Abstract
Introduction
Centhaquine (Lyfaquin®) showed significant efficacy as a resuscitative agent in animal models of haemorrhagic shock. Its safety and tolerability were confirmed in healthy human volunteers. In this study, our primary objective was to determine the safety, and the secondary objective was to assess the efficacy of centhaquine in patients with hypovolemic shock.
Methods
A prospective, multicentre, randomized phase II study was conducted in male and female patients aged 18–70 years with hypovolemic shock having systolic BP ≤ 90 mmHg. Patients were randomized in a 1:1 ratio to either the control or centhaquine group. The control group received 100 ml of normal saline infusion over 1 h, while the centhaquine group received 0.01 mg/kg of centhaquine in 100 ml normal saline infusion over 1 h. Every patient received standard of care (SOC) and was followed for 28 days.
Results
Fifty patients were included, and 45 completed the trial: 22 in the control group and 23 in the centhaquine group. The demographics of patients in both groups were comparable. No adverse event related to centhaquine was recorded in the 28-day observation period. The baseline, Injury Scoring System score, haemoglobin, and haematocrit were similar in both groups. However, 91% of the patients in the centhaquine group needed major surgery, whereas only 68% in the control group (p = 0.0526). Twenty-eight-day all-cause mortality was 0/23 in the centhaquine group and 2/22 in the control group. The percent time in ICU and ventilator support was less in the centhaquine group than in the control group. The total amount of vasopressors needed in the first 48 h of resuscitation was lower in the centhaquine group than in the control group (3.12 ± 2.18 vs. 9.39 ± 4.28 mg). An increase in systolic and diastolic BP from baseline through 48 h was more marked in the centhaquine group than in the control group. Compared with the control group, blood lactate level was lower by 1.75 ± 1.07 mmol/l in the centhaquine group on day 3 of resuscitation. Improvements in base deficit, multiple organ dysfunction syndrome (MODS) score and adult respiratory distress syndrome (ARDS) were greater in the centhaquine group than in the control group.
Conclusion
When added to SOC, centhaquine is a well-tolerated and effective resuscitative agent. It improves the clinical outcome of patients with hypovolemic shock.
Trial Registration
ClinicalTrials.gov identifier number: NCT04056065.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01760-4.
Keywords: Centhaquine, Haemorrhage, Hypovolemia, Resuscitative agent, Shock
Key Summary Points
| A multicentric, randomized, controlled trial was conducted to evaluate the safety and efficacy of centhaquine as an adjuvant to the standard of care in hypovolemic shock patients. |
| Fifty patients were randomized 1:1 to receive centhaquine or saline. Centhaquine was administered at a dose of 0.01 mg/kg in 100 ml saline and infused over 1 h. The control group received 100 ml of saline over a 1-h infusion. |
| Centhaquine was safe and well tolerated. There were no drug-related adverse events in the study. |
| Centhaquine improved blood pressure, reduced blood lactate levels, and improved base deficit. The total amount of vasopressors needed was lower in the centhaquine group than in the control group. Multiple organ dysfunction syndrome (MODS) score and acute respiratory distress syndrome (ARDS) also improved with centhaquine. |
| Although the sample size is small, most of the parameters indicate that centhaquine is likely to be a safe and effective resuscitative agent. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to: https://doi.org/10.6084/m9.figshare.14465403.
Introduction
Hypovolemic shock is a life-threatening condition due to depletion of intravascular volume by extracellular fluid loss or blood loss [1]. Patients, if left untreated, can develop ischaemic injury of vital organs, leading to multi-organ failure [1]. About 61,000 in the US and 1.9 million people worldwide die because of haemorrhagic shock every year, and many patients die within the first 6 h [2, 3]. Immediate attention and management are needed to prevent multi-organ failure and death. Infusion of fluid offers the benefit of increasing the intravascular volume, but it rapidly moves out to the extravascular space. Damage control resuscitation prevents a formed clot from being dislodged, dilutes clotting factors, and accelerates haemorrhage because of elevated blood pressure [4–6]. Blood products in a balanced ratio of plasma, platelets, and red blood cells [7] are useful [8]; however, if these measures are not adequate, vasopressors are added to resuscitate patients [9]. Common adverse effects of vasopressors include arrhythmias, fluid extravasation, and ischaemia [10, 11].
