Abstract
The low five-year survival rate for patients with acute myeloid leukemia (AML), primarily caused due to disease relapse, emphasizes the need for better therapeutic strategies. Disease relapse is facilitated by leukemic stem cells (LSCs) that are resistant to standard chemotherapy and promote tumor growth. To target AML blasts and LSCs using Natural Killer (NK) cells, we have developed a trispecific killer engager (TriKE™) molecule containing a humanized anti-CD16 heavy chain camelid single domain antibody (sdAb) that activates NK cells, an IL-15 molecule that drives NK cell priming, expansion and survival, and a single-chain variable fragment (scFv) against human CLEC12A (CLEC12A TriKE). CLEC12A is a myeloid lineage antigen that is highly expressed by AML cells and LSCs, but not expressed by normal hematopoietic stem cells (HSCs), thus minimizing off-target toxicity. The CLEC12A TriKE induced robust NK cell specific proliferation, enhanced NK cell activation and killing of both AML cell lines and primary patient derived AML blasts in vitro while sparing healthy HSCs. Additionally, the CLEC12A TriKE was able to reduce tumor burden in pre-clinical mouse models. These findings highlight the clinical potential of the CLEC12A TriKE for the effective treatment of AML.
Keywords: Natural Killer, TriKE, Tri-specific Antibodies, IL-15, Immunotherapy
Introduction
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults(1). Despite the recent approval of new drugs like venetoclax, midostaurin, gemtuzumab, and enasidenib, cure rates have only increased modestly, especially in those patients who relapse after primary therapy or those who have high risk disease(2–4). AML remains difficult to treat due to the heterogeneity of the disease, lack of specific target antigens and presence of leukemic stem cells (LSCs) that mediate relapse after conventional treatments(5,6).
Targeted immunotherapy could be a curative therapy option for AML. Current targets on AML cells include CD33, CD123, CLEC12A, FLT3, CD96, TIM3, CD135, CD244 and CD7(7). CD33 and CD123 are the most commonly targeted antigens for clinical development (8–10) but their presence on normal progenitors limit their therapeutic application (11–13). C-type lectin domain family 12 member A or CLEC12A (also called CLL-1, CD371 or hMICL) is a promising target that may overcome these limitations.
CLEC12A is expressed on up to 92% of AML blasts and its expression is stable during the course of the disease(14–16). CLEC12A is specifically expressed by leukemic stem cells (LSCs), but not nonmalignant hematopoietic stem cells (HSCs) (16,17). These factors make CLEC12A a promising target for the treatment of AML and there are several clinical agents being developed that include bispecific antibodies, CAR-T cells and antibody-drug conjugates (18–20).
Natural Killer (NK) cells are important mediators of cancer immunosurveillance due to their ability to kill malignant cells without priming. They can eliminate cancer cells through the release of cytotoxic granules triggered by interactions with natural ligands or through CD16 mediated recognition of antibody coated tumors by a process called antibody-dependent cellular cytotoxicity (ADCC)(21–23). Several studies have demonstrated the therapeutic potential of NK cells in the treatment of leukemia with 30–50% remission reported after NK cell infusions (24–26). Most significantly, NK cell therapies have better safety profiles than T cell therapies in a clinical setting. Adoptive transfer of NK cells is safe with essentially no graft-versus-host-disease (GvHD) and rare instances of cytokine release syndrome (CRS) and neurotoxicity compared to T cell based therapies(27–29).
Our lab has developed trispecific killer engager (TriKE) molecules that direct NK cell function against tumor cells(30–32). Early TriKE molecules were comprised of an anti-CD16 single chain variable fragment (scFv) that activates NK cells, a mutant IL-15 molecule that adds co-stimulation and a scFv that engages cancer targets. Our CD33 TriKE is currently in a phase I/II clinical trial for patients with high risk myelodysplastic syndromes (MDS) and refractory AML (NCT03214666). Given the potential limitations of CD33 as a target antigen in AML and the possibility of antigen escape, we developed a second generation TriKE(33) containing a humanized anti-CD16 heavy chain camelid single domain antibody (sdAb), a wild-type IL-15 moiety, and a scFv against human CLEC12A (CLEC12A TriKE) that functions better than the first generation TriKE. In this study, we have tested the efficacy of the CLEC12A TriKE in activating NK cells against AML in vitro and in vivo.
Methods
TriKE construct:
The CLEC12A TriKE construct was synthesized using previously described cloning techniques(30). The fully assembled fragment was cloned into the Minicircle DNA vector (System Biosciences, Palo Alto, CA) under the control of a CMV promoter and consisted of an ATG start codon, humanized camelid anti-CD16 sdAb(34,35), wild type human IL-15, whitlow amino acid linker, anti-human CLEC12A scFv and a 10X His tag.
TriKE production:
TriKE plasmids were transfected into Expi293 cells (Thermo Fisher, Waltham, MA) according to the manufacturer’s protocol and protein was purified using HisPur Cobalt Resin (Thermo Fisher) and Pierce centrifuge columns (Thermo Fisher). Protein was eluted using 250mM imidazole solution and desalted using Prepacked disposable PD-10 columns (GE Healthcare, Chicago, IL). Purity and size (57KDa) were determined by running sodium dodecyl sulfate polyacrylamide gel electrophoresis using Simply Blue Life Stain (Invitrogen, Carlsbad, CA).
Cell culture, Isolation of immune cells and AML patient samples:
CLEC12A expressing cancer cell lines HL-60 and THP-1 were obtained from American Type Culture Collection (ATCC), cultured as recommended and tested for mycoplasma regularly. Healthy donor blood was obtained from Memorial Blood Bank (Minneapolis, MN) or Sanquin Blood Bank (Nijmegen, Netherlands). Cells were processed from peripheral blood mononuclear cells (PBMCs) using EasySep Human NK Cell Enrichment Kits (STEMCELL Technologies, Vancouver, Canada), or CD3/CD19 depletion Selection Kits (STEMCELL Technologies). Healthy donor CD34+ cells were purified from G-CSF mobilized PBMCs or from cryopreserved bone marrow by positive selection (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). AML patient samples were obtained from the translational therapy lab (TTL) at the University of Minnesota, or the Laboratory of Hematology at the Radboudumc (protocol CMO 2013/064) after Institutional Review Board approval. All human samples were obtained following informed consent in compliance with guidelines by the Committee on the Use of Human Subjects in Research and in accordance with the Declaration of Helsinki.
