Abstract
Background/purpose
Periodontal ligament stem cells (PDLSCs)-based regeneration therapy has received attention for its potential alternative applications in hard tissue and tooth. However, the environmental diversity of oral cavity that regulates PDLSCs differentiation has made it difficult to develop. Therefore, we investigated how high calcium concentrations in the oral environment influence osteogenic differentiation of human PDLSCs (hPDLSCs).
Materials and methods
hPDLSCs collected from human molars were isolated and cultured with CaCl2. First, multi lineage differentiation potentials to osteogenic, chondrogenic, and adipogenic cells were investigated. Then, the effects of CaCl2 on both alkaline phosphatase (ALP) activity and bone mineralization were analyzed and the expression of mRNA and protein for osteogenic marker was explored. Further, luciferase assay was performed to evaluate CaCl2 could regulate the transcriptional activity on osteogenic differentiation in hPDLSCs
Results
CaCl2 treatment at normal to high concentrations showed similar suppression of ALP activity, while mineralized nodule formation was decreased by CaCl2 treatment dose-dependently without affecting proliferation or cytotoxicity in hPDLSCs. We also observed that CaCl2 treatment repressed the mRNA expression and protein abundance of osteogenic genes and transcriptional factors. Notably, repression of the Runx2 level was significant, and CaCl2 treatment inhibited Runx2-mediated transcriptional activity on the osteoblast-specific element (OSE) and ALP promoters.
Conclusion
High concentrations of calcium negatively regulate osteogenic differentiation of hPDLSCs, by repressing osteogenic gene expressions and transcriptional activity. Therefore, these conditions may be applicable to determine the physiologically appropriate concentration of calcium.
Keywords: CaCl2, Calcium, hPDLSCs, Osteogenic differentiation
Introduction
The periodontium is necessary for the maintenance of tooth function and includes the gingiva, cementum, periodontal ligament (PDL), and alveolar bone. As a specialized connective tissue fiber between alveolar bone and cementum, PDL provides supportive, nutritive, and sensory functions and remodeling in teeth.1 For remodeling, the PDL contains dental mesenchymal stem cells that can differentiate into osteoblasts, adipocytes, cementoblasts, and chondrocytes for diverse physiological roles.2 Many studies have successfully isolated human periodontal ligament stem cells (hPDLSCs) from the PDL and elucidated the therapeutic potential of hPDLSCs clinically.3 hPDLSCs mainly present on the surface of the tooth and boundary locations of the periodontium, which are important for periodontal regeneration and inflammatory response.4 Currently, various types of bone graft materials, including allografts, xenografts, and synthetic bone graft substitutes, are being developed to enhance the regeneration of periodontium after grafting.5 Specifically, stem cell research is actively being conducted, and detailed biological approaches and better understanding of hPDLSC differentiation are expected to significantly improve the rate of adhesion and bone formation performance.
Although hPDLSCs are multipotent stem cells that can differentiate into various cells, the number of cells capable of performing desired functions is limited.6 Therefore, it is considerably important to induce hPDLSCs to differentiate into osteoblasts and cementoblasts for formative function. In general, genetic, molecular, and environmental factors play a role in the determination of stem cell fate.7 The molecular genetic study of hPDLSC differentiation has progressed somewhat; however, little research has examined the role of environmental factors in the oral cavity in relation to hPDLSC differentiation.8 According to a previous report, it is only known that hypoxia in the oral cavity increases the proliferation of hPDLSCs,9 whereas other factors that affect the oral environment, such as temperature, pH, and nutritional conditions, are extremely underexamined. Therefore, if this study confirms hPDLSC differentiation by other oral environmental factors, the results will provide potent possibilities for future research.
Calcium consumed through food or nutritional supplements has various effects on our bodies.10 Among the many nutrients, calcium mediates signal transmission within various cells and has an important role in the interaction between cells or tissues.11 As an essential nutrient, calcium could regulate the survival and death of various bacteria in the oral cavity; that is, calcium can influence the oral microbiome, which is important for digestion, energy generation, inflammation, and cell fate determination.12 In addition, calcium is the main constituent of hydroxyapatite, which composes the tooth tissue, including enamel, dental pulp, cementum, and alveolar bone; therefore, it plays a crucial role in the formation and regeneration of teeth.13 Meanwhile, a high calcium concentration, pathophysiologically defined as hypercalcemia, can interfere with heart and kidney function and disorder digestive system.14 Especially in bones, the excess calcium in blood was leached from bones, subsequently it can cause bone pain and muscle weakness.15 Therefore, it is necessary to supplement the appropriate concentration of calcium needed physiologically. In this study, we aim to elucidate the role of high calcium concentrations in controlling hPDLSC differentiation mainly through Runx2 or osterix regulation.
