Abstract
Patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) have a two- to threefold greater risk of developing venous as well as arterial thrombotic events. Although such thrombotic events are more commonly seen during phases of active AAV, they are also recognized to occur during AAV in remission. Endothelial injury is a key pathogenic event in AAV. Endothelial injury can be caused by neutrophil activation and release of thrombogenic tissue factor into the circulation. Neutrophil activation further results in the formation of neutrophil extracellular traps (NETs). NETs contribute to thrombosis by expressing tissue factor. NETs have also been detected in cutaneous thrombi from patients with AAV induced by hydralazine. Activated neutrophils in AAV patients release thrombogenic microparticles loaded with tissue factor which further enhances clotting of blood. Antiphospholipid antibodies (APLs) have been detected in up to a third of AAV and might also be induced by drugs such as cocaine adulterated with levamisole and propylthiouracil, which are known to trigger AAV. Such APLs further drive the thrombosis in AAV. Once thrombogenesis occurs, the homeostatic mechanisms resulting in clot dissolution are further impaired in AAV due to anti-plasminogen antibodies. The ongoing pandemic of coronavirus disease 2019 (COVID-19) is associated with endothelial injury and NETosis, mechanisms which are in common with AAV. Reports of new-onset AAV following COVID-19 have been described in the literature, and there could be shared mechanisms driving these processes that require further evaluation.
Keywords: Anti-neutrophil cytoplasmic antibody-associated vasculitis, Antiphospholipid antibodies, Deep vein thrombosis, Embolism and thrombosis, Neutrophil extracellular traps, Vascular endothelium
The association of anti-neutrophil cytoplasmic antibody (ANCA) and ANCA-associated vasculitis with thrombosis
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) refers to a group of systemic diseases pathogenetically linked by small vessel vasculitis, presenting with myriad clinical features involving the lungs, kidneys, peripheral nerves, and other internal organs [1]. When AAV was first recognized, they were associated with early mortality in nearly 90% patients [2]. Over the past three decades, the recognition of cyclophosphamide and rituximab as potent therapies for remission induction in AAV has markedly improved the outlook, with long-term survival in nearly 90% patients [2, 3]. Longer survival in patients with AAV has brought into focus the impact of comorbid conditions on these individuals [4, 5]. Increasing attention has focused on the higher risk of venous and arterial thromboses in AAV.
The risk of venous thrombotic events [deep venous thrombosis (DVT), pulmonary thromboembolism (PTE)] as well as arterial thrombotic events (ATE) leading on to cardiovascular disease (CVD) or coronary artery disease (CAD) is increased in patients with AAV, summarized in Table 1 [6–11]. These findings have been observed across population-based observational cohorts as well as in patients recruited for participation in clinical trials of AAV. Some studies have reported a greater risk of thrombotic events in those with renal involvement due to AAV [8, 9]. This risk might be driven in part by the loss of anti-thrombotic factors in urine of patients with renal involvement, although this is more common with nephrotic syndrome rather than with the glomerulonephritis usually seen in AAV [12]. While most studies report an increased risk of venous thromboembolism (VTE) during active disease and early on during the course of AAV, studies have reported increased thrombogenic potential in peripheral blood of AAV in remission also [13].
Table 1.
Risk of thrombotic events in patients with ANCA vasculitis
| Reference number, location | Type of study | n | Key findings |
|---|---|---|---|
| [6] Germany | Retrospective cohort | 105 | - 13 VTE episodes (2 fatal due to PTE) |
| [7] North America | Population-based cohort | 58 |
- Over 6.5-year median follow-up, HR (95% CI) for CVD adjusted for age, gender, and time of recruitment to the cohort was 3.15 (1.51–6.57). HR increased when traditional CVD risk factors were adjusted for (HR 4.62, 95% CI 1.87–11.42) - HR for VTE was 3.26 [6.25 for DVT and 1.33 for PTE; increased HR for VTE and PTE not statistically significant]. Highest risk for CVD in the first 2 years following diagnosis |
| [8] USA, Netherlands | Clinical trial of rituximab in AAV | 197 |
- VTE in 8.1% individuals. Occurred early [median 1.5 (range 1–2.75) months following enrollment] - Factors associated with VTE risk on multivariable-adjusted analyses: • Cardiac involvement (HR 21.84, 95% CI 2.56–185.81) • Pulmonary hemorrhage (HR 3.91, 95% CI 1.45–10.52) • Urinary red cell casts (HR 16.46, 95% CI 3.61–75.08) • Anti-PR3 antibody positive AAV (HR 9.12, 95% CI 1.16–71.84) |
| [9] Europe | Clinical trials of AAV by the EUVAS | 417 |
