Abstract
Background/purpose
Dental pulp stem cells (DPSCs) play a crucial role in the tissue healing process through odontoblast like cell differentiation. The aim of this study was to evaluate the biocompatibility and compare the potential invitro cytotoxic effects of NeoMTA Plus, ProRootMTA and Biodentine on human dental pulp stem cells (hDPSCs).
Materials and methods
To assess the effects of NeoMTA Plus, ProRoot MTA and Biodentine extracts at 1st, 3rd and 7th d on hDPCs, cell populations was determined by flow cytometry using an Annexin V detection kit. The data were analyzed statistically using the Kruskal–Wallis test. A p < 0.05 was considered as statistically significant.
Results
All groups showed cell viability similar to that of the control group on 1st, 3rd and 7th d. Although Biodentine exhibited higher cell viability rates than the other material groups, no statistically significant differences were noted between the sampled days (p > 0.05).
Conclusion
All materials extracts are not cytotoxic and do not induce apoptosis in the hDPSCs. These results suggest that all the tested materials can lead to positive outcomes when used as reparative biomaterials.
Keywords: Cytotoxicity, Dental pulp stem cells, Mineral trioxide aggregate
Introduction
Calcium silicate cements, such as Mineral trioxide aggregate (MTA) and Biodentine, have a wide and successful range of various clinical dental applications.1,2 Tricalcium silicate cements are called dental biomaterials because of their physicochemical and biocompatibility. They have the ability to increase proliferation and odontogenic differentiation in human dental pulp cells in vitro.3 MTA is predominantly formed of portland cement and comprises of tricalcium silicate, dicalcium silicate and bismuth oxide for radiopacifier.4 MTA is used for perforation repair, root-end filling, direct pulp capping, partial or total pulpotomy methods due to its high sealing ability, biocompatibility, antibacterial activity, physical and chemical properties.5 However, the use of MTA is reported to have some disadvantages due to the long material setting time, high material cost, poor handling characteristics and the potential for discoloration of dental tissues.6,7 All the properties of MTA have been further developed to overcome the drawbacks of MTA and different bioactive endodontic cements have been introduced to the market. BioAggregate, Biodentine, BioRoot RCS, calcium–enriched mixture cement, Endo-CPM, Endocem, EndoSequence, EndoBinder, EndoSeal MTA, iRoot, MicroMega MTA, MTA Bio, MTA Fillapex, MTA Plus, NeoMTA Plus, OrthoMTA, Quick- Set, RetroMTA, Tech Biosealer and TheraCal LC have been changed by certain properties and manufactured by companies for different usage areas. It is claimed that these materials show similar properties to MTA and do not contain its disadvantages.8, 9, 10
Biodentine is a calcium silicate cement with dentin like mechanical properties, consisting of a powder and mixing liquid consisiting of water, calcium chloride (decreases the setting time) and a hydrosoluble polymer (water reducing agent), the powder part of which is composed of tricalcium silicate (Ca3SiO5) (main component), dicalcium silicate (second main component), zirconium oxide (radiopacifer) and calcium carbonate (filler component).11,12 Although biocompatibility and dental applications of Biodentine are similar to MTA, it is reported that it is easier to use in clinical practise, shorter working time and does not cause discoloration in dental tissues due to the absence of bismuth oxide.13
NeoMTA Plus was developed with similar properties to MTA. It contains tricalcium silicate, dicalcium silicate, tantalum oxide, calcium sulfate and silica. Tantalum oxide (Ta2O5) was used instead of bismuth oxide as radiopacifier. NeoMTA Plus is indicated for direct pulp capping, indirect pulp, pulpotomy, root end filling, perforation repair and apexification. Bismuth oxide has been reported to cause discoloration in dental tissues due to contact with sodium hypochlorite solution.14,15 It is stated that NeoMTA Plus does not cause discoloration in dental tissues, it has sufficient radiopacity and hydration with MTA and it shows the production of calcium hydroxide required to induce mineralized tissue formation.15,16
The aim of this study was to evaluate the biocompatibility and compare the potential invitro cytotoxic effects of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells (hDPSCs).
Material and methods
Isolation and passaging of hDPSCs
Human dental pulp tissue were isolated from extracted third molars from healthy three adult patients aged from 18 to 25 years at the Faculty of Dentistry Oral and Maxillofacial Surgery, Istanbul University, Turkey. All experiments were performed with the approval of the Ethics Committee of the Faculty of Dentistry (no: 2017/39). After extraction, the teeth were transported in dulbecco's phosphate-buffered saline (DPBS; Gibco, Grand Island, NY, USA) containing 1% penicillin and 1% streptomycin (Gibco) to the laboratory in the Department of Pediatric Allergy/Immunology, Marmara University Research Hospital. The teeth were split open then the pulp tissues were aseptically removed and isolated under sterile conditions.
