Abstract
Brunner’s gland hamartoma is a benign tumor of the duodenum, but has malignant potential with a very low risk of progression into adenocarcinoma. It is uncommon with a frequency of less than 1.0% among the primary tumors of the small intestine. In addition, its clinical manifestations are nonspecific, etiology remains unclear, and treatment strategy needs to be further refined. This literature review mainly discusses the epidemiology, clinical features, possible etiology and pathogenesis, diagnostic methods, malignant potential, treatment, and prognosis of Brunner’s gland hamartoma.
Keywords: Brunner’s gland, Duodenum, Etiology, Hamartoma, Hyperplasia
Key Summary Points
| Why carry out this study? |
| Duodenal Brunner’s gland hamartoma is a rare benign tumor, which accounts for less than 1% of the primary tumors of the small intestine, and usually does not produce clinical symptoms. |
| Its clinical manifestations are nonspecific, etiology remains unclear, and treatment strategy needs to be further refined. |
| Endoscopic biopsies are mostly negative, because the mass is often covered by intact duodenal mucosa, and the depth of biopsy is usually insufficient to reach the tumor tissue located in the submucosa. |
| This lesion has been insufficiently recognized. |
| What was learned from the study? |
| Brunner’s gland hamartoma often refers to a benign proliferative lesion of the duodenum. |
| Underlying risk factors of Brunner’s gland hamartoma include high gastric acid secretion, Helicobacter pylori infection, chronic pancreatitis, inflammatory stimulation, mucosal injury, etc. |
| Endoscopic ultrasonographic features are as follows: mucosal and submucosal involvement, variable echogenicity (sometimes mixed with hypoechoic), and multiple cystic changes inside the tumor. Endoscopic ultrasonography-guided fine-needle aspiration can improve the diagnostic accuracy of Brunner’s gland hamartoma. |
| With the growth of benign proliferative lesions of Brunner’s glands, mucosal ulcers may develop, thereby leading to the repair of gastric foveolar metaplasia with papillary architecture and then malignant transformation. |
| For asymptomatic patients with Brunner’s gland hamartoma, conservative treatment of small lesions is acceptable, while excision of large lesions is recommended to prevent bleeding and obstruction. For symptomatic patients, endoscopic or surgical resection should be considered. |
Digital Features
This article is published with digital features, including a summary slide and slide deck, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.14406650.
Introduction
Brunner’s glands are branched acinotubular glands which are mainly located in the deep mucosal or submucosal layers of the proximal duodemum [1] and their size and number are remarkably decreased at the distal duodenum [2, 3]. In some cases, Brunner’s glands extend to the proximal jejunum [4]. The main function of Brunner’s glands is to secrete alkaline substances and bicarbonate to neutralize acidic chyme and gastric acid in the stomach, and to produce and secrete urinary suppressant to inhibit gastric acid secretion [5], which can protect the integrity of duodenal mucosal epithelium and maintain an alkaline environment in the small intestine for intestinal absorption [6]. Brunner’s glands appear from the 13th to 14th weeks of embryonic development [2, 3]. Prevalence of Brunner’s glands in the duodenum is decreasing from 55.0% in infancy to 35.0% in persons aged 50 years old [3].
Brunner’s gland hyperplasia and hamartoma are benign proliferative lesions of the duodenum. Brunner’s gland hyperplasia is mostly a lesion of less than 0.5 cm, which is characterized as neutral mucin-containing glands expanding at least 50.0% of duodenal mucosa in a biopsy specimen [1, 7]; by comparison, Brunner’s gland hamartoma, also called as Brunner’s gland adenoma, is usually a lesion of greater than 0.5 cm regardless of number of lesions [2, 6], which refers to these proliferative glands involving duodenal submucosa, mixed with cystically dilated glands and smooth muscle proliferation [8]. The fundamental difference between them lies in the admixture of other benign component (smooth muscle fibers) with glands to be qualified as “hamartoma”. A majority of Brunner’s gland hamartomas are isolated pedunculated polyps, and a minority of them are sessile polyps. Their diameter varies from 1.0 to 2.0 cm, rarely larger than 5.0 cm and even up to 12.0 cm [5, 9]. Most of the lesions are located at the proximal duodenum, and their occurrence gradually decreases with an increase in the distance from the pyloric ring: 57.0% in the duodenal bulb, 27.0% in the descending part, and 7.0% in the horizontal part [4]. Histologically, Brunner’s gland hyperplasia is a single or multiple nodular lesion of excessive Brunner’s glands separated by fibrous septa. Brunner’s gland hamartoma is an isolated mass, which contains a mixture of Brunner’s glands, ducts, smooth muscle, fibrous tissue, adipose tissue, lymphocytes, etc. [2, 10, 11]. In 1934, Dr. Feyrter for the first time classified these proliferative lesions of Brunner’s glands into three types: type 1, diffuse nodular hyperplasia with sessile projections distributed among most of the duodenal area; type 2, circumscribed nodular hyperplasia with sessile projections limited to the duodenal bulb; and type 3, glandular adenoma with pedunculated or sessile polypoid mass. However, it remains unclear whether all three types undergo the same pathological process [3, 12].
Epidemiology
Brunner’s gland hamartoma is rare with a frequency of 5.0–10.0% [3, 11, 13] in benign duodenal tumors and less than 1.0% [14] in primary tumors of the small intestine. Among the patients who undergo routine esophagogastroduodenoscopy (EGD) examination, Brunner’s gland hamartoma can be found in 0.01–0.07% [14] and Brunner’s gland hyperplasia in 0.3% [15]. Brunner’s gland hamartoma has no remarkable preference for race or gender [3], but it is more common in people between 50 and 70 years old [3, 5].
Etiology and Pathogenesis
The etiology and pathogenesis of Brunner’s gland hamartoma remain unclear. The most plausible hypothesis holds that the essence of hamartoma is embryonic dysplasia of the duodenum [9, 16]. Other underlying risk factors include high gastric acid secretion, Helicobacter pylori (Hp) infection, chronic pancreatitis, inflammatory stimulation, mucosal injury, etc. (Fig. 1).
Fig. 1.
