Abstract
Gastrointestinal bleeding (GIB) accounts for a significant proportion of life-threatening bleeding cases occurring after allogeneic haematopoietic stem cell transplantation (allo-HSCT). However, data on GIB after haploidentical HSCT (haplo-HSCT) are not available. A total of 3180 patients received haplo-HSCT at Peking University People’s Hospital from January 2015 to November 2019, and GIB occurred in 188 of these patients (incidence of 5.9%). Platelet counts <30 × 109/L, viral hepatitis, acute kidney injury (AKI), gastrointestinal disease or bleeding before HSCT and sinusoidal obstruction syndrome (SOS) were determined to be significant risk factors for the occurrence of GIB after haplo-HSCT. Grade III-IV acute graft-versus-host disease (aGVHD), AKI, thrombotic microangiopathy (TMA), disseminated intravascular coagulation (DIC) and gastrointestinal disease or bleeding before HSCT were significantly related to mortality in patients with GIB after haplo-HSCT. The predictive models developed for the occurrence and mortality of GIB performed well in terms of discrimination, and they might assist clinicians with personalised strategies for GIB prevention and treatment in patients after haplo-HSCT.
Subject terms: Signs and symptoms, Risk factors
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) has been demonstrated to be the most effective therapy for various malignant as well as nonmalignant haematological diseases. However, the chance of patients receiving human leucocyte antigen (HLA)-matched donors is low because of the deficiency of sibling and unrelated donors in China [1–3]. In recent decades, our centre has successfully developed an approach for haplo-HSCT with a myeloablative regimen and granulocyte colony-stimulating factor-primed bone marrow and/or peripheral blood grafts. Numerous studies have suggested a comparable curative effect of haplo-HSCT performed using this approach for various haematological diseases [4–8]. Therefore, haplo-HSCT, with universal donor availability and a similar efficiency to HLA-matched HSCT, has gradually become a mainstream method for allo-HSCT [1, 3].
The wide use of haplo-HSCT has inevitably led to a variety of complications after transplantation [9–12], with bleeding complications due to various reasons being one of the most significant. Conditioning regimens, including antithymocyte immunoglobulin, and some posttransplant complications, such as GVHD and infection, can result in haemostatic and coagulation disturbances [13–16], thus increasing the risk of bleeding after transplantation. Numerous studies have shown that bleeding after transplantation is associated with reduced survival in patients [17–21]. The gastrointestinal tract is one of the most common bleeding sites after HSCT, and bleeding at this site is often more severe than that in other bodily locations except for pulmonary haemorrhage [18, 20]. However, most previous studies focused on overall bleeding after HSCT rather than only the gastrointestinal tract, and the analyses of these studies might not fully represent the features of GIB [17–20, 22–25]. Two other studies did focus on GIB after HSCT; however, one study targeted patients with primary systemic amyloidosis [22], and the other focused on only severe GIB [23]; both studies were relatively incomplete.
Thus, we herein focused on this topic, describing the incidence, clinical features, outcomes, risks and prognostic factors of GIB in patients receiving haplo-HSCT. Furthermore, to the best of our knowledge, predictive models for GIB after HSCT have not been developed, and we therefore developed predictive models for the occurrence and mortality of GIB after haplo-HSCT, which could have important implications for individualised prevention and therapeutic strategies for GIB following haplo-HSCT.
Methods
Patients
This was a retrospective, nested, case-control study carried out from January 2015 to November 2019. A total of 4312 consecutive patients received HSCT at Peking University People’s Hospital, among which 3180 received haplo-HSCT. Based on medical records, 214 patients were diagnosed with GIB. Patients without documented overt GIB (haematemesis, melena, or haematochezia) and those who developed overt GIB before HSCT were excluded. Finally, 188 patients with overt GIB after haplo-HSCT were included in the case group (Fig. 1). For each case, three patients without GIB among 3180 patients with haplo-HSCT were randomly selected comprise the control group in accordance with the following matching criteria: time of haplo-HSCT (±3 months) and length of follow-up (±6 months). All the patients in our study signed informed consent forms based on the Declaration of Helsinki, and this study was approved by the Institutional Review Board of Peking University.
Fig. 1. Flow chart of patient screening.
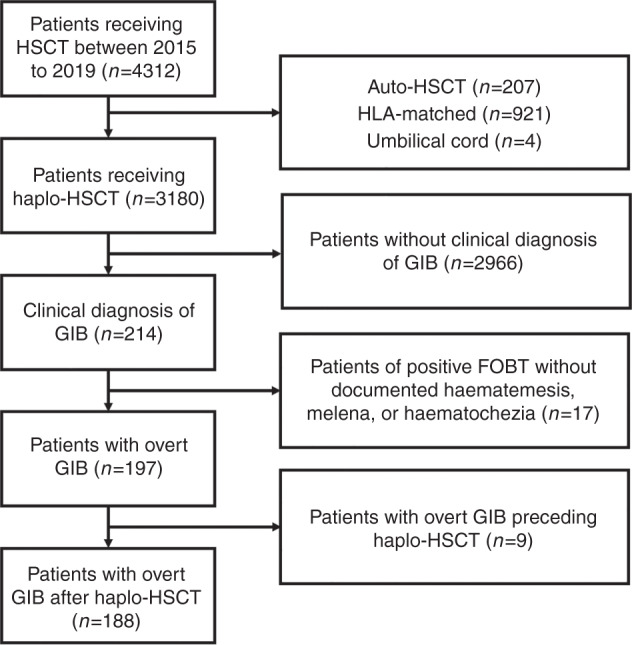
HSCT haematopoietic stem cell transplantation, allo allogenic, auto autogenic, HLA human leucocyte antigen, FOBT faecal occult blood test, GIB gastrointestinal bleeding.
Conditioning regimens and prophylaxis of GVHD
For patients with haplo-HSCT in our study, the conditioning regimens included cytarabine, busulfan, cyclophosphamide, semustine and antithymocyte immunoglobulin. Cytarabine was given at a dosage of 4 g/m2/d on days −10 and −9, and busulfan was given at a dosage of 3.2 mg/kg/d from days −8 to −6. Cyclophosphamide was administered at a dosage of 1.8 g/m2/d on days −5 and −4, and semustine was administered at a dosage of 250 mg/m2/d on day −3. Antithymocyte immunoglobulin was used at a dosage of 2.5 mg/kg/d from days −5 to −2. The prophylactic regimens for GVHD, cyclosporine, mycophenolate mofetil and short-term methotrexate as previously described [26], were administered to all patients after haplo-HSCT. The diagnoses and grades of aGVHD and chronic GVHD (cGVHD) were based on established guidelines and criteria [27].