The pulp tissues were divided into 1 mm3 pieces by applying the mechanical seperation method with the scalpel. After micromechanical digestion, colllagenase solution was prepared using 1 ml of DPBS (Gibco) and 0.003 g Type 1 collagenase (Gibco). Next, 2 ml of the collagenase solution was then added to micromechanically decomposed tissues, following which enzymatic digestion was started. After enzymatic and mechanical disintegration, the tissues were placed in 15 ml sterile Falcon tubes. Pipetting was performed in dulbecco's modified eagle medium (DMEM; Gibco) to ensure homogenization of the tissues, which were then placed in the incubator for 45 min to 1 h at 37 °C and %5 CO2. After the tubes were removed from the incubator, the cells were centrifuged at 1500 rpm for 5 min. The cells pellets were obtained, and the supernatant was aspirated. The cells were transfered to a T-25 cm2 flask containing 4 ml of culture medium consisting of DMEM (Gibco), suplemented with %15 fetal bovine (FBS; Gibco), %1 penicillin/streptomycin (Gibco). The culture medium was changed every 3 d, and the proliferation and spreading of the cells im the flask were monitored at regular intervals with an invert microscope (EVOS-AMG, Thermo Fisher Scientific, Waltham, MA, USA). The hDPSCs were seperated with %0.25 trypsin–EDTA (Gibco) when they attained %70–80 confluence. The characterization of cell cultures reaching the third passage was identified for specific surface markers and multiple differentiation potential using flow cytometry.
Flow cytometry analysis
The cell surface antigen expressions of the third-passage hDPSCs were analyzed after the cells were incubated with antibodies for human CD73 phycoerytrin (PE), CD90 PE, CD146 fluorescein isothiocyanate (FITC), CD29 allophycocyanin (APC), CD105 PE, CD45 FITC, CD34 PE, CD14 PE, CD25 APC, and CD28 PE (BD Biosciences, CA, USA) at room temperature in the dark.
The control antibodies were phycoerythrin-conjugated or fluorescein isothiocyanate-conjugated and allophycocyanin-conjugated Mouse IgG1 and Mouse IgG2 (BD Biosciences, San Diego, CA, USA). The flow cytometry outcomes were examined using a flow cytometer (BD, FACSCalibur, San Jose, CA, USA).
Differentiation of stem cells
In order to characterize mesenchymal stem cells (MSCs) in vitro according to International society for cellular therapy (ISCT) criteria, their trilenage differentiation including osteogenic, chondrogenicand adipogenic was evaluated. The ostogenic, chondrogenic and adipogenic differentiation potentials of the cells were analyzed using the differentiation kits [Stem Pro Osteogenesis Differentiation Kit, (Thermo Fisher Scientific), Stem Pro Adipogenesis Differentiation Kit (Thermo Fisher Scientific), and Stem Pro Chondrogenesis Differentiation Kit (Thermo Fisher Scientific)]. The cells were plated in 6 well plates (5 × 104) cell/well) and the differentiations mediums were applied on the cells according to the manufacturer's instructions. The differentiation medias were changed three times per week.The calcium deposits and extracellular matrix mineralization were confirmed with Alizarin Red (Sigma–Aldrich, St. Louis, MO, USA) staining assay performed after 28 days of the osteogenic stimulation. After 14 days the cell cultures were stained with Alcian Blue (Sigma–Aldrich) to assess the chondrogenic differentiation potentials of the cells and evaluated the presence of chondrocyte-like cells and proteoglycans. The Oil Red (Sigma–Aldrich) staining was used to determine the adipogenic differentiation potential of the cells on the 14th day after the application of the differentiation media. The staining cells were evaluated using a binocular microscope (Olympus, BH2-RFCA, Olympus, Tokyo, Japan).
Preparation of materials
The materials used in study and their contents are shown in Table 1. Complete dulbecco's modified eagle medium (CDMEM; Gibco) was used in the control group in this study.
Table 1.
Composition of materials evaluated.
| Materials | Manufacturer | Composition |
|---|---|---|
| ProRoot MTA | Dentsply Tulsa Dental, Hohnson City, Germany | Powder: Portland cement, bismuth oxide, calcium sulfate dihydrate, tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite Liquid: Water |
| NeoMTA Plus | Avalon Biomed, Bradenton, Florida, USA | Powder: Tricalcium silicate, dicalcium silicate, tantalum oxide Liquid: Water, proprietary polymers |
| Biodentine | Septodont, Saint Maurdes Fosses, France | Powder: Tricalcium silicate, dicalcium silicate, calcium carbonate, calcium oxide, iron oxide, zirconium oxide Liquid: Calcium chloride, hydrosoluble polymer |
| CDMEM | Gibco, Grand Island, NY, USA | %10 FBS, %1 penicillin/streptomycin, DMEM (Dulbecco's Modified Eagle Medium) |
CDMEM, complete dulbecco's modified eagle medium; DMEM, dulbecco's modified eagle medium.
ProRoot MTA (Dentsply Tulsa Dental, Hohnson City, Germany) Biodentine (Septodont, Saint Maurdes Fosses, France) and NeoMTA Plus (Avalon Biomed, Bradenton, FL, USA) were mixed according to the manufacturer's instructions under sterile conditions; then they were added to the molds (diameter 5.0 mm and height 3.0 mm) (n: 16 samples per group). After materials underwent polymerization, each discs were removed using sterile forceps, and the all discs were exposed to ultraviolet (UV) light for 30 min to prevent contamination.
