Abstract
Background
Bio-Oss® collagen (BC) has been used in clinical applications for years but the ridge preservation property of BC remains controversial. There is no animal model accurately simulates the extraction socket in people. The aim of this study was to assess the ridge preservation of a novel extraction sockets with a thin buccal plate using BC.
Materials and methods
Two beagle dogs were used to assess the characterization of the novel extraction socket. The width and height of the socket were measured and biopsies of the socket were collected for histologic examination. Four beagle dogs were used to assess the ridge preservation property of BC. BC was placed in the socket and socket left untreated was set as control group (CT). Cone-beam computed tomography analysis, histological examination, and micro-CT analysis were used to evaluate the ridge preservation.
Results
The novel extraction socket had obvious larger volume with a markedly narrow buccal wall than mandible extraction sockets. At 12 weeks, the width of the crest of the alveolar ridge preservation ratios was 34% for the CT and 82% for the BC. BC group had larger socket volume compare to CT group. BC group had a significant higher bone density in the middle and apical areas of the alveolar bone. Socket placed with BC showed significantly less vertical bone loss compared with CT group.
Conclusion
Extraction site with a significantly larger dimension and a very thin buccal plate was established. Extraction sockets filled with BC exhibit excellent maintenance of alveolar bone volume.
Keywords: Biomaterial, Bio-Oss® collagen, Bone regeneration, Extraction socket, Ridge preservation
Introduction
Extensive experimental evidence from both animal and human studies suggested that it is unavoidable for a severe alveolar bone resorption to occur after tooth extraction.1, 2, 3, 4 Significant loss of the alveolar ridge occurs within six months after tooth extraction, averaging 3.8 mm in width and 1.24 mm in height.5 The resorption of the alveolar bone is not conducive for future implant treatment and may result in devastating esthetical consequences.6,7
Ridge preservation is a procedure aiming to control alveolar bone resorption following tooth extractions.8 Ridge preservation involves the application of different bone graft materials to fill the extraction socket.9 Numerous studies demonstrated that ridge preservation could preserve more bone volume when compared with tooth extraction alone.10, 11, 12 However, there is no report showing that the bone volume of the extraction socket could be complete preserved and ridge preservation technique does not completely meet the clinical demand.13
Various bone graft materials have been used for ridge preservation.14 Bone grafts such as hydroxyapatite, bioactive glass, deproteinized bovine bone mineral (DBBM), human demineralized bone matrix and others are effective for maintaining the bone volume of extraction socket.15, 16, 17 However, there is no evidence to suggest the superiority of one bone graft over others.18 Bio-Oss® collagen (BC, Geistlich, Wolhusen, Switzerland) is a kind of xenogenic bone substitute material consists of 90% DBBM extracted from cattle and 10% highly purified porcine collagen matrix.8 BC possesses osteoconductive and biocompatibility properties and has been currently used in the clinical applications.19 However, there are concerns over the residence of DBBM particles that may influence the new bone formation.18,20 More studies are needed to evaluate the ridge preservation property of BC.
Most animal studies so far evaluate extraction socket on mandible (with very thick buccal wall), which does not reflect clinical circumstances where majority ridge preservation are needed on maxilla and at least parts of the buccal wall is missing or will resorb during socket remodeling.18,19 Today, there is no animal model that accurately simulates the extraction socket in people.
Based on these concerns, the aim of the present study was to assess the ridge preservation result of a novel extraction socket on maxilla with a thin buccal plate using BC. In this study, the characterization of the novel extraction socket and the ridge preservation property of BC were the main focus.
Material and methods
The research protocol was approved by the Ethics Committee of School and Hospital of Stomatology, Wuhan University, China. Animal experiments followed the National Institutes of Health guide for the Care and Use of Laboratory Animals. Six female beagle dogs about 12 months old and weighing about 10 kg were used for the study. The dogs were anesthetized with intramuscular administered with ketamine (20 mg/kg) and xylazine hydrochloride (1 mg/kg). In addition, the dogs received local anesthesia with articaine with adrenaline (1:100,000).
Characterization of the novel extraction socket
The novel extraction socket was located on the distal roots of the 4th maxillary premolar (4P4). 4P4 of two beagle dogs were sectioned into two parts with fissure burs. The distal roots of 4P4 were extracted atraumatically while the mesial portion was retained. The width and height of the sockets were measured with a periodontal probe and dogs were killed by injecting an overdose of xylazine hydrochloride. Block biopsies of the sockets were collected for histologic examination. Extraction socket of the distal roots of 3rd mandibular premolars (3P3), the most commonly studied mandible extraction sockets, were used as control.
