Abstract
Uterine arteriovenous malformations (AVMs) are rare, with approximately 100 described cases. They can be either congenital or acquired, with acquired AVMs mainly being associated with pregnancy related iatrogenic uterine trauma. Congenital AVMs are rarer, they originate from anomalous differentiation in the primitive capillary network, resulting in anomalous communication between the arteries and veins. In this article, we present and discuss 2 cases of uterine AVMs aged 21 and 22 with P0G2M2 and P0G1M1 respectively. Both cases presented with repeated episodes of profuse vaginal bleeding. Ultrasound (US) examination revealed classical signs of uterine arteriovenous malformation (AVM) confirmed on computerized tomography angiography (CTA) and digital subtraction angiography (DSA). The present case report highlights on the type of uterine malformations with their clinical presentation, imaging findings and management. Uterine AVM's are either congenital or acquired, clinically they are suspected if a pulsatile mass or bruit is felt in the pelvis. They may be confused with gestational related pathologies (retained products of conception, gestational trophoblastic disease), other vascular anomalies (hemangiomas), or malignancies of the uterus. In a case of suspected uterine AVM, clinical examination and diagnostic imaging, particularly quantitative ultrasound blood flow measurements, plays an important role.
Keywords: Uterine AVMs, Vascular malformations, Congenital arterial malformations, Traumatic uterine AVM
Case reports
Case 1
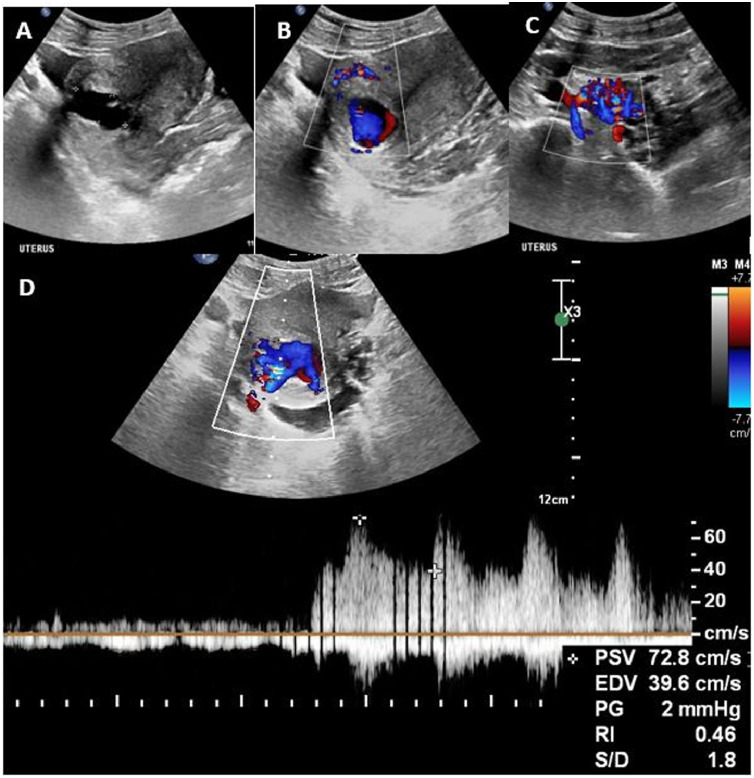
A 21-year-old P0G2M2 presented to the emergency gynecology service with a history of profuse per vaginal (PV) bleeding. She denied any past surgical procedures, dilatation and curettage (D & C) or the use of pharmacologic contraceptives. Upon examination she was anemic (hemoglobin 3.2 g/dL), apyrexial with a normal blood pressure. Transabdominal US revealed an enlarged uterus with an anechoic mass in the posterior wall of the lower uterine segment (Fig. 1a). The endometrial lining was thin with no signs of intra-uterine or extra-uterine gestation. Color Doppler US showed a hypervascular mass with (Fig. 1b) bi-directional flow (Yin-Yang phenomena), color aliasing and flow reversal, indicating possible aneurysm or AVM (Fig. 1c), with features of arteriovenous shunting, and peak systolic velocity (PSV) of 71.5 cm/s and resistive index (RI) of 0.42 on spectral Doppler (Fig. 1d). We considered a diagnosis of a uterine AVM and requested an immediate CTA.
Fig. 1.
