Abstract
Obesity is a chronic disease associated with many complications. Weight loss of 5–15% can improve many obesity-related complications. Despite the benefits of weight reduction, there are many challenges in losing weight and maintaining long-term weight loss. Pharmacotherapy can help people with obesity achieve and maintain their target weight loss, thereby reducing the risk of obesity-related complications. The prevalence of obesity in the USA has been increasing over the past few decades, and despite the availability of approved anti-obesity medications (AOMs), people with obesity may not be accessing or receiving treatment at levels consistent with the disease prevalence. Reasons for low levels of initiation and long-term use of AOMs may include reluctance of public health and medical organizations to recognize obesity as a disease, lack of reimbursement, provider inexperience, and misperceptions about the efficacy and safety of available treatments. This article aims to inform primary care providers about the mechanism of action of one class of AOMs, glucagon-like peptide 1 receptor agonists (GLP-1RAs), in weight loss and longer-term maintenance of weight loss, and the efficacy and safety of this treatment class. GLP-1RA therapy was initially developed to treat type 2 diabetes. Owing to their effectiveness in reducing body weight, once-daily subcutaneous administration of liraglutide 3.0 mg has been approved, and once-weekly subcutaneous administration of semaglutide 2.4 mg is being investigated in phase III trials, for obesity management. Considerations regarding adverse effects and contraindications for different drug classes are provided to help guide treatment decision-making when considering pharmacotherapy for weight management in patients with obesity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01710-0.
Keywords: Glucagon-like peptide 1 receptor agonist, Obesity, Pharmacotherapy, Antiobesity medication, Weight loss
Plain Language Summary
Obesity is a growing public health issue that increases the risk of developing heart disease, type 2 diabetes, and osteoarthritis. Weight loss can reduce the risk of developing these health problems but, despite this, levels of obesity remain high. Achieving and maintaining weight loss is challenging for many individuals. There is therefore a need for some patients to take medications to help them lose weight and prevent weight regain. Glucagon-like peptide 1 receptor agonists (GLP-1RAs) are a type of medication originally developed to treat type 2 diabetes, but are now being used for the treatment of obesity because they are effective at helping people to lose weight. One GLP-1RA, liraglutide, has been approved to treat obesity, and another, semaglutide, is in clinical trials. GLP-1RAs work by reducing the appetite and feelings of hunger, slowing the release of food from the stomach, and increasing feelings of fullness after eating. Most people can tolerate GLP-1RAs well. The most common side effects (nausea, vomiting, and diarrhea) are usually mild and occur in the first few weeks of treatment, reducing over time. Because of the difficulties many people face in maintaining weight loss, lifelong treatment may be needed. In clinical trials, GLP-1RAs were well tolerated and effective at helping people prevent weight regain, and may be a good option for long-term weight control and lowering patients’ chances of serious health problems.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01710-0.
Key Points
| Many people with obesity have various health complications, but in spite of the benefits of weight loss, losing and maintaining weight is challenging. |
| Anti-obesity medication can help people with obesity achieve target weight loss and help to reduce the risk of regaining weight, thereby improving obesity-related health complications. |
| Glucagon-like peptide 1 receptor agonist therapy provides an effective and well-tolerated treatment option to help people with obesity achieve and maintain weight targets. |
Digital Features
This article is published with digital features, including a plain language summary, and video animation, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14192567.
Introduction
Obesity (defined as a body mass index [BMI] ≥ 30 kg/m2 in adults [1]) is a major health concern in the USA [2] and is associated with multiple complications, including cardiovascular disease, type 2 diabetes, and osteoarthritis [3–8]. Weight loss of 5–15% is recommended to improve many of the complications of overweight/obesity, with greater improvements observed with further weight reductions [5, 9]. Even a modest weight loss of 5% has been shown to improve cardiometabolic risk factors, including reduced systolic blood pressure and plasma triglyceride concentration, and increased multi-organ insulin sensitivity and β-cell function [6]. Despite evidence that weight loss improves obesity-related complications, the age-adjusted prevalence of obesity in US adults in 2017–2018 was 42.4% [2], indicating an unmet need in the management of obesity.
Challenges for Achieving and Maintaining Weight Loss, and Pharmacotherapeutic Options for Obesity
Obesity is a chronic disease associated with high rates of relapse after achieving initial weight loss [10, 11]. As such, people with overweight or obesity may face many challenges losing weight and maintaining weight loss. These challenges can involve internal factors such as hormonal influence on the homeostatic regulation of body weight [11]. In addition, energy expenditure is decreased following diet-induced weight loss, with the implication that reduced food intake would need to be maintained in the long-term [12]. Other challenges include external factors due to an increasingly obesogenic environment. Such an environment encompasses the interrelated issues of exposure to high-density, highly caloric foods, the relatively low cost of these foods, and physical environments that limit the scope of physical activity [13]. Indeed, alterations in the levels of weight-regulating hormones along with the obesogenic environment explain why many individuals find maintaining a lower weight as challenging as the initial weight loss.
Given the risk that obesity represents to public health and the difficulty of achieving and maintaining weight loss via lifestyle changes alone, there is a need for pharmacological approaches to aid weight loss in some individuals. There are several anti-obesity medications (AOMs) currently available in the USA as an adjunct to lifestyle modification, each with differing mechanisms of action (Table 1). However, despite their availability, adoption of medications for the management of obesity remains low [14, 15]. Barriers to initiating treatment may include delayed recognition of obesity as a disease by public health and medical organizations, provider biases regarding patients with obesity, lack of reimbursement leading to out-of-pocket costs to patients, inadequate training of providers, historical safety issues with AOMs, and perceptions of patients and their caregivers regarding the efficacy of AOMs [14–17]. In the ACTION study, 3008 individuals with obesity and 606 healthcare providers were questioned on their obesity-related perceptions, attitudes, and behaviors. Only 27% of patients and 30% of healthcare providers believed prescription AOMs to be completely effective for weight management, and most survey respondents found other interventions to be more effective than AOMs [16]. Nearly all other interventions listed (including improved eating habits, exercise tracking, counseling or lifestyle modification, and visiting a dietitian) were perceived to be more effective than AOMs [16], indicating a lack of knowledge about the potential benefits of pharmacotherapy.
Table 1.