Ridge preservation surgical protocol
In four dogs, 4P4 were sectioned hemisected and the distal roots were atraumatically removed. Sockets were allocated to the following two experimental groups: (1) no treatment (CT, control group) (2) BC group. In BC groups, BC were placed to fill the socket while in CT group, blood clot was allowed to form in the empty socket (Fig. 1). CBCT analyses were taken after 2 weeks for postoperative comparison and 12 weeks for final comparison. The beagle dogs were sacrificed 12 weeks after tooth extraction, Block biopsies of 4P4 were taken for micro-CT analysis and histological examination.
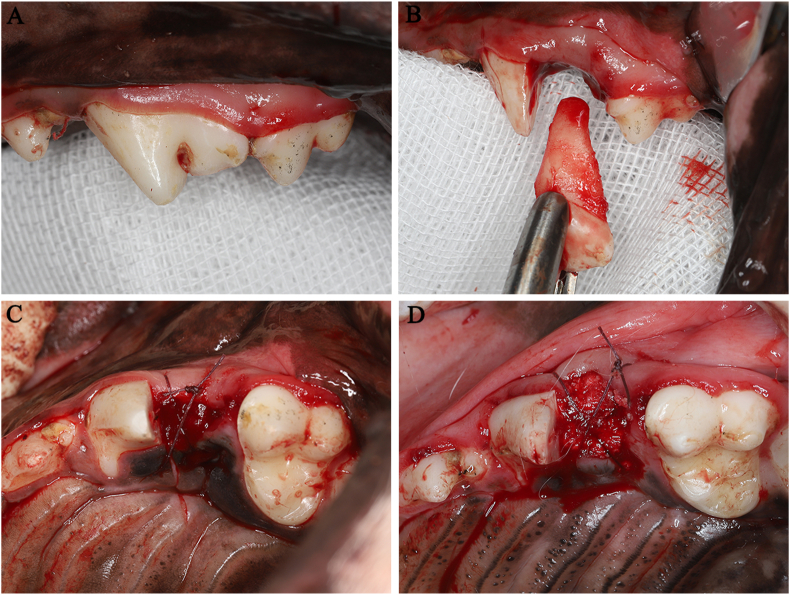
Figure 1.
Preparation of a novel extraction socket model and fill of Bio-Oss collagen. The distal root of the 4th premolar in both quadrants of the maxilla were removed (A, B). BC (D) was filled into root extraction socket. Socket left untreated (C) was set as a control.
Cone-beam computed tomography (CBCT) analysis
The CBCT images were taken with a CBCT machine (NewTom, Verona, Italy). After general anesthesia, beagle dogs underwent a low-dose CBCT imaging with voxel size 0.300 mm, tube voltage of 110 KV, current of 0.56 mA, and exposure time of 3.6 s. A comparison was made between the 2 weeks postoperative CBCT radiographs and the 12 weeks postoperative CBCT radiographs. The alveolar bone width (B–P, buccal–palatal width) was measured at the top of the center alveolar ridge.
Histological examination
The biopsies were processed according to the methods described by Araújo.19 They were dehydrated in increasing concentrations of ethanol and embedded in resin. Serial sections were cut in the B–P plane. The microtome was set at 10 μm. For each biopsy, three sections stood for the central part of the extraction socket, about 30 μm apart, were selected for histological examination. The sections were stained with Goldner trichrome staining, allowing the distinct classification of mineralized bone (green), non-mineralized osteoid (red-orange). Total socket area and the area occupied by mineralized bone (MB), non-mineralized osteoid (NMO), and bone marrow (BM) in the socket were measured. In addition, Bio-Oss® like particles (BP) in BC group were also measured.
Micro-CT analysis
The socket samples were scanned with micro-CT (Bruker Skyscan-1176, Kontich, Belgium) at 82 kV, with 18 μm pixel size. The projection image data were reconstructed using Skyscan NRecon software (Bruker, Kontich, Belgium) to create 3D images and analyzed using the Mimics 21.0 (Materialise, Leuven, Belgium). Bone density were measured as described in other study.10,14 A line between mesial and distal wall was drawn and the socket was divided into three parts at the apical, middle and coronal area with the same vertical height. Bone density of these areas were evaluated through variations of grayscale varied from 0 (transparent) to 255 (opaque) using the Mimics 21.0 software. Vertical bone loss was measured according to the methods described before.10 Briefly, a horizontal base line was drawn on the basis of bone height of the flanking teeth. The vertical bone loss was then measured at the center positions of the socket (B–P plane) with respect to the horizontal base line.