Trans-abdominal pelvic US of case 1 (a), Sagittal grayscale image of the uterus shows a cystic mass in the posterior myometrium. (b, c) On colour Doppler imaging, flow can be seen in the myometrium and in the lesion in a characteristic (b) yin-yang pattern indicating turbulent flow and a (c) tortuous network of dilated vascular channels within the parametria. (d) Spectral analysis of the same patient as in figure 1 demonstrates high velocity with low resistance within the lesion.
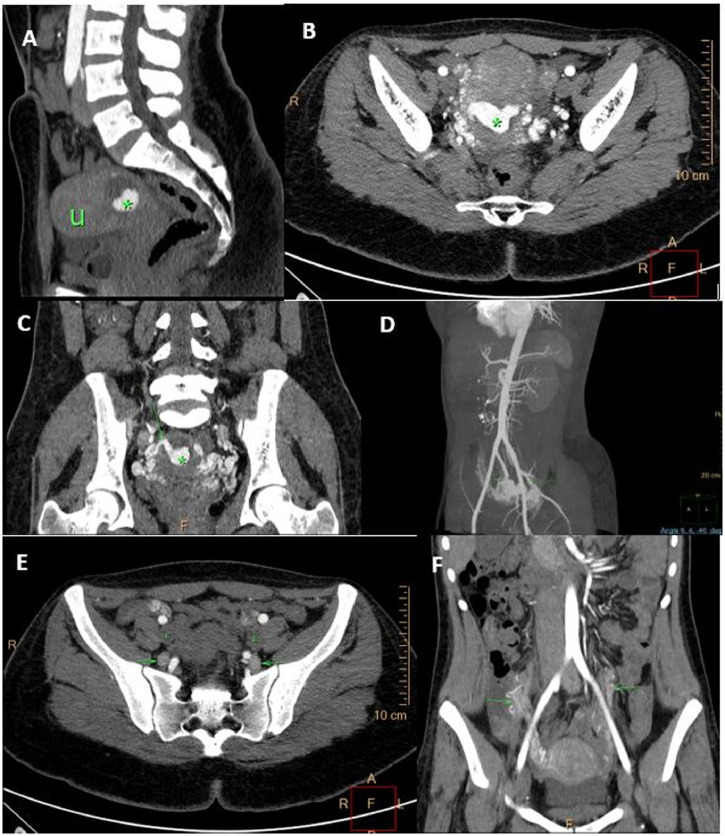
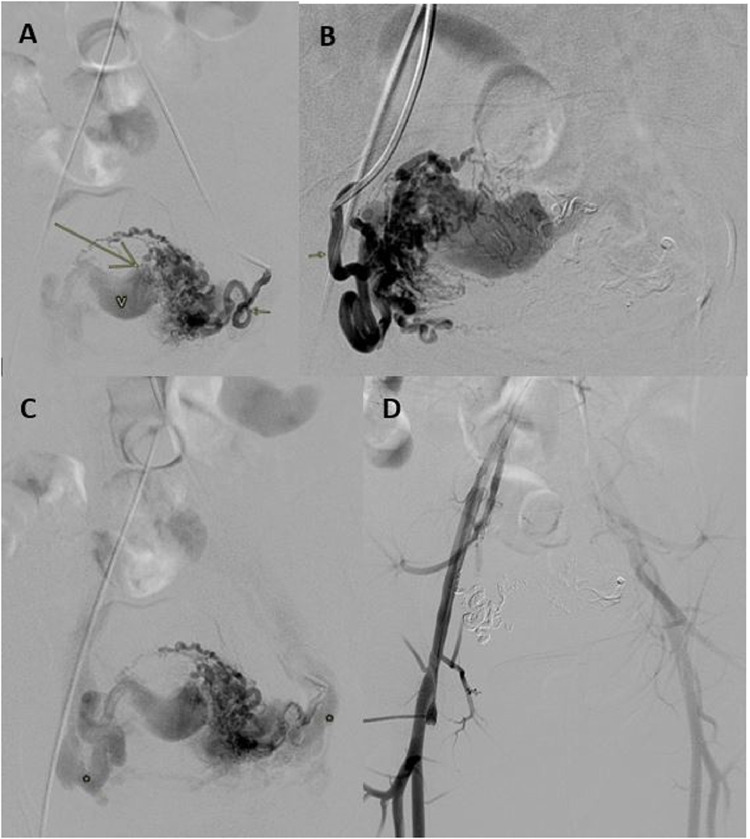
On CTA, there was a complex cystic lesion with central vivid enhancement within the posterior myometrium; multiple dilated, tortuous arterial feeders were noted, from bilateral uterine and gonadal arteries. There was early venous drainage into the bilateral internal iliac and gonadal veins via multiple dilated uterine/para-uterine veins (Fig. 2). Subsequently, she was planned for emergency endovascular intervention. Pre-embolization arteriogram of the bilateral uterine arteries confirmed the AVM (Fig. 3). On the left, the uterine artery entered the uterine substance and divided into arterioles with no true capillary phase and ended in a fistulous connection to a large Varix (Fig. 3a). The variceal pouch then connected to the pelvic veins through uterine and other pelvic veins (Fig. 3c). Similar picture noted on the right, but no fistulous jet flow. The bilateral uterine arteries were embolized and postembolization DSA run off (Fig. 3d) showed no filling of the arteriovenous shunt or significant uterine vascularity. Subsequently the patient was lost to follow-up.