Definitions
Overt GIB was defined as haematemesis, melena or haematochezia [28–30]. GIB was recognised as severe (sGIB) if it met one of the following criteria: (1) overt GIB with signs of haemorrhagic shock [23, 31], (2) overt GIB resulting in a decrease in haemoglobin of ≥2 g/dL [32, 33] or at least a 20% decrease in haematocrit levels [34–39], and (3) overt GIB requiring at least two units of packed red blood cells [23, 31–39]. Patients without any of the features described above constituted the non-severe GIB group [40].
Evaluation of GIB
Endoscopic examination was performed for patients with GIB after they agreed to participate in the study. The anatomical site and cause of GIB were identified by reviewing the endoscopic, histological and microbiological data, and the attribution of bleeding causes was based on a previous report [23]. GVHD was recognised as the single bleeding cause when typical appearance and histology were observed and cultures and immunohistochemistry analyses of viruses and fungi were negative. When evidence of GVHD and infection was found, both were regarded as the causes of bleeding. Other bleeding causes, such as gastritis and solitary ulcers, were based on the clinical and endoscopic findings.
Development of predictive models
To determine the risk and prognostic factors for GIB after haplo-HSCT, potential variables were evaluated using univariate Cox analysis, and the variables with a P value < 0.1 were then selected for backward stepwise multivariate Cox proportional hazards regression analysis. According to the previous prediction methods [41–44], predictive models for the occurrence of GIB and mortality in patients with GIB were developed based on the independent risk and prognostic factors in backward stepwise multivariate Cox analysis. The weighted point value proportional to the β regression coefficient values in multivariate regression analysis was assigned for each factor. The area under the receiver operating characteristic curve (AuROC) was used to assess the performances of the predictive models.
Statistical analysis
Continuous variables in our study are shown as medians (ranges) and were compared with Mann–Whitney U tests. Pearson’s Chi-square test and Fisher’s exact test were applied to compare categorical variables. Outcomes described by the cumulative incidence of overall survival (OS), non-relapse mortality (NRM) and relapse were evaluated by the Kaplan–Meier method and the log-rank test. OS was defined as the time of death for any reason after transplantation. NRM was defined as deceased without recurrence of primary disease following HSCT. Relapse was defined as over 5% blasts in bone marrow or the occurrence of blasts in peripheral blood or intramedullary infiltration of blasts. P values < 0.05 (two-sided) were considered statistically significant.
Results
Baseline patient characteristics
GIB occurred in 188 patients following haplo-HSCT (incidence of 5.9%). The baseline characteristics of the two groups are shown in Table 1. Patients with GIB were older than those without (median age 29 vs. 25 years, P = 0.002). A higher proportion of male patients was observed in patients with GIB, however, there were similar distribution of gender between patients with GIB and the controls. Gastrointestinal disease or bleeding before HSCT and viral hepatitis were more frequently observed in patients with GIB, and more patients with GIB experienced grade III-IV aGVHD, intestinal infection and TMA than patients in the control group. Platelet counts before the onset of bleeding in patients with GIB were lower than those in control patients (33 vs. 60 × 109/L, P = 0.000). There were no differences in underlying diseases, HLA mismatching, blood type compatibility, donor-recipient sex mismatching, engraftment time or history of alcohol intake between the two groups.
Table 1.
Baseline characteristics in patients with GIB and matched controls after haplo-HSCT.
| Variables | GIB (n = 188) | Control (n = 564) | P |
|---|---|---|---|
| Age at HSCT, years | 29 (2–68) | 25 (1–63) | 0.002 |
| Gender (n, %) | 0.297 | ||
| Male | 111 (59.0) | 358 (63.5) | |
| Female | 77 (41.0) | 206 (36.5) | |
| Time of GIB (post-HSCT) (days) | 46 (1–892) | / | |
| Underlying disease, n (%) | 0.111 | ||
| AML | 68 (36.1) | 193 (34.2) | |
| ALL | 62 (33.0) | 214 (37.9) | |
| CML | 5 (2.7) | 13 (2.3) | |
| AA | 15 (7.9) | 73 (12.9) | |
| MDS | 24 (12.8) | 47 (8.3) | |
| Lymphoma | 6 (3.2) | 8 (1.4) | |
| Others | 8 (4.3) | 16 (3.0) | |
| ABO mismatch, n (%) | 95 (50.5) | 256 (45.4) | 0.221 |
| Donor-recipient sex mismatch, n (%) | 87 (46.3) | 233 (41.3) | 0.233 |
| HLA, n (%) | 0.475 | ||
| 3/6 | 164 (87.2) | 471 (83.5) | |
| 4/6 | 21 (11.2) | 81 (14.4) | |
| 5/6 | 3 (1.6) | 12 (2.1) | |
| MNC (×108/kg, mean ± SD) | 8.61 ± 1.73 | 8.74 ± 2.36 | 0.791 |
| CD34+ (×106/kg, mean ± SD) | 2.41 ± 1.32 | 3.07 ± 4.85 | 0.002 |
| Engraftment time, d (range) | |||
| Neutrophil | 13 (8–25) | 13 (9–28) | 0.491 |
| Platelet | 18 (7–295) | 16 (7–259) | 0.114 |
| aGVHD, n (%) | 0.000 | ||
| 0–II | 84 (44.7) | 528 (93.6) | |
| III–IV | 104 (55.3) | 36 (6.4) | |
| Extensive cGVHD, n (%) | 17 (9.0) | 38 (6.7) | 0.293 |
| SOS | 2 (1.1) | 1 (0.2) | 0.156 |
| TMA | 13 (6.9) | 8 (1.4) | 0.000 |
| DIC | 8 (4.3) | 17 (3.0) | 0.411 |
| AKI | 38 (20.2) | 14 (2.5) | 0.000 |
| Intestinal infection | 62 (33.0) | 29 (5.1) | 0.000 |
| CMV viremia | 112 (59.6) | 355 (62.9) | 0.410 |
| Platelet count before GIB, 109/L | 33 (3–324) | 60 (4–356) | 0.000 |
| Diabetes mellitus | 10 (5.3) | 18 (3.2) | 0.182 |
| Hypertension | 20 (10.6) | 38 (6.7) | 0.083 |
| Viral hepatitis | 22 (11.7) | 18 (3.2) | 0.000 |
| Gastrointestinal disease or bleeding before HSCT | 19 (10.1) | 8 (1.4) | 0.000 |
| Alcohol, n (%) | 6 (3.2) | 8 (1.4) | 0.127 |
| Median follow-up time after HSCT, d (range) | 380 (34–1808) | 418 (3–1797) | 0.273 |
GIB gastrointestinal bleeding, AML acute myeloblastic leukaemia, ALL acute lymphoblastic leukaemia, CML chronic myeloid leukaemia, AA aplastic anaemia, MDS myelodysplastic syndrome, HLA human leucocyte antigen, aGVHD acute graft-versus-host disease, cGVHD chronic graft-versus-host disease, SOS sinusoidal obstruction syndrome, TMA thrombotic microangiopathy, DIC disseminated intravascular coagulation, AKI acute kidney injury, CMV cytomegalovirus, HSCT haematopoietic stem cell transplantation.