The current standard of care (SOC) is inadequate and resuscitative agents are decades old. Attempts to develop an effective resuscitative agent have not been successful. Agents that could decrease metabolic activity to reduce oxygen demand were studied [12–15], but none was promising. Haemoglobin-based blood substitutes were effective in animal models [16, 17] but failed in phase III clinical trials [18–20] and were dropped from further development.
Centhaquine (Lyfaquin®) has been found to be an effective resuscitative agent in rat, rabbit, and swine models of haemorrhagic shock [21–27]. Centhaquine significantly decreased blood lactate, increased mean arterial pressure, pulse pressure, cardiac output, and decreased mortality and increased animals' survival time with severe blood loss. Centhaquine appears to stimulate venous α2B adrenergic receptors to produce venous constriction and increase venous return to the heart, resulting in increased cardiac output and improved tissue perfusion. Centhaquine also acts on central α2A adrenergic receptors to reduce sympathetic drive and decrease arterial vascular resistance contributing to improved tissue blood perfusion [22]. Enhancing tissue blood perfusion is a significant advantage in reducing resuscitation volume and preventing extravasation of fluid and adverse effects of lung oedema. Centhaquine has no action on beta-adrenergic receptors, and therefore the risk of arrhythmias is mitigated.
We performed a double-blind, randomized, and placebo-controlled phase I clinical study (CTRI/2014/06/004647; NCT02408731) [21, 22] to assess the safety and tolerability of centhaquine. Single ascending dose (SAD) and multiple ascending dose (MAD) study with centhaquine showed that it was well-tolerated and safe in healthy male volunteers [21]. None of the subjects experienced a serious adverse event in any cohort. We observed few non-serious adverse events (hypotension, high lactic acid, fall in respiratory rate, dryness of mouth, and drowsiness) at more than ten times higher than the therapeutic dose of 0.01 mg/kg. These events were transient and resolved without sequelae and any intervention. Based on these results, we conducted a phase II trial to evaluate the safety and efficacy of centhaquine in patients with hypovolemic shock due to blood loss.
Methods
We conducted a multicentric, randomized, controlled, double-blind study primarily to determine the tolerability and safety of centhaquine given along with SOC in patients with hypovolemic shock due to blood loss with systolic blood pressure (SBP) ≤ 90 mmHg. The secondary objective was to determine the efficacy of centhaquine as a resuscitative agent.
Study Design
Patients were assessed for eligibility based on the inclusion and exclusion criteria and randomized in a 1:1 ratio either to the centhaquine group receiving centhaquine (0.01 mg/kg) by IV infusion along with SOC or to the control group receiving SOC plus saline. According to the local hospital setting's treatment guidelines, the SOC generally included endotracheal intubation, administration of fluids, blood products, and vasopressors. The study duration for an individual patient was 28 days, including two study visits. Visit 1 on day 1 included screening, randomization, treatment, and visit 2 at the end of the study (day 28 + 5). The patients randomized in this study were in a state of severe life-threatening shock. An Interactive Web Response System (IWRS) was used to randomize the eligible patient to the treatment groups. Each patient was monitored closely throughout his/her hospitalization and followed until discharge from randomization. Each patient was assessed for safety and efficacy parameters over 28 days from randomization. At baseline, we recorded various demographic data (age, gender, weight, height), chest x-ray, ECG, and vital signs. Blood tests at baseline included haematology, blood lactate, base deficit, lipid profile, kidney function tests, liver function tests, and serum electrolytes. We also noted the patient's physical examination, information about their medical history, concomitant illness, concomitant medications, and initial Glasgow coma scale (GCS) and ARDS scores.
