Summary
The family of vascular endothelial growth factors (VEGFs) includes 5 members (VEGF-A to -D, and placenta growth factor), which regulate several critical biological processes. VEGF-A exerts a variety of biological effects through high-affinity binding to tyrosine kinase receptors (VEGFR-1, -2 and -3), co-receptors and accessory proteins. In addition to its fundamental function in angiogenesis and endothelial cell biology, VEGF/VEGFR signalling also plays a role in other cell types including epithelial cells. This review provides an overview of VEGF signalling in biliary epithelial cell biology in both normal and pathologic conditions. VEGF/VEGFR-2 signalling stimulates bile duct proliferation in an autocrine and paracrine fashion. VEGF/VEGFR-1/VEGFR-2 and angiopoietins are involved at different stages of biliary development. In certain conditions, cholangiocytes maintain the ability to secrete VEGF-A, and to express a functional VEGFR-2 receptor. For example, in polycystic liver disease, VEGF secreted by cystic cells stimulates cyst growth and vascular remodelling through a PKA/RAS/ERK/HIF1α-dependent mechanism, unveiling a new level of complexity in VEFG/VEGFR-2 regulation in epithelial cells. VEGF/VEGFR-2 signalling is also reactivated during the liver repair process. In this context, pro-angiogenic factors mediate the interactions between epithelial, mesenchymal and inflammatory cells. This process takes place during the wound healing response, however, in chronic biliary diseases, it may lead to pathological neo-angiogenesis, a condition strictly linked with fibrosis progression, the development of cirrhosis and related complications, and cholangiocarcinoma. Novel observations indicate that in cholangiocarcinoma, VEGF is a determinant of lymphangiogenesis and of the immune response to the tumour. Better insights into the role of VEGF signalling in biliary pathophysiology might help in the search for effective therapeutic strategies.
Keywords: VEGF-A, VEGF/VEGFR-2 signalling, Cholangiocytes, Development, Liver repair, Polycystic liver diseases, Cholangiopathies, Anti-Angiogenic therapy
Abbreviations: ADPKD, adult dominant polycystic kidney disease; BA, biliary atresia; BDL, bile duct ligation; CCA, cholangiocarcinoma; CCl4, carbon tetrachloride; CLDs, chronic liver diseases; DP, ductal plate; DPM, ductal plate malformation; DRCs, ductular reactive cells; HIF-1α, hypoxia-inducible factor type 1α; HSCs, hepatic stellate cells; IHBD, intrahepatic bile ducts; IL-, interleukin-; LECs, lymphatic endothelial cells; LSECs, liver sinusoidal endothelial cells; MMPs, matrix metalloproteinases; mTOR, mammalian target of rapamycin; PBP, peribiliary plexus; PC, polycystin; PDGF, platelet-derived growth factor; PIGF, placental growth factor; PLD, polycystic liver diseases; SASP, senescence-associated secretory phenotype; TGF, transforming growth factor; VEGF, vascular endothelial growth factors; VEGFR-1/2, vascular endothelial growth factor receptor 1/2
Key points.
-
•
Angiogenic signalling is activated in biliary epithelial cells during development and disease.
-
•
The VEGF/VEGFR-2 axis mediates the crosstalk between the biliary epithelium and the vascular system during bile duct morphogenesis.
-
•
In polycystic liver disease, VEGF-A signalling acts in an autocrine and paracrine fashion to mediate liver cyst growth.
-
•
Angiogenic signalling is reactivated during the repair process leading to pathological neo-angiogenesis.
-
•
VEGF-A signalling contributes to the development of the tumour microenvironment in cholangiocarcinoma.
-
•
The role of anti-angiogenic therapies still needs to be explored in chronic cholangiopathies.
Introduction
The liver is a highly vascularised organ endowed with both venous capillary (sinusoids) and arterial perfusion. The latter also nourishes the biliary tree through a network of capillaries, called the peribiliary plexus (PBP).1 The biliary tree performs several important functions in digestive physiology, from bile production to liver/regeneration and repair after liver damage.2,3 The epithelial cells that line the bile ducts (i.e. cholangiocytes) are also the primary target in a variety of chronic liver diseases (CLDs), collectively called cholangiopathies.2,4 Several observations emphasise the importance of angiogenic signalling in biliary epithelial cell biology. Studies have shown that the biliary epithelium is able to secrete vascular endothelial growth factor (VEGF) and other angiogenic factors, to express their cognate receptors during development, and to reactivate this signalling axis during biliary diseases and repair.[5], [6], [7], [8] In this review, we will focus on the biological and pathobiological role of VEGF-A and its cognate receptors (VEGFRs) in the biliary tree. Studies have shown that VEGF and VEGFR signalling play a pivotal role in biliary tree development, in bile duct proliferation and biliary repair, as well as in polycystic liver disease (PLD) and cholangiocarcinoma (CCA) progression.
VEGF and VEGFR signalling
VEGF-A is the prototypical member of a growth factor family that also encompasses VEGF-B, VEGF-C, VEGF-D and the placenta growth factor (PIGF).9 The human VEGF-A gene is located on chromosome 6p21.1 and is composed of 8 exons and 7 introns. Differential splicing involving mostly exons 6 and 7 gives rise to multiple VEGF-A isoforms while exons 1-5 and exon 8 are conserved in all VEGF-A variants.10 VEGF-A isoforms are named based on the number of amino acid residues in the mature proteins. VEGF-A165 is the primary gene product found in human tissues, and the one believed to play crucial roles in endothelial cell proliferation, migration, and differentiation.9
Secreted VEGF isoforms bind to specific receptor tyrosine kinases, i.e. VEGFR-1 (Flt-1), VEGFR-2 (KDR or Flk-1) and VEGFR-3, but can also interact with non-tyrosine kinase co-receptors, such as the members of the Neuropilin receptors family (Nrp-1 and -2) and the heparan sulfate proteoglycans.11,12 Upon ligand binding, tyrosine residues present in the intracellular domain of VEGFRs undergo autophosphorylation triggering the transduction of signals through different intracellular mediators.
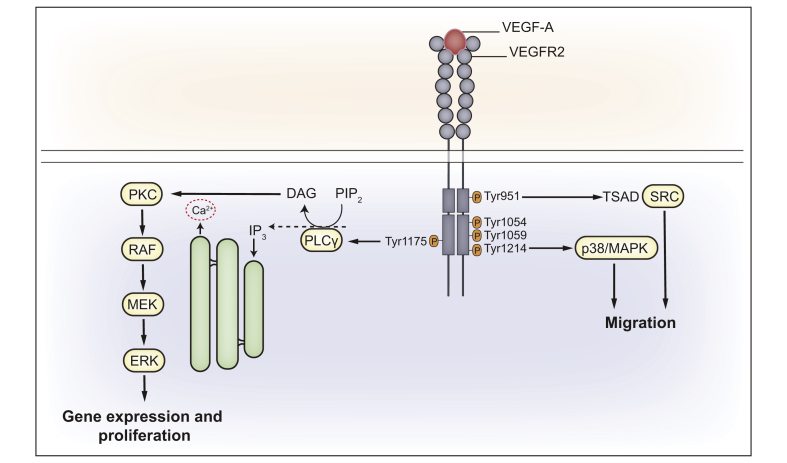
In addition to endothelial cells (ECs) and vascular smooth muscle cells, VEGFR-1 is expressed by diverse cell types including certain epithelial and tumour cells, as well as by haematopoietic stem cells, monocytes, and macrophages.9,13 VEGFR-1 has a higher affinity for PIGF, VEGF-B and for VEGF-A, compared to VEGFR-2. VEGFR-1 acts as a VEGF-A trap and is considered a negative regulator of VEGFR-2 able to suppress its pro-angiogenic effects.14 Most effects of VEGF-A are generated by its binding to VEGFR-2, which stimulates a multitude of downstream signalling events (Fig. 1). Upon ligand binding, VEGFR-2 dimerises and concomitantly cross-phosphorylates tyrosine residues on the intracellular domain,15 including Tyr951, Tyr1054, Tyr1059, Tyr1175, and Tyr1214 (in humans).16,17 Phosphorylation of these tyrosine residues activates different intracellular signalling pathways that are essential for the biology of ECs. For example, phosphorylation of tyrosine 1175 (Y1175, or Y1173 in mice) triggers phospholipase Cγ (PLCγ)-mediated activation of the ERK1/2 pathway, which has a key role during vascular development.16,18 Moreover, the same phosphorylation event leads to the induction of the PI3K/AKT/mTOR pathway, which is crucial for long-term responses such as proliferation, survival and EC migration.16 Cell motility is regulated by the activation of p38MAPK signalling, which is mediated by phosphorylation of residue Y1214 (Y1212 in mice);19 whereas phosphorylation of the Y951 residue (or Y949 in mice) triggers the induction of c-Src-mediated vascular permeability and EC migration.20 It is worth noting that different factors influence VEGFR-2 signalling regulation, including VEGFR-2 expression levels and the presence of co-receptors (such as Nrp-1 and -2).9,11 For example, VEGF-A co-binding to Nrp-1 and VEGFR-2 leads to the formation of Nrp-1/VEGFR-2 complexes and, ultimately, to ERK-1/2 pathway activation.21
Fig. 1.
VEGF-A-mediated VEGFR-2 signal transduction.
Graphic illustration of different signalling pathways elicited by the binding of VEGF-A to VEGFR-2. VEGF-A-induced phosphorylation of several tyrosine (Y) residues in the intracellular domain of the VEGFR-2 triggers multiple downstream signalling pathways. In particular, phosphorylation of Y951 residue leads to the recruitment of TSAd, which in turns binds and activates Src that acts on mediators of cell migration. Cell motility is also regulated by the Y1214 phosphorylation which causes p38MAPK activation. pY1175 residue recruits PLCγ and trigger Ca2+-dependent signalling, which leads to transcriptional control of proliferation, through PKC/RAF/MEK/ERK axis. This scheme is derived from information available for endothelial cells and adapted to the hypothetical signalling in cholangiocytes. Among the phosphorylated residues, the ones shown in red have been detected in mouse cholangiocytes (9,87 and personal unpublished data). DAG, diacylglycerol; IP3, inositol trisphosphate; PIP2, phosphatidylinositol biphosphate; PKC, protein kinase C; PLCγ, phospholipase Cγ; TSAd, T cell-specific adaptor protein; VEGF-A, vascular endothelial growth factor type A; VEGFR-2, vascular endothelial growth factor receptor type 2.