Fig. 2.
(a, b) Sagittal and axial images of a CTA of patient 1, illustrating a vascular lesion (asterisk) and multiple tortuous vessels replacing the posterior wall of the uterus (U). (c, d) The dilated tortuous vascular channels in the uterus with arterial feeders from bilateral uterine arteries are better demonstrated on the coronal multi-planar reformatted and maximal intensity projection CTA (arrows). (e, f) Axial and coronal multi-planar reformatted CT angiography shows early filling of numerous uterine and para-uterine veins with primary drainage via bilateral internal iliac veins (e) and dilated bilateral gonadal veins (f) indicated by arrows. Note no contrast opacification of the external iliac veins on arterial phase (E).
Fig. 3.
Pelvic arteriogram of patient 1, after selective left and right uterine artery injection demonstrating; (a, b) Dilated left and right uterine arteries (short arrows) feeding a central uterine Varix (V) via numerous dilated para-uterine and uterine arteries. A fistulous jet flow is noted on the left (long arrow). (c) Slightly delayed image from same arteriogram confirming early venous drainage from the right and left aspect of the uterus (asterisk). (d) Post embolization image with no uterine hypervascularity or filling of the arteriovenous shunt.
Case 2
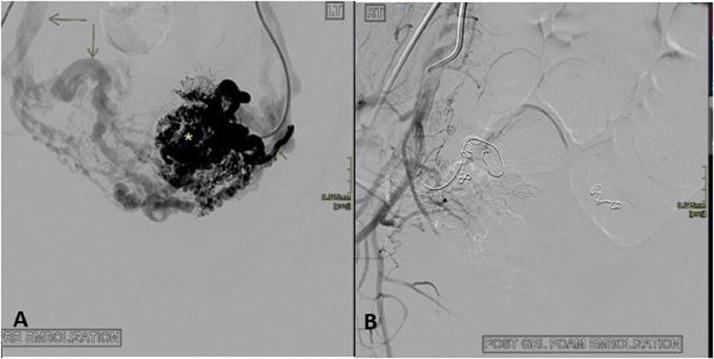
A 22-year-old P0G1M1, with a long-standing history of abnormal uterine bleeding with frequent admissions presented to the vascular surgery department with a confirmed diagnosis of uterine AVM. She had a D & C for a miscarriage 4 years prior to the index presentation. She had no known bleeding disorders. She was taking tranexamic acid and previously used a combined oral contraceptive preparation for the bleeding. On admission, she was not anemic and had no vaginal bleeding. Extensive arteriovenous shunting was seen on pre-embolization DSA (Fig. 4); there were massively dilated bilateral uterine arteries with extensive branching upon entering the uterus with early venous filling. The branching arteries were nearly the same size as the main uterine artery with a corkscrew appearance (Fig. 4a). The uterine arteries were successfully embolized with a combination of fibered and nonfibered coils (Cook), and gel foam. The patient was discharged uneventfully with a planned follow-up US examination after 1 month.
Fig. 4.
Uterine AVM (Patient 2) (a) Angiogram after selective left uterine artery injection demonstrating simultaneous contrast opacification of intra-uterine arteries (asterisk) and early venous return via dilated and tortuous veins (long arrows), indicating brisk arteriovenous shunting. (b) Arteriogram after embolization demonstrates successfully embolized AVM.
