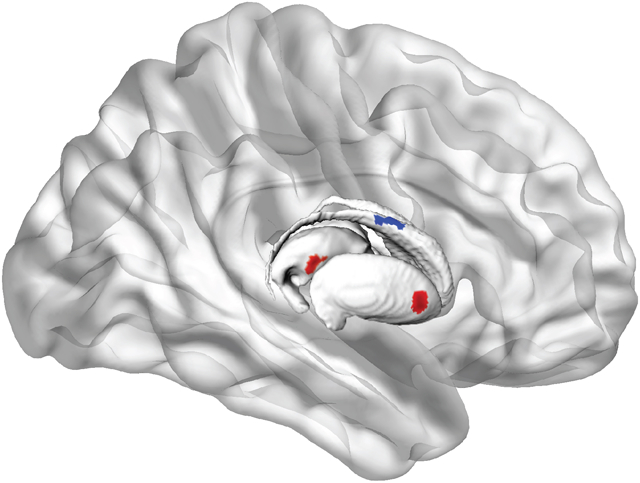
Morphometry results are presented in Figure 2. Red indicates regions of negative associations with cSBP (inward deformity associated with increased cSBP), and blue indicates regions of positive associations with cSBP (outward deformity associated with increased cSBP). Negative associations were observed for the left lateral caudate head and lateral nucleus accumbens, right medial and lateral putamen, lateral thalamus and lateral anterior pallidum. A positive association was also found on the right lateral caudate body. Because this region abuts the ventricles, it is highly susceptible to WMH. Visual inspection confirmed that observed positive associations were, in fact, the result of segmentation errors by the automated pipeline. This pipeline uses T1 weighted images to segment subcortical structures, and since WMH appear hypointense on T1 weighted images, they are misclassified as gray matter.
Figure 2. Surface deformities related to cumulative SBP.
a) left lateral basal ganglia/thalamus, b) right lateral basal ganglia/thalamus, and occluded structures of c) left lateral caudate, nucleus accumbens, thalamus d) right lateral pallidum, e) right medial putamen, f) the basal ganglia in situ. Scale shows standardized regression coefficients.
A sensitivity analysis additionally covarying handedness replicated Figure 2, without the pallidum, thalamus or lateral putamen clusters, or the positive caudate cluster. There were no associations between cDBP and morphometry, after covariate adjustment, at the FWE corrected threshold.
Exploring gait in midlife and cSBP-related morphological changes
Gait velocity in the entire sample ranged from 77 to 181cm/second (125± 19). Cadence ranged from 79 to133 steps/minute (107±10). Stride width ranged from −2 to 17cm (8±3).
Bivariate correlations between inward clusters and gait measures are presented in Supplemental Table S1. Positive correlations were observed between velocity and the large nucleus accumbens and thalamic clusters, and negative correlations were found between stride width and caudate, pallidum and medial putamen clusters. These four clusters were subsequently examined as potential mediators of the relationship between cSBP and gait. WMH volume was not correlated with any gait measures (p> .05). Partial correlations found cBP were not correlated with gait measures after controlling for cardiovascular risk factors (Supplemental Table S2). However, the total effect of X (cSBP) on Y (gait) should not be a prerequisite for searching for indirect (mediating) effects28.