Materials and methods
Primary cell culture
According to the protocol approved by the Institutional Review Board (IRB No. WKDIRB-201708-02) of the dental clinic of Wonkwang University, the third human molars were collected from three healthy young females (15–23 years old). After gently separating the PDL from the extracted molar, the separated PDL was digested in 3 mg/mL collagenase type I solution (Worthington Biochem, Freehold, NJ, USA) and 4 mg/mL dispase (Boehringer, Mannheim, Germany). For single-cell suspension, whole cells were passed through the 40-mm strainer (Falcon BD Labware, Franklin Lakes, NJ) and were incubated in alpha-modified Eagle's medium (αMEM; Gibco BRL, Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco BRL) and 1% penicillin-streptomycin antibiotics (Thermo Fisher Scientific, Waltham, MA, USA) in a 37 °C, 5% CO2 incubator. The medium was changed after the first 24 h and every three days thereafter. Colonies of hPDLSCs were randomly selected and incubated separately. All primary cells within passage 2 or 3 were used in this study.
Flow cytometric analysis
Characterization and comparison of the mesenchymal stem cell (MSC) phenotypes of hPDLSCs were performed by examining expression of MSC-associated surface markers at passage 2 using flow cytometry as previously described. In brief, 1.0 × 106 cells were fixed for 10 min with 3.7% paraformaldehyde and resuspended in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA; ICN Biomedicals, Aurora, OH, USA) for 30 min for blocking. Next, cells were incubated with specific antibodies for CD34, CD13, CD90, or CD146 (BD Biosciences, San Jose, CA, USA) at 4 °C for 1 h, followed by incubation with fluorescent secondary antibodies at room temperature for 1 h. The percentage of cells positive for each marker was measured with a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) and analyzed with Cell Quest Pro software (Becton Dickinson Immunocyte Measurement System).
Cytotoxicity assay
Cell toxicity was tested using 3-(4, 5-dimethyltiazzol-2-day)-2, 5-diphenyl tetrazoleum bromide (MTT, Thermo Fisher Scientific) assays. hPDLSCs were cultured with various concentrations of CaCl2 on a 96-well plate. After incubation for 1, 2, 7 or 14 days, 20 μL of MTT (5 mg/mL) solution was added to the 96-well plate, and the final concentration was set to 0.5 mg/mL. After incubating the cells for 1 h, the top solution was removed from each well, and isopropanol (100 L) was added to dissolve the MTT crystals. Quantitative analysis of samples dissolved in isopropanol was conducted at 490 nm using SpectraMax 190 (Molecular Devices, San Jose, CA, USA).
Osteogenic differentiation and alkaline phosphatase (ALP) and Alizarin red S (ARS) staining
For osteogenic differentiation, hPDLSCs were cultured in osteogenic differentiation medium (DM) with 50 mg/mL ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone (Sigma–Aldrich, St. Louis, MO, USA) for 1 week (ALP staining) and 2 weeks (ARS staining). After fixing the differentiated hPDLSCs for 5 min using 4% paraformaldehyde solution, ALP activity was measured using BCIP/NBT color development substrate (Sigma–Aldrich), and mineralized nodules was detected with ARS solution (Sigma–Aldrich). Samples dissolved in dimethyl sulfoxide were quantitatively analyzed using spectrophotometric measurements obtained at absorption wavelengths of 480 nm (ALP staining) or 405 nm (ARS staining) by Spectral Max 190 (Molecular Devices). Cells were observed using a microscope (Olympus, CKX53, Tokyo, Japan).