The direct contact test was used to obtain material extraction fluids. Since the surface/medium ratio was 3 cm2/ml for disc shaped samples with a thickness of more than 1 mm specified in ISO (International Organization for Standardization) 10993-1217 standards while preparing the release fluids of solid form materials, the discs of each material were placed into 15 ml of DMEM and incubated for 24 h at 37 °C in a humidified 5% CO2 environment. The material solutions were passed through the 0.22 μm filter twice to remove the remaining small particles, and the material extracts were obtained for the cell viability tests (ISO 19993-5).18
Cell viability tests
Cell viability was determined using a flow cytometry assay. Human dental pulp stem cells (hDPCs) were plated onto 48-well plates and the culture medium was added. After 24 h, the culture medium was removed. The extraction materials were enumerated by someone outside the researcher team and randomly applied to the cells. The cells were exposed for 1 day, 3 days, and 7 days obtain the experimental material extracts (ProRoot MTA, NeoMTA Plus, Biodentine).
The Annexin V-FITC Apoptosis Detection Kit (BD Biosciences) was used to read the effects of material extracts on the viable, necrotic, early and late apoptotic cell ratios. The five cell viability experiments repeated independently from each other, and the average of the obtained values was evaluated.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 22 (IBM SPSS Inc.). The Kruskal–Wallis test was used to study the differences among the groups and days in the non-normally distributed data. p < 0.05 was considered as the significance level.
Results
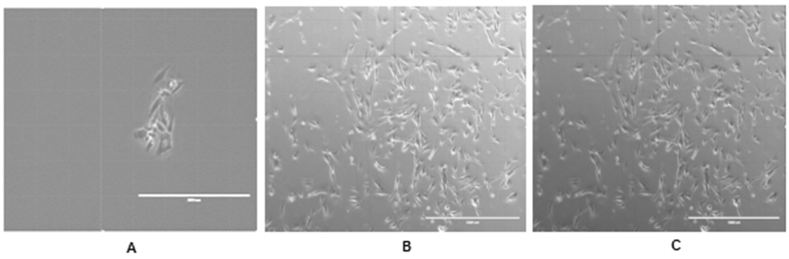
The confluent cells in the plates exhibited a spindle-shaped fibroblast-like morphology. From the first passage to the third passage, the confluent structures of the cells and their contact with each other increased (Fig. 1A, B, C).
Figure 1.
Morphological appearance of hDPSCs, (x10); A) hDPSCs on 1st passage B) hDPSCs on 2nd passage C) hDPSCs on 3rd passage.
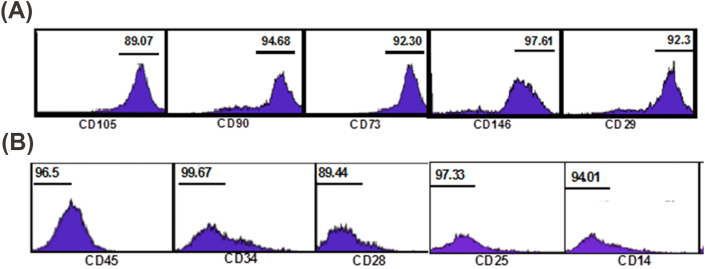
Then, the cells in the third passage were examined to determine their immunnophenotype and differentiation potentials. The cells demonstrated a positive expression for mesenchymal stem cell (MSC) markers (CD105, CD146, CD90, CD29, CD73) (Fig. 2A); the staining for hematopoietic stem cell markers was negative (CD45, CD34, CD25, CD28,CD14) (Fig. 2B).
Figure 2.
(A): Representative flow cytometry analysis of mesenchymal cell surface markers on hDPSCs. (B): Representative flow cytometry analysis of hematopoietic cell surface markers on hDPSCs.
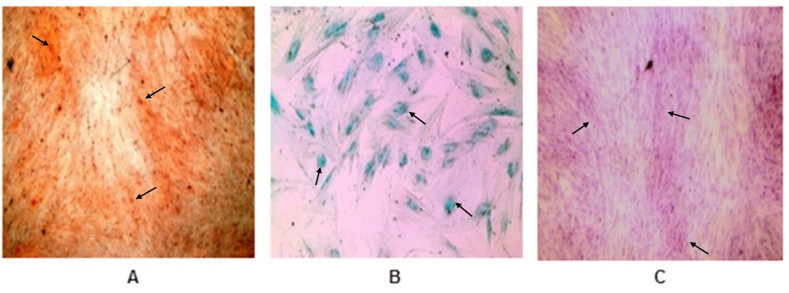
The hDPCCs showed the potential for osteogenic, chondrogenic, and adipogenic differentiation after stimulation with the differentiation medium. When osteogenic differentiation was evaluated, the presence of mineralized calcium nodules stained in an orange-red color were detected (Fig. 3A). In the chondrogenic differentiation, proteoglycan and chondrocyte-like cells stained a blue-turquoise color were observed (Fig. 3B). In the adipojenic differentiation, the presence of oil droplets stained in a red color was observed (Fig. 3C).
Figure 3.
Differentiation analysis in hDPSCs; A) Differentiation of hDPSCs into osteoblasts was confirmed by Alizarin Red staining, (x40); B) Differentiation of hDPSCs into chondrocytes was confirmed by Alcian Blue staining, (x40); C) Differentiation of hDPSCs into adipocytes was confirmed by Oil Red O staining, (x40).