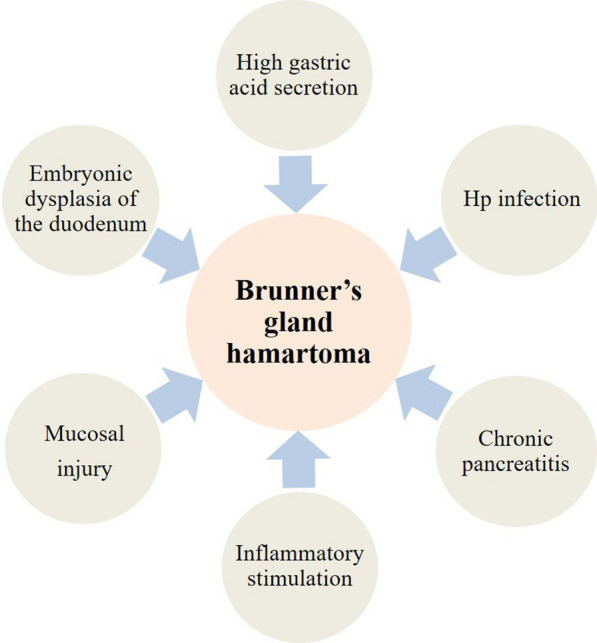
Underlying risk factors of Brunner’s gland hamartoma
It is often believed that high gastric acid secretion can stimulate glandular hyperplasia, considering that Brunner’s glands can secrete alkaline mucus [3, 5, 17]. Among the patients with duodenal ulcer, Brunner’s glands usually become proliferatively thickened, especially near the ulcers [18], suggesting that excessive secretion of gastric acid may play a role in the pathogenesis of Brunner’s gland hamartoma. However, the conclusion is a bit controversial. A study involving 20 patients with confirmed Brunner’s gland hamartoma or hyperplasia revealed that only 45.0% of these patients had hyperchlorhydria [19]. In addition, acid inhibitors could not eliminate this lesion [20]. Therefore, the causal relationship between high gastric acid secretion and these lesions should be further confirmed.
Another hypothesis is that Hp infection may contribute to the pathogenesis of Brunner’s gland hamartoma. Three studies found that patients with Brunner’s gland hyperplasia or hamartoma have a high positive Hp infection rate of 56.6–71.0% [21–23], but another study reported that none had Hp infection [10] (Table 1). Thus, it is necessary to further analyze the relationship of Hp infection with Brunner’s gland hamartoma.
Table 1.
Helicobacter pylori (Hp) infection in Brunner's gland proliferating lesions
| First author (year) |
Number of patients in total | Hp infection (+) | |
|---|---|---|---|
| Number of patients | Frequency | ||
| Destek (2019) | 18 | 12 | 67.0% |
| Kim (2012) | 25 | 0 | 0 |
| Sakurai (2005) | 129 | 73 | 56.6% |
| Kovacević (2001) | 7 | 5 | 71.0% |
Stolte et al. [24] put forward chronic pancreatitis as a contributing factor of Brunner’s gland hyperplasia in 1981. Pathological analyses found that 75.7% of patients with chronic pancreatitis had diffuse nodular hyperplasia of Brunner’s gland [24]. This might be attributed to adaptive response to pancreatic exocrine insufficiency [25]. In addition, Brunner’s gland hamartoma has vast lymphocyte infiltration on histology, which supports the “inflammatory hypothesis” that the lesion may be secondary to inflammatory stimulation [25]. But the presence of lymphocytes in the normal gastrointestinal submucosa compromises this hypothesis [5].
Brunner’s gland hamartoma is often associated with gastric foveolar metaplasia, which is an indispensable mechanism of mucosal repair in duodenal ulcer lesions. Therefore, it is considered that repeated mucosal injury activates mucosal repair and promotes the occurrence of this disease. Mechanical stimulation, Hp infection, and a highly acidic environment in the duodenum may cause mucosal injury together [26].
Collectively, this disease may be associated with multiple risk factors, which still need to be supported by more epidemiological evidence and strict pathological confirmation.
Clinical Manifestations
Most patients with Brunner’s gland hamartoma are asymptomatic, and the clinical manifestations of symptomatic patients are nonspecific, including dyspepsia, abdominal distension, abdominal pain, nausea, vomiting, gastrointestinal bleeding and obstruction, iron deficiency anemia, etc. [16, 17, 27] (Table 2). Among them, gastrointestinal bleeding and obstruction are the major causes for seeking medical treatment [28].
Table 2.
Main clinical features of patients with Brunner's gland hamartoma: an overview of literature
| First author (year) | Sex | Age (years) | Complaint | Location | Shape | Size (cm) | Diagnosis | Treatment | Complication | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Walden (1998) | Male | 28 | Melena, exertional dyspnea | Duodenal bulb | Pedunculated polyp | 4.0 × 2.5 × 2.0 | Brunner's gland hamartoma | Endoscopic resection | Uneventful | Asymptomatic with 14 months follow-up |
| Rocco (2006) | Female | 58 | Epigastric discomfort | Duodenal bulb | Pedunculated polyp | 3.0 × 4.0 | Brunner's gland hamartoma | Endoscopic resection | Uneventful | Asymptomatic with 6 months follow-up |
| Gao (2004) | Male | 32 | Epigastric discomfort, melena | Duodenal bulb | Pedunculated polyp | 3.5 × 3.0 × 2.0 | Brunner's gland hamartoma | Surgical resection | Uneventful | Asymptomatic at postoperative period |
| Rana (2019) | Male | 76 | Vomiting, melena | Duodenal descending | Pedunculated polyp with multiple ulcers on surface | 10.0–12.0 × 3.5 × 1.5 | Brunner's gland hamartoma | Endoscopic resection | NA | NA |
| Peloso (2017) | Male | 72 | Epigastric pain, vomiting | Duodenal descending-horizontal junction | Broad-based lesion | 4.0 | Brunner's gland hamartoma | Polyp excision via duodenotomy | Uneventful | Asymptomatic at postoperative period |
| Akaki (2014) | Male | 26 | Melena, anemia | Gastroduodenal junction | Pedunculated polyp | 6.4 × 3.0 | Brunner's gland hamartoma | Distal gastrectomy | Uneventful | Asymptomatic with 1 year follow-up |
| Tan (2002) | Male | 70 | Melena, anemia | Duodenal descending | Pedunculated polyp | 10.0 × 2.0 × 1.5 | Brunner's gland hamartoma | Laparotomy via a transduodenal approach | Uneventful | Asymptomatic with 2 years follow-up |
| Martinez (2014) | Male | 60 | Epigastric discomfort, vomiting | Duodenal bulb | Subepithelial mass | 3.0 × 4.0 | Brunner's gland hamartoma | Surgical resection | NA | NA |
| Hizawa (2002) | Female | 62 | NA | Duodenal bulb | Broad-based lesion | 0.7 | Brunner's gland hamartoma | Endoscopic resection | Uneventful | NA |
| Female | 71 | Broad-based lesion | 1.8 | |||||||
| Male | 63 | Sessile polyp | 1.5 | |||||||
| Male | 65 | Sessile polyp with surface dimples | 1.5 | |||||||
| Male | 34 | Sessile polyp with surface dimples | 2.0 | |||||||
| Female | 36 | Pedunculated polyp | 2.0 | |||||||
