Abstract
Background
Ventilator-associated pneumonia (VAP) is an important diagnosis in critical care. VAP research is complicated by the lack of agreed diagnostic criteria and reference standard test criteria. Our aim was to review which reference standard tests are used to evaluate novel index tests for suspected VAP.
Methods
We conducted a comprehensive search using electronic databases and hand reference checks. The Cochrane Library, MEDLINE, CINHAL, EMBASE, and web of science were searched from 2008 until November 2018. All terms related to VAP diagnostics in the intensive treatment unit were used to conduct the search. We adopted a checklist from the critical appraisal skills programme checklist for diagnostic studies to assess the quality of the included studies.
Results
We identified 2441 records, of which 178 were selected for full-text review. Following methodological examination and quality assessment, 44 studies were included in narrative data synthesis. Thirty-two (72.7%) studies utilised a sole microbiological reference standard; the remaining 12 studies utilised a composite reference standard, nine of which included a mandatory microbiological criterion. Histopathological criteria were optional in four studies but mandatory in none.
Conclusions
Nearly all reference standards for VAP used in diagnostic test research required some microbiological confirmation of infection, with BAL culture being the most common reference standard used.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01560-0.
Keywords: Ventilator-associated pneumonia, Diagnostics, Intensive care, Critical care
Take home message
This comprehensive systematic review assesses the reference standard tests used to evaluate novel index tests for suspected VAP in ICU over 10 years period (2008 until 2018) and included high-quality studies with low risk of bias.
BAL culture is the most common reference standard used for VAP diagnostic in ICU and almost all reference standards required some microbiological confirmation of infection.
Background
Ventilator-associated pneumonia (VAP) refers to inflammation of the lung parenchyma caused by infectious agents acquired specifically while receiving invasive mechanical ventilation [1, 2]. VAP is a preventable nosocomial complication which potentially contributes to avoidable mortality and morbidity [3, 4]. Therefore, it is considered a clinically and epidemiologically important measure of the quality of care [5, 6]. It contributes to additional resource consumption, adding time and expense to an intensive care stay, accounting for a large proportion of all antibiotic prescriptions [7]. VAP is considered to be responsible for an additional cost of approximately $40,000 per episode in the US [8, 9] and around £9000 in the UK [10]. The contribution of an episode of VAP to mortality is difficult to definitively ascertain because of the high number and severity of confounders amongst the at-risk population [4, 11–13]. This attributable mortality has been reported from high to neutral or near-neutral [11–13].
Throughout recent decades investigators have not adopted a fixed set of criteria or a fixed definition for VAP [14]. This lack of a reference standard has led to an inability to make comparisons across study sets and uncertainty about VAP incidence [15]. The incidence of VAP varies widely in different studies depending on the diagnostic criteria used, type of intensive therapy unit (ITU), and patient population [16, 17].
Existing literature reports that the incidence of VAP varies widely between 4.0% and 28.8% of the at-risk population [8, 18–24], with an event rate between 1.4 and 16.5 per 1000 ventilator days [1, 25–27]. As VAP rates have become an important quality indicator, the Centre for Disease Control and the European Centre for Disease Control use their own precise case definitions to identify VAP events [28, 29]. Both definitions return similar VAP rates making them adequate for surveillance purposes and benchmarking of critical care units internationally [1]. However, due to the lack of concordance between these two definitions, they do not make ideal reference standards [1, 28], and further highlight the difficulty in achieving consensus in diagnosing VAP. Microbiological samples, especially quantitative culture of bronchoalveolar lavage (BAL), are considered to be integral to the diagnosis of VAP [30, 31]. However, a systematic review of diagnostic methods in 2008 found that microbiological methods did not contribute to the accuracy of diagnosis over clinical criteria and all respiratory sampling methods were equivalent [32]. The continuing lack of an agreed reference standard hampers research into novel diagnostic methods. The aim of this review was to identify what reference standards have been used in diagnostic evaluation research for VAP.
Methods
The protocol for this review was published in PROSPERO (International Prospective Register of Systematic Reviews) under registration CRD42019125449 [33].
Search strategy
A comprehensive search strategy was developed by one of the authors (BA). The Cochrane Library, PubMed (MEDLINE), CINHAL, EMBASE, and web of science were electronically searched from January 2008 until November 2018. We limited our search to studies published after 2008 following a comprehensive systematic review of diagnostic methods [32]. Medical Subject Headings (MeSH) and search terms were used to interrogate the databases. The 3 concepts used for the searches were VAP, diagnostics, and ITU (for search terms see Additional file 1). No restriction on publication language was applied. In addition, electronic searching of Google and hand searching through an examination of the reference list of the published articles were also used to identify additional publications (an example of MEDLINE search is provided in Additional file 1).
Review strategy
All records were independently reviewed by the lead author (BA) and another author (PM or JG) and disagreement was resolved by a third independent adjudicator (PM or JG). Initially, titles and abstracts review of all records, then full-text reviews were conducted against the inclusion/exclusion criteria. Studies included in the review fulfilled the following criteria: (1) adult ventilated patients of any gender, (2) ITU settings, (3) suspected VAP as defined in this study (after 48 h on the ventilator), (4) focused on the diagnostic procedures of VAP (clinical markers, biomarkers, chest x-ray, chest ultrasound (U/S), lung biopsy, BAL and mini-BAL, protected specimen brush (PSB), blind PSB, Endotracheal Aspirate (ETA)). Studies were excluded from the review if they: (1) were animal studies, (2) included patients under the age of 18 years old, (3) focused on the surveillance of VAP, (4) compared the diagnosis of VAP against another illness diagnostic, (5) were feasibility studies, (6) included participants who were already diagnosed with VAP, (7) investigated VAP treatment effectiveness by monitoring biomarkers or other diagnostics, (8) evaluated risk factors to predict VAP, (9) were procedures used to predict the mortality in VAP, (10) were case-controlled studies. All papers that passed the full-text review and those that had some diagnostic technical terms were examined by an ITU clinician (THC) to confirm their clinical relevance to the research question.
Quality assessment and data extraction
A team of 12 reviewers (systematic reviewers, clinicians, methodologists, health economists) from the University of Northumbria, Newcastle University, The Newcastle Upon Tyne Hospitals NHS Foundation Trust, and the University of Edinburgh were involved in the quality assessment and data extraction process. All included papers were quality assessed and the data were extracted by two authors independently. Any disagreement was discussed between both reviewers in the first instance. The further disagreement was resolved by a third reviewer. The quality assessment scoring checklist was adopted from the Critical Appraisal Skills Programme (CASP) checklist for diagnostic studies [34], which is one of the well-recognised methodological quality or risk of bias assessment tools for primary and secondary medical studies [35, 36] and has been used to assess the quality of diagnostic studies in systematic reviews [37–39]. The quality assessment scoring checklist contains 8 questions from the overall 12 questions in the CASP checklist. Questions from section C in the CASP for diagnostics checklist “will the results help locally?” were not included in our scoring as the main aim of the review was not related to the local application of the diagnostic procedures. Studies were assigned a score of ‘1’ for each item of the checklist if they were considered to meet the aspect of this item and ‘0’ if not. A total score for each study was calculated by summing the item scores. The maximum possible final score was 8. Any study that scored ‘0’ for the first or the second question or scored less than ‘5’ out of 8 in total was excluded. According to CASP guide for diagnostic studies, if the answer to question 1 or 2 while critically appraising a study was “no”, then it is not worth continuing. That leaves 6 questions out of the total 8 we used in our quality assessment. Taking in consideration that these questions are equally as important but less important than the first 2 question, we determined that a study must fulfil the quality of at least half of these 6 points (score 3 out of 6) to be consider for the review. Therefore, this threshold was derived through reviewer consensus that studies scoring less than 5 out of 8 were not of sufficient quality to adequately address the research question.
A standardised data extraction form was developed by three authors (AJA, BA, THC) and reviewed by all authors (for quality assessment and data extraction form see Additional file 2). We recorded and present study country of origin, study size, male: female ratio or enrolled participants, index test(s) under investigation, reference standard used to define VAP, and test characteristics. Although test characteristics for the index test are not relevant to the aims of this review, we present them herein because several of the index tests are also used as reference standards. Test characteristics are taken directly from the studies or calculated using data contained within the studies. Where multiple test characteristics are presented in the original paper, we selected those highlighted by the original authors or those which reflect the comparison best, or those which indicate the best performance. Where BAL was conducted, we recorded the details of the lavage procedure.
A narrative data synthesis approach was used to report the results from reviewed studies. Due to the large variation in practice, processes, and reference standards, a meta-analysis of diagnostic accuracy was not conducted.