-VTE in 9.8% - Factors associated with VTE risk on multivariable-adjusted analyses: • Increasing CRP levels • Cutaneous or gastrointestinal involvement • Impaired renal function at baseline |
| [10] UK | Retrospective cohort | 204 |
- Follow-up 1088 person-years - Incidence of ATE of 2.67 [CAD 1.56, stroke 1.1] per 100 person-years and VTE of 1.47 [DVT 0.83, PTE 0.64] per 100 person-years - AAV had 15 times higher risk of CAD, 11 times greater risk of stroke and 20 times greater risk of VTE versus the general population - The risk of vascular thrombotic events was highest in the first year following diagnosis - ATE (but not VTE) associated with more risk of mortality |
| [11] Russia | Retrospective cohort | 357 |
- VTE in 8.4% patients - Most occurred in the first year following diagnosis |
95% CI, 95% confidence intervals; AAV, ANCA-associated vasculitis; ANCA, anti-neutrophil cytoplasmic antibody; ATE, arterial thrombotic events; CAD, coronary artery disease; CRP, C-reactive protein; CVD, Cardiovascular disease; DVT, deep venous thrombosis; EUVAS, European Vasculitis Society; HR, hazard ratio; PR3, proteinase 3; PTE, pulmonary thromboembolism; VTE, venous thrombotic events
A systematic review of observational studies confirmed the increased risk of CVD events [risk ratio (RR) of 1.65, 95% CI 1.23–2.22] in AAV [14]. Another systemic review reported an incidence of VTE in 12.4% (95% CI 8.8–17.2) AAV patients at a mean follow-up of 5.2 years, pooled across 21 studies. Of these VTE, 63.4% were DVT and 26.3% PTE. Repeated episodes of VTE were reported in 10% patients. On meta-regression, greater risk of VTE was seen with anti-myeloperoxidase (anti-MPO) ANCA but not anti-PR3 ANCA. Increased baseline disease activity measured by the Birmingham Vasculitis Activity Score (BVAS) and renal involvement portended greater risk of VTE. A longer duration of follow-up also associated with increased VTE [15].
Venous thromboses might be presenting features of AAV [16, 17]. The presence of ANCA also increases the risk of thrombosis in other vasculitides such as Behcet’s disease and thromboangiitis obliterans [18, 19]. Understanding the pathogenic factors driving the heightened risk of arterial and venous thromboses in AAV may help identify newer therapeutic approaches to ameliorate such risk. In this article, we overview the mechanisms underlying thrombosis in AAV.
Search strategy
The literature search was conducted in line with recommendations for comprehensive and systematic bibliographic searches for narrative reviews [20]. The Scopus database and MEDLINE/PubMed were searched through using the terms “thrombosis” and “ANCA vasculitis” on March 3, 2021. We retrieved 246 documents. These references were manually searched to identify relevant original articles and case reports discussing the epidemiological and pathophysiological aspects of thrombosis in AAV. Reference lists of notable review articles were also screened to identify additional relevant articles. Forty-one such articles were retrieved and reviewed in context. Cross-references to these articles were searched for clarification wherever the same was required. Additional articles were also selected based in the prior knowledge of the authors. The information was synthesized under the headings described below.
Neutrophil extracellular traps associate with both disease activity and thrombosis in AAV
Neutrophil activation has been recognized as a key pathogenic feature of AAV [21]. Neutrophils in AAV, particularly when they are primed by inflammatory cytokines like tumor necrosis factor alpha (TNF), increasingly express proteinase-3 (PR-3) and myeloperoxidase (MPO) granules on their surface. These granules are recognized by anti-PR3 or anti-MPO antibodies and result in neutrophil degranulation [21]. In the past decade, the generation of neutrophil extracellular traps (NETs) following de-condensation of chromatin has been recognized as a key pathological feature in renal biopsies from patients with glomerulonephritis associated with AAV [22]. These NETs serve as a persistent source of autoantigens, i.e., proteinase-3 and myeloperoxidase, enabling their presentation to myeloid dendritic cells [23, 24]. Increased NETosis has been identified in patients with active AAV compared to inactive AAV [25]. NETosis has been identified as a pathological feature in drug-induced AAV triggered by exposure to cocaine [26] and hydralazine [27]. NETs have been identified in post-mortem venous thrombi of patients with AAV. Histone citrullination is a key step driving the generation of NETs by causing unfolding of chromatin. NETs were more abundant in post-mortem examination of venous thrombi derived from AAV as opposed to those from patients with sepsis or other causes of pulmonary thromboembolism, and these NETS also demonstrated increased histone citrullination [28]. Thus, NETs might play a role in the thrombosis associated with AAV [29].