| Kostalas (2016) | Female | 52 | Mildly deranged liver-function tests, weight loss | Duodenal bulb and descending | A solid mass | NA | Brunner's gland hamartoma | Pancreatoduodenectomy | NA | NA |
| Petersen (2008) | Female | 56 | Epigastric pain, nausea, vomiting, weight loss, anorexia | Pylorus-duodenal descending | Submucosal long tubular mass | 8.5 × 7.0 | Brunner's gland hamartoma | Billroth I procedure | Uneventful | Asymptomatic at postoperative period |
| Gourtsoyiannis (1990) | Male | 74 | Melena, weakness, dizziness | Duodenal descending | Pedunculated polyp | 5.0 × 3.0 × 4.0 | Brunner's gland hamartoma | Surgical resection | NA | NA |
| Takeuchi (2015) | Female | 52 | A sticking sensation when eating | Duodenal bulb | Pedunculated polyp | 5.0 × 3.0 | Brunner's gland hamartoma | Laparoscopic partial duodenectomy | NA | NA |
| Male | 67 | Epigastric discomfort | 6.0 × 3.5 | Laparoscopic and endoscopic tumor resection | ||||||
| Female | 52 | Asymptomatic | 3.5 × 2.7 | Laparoscopic partial duodenectomy | ||||||
| Kitagawa (2018) | Female | 64 | Anemia | Duodenal bulb | Pedunculated polyp | 7.0 | Brunner's gland hamartoma | Endoscopic mucosal resection | NA | NA |
| Jung (2013) | Male | 45 | Melena | Pyloric ring | Pedunculated polyp | 4.8 × 3.2 | Brunner's gland hamartoma | Piecemeal endoscopic mucosal resection | NA | No relapse with 5 months follow-up |
NA not available
Melena and hematemesis may occur when there is ulceration or tumor vascular erosion, and these manifestations are related to the size and location of lesions [25]. The average size of hamartoma is 2.8 cm in patients who develop gastrointestinal bleeding [28]. Lesions located at descending and horizontal parts of the duodenum have a higher bleeding tendency as compared to those located at the bulb part, probably as a result of higher pressure from digestive tract movement and vascular damage in the descending and horizontal parts [28, 29].
Gastrointestinal obstruction occurs when the nodules of Brunner’s gland hyperplasia are diffuse or the size of a single hamartoma is large enough (the average diameter should be greater than 2.1 cm) [28], which can present with abdominal distension, abdominal pain, nausea, vomiting, and weight loss [1, 30]. Generally, it is more related to large hamartoma than diffuse hyperplasia. Krishnamurthy et al. [11] reviewed 16 cases with gastrointestinal obstruction caused by these lesions, and found that 81.3% (13/16) of them were from Brunner’s gland hamartoma and the remaining cases were from Brunner’s gland hyperplasia. There are also a few cases of diffuse nodular hyperplasia with involvement of pylorus causing pyloric obstruction [11, 13] and giant hamartoma causing gastroduodenal intussusception [31].
If Brunner’s gland hamartoma involved the duodenal ampulla, biliary obstruction would develop, which can present with jaundice, acute pancreatitis, and dilatation of the common bile duct and pancreatic duct [2, 3]. These lesions are similar to peri-ampullary or pancreatic malignancy [32].
Diagnostic Approaches
EGD can provide direct visualization and accurate location of Brunner’s gland hamartoma [25]. But there is still a missed diagnosis of this lesion on EGD, especially when it is located at the posterior wall of the duodenal bulb, transitional part, and the beginning of the descending part.
Endoscopic ultrasonography (EUS) has been increasingly used to evaluate the origin, extent, and vascular distribution of suspected lesions. On EUS, Brunner’s gland hamartoma is shown as inhomogeneous solid or cystic mass in the submucosa [25, 33]. There are some endoscopic ultrasonographic features, as follows: mucosal and submucosal involvement; variable echogenicity (sometimes mixed with hypoechoic); and multiple cystic changes inside the tumor [30, 34, 35]. EUS-guided fine-needle aspiration can improve the diagnostic accuracy, but needs high technical requirements for the operators [36–38].
Barium X-rays and computed tomography (CT) scans can be complementary approaches to decrease the rate of missed diagnoses. Barium X-ray examination is noninvasive and safe, but sometimes it may not be easy for small lesions. For larger lesions, the findings of Brunner’s gland hamartoma are nonspecific with smooth and sessile or pedunculated polypoid-filling defects in the duodenum [3, 5, 6] without evidence of duodenal wall stiffening [34]. Bleeding spots, erosion, or superficial ulcers on the surface of the lesion are rarely seen [39]. Hypotonic duodenography is considered to be appropriate to examine the changes of lesion surface [9, 39]. As for diffuse nodular Brunner’s gland hyperplasia, there are multiple small filling defects in the duodenum shown on barium X-ray examination [29].
Large Brunner’s gland hamartoma can be detected by CT [17]. CT is considered as the first choice in many cases. It is helpful to confirm the absence of extraluminal extension and to define its relationship with adjacent structures, such as pancreas, common bile duct, and blood vessels [25, 31]. The internal cysts and pedicles shown on CT may be conducive to a diagnosis of Brunner’s gland hamartoma, which are especially useful in patients who are intolerant to EGD [40]. A differential diagnosis of Brunner’s gland hamartoma should be made with isolated duodenal masses, such as leiomyoma, gastrointestinal stromal tumor, lymphoma, neuroendocrine tumor, pyloric mucosal prolapse, or Peutz-Jeghers polyps [21, 31, 34]. Brunner’s gland hamartoma should be considered if contrast-enhanced CT reveals some specific imaging features, including a mass with central low attenuation in the bulb and descending parts of the duodenum, circumjacent enhancement, and/or internal small cystic change [35], which indicate solid proliferation of Brunner’s gland, superficial duodenal mucosa, and internal cysts on histology, respectively [41].
