Abstract
During the highly infectious pandemic of coronavirus disease 2019 (COVID-19), artificial intelligence (AI) has provided support in addressing challenges and accelerating achievements in controlling this public health crisis. It has been applied in fields varying from outbreak forecasting to patient management and drug/vaccine development. In this paper, we specifically review the current status of AI-based approaches for patient management. Limitations and challenges still exist, and further needs are highlighted.
Keywords: Coronavirus disease 2019, Artificial intelligence, COVID-19(Coronavirus disease 2019)
1. Introduction
Severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) was identified in December 2019 [1], followed by transmission around the world. The public health emergency rapidly developed into a pandemic with global involvement and was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [2]. Although authorities in various countries launched different policies to contain the pandemic, the daily new confirmed cases and deaths still increased, causing more than 34,495,176 infections and 1025,729 deaths by October 4, 2020 [3]. A surge in medical burden within a short time, shortages of medical resources and health workforce, weak public health surveillance systems, and inefficient screening and triage routine were challenging public health systems, emphasizing a need for better methods to control the public health crisis.
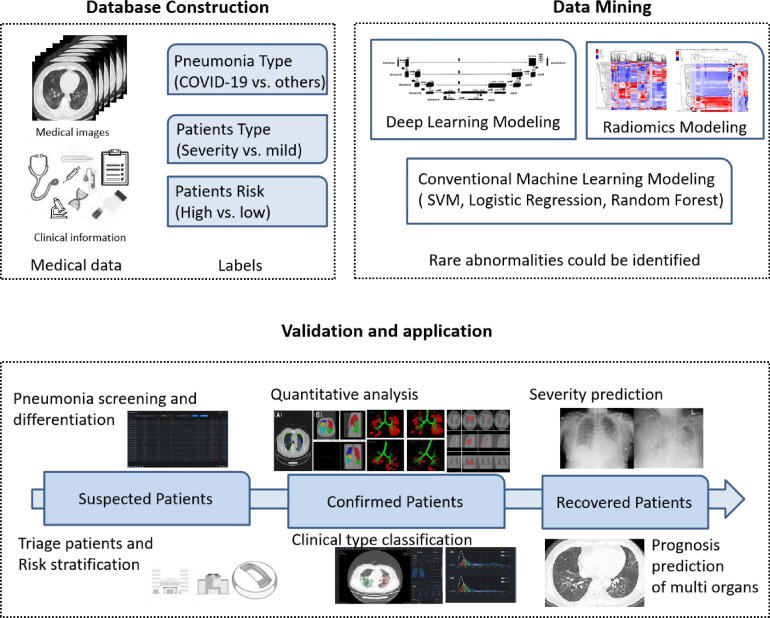
Artificial intelligence (AI) can actively learn and analyze different kinds of data without complete knowledge. As shown in Figure 1 , rare abnormalities can be identified after a huge amount of data mining, and evidence or suspicions might be provided for differentiation/classification. AI technology has successfully exhibited its promising potential in medical and health care fields and is altering the way health services are delivered. Large datasets and various computational algorithms facilitated the development of AI. On January 31, 2020, Wu et al. warned of a global outbreak of COVID-19 as assessed by a susceptible-exposed-infectious-recovered metapopulation model [4], and a multivariate prediction model was used for adjusting dynamic mitigation and suppression interventions [5]. Additionally, with its success in aggregating and mining big data, AI demonstrates beneficial contributions in areas such as patient monitoring [6], disease diagnosis [7], [8], [9], prognostic prediction [7,10,11], drug and vaccine development [12], etc. The latest studies have clarified the crucial supporting role of AI-based models in the control of the COVID-19 pandemic [13], [14]. The purpose of this review was to comprehensively assess current applications of AI-based tools in patient management during the public health crisis. Moreover, additional research needs are highlighted.
Fig. 1.
An example of the workflow of artificial intelligence (AI). COVID-19: coronavirus disease 2019; SVM: support vector machine.