Endothelial cell injury and triggering of coagulation cascade in AAV
The endothelial lining is critical towards homeostasis that prevents circulating blood from clotting. The intact endothelial lining separates circulating blood from tissue factor (TF) [30]. The damaged endothelium releases von Willebrand factor (VWF), which in turn initiates platelet activation and thrombosis [31]. Exposure to tissue factor further initiates activation of clotting cascade via factor VIIa. In inflammatory states, circulating tissue factor may be further expressed on monocytes, platelets, and possibly neutrophils and eosinophils in disease states. The cellular sources of TF also release this into the circulation in the form of microparticles containing tissue factor [30]. The measurement of tissue factor pathway inhibitor, which is an anticoagulant response of the body following the activation of tissue factor, might indicate activation of tissue factor pathway (and thereby thrombosis) [32]. The activation of circulating factors like factor VIII further drives the coagulation cascade [33]. The cleavage of prothrombin to thrombin by factor Xa is a critical step driving the conversion of fibrinogen of fibrin, which forms the bulk of the clot. The prothrombin fragments generated during this step therefore indicate cleavage of prothrombin to thrombin. Higher levels of plasma fibrinogen denote greater thrombogenic potential [34]. The endogenous thrombin generation potential of plasma indicates potential for thrombogenesis [35]. Clot formation is normally followed by fibrinolysis, driven by conversion of plasminogen to plasmin by the enzyme tissue plasminogen activator (tPA) in the presence of fibrin [36]. Fibrinolysis is indicated by circulating levels of D-dimer [37].
Anti-endothelial cell antibodies (AECA) have been identified in various disease states, including in AAV. Circulating AECA were detectable in 10/11 patients with propylthiouracil-induced AAV during active disease but in only 3/10 patients from the same cohort during remission. Interestingly, such AECA were not detectable in individuals with propylthiouracil-induced ANCA without clinical features of vasculitis or in healthy controls, suggesting that they might have a role in driving AAV in this situation [38]. In another study involving 6 patients with GPA who were positive for anti-PR3 ANCA, AECA were not detected. However, anti-PR3 ANCA from the serum of such individuals was able to bind in vitro to human umbilical vein endothelial cells (HUVEC). Such binding was noted despite the lack of expression of proteinase-3 on HUVEC, suggesting interaction of the anti-proteinase 3 antibodies with other antigenic targets in the HUVEC. When endothelial cells were lysed from the HUVEC culture and treated with anti-proteinase 3 antibodies, no such binding was observed. This denoted that the antigens on the endothelial cells which were bound to by anti-proteinase 3 antibodies were only expressed during the native conformation of endothelial cells [39]. Thus, the binding of ANCA to endothelial cells has the potential to cause endothelial injury, which might activate the coagulation cascade. In another experiment, in vitro exposure of HUVEC to neutrophils primed by anti-proteinase 3 antibodies and TNF induced endothelial injury and cell death, which was ameliorated by inhibition of myeloperoxidase [40]. These studies indicate the potential for endothelial injury in patients with AAV, both due to ANCA and other circulating antibodies such as AECA, which might be the first step triggering thrombogenesis in these individuals.
The activation of coagulation cascade is detectable in patients with AAV even without clinically apparent thrombotic events. A study compared 21 patients with renal AAV with 20 control subjects with mild renal injury and another 20 with more severe renal injury without AAV. The levels of prothrombin fragments in plasma as well as D-dimer were higher in patients with active AAV than those in remission. These parameters negatively correlated with the estimated glomerular filtration rate. Furthermore, in both active AAV and AAV in remission, the levels of factor VIII activity, VWF antigen, as well as VWF activity were elevated. When compared with disease controls, plasma prothrombin fragments, D-dimer, fibrinogen, VWF antigen, and VWF activity were elevated in AAV compared to those controls with either mild or severe renal disease. Factor VIII activity was elevated in individuals with AAV compared to those with mild renal disease only [41].
Patients with AAV in remission also have increased thrombotic risk. Increased endogenous thrombin generation potential in platelet poor plasma was noted in 27 patients with AAV in remission when compared with 36 healthy controls. In the same cohort, elevated factor VIII activity and increased levels of tissue factor pathway inhibitor were noted in AAV in remission than in healthy controls, suggesting pro-thrombotic potential in AAV in remission also. On follow-up, four individuals from this cohort developed VTE, despite persistent remission during VTE in 3/4 individuals [13].
Aberrant tissue factor activation is one of the mechanisms driving thrombosis in AAV. Neutrophils from patients with AAV have been found to express tissue factor as well as release microparticles containing tissue factor. Furthermore, the NETs generated from such neutrophils have been found to contain tissue factor. Increased circulating levels of tissue factor–containing neutrophil microparticles and higher levels of circulating deoxyribonucleic acid (DNA, which indicates ongoing aberrant NETosis, have been found in active but not in those with inactive AAV. When neutrophils isolated from healthy individuals are treated in vitro with either serum from AAV patients of immunoglobulin G fraction derived from such serum, they are activated to form NETs which express tissue factor, as well as release tissue factor–containing microparticles. Such microparticles resulted in elevated levels of thrombin–antithrombin complex in serum, indicating the activation of the coagulation cascade [42]. In another study, the tissue factor activity in circulating microparticles in 12 patients with AAV with VTE was elevated when compared with 29 patients with AAV without VTE and 70 healthy controls. However, the levels of microparticles containing tissue factor did not differ between AAV patients with active disease or remission. Tissue factor expression on leukocytes is associated with significantly increased hazard of VTE both in patients with active AAV as well as in remission. This study had serial samples of AAV patients enrolled in the cohort, and the investigators were able to identify increased levels of tissue factor–containing microparticles in plasma in the months preceding VTE [43]. Thus, tissue factor–expressing microparticles derived from neutrophils appear to influence VTE risk in active AAV as well as AAV in remission.
