Abstract
Background/purpose
Curcumin has anti-inflammatory impacts and was suggested as an inflammatory disease therapy. This study aimed to investigate the implications of curcumin gel on experimental periodontitis (EPD) and alveolar bone loss in rats.
Materials and methods
In this study, twenty-four male Wistar rats were divided equally into four groups: negative control (with no EPD); positive control (EPD induced around lower centrals without treatment); control-treated group: EPD treated with chlorhexidine; and test EPD group treated with curcumin. After 30 days, the serum concentrations of RANKL and IL-1β were measured via ELISA. All animals were sacrificed, and mandibular central incisors with the periodontium were removed. The lingual probing depth and radiographical alveolar bone loss were measured, then samples processed for routine preparation of H&E stained sections and histologically assessed for counting inflammatory cells, osteoclasts, and PDL width.
Results
A significant decrease in the inflammatory cells infiltration, probing depth, and osteoclast numbers with the improvement of PDL associated with a reduction in RANKL and IL-1β serum concentration were seen in both EPD treated groups.
Conclusion
Curcumin is as effective as chlorhexidine in treating experimental periodontitis in rats. It was demonstrated to stop bone destruction related to periodontitis by regulating the RANKL and IL-1β markers level in the blood.
Keywords: Curcumin gel, Experimental periodontitis, IL-1β, RANKL
Introduction
Periodontitis is an immune-inflammatory disease presented by clinical attachment and alveolar bone loss as a consequence of the host response to the presence of polymicrobial subgingival oral biofilms.1 As one of the most widespread inflammatory conditions affecting the adult population, this disease is still a significant public health burden throughout the world.2 It is one of the most commonly known diseases in humans. The progression of the disease is identified with the colonization of main microorganisms such as Porphyromonas gingivalis.3
Root surface debridement is the “gold standard” mechanical therapy used for the treatment of periodontal diseases,4 but it achieves limited effects on some pathogenic species when used alone. Therefore, a complete elimination of subgingival bacteria is often not accomplished. This may be because some of these species may stay in soft tissues, dentinal tubules, or root surface defects, resulting in failure of therapy.5
Localized antimicrobial therapy has aroused remarkable interest due to the site-specific nature of periodontal diseases; the suggestion being that antimicrobial agent concentration decreases the side effects of systemic antimicrobial remedies. Local drug delivery's main advantage is involvement of minimal systemic participation, improved patient compliance, and least discomfort. Local drug supply releases the antimicrobial agent at an unchanged pharmacological level for an extended period of time.6 Due to the relatively secure nature of herbal extracts and the disadvantages and complications of the many chemical and artificial drugs, crops still provide a very significant supply of medication in several nations globally, despite the substantial advancements in bioscience. The use of assorted herbal or ayurvedic drugs for oral and dental health has recently received revived interest and these come in the form of mouth rinses, gels, and chips.7
“Curcumin” is the main active ingredient of turmeric (Curcuma longa), an Indian spice extracted from rhizomes, a perennial member of the Zingiberaceae family. It was recognized in 1910 by Lampe and Milobedzka for its vivacious yellow color.8 The composition and percentage of the separate biologically active components in the rhizome extract of the curcumin were variable.9 A significant number of published researches mentioned the anti-inflammatory and wound healing, anti-microbial and anti-neoplastic properties of curcumin, in both In-vitro and In-vivo studies. It was tested with several diseases, such as such as diabetes, neurological disturbances, cancer, auto-immune conditions and chronic inflammatory conditions including Crohn's disease, rheumatoid arthritis and periodontal disease.10,11 It has also been suggested that the relationship between natural goods and preventive care can reduce the elevated incidence of conditions that affect the dental component, such as periodontal disease.12
For trabecular bone, which is nearer to the cytokine-rich marrow than the cortical bone, cytokine regulation is probably essential.13 In relation to their impacts on bone cells, many potent osteotropic cytokines, such as IL-1, IL-6, TNF-α, and TGF-β, mediate a variety of effects in the body. The production of cytokines by osteoblasts and bacteria and lipopolysaccharides is controlled by different hormones and cytokines.14 The pro-inflammatory cytokines IL-1β engage in stimulating osteoclastic resorption in periodontitis, upregulate matrix metalloproteinases and downregulate metalloproteinase manufacturing tissue inhibitors. It is well documented that IL-1β is involved in the pathogenesis of inflammation-induced bone resorption.15
RANKL, a polypeptide of 314 amino acids, is a member of the TNF cytokine family. RANKL expression and activation is essential for the absorption and metabolism of alveolar bone.16 RANKL binds to RANK on osteoclast precursors and induces osteoclast formation.17 RANKL is expressed in many cell types, including osteoblasts, lymphocytes, and osteocytes.18 It is considered the primary regulator of bone metabolism and is also regarded as essential for the mechanism of periodontal destruction in periodontitis in which mechanical forces or bacterial challenges in periodontitis are caused in periodontal fibroblasts.19
Due to the lack of information about the impacts of local application of curcumin on experimental periodontitis (EPD) inhibition, our research was directed to evaluate the effects of curcumin gel in treating EPD under microscope and its ability to minimize the amount of alveolar bone loss as assessed by radiographical and molecular levels.
Material and methods
Animals and experimental design
Twenty-four male Wistar albino rats aged (3–4 months) and weighing 250–300 g were used in this study, following the principles of laboratory animal care (NIH publication 85-23, 1985).20 The study was approved by the Ethics Committee for Animal Research, College of Dentistry of Sulaimani University, Iraq (292/2016). The rats were allowed to acclimate for one week prior to the first procedure in temperature-controlled rooms, inside plastic cages identified by their group types and periods. The cages were cleaned daily. A regular rodent pellet diet and water were provided ad libitum throughout the experiment.
Rats were randomly and equally allocated into four groups as follows: Negative control: rats with normal periodontium (NP) and not subjected to EPD or treatment; Positive control: rats with EPD and without treatment; Control-treatment (CHX-T-EPD): rats with EPD and treated with 0.2% chlorhexidine gel; Test-treatment (Cu-T-EPD): rats with EPD and treated with 12.5 μg/ml curcumin gel.
The gel was administered one time in a day, via local application of materials through a syringe with a blunt end, for 30 days (i.e., from day 0 to day 30). The rats were evaluated daily throughout the experiment to ascertain for sutures and attainable clinical or toxicological symptoms.