GIB after haplo-HSCT
The median time for the first GIB event was 46 days (range, 1–892 days) after haplo-HSCT. GIB presented as melena (17 patients, 9.0%), haematemesis (37 patients, 19.7%) and haematochezia (130 patients, 69.1%), with four patients (2.1%) presenting with both haematemesis and melena. Endoscopic evaluation was conducted for four patients with melena or haematemesis and for 95 patients with haematochezia. Among patients receiving endoscopic evaluation, GIB was detected in the oesophagus (1/99), duodenum (2/99), stomach (1/99), small intestine (29/99), colon (33/99), rectum (3/99) and at multiple sites (28/99). No bleeding foci were detected in two patients. The causes of GIB were diverse, with the most common single cause of bleeding being GVHD (56/99), followed by solitary ulcers (6/99), infection (3/99) and gastritis (1/99). Multiple bleeding causes were found in 26 patients; GVHD and gut infection both contributed to the occurrence of GIB in 25 patients, and one patient bled due to GVHD and TMA. The causes of bleeding in seven patients were not determined.
Among patients with GIB, 102 patients (54.3%) experienced severe GIB. The clinical characteristics of patients with severe and non-severe GIB as well as the latest laboratory data before GIB onset were shown in Table S1. The two groups were similar in terms of age at HSCT, sex, time of GIB onset, history of alcohol intake and some comorbidities. Compared with those of patients with non-severe GIB, the platelet counts of patients with severe GIB were significantly lower, and the prothrombin time/international normalised ratio was significantly longer. Some biochemical indicators, including total bilirubin and glucose, were present at significantly higher concentrations in patients with severe GIB than in those with non-severe GIB, while the concentration of albumin was lower in patients with severe GIB.
Outcomes
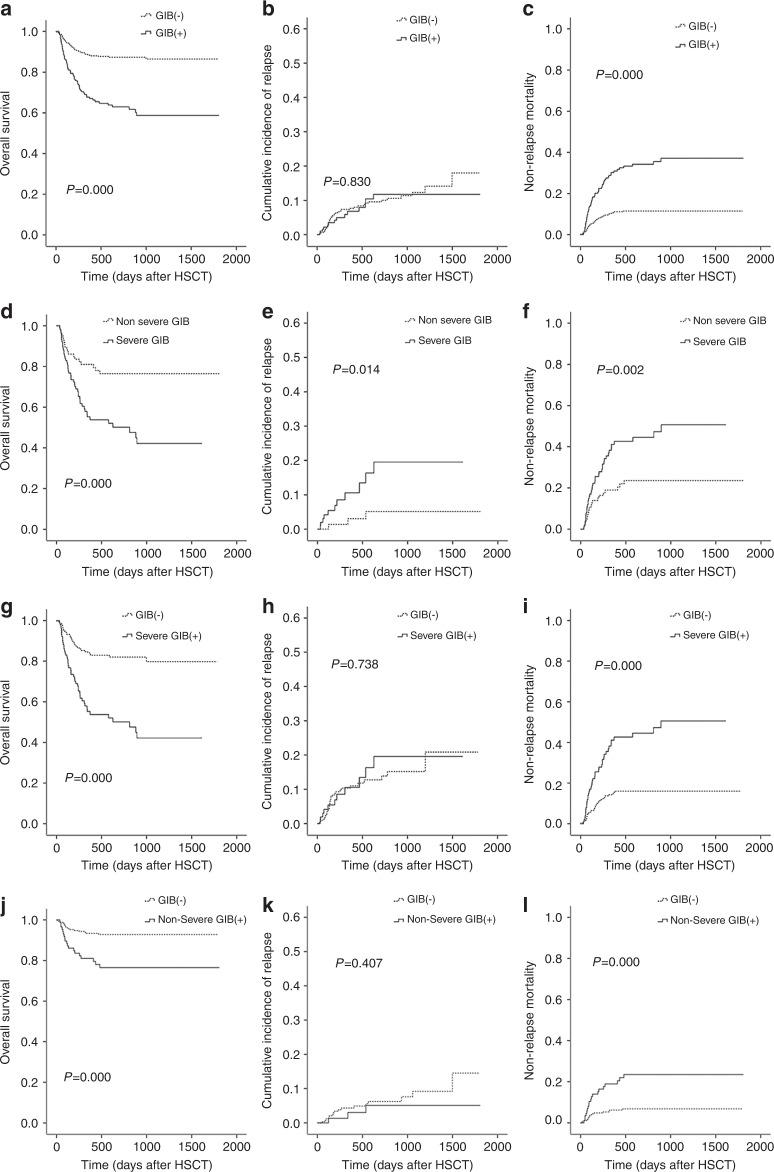
The OS of patients with GIB was significantly reduced (P = 0.000, Fig. 2a), and the NRM was higher in these patients than in those without GIB (P = 0.000, Fig. 2c). The cumulative incidence of relapse was not different between patients with GIB and the control group (P = 0.765, Fig. 2b). Among patients with GIB, the OS of patients with severe GIB was significantly lower (P = 0.000, Fig. 2d) and the NRM (P = 0.000, Fig. 2f) and cumulative incidence of relapse (P = 0.000, Fig. 2e) were higher than in those without severe GIB. Notably, both severe GIB and non-severe GIB significantly decreased OS (Fig. 2g, j) and increased the NRM (Fig. 2i, l) in patients after haplo-HSCT.
Fig. 2. Clinical outcomes of patients with GIB after haplo-HSCT.
Overall survival (a), incidence of relapse (b) and non-relapse mortality (c) for patients with GIB and without GIB after haplo-HSCT. The comparison of overall survival (d), incidence of relapse (e) and non-relapse mortality (f) of patients with severe GIB and non-severe GIB. The impact of severe GIB on overall survival (g), incidence of relapse (h) and non-relapse mortality (i) after haplo-HSCT. The impact of non-severe GIB on overall survival (j), incidence of relapse (k) and non-relapse mortality (l) after haplo-HSCT.
Predictors for the occurrence of GIB
The univariate and multivariate analysis results are presented in Table 2. According to the univariate analysis results, age > 30 years, SOS, AKI, platelet counts <30 × 109/L, viral hepatitis and gastrointestinal disease or bleeding before HSCT were significantly related to the occurrence of GIB after haplo-HSCT. In the multivariate analysis, the independent risk factors for GIB included platelet counts <30 × 109/L, viral hepatitis, AKI, gastrointestinal disease or bleeding before HSCT and SOS.
Table 2.