2. Current research on AI in COVID-19 patient management
AI-based techniques are broadly applied in four categories, including patient tracing and monitoring, screening and diagnosis, characterization and severity assessment, and prognostic prediction.
2.1. Tracing and monitoring
Disrupting the chain of transmission and timely quarantining of infected individuals are essential for pandemic control. AI-based smartphone apps have facilitated implementing real-time tracing technologies. By integrating Global Positioning System (GPS), social media, and geospatial AI, real-time spatiotemporal trajectory data were gathered via a smartphone so that every individual was traced and monitored accurately [6]. This type of effort is crucial in containing COVID-19, especially because of its high infectivity. Although there were concerns about confidentiality and privacy, these apps were widely accepted and supported, and a variety of similar smartphone apps were used in different countries [15], [16], [17], [18].
Because automatic contact tracing is a mobile phone app-based intervention, several caveats should be considered. It is vital to get near-perfect compliance, without people fearing isolation or quarantine after those with confirmed cases have been contacted. Moreover, various apps or models were developed and applied in different countries. A universal platform or network for sharing contact tracing information might be of more benefit for containing a global pandemic. Although transmission from presymptomatic and asymptomatic carriers has been widely proven [12,19], current app-based contact tracing is still challenging [20], [21].
2.2. Screening and diagnosis
Because of the high infectivity of COVID-19, early detection and diagnosis is of great importance. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing is the standard of reference for confirming a COVID-19 diagnosis. Nevertheless, testing is limited and time consuming. The uncertain sensitivity of RT-PCR testing, varying from 37% to 71% as reported in early studies [22], [23], [24], is another important impediment for early detection of COVID-19.
In addition to RT-PCR testing, thoracic imaging is an important component of COVID-19 diagnosis. However, the sensitivity and specificity of COVID-19 recognition in chest imaging varied among radiologists [25]. Most AI-based radiological diagnostic systems, based on radiographs or CT images, reportedly have high sensitivity and specificity or high accuracy, comparable to diagnosis by senior radiologists [7,8,[26], [27], [28], [29]]. Moreover, assisted by AI, radiologists’ performance in distinguishing COVID-19 was optimized, with a better test accuracy (90% vs. 85%, P<0.001), sensitivity (88% vs. 79%, P<0.001) and specificity (91% vs. 88%, P = 0.001) [7, 26].
AI-driven diagnostic systems/models mainly refer to dual classification problems (including COVID-19 vs. healthy control, COVID-19 vs. non-COVID-19, and COVID-19 vs. other pneumonia), three-class classification problems (including COVID-19 vs. other pneumonia vs. healthy control, etc.) and multiclass classification problems (including COVID-19 vs. viral pneumonia vs. bacterial pneumonia vs. healthy control, etc.). Because COVID-19 infection could not be ruled out in a proportion of patients because of lack of RT-PCR testing early in the pandemic, several studies recruited previous non-COVID-19 cases for model training [26, [30], [31], [32]]. They compensated the limited sample size as well as avoided falsely assigning patients with COVID-19 to the non-COVID-19 group. Nevertheless, the value of the proposed model might be diminished, because coinfection with SARS-CoV-2 and other respiratory pathogens has been confirmed [33,34]. Several studies shared this problem. Minaee et al. [28] compared efficacies of four convolutional neural networks (CNNs) (including ResNet18, ResNet50, SqueezeNet, and DenseNet-121) used to detect COVID-19 in chest radiographs. Although the average sensitivity rate was around 98% and the specificity was around 90%, the deployment of the proposed model was limited. Another study combined the discrimination deep learning model and the localization deep learning model for COVID-19 identification. By training on both a community-acquired pneumonia (CAP) and a COVID-19 radiograph dataset from the Radiological Society of North America (RSNA), the proposed model achieved an accuracy of 98.71% [29]. Since differentiating COVID-19 from other viral pneumonias in flu season was a big challenge for junior radiologists, it was hoped that a 3D CNN-based model, based on CT images from multicenters during the pandemic period, successfully differentiated COVID-19 from influenza-A viral pneumonia and healthy controls (accuracy rate: 86.7%) [35]. It would truly aid in the clinic, but its application is still limited by the overlap of radiological manifestations in multiple diseases as well as the small sample size.