Patient Population
In this study, the patients were both males and females aged 18–70 years, with hypovolemic shock due to blood loss with SBP ≤ 90 mmHg at presentation and continued receiving standard shock treatment, having body weight 45–85 kg. The female patients included were either not of childbearing potential, defined as postmenopausal for at least 1 year or surgically sterile, or if of childbearing potential, they agreed to use effective contraception through the study. Patients with postpartum haemorrhage were included. Exclusion criteria were patients with (1) a terminal illness (any other terminal illness developed during the 28-day observation period which was not associated with hypovolemic shock); (2) severe brain injury (GCS < 8); (3) type of injury not known; (4) inability to obtain intravenous access; (5) known pregnancy; (6) cardiopulmonary resuscitation before randomization; (7) the presence of a do not resuscitate order; (8) taking beta-adrenergic antagonists; (9) untreated tension pneumothorax; (10) untreated cardiac tamponade; (11) bilateral absent pupillary light reflex (both pupils fixed and dilated); (12) participating in another interventional study; (13) systemic diseases which were present before having trauma, such as cancer, chronic renal failure, liver failure, decompensated heart failure, or AIDS.
Consent
We took informed consent from every patient. For patients who were not fit to give consent at the time of initiation of treatment, their legally authorized representative (LAR) gave the consent, and we took re-consent of the patients as soon as their condition allowed. The investigator informed the patient/LAR in writing and audio-visual recording about all aspects of the study relevant to deciding whether to participate. The informed consent form included all the elements required by ICH-GCP recommendations and schedule Y.
Treatment Regimen
Centhaquine (Lyfaquin®; lyophilized centhaquine citrate injection 1.0 mg) manufactured by Pharmazz India Private Limited at Gufic Biosciences Limited was provided to the investigators at the participating sites. Patients who met the eligibility criteria were randomized 1:1 to the centhaquine or control groups. Throughout the study, all patients in both groups received the best SOC for hypovolemic shock according to local institutional standard practice, including fluid resuscitation with crystalloids/colloids, blood products, and vasopressors. Centhaquine or placebo (normal saline) was administered intravenously after randomization to hypovolemic shock patients, an add-on to SOC, and all patients continued receiving standard treatment for hypovolemic shock. In the centhaquine group, intravenous infusion of centhaquine at a dose of 0.01 mg/kg body weight was carried out over 1 h in 100 ml normal saline. The next dose of centhaquine was administered if SBP fell or remained below or equal to 90 mmHg, but not before 4 h of the previous dose, and the total number of doses did not exceed three per day. Centhaquine administration, if needed, was continued for 2 days post-randomization. A minimum of one dose and a maximum of six doses of centhaquine were administered within the first 48 h post-randomization. Similar treatment was carried out in the control group using an equal volume of normal saline administered as an intravenous infusion over 1 h in 100 ml of normal saline post-randomization. Specific intravenous treatments and dose selection were based on preclinical and phase I safety and tolerability studies [21, 22].
Data Safety Monitoring Board
An independent data safety monitoring board consisting of a clinician with experience in critical care medicine, a biostatistician, and a clinical pharmacologist monitored the trial's safety and efficacy. The data safety monitoring board reviewed each subject's safety data from the study and all serious adverse events, regardless of attribution, contemporaneously with submissions to the sponsor and investigator.
Randomization and Blinding
Patients were randomized 1:1 to either centhaquine or placebo using block randomization. A statistician prepared the randomization list using a validated computer program, the statistical analysis system SPSS. An interactive web response system (IWRS) method containing randomization codes was used to randomize eligible patients to the treatment groups. The patients and all relevant personnel involved with the conduct and interpretation of the study (including the investigator, investigational site personnel, and the sponsor or designee's staff) were blinded to the identity of the assigned study drug (centhaquine/placebo) and the randomization codes. The biostatistician/unblinded pharmacist was independent of the study team. An unblinded monitor independent of the monitoring team monitored the dispensing activity. The final randomization list was kept strictly confidential and accessible only by authorized people per sponsor until completion of the study. Emergency unblinding through IWRS was available.