Probing depth measurement
A pre-experimental clinical examination was conducted after anesthetizing the animals (using IM injection of a mixture of ketamine hydrochloride 3.3 ml and xylazine hydrochloride 2 ml, in a dose of 0.05 ml/kg of body weight) to exclude animals with periodontal probing depths surpassing 0.5 mm.21 The Probing Depth was indicated as the measured distance from the gingival margin to the base of pocket by using a Williams periodontal probe. The measurements were done at the beginning before the placement of sutures and at the end of the experiment before the scarification of the animals.
Induction of experimental periodontitis (EPD) in the rat model
Experimental periodontitis was induced by a combination of ligature and application of P. gingivalis as follows: After anesthetizing the animals, ligatures in 8-shape with 4/0 non-resorbable sterile silk suture were placed in the cervical area of lower incisor teeth, resulting in a subgingival position lingually and a supragingival position labially. As previously described, this ligature induced gingival irritants and promoted the accumulation of plaque and, subsequently, development of periodontal disease.22
Preparation and application of P. gingivalis slurry
The preserved clinical isolated strain of P. gingivalis was grown at 37 °C for 2 days on Columbia agar plates supplemented with %5 Human blood, 5 μg/ml of Hemin (Sigma Aldrich, Shanghai, China), and 1 μg/ml vitamin K1 (Himedia, Mumbai, India) under anaerobic conditions by using anearopack system (Mitsubishi Gas Chemical, Katsushika, Japan). The largest colonies were then selected and anaerobically sub-cultured at 37 °C for 48 h in nutrient broth (Himedia, Mumbai, India) supplemented with hemin (Sigma Aldrich) and vitamin k1 (Himedia). After applying PBS, spectrophotometry with 624 nm wavelength was used to make a bacterial concentration of up to 1 × 106 CFU; then, the bacteria were mixed with 4% sodium carboxymethyl cellulose (Panreac, Barcelona, Spain). Finally, 0.03 ml of live P. gingivalis with a concentration of 1 × 106 CFU/ml were locally applied to the rats in the gingival sulcus bottom of the left and right mesial and lingual sides of their incisor teeth. The same procedure was repeated every three days for two weeks.
Treatment of periodontitis
The therapeutic mucoadhesive gels were administered intragingivally for the two EPD groups one day after induction and thereafter daily.
Chlorhexidine gel
In the control-treatment group, rats received 0.2% chlorhexidine gel (Kin Company, Barcelona, Spain) that consisted of the following ingredients: Sorbitol, Aqua, PEG-60 Hydrogenated Castor Oil, Hydroxyethylcellulose, Aroma, Chlorhexidine Digluconate (0.20%), Sodium Methylparaben, Methyl Salicylate, Menthol, Citric Acid, Eugenol, d-Limonene.
Curcumin gel
In the test-treatment group, rats were treated with 12.5 μg/ml curcumin gel23 that consisted of Curcumin powder: 95% Curcumin (Bulk Supplements Pure Curcumin 95% Natural Turmeric Extract Powder), Potassium sorbate (Analitik Kimya Ve Lab. Cih. San. Tic. Ltd. Sti. Istanbul, Turkey), Propylene glycol (Pharmaco-Aaper, Bengaluru, India), Metalose 90SH 10000 (Shin-Etsu Chemical Co., Chiyoda, Japan) and purified water. The gel was formulated by Awa Medica Company, Hawler, Kurdistan Region, Iraq.
Enzyme-linked immunosorbent assay (ELISA) for the RANKL and IL-1β
Blood was drawn (3 ml) from each rat by intracardiac puncture just before being sacrificed. The blood samples were transferred into empty tubes, centrifuged at 3000 g for 10 min, and dissociated to serum. The acquired serum samples were kept in tubes at −80 °C until analysis. IL- 1β and RANKL (E0119Ra and E0289Ra, respectively; Bioassay Technology Laboratory, Shanghai, China) levels were analyzed by ELISA following the manufacturer's instructions.
Collection of the teeth and periodontal tissue samples
At the end of the experiment, all animals were anesthetized and sacrificed by cervical dislocation 24 h after the last local treatment application. The mandibles were resected and bisected from both sides posterior to the incisor teeth and then processed routinely to prepare H&E stained tissue sections.
Radiographical measurement of alveolar bone loss
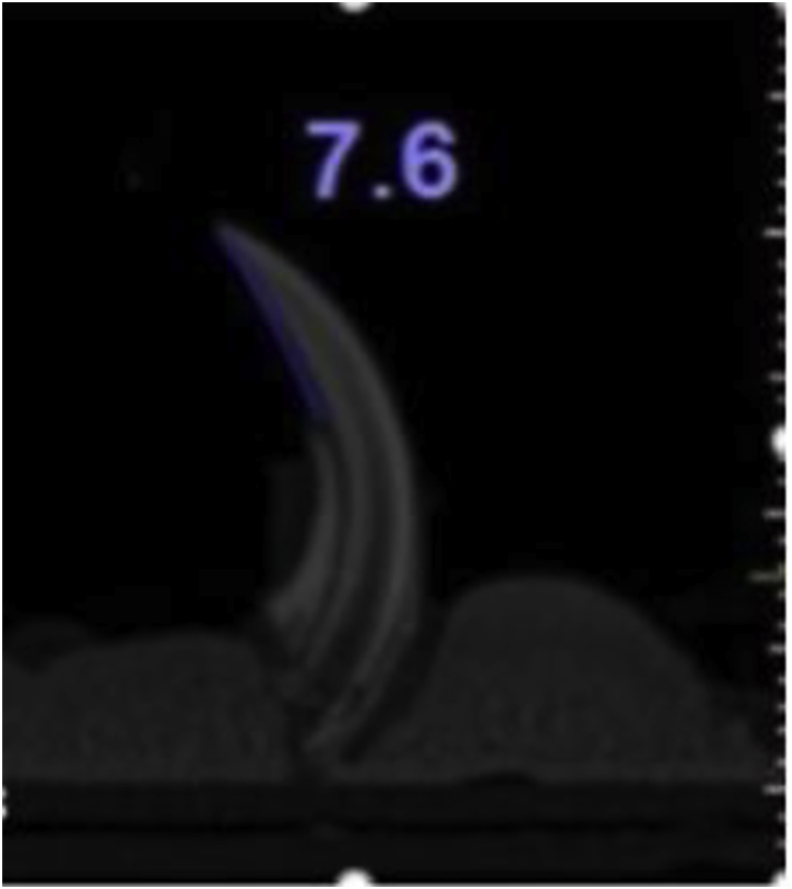
The harvested fixed samples were fitted perpendicularly on heavy body impression materials. Then they were exposed to CBCT using a tomography scanner Model-Cat (Imaging Sciences International) with an exposure area of 6 cm, an exposure time of 40 s, and voxel of 0.2 mm at a maximum resolution. Sagittal cuts of the digital images were made at the center of the mesiodistal width and analyzed by the i-Cat Cone-Beam 3D program for Dental Imaging System, version 3.1.62. The linear distance between the tip of the crown and the alveolar bone crest was measured,24 with measurements being made along the axis of the crown in the midlingual side (Fig. 1).