Mechanism of action and efficacy of anti-obesity medications currently available in the USA [10, 21, 23, 37, 39, 43, 46, 47, 57, 62–65, 69–78]
| Anti-obesity medication | Type of agent/mechanism of action [23] | Trial information | Percentage of patients achieving categorial weight loss at 1 year | |||
|---|---|---|---|---|---|---|
| ≥ 5% (or > 5%) |
≥ 10% (or > 10%) |
Percentage of patients achieving weight loss maintenance at 2 years | ||||
| Liraglutide |
· GLP-1RA · Reduces appetite and food cravings [21] · Increases satiety · Alters food preference and reward pathways [21] |
Astrup et al., 2009; Astrup et al., 2012: placebo-controlled, randomized, 20-week trial for liraglutide (1.2, 1.8, 2.4, and 3.0 mg QD) with open-label comparator (orlistat 120 mg TID) + 84-week extension in patients with BMI 30–40 kg/m2 Patients were on a 500-kcal/day energy-deficient diet and increased their physical activity |
(Liraglutide 3.0 mg; orlistat; placebo) 73%; 44%; 28% (liraglutide vs. placebo or orlistat, p ≤ 0.0001) |
(Liraglutide 3.0 mg; orlistat; placebo) 37%; 14%; 10% |
(Liraglutide 2.4/3.0 mg vs. orlistat) ≥ 5% weight loss: 52% vs. 29% (p < 0.001) ≥ 10% weight loss: 26% vs. 16% (p = 0.04) |
|
|
Pi-Sunyer et al., 2015: placebo-controlled, double-blind, randomized, 56-week trial of liraglutide 3.0 mg QD in patients with BMI ≥ 30 kg/m2 (or ≥ 27 kg/m2 with dyslipidemia or hypertension) Patients received counseling on lifestyle modification |
(Liraglutide 3.0 mg; placebo) 63%; 27% (p < 0.001) |
(Liraglutide 3.0 mg; placebo) 33%; 11% (p < 0.001) |
NR | |||
|
Davies et al., 2015: placebo-controlled, randomized, double-blind, parallel-group 56-week trial of liraglutide 1.8 and 3.0 mg QD in patients with BMI ≥ 27 kg/m2 with diabetes taking 0–3 OADs Patients were on a 500-kcal/day energy-deficient diet and increased their physical activity |
(Liraglutide 3.0 mg; placebo) 54%; 21% (p < 0.001) |
(Liraglutide 3.0 mg; placebo) 25%; 7% (p < 0.001) |
NR | |||
|
Wadden et al., 2013: placebo-controlled, double-blind, randomized, 56-week trial of liraglutide 3.0 mg QD in patients with BMI ≥ 30 (or ≥ 27 with comorbidity) kg/m2 after low-calorie-diet-induced weight loss Patients received diet and exercise counseling |
(Liraglutide 3.0 mg; placebo)a 51%; 22% (p < 0.0001) |
(Liraglutide 3.0 mg; placebo)a 26%; 6% (p < 0.0001) |
NR | |||
| Wadden et al., 2020b: placebo-controlled, double-blind, randomized, 56-week trial of liraglutide 3.0 mg QD plus IBT in patients with BMI ≥ 30 kg/m2 |
(Liraglutide 3.0 mg; placebo) 62%; 34% (p = 0.0003) |
(Liraglutide 3.0 mg; placebo) 31%; 20% (p = 0.0469) |
NR | |||
| Garvey et al., 2020: placebo-controlled, double-blind, randomized, 56-week trial of liraglutide 3.0 mg QD plus IBT in patients with BMI of ≥ 27 kg/m2 and diabetes treated with basal insulin and ≤ 2 OADs |
(Liraglutide 3.0 mg; placebo) 52%; 24.0% (p < 0.0001) |
(Liraglutide 3.0 mg; placebo) 23%; 7% (p < 0.0001) |
NR | |||
| Naltrexone-bupropion |
· Naltrexone: opioid antagonist · Bupropion: aminoketone antidepressant [62] ⋅ Suppresses appetite |
Greenway et al., 2010: placebo-controlled, double-blind, randomized, 56-week trial of naltrexone-bupropion (NB16 and NB32b) BID in patients with BMI ≥ 30 (or ≥ 27 with comorbidity) to 45 kg/m2 Patients were on a mild hypocaloric diet and exercise |
(NB16; NB32; placebo) 39%; 48%; 16% (NB16/NB32 vs. placebo, both p < 0.0001) |
(NB16; NB32; placebo) 20%; 25%; 7% (NB16/NB32 vs. placebo, both p < 0.0001) |
NR | |
|
Apovian et al., 2013: placebo-controlled, double-blind, randomized, 56-week trial of naltrexone-bupropion (NB32) BID in patients with BMI ≥ 30 (or ≥ 27 with controlled hypertension and/or dyslipidemia) to 45 kg/m2 Patients were on a 500-kcal/day energy-deficient diet, increased physical activity, and behavioral modification advice |
(NB32; placebo) 51%; 17% (p < 0.001) |
(NB32; placebo) 28%; 6% (p < 0.001) |
NR | |||
| Wadden et al., 2011: placebo-controlled, double-blind, randomized, 56-week trial of naltrexone-bupropion (NB32) QD and BMOD in patients with BMI ≥ 30 (or ≥ 27 with controlled hypertension and/or dyslipidemia) to 45 kg/m2 |
(NB32; placebo) 66%; 43% (p < 0.001) |
(NB32; placebo) 42%; 20% (p < 0.001) |
NR | |||
|
Hollander et al., 2013: placebo-controlled, double-blind, randomized, 56-week trial of naltrexone-bupropion (NB32) QD in patients with BMI ≥ 27 and ≤ 45 kg/m2 and type 2 diabetes treated with or without OADs Patients were on a 500-kcal/day energy-deficient diet, dietary counseling and advice on behavioral modification, including instructions to increase physical activity |
(NB32; placebo) 45%; 19% (p < 0.001) |
(NB32; placebo) 19%; 6% (p < 0.001) |
NR | |||
| Orlistat |
· Reversible inhibitor of gastrointestinal lipases [65] · Inhibits fat absorption |
Hauptman et al., 2000: placebo-controlled, double-blind, randomized, 2-year trial of orlistat (60 and 120 mg TID) in patients with BMI 30–44 kg/m2 Patients were on an energy-deficient diet |
(Orlistat 60 mg; orlistat 120 mg; placebo) 49%; 51%; 31% (Orlistat 60 mg/120 mg vs. placebo, both p < 0.001) |
(Orlistat 60 mg; orlistat 120 mg; placebo) 24%; 29%; 11% (Orlistat 60 mg/120 mg vs. placebo, both p < 0.001) |