Outcome Assessment
Safety Assessment
All patients who received treatment were included in the safety analysis. Safety was assessed during treatment and the post-treatment follow-up period based on adverse events, physical examination, vital signs, ECG, and clinical laboratory parameters as per protocol. A complete set of haematological, biochemical, and organ function tests (complete blood count, blood glucose, lipid profile, serum electrolytes, liver function test, and kidney function test) was performed. Adverse events that occurred or worsened during treatment or post-treatment were recorded. All adverse events were coded by preferred term and system organ class using the latest version of the Medical Dictionary for Regulatory Activities. All patients were followed up for safety assessment at visit 1 (from day 1 to day 7 or discharge, whichever is earlier) and visit 2 (day 28 + 5).
Efficacy Assessment
The efficacy of centhaquine in patients of hypovolemic shock was assessed using (1) mortality through day 28 days; (2) days in ICU and on a ventilator; (3) total fluids and blood products requirement during first 48 h; (4) the total amount of vasopressor infused in first 48 h; (5) the number of doses of centhaquine administered in first 48 h post-randomization; (6) haemodynamic variables mean through 48 h; (7) blood haematocrit and haemoglobin mean through 48 h; (8) blood lactate and base deficit mean through day 3; (9) coagulation parameter mean through 28 days. Additionally, MODS, ARDS, and GCS means through 28 days were recorded.
Sample Size Estimate
The data obtained from earlier clinical studies were considered for sample size determination for this phase II study. Trauma Center registry data suggest that 30-day mortality under the SOC protocol is between 16.4 and 29.2% [28]. A similar estimate of mortality (26%) was for the control group of the Resuscitation Outcomes Consortium pre-hospital hypertonic saline trial [29]. If we assume 18% mortality in the control group, a sample size of a minimum of 15 per group was required to achieve 80% power to detect a clinically significant (at 5% significant level) reduction in mortality of 66% (9.6% mortality) in the study group [30]. We further considered a 20% loss to follow-up, and with this, a total of 36 patients (18 in each group) was required. To increase the study's power, we increased the sample size to 50 patients (25 patients in each group).
Data Analysis
The results of the trial are presented as mean ± SEM. Unpaired t-test with Welch's correction was used to analyse data sets with unequal variances. The unpaired t-test was used to compare the discrete variables between the two data sets at baseline and follow-up. Non-parametric analysis was carried out using Kruskal-Wallis one-way ANOVA without assuming equal variances, and Tukey's multiple comparisons test estimated the significance of differences. A chi-square test was used to compare the groups. Baptista-Pike method was used to calculate the odds ratio. A P value < 0.05 was considered significant at a 95% confidence level and 0.10 at a 90% confidence level. Demographic variables (age, weight, height, body surface area, and body mass index) and patient characteristics were summarized descriptively by treatment assignments. Continuous variables, such as patient age at enrolment, number of non-missing observations (n), mean, and SEM, were tabulated by treatment assignment. All available data were used in the analyses. Each group was summarized individually. Unavailable data were assessed as "missing values" and only the observed population was evaluated. The statistical analysis was processed with GraphPad Prism 8.1.1 (GraphPad, San Diego, CA, USA).
Regulatory Oversight
The study was performed according to the Declaration of Helsinki principles, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for Good Clinical Practice (ICH-GCP), and local regulatory requirements. The study protocol (PMZ-02, version 2.0/dated 10 March 2016) was approved by the Drugs Controller General of India, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India (DCGI CT NOC. no.: CT/ND/37/2016). Each institutional ethics committee also reviewed and approved the study protocol before initiating patient enrolment. The trial was registered at the Clinical Trials Registry, India (CTRI/2017/03/008184), and the United States National Library of Medicine, ClinicalTrials.gov (NCT04056065). Each site's ethics committee was informed of any protocol deviation, amendment, subject exclusion or withdrawal, and serious adverse events.