Chondrogenic and adipogenic differentiation
To induce chondrogenic and adipogenic differentiation, hPDLSCs was cultured in StemPro Chondrogenic and Adipogenic differentiation media (Gibco BRL), respectively, using appropriate nutritional supplements to induce cartilage and adipocyte formation. After 3 weeks, the differentiated hPDLSCs was fixed for 5 min using 4% paraformaldehyde solution and stained with 1% Alcian Blue (Sigma–Aldrich, for cartilage staining) and 0.3% Oil Red O dye (Sigma–Aldrich, for lipid droplet staining) Cells were observed using a microscope (Olympus, CKX53).
Quantitative PCR
Whole-cell RNA was obtained using TRIzol Reagent (Thermo Fisher Scientific) as directed by the manufacturer. Oligo (dT) primers and ReverTra Ace qPCR Reverse Transcriptase (RT) Master Mix (Toyobo, Osaka, Japan) were used to synthesize cDNA. Real-time PCR was performed using the Thunderbird SYBR qPCR Master Mix (Toyobo) on a StepOne Real-Time PCR System (Molecular Devices) using the following conditions: Samples were held at 95 °C for 30 s, followed by 95 °C for 30 s, 60 °C for 5 s, and 95 °C for 5 s. The expression levels of genes of interest were normalized to the expression of GAPDH (glyceraldehyde 3-phosphate dehydrogenase). The primer sequences for PCR were as follows: Alp, forward (F), 5′-ATT GCC CTG AAA CTC CAA AAC C-3′, and reverse (R), 5′-CCT CTG GTG GCA TCT CGT TAT C-3′; Dentin sialophosphoprotein (Dspp), F, 5′-TGC TGG AGC CAC AAA C-3′, and R, 5′-AAA CCC TAT GCA ACC TTC-3′; collagen, type I, alpha 1 (Col1α1), F, 5′-TCT CCACTC TTC TAG GTT CCT-3′, and R, 5′-TTG GGT CAT TTCCAC ATG C-3′; Runx2, F, 5′-AGC AAC AGC AAC AGC AG-3′, and R, 5′-GTA ATC TGA CTC TGT CCT TG-3′; Osterix, F, 5′-GGG TTA AGG GGA GCA AAG TCA GAT-3′, and R, 5′-CTG GGG AAA GGA GGC ACA AAG AAG-3′; and GAPDH, F, 5′-ACC ACA GTC CAT GCC ATC AC-3′, and R, 5′-TCC ACC CTG TTG CTG TA-3′.
Western blot analysis
The hPDLSCs (3.0 × 105 cells/well) were seeded in a 6-well plate and incubated for 2 weeks. Cell pellets were washed with PBS and lysed in ice-cold RIPA lysis and extraction buffer (Thermo Fisher Scientific). Protein concentrations of cell lysates were measured by a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of cell extracts (20 μg of protein) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membrane (GE Healthcare, Buckinghamshire, UK). After blocking with 5% skim milk, the membranes were incubated with primary antibodies against Runx2, α-tubulin (Cell Signaling, Danvers, MA, USA), Col1α1, and osterix (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific). Blots were finally visualized using an enhanced chemiluminescence kit (GE Healthcare).
Transient transfection and luciferase reporter assay
For transient transfection, polyethylenimine (PEI; Polysciences, Warrington, PA, USA) was used. The hPDLSCs were plated in 24-well plates 1 day before transfection at a density of 2 × 104 cells/well. The cells were co-transfected with luciferase reporter gene (6 × osteoblast-specific element [OSE]-Luc, ALP-Luc, BSP-Luc, or OC-Luc) and a CMV promoter-driven β-galactosidase reporter (pCMV-β-gal) along with Runx2 or osterix. After 18 h, the cells were treated with the indicated concentration of CaCl2 for 24 h. The Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) was used to measure the luciferase activity, and all experiments were performed in triplicate.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5.03 software (La Jolla, CA, USA). The results were expressed as the means ± SD. We determined the statistical significance using one-way analysis of variance (ANOVA) with the Tukey–Kramer test.