Data analysis
Statistical analyses were performed using the SPSS version 25 software (IBM Software, New York, USA). The values for the parameters were measured and presented as the means ± standard deviation. Two sample t test was used to analyze experimental data.
Results
Result of the characterization of the extraction socket
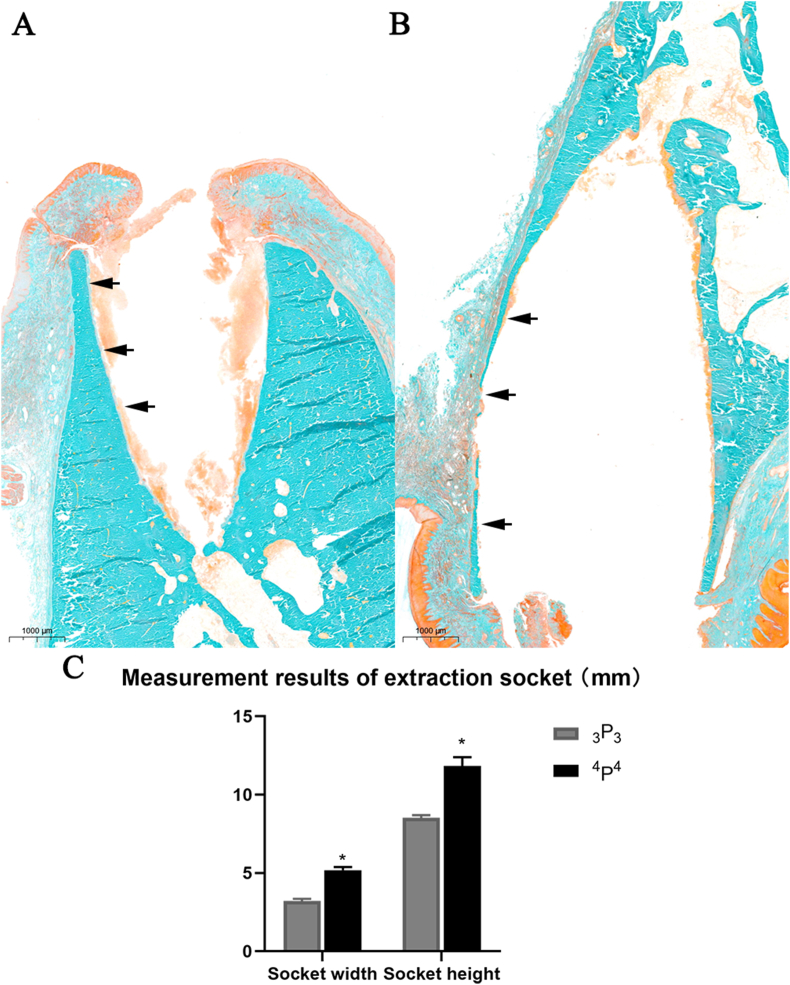
Compared to 3P3(A), the extraction socket of 4P4(B) had obvious larger volume. The buccal bone wall of 4P4 was markedly narrow than 3P3 counterpart (Fig. 2). The mean socket width and socked depth of 4P4 was 5.2 ± 0.2 mm and 11.8 ± 0.6 mm. The corresponding value for 3P3 was 3.2 ± 0.1 mm and 8.5 ± 0.2 mm. There was a significant difference (P < 0.05) between the two groups (Fig. 2, C).
Figure 2.
Histological examination results of 3P3 (A) and 4P4 (B) and measurement results of extraction socket dimensions (C). The arrows indicate buccal wall. Original magnification: 1×, ∗P < 0.05.
CBCT analysis result
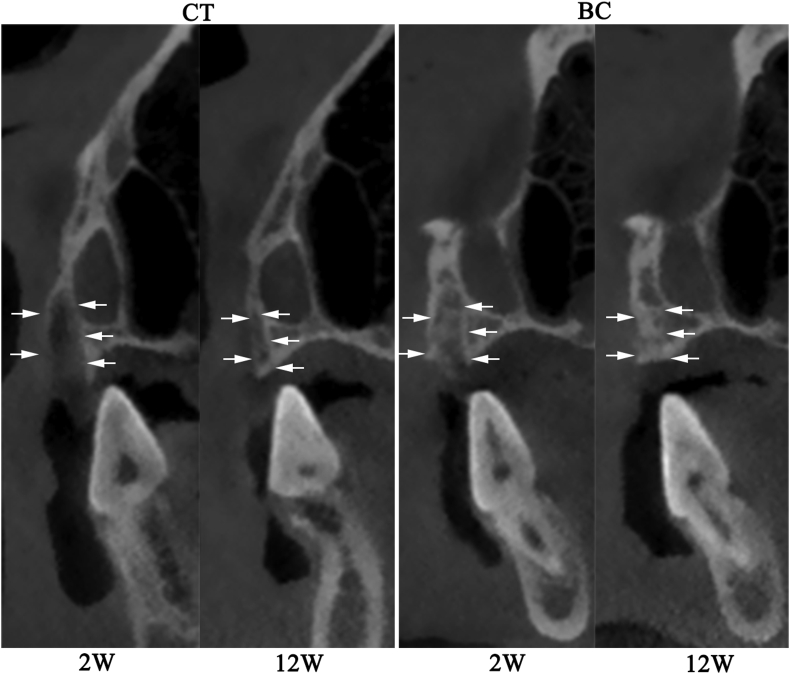
The CBCT results showed that BC group result in greater bone volume compared with CT group. The structure of alveolar bone in CT group was clear high-density edges and internal low-density shadow (12 weeks). The original buccal bone wall disappeared at 12 weeks in two groups (Fig. 3). The width of the crest of the alveolar ridge (WD) was presented in Table 1.