(Orlistat 60 mg; orlistat 120 mg; placebo) ≥ 5% weight loss: 34%; 34%; 24% (Orlistat 60 mg vs. placebo: p = 0.03; orlistat 120 mg vs. placebo: p = 0.02) ≥ 10% weight loss: 15%; 19%; 7% (Orlistat 60 mg vs. placebo: p = 0.008; orlistat 120 mg vs. placebo: p = 0.001) |
|
|
Rössner et al., 2000: placebo-controlled, double-blind, randomized, 2-year trial of orlistat (60 and 120 mg) TID in patients with BMI 28–43 kg/m2 Patients were on a 600-kcal/day energy-deficient diet |
NR |
(Orlistat 60 mg; orlistat 120 mg; placebo) 31%; 38%; 19% (Orlistat 60 mg vs. placebo: p = 0.002; orlistat 120 mg vs. placebo: p = 0.001) |
(Orlistat 60 mg; orlistat 120 mg; placebo) > 10% weight loss: 29%; 28%; 19% (Orlistat 60 mg/120 mg vs. placebo: both p < 0.05) |
|||
| Phentermine |
· Phentermine: sympathomimetic amine anorectic [63] · Suppresses appetite |
Kang et al., 2010: placebo-controlled, double-blind, randomized, 12-week trial of phentermine 30 mg QD in patients with obesity and controlled diabetes, hypertension, and dyslipidemia |
(Phentermine; placebo) 96%; 21% (p < 0.001) |
(Phentermine; placebo) 63%; 5% (p < 0.001) |
NR | |
| Phentermine-topiramate |
· Phentermine: sympathomimetic amine anorectic [64] · Topiramate: anti-epileptic drug · Suppresses appetite |
Allison et al., 2012: placebo-controlled, randomized, 56-week trial of PT (3.75/23 mg or 15/92 mg) QD added to a reduced-energy diet in patients with BMI ≥ 35 kg/m2 Patients were advised to follow a 500-kcal/day energy-deficient diet and received standardized diet and lifestyle-modification counseling |
(PT 3.75/23 mg; PT 15/92 mg; placebo) 45%; 67%; 17% (PT 3.75/23 mg/15/92 mg vs. placebo, both p < 0.0001) |
(PT 3.75/23 mg; PT 15/92 mg; placebo) 19%; 47%; 3% (PT 3.75/23 mg/15/92 mg vs. placebo, both p < 0.0001) |
NR | |
|
Gadde et al., 2011; Garvey et al., 2012: placebo-controlled, double-blind, randomized, 108-week trial of PT (7.5/46 mg or 15/92 mg) QD in patients with BMI 27–45 kg/m2 and cardiometabolic disease Patients received standardized diet and lifestyle-modification counseling |
(PT 7.5/46 mg; PT 15/92 mg; placebo) 62%; 70%; 21% (PT 5/46 mg/15/92 mg vs. placebo, both p < 0.0001) |
(PT 7.5/46 mg; PT 15/92 mg; placebo) 37%; 48%; 7% (PT 5/46 mg/15/92 mg vs. placebo, both p < 0.0001) |
(PT 7.5/46 mg; PT 15/92 mg; placebo) ≥ 5% weight loss: 75%; 79%; 30% ≥ 10% weight loss: 50%; 54%; 12% (PT 7.5/46 mg/15/92 mg vs. placeb, both p < 0.0001) |
|||
Percentages are rounded up to one decimal place
BID two times a day, BMI body mass index, BMOD intensive behavior modification, GLP-1RA glucagon-like peptide 1 receptor agonist, IBT intensive behavioral therapy, NR not reported, OAD oral antihyperglycemic drug, PT phentermine-topiramate, QD once-daily, TID three times per day
aBased on patients achieving ≥ 5% weight loss during the run-in period
bNB16: sustained-release naltrexone 16 mg per day plus sustained-release bupropion 360 mg per day combined in fixed-dose tablets; NB32: sustained-release naltrexone 32 mg per day plus sustained-release bupropion 360 mg per day combined in fixed-dose tablets
Development of Glucagon-Like Peptide 1 Receptor Agonists for the Management of Obesity
Glucagon-like peptide 1 receptor agonists (GLP-1RAs), including liraglutide and semaglutide, were initially developed for the treatment of type 2 diabetes but were found to be effective not only in reducing blood glucose levels but body weight as well [18–20]. Consequently, once-daily subcutaneous administration of liraglutide 3.0 mg was developed for the treatment of obesity [21] and once-weekly subcutaneous administration of semaglutide 2.4 mg is currently being investigated in phase III trials for this indication [22]. In contrast to other AOMs, which either suppress appetite or inhibit fat absorption [23], GLP-1RAs reduce body weight in a number of ways, decreasing appetite and hunger, and increasing satiety, resulting in reduced energy intake [24–26]. The purpose of this review is to further elucidate the mechanism of action of GLP-1RAs in helping individuals with overweight or obesity to achieve and maintain weight loss.
We searched PubMed and Embase databases using the terms glucagon-like peptide receptor agonists; obesity; anti-obesity; weight; overweight; bodyweight; overweight; anti-obesity agents; appetite; food intake regulation; caloric; satiety; gastric emptying; energy intake; craving; cravings; eating control; safety; tolerability; tolerated; adverse; hypoglycemia; nausea; diarrhea; vomiting; gastrointestinal; long-term; durable; maintain*; sustain*. Records were limited to those in English language (N = 247). Records were excluded during screening if they were press releases, news reports, not relevant drug/indication/population, preclinical study, reviews, case reports, not a randomized trial, or not in humans. Searches last updated November 26, 2020 (N = 16). Supplementary searches were performed to identify overview of approved AOMs and background information. American Association of Clinical Endocrinologists and American College of Endocrinology, and European guidelines for obesity were hand searched for relevant data.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Effects of GLP-1RAs on Appetite, Satiety and Hunger, and Gastric Emptying
GLP-1RAs are attractive agents for the management of obesity owing to the actions of GLP-1 on appetite and energy intake.