Discussion
Uterine AVMs can be either congenital or acquired. Congenital AVM's arise from anomalous differentiation in the primitive capillary network, resulting in abnormal communication between the arteries and veins [1], [2]. AVMs may be confused with retained products of conception, gestational trophoblastic disease (GTD), uterine pseudo-aneurysms, hemangiomas, varicosities and malignancies of the uterus such as sarcomas [3]. Uterine AVMs typically have multiple intra- and extra-uterine feeding arteries and draining veins with an intervening nidus, and may extend extrauterine. Histopathologically, AVMs may be classified as cirsoid or cavernous depending on the number and diameter of the intra-lesional vessels. Cirsoid AVMs have multiple, dilated vessels with a corkscrew appearance, resembling varicose veins with interconnecting fistula. Cavernous type includes a single arterial vessel feeding many small connecting fistulas [2,[4], [5]]. The congenital AVMs often present with severe vaginal bleeding and do not respond to conventional therapy, while acquired ones present with symptoms that appear slowly or suddenly [5,6]. In present case report, we speculate that the second patient most likely had a congenital AVM as indicated by the presence of “Corkscrew” uterine vessels on DSA and multiple extra uterine arterial feeder (Fig. 4a), bleeding most likely was initiated by the pregnancy and uterine evacuation.
Acquired uterine AVMs are more common and are characterized by abnormal communication between uterine arteries and the myometrial venous plexus, deep within the myometrium and endometrium. Arterial blood is usually supplied from uni- or bilateral uterine arteries rather than extrauterine arteries and may not have an intervening nidus [2,7]. Acquired AVMs are largely complications associated with uterine surgery, D&C including spontaneous or therapeutic abortion, gestational trophoblastic disease (GTD), endometrial cancer and infection. Bleeding occurs when the endothelium of the AVM vessels is disrupted as in a curettage. Chronic blood loss, associated with AVMs, may also cause abdominal pain, dyspareunia and anemia [2,[5], [6]].
Initially, AVMs are suspected if a pulsatile mass or bruit is felt in the pelvis [8]. Clinically a differential diagnosis should include retained products of conception, GTD and postpartum uterine pseudoaneurysm [5,8]. GTD and postpartum uterine pseudoaneurysm can be excluded if beta-hCG levels are normal [5,8]. In this case report, case one had dropping beta-hCG levels, while the case 2 had normal levels. During the postpartum period, AVMs should also be differentiated from subinvolution of the placental bed, and postpartum uterine pseudoaneurysms. Subinvolution of the placental bed present as hypervascular lesions that do not have fistulous connections between arteries and veins. In contrast to AVM, pseudoaneurysm is a saccular dilatation of the uterine artery, without communication with the venous system [5,7].
Initially, AVMs are investigated using trans-abdominal or transvaginal US. On grayscale US, uterine AVMs commonly present as a heterogeneous, ill-defined mass, with multiple, hypoechoic cystic or serpiginous structures of variable size and may or may not show endometrial and myometrial thickening. Color Doppler US will reveal intense flow with color aliasing and flow reversal. Duplex Doppler will typically demonstrate a high peak systolic velocity (PSV;40-96 cm/s,) arteriovenous shunting with to and fro flow and low resistance index (RI) 0.25–0.55 [2,5,[8], [9]]. Normal myometrium usually has a PSV of 9-44 cm/s and a RI ranging from 0.6 to 0.8 [10]. Blood flow velocity and RI, together with clinical presentation can be used as tools to triage patients at different levels of risk and assist in the selection of the appropriate treatment. A correct sonographic diagnosis is thus very important [9,11].
Treatment is definite when PSV >83 cm/s and should be considered when PSV is more than 60–70 cm/sec. Patients with PSV between 40 and 60 cm/sec present a treatment dilemma and may be managed expectantly provided they do not have prolonged or severe bleeding. Patients are low risk if they have a PSV <40 cm/s and can be managed conservatively [9,11].
Following US, 3-dimensional CTA can be used to define the extent of involvement by the AVM and assess extrauterine involvement. CTA being quick and noninvasive obviates the need for conventional angiography in patients with normal renal function. CTA can also help to delineate the feeding uterine arteries prior to any intervention [[7], [8],12]. Although CTA can accurately identify abnormal flow dynamics in AVMs with optimal bolus and scan timing, dynamic contrast-enhanced MR can more consistently capture these abnormalities. MRI, when available, is able to better characterize the pelvic organs compared to CT. Hence, dynamic contrast-enhanced MRI may be the preferred cross-sectional imaging modality [2,8,10]. Unfortunately, MRI is not always readily accessible, is costly and has longer acquisition time compared to CT. For unstable patients, who are bleeding heavily, CT thus is a better diagnostic technique [2,8]. Both patients in our case series required urgent intervention and did not undergo an MRI examination. Following preliminary investigation, DSA due its invasive nature is reserved for patients requiring embolization [13], [14].