Results
Studies identified
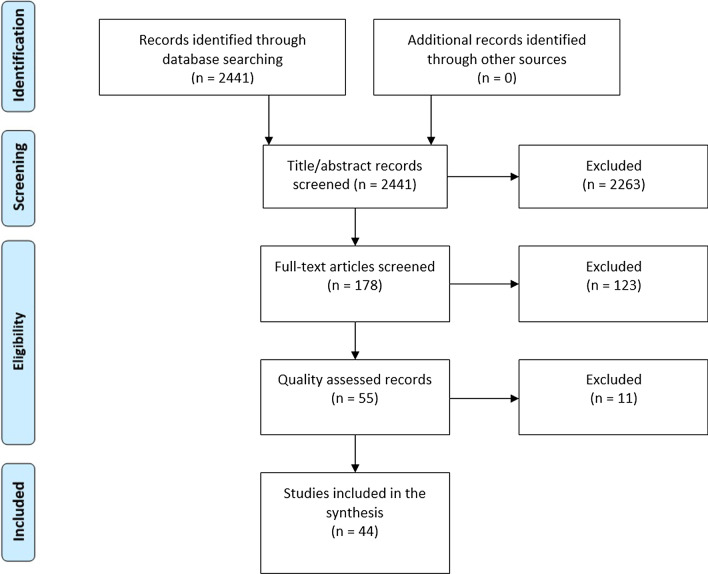
The searches identified a total of 2441 articles. Records that were not published in English were translated to English using Google translator. 2263 articles were excluded on the basis of title and abstract and a further 123 on the basis of full-text screening were excluded as not clinically relevant to the inclusion criteria or meeting at least one of the exclusion criteria, leaving 55 articles for quality assessment (see Fig. 1 for PRISMA flow chart).
Fig. 1.
PRISMA systematic review flow chart.
Adapted from: Moher et al. [96]
Quality assessment and data extraction
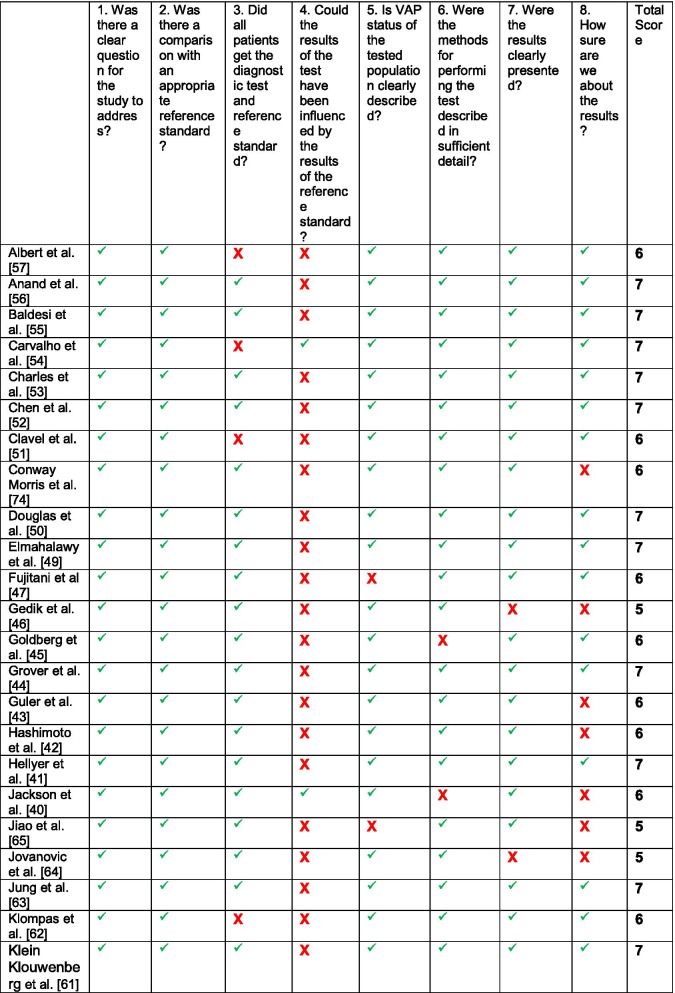
Of the 55 studies examined in the quality assessment stage, 11 studies were excluded due to either scored ‘0’ for the first or the second question or scored less than ‘5’ out of’8’ in total score, leaving 44 studies included in this review [40–83]. All scored were agreed by at least two reviewers and reviewed by the principal investigator (PI). The lowest score assigned to any included study was ‘5’ out of ‘8’. Three studies scored 8/8, 24 studies scored 7/8, 11 studies scored 6/8, and six studies scored 5/8 (see Table 1).
Table 1.
Quality assessment checklist scores
green tick = acceptable quality, red box = unacceptable quality
As expected, all papers suffered from bias in their accuracy estimates of the index test from the use of an imperfect reference standard comparison, a well-known issue with comparative diagnostic accuracy studies [84]. The results of the quality assessment reviews conducted using the form adopted from CASP diagnostic study checklist showed that five papers [51, 54, 57, 62, 82] suffered from verification bias: not all patients received testing by both the index and the reference standard. In 38 papers [41–47, 49–53, 55–59, 61–68, 70, 71, 73–82], the results of the index test could have been influenced by the reference standard result. This means that there was no evidence blinding or the tests being performed independently. The VAP status for all participants in the study was not clearly defined in two papers [47, 65]. The methodology description was not described in detail in three papers [40, 45, 82] and the results of the study were not clearly presented in five papers [46, 64, 67, 69, 75]. There was a lack of certainty regarding the results of the study on 11 occasions [40, 42, 43, 46, 58, 64, 65, 67, 68, 74, 75]. CASP diagnostic study checklist guide was followed in assessing all points.
The disagreement was solved through discussion between both reviewers on 20 papers, and adjudication of a third reviewer in one paper. Forty-three of the studies were cohort studies (38 of which were prospective studies) and one was a secondary analysis of data from a randomised controlled trial (RCT) [57]. All studies were published between 2008 and 2018 with a wide geographical spread; four studies conducted in the UK [41, 44, 72, 74], eight in the USA [40, 45, 47, 50, 56, 62, 67, 75], seven in France [51, 53, 55, 63, 70, 73, 78], five in Netherland [48, 61, 66, 77, 79], three each in both China [52, 65, 68] and Turkey [43, 46, 82], two each in Egypt [49, 76], Brazil [54, 81], and Italy [71, 83], and eight in other countries [42, 57–60, 64, 69, 80]. The median sample size was 180 recruited participants, with the 21 being the lowest [58] and 2080 being the highest number of participants [61] (see Tables 2, 3).
Table 2.