GLP-1 is released from the L cells in the gut in response to energy intake, and facilitates a multitude of physiological actions, including a delay in gastric emptying [27]. In pharmacology trials, GLP-1RA treatment has been shown to delay gastric emptying within the first postprandial hour [25, 28], although overall gastric emptying did not appear to be affected [28], suggesting additional mechanisms of action in GLP-1RA-mediated weight loss.
In the central nervous system, GLP-1 receptors are located in the hypothalamus, which is involved in regulating food intake [24, 29, 30]. Coveleski et al. found that acute administration of the GLP-1RA exenatide resulted in reduced feelings of hunger in eight women with obesity. The reduced feelings of hunger were associated with an increase in functional connectivity of the nucleus tract solitaries with the hypothalamus and thalamus [31]. In addition, murine models show that liraglutide can access specific brain areas relevant for appetite regulation, binding GLP-1 receptors on proopiomelanocortin and cocaine- and amphetamine-regulated transcript (POMC/CART)-expressing arcuate nucleus neurons [32]. GLP-1 directly stimulates POMC/CART neurons and indirectly inhibits neuropeptide Y (NPY) and agouti-related peptide (AgRP) to increase measures of satiety and decrease hunger [32]. These effects of GLP-1 can lead to reduced energy intake [27], thereby facilitating weight loss (Fig. 1).
Fig. 1.
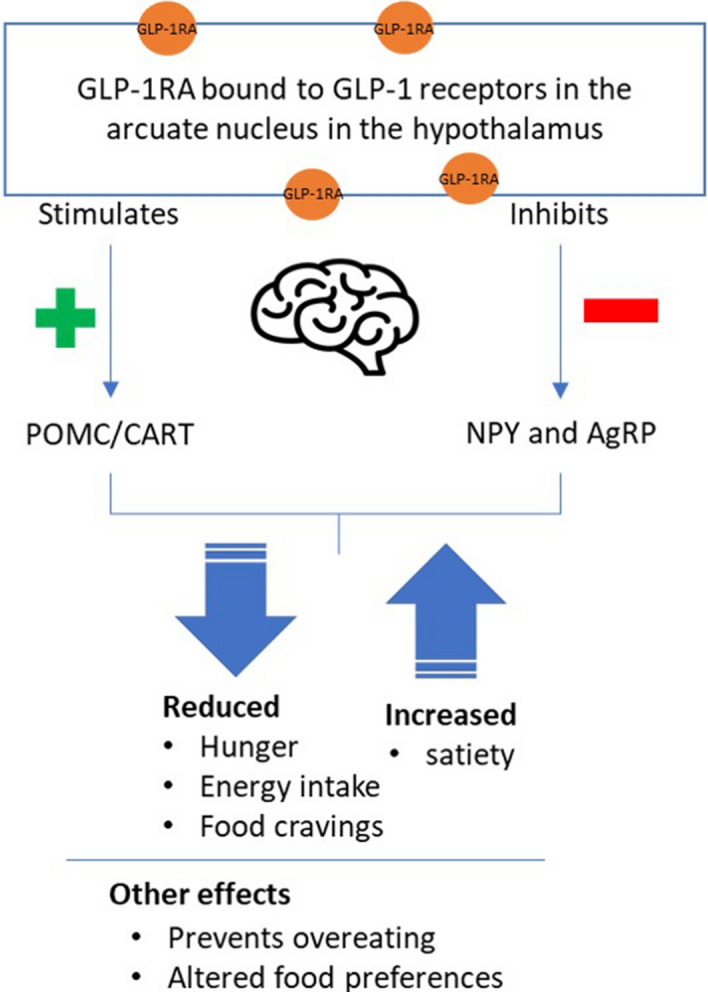
Overview of the actions of GLP-1 in the central nervous system [27, 29, 32, 41]. AgRP agouti-related peptide, CART cocaine- and amphetamine-regulated transcript, GLP-1RA glucagon-like peptide 1 receptor agonist, NPY neuropeptide Y, POMC proopiomelanocortin
Overview of the actions of GLP-1 in the central nervous system. (MP4 3429 kb)
Studies investigating the mechanism of action of GLP-1RA therapy for causing weight loss provide evidence that GLP-1RA treatment is associated with reductions in appetite and hunger, lower preference for energy-dense foods, alteration in food reward pathways, decrease in food cravings, and improvement in eating control (Table 2) [25, 26, 33, 34].
Table 2.