Risk factors for GIB after haplo-HSCT.
| Variables | Univariate P value | Multivariate | ||
|---|---|---|---|---|
| HR | 95% | P value | ||
| Age > 30 | 0.004 | 1.069 | 0.786–1.454 | 0.670 |
| Male | 0.212 | |||
| ABO mismatch | 0.147 | |||
| Donor-recipient sex mismatch | 0.199 | |||
| SOS | 0.038 | 6.105 | 1.471–25.341 | 0.013 |
| DIC | 0.097 | 1.301 | 0.633–2.675 | 0.474 |
| AKI | 0.000 | 3.115 | 2.110–4.599 | 0.000 |
| CMV viremia | 0.148 | |||
| Platelet counts < 30 × 109/L | 0.000 | 2.408 | 1.785–3.248 | 0.000 |
| Viral hepatitis | 0.000 | 1.965 | 1.244–3.102 | 0.004 |
| Gastrointestinal disease or bleeding before HSCT | 0.000 | 3.780 | 2.334–6.122 | 0.000 |
| Diabetes mellitus | 0.153 | |||
| Alcohol | 0.092 | 1.384 | 0.605–3.168 | 0.441 |
GIB gastrointestinal bleeding, SOS sinusoidal obstruction syndrome, DIC disseminated intravascular coagulation, AKI acute kidney injury, CMV cytomegalovirus, HSCT haematopoietic stem cell transplantation.
Predictive model for GIB
According to the above findings, a predictive model for GIB was developed based on the independent risk factors and β-coefficients that were obtained by multivariable Cox regression analysis (Table 3). Thus, the total score of each patient, ranging from 0 to 9 points, was determined. On the basis of each their scores, we categorised the 752 patients into three groups for GIB prediction: low risk (0–1 point), intermediate risk (2–3 points) and high risk (4–9 points). There were 667 patients in the low-risk group, 73 patients in the intermediate-risk group and 12 patients in the high-risk group; the corresponding GIB rates of each group were 19.5%, 63.0% and 100%, respectively (Table S2). The estimations of GIB occurrence following haplo-HSCT in each group are presented in Fig. 3. The mortality rate of patients after haplo-HSCT was 12.9% in the low-risk group, 43.8% in the intermediate-risk group and 83.3% in the high-risk group (Fig. 4, P = 0.000). The performance of our predictive model was good in terms of discrimination (AuROC = 0.705, 95% CI, 0.659–0.752, P = 0.000, Fig. 5). Furthermore, the nomogram plot for the predictive model was presented as Fig. S1.
Table 3.
Predictive model for GIB after haplo-HSCT.
| Variables | HR (95% CI) | P value | Multivariate regression coefficient | Points |
|---|---|---|---|---|
| Platelet count < 30 × 109/L | 2.433 (1.811–3.268) | 0.000 | 0.889 | 1 |
| Viral hepatitis | 1.966 (1.248–3.098) | 0.004 | 0.676 | 1 |
| AKI | 3.256 (2.237–4.739) | 0.000 | 1.180 | 2 |
| Gastrointestinal disease or bleeding before HSCT | 3.770 (2.327–6.107) | 0.000 | 1.327 | 2 |
| SOS | 6.323 (1.542–25.922) | 0.010 | 1.844 | 3 |
GIB gastrointestinal bleeding, AKI acute kidney injury, HSCT haematopoietic stem cell transplantation, SOS sinusoidal obstruction syndrome.
Fig. 3. Estimation of GIB occurrence stratified by the predictive model.
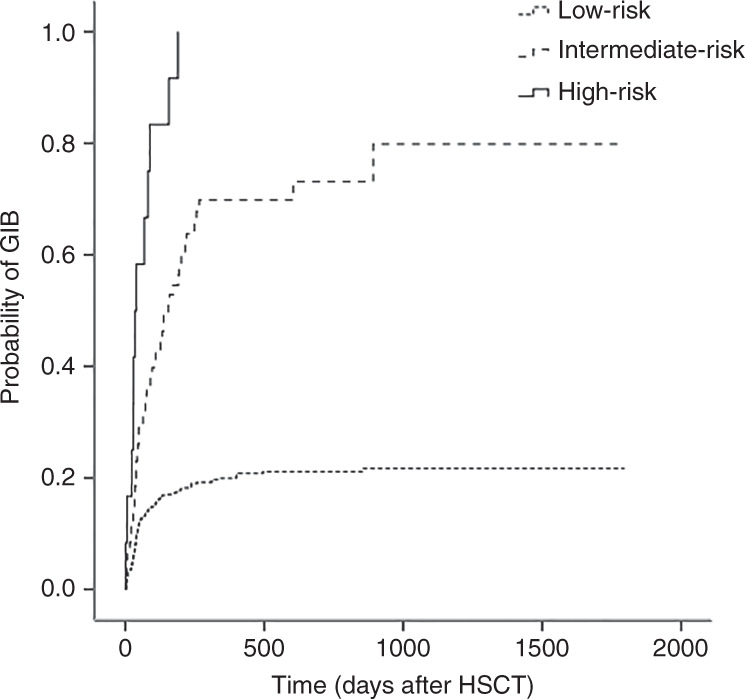
Probability of gastrointestinal bleeding (GIB) after haploidentical haematopoietic stem cell transplantation (haplo-HSCT) among patients of low-risk, intermediate-risk and high-risk group stratified by the predictive model.
Fig. 4. Survival of patients stratified by the predictive model for GIB.

Overall survival after haploidentical haematopoietic stem cell transplantation (haplo-HSCT) of patients of low-risk, intermediate-risk and high-risk groups stratified by the predictive model for gastrointestinal bleeding (GIB).
Fig. 5. Performance of the predictive model on discriminating GIB following haplo-HSCT.

The area under the receiver operating characteristic curve (AuROC) of the predictive model for GIB following haploidentical haematopoietic stem cell transplantation (haplo-HSCT).
Prognostic factors
Among patients with GIB, 66 patients (35.1%) experienced death during the study. To further identify the prognostic factors for patients with GIB, age, sex, transplant complications prior to GIB, laboratory data on the occurrence of GIB and comorbidities were analysed. In multivariate analysis, grade III–IV aGVHD, AKI, TMA, DIC and gastrointestinal disease or bleeding before HSCT were significantly related to mortality in patients with GIB after haplo-HSCT (Table 4).
Table 4.