While studied mostly in the context of vascular physiology, the effects of VEGF are not confined to ECs. Several non-EC types that express VEGFRs are involved in wound repair and include macrophages,22 neutrophils,23 pericytes,24 and other mesenchymal cells.25 Furthermore, VEGF is expressed by cholangiocytes during development (ductal plate cells), and in disease conditions, as observed in reactive cholangiocytes, in cystic cholangiocytes of PLDs, and in tumoural cholangiocytes of CCA.[26], [27], [28], [29] The source and locations of VEGF/VEGFR-2 in the hepatic population in biliary health and disease are summarised in Table 1.
Table 1.
Liver cell atlas on the sources and locations of VEGF/VEGFRs.
| Liver cells | VEGF signalling in homeostasis | Ref. | VEGF signalling in disease | Ref. |
|---|---|---|---|---|
| Hepatocytes | SEC structural development in liver organogenesis (VEGF secretion) Liver regeneration (PHx) (VEGF secretion, VEGFR-1 expression). Homeostasis of hepatic vascular system (VEGF secretion). |
135,136 | Hypoxia (VEGF secretion). Biliary atresia (hypoxia mediated): VEGF-A expression |
67,65 |
| Cholangiocytes | Bile duct morphogenesis and PBP development (VEGF-A secretion, VEGFR-2 expression). | 29 | Experimental cholestasis (i.e. BDL): cholangiocyte proliferation and PBP expansion (VEGF-A secretion, VEGFR-2 expression). Biliary atresia: VEGF-A, VEGFR-1/-2 expression. DPM: VEGF-A expression. ADPKD: Cyst growth, ECs proliferation (VEGF-A secretion, VEGFR-1 and -2 expression). CCA: ECM remodelling, TAM recruitment (VEGF-A secretion). |
86,65,46, 8,116 |
| Liver sinusoidal ECs | Maturation in liver organogenesis (VEGFR-2 expression). Liver regeneration (PHx) (VEGFR-1, VEGFR-2 expression). |
135,136 | PBC: VEGF-A, VEGFR-2 expression. | 67 |
| Macrovascular ECs | Biliary atresia: VEGF-A expression. | 65 | ||
| Stellate cells and myofibroblasts |
In vitro activation (VEGF secretion). Vascular remodelling (VEGF secretion). |
137,138 | Hypoxia (VEGF secretion). Fibrogenesis (VEGF secretion). |
139 |
| Kupffer cells | Liver regeneration (VEGFRs expression). | 136 | Fibrosis resolution (VEGF secretion). | 140 |
| Hepatic progenitor cells | Niche expansion in PBC: VEGF-A, VEGFR-1/-3 expression). | 28 | ||
| Lymphatic endothelial cells | Sprouting lymphangiogenesis (VEGFR3 expression and VEGF-C secretion). | 141 | Fibrogenesis (VEGF-C and VEGF-D secretion), tumour lymphangiogenesis in CCA (VEGFR2 and VEGFR3 expression). |
141,113 |
ADPKD, autosomal dominant polycystic kidney disease; BDL, bile-duct ligation; CCA, cholangiocarcinoma; DPM, ductal plate malformation; ECs, endothelial cells; PBP, peribiliary plexus; PHx, partial hepatectomy; VEGF, vascular growth factor; VEGFR, vascular growth factor receptor.
Angiogenic signalling and biliary development
Bile ducts are closely associated with the arterial vasculature both anatomically and functionally. The portal triad is composed of the intrahepatic bile ducts (IHBDs), a branch of the portal vein and 1 or 2 branches of the hepatic artery, all running in a parallel fashion.1 IHBDs are also surrounded by the PBP, a capillary network that meets the metabolic and functional needs of cholangiocytes.13 Moreover, the PBP enables the exchange of signals between biliary epithelial cells and different vascular cell types, such as endothelial and mural cells,30 in an association which begins at the earliest stages of liver development.29
A brief description of the development of the intrahepatic biliary system is required here, as during repair from biliary damage, the liver exploits morphogenetic signalling mechanisms similar to those operating during biliary development. Briefly, IHBD development revolves around the establishment and subsequent remodelling of the ductal plate (DP), a peri-portal embryonic structure formed by the differentiation of hepatoblasts into immature cholangiocytes, driven by clues generated by the portal vasculature and the mesenchyme.31,32 This event requires finely regulated epithelial-mesenchymal interactions, and the cooperation among several signalling networks and morphogenetic signals. For example, in the early stages of DP formation, cells in the mesenchyme surrounding the portal vein express Jagged-1, a Notch ligand able to stimulate downstream signalling in DP cells that promote further differentiation into the biliary lineage.33,34 Instructed by these and other signals, the single-layered DP surrounding the portal mesenchyma eventually duplicates into double-layered structures, which later become incorporated into the portal space. A plethora of growth factors and morphogenetic cues are involved in the phases of formation, duplication, and incorporation of the developing bile ducts.
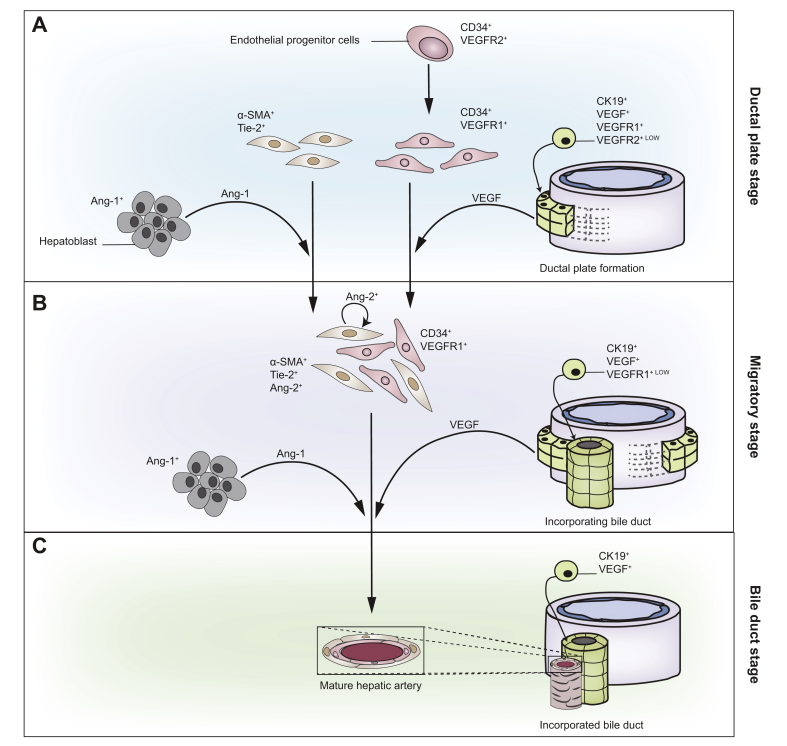
VEGF/VEGFR-2 signalling links bile duct morphogenesis to the development of the PBP stemming from the branches of the hepatic artery in contiguity to the DP (Fig. 2).35 Previous studies from our group have shown that in human foetal liver at different gestational ages, VEGF-A and other angiogenic growth factors, such as angiopoietins 1 and 2 (Ang-1, Ang-2), together with their cognate receptors (VEGFR-1, VEGFR-2, and Tie-2) are differentially expressed by different immature developing structures.29 Ang-1/2 represent a second group of tyrosine kinase receptor ligands, which primarily play a role in developmental vascular remodelling and angiogenesis. Ligand binding to Tie-2 receptors expressed by ECs elicits opposite balanced effects on the activation level of the Tie-2 receptor.29
Fig. 2.
VEGF-A signalling in biliary development.
Angiogenetic factors regulate the anatomical and functional relationship between developing bile ducts and the forming branches of the hepatic artery throughout 3 different maturation stages (A-C). Of note, VEGF-A is secreted by ductal plate cells and is widely expressed in developing liver. During the ductal plate stage (A) VEGF-A recruits VEGFR-2-positive endothelial cell precursors to the portal mesenchyme close to the ductal plates where they cluster as VEGFR-1-positive endothelial cells. On the other hand, portal myofibroblast-derived mural cells expressing Tie-2, the receptor for Ang-1 secreted by hepatoblasts, are recruited into the portal space. During the so-called migratory stage (B), mural and endothelial precursors, assemble as an immature hepatic artery structures characterised by the absence of a recognisable lumen. Ang-2, released by mural cells, remodels the hepatic artery by acting through an autocrine loop mechanism. In parallel, the forming ductal tubular structures are integrated within the mesenchyme of the forming portal space, as VEGFR-1-positive endothelial cells also migrate to develop the vascular peribiliary plexus. During the bile duct stage (C), the incorporated immature ductal tubules mature as bile ducts along with the maturation of the hepatic artery in close proximity to the biliary network. The ability of cholangiocytes to secrete and express angiogenic factors and receptors returns during biliary damage repair. VEGF-A, vascular endothelial growth factor type A; VEGFR-1/2, vascular endothelial growth factor receptor type 1/2.