Proliferation Assay:
PBMCs (n=6) were labeled with CellTrace Violet (Thermo Fisher), harvested after 7 days and dilution of the dye was assessed by flow cytometry on an LSRII machine (BD Biosciences, East Rutherford, NJ). NK (LiveDead-/CD56+/CD3- ) and T cells (LiveDead-/CD56-/CD3+) were identified using Live/Dead Near-IR (Thermo Fisher), PE-CY7 conjugated anti-CD56 (318318, BioLegend, San Diego, CA), and PE-CF594 conjugated anti-CD3 (562280, BD Biosciences). Analysis of flow cytometric data was performed using FlowJo software (Ashland, OR). For in vitro experiments, we used 6 biological replicates for the experiments as that is the minimum number required for non-parametric testing of samples with a normal distribution.
Function Assay Measuring Degranulation (CD107a), activation (CD69) and Cytokine Production (IFNγ and TNFα):
Assessment of NK cell function by flow cytometry was carried out as previously described(30). Briefly, PBMCs (n=6) and targets cells were co-cultured and stained with FITC conjugated anti-CD107a (93937, BioLegend). An hour later, Golgi Stop and Golgi Plug (BD Biosciences) was added and cells were incubated for 3 hours. Cells were stained with Live/Dead Fixable Aqua Staining Kit (Thermo Fisher), PE-CY7 conjugated anti-CD56 and PE-CF594 conjugated anti-CD3, PE conjugated anti-CD69 (310906 (FN50), BioLegend), fixed and permeabilized. Permeabilized cells were stained with BV650 conjugated IFNγ (93705 (4S.B3), BioLegend) and BV421 conjugated TNFα (562783 (Mab11), BD Biosciences).
Real Time Tumor Killing:
HL-60 and THP-1 cells were labeled using CellTrace Far Red Proliferation Kit (Thermo Fisher). Enriched NK cells were plated with targets, indicated treatments and Caspase-3/7 Green Apoptosis Assay reagent (Sartorius Inc., Göttingen, Germany) for reading every 30 minutes in an IncuCyte S3 machine (Sartorius Inc.). A time-dependent graph of the percentage live HL-60 and THP-1 targets (CellTrace Far Red+, Caspase3/7-) was plotted to represent killing after normalization to targets alone.
Killing assay with primary AML blasts:
Allogeneic NK cells and AML blasts were co-cultured in the presence of treatments in IMDM supplemented with 10% human serum (Sanquin blood bank), GM-CSF (Immunotools, Friesoythe, Germany), G-CSF (Amgen, Thousand Oaks, CA), IL-3 (Immunotools), SCF (Immunotools) and Flt3L (Immunotools) in triplicate. To determine killing of AML blasts, cells were labeled with CFSE on day 0. After 48 hours 7AAD was added and cells were quantified by FC500 flow cytometry analyzer (Beckman Coulter, Brea, CA). CLEC12A or CD33 expression on remaining AML blasts, was determined with FITC conjugated anti-CD34 (343604, Biolegend), PE conjugated anti-CLEC12A (353604, Biolegend), APC conjugated anti-CD33 (A70200, Beckman Coulter), BV510 conjugated anti-CD45 (304036, Biolegend) and sytox blue (Thermo Fisher) and analysis on a Gallios flow cytometer (Beckman Coulter). Cell number was calculated as cells x %CD45+ cells x %CD34+ cells x %CLEC12A+/− CD33+/− cells.
To evaluate LSCs, refractory AML bone marrow samples were cultured with enriched NK cells and % of live LSCs remaining was analyzed in a flow cytometry-based function assay using the Live/Dead Fixable Aqua Staining Kit, FITC conjugated CD38 (303504 (HIT2), Biolegend), PE conjugated CLEC12A, APC conjugated CD34 (340441, Biolegend), BV711 conjugated CD33 (366624, Biolegend), and BV605 conjugated CD45 (304042, Biolegend). The % live LSCs was calculated relative to the total CD38-CD34+ cells in the tumor alone condition using a previously described gating strategy(36).
In Vivo Models:
Mouse experiments were performed following the guidelines of Institutional Animal Care and Use Committee at the University of Minnesota with female mice being used for all experiments. The HL-60luc/Human NK cell Xenogeneic NOD/SCID IL2Rgnull (NSG) mouse model was used with a sample size of 5 per group that was previously described to provide enough statistical power (30,33). Briefly, 8–10 week old mice were irradiated (225 cGy), intravenously (IV) injected with 7.5×105 HL-60luc cells, injected IV with 1×106 CD3/CD19 depleted human NK Cells three days later and treated for three weeks intraperitoneally (IP). All mice were injected with tumors and broken up into groups prior to evaluation of tumor load. At day 7, 14 and 21 tumor load was assessed after luciferin injection using bioluminescent imaging.
For the patient-derived xenograft (PDX) model, 6–8 week old NSG-SGM3 mice were irradiated (125 cGy) and injected IV the next day with 2×106 primary AML cells. Progress of AML engraftment was monitored with weekly bleeds to determine proportion of CD45+/CD33+ blast cells until detection of at least 1% human AML in blood was achieved (in about 6 weeks). Mice were randomized into 4 groups containing mice with equal means of percent blasts in the blood per group. The relevant groups received 3×106 CD3/CD19-depleted human NK Cells and subsequently received IP treatments of TriKE for 3 weeks. Mice were harvested on Day 21 and blood was stained to determine CD45+/CD56+/CD3- NK cell expansion. Bone marrow was stained with BV605 conjugated CD45, FITC conjugated CD34, and APC conjugated CD33 (303408, Biolegend) to determine CD45+/CD33+ AML blasts. No mice were excluded in the study. Our pre-established criteria only exclude mice that have to be removed from the study for non-study related events. No blinding was carried out for these studies
Statistical Analysis:
GraphPad Prism (GraphPad Prism Software, Inc, La Jolla, CA) was used to generate graphs with error bars showing mean SEM, and to calculate statistical significance as *P<0.05, **P< 0.01, ***P<0.001, and ****P<0.0001. All the data met the assumptions of the test and the variance was similar between the groups that were being statistically compared.