Prognostic factors of patients with GIB after haplo-HSCT.
| Variables | Patients (n, %) | Univariate P value | Multivariate | |||
|---|---|---|---|---|---|---|
| Death (−) | Death(+) | HR | 95% | P value | ||
| Age > 30 | 54 (44.3) | 37 (56.1) | 0.045 | 1.427 | 0.833–2.444 | 0.195 |
| Male | 68 (55.7) | 43 (65.2) | 0.296 | |||
| Transplant complications prior to GIB | ||||||
| III–IV aGVHD | 60 (49.2) | 44 (66.7) | 0.004 | 2.302 | 1.340–3.952 | 0.003 |
| Extensive cGVHD | 11 (9.0) | 6 (9.1) | 0.566 | |||
| TMA | 2 (1.6) | 11 (16.7) | 0.000 | 5.539 | 2.745–11.179 | 0.000 |
| SOS | 0.120 | |||||
| DIC | 1 (0.8) | 7 (10.6) | 0.000 | 4.535 | 1.963–10.478 | 0.000 |
| AKI | 16 (13.1) | 22 (33.3) | 0.000 | 2.621 | 1.510–4.548 | 0.001 |
| Intestinal infection | 41 (33.6) | 21 (31.8) | 0.934 | |||
| CMV viremia | 67 (54.9) | 45 (68.2) | 0.087 | 1.306 | 0.749–2.277 | 0.346 |
| Gastrointestinal disease or bleeding before HSCT | 7 (5.7) | 12 (18.2) | 0.002 | 3.748 | 1.925–7.296 | 0.000 |
| Laboratory data at the occurrence of GIB | ||||||
| Hb < 80 g/L | 59 (48.4) | 38 (57.6) | 0.056 | 1.319 | 0.785–2.215 | 0.296 |
| Platelet count < 30 × 109/L | 58 (47.5) | 34 (51.5) | 0.421 | |||
| Platelet count < 20 × 109/L | 33 (27.0) | 26 (39.4) | 0.083 | 1.390 | 0.802–2.410 | 0.241 |
| Comorbidities | ||||||
| Viral hepatitis | 14 (11.5) | 8 (12.1) | 0.659 | |||
| Hypertension | 9 (7.4) | 11 (16.7) | 0.109 | |||
| Diabetes mellitus | 5 (4.1) | 5 (7.6) | 0.422 | |||
| Alcohol | 4 (3.3) | 2 (3.0) | 0.997 | |||
GIB gastrointestinal bleeding, aGVHD acute graft-versus-host disease, cGVHD chronic graft-versus-host disease, SOS sinusoidal obstruction syndrome, TMA thrombotic microangiopathy, DIC disseminated intravascular coagulation, AKI acute kidney injury, CMV cytomegalovirus, HSCT hematopoietic stem cell transplantation.
Predictive model for mortality
Based on the prognostic factors we identified and their β-coefficients obtained by multivariable Cox regression analysis, we established a predictive model for mortality in patients with GIB after haplo-HSCT (Table 5), with scores ranging from 0 to 7 points. Estimation of death was therefore divided into three groups: low risk (0–1 points), intermediate risk (2–3 points) and high risk (4–7 points); the mortality rates of each group were 25.3%, 60.6% and 100%, respectively (Fig. 6). The performance of our predictive model for mortality was good in terms of discrimination (AuROC = 0.728, 95% CI, 0.651–0.805, P = 0.000, Fig. 7). Furthermore, the nomogram plot for the predictive model was presented as Fig. S2.
Table 5.
Predictive model for mortality of patients with GIB after haplo-HSCT.
| Variables | HR (95% CI) | P value | Multivariate regression coefficient | Points |
|---|---|---|---|---|
| III–IV aGVHD | 2.347 (1.390–3.965) | 0.001 | 0.853 | 1 |
| AKI | 2.957 (1.748–5.001) | 0.000 | 1.084 | 1 |
| Gastrointestinal disease or bleeding before HSCT | 3.438 (1.805–6.550) | 0.000 | 1.235 | 1 |
| TMA | 6.040 (3.075–11.864) | 0.000 | 1.798 | 2 |
| DIC | 4.736 (2.102–10.670) | 0.000 | 1.555 | 2 |
GIB gastrointestinal bleeding, aGVHD acute graft-versus-host disease, TMA thrombotic microangiopathy, DIC disseminated intravascular coagulation, AKI acute kidney injury, HSCT haematopoietic stem cell transplantation.
Fig. 6. Estimation of mortality of patients with GIB stratified by the predictive model.
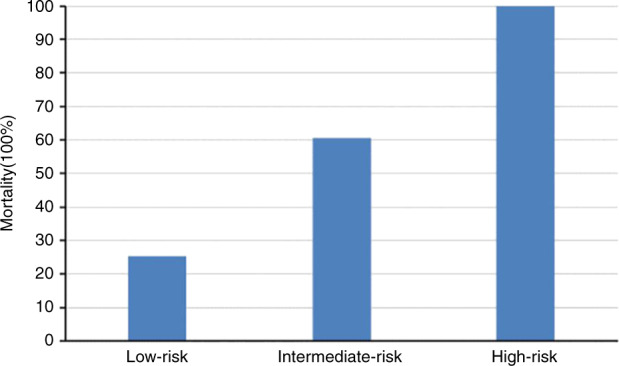
Probability of mortality of patients with gastrointestinal bleeding (GIB) after haploidentical haematopoietic stem cell transplantation (haplo-HSCT) stratified by the predictive model.
Fig. 7. Performance of the predictive model on discriminating mortality of patients with GIB following haplo-HSCT.
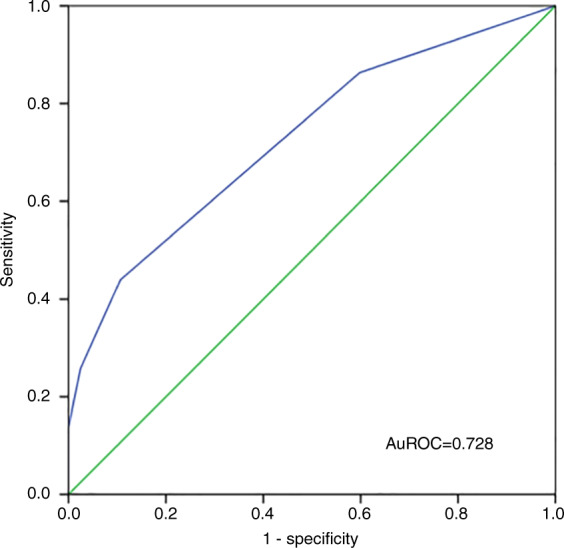
The area under the receiver operating characteristic curve (AuROC) of the predictive model for mortality of patients with gastrointestinal bleeding (GIB) following haploidentical haematopoietic stem cell transplantation (haplo-HSCT).
Discussion
GIB accounts for a significant proportion of bleeding events, especially in cases of life-threatening bleeding after allo-HSCT [18]. As haplo-HSCT is becoming the main method of transplantation [1, 3], systemic investigations of GIB following haplo-HSCT are needed.