Results
Patient Enrolment and Demographics
A total of 137 patients were assessed in seven clinical sites across India, and 50 patients met the eligibility criteria. All patients received standard treatment for hypovolemic shock and were randomly assigned to either the control group (N = 26) who received standard treatment for shock and normal saline or the centhaquine group (N = 24) that received standard treatment for shock along with centhaquine. From the control group, 22 patients completed the study (2 patients withdrawn by the investigator, 2 patients withdrew the consent), and from the centhaquine group, 23 patients completed the study (1 patient withdrawn by the investigator) (Fig. 1). Demographics and baseline characteristics of patients were comparable between the two groups. More male than female patients were included in the control and centhaquine groups, and their proportion was similar in both groups (Table 1). Age, body weight, height, BMI, and BSA were similar in each group (Table 1).
Fig. 1.
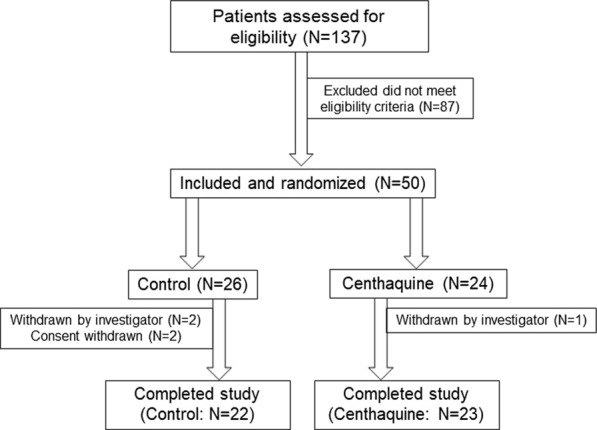
Patient enrolment, randomization, and trial completion
Table 1.
Baseline characteristics of patients
| Control (N = 22) | Centhaquine (N = 23) | |
|---|---|---|
| Age (years) | 35.82 ± 3.24 | 42.13 ± 2.90 |
| Body weight (kg) | 64.27 ± 2.42 | 64.78 ± 2.79 |
| Body mass index (kg/m2) | 22.65 ± 0.76 | 23.06 ± 0.76 |
| Body surface area (m2) | 1.73 ± 0.03 | 1.72 ± 0.04 |
| Sex | ||
| Men | 16 (72.72%) | 18 (78.26%) |
| Women | 06 (27.27%) | 05 (21.74%) |
| Reason for hypovolemic shock | ||
| Trauma | 14 (63.64%) | 15 (65.23%) |
| Post-surgery | 03 (13.64%) | 02 (8.69%) |
| Molar/ectopic pregnancy/uterine rupture/hysterectomy | 03 (13.64%) | 02 (8.69%) |
| Gastrointestinal bleeding | 02 (9.09%) | 04 (17.39%) |
| Clinical factors | ||
| Systolic blood pressure (mmHg) | 90.45 ± 2.28 | 87.36 ± 1.85 |
| Diastolic blood pressure (mmHg) | 59.64 ± 3.17 | 57.64 ± 1.50 |
| Heart rate (beats/min) | 94.36 ± 5.09 | 98.91 ± 5.07 |
| Respiratory rate (breaths/min) | 20.73 ± 0.77 | 21.23 ± 0.93 |
| Body temperature (°C) | 36.73 ± 0.07 | 36.61 ± 0.06 |
| Blood lactate (mmol/l) | 4.30 ± 0.96 | 4.34 ± 0.78 |
| Base deficit (momol/l) | − 7.40 ± 1.42 | − 5.78 ± 1.22 |
| Haemoglobin (g/dl) | 9.38 ± 0.71 | 8.73 ± 0.55 |
| Haematocrit (%) | 28.79 ± 2.11 | 26.43 ± 0.96 |
| Creatinine (mg/dl) | 0.94 ± 0.10 | 1.13 ± 0.10 |
| Blood urea nitrogen (mg/dl) | 12.46 ± 1.06 | 15.24 ± 1.61 |
| Glomerular filtration rate (ml/min/1.73 m2) | 114.08 ± 10.47 | 90.86 ± 9.72 |
| Injury severity score | 20.63 ± 2.45 | 23.14 ± 3.30 |
| Glasgow coma scale | 13.95 ± 0.44 | 13.78 ± 0.45 |
| Adult respiratory distress syndrome | 0.25 ± 0.11 | 0.12 ± 0.05 |
| pH | 7.32 ± 0.03 | 7.36 ± 0.02 |
| pCO2 (mmHg) | 36.11 ± 2.87 | 33.58 ± 1.53 |
| paO2 (mmHg) | 93.92 ± 9.67 | 98.50 ± 13.57 |
Data are presented as mean ± standard error of the mean
Patient Assessment at the Time of Inclusion
The Injury Scoring System (ISS) values of patients in the control and centhaquine groups were measured on day 1 (baseline). The ISS value indicates the severity of an injury which was a little higher in centhaquine group patients (23.14 ± 3.30) compared to controls (20.63 ± 2.45), although the difference did not reach the level of statistical significance. Baseline levels of haemoglobin (control 9.38 ± 0.71 g/dl vs. centhaquine 8.73 ± 0.55 g/dl) and haematocrit (control 28.79 ± 2.11% vs. centhaquine 26.71 ± 1.81%) were almost similar in control and centhaquine groups patients. The level of consciousness (GCS) of patients in both groups was similar (control 13.95 ± 0.44 vs. centhaquine 13.78 ± 0.45). Table 2 shows the case details of individual patients enrolled in each cohort.