Defective fibrinolytic activity might aid clot persistence in AAV
The fibrinolytic activity is also defective in patients with AAV. Anti-plasminogen antibodies have also been detected in AAV and associate with increased risk of VTE in AAV patients in remission [43]. While anti-proteinase 3 antibody is well recognized in AAV, another antibody commonly found in such individuals recognizes the protein derived from the complementary strand of the ribonucleic acid encoding proteinase 3 (anti-cPR3 antibody). This antibody binds with plasminogen and, in vitro, delays the process of fibrinolysis, resulting in persistence of the clot [44]. A study identified elevated levels of anti-plasminogen antibodies in patients with anti-PR3 (but not anti-MPO AAV) when compared with healthy individuals or those with DVT without AAV. In this particular study, five out of nine patients who developed future VTE had anti-plasminogen antibodies along with anti-PR3 antibodies [44].
Antiphospholipid antibodies associate with thrombotic risk in AAV
Antiphospholipid antibodies (APL) include anticardiolipin antibodies (ACLA), antibodies to beta-2 glycoprotein 1, and lupus anticoagulant (LAC). Individuals with APLs or with antiphospholipid antibody syndrome have a greater risk of thrombotic events. The prevalence of antiphospholipid antibodies in AAV is variable. In a large North American cohort of patients with multiple autoimmune diseases, ACLA were detectable in 3.8% patients with AAV [45]. In another cohort of 116 AAV patients from the UK, persistent LAC or ACLA or a diagnosis of concomitant antiphospholipid antibody syndrome was noted in nearly a third of patients. Such patients accrued greater vascular damage in the longer term when compared to other patients in the cohort [46]. Another Korean cohort of 138 AAV patients reported APLs in 13% patients. Patients with persistently detectable APLs in the context of AAV had 2.9 times greater hazard of developing venous thrombotic events than those without [47]. APLs have also been identified in patients with AAV or ANCA triggered by cocaine/levamisole [48] or propylthiouracil [49]. Thus, APLs might be a contributory factor towards thrombosis in AAV. Increasingly, NETosis is being recognized to play a role in the pathogenesis of antiphospholipid antibody syndrome [50, 51], and this might form a common link between APLs and AAV.
Hypertrophic pachymeningitis, AAV, and cortical venous sinus thrombosis
A rare manifestation of AAV is hypertrophic pachymeningitis. This disorder might be associated with the development of cortical venous sinus thromboses. The mechanism driving such thrombosis is unclear. It has been proposed that vascular inflammation in the region adjoining the area of hypertrophic pachymeningitis might drive such thrombosis [52–54].
Thrombosis in drug-induced AAV
Various drugs are known to induce circulating ANCA as well as AAV [55]. Cocaine adulterated with levamisole (cocaine/levamisole)-induced vasculitis is characterized by retiform purpura and cutaneous necrotic lesions with demonstrable vascular thrombi in skin biopsies [56–58]. Lupus anticoagulant positivity has been identified in nearly one-half patients, and IgM ACLA have been identified in about two-thirds of patients with cocaine-/levamisole-induced AAV. The presence of APLs might be one of the mechanisms driving vascular thrombosis in this setting [48]. Propylthiouracil is another drug associated with AAV. Cutaneous necrosis accompanied by vascular thromboses, cerebral infarcts, as well as DVT has been reported in such patients. ACLA and LAC have also been detected in such individuals [59, 60]. Hydralazine is another cause of drug-induced AAV. Cutaneous vascular thromboses have been identified in hydralazine-induced AAV. Demonstrable NETs in such thrombi possibly indicate a role for NETosis in driving vascular thromboses in this setting [27].
Infections triggering thrombosis and endothelial activation and AAV
Patients with AAV on immunosuppressive therapy have a greater predisposition to develop opportunistic infections such as with cytomegalovirus. Cytomegalovirus infection directly causes endotheliitis by replicating in the endothelial cells, which might trigger vascular thrombosis resulting in DVT and PTE [61]. Infective endocarditis is another trigger of endothelial injury which might be associated with detectable anti-neutrophilic cytoplasmic antibodies, particularly to proteinase-3 [62, 63]. Such presentations might mimic AAV and, however, need to be carefully distinguished to avoid immunosuppressive therapy which might result in flare-up of bacterial endocarditis.
Immunothrombosis in the context of COVID-19 and AAV: is there a relationship?
In the context of the ongoing coronavirus disease 19 (COVID-19) pandemic, thrombotic manifestations occurring as a consequence of endotheliitis have been well recognized [64]. One of the mechanisms driving such thrombosis in COVID-19 is the activation of neutrophils resulting in the formation of NETs [65, 66]. Case reports of AAV occurring after diagnosis of COVID-19 have also been reported in the literature [67–72]. Although demonstrable vascular thromboses could not be identified in any of these reports, it is known that vascular thromboses might follow diagnosis of AAV. Therefore, it is possible that the mechanisms driving immunothrombosis might be similar to those driving the induction of AAV in these individuals (Fig. 1).