The study of Karthik et al. [36] was more impressive. By integrating channel shuffled dual-branched (CSDB) CNN and distinctive filter learning (DFL), the proposed model attained an accuracy of 99.8% in detecting COVID-19 or a particular type of pneumonia (viral pneumonia or bacterial pneumonia) on radiographs. It might perform well in a realistic scenario, but lack of external validation would curb its wide deployment.
Other common weaknesses in early studies concern (1) limited sample size, which might affect the robustness of models and increase the risk of overfitting [35,[37], [38]]; (2) training and testing images belonging to one or limited datasets and lack of heterogeneity of data sources, which might hinder generalization of the proposed system [39]; (3) studies that were mostly retrospectively designed, and the efficacy of only a few models was prospectively validated in clinic; (4) only a few studies reported on the applicable scope of the proposed models (such as the prevalence), although it is of great importance for deployment; and (5) lack of external dataset validation or validated with dataset containing few COVID-19 cases, which might not reveal the real performance of models.
In order to overcome limited training data, Oh et al. [40] introduced a novel patch-based deep neural network architecture. Each COVID-19 radiograph was randomly divided into numerous small patches for model training. Regarding the study of Al, Waisy et al. tried to employ pre-trained deep learning models and a high-resolution network model for the proposed system [41]. In addition, a transfer learning technique and large datasets of preprocessed radiographs were adopted to ensure the stability and generalizability of the system.
Researchers have also focused on joint AI models integrating CT images and clinical information for rapid diagnosis. One study proposed a joint model based on a multicenter dataset of 905 cases. It achieved a better performance than models based on CT images or clinical information only, obtaining 84.3% sensitivity (95% CI: 77.1%- 90.0%), 82.8% specificity (95% CI: 75.6%−88.5%) and 0.92 area under the curve (AUC) (95% CI: 0.887–0.948) [8]. Additionally, Nikolas et al. introduced an AI system for rapid diagnosis based on a chest CT score [27,42]. The AUC was 0.95 on test dataset and 0.88 on the external validation dataset. Regardless of good performance, they were also either limited by small sample size or the homogeneity of data source.
Harmon et al. [9] considered the heterogeneity of data sources and developed a deep learning model based on a diverse multinational cohort of 1280 patients, maintaining an accuracy of 90.8% and sufficient generalizability. Another study proposed an AI system for a three-class classification problem. A 3D classification network was employed and the proposed system was based on 6752 CT scans from 4154 patients, obtaining an overall performance of 92.49% accuracy and AUC of 0.9813 (95% CI: 0.9691–0.9902) [7]. Across races and prevalence, validation was conducted with an internal cohort and one retrospective and three prospective external cohorts.
Other authors highlighted a chest CT-based deep learning algorithm for medical triage [43]. The proposed model utilized U-Net and was based on a dataset of 2447 patients. Multiple external validations were used to assess the performance of the algorithm. Comparing to radiological reports, the model achieved a high degree of accuracy. Authors emphasized the great importance of reducing reporting time with an accurate diagnostic AI system in patient management.
Applications of thoracic imaging have been controversial, and the value of imaging tests has been diminished by risk of radiation exposure and potential transmission to uninfected medical staff [44]. Because symptoms of COVID-19 are nonspecific and less than half of patients present with fever before admission [45], studies on identification of reliable biomarkers for disease screening are difficult. Wagner et al. [46] introduced a clinical symptom-based augmented intelligence platform to predict impending COVID-19 onset. Another study enabled preliminary diagnosis of COVID-19 with cough feature-based AI model [47]. Banerjee et al. [48] applied random forest machine learning and artificial neural networks to identify an altered immune cell profile as a rapid diagnosis tool for COVID-19. The model was based on 598 cases, achieving an AUC of 0.81 and 0.85 in regular ward and in the community, respectively, with a false negative rate of 50%. These insights might provide evidence for risk stratification of individuals out of hospital, but further multicenter large-scale validation is required before real-life application. In addition, Naeem et al. [49] suggested a genomic signal processing-based automatic diagnostic system for accurate COVID-19 diagnosis. It was executed by detecting DNA features, and the entire process was accomplished within several minutes. Although the accuracy results were perfect, its specific technology hinders deployment in most countries and regions, especially the low and middle-income countries.