Studies comparing index test to a microbiological reference standard
| References | Study population | Index test | Reference test | Results (95% confidence interval where available) | |||
|---|---|---|---|---|---|---|---|
| Country | n = | Mean age | M:F ratio | ||||
| Albert et al. [57] | Canada | 705 | 58.9 | 2.9 | Gram stain of either ETA or BAL | same sample (ETA or BAL) positive culture result for pathogenic bacteria any quantity |
Sens = 74% Spec = 72% κ = 0.36 (0.31–0.40) |
| Anand et al. [56] | USA | 105 | 51.5 | 1.4 | sTREM-1 ELISA in BAL | BAL culture > 103 CFU/mL |
Sens = 42.1% Spec = 75.6% |
| Baldesi et al. [55] | France | 127 | 62.5 | 2.3 | BAL culture > 104 CFU/mL (uncorrected for dilution factor) | BAL culture > 104 CFU/mL (corrected for dilution factor using plasma/BAL urea) |
Sens = 97.7% Spec = 98% |
| Carvalho et al. [54] | Brazil | 22 | 59.41 | comparison of three sampling techniques (ETA, miniBAL, and BAL) | No mention which is considered index test or reference standard |
Sens = NR Spec = NR |
|
| Clavel et al. [51] | France | 120 | 65 | 3.1 | qPCR from BAL equivalent to > 104 CFU/mL and qPCR from ETA equivalent to > 106 CFU/mL | BAL culture > 104 CFU/mL or ETA culture > 106 CFU/mL |
qPCR from BAL Sens = 89.2% Spec = 97.1% qPCR from ETA Sens = 71.8% Spec = 96.6% |
| Conway Morris et al. [74] | UK | 72 | 57 | 1.5 | BAL IL-1β and IL-8 | BAL culture > 104 CFU/mL |
Sens = 94% Spec = 64% |
| Douglas et al. [50] | USA | 33 | 55 | N/R | Mini BAL automated microscopy | Mini BAL culture > 104 CFU/mL |
Sens = 100% Spec = 97% |
| Elmahalawy et al. [49] | Egypt | 40 | 45.4 | 3.0 | Pentraxin 3 ELISA in BAL | BAL culture > 104 CFU/mL |
Sens = 96.7% Spec = 100% AUROCC = 0.966 (0.985 to 1) |
| Fujitani et al. [47] | USA | 256 | 57.5 | 2.1 | ETA culture | Mini BAL culture > 104 CFU/mL |
Sens = 65.4% Spec = 56.1% κ = 0.22 (0.17–0.27) |
| Gedik et al. [46] | Turkey | 31 | 56 | 1.4 | ETA culture > 105 CFU/mL | Mini BAL culture > 104 CFU/mL | κ = 0.804, p = 0.005 |
| Goldberg et al. [45] | USA | 229 | 49 | 2.0 | Gram stain of BAL | BAL culture > 105 CFU/mL |
Sens = 90% Spec = 67% |
| Guler et al. [43] | Turkey | 50 | 52 | 2.1 | Modified CPIS | BAL culture > 104 CFU/mL |
CPIS > 7 Sens = 80% Spec = 17% CPIS > 6 Sens = 76% Spec = 15% CPIS ≥ 6 Sens = 80.7% Spec = 16.6% CPIS ≥ 5 Sens = 80% Spec = 10% |
| Hashimoto et al. [42] | Japan | 51 | 41 | 1.4 | Semi-quantitative Gram stain or culture of ETA or BAL (not otherwise specified) | ETA culture > 106 CFU/mL |
Gram stain Sens = 95% Spec = 61% κ = 0.59 (0.45–0.72) Culture Sens = 96% Spec = 40% κ = 0.4 (0.25–0.54) |
| Hellyer et al. [41] | UK | 150 | 56.3 | 2.9 | BAL IL-1β and IL-8 | BAL culture > 104 CFU/mL |
Sens = 100% Spec = 44.3% |
| Jackson et al. [40] | USA | 73 | 52 | 3.5 | BAL—unilateral | BAL—bilateral |
Sens = 73% Spec = 82% |
| Jiao et al. [65] | China | 92 | 48 | 1.0 |
Serum PCT Serum IL-6 Serum CRP |
BAL culture > 104 CFU/mL |
PCT Sens = 91% Spec = 71% IL-6 Sens = 58% Spec = 64% CRP Sens = 52% Spec = 74% |
| Jung et al. [63] | France | 57 | 61 | 2.2 | BAL Procalcitonin | BAL culture > 104 CFU/mL |
Sens = 94% Spec = 75% |
| Kneidinger et al. [60] | Austria | 132 | 62 | 2.1 | BAL culture > 104 CFU/mL after 24 h storage at 4 °C and after storage at − 80 °C | BAL culture > 104 CFU/mL after immediate culture |
Immediate culture vs delayed culture (4 °C) Sens = 96.5% Spec = 100% Immediate culture vs delayed culture (− 80 °C) Sens = 50.4% Spec = 100% |
| Kwon et al. [59] | Korea | 64 | 65 | 2.6 | qPCR of BAL fluid or bronchial washings | BAL culture > 104 CFU/mL or bronchial washing > 105 CFU/mL |
Sens = 88.9% Spec = 88.9% |
| Leo et al. [58] | Mexico | 21 | 42 | 2.0 | mini BAL (using cut NG tube) > 104 CFU/mL | BAL culture > 104 CFU/mL |
Sens = 92.8% Spec = 85% |
| Linssen et al. [67] | USA | 117 | N/R | N/R | BAL PCT and CRP | BAL culture > 104 CFU/mL or > 2% cells with intracellular organisms |
PCT AUCROCC = 0.448 CRP AUCROCC = 0.477 |
| Linssen et al. [75] | USA | 282 | 60.3 | 2.1 | Various BAL cytological parameters including total cell count, alveolar macrophages, lymphocytes, neutrophils, eosinophils, mast cells, infected cells; in isolation or in combination | BAL culture > 104 CFU/mL |
AUROCC for various cytological parameters Total cell count = 0.647 Alveolar macrophages = 0.313 Lymphocytes = 0.381 Neutrophils = 0.705 Eosinophils = 0.589 Mast cells = 0.557 Infected cells (%) = 0.904 AUROCC for combinations of cytological parameters Infected cells (%) + PMNs (%) = 0.892 Infected cells (%) + Total cell count = 0.890 |
| Luna et al. [69] | Argentina | 283 | 71.3 | 1.1 | ETA culture > 103 CFU/mL | BAL culture > 104 CFU/mL |
Sens = NR Spec = NR |
| Luyt et al. [70] | France | 41 | 60 | 2.4 | serum PCT 1st day of clinical suspicion of VAP | BAL culture > 104 CFU/mL |
Sens = 72% Spec = 24% |
| Mongodi et al. [73] | France | 99 | 66 | 3.7 | Vplus-Eagram (ventilator-associated pneumonia lung ultrasound score and gram stain of ETA) | BAL culture > 104 CFU/mL |
Sens = 81% Spec = 41% |
| Oudhuis et al. [66] | Netherlands | 207 | 62 | 1.7 | ETA culture > 105 CFU/mL | BAL culture > 104 CFU/mL or > 2% cells with intracellular organisms |
Sens = 65% Spec = 48% |
| Refaat et al. [76] | Egypt | 66 | NR | NR | BAL composite biomarker (CRP, PCT, neutrophils, MIF, sTREM-1, suPAR) | BAL culture > 10 CFU/mL |
Sens = 76% Spec = 62% |
| Schnabel et al. [48] | Netherlands | 100 | 61.3 | 2.3 | VOC in exhaled breath | BAL culture > 104 CFU/mL or > 2% cells with intracellular organisms |
Sens = 75.8% Spec = 73.0% |
| Scholte et al. [77] | Netherlands | 311 | NR | NR |
ETA Gram stain ETA Culture BALF Gram stain |
BAL culture > 104 CFU/mL or > 2% cells with intracellular organisms |
ETA Gram stain Sens = 65% Spec = 72% ETA Culture (any threshold) Sens = 82% Spec = 42% BALF Gram stain (> 0) Sens = 82% Spec = 66% |
| Vanspauwen et al. [79] | Netherlands | 196 | 58.8 | 1.7 | BAL Clara Cell protein 10 ELISA | BAL culture > 104 CFU/mL or > 2% cells with intracellular organisms | AUROCC of 0.586 (0.496–0.676) |
| Yagmurdur et al. [82] | Turkey | 59 | 71 | 0.73 | ETA culture > 104 CFU/mL | BAL culture > 104 CFU/mL |
Sens = 60% Spec = 39% |
| Zagli et al. [83] | Italy | 221 | 56 | 2.7 | Chest Echography and Procalcitonin Pulmonary Infection Score > 5 | ETA culture > 104 CFU/mL |
Sens = 80.5% Spec = 85.2% |
Table 3.