Fig. 1.
Immunothrombosis in COVID-19 and ANCA-associated vasculitis: shared mechanisms
Conclusion
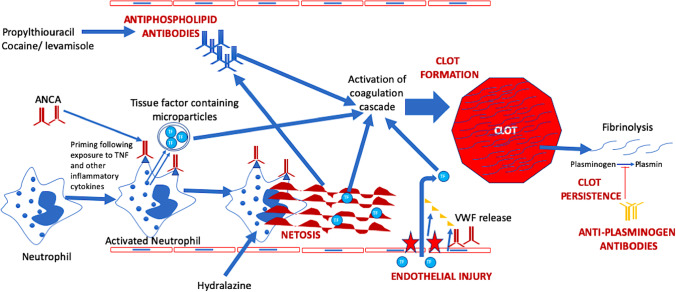
Increased risk of venous as well as arterial thrombosis has been seen in patients with AAV, both during active disease as well as during periods of quiescence. The circulatory milieu in patients with AAV is pro-thrombotic. Endothelial injury, a key pathogenic feature of AAV, also aids thrombogenesis. The presence of antiphospholipid antibodies further contributes towards thrombotic risk in a subset of AAV, particularly in secondary AAV following exposure to cocaine/levamisole. The presence of anti-plasminogen antibodies further retards the dissolution of formed clots in AAV, thereby perpetuating the persistence of clot. Platelets are known to play a role in thrombogenesis and elevated platelet counts as well as increased platelet–lymphocyte ratio associate with disease activity in AAV [73, 74]. However, there is little direct evidence to link platelets with increased thrombotic risk in AAV. Figure 2 summarizes the mechanisms driving thrombosis in the setting of AAV. Understanding the mechanisms of thrombogenesis in AAV might aid clinical management of these patients. However, clinical guidance for managing thrombosis in AAV is sparse. The authors opine that in active AAV, disease activity should be controlled with immunosuppressive agents, with anticoagulation administered until clot dissolution has been documented. In quiescent AAV, pro-thrombotic milieu is more likely to be the cause of any untriggered thrombotic event, in which case a search for APLs should be conducted. Such patients might possibly require a longer duration of anticoagulation. Data to guide the role of antiplatelet agents in this setting is sparse.
Fig. 2.
Mechanisms driving thrombosis in ANCA-associated vasculitis. ANCA, anti-neutrophil cytoplasmic antibody; TF, tissue factor; VWF, von Willebrand factor
Abbreviations
- AAV
ANCA-associated vasculitis
- ACLA
Anticardiolipin antibodies
- AECA
Anti-endothelial cell antibodies
- ANCA
Anti-neutrophil cytoplasmic antibody
- APLs
Antiphospholipid antibodies
- ATE
Arterial thrombotic events
- cPR3
Complementary Proteinase-3
- CAD
Coronary artery disease
- CRP
C-reactive protein
- COVID-19
Coronavirus disease 2019
- CVD
Cardiovascular disease
- DVT
Deep venous thrombosis
- EUVAS
European Vasculitis Society
- GPA
Granulomatosis with polyangiitis
- HR
Hazard ratio
- HUVEC
Human umbilical vein endothelial cells
- LAC
Lupus anticoagulant
- MPA
Microscopic polyangiitis
- MPO
Myeloperoxidase
- NETs
Neutrophil extracellular traps
- PE
Pulmonary embolism
- PTE
Pulmonary thromboembolism
- PR3
Proteinase-3
- RAVE
Rituximab in AAV
- RR
Risk ratio
- TF
Tissue factor
- TNF
Tumor necrosis factor alpha
- tPA
Tissue plasminogen activator
- VWF
Von Willebrand factor
- VTE
Venous thromboembolism
Author contributions
• Conception and design of the study — DPM and AYG; acquisition and analysis and interpretation of data – DPM, KNT, OZ, and AYG.
• Drafting the article — DPM and KNT; revising the article critically for important intellectual content — OZ and AYG
• Final approval of the version to be submitted — DPM, KNT, AYG and OZ
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved — DPM, KNT, AYG, and OZ
Funding
SGPGIMS Intramural Research Grant (Ref. No. PGI/DIR/RC/656/2020) to Durga Prasanna Misra for research on ANCA-associated vasculitis.
Declarations
Disclosures
None.