Previous studies provide value and promise of deploying AI-based systems for patient screening and diagnosis. It is vital for patient risk stratification, effective medical triage, and better allocation of medical resources. Deployment in the real world is still a big challenge for the reviewed studies. Open access to diverse datasets in different regions and different populations may facilitate generalizing AI systems. Close collaboration between regions and countries will ensure the cross-border access, while patient privacy and data safety should raise special concern. Because there is no need for individual sharing, federated learning-based unified AI systems/models might be a promising alternative to improve the generalizability of models [50].
2.3. Characterization and severity assessment
Medical imaging is of special importance in tissue characterization and the assessment of pathophysiological process in the body [51]. Pulmonary involvement measured by a semiquantitative visual score on chest CT has been proved highly consistent with disease severity in early studies [52], [53]. In order to avoid subjectivity, quantitative methods, such as deep learning and deep reinforcement learning-based algorithms [54] and multi-scale convolutional neural networks-based algorithms [55] have been suggested for automated quantification of CT abnormalities, as well as quantification of the severity of both lobular involvement and lung involvement. Another algorithm based on a Siamese neural network was also suggested for evaluating severity on chest radiographs by automatically calculating the pulmonary X-ray score, defined as the Euclidean distance between an image-of-interest and normal images [56].
Quantitative parameters and whole lung radiomics demonstrated a superior reliance and accuracy [57, 58]. Severe COVID-19 pneumonia was correlated with higher percentage of consolidation and larger extent of pulmonary involvement [55]. In the study of Kang et al. [59], pulmonary lesions were automatically detected and quantified by a deep learning-based system. The authors clarified correlations between pulmonary lesion extent and respiratory function, as well as clinical parameters reflecting other organ damage.
2.4. Prognostic prediction
Severity and adverse outcome prediction in the early stage of the disease is of great importance for risk stratification and allocation of intensive care medical resources, especially with limited medical resources. Clinical biomarkers and dynamic changes in laboratory parameters were noted by researchers in early observational studies. Patients’ preconditions including age and comorbidities (obesity, diabetes, hypertension, and chronic renal insufficiency) predicted high risk of intensive care unit (ICU) admission, ventilator requirements, and mortality [60]. Different combinations of clinical and laboratory parameters were used in a logistic regression model that performed well in predicting severity progression [61], [62]. D-dimer, oxygen index, neutrophil-to- lymphocyte ratio, C-reactive protein, and lactate dehydrogenase were employed in a deep neural network-based model, achieving an AUC for mortality prediction of 0.968 [63]. Another artificial neural network-based system had a similar predictive efficacy with an AUC of 0.9012 [64].
Many studies highlighted the predictive value of quantitative CT parameters in assessing condition deterioration and adverse outcomes. Quantitative disease burden on chest CT in early stages predicted severe outcomes [10,65]. With a 3D U-Net-based model, Grodecki et al. [66] showed higher odds with older age and a larger consolidation burden in upper lobes on admission in assessing poor outcomes. In another multivariable regression model, the volumes of consolidation and ground glass opacities were identified as independent predictors of condition deterioration or death and demonstrated incremental predictive efficacy when being integrated in a model based only on clinical data [67]. These findings were consistent with other studies. The Light Gradient Boosting Machine and Cox proportional-hazards regression-based prediction system were based on quantitative pulmonary lesion features of 456 patients and had an AUC of 0.8479 for predicting severe progression. Performance was significantly improved by being combined with clinical parameters, reaching an AUC of 0.9039 with a sensitivity of 86.71% and specificity of 80.00% [59]. Additionally, with respect to the evolution of the pattern of pulmonary lesions, other authors compared CT features seen on admission (day 0) and on day 4. Although a better predictive value of CT features on day 4 was shown by an AI-driven system, it was still inferior to that of changes in CT features [11].