Table 2.
Case details of patients in control and centhaquine groups
| Control (N = 22) | Centhaquine (N = 23) | ||
|---|---|---|---|
| Patient no. | Case details | Patient no. | Case details |
| 01-004 |
Male, age 67 years; case of RTA, bleeding over nose & multiple abrasion over face Event occurrence: 3 December 2017 Reporting at hospital (as per hospital records): 3 December 2017 at 2:39 p.m. Received first dose of study drug: 3 December 2017 at 5:50 p.m. Surgery/procedure during hospitalization (if any): Open reduction and internal fixation (ORIF)-plating fracture proximal tibia right + ORIF herbert screw fixation fracture talus + tension band wiring fracture medial malleolus right on 4 December 2017. Proximal humerus left ORIF performed on 7 December 2017 |
01-001 |
Male, age 53 years, case of RTA, wound at right thigh with abnormal mobility at right thigh and knee. Active bleed from wound. Thigh x-ray shows compound grade III fracture in right femur. Multiple abrasion over right side of chest and right wrist joint Event occurrence (as per hospital records): 28 May 2017 at 6:30 p.m. Reporting at hospital: 28 May 2017 at 9:45 p.m. Received first dose of study drug: 29 May 2017 at 12:52 a.m. Surgery/procedure during hospitalization (if any): ORIF of right femur performed on 29 May 2017 |
| 01-005 |
Male, age 26 years; case of RTA Event occurrence (as per hospital records): 22 December 2017 at 9:00 p.m. Reporting at hospital: 23 December 2017 at 12:05 a.m. Received first dose of study drug: 23 December 2017 at 8:45 a.m. Surgery/procedure during hospitalization (if any): Closed reduction and intramedullary nailing fracture shaft femur right performed on 23 December 2017. ORIF-plating fracture proximal humerus performed on 26 December 2017. ORIF tension band wiring fracture olecranon performed on 26 December 2017 |
01-002 |
Male, age 24 years, case of RTA. Lower limb x-ray showed fractured femur shaft Event occurrence (as per hospital records): 12 October 2017 at 9:00 p.m. Reporting at hospital: 13 October 2017 at 4:33 a.m. Received first dose of study drug: 13 October 2017 at 8:00 a.m. Surgery/procedure during hospitalization (if any): Closed reduction + intramedullary nailing |
| 01-007 |
Male, age 28 years; case of RTA Event occurrence (as per hospital records): 8 June 2018 at 7:00 p.m. Reporting at hospital: 9 June 2018 at 12:15 a.m. Received first dose of study drug: 9 June 2018 at 3:00 a.m. Surgery/procedure during hospitalization (if any): None |
01-006 |
Male, age 52 years; case of RTA, left lower limb pain and bleeding right frontal abrasion Event occurrence (as per hospital records): 14 January 2018 at 6:00 a.m. Reporting at hospital: 14 January 2018 at 8:47 a.m. Received first dose of study drug: 14 January 2018 at 1:25 p.m. Surgery/procedure during hospitalization (if any): Debridement + cemented intramedullary nail + vacuum-assisted closure performed on 14 January 2018. Exchange nail with tibia nail and debridement performed on 23 January 2018. Free anterolateral thigh flap cover and split thickness skin graft vascularized by anterior tibial artery and vein performed on 25 January 2018 |