Figure 1.
Measurement of alveolar bone loss by using CBCT, measurements being made along the axis of the crown in the midlingual side.
Histo-morphometric assessment
The fixed tissue samples were decalcified by daily exchange of 10% EDTA solution for 20 days. They were longitudinally trimmed (buccolingual) and routinely prepared to get hematoxylin and eosin stained tissue sections for histological interpretation. Quantitative assessment for inflammatory reaction in the gingival connective tissue at the lingual side was performed (at 400-fold magnification) by using a light microscope equipped with an image analyzing system (Motic, ToupTek, ToupView, ×86, 3.7.4183, and 2014). Each captured image was divided by a grid containing 16 squares. All types of inflammatory cells (polymorphonuclear (PMN) and mononuclear (MC)) were counted. The mean number for each group was calculated. The inflammatory cells were scored as follows: no reaction or score 0 (0–25 inflammatory cells), mild or score 1 (26–50 inflammatory cells), moderate or score 2 (51–75 inflammatory cells), and severe or score 3 (more than 75 inflammatory cells).25
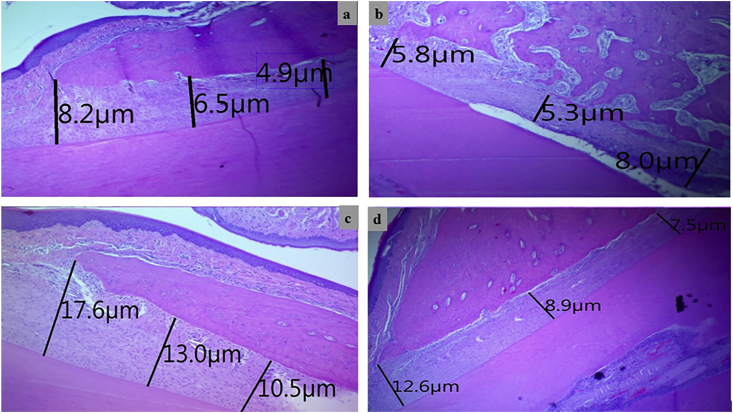
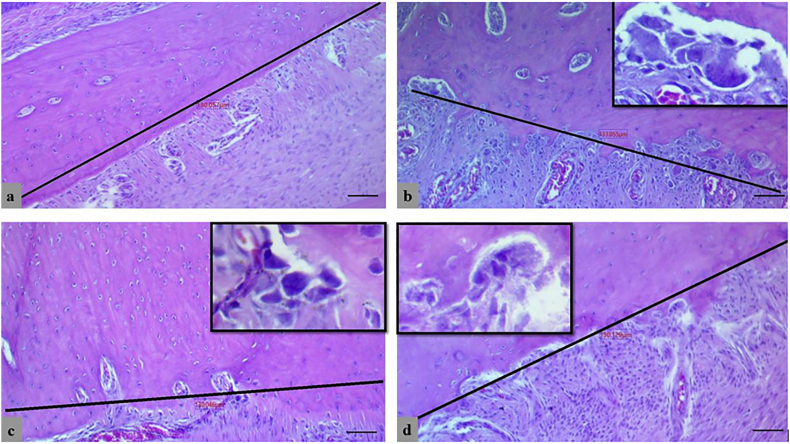
The linear measurement for the width of the PDL was estimated by the same software at three levels (at the crest, 150 μm along the bone, and point in between) perpendicularly from the alveolar bone surface to the cementum (Fig. 4).
Figure 4.
Histometric linear measurement for periodontal ligament's width at three levels in control negative group (a), EPD control-positive group (b), Chlorhexidine-treated group (c) and, Curcumin treated group (d), (H&E, scale bar 10 μm).
The osteoclasts were identified as having: multiple nuclei, ruffled border, and granular cytoplasm, in H&E stain sections. The sum of osteoclasts on the alveolar bone's surface was calculated in each section for all animals in each group along the distance of 130 μm from the alveolar crest.
Statistical analysis
Data were analyzed using the statistical software package, SPSS (version 22.0, Chicago IL, USA). The Shapiro–Wilk test was used to test the normality of data. One-way ANOVA test was carried out to ascertain the significance of differences among groups, then LSD tests were done in order to make a pairwise comparison between groups and the values were presented as mean ± standard error (SE) for parametric variables, while median with Man Whitney test was used for non-parametric variables. P ≤ 0.05 was regarded as statistically significant and used to identify differences among parametric and non-parametric variables.
Results
Histopathological findings
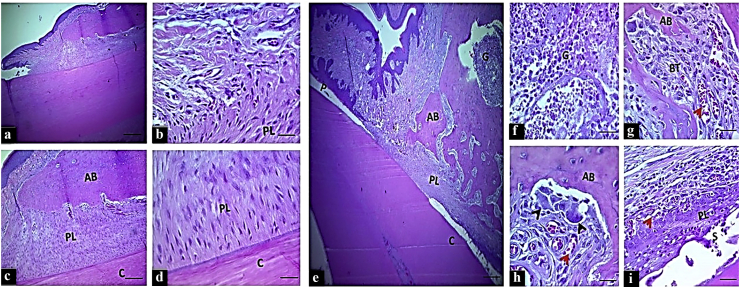
The histo-morphology of the periodontium in the negative control group NP showed typical normal structures and organization without any evidence of inflammation or bone loss (Fig. 2(a–d), whereas the predominant histologic feature in the control positive group (EPD) was severe destruction of periodontal tissue. It revealed distinct junctional epithelial disruption with pocket formation, marked mixed inflammatory cells infiltration (neutrophil, macrophages, plasma cells, and lymphocytes) in the gingival connective tissue above the crestal bone and in the bone marrow (Fig. 2(e–g). There were irregular newly formed calcified and osteoid bone trabeculae that showed marked surface irregularity, surrounded by large numbers of osteoclast, fibroblast, and inflammatory cells adjacent to congested blood vessels. The periodontal ligament was partially degenerated and did not attach to the cementum surface. It consisted of disorganized collagen fibers and active fibroblasts (Fig. 2(h and i).