Results
The CLEC12A TriKE drives NK cell proliferation.
The novel CLEC12A TriKE construct, containing a humanized anti-CD16 sdAb, a wildtype IL-15 moiety, and an anti-CLEC12A scFv, was generated in a mammalian expression system. Maximum binding was detected by ELISA with a 30nM concentration that was used in subsequent experiments (Supplementary Figure 1 A–C). To evaluate the ability of the IL-15 moiety in the CLEC12A TriKE to induce NK cell proliferation, PBMCs were CellTrace labeled and treated with recombinant human (rh) IL-15, a CLEC12A scFv or the CLEC12A TriKE at equimolar amounts for a week (30nM). Treatment with the CLEC12A TriKE drove significantly more NK cell proliferation than rhIL-15 or CLEC12A scFv (Figure 1A–C). The discrepancy between proliferation induced by the TriKE versus rhIL-15 alone can be explained by the anti-CD16 sdAb that delivers the TriKE specifically to NK cells. When T cell proliferation within the PBMCs was assessed (Figure 1D), more proliferation was observed with the rhIL-15 compared to the TriKE indicating that the TriKE acts specifically on NK cells.
Figure 1. The CLEC12A TriKE induces potent NK cell specific proliferation.
PBMCs were isolated from fresh healthy donor samples (n=6), CellTrace Violet labeled, and incubated for 7 days with no treatment or 30nM CLEC12A TriKE, rhIL-15 or anti-CLEC12A scFv. After the incubation period, cells were harvested and NK cell (CD3-, CD56+) proliferation was evaluated by flow cytometry. Representative histograms (A) and pooled data (B) showing NK cell proliferation (by CellTrace dilution) on the different treatment groups. (C) Pooled NK cell count (45 seconds at constant speed) at the time of harvest. (D) Percentage of T cell (CD3+, CD56-) proliferation (by CellTrace dilution) evaluated in the PBMCs. One-way analysis of variance (ANOVA) with repeated measures was used to calculate differences against the CLEC12A group. Error bars indicate +/− standard error of the mean. Statistical significance was determined as *P<0.05, **P<0.001, ****P<0.0001.
The CLEC12A TriKE effectively induces NK cell activation against AML cell lines.
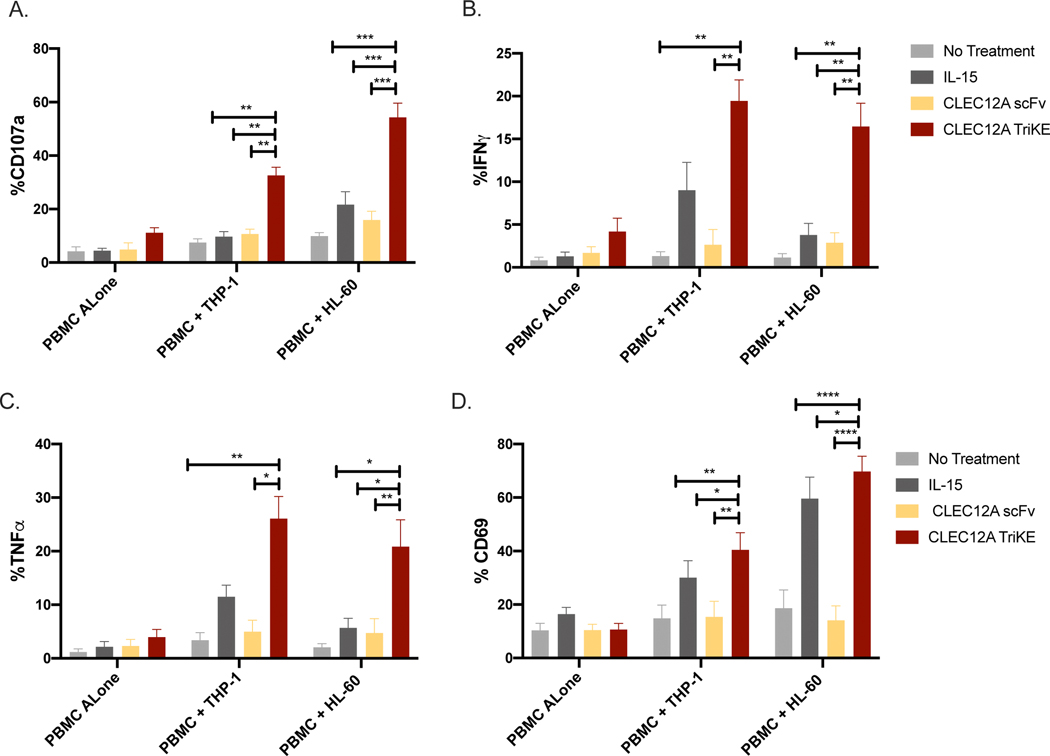
Several studies have shown that activation of NK cells with IL-15 improves CD16 mediated ADCC activity in vitro and in vivo(37,38). To test this, healthy donor PBMCs were incubated with and without HL-60 and THP-1 targets. All groups were treated with equimolar amounts of rhIL-15, CLEC12A scFv or CLEC12A TriKE (30nM). The NK cells in the PBMCs incubated with the TriKE showed significantly higher levels of degranulation, as measured by CD107a (Figure 2A). Interestingly, there were higher levels of degranulation against HL-60 targets compared to THP-1 targets which is in line with previous reports showing that HL-60 cells form more conjugates with NK cells leading to better degranulation(39). In addition, treatment with the CLEC12A TriKE significantly increased NK cell production of the inflammatory cytokines IFNγ and TNFα (Figure 2B, 2C) that can recruit other cells of the adaptive immune system. Finally, we observed increased NK cell activation, as measured by CD69 (Figure 2D) when treated with the CLEC12A TriKE compared to the conditions.
Figure 2. Functional validation of the CLEC12A TriKE.