Summary of clinical data on the effects of GLP-1RA therapy on energy intake, meal duration, appetite, satiety and hunger, food preferences, and gastric emptying [25, 26, 33]
| Agent | Comparator(s) | Trial population | Trial duration | Energy intakea | Meal duration | Appetite | Satiety | Hunger | Food preference | 1-h gastric emptying |
|---|---|---|---|---|---|---|---|---|---|---|
| Liraglutide 1.8 mg and 3.0 mgb [25] | Placebo | Individuals with obesity but without diabetes | Two treatment periods of 5 weeks with a 6–8-week washout period in between | Reduced by 16% vs. placebo(p = 0.003) | NR | Reduced vs. placebo (p = 0.0003) | Reduced vs. placebo (p = 0.002) | Reduced vs. placebo (p = 0.01) | NR | 23% lower vs. placebo (p = 0.007) |
| Semaglutide 1.0 mg [26] | Placebo | Individuals with obesity but without diabetes | 12 weeks | Reduced by approx. 35% vs. placebo (p < 0.0001) | Shorter vs. placebo (p = 0.0018) | Reduced vs. placebo (p = 0.0023) | Increased (NS) | Reduced (NS) | Lower preference for high-fat and non-sweet foods vs. placebo (p = 0.0016) | 27% lower vs. placebo (p = 0.0012) [27] |
| Semaglutide 2.4 mg [33] | Placebo | Individuals with obesity but without diabetes | 20 weeks | 35% lower vs. placebo (p < 0.0001) | NR | Reduced vs. placebo (p = 0.001) | Increased vs. placebo (p < 0.02) | Reduced vs. placebo (p < 0.02) | Reduced cravings for savory food (p < 0.02) | No clinically relevant effect vs. placebo |
EE energy expenditure, NR not reported, NS non-significant
aBased on results for ad libitum lunch
bResults are for liraglutide 3.0 mg
Clinical Trials Demonstrating Reductions in Body Weight with GLP-1RAs
Several clinical trials have reported that the effects described above resulted in larger reductions in body weight with GLP-1RA therapy compared with placebo in participants with obesity. After 5 weeks of treatment with once-daily subcutaneous administration of liraglutide 1.8 mg and 3.0 mg, estimated reductions in body weight were − 2.1 kg and − 2.5 kg, respectively, vs. − 0.3 kg with placebo [25]. In another liraglutide trial, 16-week median (interquartile range) body weight reductions were − 5.8 kg (− 6.9, − 4.45) with liraglutide 3.0 mg and − 1 kg (− 3.5, 2.53) with placebo (p < 0.003) [35]. For once-weekly subcutaneous administration of semaglutide 1.0 mg, change from baseline in mean body weight after 12 weeks was − 5.0 kg vs. + 1.0 kg with placebo [26]. A recent 20-week, phase II trial investigated the effects of subcutaneous administration of semaglutide 2.4 mg on gastric emptying, appetite, and energy intake in patients with obesity. There was no significant difference between semaglutide and placebo in gastric emptying when corrected for week-20 body weight. However, patients receiving semaglutide 2.4 mg experienced reduced hunger, and increased fullness and satiety compared with placebo (p < 0.02). Ad libitum mean energy intake was also reduced by 35% for semaglutide 2.4 mg vs. placebo (1736 vs. 2676 kJ; estimated treatment difference [ETD], − 940 kJ; p < 0.0001). Patients receiving semaglutide 2.4 mg in this trial lost 9.9% of their body weight, compared with 0.4% in those receiving placebo [33]. In a phase II study of the long-acting GLP-1RA efpeglenatide (4 mg once weekly, 6 mg once weekly, 6 mg once every 2 weeks, and 8 mg once every 2 weeks), patients with obesity and without diabetes had statistically significant reductions in body weight compared with placebo after 20 weeks of treatment (differences in least squares means were − 6.3 to – 7.2 kg; p < 0.0001) [36].
Longer-term data with GLP-1RAs include results from a phase III, 56-week study of liraglutide 3.0 mg vs. placebo in patients with obesity and without diabetes. After 56 weeks of treatment with liraglutide 3.0 mg vs. placebo, patients had mean body weight reductions of − 8.4 kg vs. − 2.8 kg (ETD − 5.6 kg; 95% confidence interval [CI] − 6.0 to − 5.1; p < 0.001) [37]. In a recently published landmark phase III study in patients with overweight or obesity (STEP 1), greater reductions in body weight were observed after 68 weeks of treatment with once-weekly semaglutide 2.4 mg vs. placebo (mean change from baseline − 14.9% vs. − 2.4%; ETD − 12.4%; 95 CI − 13.4 to − 11.5; p < 0.001) [38]. Similarly, in a 68-week phase III study comparing the effects of semaglutide 2.4 mg vs. placebo in adults with overweight or obesity without diabetes (STEP 3), mean body weight decreased 16.0% with semaglutide compared with 5.7% with placebo, both as adjunct to intensive behavioral therapy (ETD − 10.3%; 95% CI − 12.0 to − 8.6; p < 0.0001) [39]. Ongoing studies in the STEP program will provide further insights into the effects of once-weekly semaglutide 2.4 mg in a broader population [22].
In addition to the above findings, several studies have shown that GLP-1RA therapy results in larger proportions of patients achieving 5% and 10% body weight loss than with placebo (Table 1).
Safety and Tolerability of GLP-1RAs for the Treatment of Obesity
Since GLP-1RA therapy is used for the treatment of type 2 diabetes, there may be concerns about whether using an antidiabetic medication to treat obesity will increase the risk of hypoglycemia in people with obesity but without diabetes. However, the action of GLP-1 is glucose-dependent and blood glucose is only lowered by GLP-1 if concentrations are above fasting levels [40, 41]. This effect translates to a low risk of hypoglycemia in patients with type 2 diabetes treated with GLP-1RAs [42]. In individuals without diabetes, the potential for hypoglycemia with GLP-1RA treatment would therefore also be expected to be low. Indeed, Garvey et al. found that hypoglycemia was less common with liraglutide 3.0 mg than with placebo in individuals with overweight or obesity and insulin-treated type 2 diabetes [43]. Pi-Sunyer et al. also reported a low risk of hypoglycemia, with events occurring in similar proportions of patients with obesity but without diabetes who were treated with liraglutide 3.0 mg (1.3%) compared with placebo (1.0%) [37]. In a similar patient population of adults with obesity who did not have diabetes, no severe or blood glucose-confirmed symptomatic hypoglycemic events were reported with semaglutide 1.0 mg [26].
In studies of GLP-1RA therapy in individuals with type 2 diabetes, the most frequently reported adverse events tend to involve the gastrointestinal system, with nausea, vomiting, and diarrhea occurring in up to 51%, 19%, and 20% of patients, respectively [44]; however, in clinical trials for once-daily subcutaneous administration of liraglutide 3.0 mg in patients with obesity only, or with obesity and diabetes, these effects tended to be mild-to-moderate and transient. Nausea, vomiting, and diarrhea were typically the most common gastrointestinal adverse events, occurring in up to 48.4%, 23.2%, and 23.1% of participants, respectively, with events occurring predominantly during dose escalation and decreasing over time [10, 37, 43, 45–47]. Since gastrointestinal adverse effects are rarely severe and tend to diminish over time, they would therefore not be expected to cause a barrier to initiating and continuing treatment for most patients. Indeed, GLP-1RA therapy appears to be well tolerated overall, and proportions of clinical trial participants discontinuing treatment because of adverse events tend to be low (5.4–9.9%) [10, 37, 39, 43, 46, 47].