Figure 2.
The H&E stained histologic section of an incisor tooth and periodontal tissue of rat. Control negative group (from a to d): Normal histo-morphology of gingival and periodontal tissues. Control positive group (from e to i): e–f: Marked periodontal pocket (P), disruption of the junctional epithelium and existence of granulation tissue (G) at the insertion point and above the bone crest, disorganized bone trabeculae (BT). g–h: irregular bone surface with presence of osteoclasts involved in bone matrix cavity (black head arrows), and i: large space of the periodontal ligament filled with active fibroblast and disorganized tissue that did not attach to the cementum, (scale bar 10 μm in section a, and 20 μm in section b–e. AB: alveolar bone, PL: periodontal ligament and C: cementum.
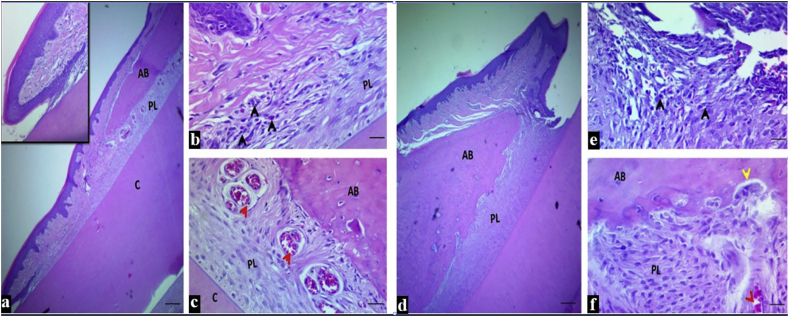
Microscopical findings of periodontal tissues around the incisor teeth in both treated groups showed: intact junctional epithelium with mild inflammatory reaction in the insertion point, a regular-surfaced, well-formed dense bone. However, the large space of uniform width of the periodontal ligament in the CHX-T-EPD group was filled with organized active fibrous tissue that was attached to a regular cementum surface (Fig. 3(a–c), unlike the Cu-T-EPD group in which the PDL consisted of less coordinated active tissue (Fig. 3(d–f).
Figure 3.
The H&E stained histologic section of an incisor tooth and periodontal tissue of rat in chlorhexidine treated group (from a to c): a-b: Intact junctional epithelium with mild inflammatory cells in the insertion point (inset), c: Regular bone surface with well-formed dense bone, a narrow periodontal ligament space of uniform width filled with organized active periodontal ligament tissue attached to a regular cementum surface and multiple dilated blood vessels (red arrow head). Curcumin treated group (from d to f): d: Intact junctional epithelium, e: With mild to moderate inflammatory cells in the insertion point. f: Irregular bone surface with resorption area as indicated by yellow arrow (osteoclast) with well-formed dense bone, a wide periodontal ligament space of uniform width filled with less organized active periodontal ligament tissue attached to a regular cementum surface and dilated blood vessels (red arrow). Scale bar 10 μm in section a, and 20 μm in section b and c. AB: alveolar bone, PL: periodontal ligament and C: cementum).
Assessment of inflammatory reaction
The total number of inflammatory cells in the EPD group was significantly higher (67.6 ± 14.15, Score 2, P = 0.0001) than their count in the NP group (12.3 ± 1.52, Score 0). The main increase was in the total PMNLs (23.3 ± 4.15) (12.2 neutrophils and 11.1 eosinophil, P = 0.001 and 0.003 respectively). Meanwhile, MCs showed a significant increase in their median from 5 to 29 (Table 1). It was predominated by macrophages (median of 23). Substantially, the total inflammatory cells count was significantly decreased after both CHX (30.7 ± 6.12, Score 1, P = 0.002) and curcumin (34.7 ± 3.26, Score 1, P = 0.006) treatments. CHX-T-EPD resulted in significant decline in neutrophil count (P = 0.0001), macrophage (P = 0.035) and plasma cell (0, P = 0.029), but not in eosinophil (P = 0.27); however, it was associated with insignificant decrease in lymphocytes (2, P = 0.063). Cu-T-EPD showed an insignificantly higher cell count than CHX-T-EPD (p = 0.72). In comparison to the EPD (control positive) group, the reduction in the differential count of each inflammatory cells type in Cu-T-EPD group was insignificant except for plasma cells (P = 0.004) and macrophage (P = 0.011) (Table 1).
Table 1.
The total and differential inflammatory cell counts with their p values in all studied groups.
| Groups | Total (mean ± SE) | PMNL cells (mean ± SE) |
Mononuclear cells (median) |
||||
|---|---|---|---|---|---|---|---|
| Neutrophil | Eosinophil | Basophil | Lymphocyte | Plasma cell | Macrophage | ||
| Normal healthy (P value; Normal healthy vs EPD) | 12.3 ± 1.52 (0.0001)a | 4.50 ± 1.09 (0.001)a | 2.60 ± 0.56 (0.003)a | 0 | 2 (0.005)b | 0 (0.002)b | 3 (0.0001)b |
| EPD (P value; EPD vs CHX -T-EPD) | 67.6 ± 14.15 (0.002)a | 12.20 ± 1.8 (0.0001)a | 11.10 ± 2.35 (0.27)a | 0 | 5 (0.063)b | 1 (0.029)b | 23 (0.035)b |
| CHX -T-EPD (P value; CHX -T-EPD vs Cu-T-EPD) | 30.7 ± 6.12 (0.72)a | 3.90 ± 0.70 (0.007)a | 8.10 ± 2.46 (0.85)a | 0 | 2 (0.089)b | 0 (0.68)b | 11 (0.679)b |
| Cu-T-EPD (P value; Cu-T-EPD vs EPD) | 34.7 ± 3.26 (0.006)a | 9.90 ± 1.9 (0.278)a | 7.60 ± 1.62 (0.203)a | 0 | 5 (0.68)b | 0 (0.004)b | 11.5 (0.011)b |
Within each row, values expressed by Mean ± SE, and P values, P ≤ 0.05 considered as significant.