(A) Frozen PBMCs from healthy donors (n=6) were incubated with no treatment or 30nM CLEC12A TriKE, rhIL-15 or anti-CLEC12A scFv to evaluate CD107a expression (as a marker of degranulation), (B) intracellular IFNγ production, (C) or intracellular TNFα expression in NK cells (CD3-, CD56+) in a 4 hour assay. The cells were evaluated with PBMCs alone or in the presence of THP-1 and HL-60 targets at a 2:1 effector to target ratio. (D) Activation of NK cells (CD3-, CD56+) in PBMCs were evaluated using CD69 expression in a 4-hour assay with PBMCs alone or in the presence of THP-1 and HL-60 targets at a 2:1 effector to target ratio. One-way analysis of variance (ANOVA) with repeated measures was used to calculate differences against the CLEC12A group. Error bars indicate +/− standard error of the mean. Statistical significance was determined as *P < .05, **P < .01, ***P < .001, and ****P < .0001.
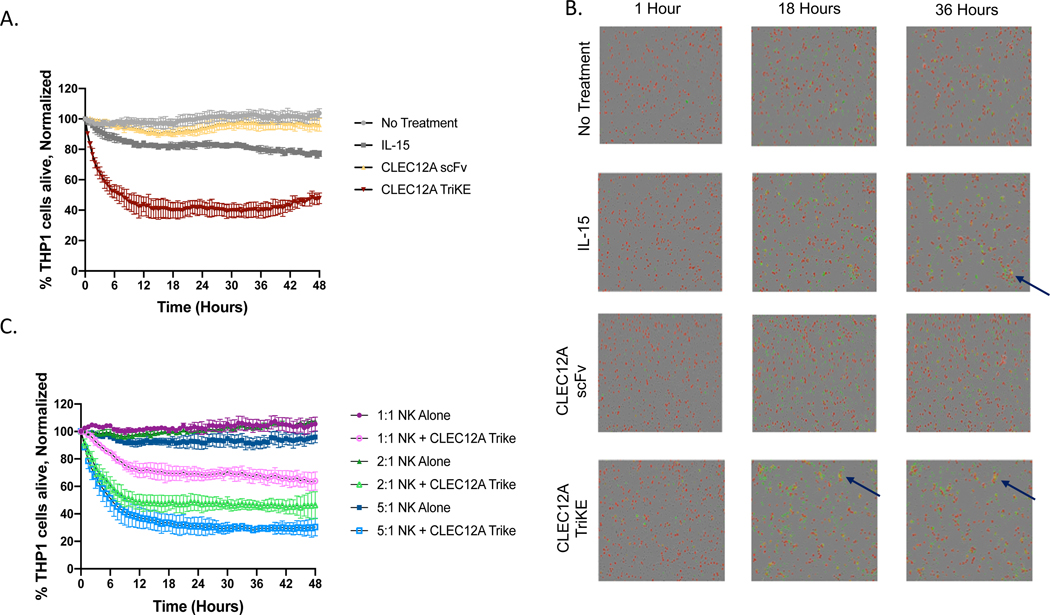
Increased tumor cell killing is observed after treatment with CLEC12A TriKE in a longitudinal killing assay.
To assess whether an increase in NK cell degranulation leads to increased killing of target cells, we incubated enriched NK cells with THP-1 cells either left untreated or treated with equimolar amounts of rhIL-15, CLEC12A scFv or CLEC12A TriKE (30nM). There were fewer THP-1 cells alive after 48 hours in the condition treated with the CLEC12A TriKE compared to the other treatments (Figure 3A). Moreover, there was also increased tumor killing against HL-60 targets (Supplementary Figure 2). Compared with rhIL-15 and CLEC12A scFv, the CLEC12A TriKE induced more killing clusters, comprised of NK cells, live THP-1 cells, and dying THP-1 cells at later time points (Figure 3B and Supplementary Figure 2B). The amount of target cell killing was directly proportional to effector to target ratio and a higher amount of NK cells induced more TriKE dependent target cell death (Figure 3C). Thus, the increase in NK cell function after TriKE treatment translates to greater killing of leukemia cells that express CLEC12A. These assays were done with a single dose of TriKE and we predict that multiple doses will further increase target cell killing.
Figure 3. CLEC12A TriKE induces target cell killing in real-time imaging assay.
Enriched NK cells were incubated with CellTrace Far Red labeled THP-1 cells at a 2:1 effector to target ratio with no treatment, 30nM CLEC12A TriKE, rhIL-15 or anti-CLEC12A scFv for 48 hours within an IncuCyte S3 imager. Dead THP-1 cells were measured using a Caspase 3/7 reagent (green). (A) Quantification of the percentage of live THP-1 tumor targets (CellTrace Far Red+, Caspase 3/7-) normalized to targets alone at the 0-hour time point. Readings were taken every 30 minutes over a 48-hour period. Representative of 3 separate experiments. (B) Representative images (original magnification 34: 2.82 mm/pixel) at 0, 18, and 36 hours showing THP-1 cells (larger red cells) and NK cells (smaller black cells). Arrows point to killing clusters where dying THP-1 cells (yellow) are apparent. (C) Quantification of the percentage of live THP-1 tumor targets at different effector to target ratios (1:1, 2:1 and 5:1).
The CLEC12A TriKE enhances NK cell mediated killing of primary AML blasts and LSCs with minimal off target toxicity.