Figure 3.
CBCT result of the extraction socket at 2 weeks and 12 weeks. The white arrows indicate the contours of the alveolar ridge.
Table 1.
Width of the crest of the alveolar ridge.
∗P < 0.05.
Control.
Bio-Oss® collagen.
Histological examination result
Because this study was performed in a novel socket with very thin buccal wall, the original buccal bone wall in all groups got lost at three months after tooth extraction (Fig. 4). Unmineralized matrix could be found on the region of bone marrow (Fig. 5). Tissue proportions of all groups were reported in Fig. 6.
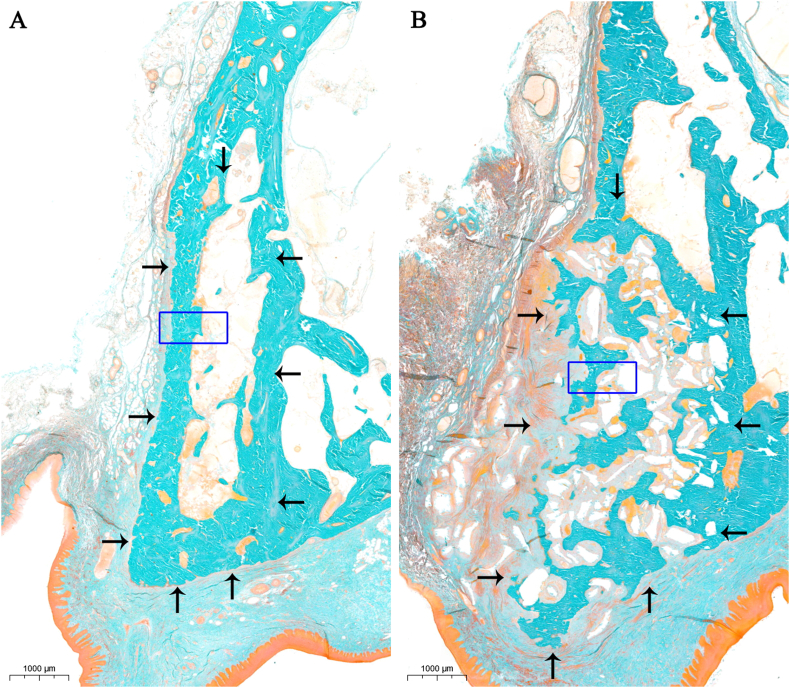
Figure 4.
Result of histological examination (A: control group, B: Bio-Oss® collagen group). The black arrows indicate the contours of the alveolar ridge. Original magnification: 1×.
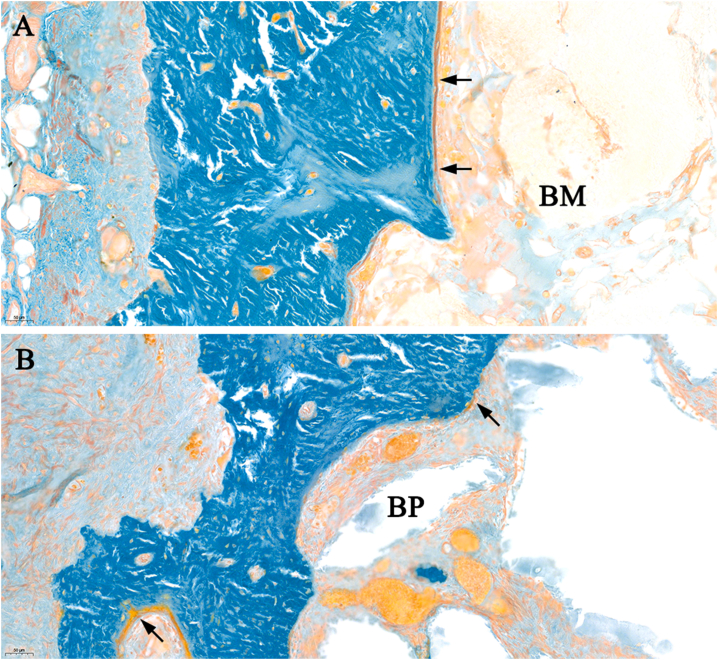
Figure 5.