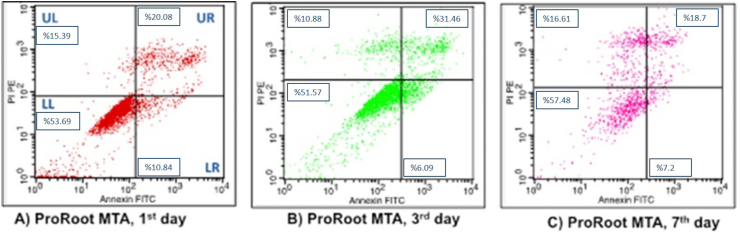
After exposing the three experimental material extracts and the control group to DMEM on the 1st, 3rd, 7th days, the flow cytometry graphics of the cells were obtained (Figure 4, Figure 5, Figure 6, Figure 7, respectively). The lower left (LL) part of the flow cytometry graphs shows the live cell ratios, the lower right (LR) part shows the early apoptotic cell ratios, the upper right (UR) part shows the late apoptotic cell ratios, and the upper left (UL) part shows the necrotic cell ratios. The averages of the datas obtained as a result of analysis with flow cytometry were indicated in the relevant areas on the graphs as percentages.
Figure 4.
Flow cytometry analysis of hDPSc after incubation in ProRoot MTA for different time intervals. (A) 1 day; (B) 3 days; (C) 7 days.
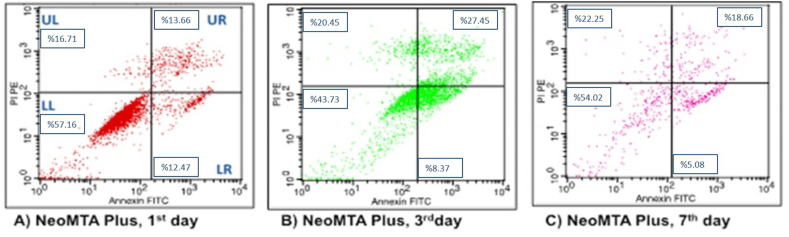
Figure 5.
Flow cytometry analysis of hDPSc after incubation in NeoMTA Plus for different time intervals. (A) 1 day; (B) 3 days; (C) 7 days.
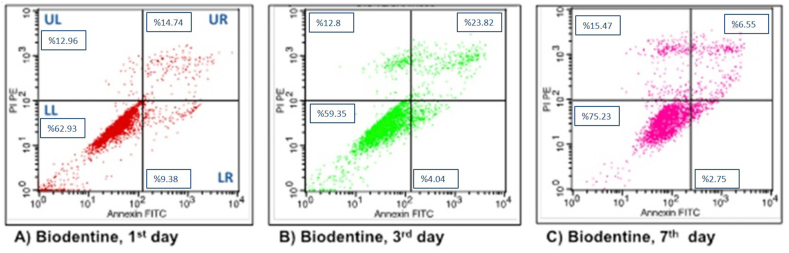
Figure 6.
Flow cytometry analysis of hDPSc after incubation in Biodentine for different time intervals. (A) 1 day; (B) 3 days; (C) 7 days.
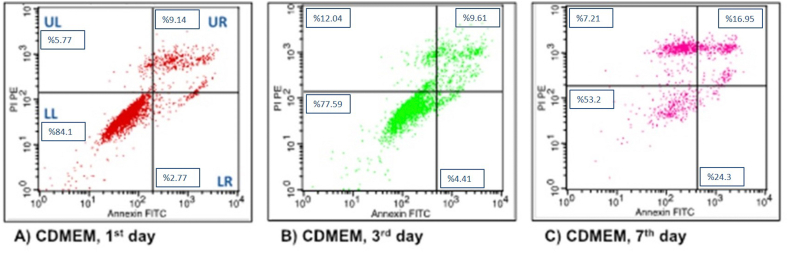
Figure 7.
Flow cytometry analysis of hDPSc after incubation in CDMEM (control group) for different time intervals. (A) 1 day; (B) 3 days; (C) 7 days.
In the ProRoot MTA group, cell viability percentage on the 7th day was found to be mathematically higher than the other days when the live cell ratios were compared with the days in flow cytometry graphs. The early apoptotic cell ratios were analyzed as high on the 1st day. While the late apoptotic cell ratios were found to be the highest on the 3rd day, the highest rates of the necrotic cells were determined on flow cytometry graphics on the 7th day (Fig. 4).
In the NeoMTA Plus group, cell viability percentage on the 3rd day was found to be mathematically lower than the other days when the live cell ratios were compared with the days in the flow cytometry graphs. The percentages of early apoptotic, late apoptotic and necrotic cells were calculated to be higher on the 1st, 3rd and 7th days, respectively, similar to the flow cytometry analysis of the ProRoot MTA group (Fig. 5).
In the Biodentine group, when the live cell ratios were compared with the flow cytometry graphs, the cell viability on the 7th day was found to be mathematically higher than the other days. The percentages of early apoptotic, late apoptotic and necrotic cells were calculated to be higher on the 1st, 3rd and 7th days, respectively, similar to the flow cytometry analysis of the ProRoot MTA group and NeoMTA Plus group (Fig. 6).