Results
hPDLSCs have multilineage differentiation potential
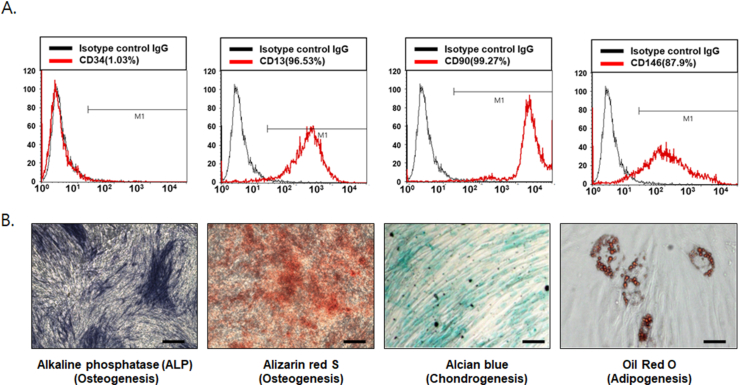
For flow cytometric analysis, we confirmed hPDLSCs using isolated cells to form single-cell-derived colonies and randomly selected three different colonies. CD13, CD90, and CD146 are estimated positive markers of mesenchymal stem cells (MSCs), whereas CD34 is an MSC-negative marker representing endothelial cells and primitive hematopoietic precursors. As a result, 96.53%, 99.27%, 87.9%, and 1.03% of hPDLSCs were positive for CD13, CD90, CD146, and CD34, respectively, indicating the presence of MSCs (Fig. 1A). The ratio of positive cells was determined by the relative strength of antibody-bound cells. To investigate the multipotent differentiation function of hPDLSCs, bone formation was first examined by ALP and ARS staining with osteogenic induction medium. After one or two weeks of osteogenic induction for ALP and ARS staining, respectively, hPDLSCs exhibited increased ALP activity and calcium nodule formation. In addition, after three weeks of incubation with adipogenic or chondrogenic medium, hPDLSCs also showed the capability of adipogenic and chondrogenic differentiation, as confirmed by Alcian blue or Oil red O staining, respectively (Fig. 1B).
Figure 1.
Characterization of human periodontal ligament stem cells (hPDLSCs) (A) As markers of mesenchymal stem cells (MSCs), CD13, CD34, CD90, and CD146 were used for fluorescence-activated cell sorting analysis of hPDLSCs. For whole-cell analysis of CD13-, CD34-, CD90-, and CD146-positive cells, the percentage of the cells positioned in the right side of the M1 gate was measured (n = 3) (B) To identify the multipotent lineage differentiation of hPDLSCs, cells were incubated with osteogenic (1 week for alkaline phosphatase [ALP] staining; 2 weeks for [Alizarin red S ARS] staining), adipogenic, and chondrogenic (3 weeks) induction medium (scale bar = 100 μm).
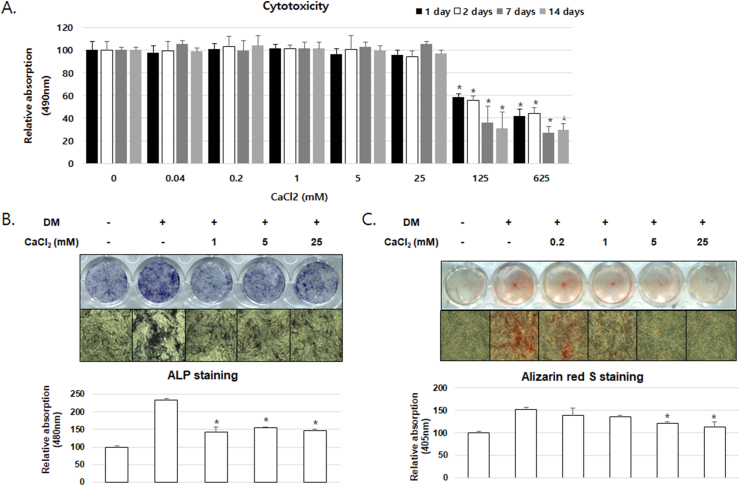
CaCl2 treatment inhibits osteoblast differentiation at non-cytotoxic concentrations of hPDLSCs
CaCl2 administered at concentrations of 125 and 625 mM was toxic to hPDLSCs after 1, 2, 7 or 14 days of culture; however, no toxicity was detected with less than 25 mM CaCl2 (Fig. 2A). Thus, we conducted further experiments at concentrations lower than 25 mM. To examine the effects of CaCl2 on osteoblast differentiation, the hPDLSCs were treated with CaCl2, ascorbic acid, dexamethasone, and β-glycerophosphate. Following 7 or 14 days of culture, ALP and ARS staining confirmed that ALP activity and mineralized nodule formation were inhibited in the CaCl2 treatment group when compared with the differentiation medium-treated group; furthermore, the absorbance of the extracted ALP and ARS was decreased by CaCl2 treatment (Fig. 2B and C). No significant difference was observed in ALP activity with CaCl2 at 1–25 mM; however, bone mineralization was repressed by CaCl2 dose-dependently.