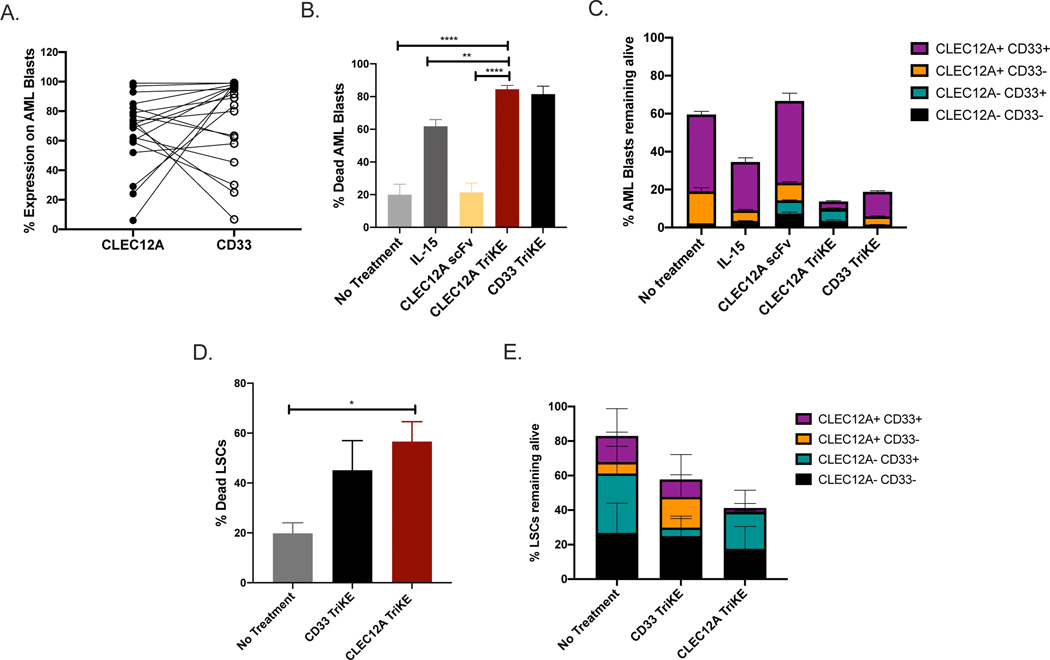
We wanted to test the efficacy of the CLEC12A TriKE against primary AML blasts since AML cells have additional mechanisms of NK cell inhibition in vivo(40). Given that the most common antigen used to target AML is CD33, we compared the effectiveness of the CLEC12A against a TriKE targeting CD33 (CD33 TriKE)(30). First, we analyzed the expression of CLEC12A and CD33 on a series of AML blasts using flow cytometry (Figure 4A and Supplementary Figure 3A). Most AML blasts express both CLEC12A and CD33 indicating that you would be able to eliminate a majority of AML cells by targeting either antigen. Next, we carried out a killing assay with AML blasts and NK cells with TriKE or control treatments (rhIL-15 and CLEC12A scFv) in equimolar amounts (30nM). Both TriKEs were able to induce more target cell death than the control treatments (Figure 4B). This killing was NK cell dependent because there was no change in AML cell viability when treated with the TriKEs or CLEC12A scFv in the absence of NK cells (Supplementary Figure 3B). Additionally, killing of AML blasts was dependent on antigen expression as the AML cells remaining after treatment with the CLEC12A TriKE were CD33 positive and vice versa (Figure 4C). We then conducted a killing assay using bone marrow samples from refractory AML patients to assess targeting of LSCs using the definition of LSC as described by Haubner et al. (Supplementary Figure 4A)(36). There were fewer LSCs remaining after treatment with the TriKEs (Figure 4D and Supplementary Figure 4B) and killing of LSCs was antigen dependent as the cells remaining after treatment with the CLEC12A TriKE were CD33 positive and vice versa (Figure 4E) further highlighting the specificity of TriKE action.
Figure 4. The CLEC12A TriKE induces killing of primary AML Blasts.
(A) Primary AML blasts (SSCh low, CD45int, CD117+, CD14-, CD34+) were assessed for expression of CD33 and CLEC12A using flow cytometry (n=20). (B) Enriched NK cells from healthy donors (n=10) were incubated with primary AML blasts with no treatment, 30nM CLEC12A TriKE, rhIL-15 or anti-CLEC12A scFv to evaluate target cell killing using flow cytometry and a live/dead marker over 48 hours. (C) The proportion of different groups of AML blasts (based on CD33 and CLEC12A expression) were tracked over 48 hours to assess specificity of the CLEC12A TriKE compared to the CD33 TriKE. (D) Enriched NK cells from healthy donors were incubated with bone marrow samples from AML patients (n=5) with no treatment, 30nM of CLEC12A TriKE or CD33 TriKE to evaluate killing of leukemic stem cells (SSCh low, CD45int, CD34+, CD38-) in a 4 hour assay at a 2:1 effector to target ratio. Combined data from the stem cell killing assay showing percentage of LSCs killed over the course of the assay. (E) The proportion of different groups of leukemic stem cells (based on CD33 and CLEC12A expression) were tracked over 4 hours to assess specificity of the CLEC12A TriKE compared to the CD33 TriKE. One-way analysis of variance (ANOVA) with repeated measures was used to calculate differences against the CLEC12A group. Error bars indicate +/− standard error of the mean. Statistical significance was determined as *P , .05, **P , .01, ***P , .001, and ****P , .0001.
To evaluate off-target toxicity, we evaluated the expression of CLEC12A and CD33 on normal bone marrow cells. While all progenitor cells expressed CD33, only the granulocyte monocyte progenitor (GMP) had high CLEC12A expression (Supplementary Figure 5A–B). We also assessed the impact of TriKE treatment on hematopoiesis using cytotoxicity assays with healthy donor bone marrow cells, followed by colony formation assays. HSCs were able to form more colonies after treatment with the CLEC12A TriKE compared to the CD33 TriKE (Supplementary Figure 5C). Overall, the CLEC12A TriKE is able to drive killing of AML blasts and LSCs while sparing healthy hematopoietic progenitor cells.
The CLEC12A TriKE mediates killing of AML cells in vivo.
We used a previously described murine xenograft model, with HL-60 cells containing a luciferase reporter gene, to compare the activity of the CLEC12A TriKE with the CD33 TriKE in vivo(30). NSG mice were injected with HL-60 cells followed 3 days later by infusion of human donor NK cells pre-activated overnight with rhIL-15. Mice then were treated with no drug (+/− NK cells in the control groups), CLEC12A TriKE, or CD33 TriKE and were imaged on day 7, 14 and day 21 to measure tumor burden (Figure 5A). At day 21, the mice treated with CLEC12A TriKE had significantly less tumor burden compared to tumor alone or tumor with NK cells (Figure 5B–C). The response was similar to that seen in the CD33 TriKE treated group. Next, we carried out a second experiment using a patient-derived xenograft model. Patient derived AML blasts were injected in conditioned mice and we waited several weeks until each mouse had at least 1% AML blasts in the bloodstream before starting treatment with NK cells alone or NK cells with CLEC12A TriKE or CD33 TriKE. The mice were treated with TriKE for 2 weeks and then sacrificed at day 21 (Figure 5D). The mice treated with CLEC12A TriKE had a lower proportion of AML blasts in the bone marrow compared to the other treatment groups (Figure 5E). We also observed a greater percentage of NK cells in the bone marrow (Figure 5F) and peripheral blood (Figure 5G) in the mice treated with CLEC12A TriKE compared to the other groups. Collectively, this data indicates that a CLEC12A TriKE based therapy can reduce tumor burden and maintain NK cell numbers in vivo.