PMNL, polymorphonuclear leukocyte; EPD, Experimental periodontitis; CHX -T-EPD, Chlorhexidine treated experimental periodontitis; Cu-T-EPD, Curcumin treated experimental periodontitis.
One-way ANOVA-LSD.
Mann–Whitney Test.
Histomorphometric result of periodontal ligament width
The linear measurement of PDL width in the EPD group showed a significant increase (29.08 ± 2.05, P = 0.0001) in comparison to the control NP group (19.06 ± 1.13). Both chlorhexidine and Curcumin treatments resulted in a critical decrease in the distance that approximated the healthy group's value (Fig. 4, Table 2).
Table 2.
The Mean and p values for: osteoclast cells, measurement of bone loss and PDL width, level of IL1 beta and RANKL expression and, Probing depth (median) in each group of the study.
| Groups | Osteoclast cell count | Alveolar bone loss | PDL width | IL-1β (pg/μl) | RANKL (pg/μl) | Probing depth |
|---|---|---|---|---|---|---|
| Normal healthy (P value; Normal healthy vs EPD) | 0.000 (0.0001)a | 6.21 ± 0.08 (0.0001)a | 19.06 ± 1.13 (0.0001)a | 1543.33 ± 194.21 (0.0001)a | 210 ± 19.32 (0.0001)a | 0.5 (0.0001)b |
| EPD (P value; EPD vs CHX -T-EPD) | 4.33 ± 0.84 (0.0001)a | 8.80 ± 0.42 (0.0001)a | 29.08 ± 2.05 (0.002)a | 8666.66 ± 299.62 (0.0001)a | 514.16 ± 44.35 (0.001)a | 3 (0.002)b |
| CHX-T-EPD (P value; CHX -T-EPD vs Cu-T-EPD) | 1.16 ± 0.47 (0.826)a | 7.00 ± 0.11 (0.532)a | 20.79 ± 2.00 (0.905)a | 4400 ± 288.67 (0.612)a | 343.33 ± 21.55 (0.591)a | 1 (0.132)b |
| Cu-T-EPD (P value; Cu-T-EPD vs EPD) | 1.33 ± 0.42 (0.001)a | 7.21 ± 0.16 (0.0001)a | 20.51 ± 1.03 (0.001)a | 4230 ± 81.44 (0.0001)a | 320 ± 29.09 (0.0001)a | 1 (0.002)b |
Within each row, values expressed by Mean ± SE, and P values, P ≤ 0.05 considered as significant.
PDL, Periodontal ligament width; IL-1β, Interleukin 1 beta; RANKL, receptor activator of nuclear factor kappa beta ligand; pg/μl, picograms/microliters; EPD, Experimental periodontitis; CHX-T-EPD, Chlorhexidine treated experimental periodontitis; Cu-T-EPD, Curcumin treated experimental periodontitis.
One-way ANOVA-LSD.
Mann–Whitney Test.
Assessment of osteoclast cell count
The healthy control periodontium did not contain osteoclasts, while the EPD group was characterized by a significantly high number of osteoclasts (4.33 ± 0.84, P = 0.0001). The curcumin gel result resembled that for chlorhexidine, with a significant drop in the number of osteoclasts (1.33 ± 0.42, P = 0.001) observed in the EPD group (Fig. 5, Table 2).
Figure 5.
Histometric imaging for counting osteoclasts (insets) at the alveolar bone surface in control negative-group (a), EPD control-positive group (b), Chlorhexidine-treated group (c) and, Curcumin-treated group (d), (H&E, scale bar 20 μm).
Assessment of probing depth (PD)
The highest median in the case of probing depth measurements (3, P = 0.0001) was seen in the gingiva of the EPD group, whereas with both types of treatment the median of the PD decreased to (1) with significant effect (P = 0.002), (Table 2).
Assessment of alveolar bone loss
The control positive EPD group significantly showed marked bone loss (8.80 ± 0.42, P = 0.0001), whereas subsequently the bone loss in both treated groups was significantly decreased (P = 0.0001) in comparison to the control positive group. In CHX-T-EPD the bone loss reached (7.00 ± 0.11) and slightly more bone loss (7.21 ± 0.16) was found in the Cu-T-EPD group, with no significant differences between the two treatments (Table 2).
ELISA assessment of IL1 beta and RANKL serum concentration
The mean concentrations of IL-1β (8666 ± 299.62) and RANKL (514.16 ± 44.35) in the EPD group revealed a highly significant (P = 0.0001) elevation in their expression in comparison to the healthy control group. While there was a highly significant (P = 0.0001) reduction in their concentration at the end of the experiment in both treated groups (Table 2).
Discussion
Periodontal diseases are latent diseases with a wide universal incidence of disease and associated morbidity and socioeconomic impacts.26 Mechanical plaque removal is the cornerstone of periodontal therapy. Since the etiology of periodontitis is complex, the response to periodontal treatment is not comprehensive. Therefore, the dentist should be aware of different attainable treatment approaches.27
Studies for drugs as host modulators in periodontal treatment have become popular.28 The discovery of a new treatment that can prevent or reduce bone loss provides possibilities for finding an agent that not only acts on inflammatory reactions but also decrease bone loss that occurred during periodontitis.
While the use of systemic medicines against periodontal disease has been shown in several published research with various side effects.29 Curcumin has been known worldwide for its many health benefits30 and clinical use of Curcumin as a suitable adjunct in the treatment of periodontitis has been reported by many researchers31, 32, 33 and several others. Therefore, the aim of the current study was to assess the impact of local application of curcumin gels in EPD in rat, depending on its different biological and medicinal properties34 and to clarify the processes by which this natural plant product acts and compared it with chlorhexidine, the well-known antimicrobial drug.