Figure 2.
Effect of CaCl2 treatment on osteogenic differentiation of hPDLSCs (A) hPDLSC viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. hPDLSCs were treated with the indicated concentrations of CaCl2 for 1, 2, 7 or 14 days. ∗P < 0.05 compared with control group; ANOVA–Bonferroni post-hoc test (B) Osteoblast differentiation was measured using alkaline phosphatase (ALP) staining after 7 days (C) Bone mineralization was measured using Alizarin Red S (ARS) staining after 14 days. The ratio of relative absorption was normalized to the non-treated group. ∗P < 0.05 compared with the DM-treated group; ANOVA-Bonferroni post-hoc test.
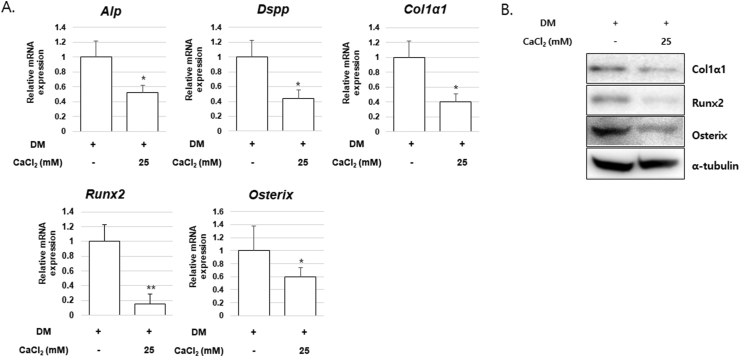
CaCl2 treatment significantly represses osteogenic gene expression
To determine the mechanisms by which CaCl2 influences osteoblast differentiation, the expression levels of osteogenic genes and transcription factors were analyzed in CaCl2-treated hPDLSCs. The mRNA expression of osteogenic differentiation markers and transcriptional factors including ALP (Alp), DSPP (dentin sialophosphoprotein, Dspp), Col1α1 (collagen, type I, alpha 1, Col1α1), Runx2 (Runx2), and Osterix (Osterix) decreased significantly after treatment with CaCl2 compared to untreated group (Fig. 3A). Furthermore, the CaCl2-treated group also showed decreased protein abundances of Col1α1, Runx2, and Osterix (Fig. 3B).
Figure 3.
Effect of CaCl2 treatment on osteogenic gene expression of hPDLSCs. hPDLSCs were cultured in osteogenic differentiation medium without or with CaCl2 (25 mM) (A) The mRNA expression of Alp, Dspp, Col1α1, Runx2, and Osterix was analyzed by real-time reverse transcription–polymerase chain reaction. ∗P < 0.05, ∗∗P < 0.01 compared with the DM-treated group; ANOVA-Bonferroni post-hoc test (B) The protein abundance was confirmed by Western blot analysis with the indicated antibodies; α-tubulin was used as a loading control.