Of note, the expression of VEGF is maintained, whereas VEGFR-2 is gradually lost after the DP stage.29 Thus, the autocrine/paracrine proliferative responses to VEGF, that are typical of the early maturation stages of the biliary epithelium, are absent in mature bile ducts. However, this signalling pathway can be reactivated in several disease conditions. The anatomical association between bile ducts and the surrounding arteries indicates that biliary and hepatic arterial development are interwoven and highlights the concept that the integrity and functionality of the bile ducts dictate arterial development. Similarly, the role of the PBP is also fundamental in the response to liver damage.36 The ductular reaction that commonly occurs in many forms of liver injury is often characterised by an increase in the number of surrounding vascular structures.[37], [38], [39]
In addition to VEGF/VEGFR-2 signalling, other morphogens, such as Wnt/β-catenin, Hedgehog, Notch, YAP/TAZ and transforming growth factor (TGF)-β regulate biliary development; their role is discussed elsewhere.[40], [41], [42], [43], [44]
VEGF signalling in polycystic liver disease
As mentioned in the previous section, IHBDs originate from hepatoblasts adjacent to the portal vein mesenchyme, thus forming the DP.45 Remodelling of the DP leads to the formation of mature bile ducts. Altered DP remodelling, also known as ductal plate malformation (DPM), results in persistence of embryonic duct features.46 The dilation of segments of IHBDs accompanied by variable degrees of fibrosis characterise different types of DPM, which are hallmarks of several congenital cholangiopathies, including PLD.46,47
PLD associated with autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations in the PKD1 or PKD2 genes, encoding the transmembrane proteins polycystin-1 (PC1) and polycystin-2 (PC2), respectively.48,49 Polycystins are located in the cilia of renal tubular and biliary epithelial cells, where they play a key role in regulating pathways related to morphogenesis, cell proliferation, and differentiation.50 In conditions of PC deficiency, cholangiocytes retain an immature, pro-proliferative and pro-secretory phenotype, which drives an altered morphogenetic programme that triggers the formation of multiple, large fluid-filled liver cysts scattered throughout the hepatic parenchyma with no connection to the biliary tree.51,52 The causal link between mutations in PCs and cystogenesis/disease progression are still the focus of extensive research. Earlier observations showed that in PLD the dysmorphic bile ducts are surrounded by hyperplastic vascular structures with an abnormal ramification resembling a “pollard willow pattern”.4,47 Similar to DP cells during development, the VEGF-A and Ang-1 are strongly upregulated in the cystic biliary epithelium of patients with PLD-ADPKD, together with their receptors VEGFR-2 and Tie-2.8,29 Moreover, the expression levels of VEGF-A and Ang-1 positively correlate with the microvascular density that surrounds the growing dysgenetic structures in ADPKD, supporting the idea that angiogenesis is crucial for cyst growth.8,29
Isolated cholangiocytes from patients with ADPKD and from rodent models of PLD showed increased VEGF-A and VEGFR-2 expression.8 Furthermore, treatment of cholangiocytes with recombinant VEGF-A induced a dose-dependent proliferative effect.8 These data indicate that cholangiocytes influence cyst growth in a paracrine and autocrine fashion via the production of pro-angiogenic factors, which in turn stimulate both the paracrine generation of the cyst vascular supply and the autocrine proliferation of the biliary epithelium.5
Hypoxia-inducible factor 1 (HIF-1α), regulates the response to tissue oxygen levels primarily by modulating the production of VEGF-A.53,54 HIF-1α can also signal in response to non-hypoxic stimuli such as growth factors, cytokines, and a variety of extracellular soluble mediators inducing its stabilisation and phosphorylation through activation of the Raf/MEK/ERK or the PI3K/AKT/tuberin/mTOR or the STAT3 signalling pathway. HIF-1α regulates an array of genes associated with energy metabolism, angiogenesis, erythropoiesis and cell proliferation.55
Increased HIF-1α-dependent production of VEGF has been demonstrated in cultured isolated cystic cholangiocytes, indicating that this may be a direct effect of the loss of PC1 or PC2 function, rather than an effect of tissue hypoxia.56 Indeed, activation of AKT/mTOR signalling induces HIF-1α-mediated VEGF-A production and VEGFR-2 expression, which further support the growing cysts in an autocrine fashion in PLD.6,7,57
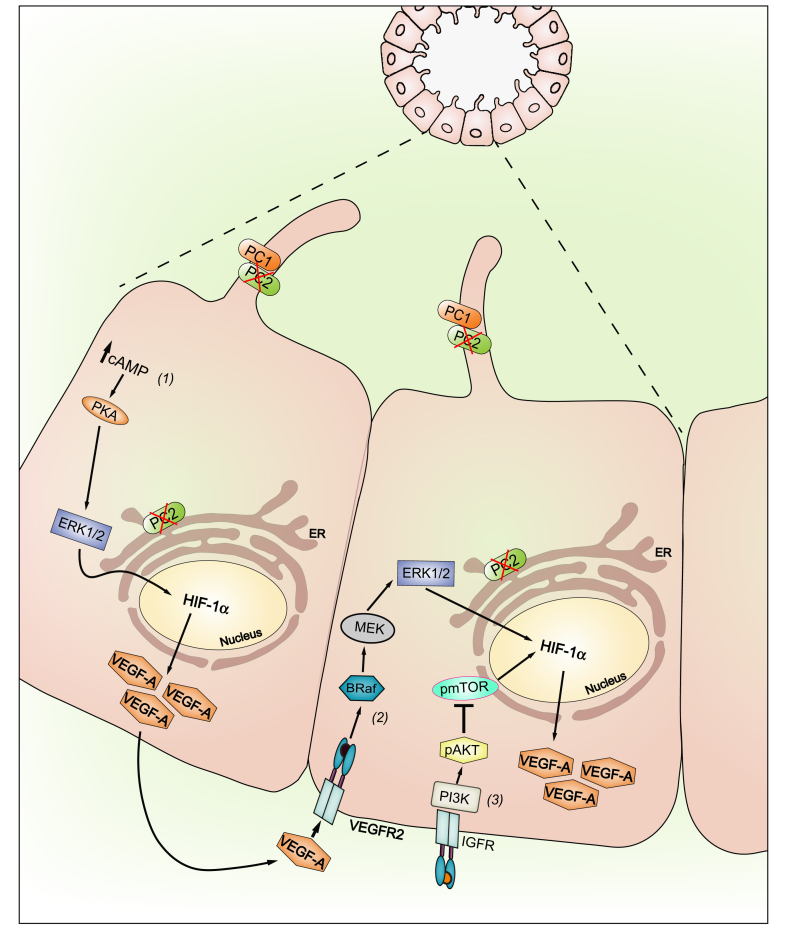
Further studies support the idea that in physiological conditions the repression of the Raf/MEK/ERK cascade is mediated by PC2. In fact, defective function of PC2 leads to Raf/MEK/ERK pathway activation and to the stimulation of cellular proliferation.58 In PC2-defective cholangiocytes, the activation of the cyclic adenosine 3’,5’-monophosphate (cAMP)/PKA-dependent Ras/Raf/ERK1/2 pathway induces the growth of liver cysts, which is further sustained by the increased activation of MEK/ERK signalling.7 A working model of VEGF-A signalling in PC2-defective cholangiocytes is presented in Fig. 3.
Fig. 3.
VEGF-A signalling in PLD-ADPKD (working model).
In PC2-defective cholangiocytes, stimulation of cAMP production drives the PKA-dependent activation of ERK1/ERK2 and the secretion of VEGF-A (1). In turn, cAMP activates the PKA–Ras–Raf–ERK pathway and stimulates VEGF-A production through an mTOR–HIF1α-mediated mechanism (2). mTOR has a central role in IGF-1-stimulated proliferation of cystic cholangiocytes. IGF-1, a growth factor secreted by the cystic epithelium and by stressed biliary epithelial cells, binds to its receptor IGF1-R and activates the PI3K/AKT/mTOR pathway; thus, mTOR stimulates proliferation through a HIF1-α/VEGF-dependent autocrine loop (3). Furthermore, VEGF-A produced by cystic cholangiocytes increases, through a paracrine route, the periductal microvascular density and bile ducts proliferation by binding to VEGFR-2. ADPKD, autosomal dominant polycystic kidney disease; HIF1α, hypoxia-inducible factor type 1α; IGF-1, insulin-like growth factor 1; IGF1-R, insulin-like growth factor 1 receptor; mTOR, mammalian target of rapamycin; PC2, polycystin-2; PLD, polycystic liver disease; VEGF-A, vascular endothelial growth factor type A; VEGFR-2, vascular endothelial growth factor receptor type 2.
The finding that cAMP/PKA/Ras/Raf/MEK/ERK1/2/VEGF cascade is overactive in the cystic epithelium is of particular interest. Indeed, an elevated intracellular concentration of cAMP is a common feature of most PLDs.59,60 Through the induction of both trans-epithelial fluid secretion and epithelial cell proliferation-dependent VEGF-A autocrine stimulation, cAMP is considered a key driver of cyst growth.59,61,62 Therefore, control of cAMP generation represents a potential therapeutic target in PLD.63,64
VEGF signalling in other cholangiopathies
Little is known about the role of VEGF in the pathogenesis of other cholangiopathies. Evidence of expression of pro-angiogenic factors in biliary atresia (BA) has been reported by Edom et al. in liver biopsies from 52 infants.65 In this cohort, VEGF-A was mainly expressed in the biliary remains of the porta hepatis of patients with BA. The expression of the cognate receptor VEGFR2 was also increased in BA liver when compared to control; however, it was still lower than in livers from patients with ischaemic cholangiopathy. Polymorphisms of the VEGF gene have also been investigated in relation to susceptibility to BA. The VEGF+936 C/T polymorphisms, particularly the C allele, may be associated with an inherited predisposition to BA.66
Among inflammatory cholangiopathies, enhanced neo-angiogenesis has been observed in primary biliary cholangitis and localised mainly in the portal area alongside inflammatory infiltrate and fibrosis.67 Furthermore, the increased expression of VEGF-A, along with Ang-1, Ang-2, and the Tie-2 receptor by ECs, suggest that angiogenesis may contribute to inflammatory cell recruitment and progression towards cirrhosis in these patients.67 Of note, a correlation has been observed between the extent of ductular reaction associated with angiogenesis and the increase in hepatic progenitor cells expressing VEGF in patients with primary biliary cholangitis.28 These results support the concept of a crosstalk between hepatic progenitor cells and ECs during liver damage that is mediated by the autocrine and paracrine effects of VEGF.