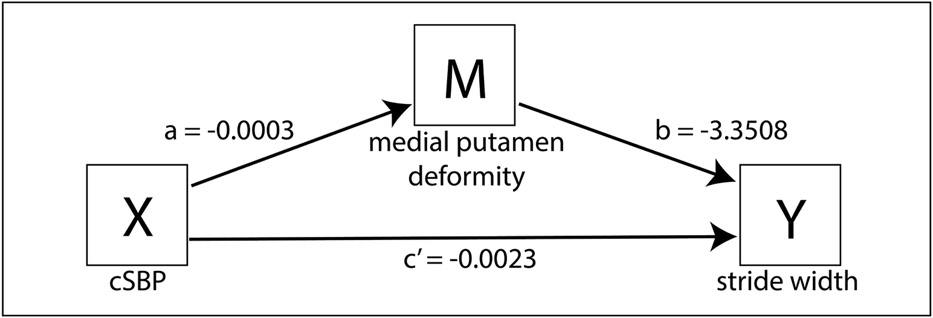
Participants with higher cSBP had greater mean inward deformity of the medial putamen (a= −0.0003, p=0.0031, CI −0.004 to −0.001), and participants with greater inward deformity of the medial putamen had wider strides (b= −3.3508, p= 0.0055, CI −5.7012 to −1.003) (Figure 3). There was no evidence that cSBP influenced stride width independently of its effect on medial putamen deformity (c’= −0.0023, p= 0.07, CI= −0.0048 to 0.0002). A bootstrap 95% confidence interval for the indirect effect of cumulative SBP on stride width through mean medial putamen deformity (ab= 0.0009) based on 5,000 bootstrap samples was above zero (0.0002 to 0.0020). These results suggest that the relationship between cSBP and stride width may be mediated through medial putamen deformity. Other deformities negatively associated with cSBP did not mediate the relationships between cSBP and gait (Supplemental Table S3).
Figure 3.
Mediation analysis: Indirect effect of cSBP on stride width through medial putamen deformity. Values are unstandardized regression coefficients. See text for statistics and interpretation.
Discussion
We show that cumulative BP exposure over 30 years from young adulthood is associated with morphological changes of the basal ganglia and thalamus in midlife. cSBP was associated with inward deformity in the left lateral caudate head and lateral nucleus accumbens, the right lateral pallidum and thalamus and the medial and lateral putamen. cDBP exposure was not associated with morphometry despite a strong relationship (r=0.80) with cSBP. In exploratory analyses, inward deformities of basal ganglia and thalamic structures predicted by longitudinal cumulative BP exposure were positively correlated with velocity and negatively correlated with stride width. Moreover, these exploratory analyses found that the relationship between cSBP and stride width was mediated by inward deformity of the medial putamen. Our findings suggest that morphometric changes of the basal ganglia and thalamus which could impact gait and mobility, may be early structural signatures of cBP exposure in the brain.
Our middle-aged sample with vascular risk factors, but without a high burden of overt hypertension, are likely at a preclinical, early stage of pathology. We argue that this accounts for the lack of correlations between cBP exposure and basal ganglia and thalamic nuclei volumes. However, the subtle morphological findings which require a precision that is not apparent on standard grey matter volumetric analysis30, reveal regional shape disparities in relation to cumulative BP exposure. Thus, our more sensitive vertex-wise analyses show that cSBP, but not cDBP exposure, across young adulthood is related to midlife inward deformity of subcortical structures known to be central to control of gait and posture. These findings are consistent with a meta-analysis where the association between high BP or hypertension and brain volume was mostly driven by SBP rather than DBP11. Our results are also consistent with Goldstein et al.31 who report that in healthy adults (aged 56-80) without a diagnosis of hypertension, those with higher BP were more likely to have brain atrophy than those with lower BP.
The inward morphometric changes related to cSBP exposure are also in line with previous studies that did not measure gait, but did examine the relationship between subcortical atrophy and hypertension. For example, these studies show thalamic atrophy in midlife and late-life adults with hypertension32 and even adults (aged 35-65) that were successfully treated for hypertension33, as well as hippocampal atrophy in otherwise healthy adults (aged 20-77) with hypertension34 and in males in late life who had uncontrolled hypertension in midlife35. While there is also data showing an absence of association between a diagnosis of hypertension and basal ganglia volumes36, in one study, SBP> 140 was associated with a steeper decline in basal ganglia volumes over 2.4 years37. Our study extends this literature and shows that cumulative BP exposure-related morphometric changes can be observed as early as midlife. In addition, our study underscores the importance of longitudinal BP measurement rather than a single measure or a clinical diagnosis of hypertension in understanding the impact of BP on brain structure. Further longitudinal data will help us determine if these early morphometric changes are related to later volume loss in these structures.