In the Control group, when the live cell ratios were compared with the flow cytometry graphs, the cell viability on the 1st day was found to be mathematically higher than the other days. While early and late apoptotic cell ratios were found to be the highest on the 7th day, the highest rates of necrotic cells were determined on flow cytometry graphics on the 3rd day (Fig. 7).
The cell viability in all tht experimental groups did not show a significant difference when the 1st, 3rd and 7th days were compared (p > 0.05) (Table 2). When the experimental and control groups were compared, the control group had the highest cell viability on the 1st and 3rd days and the Biodentine group had the highest percentage of viable cells on the 7th day, but there was no statistically significant difference between the groups based on time times (p > 0.05) (Table 2).
Table 2.
Evaluation of cell viability among 3 different days and among 4 different groups.
| Cell Viability |
p | |||
|---|---|---|---|---|
| 1st Day |
3 rd Day |
7 th Day |
||
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | ||
| Biodentine | 62.93 ± 31.45 (62.7) | 59.35 ± 33.08 (63.8) | 75.23 ± 13.38 (76.6) | 0.926 |
| ProRoot MTA | 53.69 ± 30.22 (53.7) | 51.57 ± 29.87 (53.3) | 57.48 ± 19.75 (57.6) | 0.912 |
| NeoMTA Plus | 57.16 ± 28.13 (57.1) | 43.73 ± 23.31 (36.2) | 54.02 ± 19.53 (49.5) | 0.472 |
| CDMEM |
84.1 ± 1.8 (84.9) |
77.59 ± 6.09 (77.7) |
53.2 ± 36.34 (52.9) |
0.393 |
| p | 0.091 | 0.452 | 0.608 | |
CDMEM, complete dulbecco's modified eagle medium; SD, standard deviation.
Kruskal Wallis Test ∗p < 0.05.
The early apoptotic cell rates are showed in Table 3. The lowest early apoptotic cell rates were observed in the CDMEM and Biodentine groups, respectively, but there was no statistically significant difference between the groups in terms of early apoptotic cell rates based on times (p > 0.05) (Table 3). Moreover, there was no statistically significant difference in the early apoptotic cell rates in each group based on time (p > 0.05) (Table 3).
Table 3.
Evaluation of early apoptotic cell ratios among 3 different days and among 4 different groups.
| Early Apoptotic Cell Ratios |
p | |||
|---|---|---|---|---|
| 1st Day |
3 rd Day |
7 th Day |
||
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | ||
| Biodentine | 9.38 ± 15.34 (2.5) | 4.04 ± 4.87 (2.5) | 2.75 ± 4.21 (0.9) | 0.794 |
| ProRoot MTA | 10.84 ± 17.54 (3.1) | 6.09 ± 4.73 (6.2) | 7.2 ± 12.17 (1.6) | 0.904 |
| NeoMTA Plus | 12.47 ± 18.68 (4.9) | 8.37 ± 9.82 (5.4) | 5.08 ± 4.29 (4.9) | 1.000 |
| CDMEM |
2.77 ± 1.29 (2.5) |
4.41 ± 2.49 (4.9) |
24.3 ± 25.64 (24.6) |
0.665 |
| p | 0.993 | 0.907 | 0.311 | |
CDMEM, complete dulbecco's modified eagle medium; SD, standard deviation.
Kruskal Wallis Test ∗p < 0.05.
Comparisions of the late apoptotic cell rates between the groups and the late apoptotic cell rates based on days are shown in Table 4. Although the lowest late apoptosis rates in were in the CDMEM group on the 1st and 3rd days and the Biodentine group on the 7th day, no difference was found between the days and the groups (p > 0.05) (Table 4).
Table 4.
Evaluation of late apoptotic cell ratios among 3 different days and among 4 different groups.
| Late Apoptotic Cell Ratios |
p | |||
|---|---|---|---|---|
| 1st Day |
3 rd Day |
7 th Day |
||
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | ||
| Biodentine | 14.74 ± 11.93 (12.7) | 23.82 ± 26.72 (14.4) | 6.55 ± 3.7 (6.8) | 0.551 |
| ProRoot MTA | 20.08 ± 10.84 (16.3) | 31.46 ± 27.29 (28.7) | 18.7 ± 18.68 (14.2) | 0.694 |
| NeoMTA Plus | 13.66 ± 5.23 (12.7) | 27.45 ± 19.03 (29.7) | 18.66 ± 12.57 (19.2) | 0.368 |
| CDMEM |
9.14 ± 1.77 (9.3) |
9.61 ± 3.6 (10) |
16.95 ± 13.62 (17.1) |
1.000 |
| p | 0.197 | 0.707 | 0.530 | |
CDMEM, complete dulbecco's modified eagle medium; SD, standard deviation.
Kruskal Wallis Test ∗p < 0.05.
For all the groups, no significant difference in the percentage of necrotic cells was observed based on time (p > 0.05) (Table 5). Furthermore, no statistically significant difference was found between the groups in terms of the necrotic cell rates (p > 0.05) (Table 5).
Table 5.