3. Further requirements for AI during the COVID-19 pandemic
3.1. Assisting treatment modality options or adjustment
There are diverse classes of drugs under evaluation and the efficacies of different treatment modalities might vary according to the COVID-19 manifestations and disease course in patients [68]. A novel AI-based model should automatically record every response of patients to the associated treatment modality and identify the correlation between certain kinds of alteration in patients’ condition and treatment. Moreover, with its powered data aggregating and mining ability, the proposed model is expected to provide step-by-step suggestions based on real-time conditions in each patient for clinical decision making in treatment modality options or adjustment.
3.2. Prediction of important complications
Recent studies suggest that COVID-19 patients may experience multi-organ impairment, including brain, heart, kidney, etc. [69], [70], [71], [72], [73] Comorbidities increase risk for severe complications [74]. Because medical imaging and biomarkers are important factors for outcome prediction, there may be opportunities to develop AI-driven prediction systems for important complications, such as electrocardiograph-based or ultrasound-based AI models. These would be quite helpful for patient-risk stratification and decision making in medical testing and treatment options. For example, for patients classified as high risk for cardiovascular complications, possible myocardial injury would be closely monitored and treatment that may induce myocardial damage would be avoided.
3.3. Detection of asymptomatic patients and patients with unspecific symptoms
Asymptomatic patients have been estimated to account for 17.9%−33.3% of all COVID-19 patients [19,75]. Given confirmed transmission by asymptomatic carriers to others [76,77], early identification of asymptomatic patients is crucial to contain the pandemic. However, image screening is not indicated in asymptomatic patients and patients with nonspecific symptoms, especially those who are not in high prevalence regions [44,78]. It would be more challenging to identify and quarantine them, as well as control further spread by undetected infected and nonspecific symptomatic individuals [79]. Recent research reported applications of a facial features-based AI model in coronary artery disease detection [80]. Identification of novel biomarkers with better availability for COVID-19 screening, such as respiratory pattern, might be an important direction for further investigation in AI.
3.4. Prediction of mental health
COVID-19 and its associated control measures have greatly affected people's lives as well as their mental health [81]. However, effective approaches for assessment and intervention have been lacking. In India, an AI-based smartphone app for mental health evaluation has been launched, although few studies provide further information. More attempts are required to identify risk factors and predict mental illness with AI algorithms, because it is a health issue with global relevance, and some of the mental symptoms might persist.
4. Summary
AI-based approaches have enabled accurate patient tracing and monitoring, rapid diagnosis, severity assessment, and early prognostic prediction in patient management during the COVID-19 pandemic. Although limitations and challenges still exist, the deployment of AI-based systems promises to save more lives and ameliorate the public health crisis. In the near future, more AI utilities need to be explored in different domains, including drug and vaccine development, origin-tracing research, and prediction of virus mutation.
Funding
This research was funded by National Key Research and Development Program of China (Grant No. 2020YFC0845500).
Author contributions
Lan Lan, Xinghuan Wang and Haibo Xu designed the study. Dan Xu, Wenbo Sun and Minhua Yu conducted the literature review. Lan Lan, Huijuan Hu and Feng Xiao drafted the manuscript. Haibo Xu and Xinghuan Wang critically reviewed the manuscript. All authors contributed to final approval of the version to be submitted. Editor notes Given his role as editorial board member, Xinghuan Wang had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Jing Sun and Zhuqingqing Cui
Editor notes
Given his role as editorial board member, Xinghuan Wang had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Jing Sun and Zhuqingqing Cui
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Timeline, COVID-19. https://wwww.hoint/news-room/detail/27-04-2020-who-timeline—covid-19. Published April 27, 2020. Accessed October 4, 2020.