In our study, the incidence of GIB after haplo-HSCT was 5.9%, which is lower than that in previous reports [17, 22, 24, 29]. This result might be due to the discrepancy of the underlying disease, transplantation type and therapeutic strategy for patients. The gastrointestinal tract is frequently involved in a variety of posttransplant complications, with GVHD being one of the most common after allo-HSCT, and gastrointestinal tract involvement is seen in 74% of patients with aGVHD [45] and 30% of patients with cGVHD [46]. More than half of all patients with severe gastrointestinal GVHD are reported to experience GIB [47], and other complications, such as conditioning toxicity, infection and TMA, often affect the gut [48, 49]. Thus, the causes resulting in the occurrence of GIB after HSCT vary, and the time of GIB onset is not always fixed. The time span of GIB onset was relatively large in our study, and the causes and anatomical sites of bleeding were diverse as determined by reviews of the endoscopic, histological and microbiological data of patients with GIB who underwent endoscopic examination. GVHD was demonstrated to be the most common single cause in our study, which is consistent with a previous report [23].
Bleeding, especially GIB, has been reported to correlate with a poor prognosis following HSCT [21, 50]. Similarly, we found that GIB had a profound negative influence on the OS and NRM of patients receiving haplo-HSCT. While previous studies suggested that the severity of bleeding affects patient survival [21], our results indicated that both severe and non-severe GIB after haplo-HSCT could decrease the OS and increase the NRM of patients.
In recent years, many studies have revealed risk factors for bleeding complications after HSCT, with numerous complications after HSCT, such as low platelet counts, grade III–IV aGVHD, extensive cGVHD, TMA and SOS, being related to increased risks of posttransplant bleeding[18–20]. As GIB is one of the most common manifestations of GVHD and TMA [47, 51, 52], we did not include these factors in our risk analysis. Low platelet counts and SOS were indeed identified as factors leading to GIB following haplo-HSCT in our study. Other independent risk factors for GIB identified herein included viral hepatitis, AKI and gastrointestinal disease or bleeding before HSCT. An increased risk of GIB in patients with viral hepatitis has been reported [53]. Patients positive for hepatitis viral antigens are at high risk of viral reactivation because of the long period of immunosuppression after HSCT [54, 55]. Thus, viral hepatitis was included in the risk factor analysis and it was revealed as an independent risk factor for GIB following haplo-HSCT. Impairment of renal function has been shown to increase the risk of GIB in solid organ transplantation [56, 57]. Many drugs used after HSCT are nephrotoxic, and some posttransplant complications also affect renal function; thus, AKI might become a risk factor for GIB after haplo-HSCT. Indeed, we found that AKI was related to the occurrence of GIB in patients receiving haplo-HSCT, and a history of gastrointestinal disease or bleeding has been shown to be associated with GIB in patients with other clinical backgrounds [44, 58–60]. In accordance with previous findings, gastrointestinal diseases, such as peptic ulcers and bleeding before transplantation were revealed to be independent risk factors for GIB after haplo-HSCT. Alcohol has always been known to have a substantial effect on the occurrence of GIB [61–65]. However, alcohol was not correlated with GIB after haplo-HSCT in our research, potentially because most patients entirely avoided alcohol before HSCT [66]. Until now, no study has investigated the relationship between alcohol and GIB after haplo-HSCT. As this was a retrospective single-centre study and detailed information about alcohol intake of patients was not readily available, additional large-scale multicentre studies are needed to clarify whether alcohol influences GIB after haplo-HSCT.
As GIB has a negative influence on the prognosis of patients after haplo-HSCT, it is essential to estimate the risk of its occurrence. According to the risk prediction methods used in a nested case-control study [41, 42], we developed a predictive model for GIB after haplo-HSCT. The GIB and survival rates of each risk group categorised by this model were significantly different, and the AuROC was relatively high, which indicated that the model was good for predicting the risk of GIB after haplo-HSCT when used for the derived samples; however, further external validation is needed.
In total, 66 patients (35.1%) with GIB died in our study, although bleeding was not always the direct cause of death. Once patients experience GIB, it is of great significance to identify the risk factors for mortality, and we therefore we tried to analyse the prognostic factors for patients with GIB. Grade III–IV aGVHD, AKI, TMA, DIC and gastrointestinal disease or bleeding before HSCT were associated with mortality in patients with GIB. Then, the risk model for mortality in patients with GIB was developed, and it performed well.
Some limitations also exist in our study. First, this was a single-centre retrospective study, and the results might not be completely appropriate for analyses of GIB after haplo-HSCT. Furthermore, selection bias is inevitable in nested cohort studies. In addition, multicentre prospective studies are required to validate our predictive models for GIB and mortality in patients with GIB after haplo-HSCT.
In summary, our study demonstrated that GIB is a severe complication following haplo-HSCT that negatively influences the prognosis of patients. Independent risk and prognostic factors were identified for GIB in our study to develop predictive models for the occurrence and mortality of GIB after haplo-HSCT, which might assist clinicians with personalised GIB prevention and therapeutic strategies.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Programme of China (No. 2017YFA0105503, No. 2017YFA0105500), Key Programme of National Natural Science Foundation of China (No. 81730004), National Natural Science Foundation of China (No. 81670116), Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81621001), Beijing Natural Science Foundation (No. 7171013, H2018206423) and Beijing Municipal Science and Technology Commission (No. Z171100001017084).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xueyan Sun, Yan Su, Xiao Liu
Supplementary information
The online version of this article (10.1038/s41409-020-01187-5) contains supplementary material, which is available to authorised users.