Evaluation of necrotic cell ratios among 3 different days and among 4 different groups.
| Necrotic Cell Ratios |
p | |||
|---|---|---|---|---|
| 1st Day |
3 rd Day |
7 th Day |
||
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | ||
| Biodentine | 12.96 ± 21.97 (2.6) | 12.8 ± 9.04 (11.2) | 15.47 ± 11.91 (12) | 0.368 |
| ProRoot MTA | 15.39 ± 24.76 (4.5) | 10.88 ± 5.49 (9.1) | 16.61 ± 7.53 (17.8) | 0.368 |
| NeoMTA Plus | 16.71 ± 23.44 (7.4) | 20.45 ± 15.28 (21.1) | 22.25 ± 10.48 (21.4) | 0.584 |
| CDMEM |
5.77 ± 1.09 (6.3) |
12.04 ± 7.74 (10.5) |
7.21 ± 1.01 (7.1) |
0.135 |
| p | 0.706 | 0.812 | 0.191 | |
CDMEM, complete dulbecco's modified eagle medium; SD, standard deviation.
Kruskal Wallis Test ∗p < 0.05.
Discussion
Mesenchymal stem cells (MSC) can be isolated from many different tissues. MSCs show fibroblast-like spindle morphology and have the potential to differentiate into various cells. They are also reported to carry some specific cell surface markers (CD73, CD90 and CD105).19,20
The minimum criteria for the identification of MSCs have been developed by the International Cellular Therapy Association. Accordingly, MSCs should adhere to plastic surfaces under standard culture conditions and express CD105, CD73 and CD90, no expression of CD45, CD34, CD14 and CD11b. MSCs should be able to differentiate into different cells such as osteoblasts, adipocytes and chondroblasts in vitro.21
In this study, the cultured hDPSCs were characterized by detecting specific surface antigens. The cells isolated from the pulp tissue showed a morphologically fibroblast-like spindle structure, and also potently expressed CD105, CD146, CD90, CD29 and CD73 markers immunophenotypically. Furthermore, these cells showed potential for adipogenic, osteogenic and chondrogenic triple differentiation. These cells cultured with these properties have proved to have MSC character.22
The evaluation of biocompatibility is very important because MTA-based materials are used in vital pulp therapies in the last couple decades. Dental pulp stem cells (DPSCs) play a crucial role in the tissue healing process through odontoblast like cell differentiation. DPSCs are clonogenic and capable of self-renewal and multifaceted differentiation. DPSCs respond to tooth injury by differentiating, proliferating, differentiating and adhering to odontoblast-like cells to replace lost odontoblasts, resulting in the formation and secretion of tertiary dentin, leading to dentin bridge formation. Therefore, these materials must be biocompatible with the pulp cells.23, 24, 25, 26, 27, 28, 29
According to ISO standards, cell viability tests can be evaluated at the end of the 24, 48 and 72 h. Although the cell viability test periods are generally stated as 1st day and 3rd day in the literature, the cell viability was evaluated at the end of the 7th day in this study in order to evaluate the long-term toxic effects of the materials.22
Chang et al. evaluated the biocompatibility of Biaggregate (BA), Micromega MTA (MMTA), ProRoot MTA (PMTA) and intermediate restorative material (IRM) on human pulp cells by MTT test at 1, 7 and 14 days PMTA, BA and MMTA showed equal biocompatibility, whereas IRM had a cytotoxic effect on cells when compared these materials.24
Luo et al. showed the effects of 4 different concentrations of Biodentine solutions (Biodentine 0,02 mg/ml; Biodentine 0,2 mg/ml; Biodentine 2 mg/ml; Biodentine 20 mg/ml) on hDPSCs at the end of days 1, 3, 5 and 7th days with MTT test and also they evaluated the cell viability of these experimental groıps on pulp cells at the end of 24 h with Brdu test. They demonstrated that BD 0,2 and BD 2 groups increase the proliferation of hDPSCs, but the highest concentration of BD 20 group by reducing the cell proliferation by cytotoxic effect.30
Rodrigues et al.31 studied the effects of MTA Plus and MTA Angelus on human pulp stem cells. They evaluated the cell viability by MTT test and indicated that neither experimental group had a cytotoxic effect on cells. They also examined apoptotic and necrotic cell ratios by flow cytometry. The apoptptic cell ratios in both experimental groups were similar to each other and positive control group (DMEM). They stated that MTA Plus and MTA Angelus did not induce apoptosis, but there was a small increase in necrotic cells. They also reported that this small increase in the rate of the necrosis did not affect the overall rate of dead cells (apoptosis + necrosis).31
Jung et al.32 assesed the viability of MTA, Biodentine and Bioaggregate on HDPCs by using MTT test and reported that the effects of these materials on cell viability were similar.32
Although there are many studies investigating the effects of materials such as MTA and Biodentine on the pulp cells, there are limited studies about NeoMTA Plus which is a new material which is releases to eliminate the disadvantages of MTA.
In the previous studies, it was demonstrated that MTA cements have not cytotoxic effects on dental pulp stem cells. Cell viability and bioactivity tests are important to evaluate cellular damage and biological effect of new biomaterials.33 In the present study, the effects of NeoMTA Plus, which is a new material besides MTA Angelus, ProRoot MTA on human dental pulp stem cells were investigated.