Studies comparing index test to a composite reference standard
| Reference | Study population | Index test | Reference test | Results (95% confidence intervals where available) | |||
|---|---|---|---|---|---|---|---|
| Country | n = | Mean age | M:F ratio | ||||
| Charles et al. [53] | France | 90 | 61.1 | 2.3 | Serum PCT (day of first suspicion of clinical infection) | All of (1) new lung infiltrate on the chest X-ray; (2) ETA culture > 106 CFU/mL; (3) CPIS ≥ 6 points; (4) at least 2 SIRS criteria |
Sens = 65.2% Spec = 83% |
| Chen et al. [52] | China | 49 | 54 | 1.45 |
Serum PCT serum CRP CPIS |
(1) Persistent or new invasive shadows in the lung; (2) At least two below items: temperature more than 38 °C or less than 36 °C; leucocyte count > 10 or < 4 × 10/L; purulent sputum; (3) Any of the item below: bronchoscopic aspiration or sputum specimen bacterial culture +++ or pathogenic bacteria cultured from blood |
CRP Sens = 68.0% Spec = 58.3% PCT Sens = 60.0% Spec = 87.5% CPIS Sens = 72.0% Spec = 75.0% |
| Grover et al. [44] | UK | 91 | 59 | 1.5 | 7 marker score: BAL/blood ratio mTREM-1 and mCD11b, BALF sTREM-1, IL-8 and IL-1b, and serum CRP and IL-6 |
VAP was predefined as CPIS > 5 and positive BALF microbiology. Non-VAP was predefined as CPIS score < 6 and negative microbiology |
Sens = 88.9% Spec = 100% |
| Jovanovic et al. [64] | Serbia | 39 | 47.9 | 5.5 |
Serum sCD14-ST PCT CRP Leucocyte count |
New persistent pulmonary infiltrates (not otherwise explainable) on CXR > 48 h after admission to the ICU, PLUS one systemic and two pulmonary criteria Systemic criteria Fever > 38 °C, white cell count < 4000 WBC/mm3 or > 12,000 WBC/mm3 altered mental status, with no other recognized cause (for adults older than 70 years of age) Pulmonary criteria new onset of purulent sputum (or a change in the character of the sputum, increased respiratory secretions or increased suctioning requirements), worsening gas exchange (desaturations, increased oxygen requirements or increased ventilator demand), new onset or worsening cough, and dyspnoea, tachypnoea, rales or bronchial breath sounds |
sCD14-ST AUROCC = 0.908 PCT AUROCC = 0.863 CRP AUROCC = 0.703 Leucocyte count AUROCC = 0.668 |
| Klein Klouwenberg et al. [61] | Netherlands | 2080 | 62 | 1.6 | CDC surveillance definition (2013) |
Existing local surveillance criteria divided into possible, probable, and definite VAP Possible (CPIS > 6, dubious abnormalities on radiographic examination, semi-quantitative culture from respiratory secretions—ETA or bronchoscopic aspirate) Probable (CPIS > 6, new or progressive infiltrates, consolidation, cavitation or pleural effusion, BAL culture > 104 CFU/mL or PSB culture > 103 CFU/mL OR positive blood culture with pathogen also isolated from airway culture) Definite (CPIS > 6, new or progressive infiltrates, consolidation, cavitation or pleural effusion OR radiographic evidence of lung abscess or empyema, histopathologic evidence of pneumonia (abscess with PMN concentration and positive tissue culture) OR if empyema, positive culture of aspirate)) |
Sens = 22% (for probable or definite reference standard VAP) Spec = 98% |
| Klompas et al. [62] | USA | 459 | 55.3 | 1.5 | Objective algorithm of electronic patient record (precursor of CDC surveillance definition 2013) | CDC definition (2008) |
Sens = 95 Spec = 100 |
| Liu et al. [68] | China | 162 | 61.6 | 1.5 | BAL neutrophil intracellular organisms |
(1) histopathological diagnosis performed within 7 days of bronchoscopy OR (2) BAL culture ≥ 104 CFU/mL, and responded to antibiotic therapy OR (3) rapid cavitation of the lung infiltrate on chest x-ray film or CT scan associated with responded to antibiotic therapy OR (4) positive culture of the pleural effusion, and the same microorganisms isolated from cultures of pleural effusion and lower respiratory tract secretions, and responded to antibiotic therapy OR (5) complete resolution with appropriate antibiotic therapy with no other disease explaining chest radiograph abnormality |
Sens = 94.12% Spec = 88.33% |
| Mauri et al. [71] | Italy | 82 | 59 | 2.6 | BAL fluid pentraxin 3 |
1. New and persistent radiographic infiltrates associated with at least two of the following: a. Internal body temperature > 38 °C, b. White blood cells count > 12,000 or < 4000 cells/mm3 and/or c. Purulent tracheobronchial secretions; AND 2. BAL culture > 104 CFU/mL and/or significant noncontaminant viral load |
Sens = 92% Spec = 60% |
| Medford et al. [72] | UK | 150 | 62.3 | 1.5 | ETA culture > 105 or BAL culture > 104 |
New/progressive CXR infiltrates without other obvious cause in patients mechanically ventilated for more than 4 days in the ICU and at least 2 of the following: temperature ≥ 38 °C or ≤ 35 °C, white cell count ≥ 12 or ≤ 4 × 109/L, purulent tracheobronchial secretions, with increasing oxygen requirements, computed tomography evidence of a rapidly cavitating infiltrate, positive pleural fluid culture and/or histological evidence of neutrophilic alveolitis, bronchiolitis, and consolidation in conjunction with pleural fluid microbiology, CT evidence, and histological evidence |
BAL Sens = 64.1% Spec = 83.0% ETA Sens = 42.6% Spec = 33.7% |
| Textoris et al. [78] | France | 77 | 30.75 | NR | blood Transcriptome DNA microarray analysis (HuSG9 k) | purulent bronchial sputum; body temperature more than 38 °C or less than 36 °C; leukocytes more than 10 × 109/L or less than 4 × 109/L; chest radiograph showing new or progressive infiltrates; BAL culture ≥ 104 CFU/mL, or ETA culture ≥ 106 CFU/mL |
Sens = NR Spec = NR |
| Vernikos et al. [80] | Greece | 54 | 72 | 1.3 |
1) Johansen criteria, 2) Modified CPIS > 6 REF 3) Johansen criteria combined with relative neutrophil count 4) CPIS > 6 combined relative neutrophil count 5) RPDMIa score |
CDC definition (2015) |
Johansen criteria Sens = 85.7% Spec = 73.7% Modified CPIS > 6 Sens = 62.9% Spec = 73.7% Johansen criteria combined with relative neutrophil count (20% cut off) Sens = 67.6% Spec = 81.3% CPIS > 6 combined relative neutrophil count (20% cut off) Sens = 47.1% Spec = 81.3% RPDMI score Sens = 94.3% Spec = 84.2% |
| Waltrick et al. [81] | Brazil | 168 | NR | NR | CDC definition (2013) | CPIS ≥ 7 and miniBAL culture > = 104 CFU/mL |
Sens = 37% Spec = 100% |
aRPDMI score: radiological progression, purulent secretions, duration of mechanical ventilation, immunosuppression
Reference standard
We did not consider enrolment criteria, including objective criteria for suspicion of VAP, to be part of the reference standard. Out of the 44 included studies, 32 studies (72.7%) compared an index test with a sole microbiological reference standard (see Table 2). One of these studies did not define which of the studied tests were considered index and which were considered reference [54]. Of the remaining 31 studies, culture of BAL fluid was most commonly used as the reference test, forming at least part of the reference standard in 26 (83.8%) studies [40, 41, 43, 45, 48, 49, 51, 55–60, 63, 65–67, 69, 70, 73–77, 79, 82]. BAL culture alone was the sole reference standard in 18 (58.1%) out of the 31 studies [40, 41, 43, 45, 49, 55, 56, 58, 60, 63, 65, 69, 70, 73–76, 82]. Where used, the BAL culture threshold for positivity included > 10 CFU/mL [76], > 103 CFU/mL [56], > 104 CFU/mL [40, 41, 43, 49, 55, 58, 60, 63, 65, 69, 70, 73–75, 82], and > 105 CFU/mL [45]. Three studies using BAL culture > 104 CFU/mL added an additional stipulation on BAL culture results: one requiring correction of microbial growth for plasma to BAL urea ratio [55], one requiring that lavage was bilateral [40], and one specifying culture took place immediately after lavage [60]. BAL culture was used in combination with another criterion regarding assessment of BAL on an additional eight occasions out of the 26 using BAL (30.8%); five out of these eight (62.5%) BAL culture or > 2% lavage cells containing intracellular organisms [48, 66, 67, 77, 79], one out of the eight (12.5%) BAL culture or bronchial washings culture (> 105 CFU/mL) [59], and two out of the eight (11.1%) BAL culture or ETA culture (> 106 CFU/mL [51] or no threshold specified [57]. On the remaining five out of 31 (16.1%) occasions where BAL culture was not incorporated into the microbiological reference standard, three studies used mini-BAL culture > 104 CFU/mL [46, 47, 50], one study used ETA culture > 106 CFU/mL [42]m, and one study used ETA culture > 104 CFU/mL [83], as sole reference standards.
Table 3 summarises the remaining 12/44 (27.2%) studies which compared an index test to a composite reference standard or clinical scoring system [44, 52, 53, 61, 62, 64, 68, 71, 72, 78, 80, 81]. An iteration of the CDC VAP definition was used in two out of the 12 studies (16.7%), one using the 2008 iteration for surveillance of VAP [62] and one using the 2015 iteration for surveillance of VAP (80]. One additional study [64] used a composite reference standard almost identical to the 2008 iteration of the CDC VAP surveillance criteria. The 2008 iteration of the CDC surveillance criteria does not include any microbiological assessment of respiratory samples, and non-culture methods or histopathology may supplant microbiological culture in the 2010 and 2015 iterations of the CDC surveillance criteria [28, 62, 85]. For the remaining nine studies; four out of the nine (44.4%) explicitly incorporated the clinical pulmonary infection score (CPIS) into a wider set of criteria [44, 53, 61, 81]. Seven out nine (77.8%) incorporated radiological assessment [52, 53, 64, 68, 71, 72, 78], eight out of nine (88.9%) incorporated additional clinical signs and symptoms [44, 52, 53, 61, 71, 72, 78, 81] either as part of an existing scoring system such as CPIS or de novo, and all nine studies (100%) incorporated some microbiological assessment into the combined reference standard [44, 52, 53, 64, 68, 71, 72, 78, 81]. In seven of those nine studies (77.8%) positive microbiology was a mandatory criterion for VAP diagnosis [44, 52, 53, 64, 71, 78, 81], and in two of those nine (22.2%), positive microbiological cultures were an optional criterion for VAP diagnosis [68, 72]. Amongst all included studies microbiological assessment did not form any part of the mandatory or optional criteria for VAP diagnosis in only two studies [62, 64], diagnosis of VAP included an optional microbiological assessment component in another two studies (i.e. VAP diagnosis could be made without recourse to microbiological assessment) [68, 72], and in 39 studies microbiological assessment was the sole or a mandatory component [40–53, 55–60, 63–71, 73–79, 81–83]. Four studies incorporated an optional histopathologic element into the reference standard [61, 68, 72, 80], but it was mandatory in none.