Management Strategies for Gastrointestinal Adverse Events with GLP-1RA Therapy
There are various strategies that can be employed to help manage or mitigate potential gastrointestinal adverse events when initiating a GLP-1RA for the treatment of overweight or obesity. A gradual dose-escalation is recommended for liraglutide 3.0 mg, starting with the initial dose of 0.6 mg per day for 1 week, then increasing the dose in weekly increments until the maximum therapeutic dose of 3.0 mg is reached [21]. If the patient experiences gastrointestinal adverse effects during dose escalation, uptitration can be delayed for an additional week [21]. This has been demonstrated by Gough et al. [48], who reported smaller proportions of patients experiencing gastrointestinal adverse events with a combination of insulin degludec and liraglutide (IDegLira) vs. liraglutide alone, attributing the findings to a more gradual dose escalation with IDegLira [48]. In the authors’ anecdotal experience, when further uptitration is not tolerated, but treatment effects are noted, maintaining the patient at the lower tolerated dose may be preferable to discontinuation. This strategy was used for the STEP 1 trial of semaglutide 2.4 mg [38].
Other strategies include providing counseling regarding the potential gastrointestinal adverse events that could arise, such as nausea, vomiting, and diarrhea. Patients should be informed of dietary modifications that could help reduce symptoms such as smaller portion sizes and avoiding fatty foods [49].
Associations Between Weight Loss and Gastrointestinal Adverse Events on GLP-1RA Therapy
While gastrointestinal adverse events that occur with GLP-1RAs are mainly mild-to-moderate and transient, occurring particularly during dose escalation, there may be concerns that GLP-1RA-mediated weight loss could be due to these effects. Data collected from a randomized, placebo-controlled, double-blind trial of liraglutide 3.0 mg, in which nausea was the most frequent adverse event, showed that greater weight loss was associated with transient nausea and vomiting in participants with obesity but without diabetes [50]. In a phase II study, gastrointestinal side effects were most common during semaglutide dose escalation but weight loss persisted beyond these events and continued through the 52-week trial period [51]. The fact that patients in this trial continued to lose weight after gastrointestinal adverse effects had subsided suggests that these effects were not the cause of weight loss. A retrospective analysis of the DURATION trials found that overall, greater weight loss was associated with gastrointestinal adverse events with exenatide once weekly and exenatide twice daily. Conversely, the same analysis found no difference in weight loss for exenatide once weekly or liraglutide between patients experiencing gastrointestinal adverse events and those with none in DURATION-6 [52]. Furthermore, a study of patients with type 2 diabetes who were treated with once-weekly exenatide found significant reductions in weight regardless of whether patients experienced nausea or vomiting [53]. However, patients with type 2 diabetes were included in these trials [52, 53], which could have contributed to the observed differences.
GLP-1RA-Mediated Weight Loss Maintenance
Obesity is a chronic condition, characterized by changes in weight-regulating hormones that drive weight regain following weight loss [11], therefore it is no surprise that maintaining weight loss can prove just as challenging as losing weight in the first place. Compensatory changes in the levels of weight-regulating hormones such as leptin, ghrelin, peptide YY, and gastric inhibitory peptide can counteract diet-induced weight loss, highlighting the difficulty in maintaining weight loss through diet alone [11]. Furthermore, when treated with an AOM, patients with obesity have experienced weight regain upon cessation of the AOM [10, 54]. This suggests that, to maintain weight loss, treatment for obesity should be considered chronic (as in the case of hypertension), rather than as a short course of treatment associated with acute illnesses [9, 55].
Treatment with an AOM often follows a pattern of initial weight loss that tends to level out, or “plateau”, after a period of time on treatment [54, 56, 57]. Metabolic adaptation is most likely the reason for this plateau [11] rather than poor response or resistance to the medication. Given this pattern in weight loss following treatment with an AOM, anti-obesity therapy should be conceptualized as initiating weight loss followed by establishing a new weight plateau and assisting in maintaining this weight in the long term [9, 11].
One of the mechanisms thought to be responsible for weight regain or plateau is a reduction in circulating levels of leptin after initial weight loss [11, 58]. Indeed, it has been suggested that preservation of free leptin levels is involved in GLP-1RA-mediated maintenance of weight loss [58]. In a trial in which patients with obesity were treated with or without liraglutide 1.2 mg after diet-induced body weight loss of 12%, smaller decreases in free leptin and higher levels of PYY3-36 were observed with liraglutide vs. without liraglutide [58]. In another trial, higher levels of PYY3-36 were also observed relative to baseline after 16 weeks of treatment with liraglutide 3.0 mg [34]. In addition, clinical evidence has shown that continued treatment with GLP-1RA therapy is associated with maintenance of weight loss [10, 47]. For example, after 2 years of treatment with liraglutide 2.4/3.0 mg in patients with obesity, 52% and 26% maintained at least 5% and at least 10% weight loss, respectively, compared with 29% and 16% of patients receiving orlistat [47] (Table 1). Furthermore, treatment with liraglutide 3.0 mg resulted in greater proportions of patients maintaining at least 5% weight loss over 56 weeks vs. placebo after an initial run-in period on a low-calorie diet (81.4% vs. 48.9%, respectively) [10]. These data suggest a potential for long-term benefit of liraglutide treatment in many patients, as weight loss of 5–15% has been shown to improve obesity-related complications including diabetes and cardiovascular disease risk factors [5, 9, 59]. In a cardiovascular outcomes trial of liraglutide 1.8 mg, weight loss was sustained over a median trial period of 3.5 years in patients with type 2 diabetes who had either cardiovascular disease or risk factors for cardiovascular disease [60]. Since obesity is associated with an increased risk of cardiovascular disease, the ongoing SELECT study is investigating whether once-weekly semaglutide 2.4 mg will reduce the risk of having cardiovascular events in patients with overweight or obesity and with prior cardiovascular disease [61].
Place of GLP-1RAs in the Treatment of Obesity
Currently, pharmacotherapy is recommended as an adjunct to lifestyle modification for individuals with a BMI of at least 30 kg/m2, or at least 27 kg/m2 with comorbidity [5, 9]. Achieving body weight loss of 5–15% can improve cardiometabolic parameters including prediabetes, dyslipidemia, and hypertension [5, 9]; therefore, target weight loss should be defined on the basis of the individual patient’s presentation. Adding an AOM to lifestyle modification can help patients to achieve these weight-loss targets, thereby reducing the risks of obesity-related complications [5].
Treatment Decision-Making
Treatment decision-making can be guided by the different mechanisms of action of AOMs to ascertain the suitability of the therapy for the individual patient. For instance, if patients present with symptoms such as early hunger or lack of satiety then a GLP-1RA may be appropriate compared with other available treatments that work solely by suppressing appetite or inhibiting fat absorption [23].