Highly magnified of the buccal side of the alveolar ridge (A: control group, B: Bio-Oss® collagen group). The arrows indicate the non-mineralized osteoid (red-orange). BM: bone marrow, BP: Bio-Oss® like particles. Original magnification: 20×. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
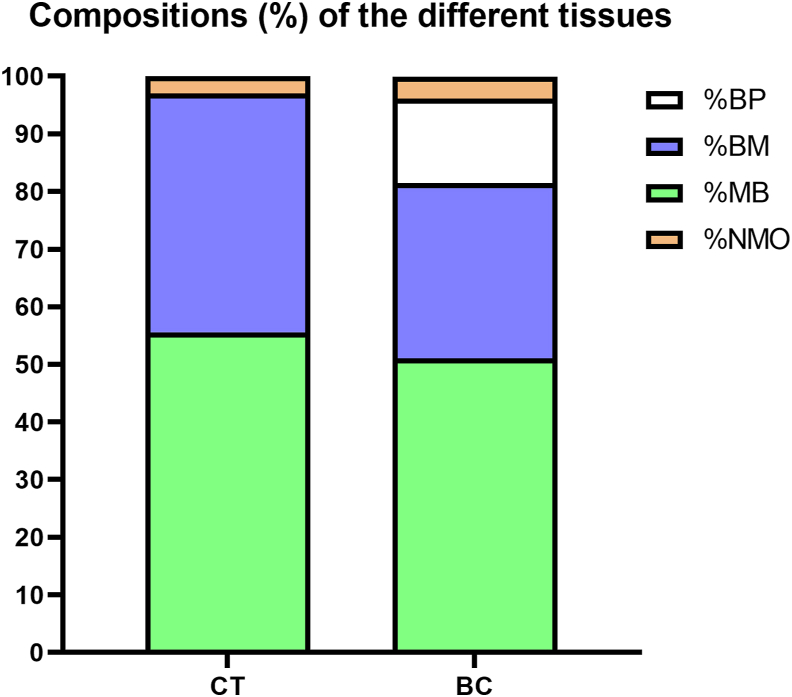
Figure 6.
Compositions (%) of the different tissues. CT: control group, BC: Bio-Oss® collagen group, BP: Bio-Oss® like particles, BM: bone marrow, MB: mineralized bone, NMO: non-mineralized osteoid.
The socket of CT group was composed of mineralized bone (MB) (55.6% ± 12%) in the peripheral and bone marrow (BM) (41.4% ± 12.3%) in the central (Figs. 4 and 6). The socket in BC group was filled by MB (51.1% ± 2.5%), bone marrow (BM) (30.4% ± 3.1%), and remnants of the BP (14.7% ± 2.8%) (Fig. 6). The majority of the spaces between BP were the newly formed bone (Figs. 4 and 5).
BC group had obvious larger socket volume compared with the CT group. No significant difference of MB% could be found between two groups. The values of BM% in BC group had a significant difference compared with the CT group (P < 0.05).