Uterine AVMs can be treated in a variety of ways, including endovascular, surgical and conservative methods. Transarterial endovascular treatment is most commonly used to treat congenital AVMs, especially when there are multiple arteriovenous shunts as in case 2 of our series. During transarterial embolization, the branches of one or both uterine arteries are embolized, which may be enough to treat the AVM [7,11]. If necessary, repeated transarterial embolizations may be performed to close off additional feeders [11]. In rare cases, transvenous embolization and ultrasound guided direct puncture procedures may be performed [7,11]. Transvenous embolization is performed via femoral or jugular access. Caution is required when using this approach to treat high flow AVMs, which may lead to nontarget delivery of embolization material to the pulmonary arteries [11]. Direct puncture procedures are performed under US guidance either percutaneously or transvaginally. Caution and a gradual approach is required to prevent tissue damage as a consequence of reflux into the capillary bed [11]. For both the transvenous and direct puncture approaches, prior arterial access is advised, during and after the procedure to better delineate the anatomy, monitor treatment progress and to set treatment endpoints. For these reasons, transarterial embolization is the first line endovascular treatment of uterine AVMs, while transvenous and direct puncture procedures remain supportive [11].
Surgical treatment, by means of hysterectomy may be performed as the first-line of treatment following failed embolization or if fertility is no longer desired. Hysterectomy is also not always desired due to the complexity of arteriovenous communications and close proximity of the abnormal vessels to major pelvic structures [11,14]. Surgically, AVMs can be completely cured by excising the nidus of the AVM [15]. Other traditionally used surgical modalities such as laparoscopic isolation, bipolar coagulation of uterine vessels, internal iliac and uterine artery ligation have proved to be counterproductive resulting in collaterals distal to the ligation, leading to recurrence and inability to embolize the AVM, which cannot be accessed [16].
Conservative medical treatment of uterine AVMs in the form of gonadotropin-releasing hormone (GnRH) agonists, is mainly reserved for stable patients. Other drugs used to successfully treat uterine AVMs include Danazol, ergot alkaloids, combined oral contraceptives and Progestins [15], [16], [17], [18]. It is possible that uterine AVM resolves spontaneously.
In conclusion, Uterine AVM's are either congenital or acquired, clinically they are suspected if a pulsatile mass or bruit is felt in the pelvis. They may be confused with, gestational (GTD and retained products of conception) and nongestational pathology (hemangiomas, varicosities, and malignancies of the uterus). In a case of suspected uterine AVM clinical examination and diagnostic imaging, particularly quantitative ultrasound blood flow measurements, plays an important role to assist the multidisciplinary team to decides on appropriate management. DSA due its invasive nature is reserved for patients requiring embolization.
Patient consent
Patient consent has been obtained.
Footnotes
Acknowledgments: The authors acknowledge Professor Samia Ahmad, Steve Biko Academic Hospital, University of Pretoria, South Africa, for her contribution of digital subtraction angiography images and Cheryl Tosh, faculty of health sciences, University of Pretoria, for language editing.
Authors declare that they have no conflict of interest or financial interests.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.02.018.
Contributor Information
Tertu Nakashololo, Email: ntertu@yahoo.com.
Zandile Dunn, Email: dunn.zandile@gmail.com.
Leon Snyman, Email: leon.snyman@me.com.
Shaakera MH Ismail, Email: sheraaz@mweb.co.za.