References
- 1.Lv M, Chang Y, Huang X. Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation. Front Med. 2019;13:45–56. doi: 10.1007/s11684-017-0595-7. [DOI] [PubMed] [Google Scholar]
- 2.Lv M, Huang XJ. Allogeneic hematopoietic stem cell transplantation in China: where we are and where to go. J Hematol Oncol. 2012;5:10. doi: 10.1186/1756-8722-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu LP, Wu DP, Han MZ, Huang H, Liu QF, Liu DH, et al. A review of hematopoietic cell transplantation in China: data and trends during 2008-2016. Bone Marrow Transpl. 2017;52:1512–8. doi: 10.1038/bmt.2017.59. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62. doi: 10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus matched-sibling transplant in adults with philadelphia-negative high-risk acute lymphoblastic leukemia: a biologically phase III Randomized Study. Clin Cancer Res. 2016;22:3467–76. doi: 10.1158/1078-0432.CCR-15-2335. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30:2055–63. doi: 10.1038/leu.2016.110. [DOI] [PubMed] [Google Scholar]
- 7.Han LJ, Wang Y, Fan ZP, Huang F, Zhou J, Fu YW, et al. Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2017;179:120–30. doi: 10.1111/bjh.14854. [DOI] [PubMed] [Google Scholar]
- 8.Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10:25. doi: 10.1186/s13045-017-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atilla E, Atilla PA, Bozdag SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45:403–11. doi: 10.1007/s15010-017-1016-1. [DOI] [PubMed] [Google Scholar]
- 10.Reisner Y, Aversa F, Martelli MF. Haploidentical hematopoietic stem cell transplantation: state of art. Bone Marrow Transpl. 2015;50:S1–5. doi: 10.1038/bmt.2015.86. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Beohou E, Labopin M, Volin L, Milpied N, Yakoub-Agha I, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair-matched comparative study of the Beijing approach with the EBMT database. Haematological. 2016;101:e352–4. doi: 10.3324/haematol.2015.140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, et al. Comparison of outcomes of hematopoietic cell transplants from T-Replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-Identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transpl. 2016;22:125–33. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Pihusch R, Holler E, Muhlbayer D, Gohring P, Stotzer O, Pihusch M, et al. The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation. Bone Marrow Transpl. 2002;30:347–54. doi: 10.1038/sj.bmt.1703640. [DOI] [PubMed] [Google Scholar]
- 14.Nevo S, Enger C, Swan V, Wojno KJ, Fuller AK, Altomonte V, et al. Acute bleeding after allogeneic bone marrow transplantation: association with graft versus host disease and effect on survival. Transplantation. 1999;67:681. doi: 10.1097/00007890-199903150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Yeh S, Liao Y, Hsu C, Chen C, Shen Y, Hsueh C, et al. Gastric bleeding due to graft-versus-host disease: discrepancy between endoscopic and histologic assessment. Am J Clin Pathol. 2004;122:919–25. doi: 10.1309/23DAL9F6P74XWJHL. [DOI] [PubMed] [Google Scholar]
- 16.Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. doi: 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 17.Labrador J, Lopez-Anglada L, Perez-Lopez E, Lozano FS, Lopez-Corral L, Sanchez-Guijo FM, et al. Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematological. 2013;98:437–43. doi: 10.3324/haematol.2012.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrador J, Lopez-Corral L, Vazquez L, Sanchez-Guijo F, Guerrero C, Sanchez-Barba M, et al. Incidence and risk factors for life-threatening bleeding after allogeneic stem cell transplant. Br J Haematol. 2015;169:719–25. doi: 10.1111/bjh.13344. [DOI] [PubMed] [Google Scholar]
- 19.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112:504–10. doi: 10.1182/blood-2007-10-117051. [DOI] [PubMed] [Google Scholar]
- 20.Pihusch R, Salat C, Schmidt E, Gohring P, Pihusch M, Hiller E, et al. Hemostatic complications in bone marrow transplantation: a retrospective analysis of 447 patients. Transplantation. 2002;74:1303–9. doi: 10.1097/00007890-200211150-00018. [DOI] [PubMed] [Google Scholar]
- 21.Nevo S, Swan V, Enger C, Wojno KJ, Bitton R, Shabooti M, et al. Acute bleeding after bone marrow transplantation (BMT)- incidence and effect on survival. A quantitative analysis in 1,402 patients. Blood. 1998;91:1469–77. doi: 10.1182/blood.V91.4.1469. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Dispenzieri A, Lacy MQ, Litzow MR, Gertz MA. High incidence of gastrointestinal tract bleeding after autologous stem cell transplant for primary systemic amyloidosis. Bone Marrow Transpl. 2001;28:381–5. doi: 10.1038/sj.bmt.1703155. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz JM, Wolford JL, Thornquist MD, Hockenbery DM, Murakami CS, Drennan F, et al. Severe gastrointestinal bleeding after hematopoietic cell transplantation, 1987–1997: incidence, causes, and outcome. Am J Gastroenterol. 2001;96:385–93. doi: 10.1016/S0002-9270(00)02342-X. [DOI] [PubMed] [Google Scholar]
- 24.Pihusch M. Bleeding complications after hematopoietic stem cell transplantation. Semin Hematol. 2004;41:93–100. doi: 10.1053/j.seminhematol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Nevo S, Enger C, Hartley E, Borinsky ME, Swan V, Fuller AK, et al. Acute bleeding and thrombocytopenia after bone marrow transplantation. Bone Marrow Transpl. 2001;27:65–72. doi: 10.1038/sj.bmt.1702717. [DOI] [PubMed] [Google Scholar]
- 26.Yan CH, Xu LP, Wang FR, Chen H, Han W, Wang Y, et al. Causes of mortality after haploidentical hematopoietic stem cell transplantation and the comparison with HLA-identical sibling hematopoietic stem cell transplantation. Bone Marrow Transpl. 2016;51:391–7. doi: 10.1038/bmt.2015.306. [DOI] [PubMed] [Google Scholar]
- 27.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15. doi: 10.1038/s41409-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuckerman GR, Prakash C, Askin MP, Lewis BS. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201–21. doi: 10.1016/S0016-5085(00)70430-6. [DOI] [PubMed] [Google Scholar]
- 29.Kaur S, Cooper G, Fakult S, Lazarus HM. Incidence and outcome of overt gastrointestinal bleeding in patients undergoing bone marrow transplantation. Dig Dis Sci. 1996;41:598–603. doi: 10.1007/BF02282348. [DOI] [PubMed] [Google Scholar]
- 30.Ramos GP, Binder M, Hampel P, Braga NM, Sunjaya D, Al BB, et al. Outcomes of endoscopic intervention for overt GI bleeding in severe thrombocytopenia. Gastrointest Endosc. 2018;88:55–61. doi: 10.1016/j.gie.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Nagata N, Sakurai T, Shimbo T, Moriyasu S, Okubo H, Watanabe K, et al. Acute severe gastrointestinal tract bleeding is associated with an increased risk of thromboembolism and death. Clin Gastroenterol H. 2017;15:1882–9. doi: 10.1016/j.cgh.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Kim K, Han BJ, Yang S, Na SY, Park S, Boo S, et al. Risk factors and outcome of acute severe lower gastrointestinal bleeding in Crohn’s disease. Digest Liver Dis. 2012;44:723–8. doi: 10.1016/j.dld.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Deutsch D, Romegoux P, Boustiere C, Sabate JM, Benamouzig R, Albaladejo P. Clinical and endoscopic features of severe acute gastrointestinal bleeding in elderly patients treated with direct oral anticoagulants: a multicentre study. Ther Adv Gastroenterol. 2019;12:321926755. doi: 10.1177/1756284819851677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoki T, Nagata N, Shimbo T, Niikura R, Sakurai T, Moriyasu S, et al. Development and validation of a risk scoring system for severe acute lower gastrointestinal bleeding. Clin Gastroenterol H. 2016;14:1562–70. doi: 10.1016/j.cgh.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 35.Strate LL, Saltzman JR, Ookubo R, Mutinga ML, Syngal S. Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol. 2005;100:1821–7. doi: 10.1111/j.1572-0241.2005.41755.x. [DOI] [PubMed] [Google Scholar]