Micro CT analysis result
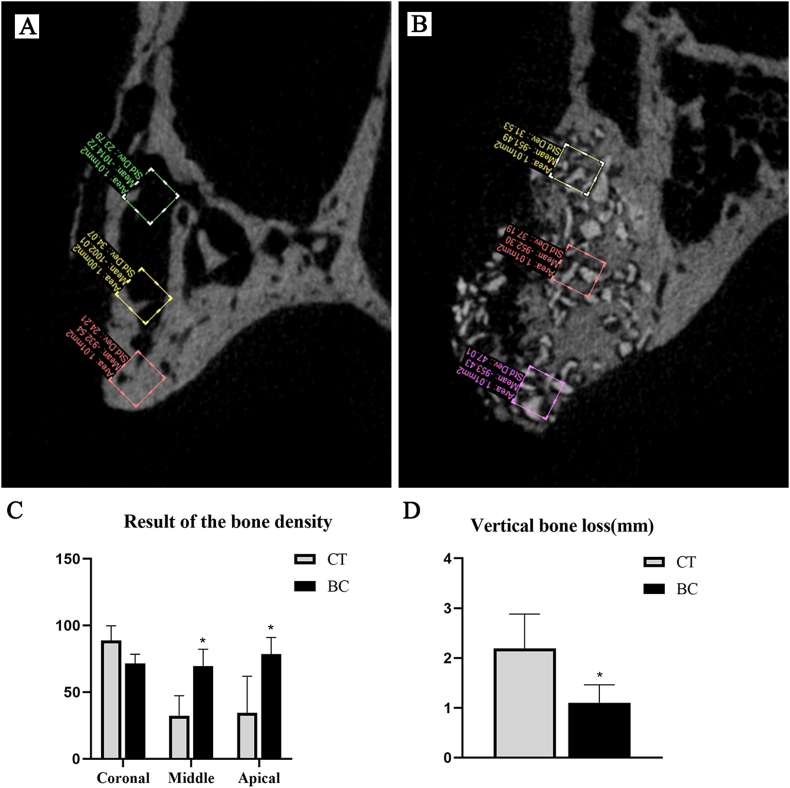
The original buccal wall in two groups disappeared 12 weeks after tooth extraction. The bone density of CT group was 88.9 ± 10.9 (coronal area), 32.3 ± 14.9 (middle area) and 34.4 ± 27.6 (apical area). The bone density for BC group was 71.4 ± 7.0 (coronal area), 69.4 ± 12.8 (middle area) and 78.6 ± 12.4 (apical area). The vertical bone loss of CT group was 2.2 ± 0.7 mm. The corresponding value for BC was 1.1 ± 0.4 mm (see Fig. 7).
Figure 7.
The result of micro-CT analysis. A: B–P plane of CT group, B: B–P plane of BC group, C: the result of the bone density, D: the result of the vertical bone loss. CT: control group, BC: Bio-Oss® collagen group. ∗P < 0.05.
BC group had a significant higher bone density in middle and apical area of the alveolar bone compare with CT group. The vertical bone loss of BC group was significant lower (P < 0.05).
Discussion
The objective of this study was to evaluate the ridge preservation result of a novel extraction sockets with a very thin buccal plate using BC. A number of studies performed on socket with thick buccal plate suggest positive outcome of ridge preservation.10,14,19 However, sites with a thick buccal plate (more than 1 mm) usually receive immediate implant placement.15 Those sockets with very thin buccal plate or partial missing buccal wall will need ridge preservation.15 In our study, we successfully established a novel extraction socket. The sockets have a significantly larger dimension and thinner buccal plate compared with the most commonly studied mandible extraction sockets. The buccal bone wall of this novel extraction socket resorbed three month later which is highly consistent with clinical findings.21 The novel extraction socket left untreated lead to severe horizontal and vertical bone resorption which is very similar to the situation in humans.5 These results suggest that this novel extraction socket highly reflects clinical circumstances and is suitable for ridge preservation research.
An adequate alveolar ridge maintenance to accommodate the implant is a prerequisite for the successful dental implantation.22 In our study, BC is able to maintain 82% of alveolar bone width while CT group is only able to maintain 37%. These results are like the results of other research that sockets treated with BC demonstrated a loss of less than 20% of the alveolar bone width in 79% of test sites, whereas in untreated sockets 79% of extract sites showed a loss of more than 20% of the alveolar bone width.23 In the cases with considerable narrow alveolar ridge, bone increment operations may be required for implantation.24
Our histology study showed that the socket of no treat control group at the 12-week was composed of new formed mineralized bone in the peripheral and bone marrow in the central. This finding is highly consistent with other studies.1,25 This phenomenon is referred to as “corticalization” of the socket which means after a series of reconstruction events the cortical bone wall was eventually developed.25 Socket filled with BC led to obviously increased bone dimension when compared to the no treatment group. This is also in agreement with the histology study of other researches.14,19 Some studies showed the adhesion of osteoblasts to the BC surface which demonstrated that BC could serve as a scaffold for osteogenic cells.11,14 However, there are concerns over the residence of DBBM particles that may influence the new bone formation.20,26 In our study, there is no significant difference of MB% could be found between BC (51.1%) and CT (55.6%). This suggests that the amount of new bone formation is not influence by the residence of DBBM particles in this novel extraction socket.
