The pandemic of Coronavirus disease 2019 (COVID-19) has resulted in a significant increase in the need for kidney replacement therapy due to the high incidence of acute kidney injury, with early reports claiming an incidence rate of 40% to 50% in critical care patients.1,2 To limit the exposure to health care workers, continuous kidney replacement therapy modalities, such as continuous veno-venous hemodialysis (CVVHD), are preferred, as these approaches can be administered by nursing staff and do not require dedicated dialysis personnel. However, lack of preparedness for pandemics and supply shortages of dialysate fluid have severely limited the ability of health care providers to deliver sufficient kidney replacement therapy for patients severely affected by COVID-19.3,4 We interacted with multiple health care providers who reported shortages in dialysate for CVVHD, and providers outside the Johns Hopkins health care system who were preparing backup plans for their depleting reserves as cases continued to increase. At the time, the solutions were limited to rationing strategies, including the reduction of treatment time, decreasing dialysate flow rate, or using noncommercial supplies of dialysate.3 In this report, we share our methodology and design for a simple, open-source 3D printed adapter that enables production and collection of dialysate for CVVHD using a Fresenius 2008T hemodialysis machine, a Fresenius Optiflux high-flux dialyzer (Fresenius, Bad Homburg, Germany) and a Baxter Exactamix total parenteral nutrition (TPN) bag (Baxter, Deerfield, Illinois, USA). The method is easily adaptable to any conventional hemodialysis (HD) machine, dialyzer, and sterile collection bag.
Results
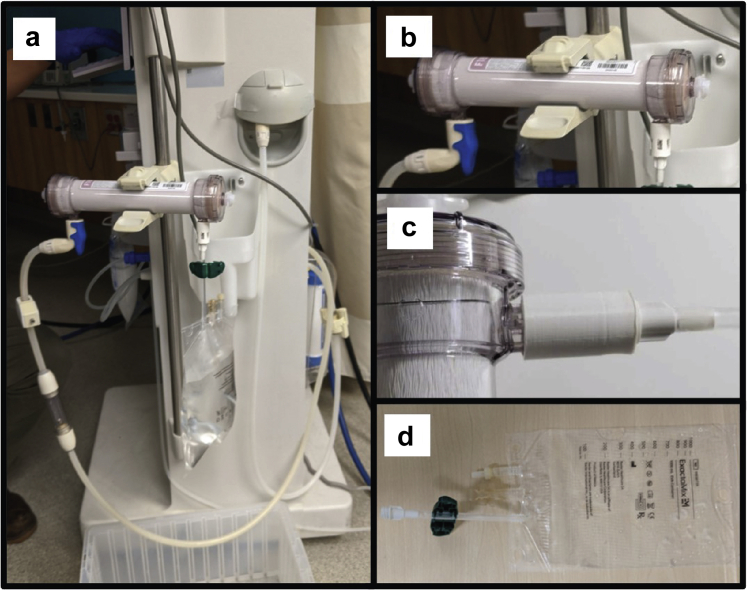
Our method facilitated CVVHD dialysate production by conventional HD machines in an aseptic manner via the implementation of a three-dimensional (3D) printed connector, which linked to TPN bags commonly available in a hospital setting (Figure 1). Detailed 3D printing instructions and files are provided in the Supplementary material (see Supplemental Document, S2) and can be downloaded here: http://bit.ly/3pKlT78. Testing of the dialysate showed that its composition was comparable to commercially available CVVHD bags (Table 1). When the dialysate flow rate is set to 800 ml/min on the HD machine, dialysate can be produced at an approximate flow rate of 400 to 500 ml/min because the flow is pulsatile. The results of culture tests (See Supplementary Document S1.3) showed that there was no growth on any of the plates throughout the experiment, suggesting that the bagged dialysate fluid was safe for use.
Figure 1.
Components required for in-house dialysate production. (a) The complete system attached to a Fresenius 2008T hemodialysis machine. (b) The Fresenius Optiflux Dialyzer. (c) close-up of the connector attached to the dialyzer effluent port (left) and total parenteral nutrition (TPN) bag inlet (right). (d) The Baxter Exactamix TPN bag. (a) and (b) show the test connector with slots used for leak tests and visual observation of the mating surfaces (please see Supplementary Document, S3).
Table 1.
Stability of electrolytes in the dialysate at room temperature and 4°C
| Analyte concentrationsa |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium, mg/dl |
Chloride, mmol/l |
Bicarbonate, mmol/l |
Glucose, mg/dl |
Potassium, mmol/l |
Lactate, mmol/l |
Magnesium, mg/dl |
Sodium, mmol/l |
|||||||||
| Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | Room Temp | 4°C | |
| Time point | ||||||||||||||||
| BL | 5.2 | 5.1 | 104 | 104 | 29.9 | 29.8 | 108 | 107 | 2.1 | 2.2 | 0 | 0 | 1.29 | 1.25 | 140 | 140 |
| ~6 h | 5.3 | 5.2 | 103 | 103 | 31.9 | 32.1 | 106 | 107 | 2.2 | 2.2 | 0 | 0 | 1.28 | 1.3 | 140 | 140 |
| ~9 h | 5.2 | 5.1 | 104 | 104 | 31.6 | 32.7 | 109 | 109 | 2.1 | 2.1 | 0 | 0 | 1.28 | 1.23 | 140 | 140 |
| ~12 h | 5.2 | 5.1 | 104 | 104 | 32.4 | 31.3 | 104 | 107 | 2.2 | 2.1 | 0 | 0 | 1.25 | 1.26 | 141 | 140 |
| ~24 h | 5 | 5 | 104 | 103 | 31.3 | 32.9 | 106 | 106 | 2.1 | 2.1 | 0 | 0 | 1.32 | 1.33 | 139 | 140 |
| Percent difference (BL–24 h), % | -3.8 | -2 | 0 | -1 | 4.7 | 10.4 | -1.9 | -0.9 | 0 | -4.5 | N/A | N/A | 2.3 | 6.4 | -0.7 | 0 |
BL, baseline; Temp, temperature; N/A, not applicable.