- 3.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. https://wwww.covid19whoint/ Published October 3, 2020. Accessed October 4, 2020.
- 4.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury R, Heng K, Shawon MSR, et al. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur J Epidemiol. 2020;35(5):389–399. doi: 10.1007/s10654-020-00649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Ding S, Xiong L. A new system for surveillance and digital contact tracing for COVID-19: spatiotemporal reporting over network and GPS. JMIR Mhealth Uhealth. 2020;8(6):e19457. doi: 10.2196/19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K, Liu X, Shen J, et al. Clinically applicable AI system for accurate diagnosis, quantitative measurements, and prognosis of COVID-19 pneumonia using computed tomography. Cell. 2020;181(6):1423–1433. doi: 10.1016/j.cell.2020.04.045. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei X, Lee HC, Diao KY, et al. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020;26(8):1224–1228. doi: 10.1038/s41591-020-0931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmon SA, Sanford TH, Xu S, et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat Commun. 2020;11(1):4080. doi: 10.1038/s41467-020-17971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296(2):E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10(12):5613–5622. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong E, Wong MU, Huffman A, et al. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front Immunol. 2020;11:1581. doi: 10.3389/fimmu.2020.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik YS, Sircar S, Bhat S, et al. How artificial intelligence may help the Covid-19 pandemic: pitfalls and lessons for the future. Rev Med Virol. 2020:e2205. doi: 10.1002/rmv.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal A, Padappayil RP, Garg C, et al. Utility of artificial intelligence amidst the COVID 19 pandemic: a review. J Med Syst. 2020;44(9):156. doi: 10.1007/s10916-020-01617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmann S, Milsom L, Zillessen H, et al. Acceptability of App-based contact tracing for COVID-19: cross-country survey study. JMIR Mhealth Uhealth. 2020;8(8):e19857. doi: 10.2196/19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abeler J, Backer M, Buermeyer U, et al. COVID-19 contact tracing and data protection can go together. JMIR Mhealth Uhealth. 2020;8(4):e19359. doi: 10.2196/19359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CM, Jyan HW, Chien SC, et al. Containing COVID-19 among 627,386 persons in contact with the Diamond Princess cruise ship passengers who disembarked in taiwan: big data analytics. J Med Internet Res. 2020;22(5):e19540. doi: 10.2196/19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S, Bhatnagar N, Gangadharan N. A case for participatory disease surveillance of the COVID-19 pandemic in India. JMIR Public Health Surveill. 2020;6(2):e18795. doi: 10.2196/18795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491):eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K, Viboud C. Impact of contact tracing on SARS-CoV-2 transmission. Lancet Infect Dis. 2020;20(8):876–877. doi: 10.1016/S1473-3099(20)30357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296(2):E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai HX, Wang R, Xiong Z, et al. Artificial intelligence augmentation of radiologist performance in distinguishing COVID-19 from pneumonia of other origin at chest CT. Radiology. 2020;296(3):E156–E165. doi: 10.1148/radiol.2020201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lessmann N, Sanchez CI, Beenen L, et al. Automated assessment of COVID-19 reporting and data system and chest CT severity scores in patients suspected of having COVID-19 using artificial intelligence. Radiology. 2021;298(1):E18–E28. doi: 10.1148/radiol.2020202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minaee S, Kafieh R, Sonka M, et al. Deep-COVID: predicting COVID-19 from chest X-ray images using deep transfer learning. Med Image Anal. 2020;65 doi: 10.1016/j.media.2020.101794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Xiao Y, Li Y, et al. Automatically discriminating and localizing COVID-19 from community-acquired pneumonia on chest X-rays. Pattern Recognit. 2021;110 doi: 10.1016/j.patcog.2020.107613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Qin L, Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296(2):E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Xiao Y, Li Y, et al. Automatically discriminating and localizing COVID-19 from community-acquired pneumonia on chest X-rays. Pattern Recognit. 2021;110 doi: 10.1016/j.patcog.2020.107613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes JC, Barbosa VadF, Santana MA, et al. IKONOS: an intelligent tool to support diagnosis of COVID-19 by texture analysis of X-ray images. Res Biomed Eng. 2020 doi: 10.1007/s42600-020-00091-7. [DOI] [Google Scholar]