In our exploratory correlation analyses, we found that inward basal ganglia and thalamic morphological deformations were associated with lower velocity and greater stride width, supporting previous findings linking elevated BP with lower gait speed38. However, in our midlife cohort, WMH volumes were not correlated with gait measurements. This finding does not support previous reports that WMH are associated with gait, even after controlling for vascular risk factor14, 39. However, these prior studies were conducted in late-life cohorts with a greater duration of exposure to cumulative vascular risk factors and a higher burden of WMH than the individuals in midlife assessed in our study. WMH likely represent more advanced injury and manifest with more severity of gait impairment. Studies that have found associations between gait speed and atrophy of the hippocampus40-42, caudate15 or putamen40 also tend to differ in demographics from the midlife sample we studied here. Our midlife sample has had a shorter exposure to vascular risk factors than cohorts in later life and less time to develop age-related changes in brain structure and function, offering the advantage of allowing us to study earlier signatures of BP exposure on the brain structures important in mobility.
Exploratory analyses found that inward deformation of the medial putamen associated with higher cSBP exposure mediated the relationship between cSBP and stride width. While this small effect isn’t likely to have immediate clinical relevance, we contend this is an early change likely to worsen with increased cSBP exposure. It’s also congruent with clinical observations. Widening of base of support is a compensatory mechanism to maintain balance in those with age-related mobility impairment and in patients with normal pressure hydrocephalus43. A study of elderly, non-disabled adults with WMH found inward left caudate deformation was associated with lower performance on the Short Physical Performance Battery16. Structural changes in the putamen have also been linked to balance and mobility in older adults44 and those diagnosed with Alzheimer’s disease45. Similarly, in older adults, reduced gray matter in the pallidum was also associated with wider step46. Stride base widening may be an early measure of compensation for at-risk adults who do not manifest overt cerebrovascular disease and/or mobility impairment.
Studying individuals in midlife provided us an opportunity to study relationships between longitudinal measures of BP, structural brain changes, and gait in an early stage of injury. Our main findings are that cSBP was associated with WMH and predicted local inward morphometric changes in deep gray nuclei. Exploratory analyses suggest that these inward shape changes in the medial putamen mediated the relationship between cSBP and stride width, even when controlling for other vascular risk including WMH volume. The complexity of age-related comorbid risk factors makes it difficult to disentangle the longitudinal sequence of structural changes that impact gait in the elderly. For example, in a late-life cohort, de Laat et al.47 suggested that WMH may lead to secondary neuronal degeneration of gray matter regions involved in gait control. Conversely, Su et al.17 conducted a mediation analysis and found that thalamic atrophy mediated the effect of WMH on walking speed in elderly adults with cerebral small vessel disease. Clearly, longitudinal data on individuals from mid-life with detailed vascular, gait and brain measures are needed before we can answer this question.
Our study has some limitations. While our focus on the basal ganglia and thalamus is supported by prior work15, our analyses did not include cortical brain regions or the cerebellum, which may also contribute to mobility and gait in midlife. We used the automated FSLDDMM pipeline, and while this approach has advantages over manual tracing methods, WMH appear to have led to some segmentation errors. Studies utilizing such pipelines in WMH-prone populations, albeit in midlife, before there is a substantial burden of WMH volume, should consider this limitation. The cross-sectional neuroimaging data precludes inference as to when the morphological changes that were associated with BP occurred. More frequent longitidunal neuroimaging data are needed to delineate the time course of these basal ganglia and thalamic morphological changes. The longitudinal BP measurements collected at 9 time points were a strength of the study, however, levels of BP control in between visits are unknown, potentially confounding results. Other measures of BP such as BP variability may also have contributions, however, we had to limit the number of analyses feasible in our sample. Unlike the main neuroimaging results, exploratory analyses linking gait to structural brain changes related to cSBP were not corrected for multiple comparisons. Therefore, while our exploratory findings offer support for previous research, they underscore the need for future larger studies powered to directly examine these relationships more closely.