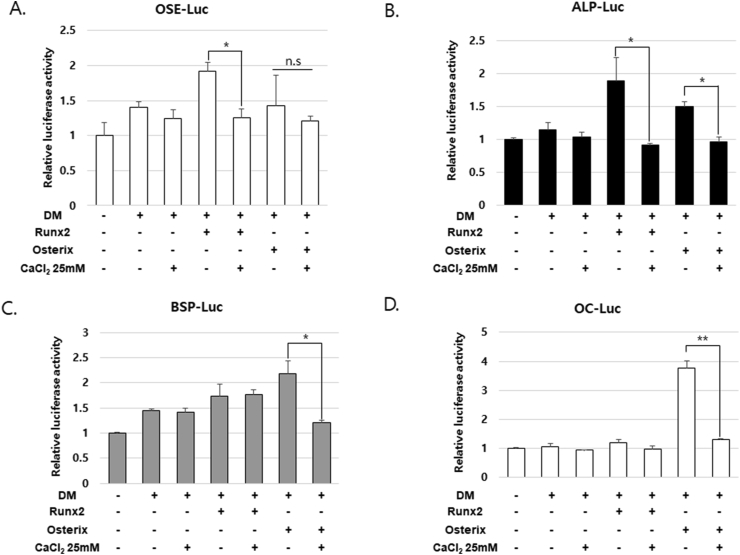
CaCl2 treatment inhibits Runx2-and osterix-mediated transcriptional activity
To further demonstrate the inhibitory effect of CaCl2 on osteogenic differentiation, we measured the transcriptional activity using a luciferase reporter gene containing Runx2-multibinding sites (6X OSE-Luc). As expected, Runx2 overexpression increased the transcriptional activity, whereas osterix overexpression showed no significant effects; additionally, CaCl2 treatment inhibited Runx2-mediated transcriptional activity to the basal level (Fig. 4A). Next, we examined the regulation of various Runx2 and osterix target gene promoters, such as the promotors of ALP (ALP-Luc), BSP (bone sialoprotein; BSP-Luc), and osteocalcin (OC-Luc). CaCl2 treatment suppressed the transcriptional activity of the ALP promoter induced by Runx2 and osterix overexpression. Furthermore, CaCl2 treatment also inhibited osterix-induced transcriptional activity of the BSP and osteocalcin promoters and slightly affected that of the ALP promoter (Fig. 4B).
Figure 4.
CaCl2-mediated suppression of Runx2-and osterix-mediated transcriptional activation (A–D) hPDLSCs were co-transfected with pCMV-β-gal, luciferase reporters (6 × OSE-Luc, ALP-Luc, BSP-Luc, or OC-Luc) along with Ruxn2 or osterix and treated with 25 mM CaCl2. After 24 h, luciferase activities were measured. ∗P < 0.05, ∗∗P < 0.01; ANOVA-Bonferroni post-hoc test.
Discussion
With the development of dental implant technology, the treatment of periodontal diseases using biomaterials combined with stem cells has achieved significant advances, which have been supported by various biological studies.16 In addition, stem cell research has been carried out in various fields with excellent results, which have been incorporated and applied in the field of dentistry.17 However, the research conducted in dentistry so far has been limited because of the different characteristics of the oral cavity environment compared to other organs.18 In particular, the oral cavity is the place where the internal body environment changes most actively due to food intake or respiration, which affects various physiological activities and pathological symptoms in the mouth.19 Nevertheless, stem cell research in dentistry has focused on elucidating the gene expression and regulatory mechanisms at the molecular level rather than influencing changes in the basal environment of the oral cavity.20 Therefore, we aimed to identify the effects of hPDLSC differentiation caused by changes in calcium concentration within the oral cavity environment.
In mammals, calcium plays central roles as a major constituent in bone and other mineralized tissues and as an intracellular messenger that mediates biological processes, including muscle contraction, glycolysis, cell growth and division, and ion transport through conversion of Ca2+ ions.21 Therefore, long-term calcium deficiency, commonly known as hypocalcemia, can lead to osteopenia, osteoporosis, brain malfunction, muscle spasm, and dental changes.22 Meanwhile, excessive intake of calcium or vitamin D due to the recovery of deficient calcium levels, side effects of some drugs, hyperparathyroidism, and lung diseases can cause hypercalcemia, which is defined as a high calcium level in the blood serum (greater than 2.6 mM).22,23 Hypercalcemia also can cause various bodily symptoms, such as bone pain, muscle weakness, and abnormal heart rhythms; however, the dental effects have not been elucidated.24 In our present study, high concentration of CaCl2 (125 and 625 mM) caused toxicity in hPDLSCs (Fig. 2A). Therefore, we conducted most experiments at a calcium concentration of 25 mM, which is high level of calcium concentrations in the blood. A previous report suggested that calcium concentrations of about 5 mM appeared to be appropriate for osteoblast differentiation and proliferation, whereas much higher concentrations (>10 mM) abrogated cell survival rather than inhibiting differentiation in mouse primary osteoblasts.25 Assuming that osteoblast differentiation and hPDLSC differentiation are not exactly same process but have considerable similarities, our results show different points. At 25 mM, CaCl2 decreased the mRNA expression levels of Alp, Dspp, Col1α1, and osterix and markedly repressed Runx2 expression (Fig. 3A). Furthermore, CaCl2 treatment downregulated the protein abundances of Col1α1, osterix, and Runx2 and ultimately inhibited ALP activity and bone mineralization (Figure 2, Figure 3B). Since sequential expression of Runx2 followed by osterix is essential for the progression of osteogenic differentiation,26 inhibitory effects on Runx2 and osterix expression by high concentrations of CaCl2 result in down-regulated osteogenic differentiation in hPDLSCs. Taken together, these results demonstrate that unlike the results of the osteoblasts in mice, high concentrations of calcium inhibit cell differentiation rather than controlling cell proliferation or survival in hPDLSCs.