Bacteria are known as the primary causative agents of periodontal disease.35 Local inflammatory response which are triggered by specific gram-negative anaerobic bacteria including host immune cell enlisting and release of cytokines (e.g; IL1 β), chemokines (e.g; CXCR2) and other signaling molecules, which will cause energizing of osteoclasts and bone destruction and finally initiation of periodontitis.36,37 The present study confirmed the significant pathogenic effect of P. gingivalis in EPD group by producing gingivitis and periodontitis. A previous study proved that P. gingivalis flora during periodontal disease frustrates the supporting tissue leading to inflammatory reaction and ultimately leading to tooth loss.29 Local CHX application as periodontal therapy significantly reduced the histopathological changes in periodontium and decreased bone loss. This finding is in accordance with previous study in which topical application of 0.2% chlorhexidine gel intended to be useful as a periodontal therapy by reducing the formation of pellicles, modifying bacterial adherence to teeth and lysis of bacterial cell walls.38
On the other hand, histologically, curcumin gel minimized the soft tissue and bone destructions caused by EPD in a better way than CHX gel that conventionally used for the treatment of periodontitis. Previous reports endorse that curcumin gel is a representative anti-microbial agent whose activities are mediated by inhibiting of P. gingivalis LPS-induced inflammatory markers.39 Furthermore, Tyagi et al. demonstrated the membrane permeabilizing activity of curcumin, which increases the efficacy of obsolete antibiotics by enhancing their cellular uptake.40
The importance of inflammatory cells within gingival tissue in the evolution of periodontal disease has been demonstrated previously.41 Although inflammatory cells play critical roles in eliminating the causes of inflammations, the activated PMNs and macrophages also generate oxygen metabolites42 and activating cytotoxic enzyme43 at higher levels in periodontal diseases.44 In the present study, EPD had a significant high inflammatory cell infiltration scored at level 2, while Cu-T-EPD group revealed predominant reduction in cell infiltration and scored by level 1. Thus it suggested curcumin gel ability to suppress the cytotoxic effects of PMNs and macrophages. Our finding was in line with previous report concerning the anti-inflammatory properties of curcumin45 that believed to be due to inhibition of NF-кB and кB-kinase activity.46
Alveolar bone loss in the EPD group evaluated histologically by increasing the number of active osteoclast cells, leading to increase gingival soft tissue component, reduce bone volumes and, significant increase in PDL width that replaced by inflamed granulation tissue. These results are very comparable to earlier research.47 However, these histopathological changes were significantly reduced after local chlorhexidine or curcumin treatments and being better in the latter. These findings were in accordance with the Hu et al. and Xiao et al. reports. They documented the cytotoxic curcumin's ability to attenuate disease process of periodontitis via suppression of the NF-κB pathway in human gingival fibroblasts and improving the histopathological features of periodontitis.48,49
The clinical probing-depth measurements referenced as criteria to monitor experimental periodontitis model induction and ability of interference,50 and in our study, PD was the deepest in EPD group (median = 3). Cu-T-EPD like CHX-T-EPD resulted in better efficacy (median 1). These results are in conjunction with the curcumin characteristics against periodontitis and probing-depth reduction that several trials have outlined.32,33,51 Curcumin may, therefore, be used for oral care and, in particular, for periodontitis due to its antimicrobial activity against periodontal bacteria.39 It has a potent anti-inflammatory and antioxidant activity,52 and also because of a it has lesser adverse effect than chlorhexidine drug.51
Pro-inflammatory cytokines can play a significant role in periodontal diseases.53 Periodontitis may be potentiated by the TNF- stimulated the release of eicosanoids and other cytokines, such as IL-1 β. IL-1 β activates neutrophils and macrophages and thereby induces the production and release of reactive oxygen species and nitric oxide, which has been implicated to be a cause of local tissue damage.54 Quantitative measurement of serum IL-1β level in this experimental research increased five-times and a half in EPD group than in its concentration in NH animals, which reflect its association with severe periodontal tissue damage. This information strengthens Baqui et al. findings that indicated lipopolysaccharide derived from P. gingivalis is a significant inflammatory stimulus and trigger of the host's immune response. It induces macrophages and monocytes to produce IL-1β, which can lead to inflammatory cascades resulting in periodontal tissue destruction.55 On the contrary, Cu-T-EPD animals dramatically lowered serum IL-1β level to its half concentration in EPD but twice that in NH animals, just like CHX-T-EPD animals. Thus it provides direct evidence to its anti-inflammatory effects by suppressing IL-1β serum level and improving periodontal condition. Besides, the potent antioxidant activity of curcumin that reduce oxidative stress and free radicals produced during inflammatory reaction56 and this findings agree with Xiao et al.49
Previous study revealed that RANKL is not only involved in physiological osteoclastogenesis, as well as in pathological bone loss.57 Consequently, interference with the differentiation, activation, and function of RANKL-induced osteoclast will lead to the abolition of periodontal bone resorption and inhibit disease progression.58 Therefore, one of the molecules that regulate the activation of osteoclasts is RANKL (receptor activator of nuclear factor kappa-B ligand), and it stimulates RANK (receptor activator of nuclear factor κB). While osteoprotegerin (OPG) binds to RANKL before it binding to RANK, and suppresses bone resorption process. Therefore, RANKL activation is essential for the absorption and metabolism of the alveolar bone, and the OPG/sRANKL ratio is an important determinant for bone resorption. Excess RANKL changes the bone metabolism balance to catabolism, resulting in periodontal bone resorption.57 Likewise, sRANKL inhibition probably reduces periodontal bone resorption.59 The EPD model in this study indicated strong association between periodontitis and boosted concentrations of RANKL. A marked bone loss and the presence of numerous osteoclasts were accompanied by the duplicating level of RANKL to that registered in NH group. Accordingly, we reported an improvement in periodontium health associated with a reduction in RANKL concentration after treating the EPD animals in control remedy (CHX gel) and testing Curcumin gel. Curcumin reduced bone resorption or osteoclastogenesis by suppression of RANKL expression60 and increased osteoprotegerin (OPG) release, resulting in high OPG/sRANKL ratio.49 Several limitations were present when the study performed such as difficulty in propagation and manipulation of rats, difficulty in getting supplements for growth of P. gingivalis, and in getting rat IL1 beta and RANKL Kits. In conclusion, curcumin caused a significant reduction in inflammation-mediated destruction of periodontal soft and hard tissues. It also modulated osteoclastogenesis and remodeling of the bone. This study provides a new anti-bone loss therapeutic option for periodontal diseases through regulation of RANKL and IL- 1β markers level in the blood of periodontitis rats induced by P. gingivalis + ligature and may influence a translational effect on the management of periodontal bone loss.