As well as taking into account the mechanism of action of each drug class, there are certain contraindications and adverse effects to consider, which could determine the suitability of the drug for individual patients. For example, naltrexone-bupropion is not suitable for patients with uncontrolled hypertension [62] and phentermine is contraindicated in patients with a history of cardiovascular disease [63]. Patients with cardiovascular risk factors may therefore find a GLP-1RA more appropriate owing to the improvements in cardiometabolic parameters that have been observed with this drug class [10, 37, 46, 47]. Naltrexone-bupropion has a black box warning for suicidal thoughts and behaviors and neuropsychiatric reactions [62]. In addition, phentermine-topiramate is contraindicated in those taking certain antidepressant drugs [64]. Therefore, liraglutide could be an alternative option to these drugs if the patient has ongoing mental health problems, although it should be avoided in patients with a history of suicidal attempts or active suicidal ideation [21]. Importantly, orlistat is contraindicated in patients with chronic malabsorption syndrome and cholestasis [65], conditions that require avoidance of fatty foods. Since GLP-1RA therapy has been shown to reduce preference for fatty foods [26], GLP-1RA therapy might be preferred for patients who would find it difficult to avoid fatty foods under normal circumstances. If other health problems such as prediabetes or polycystic ovary syndrome (PCOS) are present, GLP-1RA therapy might be preferred over other treatment options. This is due to the reduced incidence of prediabetes in patients with obesity [37, 46, 47] and improved markers for ovarian function in patients with obesity and PCOS [66], compared with placebo.
Rapid weight loss has been associated with gallbladder-related disorders including cholelithiasis and cholecystitis [67, 68], and these adverse events, while rare, have been observed more with GLP-1RAs compared with placebo in clinical trials [21, 38]. There have been post-marketing reports of acute pancreatitis with liraglutide 3.0 mg; however, incidence of this adverse event in clinical trials was very low [21]. Although rare, healthcare providers should monitor their patients for symptoms of these adverse events, and treatment should be discontinued if gallbladder- or pancreatic-related disorders are suspected [21].
Although GLP-1RAs are generally well tolerated, there are some circumstances in which this drug class is not recommended. Liraglutide has a black box warning for thyroid C cell tumors [21], therefore this therapy is contraindicated for patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.
Suboptimal Treatment Response with AOMs
Once a treatment has been initiated, it is important to consider what to do in the event of a suboptimal treatment response. After treatment initiation with liraglutide 3.0 mg, it is recommended that, if a patient has not lost at least 4% of their baseline body weight after 16 weeks, the treatment should be discontinued because it is unlikely that significant weight loss will be achieved with the medication after this time point [21]. For other AOMs, it is suggested to stop treatment if weight loss of more than 5% has not been achieved after 12 weeks of treatment [9]. If a suboptimal treatment response occurs, another therapy could be selected taking into consideration the patient’s presentation and the drug profile of the available options.
Summary
GLP-1RA-mediated weight loss is achieved through multiple pathways including effects on the central nervous system such as reduced appetite, energy intake, and hunger, increased feelings of satiety, and altered food preferences. The risk of gastrointestinal adverse events is increased with GLP-1RA therapy but these effects tend to be mild-to-moderate and transient, and are not the reason for observed weight loss. Overall, GLP-1RAs provide a highly effective and well-tolerated treatment option to help individuals with obesity achieve and maintain body weight reductions of 5–10%, thereby improving weight-related complications in addition to weight loss.
Acknowledgements
Funding
This review and the journal’s Rapid Service Fee was funded by Novo Nordisk Inc. Plainsboro, NJ. This article was supported by Novo Nordisk Inc., who performed a medical accuracy review.
Medical Writing Assistance
Medical writing and editorial support were provided by Debbie Day of Axis, a division of Spirit Medical Communications Group Limited (funded by Novo Nordisk Inc.) under the direction of the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the following during the development of this article: conception and design; analysis and interpretations; drafting of the article/critical revision for important intellectual content; and final approval of the article.
Disclosures
Angela Fitch: Served on advisory boards for Gelesis, SetPoint Health, FoundHealth, and MsMedicine; Research funding from Boehringer Ingelheim. Jamy Ard: Served on advisory boards for Novo Nordisk and Boehringer Ingelheim. Lawrence Herman: Served on an advisory board for Novo Nordisk and is on the speakers bureau for Novo Nordisk. Sharon Fruh: Served on advisory board for Novo Nordisk.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.World Health Organization. Fact sheet on obesity and overweight. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 8 Mar 2021.
- 2.Hales C, Carroll M, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020:360. [PubMed]
- 3.Cefalu WT, Bray GA, Home PD, et al. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors' expert forum. Diabetes Care. 2015;38(8):1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:74. doi: 10.1186/s13098-019-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 6.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 8.Prospective Studies Collaboration, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed]
- 9.Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–24. 10.1159/000442721. [published correction appears in Obes Facts. 2016;9(1):64] [DOI] [PMC free article] [PubMed]
- 10.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–51. 10.1038/ijo.2013.120. [Published corrections appear in Int J Obes (Lond). 2013;37(11):1514 and 2015;39(1):187]. [DOI] [PubMed]
- 11.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 12.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. 10.1056/NEJM199503093321001. [Published correction appears in N Engl J Med. 1995;333(6):399]. [DOI] [PubMed]
- 13.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin N Am. 2018;102(1):183–197. doi: 10.1016/j.mcna.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring) 2016;24(9):1955–1961. doi: 10.1002/oby.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon R, Lahiri SW. Provider practice habits and barriers to care in obesity management in a large multicenter health system. Endocr Pract. 2018;24(4):321–328. doi: 10.4158/EP-2017-0221. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity (Silver Spring) 2018;26(1):61–69. doi: 10.1002/oby.22054. [DOI] [PubMed] [Google Scholar]
- 17.Baum C, Andino K, Wittbrodt E, et al. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmacoeconomics. 2015;33(7):643–653. doi: 10.1007/s40273-015-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bode BW. Design, findings and implications of the liraglutide phase III clinical trial program. Clin Invest. 2012;2(1):59–72. doi: 10.4155/cli.11.166. [DOI] [Google Scholar]
- 19.Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1–7 trials. Diabetes Metab. 2019;45(5):409–418. doi: 10.1016/j.diabet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22(8):1263–1277. doi: 10.1111/dom.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxenda. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf. Accessed 5 Oct 2020.