Figure 5: The CLEC12A TriKE limits tumor growth in vivo.
(A) Schematic of HL060luc mouse experiment. The model was established by conditioning NSG mice (225cGy) and then injecting HL-60luc cells intravenously (7.5 × 105 cells/mouse). Three days later, 1 × 106 normal human donor NK cells (calculated from a magnetically depleted CD3/CD19 product) activated overnight with 10ng/ml rhIL-15 were infused. The CLEC12A TriKE or CD33 TriKE (20ug) was administered five days a week through the next 3 weeks of the study (15 doses total), a control group received NK cells but no treatment and we had a tumor alone group. (B) Individual mouse photoluminescence after 2-minute exposures on day 7, 14 and 21. (C) Quantification of luminescence from the four treatment groups in the HL-60luc mouse model at day 7, day 14 and day 21 after NK infusion. Each dot represents a different mouse (n=5). (D) Schematic of pdx mouse experiment. The pdx model was established by conditioning NSG SGM3 mice (125cGy) and then injecting primary AML blasts intravenously (2 × 106 cells/mouse). The tumor was allowed to grow until there was atleast 1% AML Blasts in the blood. Then 3 × 106 normal human donor NK cells (calculated from a magnetically depleted CD3/CD19 product) activated overnight with 10ng/ml rhIL-15 were infused. The CLEC12A TriKE or CD33 TriKE (20ug) was administered five days a week through the next 2 weeks of the study (10 doses total), a control group received NK cells but no treatment and we had a tumor alone group. (E) The mice were sacrificed on day 21 and the percentage of AML blasts (CD45int, CD33+) in the bone marrow from the femur was calculated by flow cytometry. Each dot represents a different mouse (n=5). The percentage of NK cells (CD3-, CD56+) was calculated in the (F) bone marrow samples and (G) peripheral blood by flow cytometry. Events were collected over 60 seconds and the number of human NK cell events was calculated. Representative dot plots are shown denoting the number of NK (CD56+CD3−) cell events within the CD45+ gate). One-way analysis of variance (ANOVA) without matched comparisons was used to calculate differences against the CLEC12A group. Error bars denote mean +/− Standard Deviation. Statistical significance was determined as *P , .05, ***P , .001, and ****P , .0001.
Discussion
For improved patient outcomes in AML, we need a potent targeted therapy that can effectively eliminate AML blasts and LSCs while sparing healthy cells. Here, we describe the first preclinical study of a unique TriKE molecule against CLEC12A. Treatment with the CLEC12A TriKE effectively redirects NK cell mediated killing of CLEC12A expressing AML cell lines as well as primary AML blasts and LSCs in an antigen dependent manner. Most significantly, the CLEC12A TriKE stimulates NK cell specific proliferation and enhances NK cell mediated killing of AML cells in vivo. In this study, we used a new and improved TriKE molecule. This latest version of the TriKE includes an anti-CD16 humanized sdAb that has higher affinity for CD16, than the previously used anti-CD16 scFv, and improves folding of the TriKE. The wild-type IL-15 molecule promotes NK cell specific proliferation while the anti-CLEC12A scFv provides directed targeting of AML blasts and LSCs expressing CLEC12A. The specific localization of the IL-15 molecule to NK cells is notable because clinical trials with systemic IL-15 treatment have reported proliferation of CD8 and gamma delta T cells leading to off-target immune effects(25,41).
CLEC12A is highly expressed on AML blasts and its expression is stable throughout the course of the disease irrespective of AML subtype(14,15,42). LSCs are chemotherapy resistant cells that are thought to be the main source of relapse in AML. CLEC12A expression is maintained during generation of LSCs and when LSCs undergo further differentiation. LSC frequency also independently predicts overall survival(43). Van Rhenen et al. reported CLEC12A+ CD34+ CD38− cells isolated from AML patients can produce leukemia in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, but CLEC12A is negative on normal CD34+ CD38− HSCs derived from bone marrow(14). This is in contrast with other antigens like CD33 or CD123 that are expressed by healthy HSCs. We were able to confirm this in our study as well. While CD33 is expressed by all progenitor cells, CLEC12A is only expressed by a subset of granulocyte macrophage progenitors and HSCs are still able to make colonies after treatment with the CLEC12A TriKE limiting the impact of this immunotherapeutic approach on normal hematopoiesis. Additionally, some activated NK cells express CD33 which could lead to fratricide(44). This might explain why there are fewer NK cells in the mice treated with CD33 TriKE compared to those treated with CLEC12A TriKE.
Targeted immunotherapy has proven to be effective and blinatumomab (BLINCYTO), an anti-CD3, anti-CD19 bispecific T cell engager (BiTE) is approved by the FDA for the treatment of patients with relapsed/refractory B-cell precursor ALL. In a phase 3 clinical trial, blinatumomab improved overall survival compared to chemotherapy. However, adverse events were reported in 99% of patients in the blinatumomab group with serious adverse events including cytokine release syndrome and neurotoxicity reported in 62% of patients(45). Recent preclinical studies have demonstrated the efficacy of an anti-CLEC12A/anti-CD3 bispecific antibody(18,46). Given the better safety profile of NK cell-based therapies compared to T cell therapies, a TriKE based treatment offers a valuable alternative to BiTEs that could be used alone or in combination with adoptive NK cell therapy. Even though targeted immunotherapy has been shown to be effective in the case of CAR T cell therapy, the emergence of antigen-negative or antigen-low tumor cells can lead to treatment escape. About 12–24% of patients treated with CAR-based therapies for B-cell malignancies, 30% for patients with CLL and 45% among patients with non-Hodgkin’s lymphoma develop tumor escape due to changes in antigen levels leading to relapse(47–49). We observe this is our study as well where escape variants in the AML blasts and LSCs remaining after TriKE treatment are antigen negative. This requires additional strategies such as a combinatorial targeting approach or activity at lower antigen levels to prevent disease relapse(50). In order to address this, we are developing dual targeting engagers for NK cell immunotherapy.