Summary and Conclusions
Our study identifies a relationship between cSBP exposure and morphology of the caudate, putamen, pallidum, thalamus and nucleus accumbens in midlife. We show that in midlife, inward deformity of these regions is associated with cSBP exposure and mobility. Morphometry may prove to be a more sensitive early brain measure of BP exposure than volume, and a useful biomarker to identify individuals at risk of functional decline. Earlier identification of at-risk individuals is essential for preventing silent brain changes which later manifest as overt cognitive and mobility impairment in our aging population.
Supplementary Material
Perspectives.
Hypertension is a well-established risk factor that negatively impacts brain health later in life. Specifically, hypertension is associated with structural changes in the brain seen on MRI as white matter hyperintenisties (WMH), mobility impairment and cognitive dysfunction in elderly people. However, we know very little about earlier brain structure and function manifestations that may be associated with higher blood pressure exposure, before there is clinical decline in brain health. Identifying early brain structural changes associated with blood pressure (BP) exposure, before the clinical onset of cognitive dysfunction and mobility impairment, is essential for understanding mechanisms and developing interventions. In this study, we examined the effect of cumulative BP exposure from young adulthood on brain structure in 144 (61 female) individuals from the Coronary Artery Risk Development in Young Adults (CARDIA) study with 9 clinical assessments between 1985 and 2015. At year 30 (Y30, 9th visit), participants (56±4 years old) completed brain MRI and gait measures (pace, rhythm, and postural control). While Y30 WMH volumes were small in this age group, they were positively correlated with cumulative BP exposure, but not gait. Higher cumulative BP exposure was also associated with negative morphometric changes seen in the caudate, putamen, nucleus accumbens, pallidum and thalamus. Moreover, a concave right medial putamen shape mediated the relationship between cumulative SBP and stride width. Our findings suggest that morphometric changes of the basal ganglia and thalamus, which could impact gait and mobility, may be early structural signatures of cumulative BP exposure in the brain.
Novelty and Significance.
1. What is new:
Morphometric changes of basal ganglia and thalamic nuclei in midlife, before substantial WMH accumulation, may be an early structural signature of brain injury in those with a higher exposure to BP during young adulthood.
2. What is relevant:
Indiviudalized, early BP control targeted to preserve brain structure is an essential step towards preventing silent brain changes which later manifest as overt cognitive and mobility impairment in our aging population.
Summary
Higher cumulative exposure to SBP starting in young adulthood is associated with morphometric changes in basal ganglia and thalamus, measured in midlife. Exploratory results suggest these morphometric changes may be related to early gait changes in a cognitively normal cohort in midlife.
Acknowledgements
This manuscript has been reviewed by CARDIA for scientific content.
Sources of Funding
This study was supported by National Institute of Neurological Disorders and Stroke (NINDS; R01-NS085002). The CARDIA Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). CARDIA was also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). Sanaz Sedaghat is supported by a Rubicon fellowship of the Netherlands Organization for Scientific Research.
Footnotes
Conflicts of Interest
Disclosures: None.