VEGF in liver regeneration and biliary repair
A remarkable property of the liver is its unique ability to regenerate and restore its original mass after tissue loss. Different pathways of liver regeneration have been identified, but the ability of differentiated hepatocytes and biliary cells to proliferate and generate new liver cells is the major mechanism. The main factors which stimulate mitogenesis in hepatocytes after partial hepatectomy are epidermal growth factor, TGF-α and hepatocyte growth factor. Proliferating hepatocytes form a small avascular cluster and produce an array of mitogenic growth factors that induce proliferation of other hepatic cells that need to be repopulated for proper tissue function, such as biliary epithelial cells, Kupffer cells, hepatic stellate cells (HSCs) and sinusoidal epithelial cells.68 Among these growth factors, VEGF is secreted by hepatocytes in the first 48–72 hours following partial hepatectomy in response to a mechanism of physiological angiogenesis and sinusoidal remodelling. Hepatocyte-derived VEGF-A induces upregulation of VEGFR-1 and VEGFR-2 in liver sinusoidal endothelial cells (LSECs) and facilitates their migration into the hepatocyte cluster, enabling the formation of capillaries.68,69 Moreover, migration of bone marrow-derived endothelial precursors into the liver is induced by increased plasma VEGF levels. The phased expression of Ang-2 plays a major role in regulating the relationships between hepatocytes and LSECs (the 2 most abundant hepatic cell populations).69 During the inductive phase of regeneration, downregulation of Ang-2 increases hepatic proliferation. At the later angiogenic phase, increased Ang-2 enables angiogenesis via VEGFR2 expression in LSECs.70
In contrast to its role in physiological angiogenesis, VEGF production in CLDs may lead to pathological neo-angiogenesis, a condition intrinsically linked with fibrosis and cirrhosis progression, and related complications such as hepatocellular damage.71 It is well known that CLDs are characterised by intrahepatic vascular remodelling with capillarisation of sinusoids, excessive fibrovascular stroma, and the development of intrahepatic shunts. Furthermore, neo-angiogenesis is known to drive the growth of tumours.72,73
Liver repair mechanisms in cholangiopathies are different to those described for other chronic liver diseases. Notably, hepatocellular damage during cholangiopathies is a late phenomenon. Furthermore, vascularisation of the biliary tract depends on the capillary bed of the hepatic artery, whereas hepatocytes are supplied by the sinusoidal circulation and engage in potent crosstalk with LSECs. Whether VEGF produced by hepatocytes and LSECs has an impact on cholangiocyte function in the later stages of biliary diseases is still unknown.
Cholangiocytes usually form a barrier epithelium involved in bile secretion and modification, but they are also highly responsive to liver damage or inflammation with morpho-functional changes leading to ductular reaction and eventually peribiliary fibrosis.34,42 The reparative response to biliary and/or hepatocellular injury is termed the “ductular reaction” and is described as the expansion of cholangiocyte-like cells arranged in cords or clusters not encircling a discernible lumen and surrounded by a polymorphic infiltrate of inflammatory, mesenchymal and vascular cells.74,75 Ductular reactive cells (DRC) may derive from mature cholangiocytes, from trans-differentiation of periportal hepatocytes76,77 or from hepatic progenitor cells. DRCs secrete an array of growth factors and cyto-/chemokines through which they establish an extensive crosstalk with the several types of cells populating the portal fibroinflammatory infiltrate.78,79 According to Desmet et al., ductular reactions can be categorised into 3 types.80 Type 1 is confined to the portal mesenchyme and derives from proliferation of mature cholangiocytes lining the pre-existing bile ducts. Type 2 and type 3 are characterised by ductular reactions extending beyond the portal mesenchyme, which associates with ductular metaplasia of hepatocytes (in type 2A prevailing in the periportal areas, while in type 2B in the centrolobular regions), or with activation of hepatic progenitor cells (type 3).80 However, given that the different types of ductular reaction frequently coexist within the same condition, it is conceivable that in clinical settings multiple mechanisms may take part, depending on the nature and the intensity of liver damage, variably affecting the biliary or the hepatocellular parenchyma.78 It is unclear whether angiogenic mechanisms are differentially involved according to the ductular reaction type. In addition, some DRCs may become senescent due to chronic inflammation. Senescent cells no longer respond to extracellular stimuli but remain metabolically active. They are characterised by the activation of a pro-inflammatory response, the so-called "senescence-associated secretory phenotype” (SASP) with secretion of soluble factors similar to those released by DRCs.81,82 Several factors produced by DRCs, including not only VEGF-A, but also platelet-derived growth factor (PDGF)-B, TGF-β2 and endothelin-171,83 have angiogenic properties. These factors work in concert with VEGF-A, which is also released by other cell elements engaged in the ductular reaction (such as the portal myofibroblasts), to maintain the intense remodelling activity that defines biliary repair.
Cholangiocyte proliferation is critical to maintain the ductal mass and for the increased formation of branched tubular structures that occur in bile duct damage.84,85 VEGF/VEGFR-2 is one of the key signals that synchronises cholangiocyte proliferation with the intense cellular crosstalk that occurs during the reparative response.1 Adaptive vascular responses induce reciprocal changes in the PBP and in the biliary epithelium. For example, bile duct ligation (BDL) triggers a substantial expansion of the PBP to fulfil the increased nutritional and functional needs of the proliferating bile ducts.86 BDL also promotes VEGF-A secretion and expression of VEGFR-2, which correlates with proliferation of cholangiocytes in concert with PBP expansion, via autocrine and paracrine mechanisms.87 In vivo administration of VEGF analogues or neutralising anti-VEGF antibodies, modulates cholangiocyte proliferation. Thus, VEGF secreted by cholangiocytes is thought to be essential for driving the adaptive changes of the PBP. In BDL rats, hepatic artery ligation causes the PBP to vanish, along with increased biliary apoptosis and decreased cholangiocyte proliferation.88 Interestingly, the administration of recombinant VEGF-A prevents these effects, again underlining the pronounced tropism of VEGF for both the bile duct and PBP.
Unfortunately, VEGF is also associated with the generation of a pathologic response with extensive production of new fibrovascular stroma, leading to progression of CLD.78,89 Chronic wound healing activation, in which angiogenesis is a key player, results in the progressive accumulation of extracellular matrix (ECM) components and the formation of regenerative nodules of parenchyma surrounded by fibrotic septa harbouring extensive angio-architectural changes.90 Several studies have highlighted the correlation between neo-vascular formation and fibrogenic progression in widely used experimental models of CLDs.91
Myofibroblasts, derived either from activated HSCs or periductal/portal fibroblasts,92 can support the pathological angiogenesis that is typical in the progression of many CLDs.36 Indeed, while quiescent HSCs in close proximity to the sinusoidal ECs in the Disse’s space contribute to physiological angiogenesis, activated myofibroblasts are a well-known target for VEGF-A, which stimulates their proliferation, motility and production of collagen. Additionally, activated myofibroblasts act as pro-angiogenetic cells that secrete VEGF-A, Ang-1, and upregulate their cognate receptors VEGFR-2 and Tie-2.93,94 Recently, Lemoinne et al. described multiple mechanisms underpinning myofibroblasts’ ability to amplify angiogenesis in vitro and in vivo. These included the formation of direct intracellular junctions with ECs and the secretion of microvesicles containing VEGF-A.95
The interplay between VEGF and Notch signalling, a morphogenetic mechanism deeply involved in liver development and liver repair has also gathered attention recently.96,97 Notch signalling via its ligands (Jagged-1, Jagged-2, Delta-like 1, 3 and 4) and the 4 transmembrane receptors (Notch 1, 2, 3, and 4), is mainly involved in the communication between neighbouring cells and in directing stem-cell self-renewal and differentiation. In the vascular system, VEGF engages in a complex and not yet completely understood relationship with Notch signalling. After organ damage, tissue hypoxia triggers VEGF secretion, that in turn promotes the formation of “tip cells”, i.e. of motile, non-proliferative and tubeless cells that connect with the highly proliferative and tube-forming “stalk cells”. Under the influence of VEGF, tip cells produce Dll4, a Notch ligand that binds Notch1 in the adjacent cells, repressing the tip phenotype and stimulating their differentiation into “stalk cells”, which proliferate and generate a tubular structure that eventually matures into a functional duct. Thus, in ECs, the crosstalk between Notch and VEGF signalling regulates sprouting and branching morphogenesis.98 It is still unclear if a similar mechanism is also adopted by the biliary tree for branching morphogenesis, and the topic is currently being investigated.
VEGF and tumour microenvironment in cholangiocarcinoma
Cholangiocarcinomas are the second most common primary liver malignancies.99,100 CCA can also be a complication of chronic biliary damage secondary to PSC, Caroli disease, intrahepatic lithiasis or fluke infestations. CCA is a challenging malignancy that still carries a very poor prognosis.101 At the time of diagnosis, most patients (>70%) are not eligible for curative liver surgery because of early dissemination, further highlighting the high invasiveness of this tumour.102
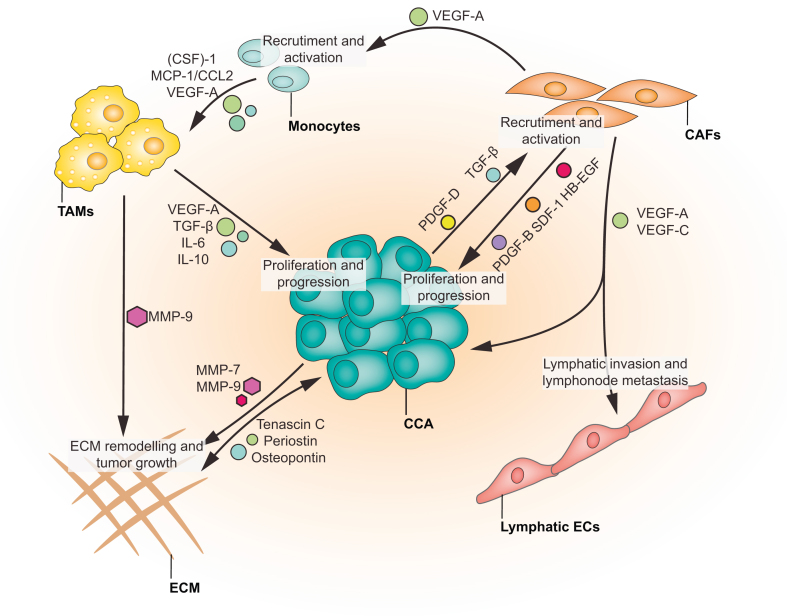
Tumour-associated angiogenesis is considered one of the fundamental mechanisms of cancer growth and metastasis.103 The secretion of pro-angiogenic factors, including VEGFs, Ang-1/2, PDGF and TGFβ, from tumour cells or from cells infiltrating the tumour microenvironment (TME), is a critical component of tumour biology104 (Fig. 4). A distinctive feature of CCA is the presence of a strongly desmoplastic TME, characterised by the presence of multiple cell types, including, but not limited to, cancer-associated fibroblasts (CAFs), immune and inflammatory cells, in particular macrophages, and vascular and lymphatic ECs.102,105 Available data indicate that this rich and polymorphic TME sustains and promotes CCA progression and its metastatic spread.106 Notably, a very recent single-cell RNA sequencing-based study has identified CAFs harbouring a microvasculature signature as the most represented subpopulation (nearly 60% of cells) in the TME of CCA.107
Fig. 4.
Contribution of VEGF-A signalling to tumour microenvironment development in CCA.