The current standard of care for AML patients is chemotherapy and even though most patients initially respond, up to 50% of patients relapse. A TriKE based treatment, specifically one that targets CLEC12A, could become an attractive targeted therapeutic option for AML. We are currently dosing patients with a CD33-Targeted TriKE (NCT03214666) but given the safety profile of CLEC12A and the fact that its expression profile is stable throughout diagnosis, treatment and relapse on leukemic blasts and LSCs, CLEC12A should be considered a highly potent and reliable alternative to CD33. As an added benefit, Myelodysplastic Syndrome (MDS) blasts and LSCs also express CLEC12A and about a third of high risk MDS patients progress to AML(51). These factors make the CLEC12A TriKE a compelling option for the treatment of AML to stimulate endogenous NK cells in low risk patients or in combination with adoptive NK cells in high risk patients.
Supplementary Material
Acknowledgements
We would like to acknowledge the Translational Therapy Laboratory, Flow Cytometry, and Imaging cores at the University of Minnesota for their services. This work was supported in part by NCI P01 CA111412 (JSM, BRB, DAV, MF), NCI P01 65493 (JSM, BRB), R35 CA197292 (JSM), P30 CA077598 (JSM, MF), R01 HL56067 (BRB), Minnesota Masonic Charities and the Killebrew-Thompson Memorial Fund. We would also like to thank Xianzheng Zhou, at New York Medical College, for use of his HL-60luc cells.
Financial Support: This work was supported in part by DoD CA150085 (MF), NCI P01 CA111412 (MF and JSM), P01 CA65493 (MF and JSM), and R35 CA197292 (MF and JSM). The TriKE™ work was also supported in part by funding provided by GT Biopharma, Inc (MF, DAV and JSM). The University of Minnesota has licensed the technology covered in this work to GT Biopharma. All conflicts have been declared and managed in accordance with the University’s conflict management plan.
Footnotes
Conflict of Interest Statement: MF, JSM and DAV consult for and hold stock options in GT Biopharma, a company which may commercially benefit from the results of this research project. These interests have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policy.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020. Jan 1;70(1):7–30. Available from: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Tamamyan G, Kadia T, Ravandi F, Borthakur G, Cortes J, Jabbour E, et al. Frontline treatment of acute myeloid leukemia in adults. Crit Rev Oncol Hematol [Internet]. 2016/12/11. 2017. Feb;110:20–34. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28109402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. J Hematol Oncol [Internet]. 2017. April 18;10(1):93. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28420416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol [Internet]. 2019;12(1):100. Available from: 10.1186/s13045-019-0774-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griessinger E, Anjos-Afonso F, Pizzitola I, Rouault-Pierre K, Vargaftig J, Taussig D, et al. A niche-like culture system allowing the maintenance of primary human acute myeloid leukemia-initiating cells: a new tool to decipher their chemoresistance and self-renewal mechanisms. Stem Cells Transl Med [Internet]. 2014/02/03. 2014. Apr;3(4):520–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24493855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollyea DA, Gutman JA, Gore L, Smith CA, Jordan CT. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica [Internet]. 2014. August;99(8):1277–84. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25082785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shang Y, Zhou F. Current Advances in Immunotherapy for Acute Leukemia: An Overview of Antibody, Chimeric Antigen Receptor, Immune Checkpoint, and Natural Killer. Front Oncol. 2019;9(September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter RB, Medeiros BC, Gardner KM, Orlowski KF, Gallegos L, Scott BL, et al. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: a phase I/II study. Haematologica [Internet]. 2013/10/18. 2014. Jan;99(1):54–9. Available from: https://pubmed.ncbi.nlm.nih.gov/24142996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein EM, Walter RB, Erba HP, Fathi AT, Advani AS, Lancet JE, et al. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood [Internet]. 2017/12/01. 2018. Jan 25;131(4):387–96. Available from: https://pubmed.ncbi.nlm.nih.gov/29196412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol [Internet]. 2014/07/06. 2014. Aug;15(9):986–96. Available from: https://pubmed.ncbi.nlm.nih.gov/25008258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricart AD. Antibody-Drug Conjugates of Calicheamicin Derivative: Gemtuzumab Ozogamicin and Inotuzumab Ozogamicin. Clin Cancer Res [Internet]. 2011. October 15;17(20):6417 LP – 6427. Available from: http://clincancerres.aacrjournals.org/content/17/20/6417.abstract [DOI] [PubMed] [Google Scholar]
- 12.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O’Connor J, et al. Phase III Randomized Multicenter Study of a Humanized Anti-CD33 Monoclonal Antibody, Lintuzumab, in Combination With Chemotherapy, Versus Chemotherapy Alone in Patients With Refractory or First-Relapsed Acute Myeloid Leukemia. J Clin Oncol [Internet]. 2005. Jun 20;23(18):4110–6. Available from: 10.1200/JCO.2005.09.133 [DOI] [PubMed] [Google Scholar]
- 13.Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R, et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs [Internet]. 2012;30(3):1121–31. Available from: 10.1007/s10637-011-9670-0 [DOI] [PubMed] [Google Scholar]
- 14.van Rhenen A, Dongen GAMS Van, Rombouts EJ, Feller N, Moshaver B, Walsum MS, et al. The novel AML stem cell – associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659–66. [DOI] [PubMed] [Google Scholar]
- 15.Larsen HØ, Roug AS, Just T, Brown GD, Hokland P. Expression of the hMICL in acute myeloid leukemia—a highly reliable disease marker at diagnosis and during follow-up. Cytom Part B Clin Cytom [Internet]. 2012. January 1;82B(1):3–8. Available from: 10.1002/cyto.b.20614 [DOI] [PubMed] [Google Scholar]
- 16.Van Rhenen A, Dongen GAMS Van, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. discrimination between normal and leukemic stem cells The novel AML stem cell – associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659–66. [DOI] [PubMed] [Google Scholar]
- 17.Morsink LM, Walter RB, Ossenkoppele GJ. Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev [Internet]. 2019;34:26–33. Available from: 10.1016/j.blre.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 18.van Loo PF, Hangalapura BN, Thordardottir S, Gibbins JD, Veninga H, Hendriks LJA, et al. MCLA-117, a CLEC12AxCD3 bispecific antibody targeting a leukaemic stem cell antigen, induces T cell-mediated AML blast lysis. Expert Opin Biol Ther [Internet]. 2019;19(7):721–33. Available from: 10.1080/14712598.2019.1623200 [DOI] [PubMed] [Google Scholar]