- 22.Kushner RF, Calanna S, Davies M, et al. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity (Silver Spring). 2020;28(6):1050–61. 10.1002/oby.22794. [DOI] [PMC free article] [PubMed]
- 23.Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84(12):951–958. doi: 10.3949/ccjm.84a.16094. [DOI] [PubMed] [Google Scholar]
- 24.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 25.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38(6):784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251. doi: 10.1111/dom.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 28.Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018;20(3):610–619. doi: 10.1111/dom.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–965. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699–713. doi: 10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coveleskie K, Kilpatrick LA, Gupta A, et al. The effect of the GLP-1 analogue exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes Sci Pract. 2017;3(4):434–445. doi: 10.1002/osp4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in subjects with obesity. Diabetes Obes Metab. 2020;23(3):754–62. 10.1111/dom.14280. [DOI] [PMC free article] [PubMed]
- 34.van Bloemendaal L, Veltman DJ, Ten Kulve JS, et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17(9):878–886. doi: 10.1111/dom.1250.6. [DOI] [PubMed] [Google Scholar]
- 35.Kadouh H, Chedid V, Halawi H, et al. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J Clin Endocrinol Metab. 2020;105(5):1552–1563. doi: 10.1210/clinem/dgz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratley RE, Kang J, Trautmann ME, et al. Body weight management and safety with efpeglenatide in adults without diabetes: a phase II randomized study. Diabetes Obes Metab. 2019;21(11):2429–2439. doi: 10.1111/dom.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. 10.1056/NEJMoa1411892. [DOI] [PubMed]
- 38.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. 10.1056/NEJMoa2032183. [DOI] [PubMed]
- 39.Wadden T, Bailey TS, Billings LK, et al. Semaglutide 2.4 mg and intensive behavioral therapy in subjects with overweight or obesity (STEP 3). Presented at the 38th annual meeting of the Obesity Society (TOS) held at ObesityWeek®, November 2–6, 2020: Oral 084.
- 40.Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23–65. doi: 10.1016/B978-0-12-800101-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Waldrop G, Zhong J, Peters M, et al. Incretin-based therapy in type 2 diabetes: an evidence based systematic review and meta-analysis. J Diabetes Complic. 2018;32(1):113–122. doi: 10.1016/j.jdiacomp.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Garvey WT, Birkenfeld AL, Dicker D, et al. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the scale insulin randomized controlled trial. Diabetes Care. 2020;43(5):1085–93. 10.2337/dc19-1745. [DOI] [PMC free article] [PubMed]
- 44.Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2015;11(3–4):202–230. doi: 10.1900/RDS.2014.11.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring). 2020;28(3):529–36. 10.1002/oby.22726 [DOI] [PMC free article] [PubMed]
- 46.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16. 10.1016/S0140-6736(09)61375-1. [Published correction appears in Lancet. 2010;375(9719):984]. [DOI] [PubMed]
- 47.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843–54. 10.1038/ijo.2011.158. [Published corrections appear in Int J Obes (Lond). 2012;36(6):890 and 2013;37(2):322]. [DOI] [PMC free article] [PubMed]
- 48.Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885–93. 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed]
- 49.Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30:202–210. doi: 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lean ME, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014;38(5):689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 52.Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: incidence and consequences. Diabetes Obes Metab. 2016;19(5):672–681. doi: 10.1111/dom.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trautmann ME, Han J, Ruggles J. Early pharmacodynamic effects of exenatide once weekly in type 2 diabetes are independent of weight loss: a pooled analysis of patient-level data. Clin Ther. 2016;38(6):1464–1473. doi: 10.1016/j.clinthera.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 54.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409. 10.1016/S0140-6736(17)30069-7. [Published correction appears in Lancet. 2017 8;389(10077):1398]. [DOI] [PubMed]
- 55.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–3077. doi: 10.1210/jc.2011-56. [DOI] [PubMed] [Google Scholar]
- 57.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iepsen EW, Lundgren J, Dirksen C, et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes (Lond) 2015;39(5):834–841. doi: 10.1038/ijo.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed]
- 61.Novo Nordisk A/S. Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT). https://clinicaltrials.gov/ct2/show/NCT03574597. Accessed 8 Mar 2021.
- 62.Contrave. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf. Accessed 5 Oct 2020.
- 63.Adipex P. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/085128s065lbl.pdf. Accessed 5 Oct 2020.
- 64.Qysmia. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf. Accessed 5 Oct 2020.
- 65.Xenical. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020766s029lbl.pdf. Accessed 5 Oct 2020.
- 66.Nylander M, Frøssing S, Clausen HV, Kistorp C, Faber J, Skouby SO. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online. 2017;35(1):121–127. doi: 10.1016/j.rbmo.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 67.Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12:1347–1352. doi: 10.1097/00042737-200012120-00015. [DOI] [PubMed] [Google Scholar]
- 68.Quesada BM, Kohan G, Roff HE, Canullán CM, Chiappetta Porras LT. Management of gallstones and gallbladder disease in patients undergoing gastric bypass. World J Gastroenterol. 2010;16(17):2075–2079. doi: 10.3748/wjg.v16.i17.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–99. 10.1001/jama.2015.9676. [Published correction appears in JAMA. 2016;315(1):90]. [DOI] [PubMed]
- 71.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–52. 10.1016/S0140-6736(11)60205-5. [Erratum in Lancet. 2011;377(9776):1494]. [DOI] [PubMed]
- 72.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. 10.1016/S0140-6736(10)60888-4. [Published corrections appear in Lancet. 2010;376(9741):594 and 2010;376(9750):1392]. [DOI] [PubMed]
- 74.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9(2):160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 75.Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–9. 10.2337/dc13-0234. [Published correction appears in Diabetes Care. 2014;37(2):587]. [DOI] [PMC free article] [PubMed]
- 76.Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12(10):876–882. doi: 10.1111/j.1463-1326.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 77.Rössner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8:49–61. 10.1038/oby.2000.8. [DOI] [PubMed]
- 78.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