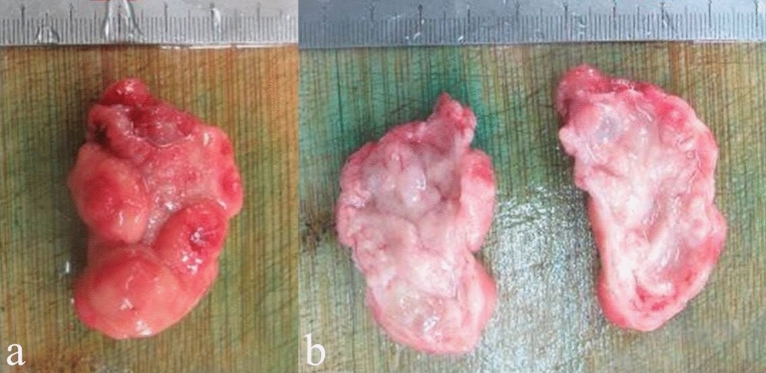
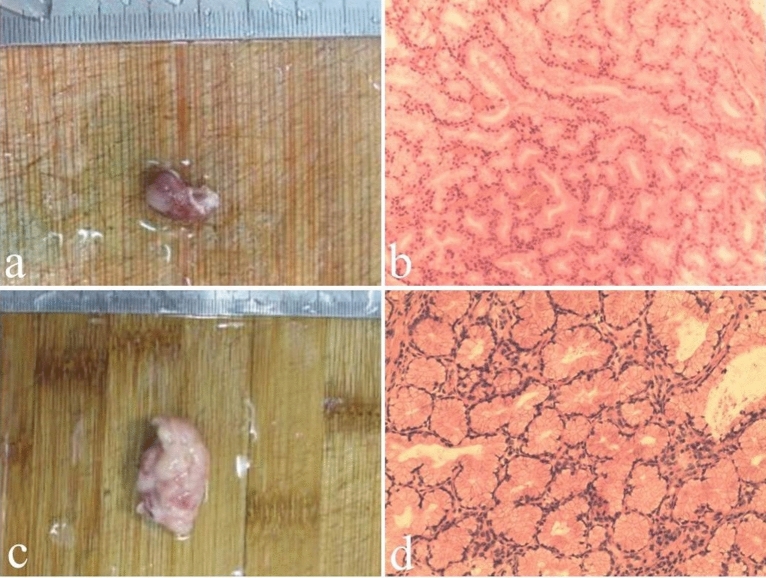
An exact diagnosis of Brunner’s gland hamartoma requires pathological evidence. On gross pathology (Fig. 2), Brunner’s gland hamartoma is generally characterized by a solid mass with well-defined boundary, smooth surface covered by normal duodenal mucosa, pink or tawny cut surface, lobules separated by fibrous septa, and internal cystic changes [6, 42]. Microscopically (Fig. 3), there is a mixture of smooth muscle, adipose tissues, large ducts, and infiltrating lymphocytes in the proliferative Brunner’s glands [1]. The cytoplasm of hyperplastic Brunner's gland cells is rich in neutral mucin, with small round nucleus located at their base in the absence of mitotic activity and atypia. Sclerotic glandular foci, which are characterized by a decreased number of Brunner’s glands with irregular structure and angulation, sparse cytoplasm, and centered nucleus, can be also seen [10]. Metachronous lesions of Brunner’s gland hamartoma have not been reported in the literature yet, but they can be observed in other gastrointestinal tract diseases, such as gastric epithelia dysplasia [43] and colorectal adenomas [44]. Immunohistochemically, Brunner’s glands always express MUC6 at different levels, and some dilated or angulated Brunner’s glands also express MUC5AC simultaneously. MUC5AC expression at the surface epithelium suggests a possibility of gastric metaplasia [10]. Notably, endoscopic biopsies are mostly negative, because the mass is often covered by intact duodenal mucosa, and the depth of biopsy is usually insufficient to reach the tumor tissue located in the submucosa [11].
Fig. 2.
Macroscopic specimen (a) and cut surface (b) of Brunner’s gland hamartoma
Fig. 3.
Macroscopic and microscopic findings of Brunner’s gland hamartoma. Case 1. A 55-year-old male patient presented with abdominal discomfort and underwent endoscopic examination showing a polyp in the duodenal bulb. a Endoscopically resected specimen of about 1.5 × 0.8 × 0.7 cm in size. b Histological examination revealing massive hyperplasia of Brunner’s glands with focal dysplasia (hematoxylin and eosin, × 100). Case 2. A 60-year-old male patient presented with melena and underwent endoscopic examination showing a polyp in the duodenal bulb with bleeding. c Endoscopically resected specimen of about 3.5 × 2.0 × 1.0 cm in size. d Histological examination revealing massive hyperplasia of Brunner’s glands mixed with smooth muscle and infiltrating inflammatory cells (hematoxylin and eosin, × 100)
If duodenal polyps were found on EGD, the physicians would differentiate the Brunner’s gland hamartoma from other types of multiple and sporadic polyps located at the duodenum according to the age of onset, distribution at the duodenum, endoscopic appearance, histological characteristics, and immunohistochemical markers (Table 3) [45–63]. Nearly all cases with familial adenomatous polyposis can be accompanied by duodenal adenomas, and some of them have extraintestinal manifestations, such as jaw and tooth abnormalities, nasopharyngeal angiofibromas, and cutaneous lesions (i.e., lipomas, fibromas, and sebaceous and epidermoid cysts) [58]. Similarly, duodenal adenomas are a relatively common manifestation of MUTYH-associated polyposis, in which cutaneous lesions, such as sebaceous gland adenomas, epitheliomas, and epithelial carcinomas, can be observed [64].
Table 3.