Micro CT result suggested that socket filled with BC had a significant higher bone density in the middle and apical area of the socket compared with control group. The higher bone density is not only because of BC but also the new formed bone in the area. Implant placed in this socket may have a better stability since a high bone density is contribute to the primary stability of implantation.27 Our study showed that socket left untreated showed significantly more vertical bone loss compared with BC. It is consistent with other studies.28 Severe vertical bone loss can pose serious problems such as increased crown-to-implant ratio and higher aesthetics concerns.29
Space maintenance for bone regeneration is one of the key factors for bone regeneration.30 BC showed a good ability to maintain alveolar bone volume. This is partly because BC offer stability for the coagulum can avoid volume reduction and BC particles act as a scaffold for new bone formation.5,19 On the contrary, blood clot in CT could not maintain the space after buccal wall absorption. This may partially explain the severe alveolar bone resorption that occur in CT. Present study showed that 14.7% of the socket volume are composed of remnants of the BP after 3 months. The fate of these remnants and long-term effects of these remnants on implant loading requires a further study.
In conclusion, a novel extraction socket with a significantly larger dimension and a very thin buccal plate was established for the study. BC filled in this novel extraction sockets exhibited excellent maintenance of alveolar bone volume.
Ethics statement
The present study is a part of the project that approved by the Ethics Committee of School and Hospital of Stomatology, Wuhan University (Ethical approval code: 2020A39). All animal care and use were performed in accordance with the ethical guidelines of the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Authorship
Conception and design of study: Qihang Fan, Hao Zeng and Bin Shi.
Acquisition of data: Qihang Fan, Hao Zeng, Tao Wu and Jing Sun.
Analysis and/or interpretation of data: Qihang Fan, Hao Zeng and Qi Yan.
Drafting the manuscript: Qihang Fan and Hao Zeng.
Revising the manuscript critically for important intellectual content: Wei Fan and Bin Shi.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgments and have given us their written permission to be named. If we have not included an Acknowledgments in our manuscript, then that indicates that we have not received substantial contributions from non-authors.
References
- 1.Cardaropoli G., Araújo M., Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30:809–818. doi: 10.1034/j.1600-051x.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- 2.Chappuis V., Engel O., Reyes M., Shahim K., Nolte L.P., Buser D. Ridge alterations post-extraction in the esthetic zone: a 3D analysis with CBCT. J Dent Res. 2013;92:195S–201S. doi: 10.1177/0022034513506713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scala A., Lang N.P., Schweikert M.T., Oliveira JAd, Rangel-Garcia I., Botticelli D. Sequential healing of open extraction sockets. An experimental study in monkeys. Clin Oral Implants Res. 2014;25:288–295. doi: 10.1111/clr.12148. [DOI] [PubMed] [Google Scholar]
- 4.Schropp L., Wenzel A., Kostopoulos L., Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restor Dent. 2003;23:313–323. [PubMed] [Google Scholar]
- 5.HaMmerle C.H.F., Araújo M.G., Simion M. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012;23:80–82. doi: 10.1111/j.1600-0501.2011.02370.x. [DOI] [PubMed] [Google Scholar]
- 6.Barone R., Clauser C., Grassi R., Merli M., Prato G.P.P. A protocol for maintaining or increasing the width of masticatory mucosa around submerged implants: a 1-year prospective study on 53 patients. Int J Periodontics Restor Dent. 1998;18:377–387. [PubMed] [Google Scholar]
- 7.Bartee B.K. Extraction site reconstruction for alveolar ridge preservation. Part 1: rationale and materials selection. J Oral Implantol. 2001;27:187–193. doi: 10.1563/1548-1336(2001)027<0187:ESRFAR>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Kassim B., Ivanovski S., Mattheos N. Current perspectives on the role of ridge (socket) preservation procedures in dental implant treatment in the aesthetic zone. Aust Dent J. 2014:48–56. doi: 10.1111/adj.12098. [DOI] [PubMed] [Google Scholar]
- 9.Canellas J.V.D.S., Ritto F.G., Figueredo C.M.D.S., Fischer R.G., Medeiros P.J.D. Histomorphometric evaluation of different grafting materials used for alveolar ridge preservation: a systematic review and network meta-analysis. Int J Oral Maxillofac Surg. 2019;49:797–810. doi: 10.1016/j.ijom.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Suttapreyasri, Srisurang, Buasod Socket preservation using platelet-rich fibrin in conjunction with epithelialized palatal free graft in minipigs. J Oral Maxil Surg. 2014;26:108–117. [Google Scholar]