As CaCl2 treatment inhibited the mRNA and protein levels of Runx2 and osterix, it can be hypothesized that transcriptional activity related to osteogenic differentiation could be regulated by calcium. In the present study, CaCl2 treatment decreased the Runx2-mediated transcriptional activity on the OSE and ALP promotors, but not the BSP and osteocalcin promoters. As Runx2 is an early-stage transcriptional factor of osteogenic differentiation, it mainly activates the ALP promoter, which was significantly inhibited by calcium, while the later stage transcriptional factor osterix mainly affected the BSP and osteocalcin promoters and was subsequently inhibited by CaCl2 treatment (Fig. 4). These results suggest that calcium not only reduces osteogenic gene expression but also inhibits transcriptional activity for osteogenic differentiation.
Dental mesenchymal stem cells, which are multipotent stem cells found in the mouth that are used to treat inflammatory diseases or regenerate periodontium, are currently in the spotlight.27,28 Elucidating the relationship between the changes in the oral cavity environment and the process of hPDLSC differentiation will aid in improving periodontium regeneration and preventing periodontitis. Although there are various restrictions on the clinical applications of stem cell differentiation, our finding may be a good approach for future clinical applications in periodontal tissue regeneration.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This work has supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1040910).
References
- 1.Cho M.I., Garant P.R. Development and general structure of the periodontium. Periodontol. 2000;24:9–27. doi: 10.1034/j.1600-0757.2000.2240102.x. [DOI] [PubMed] [Google Scholar]
- 2.Di Benedetto A., Brunetti G., Posa F. Osteogenic differentiation of mesenchymal stem cells from dental bud: role of integrins and cadherins. Stem Cell Res. 2015;15:618–628. doi: 10.1016/j.scr.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Correa N.C.R., Kuligovski C., Paschoal A.C.C. Human adipose-derived stem cells (ADSC) and human periodontal ligament stem cells (PDLSC) as cellular substrates of a toxicity prediction assay. Regul Toxicol Pharmacol. 2018;92:75–82. doi: 10.1016/j.yrtph.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P., Zhang Y., Liu Q., Ji Y., Xu X. 1,25(OH)2D3 supports the osteogenic differentiation of hPDLSCs under inflammatory conditions through inhibiting PLAP-1 expression transcriptionally. Int Immunopharm. 2020;78:105998. doi: 10.1016/j.intimp.2019.105998. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki D., Akita D., Tsurumachi N. Transplantation of mature adipocyte-derived dedifferentiated fat cells into three-wall defects in the rat periodontium induces tissue regeneration. J Oral Sci. 2017;59:611–620. doi: 10.2334/josnusd.16-0878. [DOI] [PubMed] [Google Scholar]
- 6.Romeo L., Diomede F., Gugliandolo A. Moringin induces neural differentiation in the stem cell of the human periodontal ligament. Sci Rep. 2018;8:9153. doi: 10.1038/s41598-018-27492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elcin Y.M., Inanc B., Elcin A.E. Human embryonic stem cell differentiation on periodontal ligament fibroblasts. Methods Mol Biol. 2010;584:269–281. doi: 10.1007/978-1-60761-369-5_14. [DOI] [PubMed] [Google Scholar]
- 8.Liu L., Ling J., Wei X., Wu L., Xiao Y. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endod. 2009;35:1368–1376. doi: 10.1016/j.joen.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.He Y., Jian C.X., Zhang H.Y. Hypoxia enhances periodontal ligament stem cell proliferation via the MAPK signaling pathway. Genet Mol Res. 2016;15 doi: 10.4238/gmr15048965. [DOI] [PubMed] [Google Scholar]