Different subtypes of duodenal polyposis and sporadic duodenal polyp
| Subtypes [references] |
Age of onset (years) | Common duodenal distribution | Endoscopic appearance | Histological characteristics | Immunohistochemical markers |
|---|---|---|---|---|---|
|
Brunner’s gland hamartoma |
50–70 | Duodenal bulb and descending | Pedunculated/sessile polyp | Mixture of Brunner’s glands, ducts, smooth muscle, fibrous tissue, adipose tissue, lymphocytes, etc. | MUC6 (+) |
|
Gastric heterotopia |
NA | Duodenal bulb and descending | Isolated or multiple submucosal masses | Lesions consisting of gastric glands covered by normal duodenal mucosa | β-catenin (+) |
|
Inflammatory fibroid polyp [42] |
50–80 | NA | Isolated polyp with smooth mucosa | Spindle-shaped cells proliferation with infiltration of small blood vessels and eosinophilic inflammation | Vimentin (+), CD34 (+) |
|
Lipoma |
50–80 | Duodenal descending | Isolated/rarely multiple, pedunculated/sessile, round/oval, and soft mass with normal surface mucosa, which may have areas of erosion or ulceration | Mature adipose tissue arranged in lobules | CD34 (+)a, desmin (−), S100 protein (−), STAT6 (−), SMA (−) |
|
Leiomyoma |
60–80 | NA | Lobular mass with a boundary that is well-defined/irregular/interdigitating with normal smooth muscle | Mature smooth muscle cells with hyaline degeneration, coagulative necrotic stroma, and low mitotic activity | SMA (+), desmin (+), S100 (−), Ki-67 (−), CD34 (−), HMB4 (−) |
|
Carcinoid |
No age predilection | Proximal duodenum | Intraluminal polypoid/mural mass | Endocrine secretion granules observed by a characteristic silver affinity | Serotonin (+), gastrin (+), somatostatin (+) |
|
Gastrointestinal stromal tumor |
50–65 | Duodenal descending | Smooth submucosal mass with ulceration and bleeding areas on the surface | Most are spindle cell tumors with palisade nuclei, half are mixed with skeinoid fibers, and more than 20.0% are accompanied by hemangioma-like vascular proliferation |
CD117 (+): 95.0% CD34 (+): 70.0% |
|
Lymphoma |
50–60 | Proximal duodenum | Multiple small, rough polyps or nodules | Different histological patterns: diffuse large B cell, mucosa-associated lymphoid tissue, mantle cell, and Hodgkin’s and follicular lymphoma | CD20 (+)b, CD10 (+), Bcl-2 (+), BCL6 (+), low Ki-67 index |
|
Non-ampullary sporadic adenoma |
60–90 | Distal duodenum | Isolated sessile polyp | Mostly tubular crypts with hyperchromatic, enlarged, and pseudostratified nuclei | Cytokeratin 7 (+), cytokeratin 20 (+) |
|
Familial adenomatous polyposis |
20–40 | Duodenal descending and horizontal, peri-ampullary | Multiple flat polyps | Tubular or tubulovillous crypts mixed with columnar epithelial cells, goblet cells, paneth cells, and endocrine cells, accompanied by enlarged and elongated hyperchromatic nuclei | Cytokeratin 7 (+), cytokeratin 20 (+) |
|
Peutz-Jeghers syndrome |
10–30 | NA | Isolated/multiple polypoid lesions | Branched villous structures containing smooth muscle core and multiple types of cells | Serotonin (+) |
|
Solitary Peutz-Jeghers polyp |
NA | NA | Isolated, pedunculated/rarely sessile, polypoid lesion | Branched villous structures containing smooth muscle core and multiple types of cells | Serotonin (+) |
NA not available
aThe immunohistochemical marker we describe here is the histologic pattern of spindle cell/pleomorphic lipoma
bThe immunohistochemical marker we describe here is the histologic pattern of follicular lymphoma
Malignant Potential
Brunner’s gland hamartoma or hyperplasia is usually benign [3]. However, with the growth of benign proliferative lesions of Brunner’s glands, mucosal ulcers may develop, thereby leading to the repair of gastric foveolar metaplasia with papillary architecture and then malignant transformation [22]. It has been reported that 2.1% of the 722 Brunner’s gland hyperplasia lesions evaluated had dysplasia and 0.3% invasive carcinoma [22].
Histological characteristics of dysplasia often include (1) crowded glands with slight distortion of architecture; (2) atypia cells, expanded and overlapping nuclei, and high mitotic activity on cytology; and (3) sporadically positive p53, high expression level of Ki-67/MIB-1 [10, 65].
There are several signs warning a potential malignant transformation of Brunner’s gland hamartoma. First, the size of polypoid lesions increases with a change in their morphology. Itsuno et al. reported that the lesion of Brunner’s gland hamartoma progressed from sessile polypoid to fungating ulcerated tumor during a 3-year endoscopic follow-up, its size increased from 1.4 × 1.0 cm to 4.3 × 3.1 cm, and it was finally diagnosed as adenocarcinoma [66]. Second, the submucosal tumor-like lesion is accompanied by a shallow central depression. A retrospective analysis including 25 cases with duodenal carcinoma arising from Brunner’s glands found that 13 of them had submucosal tumor-like lesions accompanied by a shallow central depression [67].
Treatment and Prognosis
For asymptomatic patients with Brunner’s gland hamartoma, conservative treatment of small lesions is acceptable, while excision of large lesions is recommended to prevent bleeding and obstruction [68]. For symptomatic patients, endoscopic or surgical resection should be considered [13].
Endoscopic resection of Brunner’s gland hamartoma has been increasingly employed [5]. Its technical success is related to the size, location, and pedicle of the lesions [1]. Its advantages include low invasiveness, high safety, low cost, and short duration of hospitalization [5, 29]. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are major endoscopic resection options for superficial non-ampullary duodenal epithelial tumors. ESD can completely remove the lesions, but has a higher risk of intraoperative and delayed perforation than EMR [69]. Such a higher incidence of delayed perforation after ESD may be due to the large ulceration produced by ESD as well as chemical stimulation of pancreatic juice and bile [34, 69]. Over-the-scope clips [70] and polyglycolic acid sheets combined with fibrin glue [71] can prevent delayed perforation by completely closing the mucosal defect.
Endoscopic resection of a giant pedunculated Brunner’s gland hamartoma is often challenging. Notably, the duodenal cavity is narrow with poor visibility. Additionally, the intestinal peristalsis can carry the mass to the distal end [4]. Therefore, it has often been considered that the head of the tumor can be pulled into the gastric antrum with a snare for further resection [4]. If the tumor is too large to pass through the pyloric ring, piecemeal EMR can be selected [68]. If the specimen is inadvertently lost to the distal duodenum, magnesium citrate can contribute to its fast passage in the stool before degradation [4].
Surgical resection, such as polypectomy, wedge duodenal resection, and partial duodenectomy plus gastrectomy, is required for complex lesions and large/sessile tumors [12]. Pancreaticoduodenectomy is usually considered for giant hamartoma and diffuse nodular hyperplasia lesions, which imitate the nature of malignancy in the pancreatic-duodenal region [27]. This consideration is potentially reasonable, because the consequence of missing an undiagnosed pancreatic cancer is much more serious than the risk of radical surgery [1, 12].