Out of all 44 included studies, 37 (84%) incorporate BAL in either the reference standard or index test [40–51, 54–60, 63, 65–79, 81, 82]. Twenty two studies (50%) included a precise lavage procedure in the methodology [40, 41, 43, 45, 48, 51, 55–58, 60, 63, 66–69, 71, 72, 74–76, 82]. Of these, 14 described an initial discard of aspirated fluid, considered to be uninformative bronchial fluid [40, 41, 43, 51, 57, 58, 60, 63, 68, 69, 71, 72, 74, 82]. The median volume of instilled fluid used to generate the discarded fluid was 20 mL (range 20 mL to 50 mL). The median total volume of instilled fluid (including that intended for discard) was 150 mL (range 80 mL to 200 mL).
Discussion
To the best of our knowledge, this is the first and most comprehensive systematic review aiming to evaluate the reference standard tests used to evaluate novel index tests for suspected VAP since the publication of Rea-Neto and colleagues systematic review of diagnostic methods in 2008 [32]. We reviewed papers comparing a novel index test against a chosen reference standard to identify what reference standards have been used in diagnostic evaluation research for VAP. To deliver a high-quality systematic review, we excluded papers with a high risk of bias and all papers included in this review fulfil at least 5 out of the 8 criteria we included from the CASP checklist.
The microbiological culture was the sole or a component criterion in the vast majority of studies. Overall, the culture of BAL fluid was the most common reference standard, with the most common growth threshold being > 104 CFU/mL. This was occasionally used in combination with another reference standard, such as the demonstration of BAL cells with intracellular organisms exceeding 2% of the total number of cells. Composite reference standards incorporating a variety of existing clinical scores, existing surveillance definitions, radiological assessments, clinical parameters, and microbiological methods including culture were used in the remaining studies. A large variation in practice, processes, and reference standards were detected, highlighting the inconsistency in the current diagnosis of VAP and making a meta-analysis of diagnostic accuracy challenging. Biological, clinical, and statistical heterogeneity makes comparisons across the different studies difficult and subjective. We display a variable and generally good quality of the papers, and the review provides an indication of what has been and is being done in this area globally with respect to the use of reference standard in the diagnostics of VAP. The line between composite criteria and a sole microbiological criterion was often blurred. Many studies in the sole microbiological criterion group had strict objective clinical and radiological enrolment criteria. Where these criteria are applied pre-enrolment and therefore applied to both index tests and reference standards we have not incorporated them into a description of the reference standard.
A key question in diagnostic accuracy research when reference standards are imperfect is whether the reference standard used to assess novel diagnostics should be ‘more inclusive’ (higher sensitivity, lower specificity) or ‘less inclusive’ (lower sensitivity, higher specificity). Using microbiological criteria alone exhibits good face validity but risks missing cases of ‘true VAP’ or including false positives through contamination (although prior specification of clinically suspected VAP reduces this risk). Importantly, both possibilities are potentially strongly influenced by operator technique/expertise, especially for BAL; this contrasts with diagnostics reliant on blood sampling or imaging. BAL culture was the most common microbiological method found in this review. The use of BAL culture is potentially problematic for several reasons. Firstly, in a recent systematic review, when compared to the reference standard of histopathological examination of lung tissue, BAL culture had a sensitivity of 71.1% and specificity of 79.6% [86] echoing previous findings that microbiological examination does not correlate well with histopathological examination [32]. Secondly, the timing and nature of prior antibiotic therapy may adversely affect sample positivity [87, 88], although this problem is conceivably solved by incorporating a criterion addressing percentage of host cells containing invading organisms, a measure not affected by prior antibiotic therapy [75]. Thirdly, the BAL procedure itself is not standardised, and the requirements for sample collection are not uniform. Whilst this may have little impact on bacterial growth, a fact confirmed by one of the included studies [55], the variety of studies that utilised bronchial discard may plausibly lead to a variety in sensitivity at detecting the causative pathogenic organism. Sole microbiological criteria also risk introducing cases of ‘false VAP’ through contamination [87], although this risk is reduced by using distal or protected specimens. Of relevance, the quality and consistency of BAL procedures are likely to be higher in studies than during routine clinical practice, which could further influence its validity.
Using composite criteria may conceivably address the problem of missing cases of ‘true VAP’, and the number or thresholds of additional criteria is not limited. Additional criteria can be made mandatory to increase specificity or made optional to increase sensitivity. Some studies in this review rely on existing surveillance definitions for VAP or use their own composite standards. The existing surveillance definitions were designed to objectively and reproducibly monitor VAP rates not to identify true VAP in a robustly sensitive and specific manner, although as a quality indicator face validity amongst clinicians is important. Other composite studies incorporated radiological assessments into the reference standard. It has been shown that chest x-ray changes are not considered integral to the diagnosis by many clinicians [89], that the performance characteristics of chest x-ray may not meet the requirements as a diagnostic standard [90–92], and that inter- and intra-observer variability is high in chest x-ray assessment [93, 94]. These issues mean that incorporation of radiology into any novel reference standard should be undertaken with caution. Many studies incorporate clinical signs which plausibly reduces the risk of false positives, and although this makes physiological sense there is minimal evidence to support this. Klompas et al. showed, in the development of the novel CDC VAP surveillance algorithm, that deterioration in oxygenation after a period of stability was associated with clinically important outcomes but the addition of other clinical measures such as abnormal temperature, abnormal white blood cell count, or purulent secretions was not [95]. However, a lack of correlation with clinically important outcomes is not the same as a lack of correlation with a true diagnosis of VAP; this issue is relevant when the decision based on the test relates to a therapy (antibiotic use) rather than prognosis.
No studies relied upon histopathological diagnosis of VAP to confirm the diagnosis. This is not surprising for practical reasons: it cannot be routinely and safely undertaken in all patients with suspected VAP either at the time of the index test or later. Histopathological analysis may also be inaccurate due to sampling artefacts, the lack of representation of a small piece of tissue, and displacement in time from the period of peak infection. It is not possible to provide certainty about the appropriate reference standard in diagnostic evaluation research for VAP following this systematic review, which simply identifies the methods chosen by researchers and confirms the lack of a standardised approach. Researchers must decide whether it is more important to be ‘more inclusive’ or ‘less inclusive’, and future comparisons may wish to employ the strategy deployed by one of the studies in this review [61]: using a graded certainty of VAP from possible to probable to definite using a composite definition.
There are three main limitations to our review. Firstly, in order to be diagnosed with VAP, a patient must be at risk of VAP, and there is no standard definition for patients at risk. For the purposes of this study, we defined those at risk of VAP as those who have undergone more than 48 h of mechanical ventilation. Secondly, many included studies enrolled only patients with suspected VAP, and this means many listed reference standards must be prefixed with “clinically suspected VAP”. This level of clinical suspicion was not systematically collected by us. This is particularly noteworthy in considering the reference standards listed in Table 3. Thirdly, although data extraction for this review was completed before the impact of Coronavirus Disease 2019 (COVID-19), the pandemic nonetheless interfered with the delivery time of this review.
Conclusion
BAL culture with a microbiological growth threshold of > 104 CFU/mL is the commonest reference standard used to examine the utility of a novel index test for VAP amongst patients who are at risk for and clinically suspected of VAP. Composite reference standards were used in approximately 25% of reviewed studies. Nearly all reference standards for VAP identified in this review required some microbiological confirmation of infection. The studies identified in this review highlight the need for a standardised approach to diagnosis VAP which may include the development of a data-driven composite reference standard from large cohort studies.
Supplementary Information
Additional file 1. MEDLINE search example.
Additional file 2. Quality assessment and data extraction form.
Acknowledgements
Not applicable.
Abbreviations
- BAL
Broncho-alveolar lavage
- CDC
Centers for disease control and prevention
- CC-10
Clara cell protein 10
- COVID-19
Coronavirus Disease 2019
- CASP
Critical appraisal skills programme
- CBA
Cytometric bead array
- ETA
Endotracheal aspirates
- ECDC
European centre for disease prevention and control
- GC-tof-MS
Gas chromatography time-of-flight mass spectrometry
- GS
Gram’s stain
- ITU
Intensive therapy unite
- LUS
Lung ultrasound
- MeSH
Medical subject headings
- CPIS
Modified clinical pulmonary infection score
- NHSN/CDC
National healthcare safety network/Center for disease control and prevention
- PCR
Polymerase chain reaction
- PSB
Protected specimen brush
- SQ
Semi quantitative
- PCT
Serum procalcitonin
- sTREM-1
Type 1 soluble triggering receptor expressed on myeloid cells
- U/S
Ultrasound
- VAE
Ventilator-associated events
- VAP
Ventilator-associated pneumonia
- VOCs
Volatile organic compounds
Authors' contributions
BA and AJA designed the review, and BA led the project. All authors contribute and approve the review design and search strategy. BA, PM, and JG conducted the title/abstract screening. The quality assessment and data extraction were conducted by BA, THC, PM, ARA, SG, JS, WSJ, BCL, AW, and MC. The data and results were summarized by BA, AJA, THC, SG, JS, WSJ, BCL, AW, MC, interpreted by THC and BA, reviewed by KD and TSW, and approved by all authors. The manuscript was drafted BA and THC, checked by KD and TSW and read and approved by all authors. All authors read and approved the final manuscript.