- 10.Juzwiak C.R., Amancio O.M., Vitalle M.S., Szejnfeld V.L., Pinheiro M.M. Effect of calcium intake, tennis playing, and body composition on bone-mineral density of Brazilian male adolescents. Int J Sport Nutr Exerc Metabol. 2008;18:524–538. doi: 10.1123/ijsnem.18.5.524. [DOI] [PubMed] [Google Scholar]
- 11.Csordas G., Thomas A.P., Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria in cardiac muscle. Trends Cardiovasc Med. 2001;11:269–275. doi: 10.1016/s1050-1738(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 12.Deo P.N., Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal A.K., Pal T.K., Mukherjee K., Pal S. Animal experimentation with tooth derived calcium hydroxyapatite based composites as bone-graft substitute biomaterials. Biomed Sci Instrum. 1997;33:561–566. [PubMed] [Google Scholar]
- 14.Sataline L., Donaghue G. Hypercalcemia, heart-block, and hyperthyroidism. J Am Med Assoc. 1970;213:1342. doi: 10.1001/jama.1970.03170340064017. [DOI] [PubMed] [Google Scholar]
- 15.Rauber C., Kihm L., Merle U. Hypercalcemia, necrotizing pancreatitis and bone lesions: a benign cause. Clin Cases Miner Bone Metab. 2017;14:245–246. doi: 10.11138/ccmbm/2017.14.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y., Zhang N., An Y.H., Wen X. Biomaterial strategies to reduce implant-associated infections. Int J Artif Organs. 2007;30:828–841. doi: 10.1177/039139880703000913. [DOI] [PubMed] [Google Scholar]
- 17.Lee Z., Dennis J.E., Gerson S.L. Imaging stem cell implant for cellular-based therapies. Exp Biol Med. 2008;233:930–940. doi: 10.3181/0709-MR-234. [DOI] [PubMed] [Google Scholar]
- 18.Surdacka A., Strzyka A.K., Rydzewska A. Changeability of oral cavity environment. Eur J Dermatol. 2007;1:14–17. [PMC free article] [PubMed] [Google Scholar]
- 19.Garg A. What's in your patient's mouth? How lifestyle, disease, and the environment affect the oral cavity. Dent Implant Update. 2010;21:20–24. [PubMed] [Google Scholar]
- 20.Kaneko T., Arayatrakoollikit U., Yamanaka Y., Ito T., Okiji T. Immunohistochemical and gene expression analysis of stem-cell-associated markers in rat dental pulp. Cell Tissue Res. 2013;351:425–432. doi: 10.1007/s00441-012-1539-9. [DOI] [PubMed] [Google Scholar]
- 21.Endo M. [Calcium ion and cell function] Tanpakushitsu Kakusan Koso. 1998;43:1529–1533. [PubMed] [Google Scholar]
- 22.Liamis G., Milionis H.J., Elisaf M. A review of drug-induced hypocalcemia. J Bone Miner Metabol. 2009;27:635–642. doi: 10.1007/s00774-009-0119-x. [DOI] [PubMed] [Google Scholar]
- 23.Luqman W.A., Przasnyski E.J., McCowen D., Reed J.W. Hypocalcemia in patients with cancer: a review. Mil Med. 1980;145:96–98. [PubMed] [Google Scholar]
- 24.Toyoshi H. [Symptom with bone metastasis--bone pain, fracture, hypercalcemia] Nihon Rinsho. 2007;65:76–81. [PubMed] [Google Scholar]
- 25.Maeno S., Niki Y., Matsumoto H. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26:4847–4855. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Fu X., Li Y., Huang T. Runx2/Osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv Sci. 2018;5:1700755. doi: 10.1002/advs.201700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatic N., Rose F., des Rieux A., White L.J. Stem cells from the dental apical papilla in extracellular matrix hydrogels mitigate inflammation of microglial cells. Sci Rep. 2019;9:14015. doi: 10.1038/s41598-019-50367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Li Y., Shi R. Generation of tooth-periodontium complex structures using high-odontogenic potential dental epithelium derived from mouse embryonic stem cells. Stem Cell Res Ther. 2017;8:141. doi: 10.1186/s13287-017-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]