CCA cells produce and secrete an array of cytokines and chemokines that allows the remodelling of the ECM and promotes an extensive crosstalk with different cells types recruited in the TME. Paracrine PDGF-D and VEGF-A signals allow the recruitment and the activation of CAFs. Once activated, CAFs secrete an array of signalling molecules (PDGF-BB, SDF-1 and HB-EGF) driving CCA progression. Moreover, VEGF-A secreted by CAFs induces recruitment of monocytes in the TME and their trans-differentiation into TAMs. TAMs contribute to CCA progression and ECM remodelling by secreting VEGF-A, TGF-B, IL-6 and -10 and MMP-9. Furthermore, in CCA, VEGF-A and -C secreted by CAFs dictated the tumour reactive stroma lymphatic invasion and the lymph node metastasis. CAFs, cancer-associated fibroblasts; CCA, cholangiocarcinoma; CSF-1, colony stimulating factor-1; ECs, endothelial cells; ECM, extracellular matrix; HB-EGF, heparin-binding EGF-like growth factor; IL-, interleukin-; MCP-1 (CCL2), monocyte chemoattractant protein-1; MMP-9, matrix metalloproteinase-9; PDGF-DD, platelet-derived growth factor-DD; SDF-1, stromal cell-derived factor-1; TAMs, tumour-associated macrophages; TGF-β, transforming growth factor-β; TME, tumour microenvironment; VEGF-A, vascular growth factor-A.
Angiogenesis, lymphangiogenesis, ECM remodelling and increased cancer cell motility are among the processes promoting tumour invasiveness. Studies in CCA have shown that CAFs are recruited into the TME by PDGF-D, which is secreted by neoplastic cholangiocytes.108 In turn, the activation of CAFs generates a pro-fibrotic and pro-angiogenic milieu that favours CCA progression. In a syngeneic orthotopic rat model of CCA, navitoclax, an inducer of the pro-apoptotic Bax protein, mediated CAF depletion, significantly reducing tumour growth and metastasis, and improving host survival.109
VEGF can also be secreted by tumour cholangiocytes likely secondary to the relatively hypoxic environment in which the tumour is localised. In cultured CCA cells, the induction of VEGF-A secretion and expression of its cognate receptors can also be mediated by other factors including insulin-like growth factor 1 (IGF1), its receptor IGFR as well as the estrogen receptor (ER) family.26,110 CCA cell proliferation induced by estrogens appears to be mediated by VEGF/VEGFR2.34 Conversely, VEGF-A induces CCA cells to secrete matrix metalloproteinase (MMP)-7 and -9, which contribute to the significant ECM remodelling that supports tumour growth and metastasis.105
CCA has traditionally been viewed as a lymphovascular tumour, especially when compared to hepatocellular carcinoma. Indeed, the TME of CCA is characterised by an extensive lymphatic bed, which favours the early spread to regional lymph nodes.111 Hence, increased lymphatic microvessel density and the overexpression of specific lymphatic markers such as podoplanin, a cell-surface mucin-like glycoprotein expressed on both lymphatic endothelial cells (LECs) and CAFs, are considered negative prognostic biomarkers in CCA.112,113 Recent studies in CCA lymphangiogenesis highlight the central role of angiogenic factors in either lymphatic invasion or lymph node metastasis. Among them, VEGF-C is implicated in the formation of the initial lymphatic vessels (small), while it cooperates with Ang-1 and Ang-2 in the formation of the terminal lymphatic vessels.114,115 Neoplastic cells secrete PDGF-D, which binds to PDGFRβ expressed on CAFs and induces ERK/NF-kB and JNK signalling.111 This cascade of events promotes the secretion of VEGF-C by CAFs and the increase in the lymphatic vasculature along with tumour cell intravasation. Our group also showed that PDGF-D secreted by CCA cells stimulated CAF-mediated secretion of VEGF-A and -C and this in turn triggered the recruitment of LECs into the TME.111 In a xenograft mouse model of CCA, this CAF/LEC paracrine loop regulates tumour-associated lymphangiogenesis and the intravasation of tumour cells, an event that was inhibited by the administration of the PDGFRβ inhibitor imatinib. Furthermore, navitoclax-induced CAF depletion was associated with a reduction in lymphatic vessels in the TME and with decreased metastasis at the regional lymph nodes.111
In addition to regulating tumour-associated lymphangiogenesis, VEGF-A stimulates monocyte recruitment into the liver, where they become tumour-associated macrophages (TAMs), acting in concert with other chemoattractant molecules variably released in the TME, such as colony stimulating factor-1 and monocyte chemoattractant protein-1 (MCP-1/CCL2).116,117 Conversely, TAMs themselves modulate the TME by secreting an array of molecules that promote cancer progression and metastasis including: tumour necrosis factor-α, interleukin (IL)-6, IL-10 and TGF-β, which promote direct tumour growth; MMPs, which participate in the dissolution and remodelling of the interstitial matrix; and VEGF-A,118 which promotes the development of newly formed vessels in the tumour.119,120
In addition to TAMs, the marked heterogeneity of the TME in CCA also involves immune cell types, encompassing several subgroups of tumour-infiltrating lymphocytes, such as CD4+, CD8+, dendritic cells, and myeloid-derived suppressor cells.121,122 Through complex interactions with immune checkpoint inhibitors, these infiltrating cells affect the response of the tumour to immune therapy.123 Recently, an immunosuppressive role of VEGF has been recognised in cancer. Although not yet studied in the specific setting of CCA, it has been shown that increased levels of VEGF induce the infiltration of TAMs and of myeloid-derived suppressor cells and inhibitory regulatory T cells.124 Inhibition of VEGF or of VEGFR-2 reprogrammes the microenvironment from immunosuppressive to immunostimulatory.125,126 Data in CCA are not yet clear, however, combination therapy with pembrolizumab (a checkpoint inhibitor) and bevacizumab (anti-VEGF) led to an overall response rate of 36% in patients with advanced hepatocellular carcinoma.127 Further data on the safety and efficacy of such combinations in patients with CCA are eagerly awaited.122
Conclusions and therapeutic implications
Our knowledge of the role of VEGF in biliary cells during physiological and pathophysiological conditions has grown significantly. Recent studies have clarified the role of VEGF/VEGFRs during biliary development, PLD, liver repair and carcinogenesis. However, several questions remain unanswered and open to investigation (Table 3).
Table 3.
Research agenda.
| Category | Priority | Time-scale |
|---|---|---|
| Basic or translational research | ||
| Animal model | Study the role of angiogenesis and anti-angiogenic treatments to target fibrosis in animal model of biliary fibrosis. | Medium-long term |
| Cell biology | Study the crosstalk between liver morphogens (i.e. Notch) and angiogenetic signalling in biliary morphogenesis during repair. | Short-medium term |
| Cell Biology | Study the regulation of VEGFR2 expression in cholangiocytes and clarification of its intracellular signalling. | Medium term |
| Cell Biology | Understand the crosstalk and cross-influences among VEGF and other pro-angiogenic pathways (Angiopoietins, Semaphorins, etc.). | Medium term |
| Liver pathophysiology | Study the role of hepatocellular production of angiogenic factors (VEGFs and angiopoietins) in cholangiopathies. | Short-medium term |
| Liver pathophysiology | Study the role of VEGF in the modulation of innate immunity. | Medium term |
| Liver pathophysiology | Determine the role of VEGF in the regulation of TAMs, and MDSCs and inhibitory Treg function in the TME of CCA. | Short-medium term |
| Disease modelling | Generate biliary organoids from patients with cholangiopathies and use to test new targets and drugs. | Medium-long term |
| Bio-engineering | Apply new biomolecular technologies to deliver anti-angiogenic treatments more specifically in a cell type/tissue. | Medium-long term |
| Bio-banking | Create biobanks of tissue, cells, organoids, nucleic acids, proteins of different cholangiopathies. | Medium-long term |
| Clinical research | ||
| Clinical trial | Design drug-repurposing studies for anti-angiogenic therapies in PLD. | Medium term |
| Clinical study | Assess the response to combination treatments with immune therapy and TKI in CCA. | Short-term |
CCA, cholangiocarcinoma; MDSCs, myeloid-derived suppressor cells; PLD, polycystic liver disease; TAMs, tumour-associated macrophages; TKI, tyrosine kinase inhibitor; TME, tumour microenvironment; VEGF, vascular growth factor; VEGFR, vascular growth factor receptor.
Despite the strong preclinical evidence available in the literature and the fact that the pathways described herein may represent potential therapeutic targets, the role of anti-angiogenic therapy in biliary diseases (Table 2) is still understudied. For example, experimental data indicate that blockade of angiogenic signalling is effective in reducing proliferation of the cystic epithelium in PLDs. Treatment of PC2-defective mice with the VEGFR-2 competitive inhibitor (SU5416), or with rapamycin, an mTOR inhibitor, led to a significant decrease in the proliferative activity of the cystic epithelium, a reduction in liver cystic area and, in the case of rapamycin, increased apoptosis of the cystic epithelium.6,7 However, we are not aware of clinical trials evaluating the effects of anti-angiogenic treatment in patients with PLD, even though there is preliminary evidence of efficacy in slowing the progression of polycystic disease in the kidney.128 There are several possible explanations for the difference in efficacy between rodent models and humans, including dosage, collateral effects, and the fact that mTOR inhibitors are given at the onset of disease in mice, whereas they are given to patients with advanced disease (see also Table 2). Discrepancies between rodent and human results are not infrequent in biomedicine. Thanks to new developments in liver cell isolation and culture, such as organoids and induced pluripotent stem cells, the prospect of obtaining patient-derived disease models for pathophysiological and pharmacological studies and personalised medicine is now a reality.129
Table 2.