The most common treatment option for Brunner’s gland adenocarcinoma of the duodenum is pancreaticoduodenectomy, followed by partial duodenectomy plus gastrectomy, partial duodenectomy, and endoscopic resection [72]. Recurrence is rare after endoscopic or surgical treatment [3, 30].
Conclusions
Brunner’s gland hamartoma is an uncommon benign tumor of the duodenum with non-specific clinical manifestations. Its mechanisms remain unclear, but may be related to high gastric acid secretion, Hp infection, chronic pancreatitis, inflammatory stimulation, and mucosal injury. Malignant transformation should be cautiously evaluated by histology combined with immunohistochemistry, especially if the size of lesion is increased and its morphology is changed. A wait-and-see strategy is employed in a majority of cases with Brunner’s gland hamartoma. If necessary, endoscopic and/or surgical resection is required. In future, it is worthwhile to carry out experimental studies to further explore the molecular characteristics of Brunner’s gland hamartoma and adenocarcinoma and to determine the potential targets for chemoprevention and regression of these lesions.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The first draft of the manuscript was written by Menghua Zhu and Xingshun Qi; Study conception and design, material preparation, data collection, and writing–review and editing were performed by all authors, including Menghua Zhu, Hongyu Li, Yanyan Wu, Yang An, Yuye Wang, Chun Ye, Dan Zhang, Rui Ma, Xuehan Wang, Xiaodong Shao, Xiaozhong Guo, and Xingshun Qi; All authors contributed to the manuscript. All authors read and approved the final manuscript.
Disclosures
Menghua Zhu, Hongyu Li, Yanyan Wu, Yang An, Yuye Wang, Chun Ye, Dan Zhang, Rui Ma, Xuehan Wang, Xiaodong Shao, Xiaozhong Guo, and Xingshun Qi have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Menghua Zhu and Hongyu Li are co-first authors.
Contributor Information
Xiaodong Shao, Email: sxdsys608@189.cn.
Xiaozhong Guo, Email: guo_xiao_zhong@126.com.
Xingshun Qi, Email: xingshunqi@126.com.
References
- 1.Dhouha B, Ahlem L, Sana BS, et al. Unexpected cause for duodenal obstruction: Brunner's gland hyperplasia. Pathologica. 2017;109(4):414–417. [PubMed] [Google Scholar]
- 2.Mayoral W, Salcedo JA, Montgomery E, et al. Biliary obstruction and pancreatitis caused by Brunner's gland hyperplasia of the ampulla of Vater: a case report and review of the literature. Endoscopy. 2000;32(12):998–1001. doi: 10.1055/s-2000-9623. [DOI] [PubMed] [Google Scholar]
- 3.Abbass R, Al-Kawas FH. Brunner gland hamartoma. Gastroenterol Hepatol (NY) 2008;4(7):473–475. [PMC free article] [PubMed] [Google Scholar]
- 4.Walden DT, Marcon NE. Endoscopic injection and polypectomy for bleeding Brunner's gland hamartoma: case report and expanded literature review. Gastrointest Endosc. 1998;47(5):403–407. doi: 10.1016/s0016-5107(98)70228-7. [DOI] [PubMed] [Google Scholar]
- 5.Rocco A, Borriello P, Compare D, et al. Large Brunner's gland adenoma: case report and literature review. World J Gastroenterol. 2006;12(12):1966–1968. doi: 10.3748/wjg.v12.i12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel ND, Levy AD, Mehrotra AK, et al. Brunner's gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation. AJR Am J Roentgenol. 2006;187(3):715–722. doi: 10.2214/AJR.05.0564. [DOI] [PubMed] [Google Scholar]
- 7.Chong KC, Cheah WK, Lenzi JE, et al. Benign duodenal tumors. Hepatogastroenterology. 2000;47(35):1298–1300. [PubMed] [Google Scholar]
- 8.Brosens LAA, Jansen M, Giardiello FM, et al. Polyps of the small intestine. Diagn Histopathol. 2011;17(2):69–79. [Google Scholar]
- 9.Gao YP, Zhu JS, Zheng WJ. Brunner's gland adenoma of duodenum: a case report and literature review. World J Gastroenterol. 2004;10(17):2616–2617. doi: 10.3748/wjg.v10.i17.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Jang SJ, Song HJ, et al. Clinicopathologic characteristics and mucin expression in Brunner's gland proliferating lesions. Dig Dis Sci. 2013;58(1):194–201. doi: 10.1007/s10620-012-2320-3. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurthy P, Junaid O, Moezzi J, et al. Gastric outlet obstruction caused by Brunner's gland hyperplasia: case report and review of literature. Gastrointest Endosc. 2006;64(3):464–467. doi: 10.1016/j.gie.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Iusco D, Roncoroni L, Violi V, et al. Brunner's gland hamartoma: 'over-treatment' of a voluminous mass simulating a malignancy of the pancreatic-duodenal area. Jop. 2005;6(4):348–353. [PubMed] [Google Scholar]
- 13.Bakir MA, AlYousef MY, Alsohaibani FI, et al. Brunner's glands hamartoma with pylorus obstruction: a case report and review of literature. J Surg Case Rep. 2020;8:rjaa191. doi: 10.1093/jscr/rjaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botsford TW, Crowe P, Crocker DW. Tumors of the small intestine. A review of experience with 115 cases including a report of a rare case of malignant hemangio-endothelioma. Am J Surg. 1962;103:358–365. doi: 10.1016/0002-9610(62)90226-x. [DOI] [PubMed] [Google Scholar]
- 15.Jung SH, Chung WC, Kim EJ, et al. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2010;16(43):5474–5480. doi: 10.3748/wjg.v16.i43.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana R, Sapkota R, Kc B, et al. Giant Brunner's gland adenoma presenting as upper gastrointestinal bleeding in 76 years old male: a case report. JNMA J Nepal Med Assoc. 2019;57(215):50–52. doi: 10.31729/jnma.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojanapu S, Mangla V, Mehrotra S, et al. Brunner's gland hyperplasia: an unusual duodenal submucosal lesion seen in four patients. J Surg Case Rep. 2018;11:riy305. doi: 10.1093/jscr/rjy305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuse Y, Tsuchihashi Y, Takamasu M, et al. Thickness of Brunner's glands and its clinical significance in duodenal ulcer disease. Scand J Gastroenterol. 1990;25(2):165–172. doi: 10.3109/00365529009107939. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Dyson WL, Fitts WT., Jr The relationship of gastric hyperacidity to hyperplasia of Brunner's glands. Arch Surg. 1969;98(5):636–639. doi: 10.1001/archsurg.1969.01340110128016. [DOI] [PubMed] [Google Scholar]