Funding
This study was funded by Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), https://carb-x.org, award Number 4500003353. AJA, JS, WJS, CL and AW were supported by the National Institute for Health Research Newcastle In Vitro Diagnostics Co-operative. The views expressed are those of the author(s) and not necessarily those of the National Institute for Health Research, the National Health Services or the Department of Health and Social Care.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Craven TH, Wojcik G, McCoubrey J, Brooks O, Grant E, Keating S, et al. Ventilator-associated pneumonia surveillance using two methods. J Hosp Infect. 2020;104(4):522–528. doi: 10.1016/j.jhin.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Fang X, Mei Q, Fan X, Zhu C, Yang T, Zhang L, et al. Diagnostic value of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in ventilator-associated pneumonia patients. Front Microbiol. 2020;11:599756. doi: 10.3389/fmicb.2020.599756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keyt H, Faverio P, Restrepo MI. Prevention of ventilator-associated pneumonia in the intensive care unit: a review of the clinically relevant recent advancements. Indian J Med Res. 2014;139(6):814–821. [PMC free article] [PubMed] [Google Scholar]
- 4.Steen J, Vansteelandt S, De Bus L, Depuydt P, Gadeyne B, Benoit DD, et al. Attributable mortality of ventilator-associated pneumonia: replicating findings, revisiting methods. Ann Am Thorac Soc. 2020;18(5):830–837. doi: 10.1513/AnnalsATS.202004-385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo SM, Palomeque AC, Li BG. The zero-VAP sophistry and controversies surrounding prevention of ventilator-associated pneumonia. Intensive Care Med. 2020;46(2):368–371. doi: 10.1007/s00134-019-05882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keneally RJ, Peterson TJ, Benjamin JR, Hawkins K, Davison D. Making ventilator associated pneumonia rate a meaningful quality marker. J Intensive Care Med. 2020. 10.1177/0885066620952763. [DOI] [PubMed]
- 7.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–644. doi: 10.1001/jama.1995.03530080055041. [DOI] [PubMed] [Google Scholar]
- 8.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(3):250–256. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 10.Luckraz H, Manga N, Senanayake EL, Abdelaziz M, Gopal S, Charman SC, et al. Cost of treating ventilator-associated pneumonia post cardiac surgery in the National Health Service: Results from a propensity-matched cohort study. J Intensive Care Soc. 2018;19(2):94–100. doi: 10.1177/1751143717740804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94(3):281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 12.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1249–1256. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 13.Bekaert M, Timsit J-F, Vansteelandt S, Depuydt P, Vésin A, Garrouste-Orgeas M, et al. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184(10):1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 14.Ego A, Preiser J-C, Vincent J-L. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147(2):347–355. doi: 10.1378/chest.14-0610. [DOI] [PubMed] [Google Scholar]
- 15.Estella A, Alvarez-Lerma F. Should the diagnosis of ventilator associated pneumonia be improved? Med Intensiva. 2011;35(9):578–582. doi: 10.1016/j.medin.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ali HS, Khan FY, George S, Shaikh N, Al-Ajmi J. Epidemiology and outcome of ventilator-associated pneumonia in a heterogeneous ICU population in Qatar. Biomed Res Int. 2016;2016:8231787. doi: 10.1155/2016/8231787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papazian L, Klompas M, Luyt C-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skrupky LP, McConnell K, Dallas J, Kollef MH. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med. 2012;40(1):281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 19.Hyllienmark P, Gårdlund B, Persson J-O, Ekdahl K. Nosocomial pneumonia in the ICU: a prospective cohort study. Scand J Infect Dis. 2007;39(8):676–682. doi: 10.1080/00365540701225728. [DOI] [PubMed] [Google Scholar]
- 20.Tejerina E, Frutos-Vivar F, Restrepo MI, Anzueto A, Abroug F, Palizas F, et al. Incidence, risk factors, and outcome of ventilator-associated pneumonia. J Crit Care. 2006;21(1):56–65. doi: 10.1016/j.jcrc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129(6):433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kollef MH. Ventilator-associated pneumonia. A multivariate analysis. JAMA. 1993;270(16):1965–1970. doi: 10.1001/jama.1993.03510160083034. [DOI] [PubMed] [Google Scholar]
- 23.Kappstein I, Schulgen G, Beyer U, Geiger K, Schumacher M, Daschner FD. Prolongation of hospital stay and extra costs due to ventilator-associated pneumonia in an intensive care unit. Eur J Clin Microbiol Infect Dis. 1992;11(6):504–508. doi: 10.1007/BF01960804. [DOI] [PubMed] [Google Scholar]
- 24.Rello J, Quintana E, Ausina V, Castella J, Luquin M, Net A, et al. Incidence, etiology, and outcome of nosocomial pneumonia in mechanically ventilated patients. Chest. 1991;100(2):439–444. doi: 10.1378/chest.100.2.439. [DOI] [PubMed] [Google Scholar]
- 25.Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Pollock DA, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39(10):798–816. doi: 10.1016/j.ajic.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Suetens C, Morales I, Savey A, Palomar M, Hiesmayr M, Lepape A, et al. European surveillance of ICU-acquired infections (HELICS-ICU): methods and main results. J Hosp Infect. 2007;65(Suppl 2):171–173. doi: 10.1016/S0195-6701(07)60038-3. [DOI] [PubMed] [Google Scholar]
- 27.De Bus L, Gadeyne B, Steen J, Boelens J, Claeys G, Benoit D, et al. A complete and multifaceted overview of antibiotic use and infection diagnosis in the intensive care unit: results from a prospective four-year registration. Crit Care. 2018;22(1):241. doi: 10.1186/s13054-018-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craven TH, Wojcik G, McCoubrey J, Brooks O, Grant E, Reilly J, et al. Lack of concordance between ECDC and CDC systems for surveillance of ventilator associated pneumonia. Intensive Care Med. 2018;44(2):265–266. doi: 10.1007/s00134-017-4993-8. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Loeches I, Torres A, Rinaudo M, Terraneo S, de Rosa F, Ramirez P, et al. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect. 2015;70(3):213–222. doi: 10.1016/j.jinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Baselski V, Klutts JS, Baselski V, Klutts JS. Quantitative cultures of bronchoscopically obtained specimens should be performed for optimal management of ventilator-associated pneumonia. J Clin Microbiol. 2013;51(3):740–744. doi: 10.1128/JCM.03383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 32.Rea-Neto A, Youssef NCM, Tuche F, Brunkhorst F, Ranieri VM, Reinhart K, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12(2):R56. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Omari B, McMeekin P, Gray J, Aleen J, Dhaliwal K, Walsh T, et al. Protocol for systematic review of studies investigating ventilator-associated pneumonia(VAP) diagnostic procedures in secondary care [Internet]. PROSPERO; CRD42019125449. 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=125449. Cited 24 Nov 2020.
- 34.https://casp-uk.net/wp-content/uploads/2018/01/CASP-Diagnostic-Checklist-2018.pdf [Internet]. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Diagnostic-Checklist-2018.pdf. Cited 24 Nov 2020.