Anti-angiogenic therapies in experimental models.
| Compound | Targets | Animal models | Study outcomes in animal models | Human disease | Study outcomes in human disease | Limitations | Ref. |
|---|---|---|---|---|---|---|---|
| Everolimus (Afinotor) | mTOR inhibitor | PCK rats Han:SPRD(Cy/+) rats |
Liver disease: prevention of liver cyst enlargement, reduction of fibrosis. Kidney disease: kidney cysts reduction at moderate dosage, amelioration of renal function loss. |
ADPKD | Kidney disease: decreased kidney volume but no recovery of renal function. | ADPKD animal models develop early and severe disease compared to humans and intervention is usually in early stage of disease. No studies were conducted in human PLD. In human studies decrease in kidney volume does not correlate with improvement in renal function. In human studies multiple side effects were reported. |
142,143 144 |
| SU5416 (Semaxanib) | VEGFR-2 selective inhibitor |
Conditional Pkd2KO mice Pkd2WS25/- |
Liver disease: Suppression of cholangiocyte proliferation, suppression of liver cyst growth Liver and kidney disease: prevention of liver cysts but not kidney cysts. |
n.a. | n.a. | n.a. | 7,145 |
| Rapamycin (Rapamune) | mTOR inhibitor | Conditional Pkd2KO mice | Suppression of cholangiocyte proliferation. Suppression of liver cyst growth. Suppression of fibrosis. Increased cholangiocyte apoptosis. |
PLD In ADPKD transplant |
Liver disease: reduction in polycystic liver volumes and a trend toward reduction in kidney volume. | The human study was retrospective | 6,146 |
| SQ22,536 | Adenylate cyclase 5 (AC5) inhibitor | Conditional Pkd2KO mice | Suppression of liver cyst growth | n.a. | n.a. | n.a. | 60 |
| Sorafenib (Nexavar) |
Multikinase inhibitor (Raf kinase, PDGF, VEGFR-2 VEGFR-3). Autophagy and apoptosis inducer. Ferroptosis activator. |
Partial portal vein ligation in rats. Common BDL in rats. |
Suppression of fibrosis. Suppression of portal pressure. |
Advanced or metastatic liver cancer and cirrhosis Clinical Trial: NCT00767468 |
No results reported | n.a. | 132 |
| anti-VEGFR1 mAb |
VEGFR-1 neutralising agents | CCl4 → mice | Suppression of fibrosis | n.a. | n.a. | n.a. | 147 |
| anti-VEGFR-2 mAb |
VEGFR-2 neutralising agents | CCl4 → mice | Suppression of angiogenesis | n.a. | n.a. | n.a. | 147 |
| SU11248 (Sunitinib) |
Multi-kinase inhibitor (VEGFR-2 PDGFRβ) |
CCl4 → rats | Suppression of fibrosis. Suppression of portal pressure. |
n.a. | n.a. | n.a. | 147 |
| Sec 5–27 | Secretin receptor inhibitor | dnTGF-βRII mice | Suppression of cholangiocyte proliferation. Suppression of angiogenesis. Suppression of fibrosis. Suppression of senescence. Reduction of inflammation. |
n.a. | n.a. | n.a. | 148 |
| ML221 | Apelin receptor (APJ) inhibitor | BDL → apelin-/- Mdr2-/- |
Suppression of cholangiocyte proliferation. Suppression of angiogenesis. Suppression of fibrosis. Suppression of senescence. Reduction of inflammation. |
n.a. | n.a. | n.a. | 149 |
| AG-012736 (Axitinib) | Selective second-generation VEGFRs inhibitor | Subcutaneous xenograft of human (NCC-BD1 and TKKK) CCA cell lines. | Growth inhibition of NCC-BD1 and TKKK. Decreased microvessel density. |
Hepatobiliary malignant tumours Clinical Trial: NCT04010071 |
-no results reported | n.a. | 150 |
ADPKD, autosomal dominant polycystic kidney disease; BDL, bile-duct ligation; CCA, cholangiocarcinoma; CCl4, carbon tetrachloride; mTOR, mammalian target of rapamycin; PDGF, platelet-derived growth factor; PDGFRβ, platelet-derived growth factor receptor-β; PLD, polycystic liver disease; TGF-βRII, transforming growth factor-β receptor 2; VEGF, vascular growth factor; VEGFR, vascular growth factor receptor.
Several studies in animal models of liver injury have shown that the extent of liver fibrosis decreases upon the inhibition of angiogenesis. It has recently been demonstrated that intravenous injection of adenovirus expressing the extracellular domain of Tie-2 was able to block Ang-1 signalling and significantly prevent both pathological angiogenesis and fibrosis in BDL mice or in animals subjected to chronic carbon tetrachloride (CCl4) treatment.130 These studies also showed that stimulation of Tie1 on LSECs plays an important role in liver fibrosis and its inhibition protects against fibrosis progression. Rapamycin can effectively reduce BDL-induced liver fibrosis and bile duct hyperplasia in rats through its inhibitory effect on mTOR, thereby reducing the production of pro-fibrotic cytokines.131
Furthermore, a study in rats with portal hypertension and cirrhosis reported the beneficial vascular effects of the tyrosine kinase inhibitor sorafenib, which is already approved for HCC. Indeed, oral administration of sorafenib reduced portal hypertension and ameliorated angiogenesis and fibrosis due to inhibition of VEGF secretion and Raf kinase signalling.132 Tugues et al. reported on the anti-fibrotic effects of the multitargeted receptor tyrosine kinase inhibitor SU11248 (sunitinib) in the chronic CCl4 rat model of CLD. The treatment of cirrhotic animals with SU11248 significantly decreased the inflammatory infiltrate, hepatic microvascular density, ECM deposition and portal pressure.133 However, as shown by a recent study from Xu et al., portal angiogenesis and sinusoid capillarisation (induced by LECT2 via the Tie1 receptor on ECs) have opposite roles in liver fibrogenesis, wherein sinusoidal capillarisation promotes fibrosis.134 This study highlights the challenges of anti-angiogenic therapy and the difficulties in predicting the end results. However, there are differences in repair mechanisms between biliary and hepatocellular diseases and strong differences in vascularisation between the biliary tree (which depends on the peribiliary capillary plexus and the hepatic artery) and the hepatocytes that are in close contact with the sinusoidal endothelial cells and the portal circulation. Hepatocellular dysfunction is a late phenomenon in cholangiopathies, suggesting that anti-angiogenic treatment should probably be applied in the early phases of biliary diseases.
While anti-angiogenic drugs may represent an alternative tool to prevent or decrease pathological angiogenesis, aberrant biliary proliferation and portal fibrosis progression, their use in the clinical setting is still not being studied. A number of issues, including the unclear risk of bleeding and decompensation, need to be thoroughly considered. More studies and clinical trials with tailored designs are needed to explore the ‘pros’ and ‘cons’ of using modulators of angiogenesis in the treatment of chronic cholangiopathies.
Financial support
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under P30 DK034989-Silvio O. Conte Digestive Diseases Research Core Center and by Award Number DK101528 to MS.
Authors’ contributions
V.M. and M.S. contributed to this paper with conception, literature review and writing the manuscript. R.F., M.C. and L.F. participated in drafting, critical revision and editing. All the authors approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest related to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found at https://doi.org/10.1016/j.jhepr.2021.100251.
Supplementary data
References
- 1.Strazzabosco M., Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159–1170. doi: 10.1016/j.jhep.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strazzabosco M., Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken) 2008;291:653–660. doi: 10.1002/ar.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer J.L. Bile formation and secretion. Compr Physiol. 2013;3:1035–1078. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabris L., Fiorotto R., Spirli C., Cadamuro M., Mariotti V., Perugorria M.J. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol. 2019;16:497–511. doi: 10.1038/s41575-019-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky K.S., McWilliams R.R., Amura C.R., Barry N.P., Doctor R.B. Liver cyst cytokines promote endothelial cell proliferation and development. Exp Biol Med (Maywood) 2009;234:1155–1165. doi: 10.3181/0903-RM-112. [DOI] [PubMed] [Google Scholar]
- 6.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51:1778–1788. doi: 10.1002/hep.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360–371 e367. doi: 10.1053/j.gastro.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabris L., Cadamuro M., Fiorotto R., Roskams T., Spirli C., Melero S. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 9.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cel Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 10.Arcondeguy T., Lacazette E., Millevoi S., Prats H., Touriol C. VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 2013;41:7997–8010. doi: 10.1093/nar/gkt539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uniewicz K.A., Fernig D.G. Neuropilins: a versatile partner of extracellular molecules that regulate development and disease. Front Biosci. 2008;13:4339–4360. doi: 10.2741/3008. [DOI] [PubMed] [Google Scholar]
- 12.Mamer S.B., Wittenkeller A., Imoukhuede P.I. VEGF-A splice variants bind VEGFRs with differential affinities. Sci Rep. 2020;10:14413. doi: 10.1038/s41598-020-71484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudio E., Franchitto A., Pannarale L., Carpino G., Alpini G., Francis H. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546–3552. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambati B.K., Nozaki M., Singh N., Takeda A., Jani P.D., Suthar T. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Xie P., Opatowsky Y., Schlessinger J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc Natl Acad Sci U S A. 2010;107:1906–1911. doi: 10.1073/pnas.0914052107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T., Yamaguchi S., Chida K., Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto T., Bohman S., Dixelius J., Berge T., Dimberg A., Magnusson P. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham S.A., Arrate M.P., Brock T.A., Waxham M.N. Interactions of FLT-1 and KDR with phospholipase C gamma: identification of the phosphotyrosine binding sites. Biochem Biophys Res Commun. 1997;240:635–639. doi: 10.1006/bbrc.1997.7719. [DOI] [PubMed] [Google Scholar]
- 19.Lamalice L., Houle F., Huot J. Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem. 2006;281:34009–34020. doi: 10.1074/jbc.M603928200. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z., Li X., Massena S., Kutschera S., Padhan N., Gualandi L. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med. 2012;209:1363–1377. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog B., Pellet-Many C., Britton G., Hartzoulakis B., Zachary I.C. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cel. 2011;22:2766–2776. doi: 10.1091/mbc.E09-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvaraj D., Gangadharan V., Michalski C.W., Kurejova M., Stosser S., Srivastava K. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell. 2015;27:780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massena S., Christoffersson G., Vagesjo E., Seignez C., Gustafsson K., Binet F. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126:2016–2026. doi: 10.1182/blood-2015-03-631572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao R., Xue Y., Hedlund E.M., Zhong Z., Tritsaris K., Tondelli B. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci U S A. 2010;107:856–861. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medal R.M., Im A.M., Yamamoto Y., Lakhdari O., Blackwell T.S., Hoffman H.M. The innate immune response in fetal lung mesenchymal cells targets VEGFR2 expression and activity. Am J Physiol Lung Cel Mol Physiol. 2017;312:L861–L872. doi: 10.1152/ajplung.00554.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancino A., Mancino M.G., Glaser S.S., Alpini G., Bolognese A., Izzo L. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156–163. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancinelli R., Onori P., Gaudio E., Franchitto A., Carpino G., Ueno Y. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med (Maywood) 2009;234:462–474. doi: 10.3181/0808-RM-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franchitto A., Onori P., Renzi A., Carpino G., Mancinelli R., Alvaro D. Expression of vascular endothelial growth factors and their receptors by hepatic progenitor cells in human liver diseases. Hepatobiliary Surg Nutr. 2013;2:68–77. doi: 10.3978/j.issn.2304-3881.2012.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabris L., Cadamuro M., Libbrecht L., Raynaud P., Spirli C., Fiorotto R. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–728. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- 30.Kono N., Nakanuma Y. Ultrastructural and immunohistochemical studies of the intrahepatic peribiliary capillary plexus in normal livers and extrahepatic biliary obstruction in human beings. Hepatology. 1992;15:411–418. doi: 10.1002/hep.1840150310. [DOI] [PubMed] [Google Scholar]