- 36.Strate LL, Orav EJ, Syngal S. Early predictors of severity in acute lower intestinal tract bleeding. Arch Intern Med. 2003;163:838. doi: 10.1001/archinte.163.7.838. [DOI] [PubMed] [Google Scholar]
- 37.Xavier SA, Machado FJ, Magalhães JT, Cotter JB. Acute lower gastrointestinal bleeding: are STRATE andBLEED scores valid in clinical practice? Colorectal Dis. 2018. 10.1111/codi.14529. [DOI] [PubMed]
- 38.Aoki T, Yamada A, Nagata N, Niikura R, Hirata Y, Koike K. External validation of the NOBLADS score, a risk scoring system for severe acute lower gastrointestinal bleeding. Plos One. 2018;13:e196514. doi: 10.1371/journal.pone.0196514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quach DT, Nguyen NT, Vo UP, Le LT, Vo CH, Ho PT, et al. Development and validation of a scoring system to predict severe acute lower gastrointestinal bleeding in vietnamese. Digest Dis Sci. 2020 doi: 10.1007/s10620-020-06253-y. [DOI] [PubMed] [Google Scholar]
- 40.Lee KK, Shah SM, Moser MA. Risk factors predictive of severe diverticular hemorrhage. Int J Surg. 2011;9:83–5. doi: 10.1016/j.ijsu.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Han W, Zhao P, Liu X, Wang J, Wang F, et al. Incidence, risk factors, outcomes, and risk score model of acute pancreatitis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Tr. 2020;26:1171–8. doi: 10.1016/j.bbmt.2019.12.721. [DOI] [PubMed] [Google Scholar]
- 42.Ganna A, Reilly M, de Faire U, Pedersen N, Magnusson P, Ingelsson E. Risk prediction measures for case-cohort and nested case-control designs: an application to cardiovascular disease. Am J Epidemiol. 2012;175:715–24. doi: 10.1093/aje/kwr374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 44.Yin MY, Ruckel S, Kfoury AG, McKellar SH, Taleb I, Gilbert EM, et al. Novel model to predict gastrointestinal bleeding during left ventricular assist device support. Circ Heart Fail. 2018;11:e5267. doi: 10.1161/CIRCHEARTFAILURE.118.005267. [DOI] [PubMed] [Google Scholar]
- 45.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14. [PubMed] [Google Scholar]
- 46.Jacobsohn DA, Kurland BF, Pidala J, Inamoto Y, Chai X, Palmer JM, et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120:2545–52. doi: 10.1182/blood-2012-04-424135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castilla-Llorente C, Martin PJ, McDonald GB, Storer BE, Appelbaum FR, Deeg HJ, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2014;49:966–71. doi: 10.1038/bmt.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuncer HH. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J Gastroenterol. 2012;18:1851. doi: 10.3748/wjg.v18.i16.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Liu X, Wang Q, He Y, Zhu X, Zhang J, et al. Thrombotic microangiopathy with concomitant GI aGVHD after allogeneic hematopoietic stem cell transplantation: Risk factors and outcome. Eur J Haematol. 2018;100:171–81. doi: 10.1111/ejh.12996. [DOI] [PubMed] [Google Scholar]
- 50.Bacigalupo A. Haemopoietic stem cell transplants: the impact of haemorrhagic complications. Blood Rev. 2003;17:S6–10. doi: 10.1016/s0268-960x(03)90001-4. [DOI] [PubMed] [Google Scholar]
- 51.Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Bietar J, Warren M, Dandoy C, Myers KC, Lane A, Wallace G, et al. Histologic features of intestinal thrombotic microangiopathy in pediatric and young adult patients after hematopoietic stem cell transplantation. Biol Blood Marrow Tr. 2015;21:1994–2001. doi: 10.1016/j.bbmt.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2013;58:40–9. doi: 10.1093/cid/cit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikulska M, Nicolini L, Signori A, Rivoli G, Del BV, Raiola AM, et al. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2014;20:O694–701. doi: 10.1111/1469-0691.12611. [DOI] [PubMed] [Google Scholar]
- 55.Mallet V, van Bommel F, Doerig C, Pischke S, Hermine O, Locasciulli A, et al. Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5) Lancet Infect Dis. 2016;16:606–17. doi: 10.1016/S1473-3099(16)00118-3. [DOI] [PubMed] [Google Scholar]
- 56.Rencuzogullari A, Binboga S, Aytac E, Rabets J, Stocchi L, Ozuner G. Incidence, management, and risk factors for lower gastrointestinal bleeding in renal transplant recipients. Transpl P. 2017;49:501–4. doi: 10.1016/j.transproceed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Fiaccadori E, Maggiore U, Clima B, Melfa L, Rotelli C, Borghetti A. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int. 2001;59:1510–9. doi: 10.1046/j.1523-1755.2001.0590041510.x. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, Tatooles AJ, et al. Incidence and Management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93:1534–40. doi: 10.1016/j.athoracsur.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 59.Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. Risk score to predict gastrointestinal bleeding after acute ischemic stroke. BMC Gastroenterol. 2014;14:130. doi: 10.1186/1471-230X-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shalev A, Zahger D, Novack V, Etzion O, Shimony A, Gilutz H, et al. Incidence, predictors and outcome of upper gastrointestinal bleeding in patients with acute coronary syndromes. Int J Cardiol. 2012;157:386–90. doi: 10.1016/j.ijcard.2010.12.081. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Pei D, Ouyang YQ, Nie X. Meta-analysis of risk and protective factors for gastrointestinal bleeding after percutaneous coronary intervention. Int J Nurs Pract. 2019;25:e12707. doi: 10.1111/ijn.12707. [DOI] [PubMed] [Google Scholar]
- 62.Kelly JP, Kaufman DW, Koff RS, Laszlo A, Wiholm BE, Shapiro S. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058–64. [PubMed] [Google Scholar]
- 63.Kaufman DW, Kelly JP, Wiholm BE, Laszlo A, Sheehan JE, Koff RS, et al. The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol. 1999;94:3189–96. doi: 10.1111/j.1572-0241.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 64.Lauffenburger JC, Rhoney DH, Farley JF, Gehi AK, Fang G. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560–8. doi: 10.1002/phar.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moukarbel GV, Signorovitch JE, Pfeffer MA, McMurray JJ, White HD, Maggioni AP, et al. Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J. 2009;30:2226–32. doi: 10.1093/eurheartj/ehp256. [DOI] [PubMed] [Google Scholar]
- 66.Nelson AM, Juckett MB, Coe CL, Costanzo ES. Illness perceptions predict health practices and mental health following hematopoietic stem cell transplantation. Psychooncology. 2019;28:1252–60. doi: 10.1002/pon.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.