References
- 1.Elias MF, Wolf PA, Dagostino RB, Cobb J, White LR. Untreated blood-pressure level is inversely related to cognitive functioning: The framingham study. Am. J. Epidemiol 1993;138:353–364 [DOI] [PubMed] [Google Scholar]
- 2.Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Hennerici M, O’Brien J, Pantoni L, Salvadori E, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D, Study L. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The ladis study. J. Neurol. Neurosurg. Psychiatry 2007;78:1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faraco G, Iadecola C. Hypertension: A harbinger of stroke and dementia. Hypertension. 2013;62:810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: The maintenance of balance, independent living, intellect, and zest in the elderly of boston study. J. Am. Geriatr. Soc 2011;59:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best JR, Liu-Ambrose T, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, Studenski S, Yaffe K, Newman AB, Rosano C, Hlth Aging Body Composition S. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J. Gerontol. Ser. A-Biol. Sci. Med. Sci 2016;71:1616–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, Pankratz VS, Geda YE, Machulda MM, Ivnik RJ, Knopman DS, Boeve BF, Rocca WA, Petersen RC. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the mayo clinic study of aging. J. Gerontol. Ser. A-Biol. Sci. Med. Sci 2013;68:929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-alzheimer’s dementia. N. Engl. J. Med 2002;347:1761–1768 [DOI] [PubMed] [Google Scholar]
- 9.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke. 2004;35:1857–1861 [DOI] [PubMed] [Google Scholar]
- 10.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436 [DOI] [PubMed] [Google Scholar]
- 11.Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C. Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. J. Hypertens 2013;31:1502–1516 [DOI] [PubMed] [Google Scholar]
- 12.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340 [DOI] [PubMed] [Google Scholar]
- 13.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The framingham heart study. Int. J. Obes 2003;27:260–268 [DOI] [PubMed] [Google Scholar]
- 14.de Laat KF, van Norden AGW, Gons RAR, van Oudheusden LJB, van Uden IWM, Bloem BR, Zwiers MP, de Leeuw FE. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41:1652–1658 [DOI] [PubMed] [Google Scholar]
- 15.Dumurgier J, Crivello F, Mazoyer B, Ahmed I, Tavernier B, Grabli D, Francois C, Tzourio-Mazoyer N, Tzourio C, Elbaz A. Mri atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage. 2012;60:871–878 [DOI] [PubMed] [Google Scholar]
- 16.Macfarlane MD, Looi JCL, Walterfang M, Spulber G, Velakoulis D, Styner M, Crisby M, Orndahl E, Erkinjuntti T, Waldemar G, Garde E, Hennerici MG, Bazner H, Blahak C, Wallin A, Wahlund LO, Grp LS. Shape abnormalities of the caudate nucleus correlate with poorer gait and balance: Results from a subset of the ladis study. Am. J. Geriatr. Psychiatr 2015;23:59–U90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su N, Liang XY, Zhai FF, Zhou LX, Ni J, Yao M, Tian F, Zhang SY, Jin ZY, Cui LY, Gong GL, Zhu YC. The consequence of cerebral small vessel disease: Linking brain atrophy to motor impairment in the elderly. Hum. Brain Mapp 2018;39:4452–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wennberg AMV, Savica R, Mielke MM. Association between various brain pathologies and gait disturbance. Dement. Geriatr. Cogn. Disord 2017;43:128–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano Y, Ning HY, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: The coronary artery risk development in young adults (cardia) study. Hypertension. 2014;64:983-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano Y, Reis JP, Levine DA, Bryan RN, Viera AJ, Shimbo D, Tedla YG, Allen NB, Schreiner PJ, Bancks MP, Sidney S, Pletcher MJ, Liu K, Greenland P, Lloyd-Jones DM, Launer LJ. Visit-to-visit blood pressure variability in young dulthood and hippocampal volume and integrity at middle age: The cardia study (coronary artery risk development in young adults). Hypertension. 2017;70:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynall RC, Zukowski LA, Plummer P, Mihalik JP. Reliability and validity of the protokinetics movement analysis software in measuring center of pressure during walking. Gait Posture. 2017;52:308–311 [DOI] [PubMed] [Google Scholar]
- 22.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. Ser. A-Biol. Sci. Med. Sci 2013;68:820–827 [DOI] [PubMed] [Google Scholar]