- 35.Ma L-L, Wang Y-Y, Yang Z-H, Huang D, Weng H, Zeng X-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 37.Williams P, Bond CM, Burton C, Murchie P. A systematic review of the use, quality and effects of pelvic examination in primary care for the detection of gynaecological cancer. J Obstet Gynaecol. 2018;38(5):737. doi: 10.1080/01443615.2018.1444410. [DOI] [PubMed] [Google Scholar]
- 38.Gomes PTM, Lima LHL, Bueno MKG, Araújo LA, Souza NM. Autism in Brazil: a systematic review of family challenges and coping strategies. J Pediatr (Rio J) 2015;91(2):111–121. doi: 10.1016/j.jped.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Smith T, Cross J, Poland F, Clay F, Brookes A, Maidment I, et al. Systematic review investigating multi-disciplinary team approaches to screening and early diagnosis of dementia in primary care—what are the positive and negative effects and who should deliver it? Curr Alzheimer Res. 2018;15(1):5–17. doi: 10.2174/1567205014666170908094931. [DOI] [PubMed] [Google Scholar]
- 40.Jackson S-R, Ernst NE, Mueller EW, Butler KL. Utility of bilateral bronchoalveolar lavage for the diagnosis of ventilator-associated pneumonia in critically ill surgical patients. Am J Surg. 2008;195(2):159–163. doi: 10.1016/j.amjsurg.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Hellyer TP, Morris AC, McAuley DF, Walsh TS, Anderson NH, Singh S, et al. Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia. Thorax. 2015;70(1):41–47. doi: 10.1136/thoraxjnl-2014-205766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto S, Shime N. Evaluation of semi-quantitative scoring of Gram staining or semi-quantitative culture for the diagnosis of ventilator-associated pneumonia: a retrospective comparison with quantitative culture. J Intensive Care. 2013;1(1):2. doi: 10.1186/2052-0492-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guler E, Kahveci F, Akalin H, Sinirtas M, Bayram S, Ozcan B. Evaluation of a clinical pulmonary infection score in the diagnosis of ventilator-associated pneumonia. SV. 2012;7(1):32–37. doi: 10.22514/SV71.042012.6. [DOI] [Google Scholar]
- 44.Grover V, Pantelidis P, Soni N, Takata M, Shah PL, Wells AU, et al. A biomarker panel (Bioscore) incorporating monocytic surface and soluble TREM-1 has high discriminative value for ventilator-associated pneumonia: a prospective observational study. PLoS ONE. 2014;9(10):e109686. doi: 10.1371/journal.pone.0109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg AE, Malhotra AK, Riaz OJ, Aboutanos MB, Duane TM, Borchers CT, et al. Predictive value of broncho-alveolar lavage fluid Gram’s stain in the diagnosis of ventilator-associated pneumonia: a prospective study. J Trauma. 2008;65(4):871–6. doi: 10.1097/TA.0b013e31818481e0. [DOI] [PubMed] [Google Scholar]
- 46.Gedik H, Yahyaoğlu M, Fincancı M. The diagnostic accuracy of endotracheal aspiration and mini-bronchoalveolar lavage cultures in the diagnosis of ventilator associated pneumonia. Nobel Med. 2010;6:68–74. [Google Scholar]
- 47.Fujitani S, Cohen-Melamed MH, Tuttle RP, Delgado E, Taira Y, Darby JM. Comparison of semi-quantitative endotracheal aspirates to quantitative non-bronchoscopic bronchoalveolar lavage in diagnosing ventilator-associated pneumonia. Respir Care. 2009;54(11):1453–1461. [PubMed] [Google Scholar]
- 48.Schnabel R, Fijten R, Smolinska A, Dallinga J, Boumans M-L, Stobberingh E, et al. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci Rep. 2015;5:17179. doi: 10.1038/srep17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elmahalawy II, Ammar AS, Fathy WM, Salama AE, Mokhtar WS. Pentraxin 3 as an early marker in diagnosis of ventilator associated pneumonia. Egypt J Chest Dis Tuberc. 2017;66(4):709–712. doi: 10.1016/j.ejcdt.2017.10.004. [DOI] [Google Scholar]
- 50.Douglas IS, Price CS, Overdier KH, Wolken RF, Metzger SW, Hance KR, et al. Rapid automated microscopy for microbiological surveillance of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2015;191(5):566–573. doi: 10.1164/rccm.201408-1468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clavel M, Barraud O, Moucadel V, Meynier F, Karam E, Ploy MC, et al. Molecular quantification of bacteria from respiratory samples in patients with suspected ventilator-associated pneumonia. Clin Microbiol Infect. 2016;22(9):812.e1–812.e7. doi: 10.1016/j.cmi.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Chen C, Yan M, Hu C, Lv X, Zhang H, Chen S. Diagnostic efficacy of serum procalcitonin, C-reactive protein concentration and clinical pulmonary infection score in Ventilator-Associated Pneumonia. Med Sci (Paris). 2018;34((Focus issue F1)):26–32. doi: 10.1051/medsci/201834f105. [DOI] [PubMed] [Google Scholar]
- 53.Charles PE, Kus E, Aho S, Prin S, Doise J-M, Olsson N-O, et al. Serum procalcitonin for the early recognition of nosocomial infection in the critically ill patients: a preliminary report. BMC Infect Dis. 2009;9:49. doi: 10.1186/1471-2334-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Carvalho EM, Massarollo PCB, Levin AS, Isern MRM, Pereira WL, Abdala E, et al. Comparative study of etiological diagnosis of nosocomial pneumonia. Braz J Infect Dis. 2008;12(1):67–74. doi: 10.1590/S1413-86702008000100015. [DOI] [PubMed] [Google Scholar]
- 55.Baldesi O, Michel F, Guervilly C, Embriaco N, Granfond A, La Scola B, et al. Bacterial ventilator-associated pneumonia: bronchoalveolar lavage results are not influenced by dilution. Intensive Care Med. 2009;35(7):1210–1215. doi: 10.1007/s00134-009-1417-4. [DOI] [PubMed] [Google Scholar]
- 56.Anand NJ, Zuick S, Klesney-Tait J, Kollef MH. Diagnostic implications of soluble triggering receptor expressed on myeloid cells-1 in BAL fluid of patients with pulmonary infiltrates in the ICU. Chest. 2009;135(3):641–647. doi: 10.1378/chest.08-1829. [DOI] [PubMed] [Google Scholar]
- 57.Albert M, Friedrich JO, Adhikari NKJ, Day AG, Verdant C, Heyland DK, et al. Utility of Gram stain in the clinical management of suspected ventilator-associated pneumonia. Secondary analysis of a multicenter randomized trial. J Crit Care. 2008;23(1):74–81. doi: 10.1016/j.jcrc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Leo A, Galindo-Galindo J, Folch E, Guerrero A, Bosques F, Mercado R, et al. Comparison of bronchoscopic bronchoalveolar lavage vs blind lavage with a modified nasogastric tube in the etiologic diagnosis of ventilator-associated pneumonia. Med Intensiva. 2008;32(3):115–120. doi: 10.1016/S0210-5691(08)70921-5. [DOI] [PubMed] [Google Scholar]
- 59.Kwon S-J, Jeon T, Seo D, Na M, Choi E-G, Son J-W, et al. Quantitative PCR for etiologic diagnosis of methicillin-resistant Staphylococcus aureus pneumonia in Intensive Care Unit. Tuberc Respir Dis (Seoul) 2012;72(3):293–301. doi: 10.4046/trd.2012.72.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kneidinger N, Warszawska J, Schenk P, Fuhrmann V, Bojic A, Hirschl A, et al. Storage of bronchoalveolar lavage fluid and accuracy of microbiologic diagnostics in the ICU: a prospective observational study. Crit Care. 2013;17(4):R135. doi: 10.1186/cc12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klein Klouwenberg PMC, van Mourik MSM, Ong DSY, Horn J, Schultz MJ, Cremer OL, et al. Electronic implementation of a novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med. 2014;189(8):947–955. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 62.Klompas M, Kleinman K, Platt R. Development of an algorithm for surveillance of ventilator-associated pneumonia with electronic data and comparison of algorithm results with clinician diagnoses. Infect Control Hosp Epidemiol. 2008;29(1):31–37. doi: 10.1086/524332. [DOI] [PubMed] [Google Scholar]
- 63.Jung B, Embriaco N, Roux F, Forel J-M, Demory D, Allardet-Servent J, et al. Microbiogical data, but not procalcitonin improve the accuracy of the clinical pulmonary infection score. Intensive Care Med. 2010;36(5):790–798. doi: 10.1007/s00134-010-1833-5. [DOI] [PubMed] [Google Scholar]
- 64.Jovanovic B, Djuric O, Markovic-Denic L, Isakovic A, Doklestic K, Stankovic S, et al. Prognostic value of presepsin (soluble CD14-subtype) in diagnosis of ventilator-associated pneumonia and sepsis in trauma patients. VSP. 2018;75(10):968–977. doi: 10.2298/VSP161104027J. [DOI] [Google Scholar]