- 33.Zhu X, Ge Y, Wu T, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D, Quinn J, Pinsky B, et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Jiang X, Ma C, et al. A deep learning system to screen novel coronavirus disease 2019 pneumonia. Engineering (Beijing) 2020;6(10):1122–1129. doi: 10.1016/j.eng.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karthik R, Menaka R, M H. Learning distinctive filters for COVID-19 detection from chest X-ray using shuffled residual CNN. Appl Soft Comput. 2021;99 doi: 10.1016/j.asoc.2020.106744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozturk T, Talo M, Yildirim EA, et al. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput Biol Med. 2020;121 doi: 10.1016/j.compbiomed.2020.103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismael AM, Şengür A. Deep learning approaches for COVID-19 detection based on chest X-ray images. Expert Syst Appl. 2021;164 doi: 10.1016/j.eswa.2020.114054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain G, Mittal D, Thakur D, et al. A deep learning approach to detect Covid-19 coronavirus with X-Ray images. Biocybern Biomed Eng. 2020;40(4):1391–1405. doi: 10.1016/j.bbe.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh Y, Park S, Ye JC. Deep learning COVID-19 features on CXR using limited training data sets. IEEE Trans Med Imaging. 2020;39(8):2688–2700. doi: 10.1109/TMI.2020.2993291. [DOI] [PubMed] [Google Scholar]
- 41.Al-Waisy AS, Al-Fahdawi S, Mohammed MA, et al. COVID-CheXNet: hybrid deep learning framework for identifying COVID-19 virus in chest X-rays images. Soft Comput. 2020:1–16. doi: 10.1007/s00500-020-05424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296(2):E97–E104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Xia C, Huang L, et al. Deep learning-based triage and analysis of lesion burden for COVID-19: a retrospective study with external validation. Lancet Digit Health. 2020;2(10):e506–e515. doi: 10.1016/S2589-7500(20)30199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020;158(1):106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner T, Shweta F, Murugadoss K, et al. Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID-19 diagnosis. Elife. 2020;9:e58227. doi: 10.7554/eLife.58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imran A, Posokhova I, Qureshi HN, et al. AI4COVID-19: AI enabled preliminary diagnosis for COVID-19 from cough samples via an app. Inform Med Unlocked. 2020;20 doi: 10.1016/j.imu.2020.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee A, Ray S, Vorselaars B, et al. Use of machine learning and artificial intelligence to predict SARS-CoV-2 infection from full blood counts in a population. Int Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naeem SM, Mabrouk MS, Marzouk SY, et al. A diagnostic genomic signal processing (GSP)-based system for automatic feature analysis and detection of COVID-19. Brief Bioinform. 2021;22(2):1197–1205. doi: 10.1093/bib/bbaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Ma L, Yang F, et al. A collaborative online AI engine for CT-based COVID-19 diagnosis. medRxiv [Preprint] 2020 doi: 10.1101/2020.05.10.20096073. [DOI] [Google Scholar]
- 51.Zhang HT, Zhang JS, Zhang HH, et al. Automated detection and quantification of COVID-19 pneumonia: CT imaging analysis by a deep learning-based software. Eur J Nucl Med Mol Imaging. 2020;47(11):2525–2532. doi: 10.1007/s00259-020-04953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan F, Ye T, Sun P, et al. Time course of lung changes at Chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaganti S, Balachandran A, Chabin G, et al. Quantification of tomographic patterns associated with COVID-19 from chest CT. ArXiv [Preprint]. 2020:arXiv:2004.01279v5. [DOI] [PMC free article] [PubMed]