Declaration of competing interest
The authors have no conflict of interest relevant to this article.
Acknowledgments
The authors express their thanks to the staff of the laboratories of College of Science biology department, veterinary research center, Histopathological Department in Shorsh General Hospital, Awa medica company, and finally extend thanks to the Ministry of Higher Education and Scientific Research, University of Sulaimani KRG, Iraq, to do this research project are also greatly acknowledged. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Aral C.A., Kesim S., Greenwell H., Kara M., Cetin A., Yakan B. Alveolar bone protective and hypoglycemic effects of systemic propolis treatment in experimental periodontitis and diabetes mellitus. J Med Food. 2015;18:195–201. doi: 10.1089/jmf.2013.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin L., Lamster I., Greenspan J., Pitts N., Scully C., Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22:609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 3.Mızrak T., Güncü G.N., Çağlayan F., Balcı T.A., Aktar G.S., İpek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–443. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 4.Cugini M., Haffajee A., Smith C., Kent R., Jr., Socransky S. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–36. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 5.Colombo A.P.V., Teles R.P., Torres M.C. Effects of non-surgical mechanical therapy on the subgingival microbiota of Brazilians with untreated chronic periodontitis: 9-month results. J Periodontol. 2005;76:778–784. doi: 10.1902/jop.2005.76.5.778. [DOI] [PubMed] [Google Scholar]
- 6.Greenstein G., Polson A. The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998;69:507–520. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 7.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.-M. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 9.Hayakawa H., Minaniya Y., Ito K., Yamamoto Y., Fukuda T. Difference of curcumin content in Curcuma longa L.(Zingiberaceae) caused by hybridization with other Curcuma species. Am J Plant Sci. 2011;2:111. [Google Scholar]
- 10.Abdollahi E., Momtazi A.A., Johnston T.P., Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol. 2018;233:830–848. doi: 10.1002/jcp.25778. [DOI] [PubMed] [Google Scholar]
- 11.Kunnumakkara A.B., Bordoloi D., Padmavathi G. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juiz P.J., Alves R.J., Barros T.F. Use of natural products as adjuvant in the treatment of periodontal disease. Rev Bras Farmacogn. 2010;20:134–139. [Google Scholar]
- 13.Oates T., Cochran D.L. Bone cell interactions and regulation by inflammatory mediators. Curr Opin Periodontol. 1996;3:34–44. [PubMed] [Google Scholar]
- 14.Bord S., Horner A., Hembry R., Reynolds J., Compston J. Production of collagenase by human osteoblasts and osteoclasts in vivo. Bone. 1996;19:35–40. doi: 10.1016/8756-3282(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande N., Deshpande A. Immunoregulation in periodontal disease. Indian J Dent. 2013;4:35–37. [Google Scholar]
- 16.Walsh N.C., Alexander K., Manning C.A. Activated human T cells express alternative mRNA transcripts encoding a secreted form of RANKL. Gene Immun. 2013;14:336. doi: 10.1038/gene.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofbauer L.C., Heufelder A.E. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 18.Pacios S., Xiao W., Mattos M. Osteoblast lineage cells play an essential role in periodontal bone loss through activation of nuclear factor-kappa B. Sci Rep. 2015;5:166–194. doi: 10.1038/srep16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostancı N., Ilgenli T., Emingil G. Differential expression of receptor activator of nuclear factor-κB ligand and osteoprotegerin mRNA in periodontal diseases. J Periodontal Res. 2007;42:287–293. doi: 10.1111/j.1600-0765.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 20.Health NIo . NIH Publication; 1985. Guide for the care and use of laboratory animals; pp. 85–123. [Google Scholar]
- 21.Verzeletti G.N., Gaio E.J., Linhares D.S., Rösing C.K. Effect of obesity on alveolar bone loss in experimental periodontitis in Wistar rats. J Appl Oral Sci. 2012;20:218–221. doi: 10.1590/S1678-77572012000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ionel A., Lucaciu O., Moga M. Periodontal disease induced in Wistar rats-experimental study. Hum Vet Med. 2015;7:90–95. [Google Scholar]
- 23.Sha A.M., Garib B.T. Antibacterial effect of curcumin against clinically isolated Porphyromonas gingivalis and connective tissue reactions to curcumin gel in the subcutaneous tissue of rats. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6810936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford J.M., Taubman M.A., Smith D.J. The natural history of periodontal bone loss in germfree and gnotobiotic rats infected with periodontopathic microorganisms. J Periodontal Res. 1978;13:316–325. doi: 10.1111/j.1600-0765.1978.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 25.Azeez S.H., Gaphor S.M. Evaluation of antibacterial effect against Porphyromonas gingivalis and biocompatibility of essential Oil extracted from the gum of Pistacia atlantica kurdica. BioMed Res Int. 2019;2019 doi: 10.1155/2019/9195361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buset S.L., Walter C., Friedmann A., Weiger R., Borgnakke W.S., Zitzmann N.U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol. 2016;43:333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 27.Shaddox L.M., Walker C.B. Treating chronic periodontitis: current status, challenges, and future directions. Clin Cosmet Invest Dent. 2010;2:79. [PMC free article] [PubMed] [Google Scholar]
- 28.Bartold P.M., Cantley M.D., Haynes D.R. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000. 2010;53:55–69. doi: 10.1111/j.1600-0757.2010.00347.x. [DOI] [PubMed] [Google Scholar]
- 29.Menezes A.M., Rocha F.A.C., Chaves H.V., Carvalho C.B., Ribeiro R.A., Brito G.A.C. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:1901–1909. doi: 10.1902/jop.2005.76.11.1901. [DOI] [PubMed] [Google Scholar]