Disclaimer
All the figures used in the paper are original and not copied from elsewhere.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Misra DP, Naidu G, Agarwal V, Sharma A. Vasculitis research: current trends and future perspectives. Int J Rheum Dis. 2019;22(Suppl 1):10–20. doi: 10.1111/1756-185x.13370. [DOI] [PubMed] [Google Scholar]
- 3.Misra DP, Naidu GSRSNK, Sharma A. Recent advances in the management of antineutrophil cytoplasmic antibody-associated vasculitis. Indian J Rheumatol. 2019;14:218–228. doi: 10.4103/injr.injr_141_19. [DOI] [Google Scholar]
- 4.Ahmed S, Gasparyan AY, Zimba O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int. 2021;41:243–256. doi: 10.1007/s00296-020-04764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra DP, Shenoy SN. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int. 2017;37:151–167. doi: 10.1007/s00296-016-3435-1. [DOI] [PubMed] [Google Scholar]
- 6.Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken) 2006;55:146–149. doi: 10.1002/art.21704. [DOI] [PubMed] [Google Scholar]
- 7.Berti A, Matteson EL, Crowson CS, Specks U, Cornec D. The incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2019;46:1243. doi: 10.3899/jrheum.181351. [DOI] [PubMed] [Google Scholar]
- 8.Kronbichler A, Leierer J, Shin JI, et al. Association of pulmonary hemorrhage, positive proteinase 3, and urinary red blood cell casts with venous thromboembolism in antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheumatol. 2019;71:1888–1893. doi: 10.1002/art.41017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronbichler A, Leierer J, Leierer G, et al. Clinical associations with venous thromboembolism in anti-neutrophil cytoplasm antibody-associated vasculitides. Rheumatology (Oxford) 2017;56:704–708. doi: 10.1093/rheumatology/kew465. [DOI] [PubMed] [Google Scholar]
- 10.Kang A, Antonelou M, Wong NL, et al. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2019;46:285–293. doi: 10.3899/jrheum.170896. [DOI] [PubMed] [Google Scholar]
- 11.Novikov P, Makarov E, Moiseev S, Meshkov A, Strizhakov L. Venous thromboembolic events in systemic vasculitis. Ann Rheum Dis. 2015;74:e27. doi: 10.1136/annrheumdis-2014-206849. [DOI] [PubMed] [Google Scholar]
- 12.Al-Azzawi HF, Obi OC, Safi J, Song M. Nephrotic syndrome-induced thromboembolism in adults. Int J Crit Illn Inj Sci. 2016;6:85–88. doi: 10.4103/2229-5151.183019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilhorst M, Winckers K, Wilde B, Van Oerle R, Ten Cate H, Tervaert JWC. Patients with antineutrophil cytoplasmic antibodies associated vasculitis in remission are hypercoagulable. J Rheumatol. 2013;40:2042–2046. doi: 10.3899/jrheum.130200. [DOI] [PubMed] [Google Scholar]
- 14.Houben E, Penne EL, Voskuyl AE, et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology (Oxford) 2018;57:555–562. doi: 10.1093/rheumatology/kex338. [DOI] [PubMed] [Google Scholar]
- 15.Hansrivijit P, Trongtorsak A, Gadhiya KP, et al. Incidence and risk factors of venous thromboembolism in ANCA-associated vasculitis: a metaanalysis and metaregression. Clin Rheumatol. 2021 doi: 10.1007/s10067-021-05589-8. [DOI] [PubMed] [Google Scholar]
- 16.Ino Y, Baba A, Shinozaki M, et al. A case of ANCA-associated vasculitis with total thrombotic occlusion of the inferior vena cava. Respiration and Circulation. 2002;50:101–104. [Google Scholar]
- 17.Pumerantz AW, Stout BJ, Tracy CL. Granulomatosis with polyangiitis presenting as acute compartment syndrome. J Clin Rheumatol. 2016;22:225–228. doi: 10.1097/RHU.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Dai Y, Lai J, Fan Y. Study about correlation of anti-neutrophil cytoplasmic antibodies and anticardiolipin antibodies with thromboangiitis obliterans. Vascular. 2013;21:363–368. doi: 10.1177/1708538113478742. [DOI] [PubMed] [Google Scholar]
- 19.Kim MK, Kwon HC, Song JJ, Park YB, Lee SW. Antineutrophil cytoplasmic antibody positivity is associated with vascular involvement in Behçet’s disease. Yonsei Med J. 2021;62:149–158. doi: 10.3349/ymj.2021.62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–1417. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 21.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol. 2019;15:91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 22.Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panda R, Krieger T, Hopf L, et al. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front Immunol. 2017;8:439. doi: 10.3389/fimmu.2017.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa D, Shida H, Tomaru U, et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol. 2014;25:990–997. doi: 10.1681/asn.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Söderberg D, Kurz T, Motamedi A, Hellmark T, Eriksson P, Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 2015;54:2085–2094. doi: 10.1093/rheumatology/kev217. [DOI] [PubMed] [Google Scholar]