- 55.Cheng Z, Qin L, Cao Q, et al. Quantitative computed tomography of the coronavirus disease 2019 (COVID-19) pneumonia. Radiol Infect Dis. 2020;7(2):55–61. doi: 10.1016/j.jrid.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li MD, Arun NT, Gidwani M, et al. Automated assessment of COVID-19 pulmonary disease severity on chest radiographs using convolutional Siamese neural networks. Radiol Artif Intell. 2020;2(4) doi: 10.1148/ryai.2020200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin X, Min X, Nan Y, et al. Assessment of the severity of coronavirus disease: quantitative computed tomography parameters versus semiquantitative visual score. Korean J Radiol. 2020;21(8):998–1006. doi: 10.3348/kjr.2020.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Homayounieh F, Ebrahimian S, Babaei R, et al. CT radiomics, radiologists and clinical information in predicting outcome of patients with COVID-19 pneumonia. Radiol Cardiothorac Imaging. 2020;2(4) doi: 10.1148/ryct.2020200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wollenstein-Betech S, Cassandras CG, Paschalidis IC. Personalized predictive models for symptomatic COVID-19 patients using basic preconditions: hospitalizations, mortality, and the need for an ICU or ventilator. Int J Med Inform. 2020;142 doi: 10.1016/j.ijmedinf.2020.104258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen C, Wang H, Liang Z, et al. Predicting illness severity and short-term outcomes of COVID-19: a retrospective cohort study in China. Innovation (N Y) 2020;1(1) doi: 10.1016/j.xinn.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji M, Yuan L, Shen W, et al. A predictive model for disease progression in non-severely ill patients with coronavirus disease 2019. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01234-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu JS, Ge P, Jiang C, et al. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J Am Coll Emerg Physicians Open. 2020;1(6):1364–1373. doi: 10.1002/emp2.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdulaal A, Patel A, Charani E, et al. Prognostic modeling of COVID-19 using artificial intelligence in the United Kingdom: model development and validation. J Med Internet Res. 2020;22(8):e20259. doi: 10.2196/20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matos J, Paparo F, Mussetto I, et al. Evaluation of novel coronavirus disease (COVID-19) using quantitative lung CT and clinical data: prediction of short-term outcome. Eur Radiol Exp. 2020;4(1):39. doi: 10.1186/s41747-020-00167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Q, Wang Y, Huang S, et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics. 2020;10(12):5641–5648. doi: 10.7150/thno.46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grodecki K, Lin A, Cadet S, et al. Quantitative burden of COVID-19 pneumonia on chest CT predicts adverse outcomes: a post-hoc analysis of a prospective international registry. Radiol Cardiothorac Imaging. 2020;2(5) doi: 10.1148/ryct.2020200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 68.Sokolowska M, Lukasik ZM, Agache I, et al. Immunology of COVID-19: mechanisms, clinical outcome, diagnostics, and perspectives: a report of the European Academy of Allergy and Clinical Immunology (EAACI) Allergy. 2020;75(10):2445–2476. doi: 10.1111/all.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klironomos S, Tzortzakakis A, Kits A, et al. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297(3):E324–E334. doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B, Li R, Lu Z, et al. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akl EA, Blažić I, Yaacoub S, et al. Use of chest imaging in the diagnosis and management of COVID-19: a WHO rapid advice guide. Radiology. 2021;298(2):E63–E69. doi: 10.1148/radiol.2020203173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin S, Li Z, Fu B, et al. Feasibility of using deep learning to detect coronary artery disease based on facial photo. Eur Heart J. 2020;41(46):4400–4411. doi: 10.1093/eurheartj/ehaa640. [DOI] [PubMed] [Google Scholar]
- 80.Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7(9):813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ransing R, Nagendrappa S, Patil A, et al. Potential role of artificial intelligence to address the COVID-19 outbreak-related mental health issues in India. Psychiatry Res. 2020;290 doi: 10.1016/j.psychres.2020.113176. [DOI] [PubMed] [Google Scholar]