- 30.Hewlings S.J., Kalman D.S. Curcumin: a review of its' effects on human health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma V., Kalsi D.S. Effects of topical application of Curcuma longa extract in the treatment of early periodontal diseases. Indian J Dent Sci. 2016;8:118. [Google Scholar]
- 32.Dave D.H., Patel P., Shah M., Dadawala S.M., Saraiya K., Sant A.V. Comparative evaluation of efficacy of oral curcumin gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis. Adv Hum Biol. 2018;8:79. [Google Scholar]
- 33.Jalaluddin M., Jayanti I., Gowdar I.M., Roshan R., Varkey R.R., Thirutheri A. Antimicrobial activity of Curcuma longa L. extract on periodontal pathogens. J Pharm BioAllied Sci. 2019;11:S203. doi: 10.4103/JPBS.JPBS_295_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ximénez-Fyvie L.A., Haffajee A.D., Socransky S.S. Microbial composition of supra-and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol. 2000;27:722–732. doi: 10.1034/j.1600-051x.2000.027010722.x. [DOI] [PubMed] [Google Scholar]
- 36.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immune and regulatory functions of neutrophils in inflammatory bone loss. Hajishengallis G., Moutsopoulos N.M., Hajishengallis E., Chavakis T., editors. Semin Immunol. 2016;28:146–158. doi: 10.1016/j.smim.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eid H., Gouda M., Al-Abdaly M. Evaluation of topical application of ChloSite (chlorhexidine gel) in management of chronic periodontitis. Egypt Dent J. 2010;56:1069–1075. [Google Scholar]
- 39.Chen D., Nie M., Fan M.-W., Bian Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82:264. doi: 10.1159/000161127. [DOI] [PubMed] [Google Scholar]
- 40.Tyagi P., Singh M., Kumari H., Kumari A., Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS One. 2015;10 doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cekici A., Kantarci A., Hasturk H., Van Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan G.W., Sarembock I.J., Linden J. The role of inflammation in vascular diseases. J Leukoc Biol. 2000;67:591–602. doi: 10.1002/jlb.67.5.591. [DOI] [PubMed] [Google Scholar]
- 43.eri S.O., Sener G., Yuksel M. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol. 2005;187:399–406. doi: 10.1677/joe.1.06432. [DOI] [PubMed] [Google Scholar]
- 44.Pinto S.H., Pinto L., Cunha G., Chaves M., Santos F., Rao V. Anti-inflammatory effect of α, β- amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology. 2008;16:48–52. doi: 10.1007/s10787-007-1609-x. [DOI] [PubMed] [Google Scholar]
- 45.Grynkiewicz G., Ślifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59:201–212. [PubMed] [Google Scholar]
- 46.Shakibaei M., Mobasheri A., Buhrmann C. Curcumin synergizes with resveratrol to stimulate the MAPK signalin.g pathway in human articular chondrocytes in vitro. Genes Nutr. 2011;6:171. doi: 10.1007/s12263-010-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y., Kang S., Kim J. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J Periodontal Res. 2012;47:800–810. doi: 10.1111/j.1600-0765.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- 48.Hu P., Huang P., Chen M.W. Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NF-κB pathway in lipopolysaccharide-stimulated human gingival fibroblasts. Cell Biol Int. 2013;37:443–448. doi: 10.1002/cbin.10050. [DOI] [PubMed] [Google Scholar]
- 49.Xiao C.J., Yu X.J., Xie J.L., Liu S., Li S. Protective effect and related mechanisms of curcumin in rat experimental periodontitis. Head Face Med. 2018;14:12. doi: 10.1186/s13005-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yimam M., Brownell L., Do S.G. Protective effect of UP446 on ligature-induced periodontitis in beagle dogs. Dent J. 2019;7:33. doi: 10.3390/dj7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anitha V., Rajesh P., Shanmugam M., Priya B.M., Prabhu S., Shivakumar V. Comparative evaluation of natural curcumin and synthetic chlorhexidine in the management of chronic periodontitis as a local drug delivery: a clinical and microbiological study. Indian J Dent Res. 2015;26:53. doi: 10.4103/0970-9290.156806. [DOI] [PubMed] [Google Scholar]
- 52.Muglikar S., Patil K.C., Shivswami S., Hegde R. Efficacy of curcumin in the treatment of chronic gingivitis: a pilot study. Oral Health Prev Dent. 2013;11:81–86. doi: 10.3290/j.ohpd.a29379. [DOI] [PubMed] [Google Scholar]
- 53.Lima V., Vidal F.D., Rocha F.A.C., Brito G.A.C., Ribeiro R.A. Effects of tumor necrosis factor-α inhibitors pentoxifylline and thalidomide on alveolar bone loss in short-term experimental periodontal disease in rats. J Periodontol. 2004;75:162–168. doi: 10.1902/jop.2004.75.1.162. [DOI] [PubMed] [Google Scholar]
- 54.Assuma R., Oates T., Cochran D., Amar S., Graves D. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 55.Baqui A., Meiller T.F., Chon J.J., Turng B.F., Falkler W.A. Granulocyte-macrophage colonystimulating factor amplification of interleukin-1β and tumor necrosis factor alpha production in THP-1 human monocytic cells stimulated with lipopolysaccharide of oral microorganisms. Clin Diagn Lab Immunol. 1998;5:341–347. doi: 10.1128/cdli.5.3.341-347.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hitchon C.A., El-Gabalawy H.S. Infection and rheumatoid arthritis: still an open question. Curr Opin Rheumatol. 2011;23:352–357. doi: 10.1097/BOR.0b013e3283477b7b. [DOI] [PubMed] [Google Scholar]
- 57.Kayal R.A. The role of osteoimmunology in periodontal disease. BioMed Res Int. 2013;2013 doi: 10.1155/2013/639368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mau L.P., Cheng W.C., Chen J.K., Shieh Y.S., Cochran D.L., Huang R.Y. Curcumin ameliorates alveolar bone destruction of experimental periodontitis by modulating osteoclast differentiation, activation and function. J Funct Foods. 2016;22:243–256. [Google Scholar]
- 59.Sasaki T. Differentiation and functions of osteoclasts and odontoclasts in mineralized tissue resorption. Microsc Res Tech. 2003;61:483–495. doi: 10.1002/jemt.10370. [DOI] [PubMed] [Google Scholar]
- 60.Guimarães M.R., de Aquino S.G., Coimbra L.S., Spolidorio L.C., Kirkwood K.L., Rossa C., Jr. Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. J Innate Immun. 2012;18:155–163. doi: 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]