- 23.Wilson J, Allcock L, McArdle R, Taylor J-P, Rochester L. The neural correlates of discrete gait characteristics in ageing: A structured review. Neuroscience and Biobehavioral Reviews. 2019, 100: 344–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AR, Wang L, Beg MF. Freesurfer-initiated fully-automated subcortical brain segmentation in mri using large deformation diffeomorphic metric mapping. Neuroimage. 2008;41:735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg MF, Miller MI, Trouve A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int. J. Comput. Vis 2005;61:139–157 [Google Scholar]
- 26.Chung MK, Worsley KJ, Nacewicz BM, Dalton KM, Davidson RJ. General multivariate linear modeling of surface shapes using surfstat. Neuroimage. 2010;53:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor JE, Worsley KJ. Detecting sparse signals in random fields, with an application to brain mapping. J. Am. Stat. Assoc 2007;102:913–928 [Google Scholar]
- 28.Hayes AF. Introduction to mediation, moderation and conditional process analysis: A regression-based approach. New York: Guilford Press; 2018. [Google Scholar]
- 29.Kreisel SH, Blahak C, Bazner H, Inzitari D, Pantoni L, Poggesi A, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O’Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG, Grp LS. Deterioration of gait and balance over time: The effects of age-related white matter change - the ladis study. Cerebrovasc. Dis 2013;35:544–553 [DOI] [PubMed] [Google Scholar]
- 30.Mamah D, Barch DM, Csernansky JG. Neuromorphometric measures as endophenotypes of schizophrenia spectrum disorders. In: Ritsner MS, ed. Neuropsychiatric biomarkers, endophenotypes, and genes: Promises, advances and challenges. Netherlands: Springer; 2009:87–122. [Google Scholar]
- 31.Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology. 2002;59:713–719 [DOI] [PubMed] [Google Scholar]
- 32.Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417 [DOI] [PubMed] [Google Scholar]
- 33.Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, Aizenstein H, Alzheimers Dis N. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J. Hum. Hypertens 2012;26:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005;15:1676–1689 [DOI] [PubMed] [Google Scholar]
- 35.Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: The honolulu asia aging study. Hypertension. 2004;44:29–34 [DOI] [PubMed] [Google Scholar]
- 36.Rodrigue KM, Haacke EM, Raz N. Differential effects of age and history of hypertension on regional brain volumes and iron. Neuroimage. 2011;54:750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong LW, Forsberg LE, Vidal JS, Sigurdsson S, Zijdenbos AP, Garcia M, Eiriksdottir G, Gudnason V, van Buchem MA, Launer LJ. Different susceptibility of medial temporal lobe and basal ganglia atrophy rates to vascular risk factors. Neurobiol. Aging 2014;35:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosano C, Longstreth WT, Boudreau R, Taylor CA, Du Y, Kuller LH, Newman AB. High blood pressure accelerates gait slowing in well-functioning older adults over 18-years of follow-up. J. Am. Geriatr. Soc 2011;59:390–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosano C, Brach J, Longstreth WT, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60 [DOI] [PubMed] [Google Scholar]
- 40.Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, Srikanth VK. Brain structural change and gait decline: A longitudinal population-based study. J. Am. Geriatr. Soc 2013;61:1074–1079 [DOI] [PubMed] [Google Scholar]
- 41.Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: A quantitative volumetric analysis of brain mri. Neuroradiology. 2015;57:851–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky S, Harris T, Yaffe K, Satterfield S, Studenski S, Rosano C. Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology. 2017;89:336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee PH, Yong SW, Ahn YH, Huh K. Correlation of midbrain diameter and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Journal of neurology. 2005;252:958–963 [DOI] [PubMed] [Google Scholar]
- 44.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J. Gerontol. Ser. A-Biol. Sci. Med. Sci 2007;62:1048–1055 [DOI] [PubMed] [Google Scholar]
- 45.Lee YW, Lee H, Chung IS, Yi HA. Relationship between postural instability and subcortical volume loss in alzheimer’s disease. Medicine (Baltimore). 2017;96:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. Ser. A-Biol. Sci. Med. Sci 2008;63:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Laat KF, Reid AT, Grim DC, Evans AC, Kotter R, van Norden AGW, de Leeuw FE. Cortical thickness is associated with gait disturbances in cerebral small vessel disease. Neuroimage. 2012;59:1478–1484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.