- 26.Lood C, Hughes GC. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford) 2017;56:638–643. doi: 10.1093/rheumatology/kew256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magro CM, Momtahen S, Harp J. The distinctive histopathology of hydralazine-associated ANCA positive vasculitis: in vivo demonstration of NETosis. Eur J Dermatol. 2017;27:91–92. doi: 10.1684/ejd.2016.2881. [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa D, Tomaru U, Yamamoto C, Jodo S, Ishizu A (2012) Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front Immunol 3:333. 10.3389/fimmu.2012.00333 [DOI] [PMC free article] [PubMed]
- 29.Barnado A, Crofford LJ, Oates JC. At the bedside: neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol. 2016;99:265–278. doi: 10.1189/jlb.5BT0615-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: which? where? when? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nightingale T, Cutler D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. J Thromb Haemost: JTH. 2013;11(Suppl 1):192–201. doi: 10.1111/jth.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mast Alan E. Tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2016;36:9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavin SI. Factor VIII: structure and function in blood clotting. Am J Hematol. 1984;16:297–306. doi: 10.1002/ajh.2830160312. [DOI] [PubMed] [Google Scholar]
- 34.Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler Thromb Vasc Biol. 2017;37:e13–e21. doi: 10.1161/ATVBAHA.117.308564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chantarangkul V, Clerici M, Bressi C, Tripodi A. Standardization of the endogenous thrombin potential measurement: how to minimize the effect of residual platelets in stored plasma. Br J Haematol. 2004;124:355–357. doi: 10.1046/j.1365-2141.2003.04771.x. [DOI] [PubMed] [Google Scholar]
- 36.Brandt JT. Plasminogen and tissue-type plasminogen activator deficiency as risk factors for thromboembolic disease. Arch Pathol Lab Med. 2002;126:1376–1381. doi: 10.5858/2002-126-1376-PATTPA. [DOI] [PubMed] [Google Scholar]
- 37.Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845%JBlood. [DOI] [PubMed] [Google Scholar]
- 38.Yu F, Zhao MH, Zhang YK, Zhang Y, Wang HY. Anti-endothelial cell antibodies (AECA) in patients with propylthiouracil (PTU)-induced ANCA positive vasculitis are associated with disease activity. Clin Exp Immunol. 2005;139:569–574. doi: 10.1111/j.1365-2249.2005.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Bandt M, Meyer O, Dacosta L, Elbim C, Pasquier C. Anti-proteinase-3 (PR3) antibodies (C-ANCA) recognize various targets on the human umbilical vein endothelial cell (HUVEC) membrane. Clin Exp Immunol. 1999;115:362–368. doi: 10.1046/j.1365-2249.1999.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonelou M, Michaëlsson E, Evans RDR, et al. Therapeutic myeloperoxidase inhibition attenuates neutrophil activation, anca-mediated endothelial damage, and crescentic GN. J Am Soc Nephrol. 2020;31:350–364. doi: 10.1681/ASN.2019060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmela A, Ekstrand A, Joutsi-Korhonen L, Räisänen-Sokolowski A, Lassila R. Activation of endothelium, coagulation and fibrinolysis is enhanced and associates with renal anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2015;30:i53–i59. doi: 10.1093/ndt/gfu379. [DOI] [PubMed] [Google Scholar]
- 42.Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73:1854–1863. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 43.Mendoza CE, Brant EJ, McDermott ML, et al. Elevated microparticle tissue factor activity differentiates patients with venous thromboembolism in anti-neutrophil cytoplasmic autoantibody vasculitis. Kidney Int Rep. 2019;4:1617–1629. doi: 10.1016/j.ekir.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bautz DJ, Preston GA, Lionaki S, et al. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J Am Soc Nephrol. 2008;19:2421–2429. doi: 10.1681/ASN.2008030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkel PA, Chang Y, Pierangeli SS, Convery K, Harris EN, Polisson RP. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576–583. doi: 10.1016/s0002-9343(96)00335-x. [DOI] [PubMed] [Google Scholar]
- 46.Jordan N, D'Cruz DP. Association of lupus anticoagulant with long-term damage accrual in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken) 2016;68:711–715. doi: 10.1002/acr.22723. [DOI] [PubMed] [Google Scholar]
- 47.Yoo J, Ahn SS, Jung SM, Song JJ, Park YB, Lee SW. Persistent antiphospholipid antibodies are associated with thrombotic events in ANCA-associated vasculitis: a retrospective monocentric study. Nefrologia. 2019;39:395–401. doi: 10.1016/j.nefro.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7–10. doi: 10.1007/s11239-012-0711-0. [DOI] [PubMed] [Google Scholar]