- 11.Araújo M., Linder E., Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2010;20:1–6. doi: 10.1111/j.1600-0501.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 12.Iasella J.M., Greenwell H., Miller R.L. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74:990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 13.Araújo M.G., Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32:212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 14.Araújo M., Linder E., Wennstrm J., Lindhe J. The influence of Bio-Oss collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodont Rest. 2008;28:123–135. [PubMed] [Google Scholar]
- 15.Darby I., Chen S., Poi R.D. Ridge preservation: what is it and when should it be considered. Aust Dent J. 2008;53:11–21. doi: 10.1111/j.1834-7819.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang R.E., Lang N.P. Ridge preservation after tooth extraction. Clin Oral Implants Res. 2012;23(Suppl 6):147–156. doi: 10.1111/j.1600-0501.2012.02560.x. [DOI] [PubMed] [Google Scholar]
- 17.Machtei E.E., Mayer Y., Horwitz J., Zigdon-Giladi H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: clinical and histological findings. Clin Implant Dent Relat Res. 2018;21:14–20. doi: 10.1111/cid.12707. [DOI] [PubMed] [Google Scholar]
- 18.Fischer K.R., Gtz W., Kauffmann F., Schmidlin P.R., Friedmann A. Ridge preservation of compromised extraction sockets applying a soft cortical membrane: a canine proof-of-principle evaluation. Ann Anat. 2020;231:151524. doi: 10.1016/j.aanat.2020.151524. [DOI] [PubMed] [Google Scholar]
- 19.Araújo M.G., Lindhe J. Ridge preservation with the use of Bio-Oss collagen: a 6-month study in the dog. Clin Oral Implants Res. 2010;20:433–440. doi: 10.1111/j.1600-0501.2009.01705.x. [DOI] [PubMed] [Google Scholar]
- 20.Lindhe J., Cecchinato D., Donati M., Tomasi C., Liljenberg B. Ridge preservation with the use of deproteinized bovine bone mineral. Clin Oral Implants Res. 2014;25:786–790. doi: 10.1111/clr.12170. [DOI] [PubMed] [Google Scholar]
- 21.Calvo-Guirado J.L., Benítez-García J.A., Maté Sánchez de Val J.E. Socket-shield technique: the influence of the length of the remaining buccal segment of healthy tooth structure on peri-implant bone and socket preservation. A study in dogs. Ann Anat. 2018;221:84–92. doi: 10.1016/j.aanat.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Fang C.H., Lin Y.W., Lin F.H., Sun J.S., Chang Z.C. Biomimetic synthesis of nanocrystalline hydroxyapatite composites: therapeutic potential and effects on bone regeneration. Int J Mol Sci. 2019;20:6002–6017. doi: 10.3390/ijms20236002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevins M., Camelo M., Paoli S.D., Friedland B., Wagenberg B. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodont Rest. 2006;26:19–29. [PubMed] [Google Scholar]
- 24.Zhang X., Li Y., Ge Z., Zhao H., Miao L., Pan Y. The dimension and morphology of alveolar bone at maxillary anterior teeth in periodontitis: a retrospective analysis—using CBCT. Int J Oral Sci. 2020;12:4–12. doi: 10.1038/s41368-019-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi H., Fujii N., Futami T., Taguchi N., Kusakari H., Maeda T. A histochemical investigation of the bone formation process by guided bone regeneration in rat jaws. Effect of PTFE membrane application periods on newly formed bone. J Periodontol. 2000;71:341–352. doi: 10.1902/jop.2000.71.3.341. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H., Jingchao H., Li Z. Histological analysis of socket preservation using DBBM: a systematic review and meta-analysis. J Stomatol Oral and Maxillofac Surg. 2020;121:729–735. doi: 10.1016/j.jormas.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Pan C.Y., Liu P.H., Tseng Y.C., Chou S.T., Chang H.P. Effects of cortical bone thickness and trabecular bone density on primary stability of orthodontic mini-implants. J Dent Sci. 2019;14:383–388. doi: 10.1016/j.jds.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunert-Keil C., Gredes T., Heinemann F., Dominiak M., Botzenhart U., Gedrange T. Socket augmentation using a commercial collagen-based product — an animal study in pigs. Mater Sci Eng C Mater Biol Appl. 2015;46:177–183. doi: 10.1016/j.msec.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Llanos A., Sapata V., Jung R. Comparison between two bone substitutes for alveolar ridge preservation after tooth extraction: cone beam computed tomography results of a non-inferiority randomized controlled trial. J Clin Periodontol. 2019;46:373–381. doi: 10.1111/jcpe.13079. [DOI] [PubMed] [Google Scholar]
- 30.Chang H.H., Yeh C.L., Wang Y.L. Neutralized dicalcium phosphate and hydroxyapatite biphasic bioceramics promote bone regeneration in critical peri-implant bone defects. Materials. 2020;13:823–841. doi: 10.3390/ma13040823. [DOI] [PMC free article] [PubMed] [Google Scholar]