Tanomaru-Filho et al.,34 researched the biocompatibility of the NeoMTA Plus, MTA Angelus and the tricalcium silicate cement containing the tantalum oxide with MTT test. They formed the materials in different dilutions and measured the cell viability after 24 h. According to the test results, they reported that NeoMTA Plus, MTA Angelus and the tricalcium silicate cement were biocompatible on hDPSCs.34
Tomas Catala et al.35 researched the effects of the NeoMTA Plus, MTA Angelus and MTA Repair HP on dental pulp stem cells by using MTT test on days 1, 3 and 7. As a result they reported that there was a high level of cell proliferation and binding in all three materials stated.35
In these studies about NeoMTA Plus, the only cell viability was examined using MTT test. In this study, the cell viability as well as the dead cell rates (early apoptotic, late apoptotic and necrotic cell rates) were evaluated differently from other studies using flow cytometry analysis.
The results of this study showed that the all groups exhibited the similar cell viability and demonstrated low percentage of early apoptotic, late apoptotic and necrotic cells. At all time intervals, the cell viability and dead cell ratios didn't show statistical significant difference between all experimental groups. When the datas presented in the tables at the end of the study are evaluated, some ‘SD’ values can be seen as higher than the ‘Mean’ values due to the nonparametric distribution of the datas.
The main composition of the MTA material is tricalcium silicate, tricalcium oxide, tricalcium aluminate, silicate oxide and bizmuth oxide. The most important difference of NeoMTA Plus is that it does not contain bismuth oxide and contains a important amount of tantalum pentoxide. NeoMTA Plus is bismuth free and contains a significant amount of tantalum pentoxide. Tantalum has been used for orthopedic plates, membranes and plates due to its inertness.
The results of this study showed that tantalum did not affect the biocompatibility of these cements. This result is consistent with the results of the studies of Tanomaru et al. and Tomas Catala et al. The effect of NeoMTA Plus on cell viability in these two studies was found to be similar to our study when compared with MTA and Biodentine.34,35
Tantalum oxide has been reported to have adequate physicochemical properties such as sufficient radiopacity and calcium hydroxide production and also does not discolorize on dental tissues.36
Liu et al. showed that iRootBP Plus a tricalcium silicate-based cement which contains Ta2O5 promoted proliferation of hDPCs.37 The present study confirmed that NeoMTA Plus (tricalcium silicate based material associated with tantalum oxide) showed biocompatibility and similar cell viability compared with Biodentine, ProRoot MTA and DMEM.
This study has some limitations due to in vitro conditions not being able to fully reflect the mouth. Therefore, there is a need for support by in vivo experiments.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by the Research Fund of Istanbul University, Project no:26391.
References
- 1.Vitti R.P., Prati C., Sinhoreti M.A.C. Chemical-pyhsical properties of experimental root canal sealers based on butyl ethylene glycol disaliscylate and MTA. Dent Mater. 2013;20:1287–1294. doi: 10.1016/j.dental.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Huang S.C., Wu B.C., Kao C.T., Huang C.J., Shie M.Y. Role of the p38 pathway in mineral trioxide aggregate induced cell viability and angiogenesis related proteins of dental pulp cell in vitro. Int Endod J. 2014;48:236–245. doi: 10.1111/iej.12305. [DOI] [PubMed] [Google Scholar]
- 3.Peng W., Liu W., Zhai W. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37:1240–1246. doi: 10.1016/j.joen.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Filho M.T., Andrade A.S., Rodrigues E.M. Biocompatibility and mineralized nodule formation of NeoMTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017;2017:1–9. doi: 10.1111/iej.12780. [DOI] [PubMed] [Google Scholar]
- 5.Parirokh M., Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: clinical application, drawbacks, and mechanism of action. J Endod. 2010;36:3. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Ber B.S., Hatton J.F., Stewart G.P. Chemical modification of ProRoot MTA to improve handling characteristics and decrease setting time. J Endod. 2007;33:1231–1234. doi: 10.1016/j.joen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Nowicka A., Lipski M., Parafiniuk M. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–747. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Parirokh M., Torabinejad M., Dummer P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview-Part I: vital pulp therapy. Int Endod J. 2017;51:177–205. doi: 10.1111/iej.12841. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto S., Han L., Noiri Y., Okiji T. Evaluation of the Ca ion release, pH and surface apatite formation of a prototype tricalcium silicate cement. Int Endod J. 2017;52:73–82. doi: 10.1111/iej.12737. [DOI] [PubMed] [Google Scholar]