- 49.Gaburri PD, Chebli JM, Attalla A, et al. Colonic ulcers in propylthiouracil induced vasculitis with secondary antiphospholipid syndrome. Postgrad Med J. 2005;81:338–340. doi: 10.1136/pgmj.2004.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng H, Yalavarthi S, Kanthi Y, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol. 2017;69:655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo Y, Yalavarthi S, Gockman K, et al. Anti-neutrophil extracellular trap antibodies and impaired neutrophil extracellular trap Degradation in antiphospholipid syndrome. Arthritis Rheumatol. 2020;72:2130–2135. doi: 10.1002/art.41460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Zhang W, Jing J, et al. The clinical and imaging features of hypertrophic pachymeningitis: a clinical analysis on 22 patients. Neurol Sci. 2019;40:269–274. doi: 10.1007/s10072-018-3619-4. [DOI] [PubMed] [Google Scholar]
- 53.Di Stefano V, Dono F, De Angelis MV, Onofrj M (2019) Hypertrophic pachymeningitis and cerebral venous thrombosis in myeloperoxidase-ANCA associated vasculitis. BMJ Case Rep 12:bcr-2018-226780. 10.1136/bcr-2018-226780 [DOI] [PMC free article] [PubMed]
- 54.Kuribayashi T, Manabe Y, Fujiwara S, Omote Y, Narai H, Abe K. Combined hypertrophic pachymeningitis and cerebral venous thrombosis in a case of granulomatosis with polyangiitis. Case Rep Neurol. 2019;11:252–255. doi: 10.1159/000502284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misra D, Patro P, Sharma A. Drug-induced vasculitis. Indian. J Rheumatol. 2019;14:3–9. doi: 10.4103/0973-3698.272156. [DOI] [Google Scholar]
- 56.Jenkins J, Babu K, Hsu-Hung E, Robinson-Bostom L, Kroumpouzos G. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:e14–e16. doi: 10.1016/j.jaad.2010.09.778. [DOI] [PubMed] [Google Scholar]
- 57.Pérez MRG, Ortiz-González VL, Betancourt M, Mercado R. Cocaine-induced vasculitis: is this a new trend? Open Access Rheumatol. 2013;5:77–80. doi: 10.2147/OARRR.S51524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veronese FV, Dode RSO, Friderichs M et al (2016) Cocaine/levamisole-induced systemic vasculitis with retiform purpura and pauci-immune glomerulonephritis. Braz J Med Biol Res 49:e5244. 10.1590/1414-431X20165244 [DOI] [PMC free article] [PubMed]
- 59.Ohta K, Shimizu M, Yokoyama T, et al. Analysis of MPO-ANCA subtypes in a patient with propylthiouracil-induced vasculitis with multiple complications. Clin Nephrol. 2007;68:315–321. doi: 10.5414/cnp68315. [DOI] [PubMed] [Google Scholar]
- 60.Antonucci A, Bardazzi F, Iozzo I, Patrizi A. Necrotizing vasculitis in a patient affected by autoimmune hyperthyroidism treated with propylthiouracil. Dermatol Ther. 2010;23:S41–S43. doi: 10.1111/j.1529-8019.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 61.Wolf G, Porth J, Stahl RA (2001) Thrombosis associated with cytomegalovirus infection in patients with ANCA-positive vasculitis. Am J Kidney Dis 38:E27. 10.1053/ajkd.2001.29576 [DOI] [PubMed]
- 62.Chirinos JA, Corrales-Medina VF, Garcia S, Lichtstein DM, Bisno AL, Chakko S. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 63.Misra DP, Chowdhury AC, Edavalath S, Aggarwal A, Kumar S, Agarwal V. Endocarditis: the great mimic of rheumatic diseases. Trop Doct. 2016 doi: 10.1177/0049475515624031[inpress]. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed S, Zimba O, Gasparyan AY. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin Rheumatol. 2020;39:2529–2543. doi: 10.1007/s10067-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakazawa D, Ishizu A (2020) Immunothrombosis in severe COVID-19. EBioMedicine 59:102942. 10.1016/j.ebiom.2020.102942 [DOI] [PMC free article] [PubMed]
- 67.Uppal NN, Kello N, Shah HH, et al. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5:2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jalalzadeh M, Valencia-Manrique JC, Boma N, Chaudhari A, Chaudhari S. Antineutrophil cytoplasmic antibody-associated glomerulonephritis in a case of scleroderma after recent diagnosis with COVID-19. Cureus. 2021;13:e12485. doi: 10.7759/cureus.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martín Navarro JA, Cintra Cabrera M, Lucca Proccacini F, et al. More difficult still: treating severe rapidly progressive glomerulonephritis in the context of COVID-19 pneumonia. Nefrologia. 2021 doi: 10.1016/j.nefro.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powell WT, Campbell JA, Ross F, Peña Jiménez P, Rudzinski ER, Dickerson JA. Acute ANCA vasculitis and asymptomatic COVID-19. Pediatrics. 2021 doi: 10.1542/peds.2020-033092. [DOI] [PubMed] [Google Scholar]
- 71.Selvaraj V, Moustafa A, Dapaah-Afriyie K, Birkenbach MP (2021) COVID-19-induced granulomatosis with polyangiitis. BMJ Case Rep 14:e242142. 10.1136/bcr-2021-242142 [DOI] [PMC free article] [PubMed]
- 72.Ahmed S, Zimba O, Gasparyan AY (2021) COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol 1–9. 10.1007/s10067-021-05691-x [DOI] [PMC free article] [PubMed]
- 73.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Misra DP, Agarwal V. Innate immune cells in the pathogenesis of primary systemic vasculitis. Rheumatol Int. 2016;36:169–182. doi: 10.1007/s00296-015-3367-1. [DOI] [PubMed] [Google Scholar]