- 20.Spellberg MA, Vucelic B. A case of Brunner's glands hyperplasia with diarrhea responsive to cimetidine. Am J Gastroenterol. 1980;73(6):519–522. [PubMed] [Google Scholar]
- 21.Destek S, Gul VO. Brunner's gland hyperplasias and hamartomas in association with Helicobacter pylori. Can J Gastroenterol Hepatol. 2019;2019(6340565):6. doi: 10.1155/2019/6340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai T, Sakashita H, Honjo G, et al. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: possible precursor lesions for Brunner gland adenocarcinoma. Am J Surg Pathol. 2005;29(11):1442–1448. doi: 10.1097/01.pas.0000180449.15827.88. [DOI] [PubMed] [Google Scholar]
- 23.Kovacević I, Ljubicić N, Cupić H, et al. Helicobacter pylori infection in patients with Brunner's gland adenoma. Acta Med Croatica. 2001;55(4–5):157–160. [PubMed] [Google Scholar]
- 24.Stolte M, Schwabe H, Prestele H. Relationship between diseases of the pancreas and hyperplasia of Brunner's glands. Virchows Arch A Pathol Anat Histol. 1981;394(1–2):75–87. doi: 10.1007/BF00431666. [DOI] [PubMed] [Google Scholar]
- 25.Peloso A, Viganò J, Vanoli A, et al. Saving from unnecessary pancreaticoduodenectomy. Brunner's gland hamartoma: case report on a rare duodenal lesion and exhaustive literature review. Ann Med Surg Lond. 2017;17:43–49. doi: 10.1016/j.amsu.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akaki M, Taniguchi S, Hatakeyama K, et al. Duodenal mucosal damage is associated with proliferative activity of Brunner's gland hamartoma: a case report. BMC Gastroenterol. 2014;14:14. doi: 10.1186/1471-230X-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WC, Yang HW, Lee YJ, et al. Brunner's gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area. J Korean Med Sci. 2008;23(3):540–543. doi: 10.3346/jkms.2008.23.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine JA, Burgart LJ, Batts KP, et al. Brunner's gland hamartomas: clinical presentation and pathological features of 27 cases. Am J Gastroenterol. 1995;90(2):290–294. [PubMed] [Google Scholar]
- 29.Tan YM, Wong WK. Giant Brunneroma as an unusual cause of upper gastrointestinal hemorrhage: report of a case. Surg Today. 2002;32(10):910–912. doi: 10.1007/s005950200179. [DOI] [PubMed] [Google Scholar]
- 30.Martinez MA, Zyromski NJ, Luz LP. An unusual case of large symptomatic Brunner's gland adenoma: endoscopic ultrasound imaging. Endosc Ultrasound. 2015;4(3):266–267. doi: 10.4103/2303-9027.163021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen JM, Felger TS, Goldstein JD. Gastroduodenal intussusception secondary to a giant brunner gland hamartoma. Gastroenterol Hepatol (NY) 2008;4(7):471–473. [PMC free article] [PubMed] [Google Scholar]
- 32.Kostalas M, Jackson P, Karanjia N. Brunner's gland hamartoma: a cause of the double-duct sign. Ann R Coll Surg Engl. 2016;98(6):e92–e93. doi: 10.1308/rcsann.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hizawa K, Iwai K, Esaki M, et al. Endosonographic features of Brunner's gland hamartomas which were subsequently resected endoscopically. Endoscopy. 2002;34(12):956–958. doi: 10.1055/s-2002-35849. [DOI] [PubMed] [Google Scholar]
- 34.Satoh T, Matsubayashi H, Takizawa K, et al. Giant Brunner's gland hyperplasia of the duodenum diagnosed by endoscopic ultrasonography-guided fine needle biopsy and treated by laparoscopic endoscopic cooperative surgery. Intern Med. 2019;58(14):2009–2013. doi: 10.2169/internalmedicine.2477-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou SR, Ullah S, Liu YY, et al. Brunner's gland adenoma: lessons learned for 48 cases. Digest Liver Dis. 2021;53(1):134–136. doi: 10.1016/j.dld.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: clinical aspects to improve the diagnosis. World J Gastroenterol. 2016;22(2):628–640. doi: 10.3748/wjg.v22.i2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang JY, Hebert-Magee S, Navaneethan U, et al. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87(6):1432–1438. doi: 10.1016/j.gie.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Cotton J, Bavi P, Ghorab Z et al. Pre-operative diagnosis of a Brunner's gland hamartoma by endoscopic ultrasound-guided fine needle aspiration. Cytopathology. 2021. In Press. [DOI] [PubMed]
- 39.Gourtsoyiannis NC, Zarifi M, Gallis P, et al. Radiologic appearances of Brunner's gland adenoma: a case report. Eur J Radiol. 1990;11(3):188–190. doi: 10.1016/0720-048x(90)90053-e. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi M, Cho H, Sugimoto M, et al. CT and MRI findings for Brunner's gland hamartoma: report of three cases. Jpn J Radiol. 2015;33(6):375–379. doi: 10.1007/s11604-015-0425-2. [DOI] [PubMed] [Google Scholar]
- 41.Hur S, Han JK, Kim MA, et al. Brunner's gland hamartoma: computed tomographic findings with histopathologic correlation in 9 cases. J Comput Assist Tomogr. 2010;34(4):543–547. doi: 10.1097/RCT.0b013e3181d472dc. [DOI] [PubMed] [Google Scholar]
- 42.Kitagawa Y, Osumi H, Kawachi H, et al. Giant duodenal Brunner's gland hamartoma successfully treated via endoscopic mucosal resection. Arab J Gastroenterol. 2018;19(3):125–129. doi: 10.1016/j.ajg.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Park WY, Lee SJ, Kim YK, et al. Occurrence of metachronous or synchronous lesions after endoscopic treatment of gastric epithelia dysplasia- impact of histologic features of background mucosa. Pathol Res Pract. 2018;214(1):95–99. doi: 10.1016/j.prp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Laish I, Sergeev I, Stein A, et al. Risk of metachronous advanced lesions after resection of diminutive and small, non-advanced adenomas. Clin Res Hepatol Gastroenterol. 2019;43(2):201–207. doi: 10.1016/j.clinre.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Eguchi K, Aoyagi K, Nimura S, et al. Diagnostic value of endoscopic and endoscopic ultrasound characteristics of duodenal submucosal tumour-like heterotopic gastric mucosa. Can J Gastroenterol. 2011;25(7):365–367. doi: 10.1155/2011/104815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakagawa M, Kitazawa R, Kondo T, et al. Duodenal gastric heterotopia, sporadic or fundic gland polyp-associated, frequently carries β-catenin mutation. Virchows Archiv Int J Pathol. 2014;465(3):253–256. doi: 10.1007/s00428-014-1612-8. [DOI] [PubMed] [Google Scholar]