The hemodialysis machine dialysate settings were: sodium 140 mEq/l , potassium 2.0 mEq/l, calcium 2.5 mEq/l, and bicarbonate 30 mEq/l.
Chemical and Microbiology Testing
We successfully produced dialysate for CVVHD using a Fresenius 2008T HD machine and stored it in a Baxter Exactamix TPN bag. Dialysate produced and collected using our method remained adequately stable for at least 24 hours when stored at room temperature or in a 4°C refrigerator. Concentrations of eight analytes that we monitored periodically over a 24-hour period at room temperature and 4°C are shown in Table 1. Over the first 12 hours, no analyte concentration increased or decreased from the baseline by more than 5%. After 24 hours, this was true for all analytes except for the refrigerated bicarbonate measurement which increased by approximately 10%. We observed no aerobic growth at 48 hours and no anaerobic growth at 96 hours in samples collected at baseline and stored at room temperature and at 4°C.
Connector Sterilization and Leak Test
Connectors printed on a desktop printer (Ultimaker 3, Dynamism, Chicago, Illinois, USA) using polycarbonate material successfully underwent seven ethylene oxide sterilization cycles with no significant changes in their form or fit with the dialyzer and the TPN bag. Microscopic observation revealed no leakage of fluid inside the connector as the fluid passed directly from the dialyzer outlet to the TPN bag inlet, without making contact with the inside walls of the connector (Supplementary Figure 1).
Discussion
We developed a reliable and aseptic method to capture dialysate for CVVHD treatments that is prepared by the conventional dialysate machine using a 3D-printed connector applied to a dialyzer. At a rate of 400 ml/min, one 5-l CVVHD bag can be prepared approximately every 15 minutes, thus allowing potential preparation of 4 to 5 bags/h from one HD machine. Based on laboratory analyses, the dialysate produced by this method should be used within 12 hours of making it to ensure electrolyte stability, with intermittent testing for any bacterial contamination. Although the results are promising, the test does not prove that the fluid is sterile. Therefore, we only suggest using this method is appropriate for dialysate production. This method is not recommended for continuous veno-venous hemofiltration or continuous veno-venous hemodiafiltration infusion as sterility of the generated product cannot be guaranteed. Although the fluid cannot be used for infusion, improved sterility can potentially be achieved by the addition of an ultrafilter in the dialysate water line to reduce bacterial content and endotoxin.5 Also, the dialysate produced through this method should not be used with citrate anticoagulation due to the high calcium and bicarbonate content.
Our approach has significant strengths. This method of preparing CVVHD dialysate is rapid, inexpensive, and uses the setup of a conventional HD center to produce the dialysate. In addition, it does not require any specialized training, and dialysis technicians or any health care worker can be trained to follow the procedure to prepare and collect dialysate. This method would be suitable in different health care settings and may be applicable in natural disasters or areas subject to political instability where transportation, shipping, or supply chain is interrupted.
The key limitation of this approach is the requirement for 3D-printed connectors that work across different dialyzers and collection bags. However, our design can be modified to accommodate different dialyzer fittings. With the increase of third-party medical and desktop 3D-printing services, we believe this limitation can be easily overcome. We recommend each center have their supply of a few connectors available for any future emergencies. There are other approaches available which do not use connectors that are aimed as alternatives to replacing traditional dialysate procurement,6 but may not satisfy the need for a quick, intuitive method that can be used without significant training, as needed in emergency use cases. Comparatively, the use of a sterilizable connector creates a simple and replicable workflow that significantly reduces the chance of user error.
Disclosure
All the authors declared no conflict of interest. The technology reported in this manuscript is currently undergoing the Johns Hopkins University invention disclosure process. The adapter used in this method is not a United States Food and Drug Administration–approved medical device and is not recommended for any clinical use. This approach is presented as a potential emergency dialysate production method and not meant to be a replacement for routine dialysate needs.
Acknowledgments
The project was supported by Center for Bioengineering Innovation and Design (CBID) at Johns Hopkins University through a coronavirus disease 2019 grant.
Footnotes
Supplementary Methods
Instructions for 3D Printing
Performance under Sterilization and Leak Test
Supplementary Material
Supplementary Methods
Instructions for 3D Printing
Performance under Sterilization and Leak Test
References
- 1.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15:880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ICNARC ICNARC report on COVID-19 in critical care. ICNARC COVID-19 Study Case Mix Program Database. 2020. https://www.icnarc.org/
- 3.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the inpatient dialysis unit managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol. 2020;15:720–722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahase E. COVID-19: increasing demand for dialysis sparks fears of supply shortage. BMJ. 2020;369:m1588. doi: 10.1136/bmj.m1588. [DOI] [PubMed] [Google Scholar]
- 5.Di Iorio B., Di Micco L., Bruzzese D. Ultrapure dialysis water obtained with additional ultrafilter may reduce inflammation in patients on hemodialysis. J Nephrol. 2017;30:795–801. doi: 10.1007/s40620-017-0422-x. [published correction appears in J Nephrol. 2017 Oct 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leblanc M., Moreno L., Robinson O.P., Tapolyai M., Paganini E.P. Bicarbonate dialysate for continuous renal replacement therapy in intensive care unit patients with acute renal failure. Am J Kidney Dis. 1995;26:910–917. doi: 10.1016/0272-6386(95)90055-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.