- 65.Jiao J, Wang M, Zhang J, Shen K, Liao X, Zhou X. Procalcitonin as a diagnostic marker of ventilator-associated pneumonia in cardiac surgery patients. Exp Ther Med. 2015;9(3):1051–1057. doi: 10.3892/etm.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oudhuis GJ, Beuving J, Bergmans D, Stobberingh EE, ten Velde G, Linssen CF, et al. Soluble Triggering Receptor Expressed on Myeloid cells-1 in bronchoalveolar lavage fluid is not predictive for ventilator-associated pneumonia. Intensive Care Med. 2009;35(7):1265–1270. doi: 10.1007/s00134-009-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linssen CFM, Bekers O, Drent M, Jacobs JA. C-reactive protein and procalcitonin concentrations in bronchoalveolar lavage fluid as a predictor of ventilator-associated pneumonia. Ann Clin Biochem. 2008;45(Pt 3):293–298. doi: 10.1258/acb.2007.007133. [DOI] [PubMed] [Google Scholar]
- 68.Liu C, Du Z, Zhou Q, Hu B, Li Z, Yu L, et al. Microscopic examination of intracellular organisms in bronchoalveolar lavage fluid for the diagnosis of ventilator-associated pneumonia: a prospective multi-center study. Chin Med J. 2014;127(10):1808–1813. [PubMed] [Google Scholar]
- 69.Luna CM, Sarquis S, Niederman MS, Sosa FA, Otaola M, Bailleau N, et al. Is a strategy based on routine endotracheal cultures the best way to prescribe antibiotics in ventilator-associated pneumonia? Chest. 2013;144(1):63–71. doi: 10.1378/chest.12-1477. [DOI] [PubMed] [Google Scholar]
- 70.Luyt C-E, Combes A, Reynaud C, Hekimian G, Nieszkowska A, Tonnellier M, et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34(8):1434–1440. doi: 10.1007/s00134-008-1112-x. [DOI] [PubMed] [Google Scholar]
- 71.Mauri T, Coppadoro A, Bombino M, Bellani G, Zambelli V, Fornari C, et al. Alveolar pentraxin 3 as an early marker of microbiologically confirmed pneumonia: a threshold-finding prospective observational study. Crit Care. 2014;18(5):562. doi: 10.1186/s13054-014-0562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medford ARL, Husain SA, Turki HM, Millar AB. Diagnosis of ventilator-associated pneumonia. J Crit Care. 2009;24(3):473.e1–6. doi: 10.1016/j.jcrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Mongodi S, Via G, Girard M, Rouquette I, Misset B, Braschi A, et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest. 2016;149(4):969–980. doi: 10.1016/j.chest.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Conway Morris A, Kefala K, Wilkinson TS, Moncayo-Nieto OL, Dhaliwal K, Farrell L, et al. Diagnostic importance of pulmonary interleukin-1beta and interleukin-8 in ventilator-associated pneumonia. Thorax. 2010;65(3):201–207. doi: 10.1136/thx.2009.122291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linssen CFM, Jacobs JA, Schouten JSAG, van Mook WNKA, Ramsay G, Drent M. Influence of antibiotic therapy on the cytological diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34(5):865–872. doi: 10.1007/s00134-008-1015-x. [DOI] [PubMed] [Google Scholar]
- 76.Refaat A, Affara N, Abdel-fatah W, Hussein T, El-gerbi M. Diagnostic accuracy of inflammatory biomarkers in bronchoalveolar lavage from patients with ventilator-associated pneumonia. Egypt J Chest Dis Tuberc. 2014;63(3):723–730. doi: 10.1016/j.ejcdt.2014.03.003. [DOI] [Google Scholar]
- 77.Scholte JBJ, van Dessel HA, Linssen CFM, Bergmans DCJJ, Savelkoul PHM, Roekaerts PMHJ, et al. Endotracheal aspirate and bronchoalveolar lavage fluid analysis: interchangeable diagnostic modalities in suspected ventilator-associated pneumonia? J Clin Microbiol. 2014;52(10):3597–3604. doi: 10.1128/JCM.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Textoris J, Loriod B, Benayoun L, Gourraud P-A, Puthier D, Albanèse J, et al. An evaluation of the role of gene expression in the prediction and diagnosis of ventilator-associated pneumonia. Anesthesiology. 2011;115(2):344–352. doi: 10.1097/ALN.0b013e318225ba26. [DOI] [PubMed] [Google Scholar]
- 79.Vanspauwen MJ, Linssen CFM, Bruggeman CA, Jacobs JA, Drent M, Bergmans DCJJ, et al. Clara cell protein in bronchoalveolar lavage fluid: a predictor of ventilator-associated pneumonia? Crit Care. 2011;15(1):R14. doi: 10.1186/cc9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vernikos P, Kampolis CF, Konstantopoulos K, Armaganidis A, Karakitsos P. The role of bronchoscopic findings and bronchoalveolar lavage fluid cytology in early diagnosis of ventilator-associated pneumonia. Respir Care. 2016;61(5):658–667. doi: 10.4187/respcare.04265. [DOI] [PubMed] [Google Scholar]
- 81.Waltrick R, Possamai DS, de Aguiar FP, Dadam M, de Souza Filho VJ, Ramos LR, et al. Comparison between a clinical diagnosis method and the surveillance technique of the Center for Disease Control and Prevention for identification of mechanical ventilator-associated pneumonia. Rev Bras Ter Intensiva. 2015;27(3):260–265. doi: 10.5935/0103-507X.20150047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yagmurdur H, Tezcan AH, Karakurt O, Leblebici F. The efficiency of routine endotracheal aspirate cultures compared to bronchoalveolar lavage cultures in ventilator-associated pneumonia diagnosis. Niger J Clin Pract. 2016;19(1):46–51. doi: 10.4103/1119-3077.164327. [DOI] [PubMed] [Google Scholar]
- 83.Zagli G, Cozzolino M, Terreni A, Biagioli T, Caldini AL, Peris A. Diagnosis of ventilator-associated pneumonia: a pilot, exploratory analysis of a new score based on procalcitonin and chest echography. Chest. 2014;146(6):1578–1585. doi: 10.1378/chest.13-2922. [DOI] [PubMed] [Google Scholar]
- 84.Umemneku Chikere CM, Wilson K, Graziadio S, Vale L, Allen AJ. Diagnostic test evaluation methodology: a systematic review of methods employed to evaluate diagnostic tests in the absence of gold standard—an update. PLoS ONE. 2019;14(10):e0223832. doi: 10.1371/journal.pone.0223832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lalwani S, Mathur P, Tak V, Janani S, Kumar SI, Bagla R, et al. Diagnosis of ventilator-associated pneumonia: comparison between ante-mortem and post-mortem cultures in trauma patients. Indian J Med Microbiol. 2014;32(3):294–300. doi: 10.4103/0255-0857.136572. [DOI] [PubMed] [Google Scholar]
- 86.Fernando SM, Tran A, Cheng W, Klompas M, Kyeremanteng K, Mehta S, et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020;46(6):1170–1179. doi: 10.1007/s00134-020-06036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris AC, Kefala K, Simpson AJ, Wilkinson TS, Everingham K, Kerslake D, et al. Evaluation of the effect of diagnostic methodology on the reported incidence of ventilator-associated pneumonia. Thorax. 2009;64(6):516–522. doi: 10.1136/thx.2008.110239. [DOI] [PubMed] [Google Scholar]
- 88.Kamel T, Helms J, Janssen-Langenstein R, Kouatchet A, Guillon A, Bourenne J, et al. Benefit-to-risk balance of bronchoalveolar lavage in the critically ill. A prospective, multicenter cohort study. Intensive Care Med. 2020;46(3):463–474. doi: 10.1007/s00134-019-05896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Browne E, Hellyer TP, Baudouin SV, Conway Morris A, Linnett V, McAuley DF, et al. A national survey of the diagnosis and management of suspected ventilator-associated pneumonia. BMJ Open Respir Res. 2014;1(1):e000066. doi: 10.1136/bmjresp-2014-000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butler KL, Sinclair KE, Henderson VJ, McKinney G, Mesidor DA, Katon-Benitez I, et al. The chest radiograph in critically ill surgical patients is inaccurate in predicting ventilator-associated pneumonia. Am Surg. 1999;65(9):805–9. [PubMed] [Google Scholar]
- 91.Wunderink RG, Woldenberg LS, Zeiss J, Day CM, Ciemins J, Lacher DA. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest. 1992;101(2):458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 92.Lefcoe MS, Fox GA, Leasa DJ, Sparrow RK, McCormack DG. Accuracy of portable chest radiography in the critical care setting. Chest. 1994;105(3):885–887. doi: 10.1378/chest.105.3.885. [DOI] [PubMed] [Google Scholar]
- 93.Beards SC, Jackson A, Hunt L, Wood A, Frerk CM, Brear G, et al. Interobserver variation in the chest radiograph component of the lung injury score. Anaesthesia. 1995;50(11):928–932. doi: 10.1111/j.1365-2044.1995.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 94.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5):1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 95.Klompas M, Magill S, Robicsek A, Strymish JM, Kleinman K, Evans RS, et al. Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med. 2012;40(12):3154–3161. doi: 10.1097/CCM.0b013e318260c6d9. [DOI] [PubMed] [Google Scholar]
- 96.Moher D, Liberati A, Tatzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 2009; 6(6):e1000097. 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. MEDLINE search example.
Additional file 2. Quality assessment and data extraction form.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.