- 47.Culver EL, McIntyre AS. Sporadic duodenal polyps: classification, investigation, and management. Endoscopy. 2011;43(2):144–155. doi: 10.1055/s-0030-1255925. [DOI] [PubMed] [Google Scholar]
- 48.Pei MW, Hu MR, Chen WB, et al. Diagnosis and treatment of duodenal lipoma: a systematic review and a case report. J Clin Diagn Res. 2017;11(7):Pe01–pe5. doi: 10.7860/JCDR/2017/27748.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zirpe D, Wani M, Tiwari P, et al. Duodenal lipomatosis as a curious cause of upper gastrointestinal bleed: a report with review of literature. J Clin Diagn Res. 2016;10(5):Pe01–4. doi: 10.7860/JCDR/2016/19851.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wroński K, Kaczor J, Masłowski Z. Duodenal leiomyoma: a rare case report. Magy Seb. 2017;70(3):232–234. doi: 10.1556/1046.70.2017.3.3. [DOI] [PubMed] [Google Scholar]
- 51.Rice DC, Bakaeen F, Farley DR, et al. Surgical management of duodenal leiomyomas. World J Surg. 2001;25(5):562–566. doi: 10.1007/s002680020083. [DOI] [PubMed] [Google Scholar]
- 52.Levy AD, Taylor LD, Abbott RM, et al. Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology. 2005;237(3):967–972. doi: 10.1148/radiol.2373041863. [DOI] [PubMed] [Google Scholar]
- 53.Chejfec G, Falkmer S, Askensten U, et al. Neuroendocrine tumors of the gastrointestinal tract. Pathol Res Pract. 1988;183(2):143–154. doi: 10.1016/S0344-0338(88)80042-6. [DOI] [PubMed] [Google Scholar]
- 54.Taskovska M, Omejc M, Grosek J. Small gastrointestinal stromal tumour of the duodenum causing a life-threatening bleeding: a case report and review of the literature. Int J Surg Case Rep. 2019;57:160–162. doi: 10.1016/j.ijscr.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Weindorf SC, Smith LB, Owens SR. Update on gastrointestinal lymphomas. Arch Pathol Lab Med. 2018;142(11):1347–1351. doi: 10.5858/arpa.2018-0275-RA. [DOI] [PubMed] [Google Scholar]
- 57.Wagner PL, Chen YT, Yantiss RK. Immunohistochemical and molecular features of sporadic and FAP-associated duodenal adenomas of the ampullary and nonampullary mucosa. Am J Surg Pathol. 2008;32(9):1388–1395. doi: 10.1097/PAS.0b013e3181723679. [DOI] [PubMed] [Google Scholar]
- 58.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zádorová Z, Hajer J, Mandys V. Multiple adenomatous duodenal polyposis. Case Rep Gastrointest Med. 2013;2013:181704. doi: 10.1155/2013/181704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan SX, Wang GH, Zhong J, et al. Peutz-Jeghers syndrome with intermittent upper intestinal obstruction: a case report and review of the literature. Medicine. 2017;96(17):e6538. doi: 10.1097/MD.0000000000006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Silva WS, Pathirana AA, Gamage BD, et al. Extra-ampullary Peutz-Jeghers polyp causing duodenal intussusception leading to biliary obstruction: a case report. J Med Case Rep. 2016;10:196. doi: 10.1186/s13256-016-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krstić M, Katić V, Stojnev S, et al. Peutz-Jeghers syndrome: quantitative study on enterochromaffin cells in hamartomatous intestine polyps. Srp Arh Celok Lek. 2013;141(9–10):602–607. doi: 10.2298/sarh1310602k. [DOI] [PubMed] [Google Scholar]
- 63.Iwamuro M, Aoyama Y, Suzuki S, et al. Long-term outcome in patients with a solitary Peutz-Jeghers polyp. Gastroenterol Res Pract. 2019;2019:8159072. doi: 10.1155/2019/8159072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampson JR, Jones N. MUTYH-associated polyposis. Best Pract Res Clin Gastroenterol. 2009;23(2):209–218. doi: 10.1016/j.bpg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Fujimaki E, Nakamura S, Sugai T, et al. Brunner's gland adenoma with a focus of p53-positive atypical glands. J Gastroenterol. 2000;35(2):155–158. doi: 10.1007/s005350050029. [DOI] [PubMed] [Google Scholar]
- 66.Itsuno M, Makiyama K, Omagari K, et al. Carcinoma of duodenal bulb arising from the Brunner's gland. Gastroenterol Jpn. 1993;28(1):118–125. doi: 10.1007/BF02775012. [DOI] [PubMed] [Google Scholar]
- 67.Ohta Y, Saitoh K, Akai T, et al. Early primary duodenal carcinoma arising from Brunner's glands synchronously occurring with sigmoid colon carcinoma: report of a case. Surg Today. 2008;38(8):756–760. doi: 10.1007/s00595-007-3707-1. [DOI] [PubMed] [Google Scholar]
- 68.Jung Y, Chung IK, Lee TH, et al. Successful endoscopic resection of large pedunculated Brunner's gland hamartoma causing gastrointestinal bleeding arising from the pylorus. Case Rep Gastroenterol. 2013;7(2):304–307. doi: 10.1159/000354138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kakushima N, Kanemoto H, Tanaka M, et al. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20(35):12501–12508. doi: 10.3748/wjg.v20.i35.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori H, Shintaro F, Kobara H, et al. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2013;25(4):459–461. doi: 10.1111/j.1443-1661.2012.01363.x. [DOI] [PubMed] [Google Scholar]
- 71.Doyama H, Tominaga K, Yoshida N, et al. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;26(Suppl 2):41–45. doi: 10.1111/den.12253. [DOI] [PubMed] [Google Scholar]
- 72.Iwamuro M, Kobayashi S, Ohara N, et al. Adenocarcinoma in situ arising from Brunner's gland treated by endoscopic mucosal resection. Case Rep Gastrointest Med. 2017;2017:7916976. doi: 10.1155/2017/7916976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.