Appendix. Supplementary materials
References
- 1.Hoffman M.K., Meilstrup J.W., Shackelford D.P., Kaminski P.F. Arteriovenous malformations of the uterus: an uncommon cause of vaginal bleeding. Obstet Gynecol Surv. 1997;52(12):736–740. doi: 10.1097/00006254-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Huang M.W., Muradali D., Thurston W., Burns P.N., Wilson S.R. Uterine arteriovenous malformations: Gray-scale and Doppler US features with MR imaging correlation. Radiology. 1998;206(1):115–123. doi: 10.1148/radiology.206.1.9423660. [DOI] [PubMed] [Google Scholar]
- 3.Kim T.-H., Lee H.-H. Presenting features of women with uterine arteriovenous malformations. Fertil Steril. 2010;94(6):2330. doi: 10.1016/j.fertnstert.2010.03.031. e7-e10. [DOI] [PubMed] [Google Scholar]
- 4.Frencken V.A., Landman G.H. Cirsoid aneurysm of the uterus: Specific arteriographic diagnosis; report of a case. Am J Roentgenol Radium Ther Nucl Med. 1965;95(3):775–781. doi: 10.2214/ajr.95.3.775. [DOI] [PubMed] [Google Scholar]
- 5.Polat P.N., Suma S., Kantarcý M., Alper F., Levent A.N. Color Doppler US in the evaluation of uterine vascular abnormalities. Radiographics. 2002;22(1):47–53. doi: 10.1148/radiographics.22.1.g02ja0947. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado J., Perez C., Rodriguez W. AJR teaching file: profuse vaginal bleeding seven weeks following induced abortion. Am J Roentgenol. 2008;191(6_supplement):S79–S82. doi: 10.2214/AJR.07.7044. [DOI] [PubMed] [Google Scholar]
- 7.Cura M., Martinez N., Cura A., Dalsaso T.J., Elmerhi F. Arteriovenous malformations of the uterus. Acta Radiol. 2009;50(7):823–829. doi: 10.1080/02841850903008792. [DOI] [PubMed] [Google Scholar]
- 8.Farias M.S., Santi C.C., Lima A.A.A.D.A., Teixeira S.M., Biase T.C.G.D. Radiological findings of uterine arteriovenous malformation: a case report of an unusual and life-threatening cause of abnormal vaginal bleeding. Radiologia Brasileira. 2014;47(2):122–124. doi: 10.1590/S0100-39842014000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman D., Wauters J., Van Calenbergh S., Van Schoubroeck D., Maleux G., Van Den Bosch T. Color Doppler imaging is a valuable tool for the diagnosis and management of uterine vascular malformations. Ultrasound Obstet Gynecol. 2003;21(6):570–577. doi: 10.1002/uog.159. [DOI] [PubMed] [Google Scholar]
- 10.Hashim H., Nawawi O. Uterine arteriovenous malformation. Malay J Med Sci: MJMS. 2013;20(2):76. [PMC free article] [PubMed] [Google Scholar]
- 11.Sridhar D., Vogelzang R.L. Diagnosis and treatment of uterine and pelvic arteriovenous malformations. Endovasc Today. 2018;17:1–5. [Google Scholar]
- 12.Aiyappan S.K., Ranga U., Veeraiyan S. Doppler sonography and 3d CT angiography of acquired uterine arteriovenous malformations (AVMS): report of two cases. J Clin Diagn Res. 2014;8(2):187. doi: 10.7860/JCDR/2014/6499.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timor-Tritsch I.E., Haynes M.C., Monteagudo A., Khatib N., Kovács S. Ultrasound diagnosis and management of acquired uterine enhanced myometrial vascularity/arteriovenous malformations. Am J Obstet Gynecol. 2016;214(6):731. doi: 10.1016/j.ajog.2015.12.024. e1-e10. [DOI] [PubMed] [Google Scholar]
- 14.Karadag B., Erol O., Ozdemir O., Uysal A., Alparslan A.S., Gurses C., et al. Successful treatment of uterine arteriovenous malformation due to uterine trauma. Case Rep Obstet Gynecol. 2016; 2016:3. doi: 10.1155/2016/1890650. [DOI] [PMC free article] [PubMed]
- 15.Katimada Annaiah T., Kodakkattil Sreenivasan S. Uterine arteriovenous malformations: clinical implications. Obstet Gynaecol. 2015;17(4):243–250. [Google Scholar]
- 16.Szpera-Goździewicz A., Gruca-Stryjak K., Bręborowicz G.H., Ropacka-Lesiak M. Acquired uterine arteriovenous malformation - a diagnostic dilemma. Ginekol Pol. 2018;89(4):227–228. doi: 10.5603/GP.a2018.0039. [DOI] [PubMed] [Google Scholar]
- 17.Vilos G., Vilos A., Rafea B., Al-Shaikh G., Sabr Y. Resolution of uterine arterio-venous malformation followed by uneventful pregnancy after administration of gonadotropin releasin g hormone agonist concomitantly with an aromatase inhibitor an d tranexamic acid. Int J Womens Health Wellness. 2017;3(3):1–3. [Google Scholar]
- 18.Khatree M., Titiz H. Medical treatment of a uterine arteriovenous malformation. Aust N Z J Obstet Gynaecol. 1999;39(3):378–380. doi: 10.1111/j.1479-828x.1999.tb03424.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.