- 10.Valles M., Roig M., Sindreu F.D., Martinez S., Mercade M. Color stability of teeth restored with biodentine: a 6 month in vitro study. J Endod. 2015;41:1157–1160. doi: 10.1016/j.joen.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Daltoe M.O., Paula Silva F.W.G., Faccioli L.H., Gaton Hernandez P.M., Rossi A.D., Silva L.A.B. Expression of mineralization markers during pulp response to biodentine and mineral trioxide aggregate. J Endod. 2016;42:596–603. doi: 10.1016/j.joen.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri J., Sorrentino F., Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, biodentine and MTA Angelus. Dent Mater. 2013;29:580–593. doi: 10.1016/j.dental.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Koubi G., Colon P., Franquin J.C. Clinical evaluation of the performance and safety of a new dentine substitute, biodentine, in the restoration of posterior teeth-a prospective study. Clin Oral Invest. 2013;17:243–249. doi: 10.1007/s00784-012-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilleri J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J Endod. 2014;40:436–440. doi: 10.1016/j.joen.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri J. Staining potential of Neo MTA Plus, MTA Plus and biodentine used for pulpotomy procedures. J Endod. 2015;41:1139–1145. doi: 10.1016/j.joen.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Siboni F., Taddei P., Prati C., Gandolfi M.G. Properties of NeoMTA Plus and MTA Plus cements for endodontics. Int Endod J. 2017;50:83–94. doi: 10.1111/iej.12787. [DOI] [PubMed] [Google Scholar]
- 17.Biological evaluation of Medical Devices-Part 12: sample preparation and reference materials. International organization for standardization; Geneva,Switzerland: 2007. ISO:10993-12. [Google Scholar]
- 18.Biological evaluation of medical devices-Part 5:test for in vitro cytotoxicity. International organization for standardization; Geneva,Switzerland: 2009. ISO:10993-5. [Google Scholar]
- 19.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo B.M., Miura M., Gronthos S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 21.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Garcia S., Pecci-Lloret M.P., Pecci-Lloret M.R. In vitro evaluation of the biological effects of ACTIVA Kids BioACTIVE restorative, Ionolux, and Riva Light Cure on human dental pulp stem cells. Mater. 2019;12:3694. doi: 10.3390/ma12223694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Z., Peng B., Jiang H., Bian Z., Yan P. Effect of bioaggregate on mineral-associated gene expression in osteoblast cells. J Endod. 2010;36:1145–1148. doi: 10.1016/j.joen.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Chang S.W., Lee S.Y., Kum K.Y., Kim E.C. Effects of ProRoot MTA, bioaggregate, and micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endod. 2014;40:113–118. doi: 10.1016/j.joen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Takeda T., Tezuka Y., Horiuchi M. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008;87:676–681. doi: 10.1177/154405910808700716. [DOI] [PubMed] [Google Scholar]
- 26.Alliot-Licht B., Bluteau G., Magne D. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- 27.Kitasako Y., Ikeda M., Tagami J. Pulpal responses to bacterial contamination following dentin bridging beneath hard-setting calcium hydroxide and self etching adhesive resin system. Dent Traumatol. 2008;24:201–206. doi: 10.1111/j.1600-9657.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 28.Williams D.W., Wu H., Oh J.E. 2- Hydroxyethyl methacrylate inhibits migration of dental pulp stem cells. J Endod. 2013;39:1156–1160. doi: 10.1016/j.joen.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Anto` V., Di Caprio M.P., Ametrano G., Simeone M., Rengo S., Spagnuolo G. Effect of mineral trioxide aggregate on mesenchymal stem cells. J Endod. 2010;36:1839–1843. doi: 10.1016/j.joen.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z., Li D., Kohli M.R., Yu Q., Kim S., He W.X. Effect of biodentine on the proliferation, migration and adhesion of human dental pulp stem cells. J Dent. 2014;42:490–497. doi: 10.1016/j.jdent.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues E.M., Cornelio A.L.G., Mestieri L.B. Human dental pulp cells response to mineral trioxide aggregate (MTA) and MTA Plus: cytotoxicity and gene experession analysis. Int Endod J. 2017;50:780–789. doi: 10.1111/iej.12683. [DOI] [PubMed] [Google Scholar]
- 32.Jung J.Y., Woo S.M., Lee B.N., Koh J.T., Nör J.E., Hwang Y.C. Effect of biodentine and bioggregate on odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp cells. Int Endod J. 2015;48:177–184. doi: 10.1111/iej.12298. [DOI] [PubMed] [Google Scholar]
- 33.Gomes-Cornelio A.L., Rodrigues E.M., Salles L.P. Bioactivity of MTA Plus, Biodentine and an experimental calcium silicate-based cement on human osteoblast-like cells. Int Endod J. 2017;50:39–47. doi: 10.1111/iej.12589. [DOI] [PubMed] [Google Scholar]
- 34.Tanomaru-Filho M., Andrade A.S., Rodrigues E.M. Biocompatibility and mineralized nodule formation of NeoMTA Plus and an experimental tricalcium silicate cement containing tantalum oxide. Int Endod J. 2017;50:31–39. doi: 10.1111/iej.12780. [DOI] [PubMed] [Google Scholar]
- 35.Tomas Catala C.J., Collado Gonzalez M., Garcia Bernal D. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int Endod J. 2017;50:63–72. doi: 10.1111/iej.12859. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri J., Sorrentino F., Damidot D. Characterization of un-hydrated and hydrated BioAggregateTM and MTA AngelusTM. Clin Oral Invest. 2015;19:689–698. doi: 10.1007/s00784-014-1292-4. [DOI] [PubMed] [Google Scholar]
- 37.Liu S., Wang S., Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod. 2015;41:652–657. doi: 10.1016/j.joen.2014.12.009. [DOI] [PubMed] [Google Scholar]