- 31.Lemaigre F.P. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Carpentier R., Suner R.E., van Hul N., Kopp J.L., Beaudry J.B., Cordi S. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. 1438 e1431-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann J.J., Zovein A.C., Koh H., Radtke F., Weinmaster G., Iruela-Arispe M.L. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorent K., Yeo S.Y., Oda T., Chandrasekharappa S., Chitnis A., Matthews R.P. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 35.Libbrecht L., Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cel Dev Biol. 2002;13:389–396. doi: 10.1016/s1084952102001258. [DOI] [PubMed] [Google Scholar]
- 36.Thomson J., Hargrove L., Kennedy L., Demieville J., Francis H. Cellular crosstalk during cholestatic liver injury. Liver Res. 2017;1:26–33. doi: 10.1016/j.livres.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crosby H.A., Hubscher S.G., Joplin R.E., Kelly D.A., Strain A.J. Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology. 1998;28:980–985. doi: 10.1002/hep.510280412. [DOI] [PubMed] [Google Scholar]
- 38.Kinugasa Y., Nakashima Y., Matsuo S., Shono K., Suita S., Sueishi K. Bile ductular proliferation as a prognostic factor in biliary atresia: an immunohistochemical assessment. J Pediatr Surg. 1999;34:1715–1720. doi: 10.1016/s0022-3468(99)90652-8. [DOI] [PubMed] [Google Scholar]
- 39.Sclair S.N., Fiel M.I., Wu H.S., Doucette J., Aloman C., Schiano T.D. Increased hepatic progenitor cell response and ductular reaction in patients with severe recurrent HCV post-liver transplantation. Clin Transpl. 2016;30:722–730. doi: 10.1111/ctr.12740. [DOI] [PubMed] [Google Scholar]
- 40.Lee D.H., Park J.O., Kim T.S., Kim S.K., Kim T.H., Kim M.C. LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nat Commun. 2016;7:11961. doi: 10.1038/ncomms11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordi S., Godard C., Saandi T., Jacquemin P., Monga S.P., Colnot S. Role of beta-catenin in development of bile ducts. Differentiation. 2016;91:42–49. doi: 10.1016/j.diff.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monga S.P. Hepatic regenerative medicine: exploiting the liver's will to live. Am J Pathol. 2014;184:306–308. doi: 10.1016/j.ajpath.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirose Y., Itoh T., Miyajima A. Hedgehog signal activation coordinates proliferation and differentiation of fetal liver progenitor cells. Exp Cel Res. 2009;315:2648–2657. doi: 10.1016/j.yexcr.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Schaub J.R., Huppert K.A., Kurial S.N.T., Hsu B.Y., Cast A.E., Donnelly B. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature. 2018;557:247–251. doi: 10.1038/s41586-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ober E.A., Lemaigre F.P. Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 2018;68:1049–1062. doi: 10.1016/j.jhep.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Raynaud P., Carpentier R., Antoniou A., Lemaigre F.P. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cel Biol. 2011;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Desmet V.J. Congenital diseases of intrahepatic bile ducts: variations on the theme "ductal plate malformation. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 48.Cornec-Le Gall E., Torres V.E., Harris P.C. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29:13–23. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strazzabosco M., Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140:1855–1859. doi: 10.1053/j.gastro.2011.04.030. 1859 e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masyuk A.I., Masyuk T.V., LaRusso N.F. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gevers T.J., Drenth J.P. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:101–108. doi: 10.1038/nrgastro.2012.254. [DOI] [PubMed] [Google Scholar]
- 52.Masyuk T., Masyuk A., LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25:265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Germain S., Monnot C., Muller L., Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol. 2010;17:245–251. doi: 10.1097/MOH.0b013e32833865b9. [DOI] [PubMed] [Google Scholar]
- 54.Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 55.Koyasu S., Kobayashi M., Goto Y., Hiraoka M., Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: two decades of knowledge. Cancer Sci. 2018;109:560–571. doi: 10.1111/cas.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariotti V., Strazzabosco M., Fabris L., Calvisi D.F. Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1254–1261. doi: 10.1016/j.bbadis.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torrice A., Cardinale V., Gatto M., Semeraro R., Napoli C., Onori P. Polycystins play a key role in the modulation of cholangiocyte proliferation. Dig Liver Dis. 2010;42:377–385. doi: 10.1016/j.dld.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Spirli C., Morell C.M., Locatelli L., Okolicsanyi S., Ferrero C., Kim A.K. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56:2363–2374. doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 59.Strazzabosco M., Fiorotto R., Melero S., Glaser S., Francis H., Spirli C. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spirli C., Mariotti V., Villani A., Fabris L., Fiorotto R., Strazzabosco M. Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease. J Hepatol. 2017;66:571–580. doi: 10.1016/j.jhep.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spirli C., Locatelli L., Fiorotto R., Morell C.M., Fabris L., Pozzan T. Altered store operated calcium entry increases cyclic 3',5'-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology. 2012;55:856–868. doi: 10.1002/hep.24723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 62.Banales J.M., Masyuk T.V., Gradilone S.A., Masyuk A.I., Medina J.F., LaRusso N.F. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Aerts R.M.M., van de Laarschot L.F.M., Banales J.M., Drenth J.P.H. Clinical management of polycystic liver disease. J Hepatol. 2018;68:827–837. doi: 10.1016/j.jhep.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 64.Masyuk T.V., Radtke B.N., Stroope A.J., Banales J.M., Gradilone S.A., Huang B. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edom P.T., Meurer L., da Silveira T.R., Matte U., dos Santos J.L. Immunolocalization of VEGF A and its receptors, VEGFR1 and VEGFR2, in the liver from patients with biliary atresia. Appl Immunohistochem Mol Morphol. 2011;19:360–368. doi: 10.1097/PAI.0b013e3182028a8e. [DOI] [PubMed] [Google Scholar]
- 66.Lee H.C., Chang T.Y., Yeung C.Y., Chan W.T., Jiang C.B., Chen W.F. Genetic variation in the vascular endothelial growth factor gene is associated with biliary atresia. J Clin Gastroenterol. 2010;44:135–139. doi: 10.1097/MCG.0b013e3181b152c2. [DOI] [PubMed] [Google Scholar]
- 67.Medina J., Sanz-Cameno P., Garcia-Buey L., Martin-Vilchez S., Lopez-Cabrera M., Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124–131. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu H., Mitsuhashi N., Ohtsuka M., Ito H., Kimura F., Ambiru S. Vascular endothelial growth factor and angiopoietins regulate sinusoidal regeneration and remodeling after partial hepatectomy in rats. World J Gastroenterol. 2005;11:7254–7260. doi: 10.3748/wjg.v11.i46.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato T., El-Assal O.N., Ono T., Yamanoi A., Dhar D.K., Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690–698. doi: 10.1016/s0168-8278(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 70.Hu J., Srivastava K., Wieland M., Runge A., Mogler C., Besemfelder E. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 71.Cannito S., Milani C., Cappon A., Parola M., Strazzabosco M., Cadamuro M. Fibroinflammatory liver injuries as preneoplastic condition in cholangiopathies. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19123875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blechacz B., Gores G.J. Tumor-specific marker genes for intrahepatic cholangiocarcinoma: utility and mechanistic insight. J Hepatol. 2008;49:160–162. doi: 10.1016/j.jhep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosmorduc O., Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258–270. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 74.Roskams T.A., Theise N.D., Balabaud C., Bhagat G., Bhathal P.S., Bioulac-Sage P. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 75.Gouw A.S., Clouston A.D., Theise N.D. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 76.Sato K., Marzioni M., Meng F., Francis H., Glaser S., Alpini G. Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology. 2019;69:420–430. doi: 10.1002/hep.30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko S., Russell J.O., Molina L.M., Monga S.P. Liver progenitors and adult cell plasticity in hepatic injury and repair: knowns and unknowns. Annu Rev Pathol. 2020;15:23–50. doi: 10.1146/annurev-pathmechdis-012419-032824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabris L., Spirli C., Cadamuro M., Fiorotto R., Strazzabosco M. Emerging concepts in biliary repair and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2017;313:G102–G116. doi: 10.1152/ajpgi.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strazzabosco M., Fiorotto R., Cadamuro M., Spirli C., Mariotti V., Kaffe E. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1374–1379. doi: 10.1016/j.bbadis.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desmet V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 81.Roskams T.A., Libbrecht L., Desmet V.J. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 82.Zhang B., Fu D., Xu Q., Cong X., Wu C., Zhong X. The senescence-associated secretory phenotype is potentiated by feedforward regulatory mechanisms involving Zscan4 and TAK1. Nat Commun. 2018;9:1723. doi: 10.1038/s41467-018-04010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams D.H. Biliary epithelial cells: innocent victims or active participants in immune-mediated liver disease? J Lab Clin Med. 1996;128:528–530. doi: 10.1016/s0022-2143(96)90123-7. [DOI] [PubMed] [Google Scholar]