- 19.Jiang YP, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, et al. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018;2(14):1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laborda E, Mazagova M, Shao S, Wang X, Quirino H, Woods AK, et al. Development of a chimeric antigen receptor targeting c-type lectin-like molecule-1 for human acute myeloid leukemia. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol [Internet]. 2008;9(5):503–10. Available from: 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 22.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene [Internet]. 2008;27(45):5932–43. Available from: 10.1038/onc.2008.267 [DOI] [PubMed] [Google Scholar]
- 23.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol [Internet]. 2006;6(12):940–52. Available from: 10.1038/nri1983 [DOI] [PubMed] [Google Scholar]
- 24.Miller JS, Soignier Y, Panoskaltsis-mortari A, Mcnearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–8. [DOI] [PubMed] [Google Scholar]
- 25.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood [Internet]. 2018. June 7;131(23):2515 LP – 2527. Available from: http://www.bloodjournal.org/content/131/23/2515.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Björklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin Cancer Res [Internet]. 2018 Apr 15;24(8):1834 LP – 1844. Available from: http://clincancerres.aacrjournals.org/content/24/8/1834.abstract [DOI] [PubMed] [Google Scholar]
- 27.Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a Phase I clinical study. Immunotherapy [Internet]. 2009. September 1;1(5):753–64. Available from: 10.2217/imt.09.47 [DOI] [PubMed] [Google Scholar]
- 28.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood [Internet]. 2010/03/16. 2010 May 27;115(21):4293–301. Available from: https://pubmed.ncbi.nlm.nih.gov/20233969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu E, Marin D, Banerjee P, MacApinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin Cancer Res [Internet]. 2016/02/04. 2016. Jul 15;22(14):3440–50. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26847056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felices M, Kodal B, Hinderlie P, Kaminski MF, Cooley S, Weisdorf DJ, et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv [Internet]. 2019. March 26;3(6):897–907. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30890546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarhan D, Brandt L, Felices M, Guldevall K, Lenvik T, Hinderlie P, et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv [Internet]. 2018. June 26;2(12):1459–69. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29941459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felices M, Lenvik TR, Kodal B, Lenvik AJ, Hinderlie P, Bendzick LE, et al. Potent Cytolytic Activity and Specific IL15 Delivery in a Second-Generation Trispecific Killer Engager. Cancer Immunol Res [Internet]. 2020 Jul 13; Available from: http://cancerimmunolres.aacrjournals.org/content/early/2020/08/05/2326-6066.CIR-19-0837.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem. 2009;284(5):3273–84. [DOI] [PubMed] [Google Scholar]
- 35.Behar G, Sibéril S, Groulet A, Chames P, Pugnière M, Boix C, et al. Isolation and characterization of anti-FcγRIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Eng Des Sel [Internet]. 2007. December 11;21(1):1–10. Available from: 10.1093/protein/gzm064 [DOI] [PubMed] [Google Scholar]
- 36.Haubner S, Perna F, Köhnke T, Schmidt C, Berman S, Augsberger C, et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia [Internet]. 2019;33(1):64–74. Available from: 10.1038/s41375-018-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moga E, Cantó E, Vidal S, Juarez C, Sierra J, Briones J. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Exp Hematol. 2011;39(11):1064–71. [DOI] [PubMed] [Google Scholar]
- 38.Rosario M, Liu B, Kong L, Collins LI, Schneider SE, Chen X, et al. The IL-15-Based ALT-803 Complex Enhances FcγRIIIa-Triggered NK Cell Responses and In Vivo Clearance of B Cell Lymphomas. Clin Cancer Res [Internet]. 2015/09/30. 2016. Feb 1;22(3):596–608. Available from: https://pubmed.ncbi.nlm.nih.gov/26423796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khaznadar Z, Henry G, Setterblad N, Agaugue S, Raffoux E, Boissel N, et al. Acute myeloid leukemia impairs natural killer cells through the formation of a deficient cytotoxic immunological synapse. Eur J Immunol. 2014;44(10):3068–80. [DOI] [PubMed] [Google Scholar]
- 40.Lion E, Willemen Y, Berneman ZN, Van Tendeloo VFI, Smits ELJ. Natural killer cell immune escape in acute myeloid leukemia. Leukemia [Internet]. 2012;26(9):2019–26. Available from: 10.1038/leu.2012.87 [DOI] [PubMed] [Google Scholar]
- 41.Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res. 2018;24(7):1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker ABH, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res [Internet]. 2004;64(22):8443–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15548716%5Cnhttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15548716 [DOI] [PubMed] [Google Scholar]
- 43.Zeijlemaker W, Grob T, Meijer R, Hanekamp D, Kelder A, Carbaat-Ham JC, et al. CD34+CD38− leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia [Internet]. 2019;33(5):1102–12. Available from: 10.1038/s41375-018-0326-3 [DOI] [PubMed] [Google Scholar]
- 44.Eckel AM, Cherian S, Miller V, Soma L. CD33 expression on natural killer cells is a potential confounder for residual disease detection in acute myeloid leukemia by flow cytometry. Cytom Part B - Clin Cytom. 2019;(April):1–5. [DOI] [PubMed] [Google Scholar]
- 45.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood [Internet]. 2016/12/01. 2017. Feb 2;129(5):609–18. Available from: https://pubmed.ncbi.nlm.nih.gov/27908880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med [Internet]. 2018. December 1;380(1):45–56. Available from: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies [Internet]. Vol. 10, Frontiers in Immunology. 2019. p. 2664. Available from: 10.3389/fimmu.2019.02664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med [Internet]. 2017/12/10. 2017. Dec 28;377(26):2531–44. Available from: https://pubmed.ncbi.nlm.nih.gov/29226797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, et al. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell [Internet]. 2017. October 9;32(4):506–519.e5. Available from: https://pubmed.ncbi.nlm.nih.gov/29017060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toft-Petersen M, Nederby L, Kjeldsen E, Kerndrup GB, Brown GD, Hokland P, et al. Unravelling the relevance of CLEC12A as a cancer stem cell marker in myelodysplastic syndrome. Br J Haematol. 2016;175(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.