- 84.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 85.Banales J.M., Huebert R.C., Karlsen T., Strazzabosco M., LaRusso N.F., Gores G.J. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Franchitto A. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–317. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 87.Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 88.Alpini G., Glaser S.S., Ueno Y., Pham L., Podila P.V., Caligiuri A. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 89.Bocca C., Novo E., Miglietta A., Parola M. Angiogenesis and fibrogenesis in chronic liver diseases. Cell Mol Gastroenterol Hepatol. 2015;1:477–488. doi: 10.1016/j.jcmgh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fabris L., Cadamuro M., Cagnin S., Strazzabosco M., Gores G.J. Liver matrix in benign and malignant biliary tract disease. Semin Liver Dis. 2020;40:282–297. doi: 10.1055/s-0040-1705109. [DOI] [PubMed] [Google Scholar]
- 91.Srivastava A., Shukla V., Tiwari D., Gupta J., Kumar S., Kumar A. Targeted therapy of chronic liver diseases with the inhibitors of angiogenesis. Biomed Pharmacother. 2018;105:256–266. doi: 10.1016/j.biopha.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 92.Gupta V., Gupta I., Park J., Bram Y., Schwartz R.E. Hedgehog signaling demarcates a niche of fibrogenic peribiliary mesenchymal cells. Gastroenterology. 2020;159:624–638 e629. doi: 10.1053/j.gastro.2020.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaparro M., Sanz-Cameno P., Trapero-Marugan M., Garcia-Buey L., Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007;6:208–213. [PubMed] [Google Scholar]
- 94.Paternostro C., David E., Novo E., Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281–288. doi: 10.3748/wjg.v16.i3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemoinne S., Cadoret A., Rautou P.E., El Mourabit H., Ratziu V., Corpechot C. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- 96.Boulter L., Govaere O., Bird T.G., Radulescu S., Ramachandran P., Pellicoro A. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fiorotto R., Raizner A., Morell C.M., Torsello B., Scirpo R., Fabris L. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–130. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanco R., Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banales J.M., Marin J.J.G., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020 doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banales J.M., Cardinale V., Carpino G., Marzioni M., Andersen J.B., Invernizzi P. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 101.Tyson G.L., El-Serag H.B. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banales J.M., Cardinale V., Macias R.I.R., Andersen J.B., Braconi C., Carpino G. Cholangiocarcinoma: state-of-the-art knowledge and challenges. Liver Int. 2019;39(Suppl 1):5–6. doi: 10.1111/liv.14101. [DOI] [PubMed] [Google Scholar]
- 103.Tamma R., Annese T., Ruggieri S., Brunetti O., Longo V., Cascardi E. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur J Clin Invest. 2019;49 doi: 10.1111/eci.13087. [DOI] [PubMed] [Google Scholar]
- 104.Cadamuro M., Stecca T., Brivio S., Mariotti V., Fiorotto R., Spirli C. The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1435–1443. doi: 10.1016/j.bbadis.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabris L., Perugorria M.J., Mertens J., Bjorkstrom N.K., Cramer T., Lleo A. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):63–78. doi: 10.1111/liv.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue K., Makuuchi M., Takayama T., Torzilli G., Yamamoto J., Shimada K. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498–505. doi: 10.1067/msy.2000.104673. [DOI] [PubMed] [Google Scholar]
- 107.Zhang M., Yang H., Wan L., Wang Z., Wang H., Ge C. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 108.Cadamuro M., Morton S.D., Strazzabosco M., Fabris L. Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies. Transl Gastrointest Cancer. 2013;2:130–144. doi: 10.3978/j.issn.2224-4778.2013.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mertens J.C., Fingas C.D., Christensen J.D., Smoot R.L., Bronk S.F., Werneburg N.W. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alvaro D., Barbaro B., Franchitto A., Onori P., Glaser S.S., Alpini G. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–888. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cadamuro M., Brivio S., Mertens J., Vismara M., Moncsek A., Milani C. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70:700–709. doi: 10.1016/j.jhep.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Obulkasim H., Shi X., Wang J., Li J., Dai B., Wu P. Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncol Lett. 2018;15:137–146. doi: 10.3892/ol.2017.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roy S., Glaser S., Chakraborty S. Inflammation and progression of cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. Front Med (Lausanne) 2019;6:293. doi: 10.3389/fmed.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao R., Chang Y., Liu Z., Liu Y., Guo S., Yu J. Effect of vascular endothelial growth factor-C expression on lymph node metastasis in human cholangiocarcinoma. Oncol Lett. 2015;10:1011–1015. doi: 10.3892/ol.2015.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aishima S., Nishihara Y., Iguchi T., Taguchi K., Taketomi A., Maehara Y. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21:256–264. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 116.Mantovani A., Germano G., Marchesi F., Locatelli M., Biswas S.K. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 117.Hasita H., Komohara Y., Okabe H., Masuda T., Ohnishi K., Lei X.F. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sica A., Allavena P., Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 119.Thanee M., Loilome W., Techasen A., Namwat N., Boonmars T., Pairojkul C. Quantitative changes in tumor-associated M2 macrophages characterize cholangiocarcinoma and their association with metastasis. Asian Pac J Cancer Prev. 2015;16:3043–3050. doi: 10.7314/apjcp.2015.16.7.3043. [DOI] [PubMed] [Google Scholar]
- 120.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 121.Martin-Sierra C., Martins R., Laranjeira P., Abrantes A.M., Oliveira R.C., Tralhao J.G. Functional impairment of circulating FcepsilonRI(+) monocytes and myeloid dendritic cells in hepatocellular carcinoma and cholangiocarcinoma patients. Cytometry B Clin Cytom. 2019;96:490–495. doi: 10.1002/cyto.b.21777. [DOI] [PubMed] [Google Scholar]
- 122.Loeuillard E., Conboy C.B., Gores G.J., Rizvi S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019;1:297–311. doi: 10.1016/j.jhepr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lim Y.J., Koh J., Kim K., Chie E.K., Kim B., Lee K.B. High ratio of programmed cell death protein 1 (PD-1)(+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol. 2015;117:165–170. doi: 10.1016/j.radonc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Takeya M., Komohara Y. Role of tumor-associated macrophages in human malignancies: friend or foe? Pathol Int. 2016;66:491–505. doi: 10.1111/pin.12440. [DOI] [PubMed] [Google Scholar]
- 125.Mizrahi J.D., Overman M.J. Bevacizumab as a chemoprotectant: reducing oxaliplatin induced hepatic sinusoidal injury. Oncotarget. 2018;9:34857–34858. doi: 10.18632/oncotarget.26207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arkenau H.T., Martin-Liberal J., Calvo E., Penel N., Krebs M.G., Herbst R.S. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF) Oncologist. 2018;23:1407. doi: 10.1634/theoncologist.2018-0044. e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kudo M. A paradigm change in the treatment strategy for hepatocellular carcinoma. Liver Cancer. 2020;9:367–377. doi: 10.1159/000507934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tao Y., Kim J., Yin Y., Zafar I., Falk S., He Z. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int. 2007;72:1358–1366. doi: 10.1038/sj.ki.5002550. [DOI] [PubMed] [Google Scholar]
- 129.Fiorotto R., Amenduni M., Mariotti V., Fabris L., Spirli C., Strazzabosco M. Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta Mol Basis Dis. 2019;1865:920–928. doi: 10.1016/j.bbadis.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taura K., De Minicis S., Seki E., Hatano E., Iwaisako K., Osterreicher C.H. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 131.Kim Y.J., Lee E.S., Kim S.H., Lee H.Y., Noh S.M., Kang D.Y. Inhibitory effects of rapamycin on the different stages of hepatic fibrosis. World J Gastroenterol. 2014;20:7452–7460. doi: 10.3748/wjg.v20.i23.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mejias M., Garcia-Pras E., Tiani C., Miquel R., Bosch J., Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 133.Tugues S., Fernandez-Varo G., Munoz-Luque J., Ros J., Arroyo V., Rodes J. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 134.Xu M., Xu H.H., Lin Y., Sun X., Wang L.J., Fang Z.P. LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell. 2019;178:1478–1492 e1420. doi: 10.1016/j.cell.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 135.Carpenter B., Lin Y., Stoll S., Raffai R.L., McCuskey R., Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development. 2005;132:3293–3303. doi: 10.1242/dev.01902. [DOI] [PubMed] [Google Scholar]
- 136.Fernandez M., Semela D., Bruix J., Colle I., Pinzani M., Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 137.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 138.Lee J.S., Semela D., Iredale J., Shah V.H. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 139.Coulon S., Heindryckx F., Geerts A., Van Steenkiste C., Colle I., Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 140.Kantari-Mimoun C., Castells M., Klose R., Meinecke A.K., Lemberger U.J., Rautou P.E. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology. 2015;61:2042–2055. doi: 10.1002/hep.27635. [DOI] [PubMed] [Google Scholar]
- 141.Jha S.K., Rauniyar K., Jeltsch M. Key molecules in lymphatic development, function, and identification. Ann Anat. 2018;219:25–34. doi: 10.1016/j.aanat.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Temmerman F., Chen F., Libbrecht L., Vander Elst I., Windmolders P., Feng Y. Everolimus halts hepatic cystogenesis in a rodent model of polycystic-liver-disease. World J Gastroenterol. 2017;23:5499–5507. doi: 10.3748/wjg.v23.i30.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu M., Wahl P.R., Le Hir M., Wackerle-Men Y., Wuthrich R.P., Serra A.L. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–259. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 144.Walz G., Budde K., Mannaa M., Nurnberger J., Wanner C., Sommerer C. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 145.Amura C.R., Brodsky K.S., Groff R., Gattone V.H., Voelkel N.F., Doctor R.B. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/-) mice. Am J Physiol Cel Physiol. 2007;293:C419–428. doi: 10.1152/ajpcell.00038.2007. [DOI] [PubMed] [Google Scholar]
- 146.Qian Q., Du H., King B.F., Kumar S., Dean P.G., Cosio F.G. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–638. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshiji H., Kuriyama S., Yoshii J., Ikenaka Y., Noguchi R., Hicklin D.J. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347–1354. doi: 10.1136/gut.52.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy L., Francis H., Invernizzi P., Venter J., Wu N., Carbone M. Secretin/secretin receptor signaling mediates biliary damage and liver fibrosis in early-stage primary biliary cholangitis. FASEB J. 2019;33:10269–10279. doi: 10.1096/fj.201802606R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen L., Zhou T., White T., O'Brien A., Chakraborty S., Liangpunsakul S. The apelin-apelin receptor axis triggers cholangiocyte proliferation and liver fibrosis during mouse models of cholestasis. Hepatology. 2020 doi: 10.1002/hep.31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takahashi H., Ojima H., Shimizu H., Furse J., Furukawa H., Shibata T. Axitinib (AG-013736), an oral specific VEGFR TKI, shows potential therapeutic utility against cholangiocarcinoma. Jpn J Clin Oncol. 2014;44:570–578. doi: 10.1093/jjco/hyu045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.