| 02-001 |
Male, age 31 years; case of RTA with blunt trauma abdomen with splenic laceration grade III with moderate haemoperitoneum with hepatitis Event occurrence (as per hospital records): 29 May 2017 at 10:00 a.m. Reporting at hospital: 29 May 2017 at 11:45 a.m. Received first dose of study drug: 29 May 2017 at 3:50 p.m. Surgery/procedure during hospitalization (if any): None |
01-008 |
Male, age 28 years; case of RTA, compound fracture right tibia fibula with posterior tibial artery injury Event occurrence (as per hospital records): 24 July 2018 at 6:00 p.m. Reporting at hospital: 24 July 2018 at 7:00 p.m. Received first dose of study drug: 24 July 2018 at 9:20 p.m. Surgery/procedure during hospitalization (if any): Exploration and repair of posterior tibia artery performed on 24 July 2018 Debridement intramedullary nailing posterior tibia arterial repair, cemented implantation performed on 26 July 2018. Debridement with skin grafting over raw area medial side of right leg performed on 27 July 2018 |
| 02-004 |
Female, age 31 years; case of ruptured ectopic pregnancy with pain in lower abdomen. Intraoperative finding: haemoperitoneum 800 mL dark altered blood present Event occurrence (as per hospital records): 25 January 2018 at 2:45 p.m. Reporting at hospital: 25 January 2018 at 2:45 p.m. Received first dose of study drug: 25 January 2018 at 5:30 p.m. Surgery/procedure during hospitalization (if any): Haemoperitoneum 800 mL dark altered blood was present (intraoperative finding). Right tube salpingectomy and left side tubal ligation performed on 25 January 2018 |
02-002 |
Male, age 50 years; case of RTA, injuries over right upper and lower limb with active bleeding and swelling and presented with inability to walk to bear weight on bilateral lower limb. Open grade IIIA fracture femur with closed fracture distal femur with intraarticular extension. Pubic diastasis with fracture superior pubic rami with sacroiliac joint disruption with soft tissue injury over bilateral feet Event occurrence (as per hospital records): 30 June 2017 at 4:05 p.m. Reporting at hospital: 30 June 2017 at 4:05 p.m. Received first dose of study drug: 30 June 2017 at 7:00 p.m. Surgery/procedure during hospitalization (if any): Closed reduction and internal fixation of fracture femur with Sirus femur nail. Closed reduction and internal fixation of fracture distal femur. Stabilization of pubic diastasis with pelvic external fixator |
| 02-005 |
Male, age 56 years; case of RTA with multiple ortho injuries, open fracture bilateral lower limb and compression fracture of D3, D4 and D7 vertebra Event occurrence (as per hospital records): 21 February 2018 at 5:00 p.m. Reporting at hospital: 21 February 2018 at 7:40 p.m. Received first dose of study drug: 21 February 2018 at 9:40 p.m. Surgery/procedure during hospitalization (if any): Close reduction and stabilization of fracture femur with application of spanning external fixator across knee. Close reduction and stabilization of fracture bilateral distal both bone leg with across ankle external fixator bilateral performed on 28 February 2018 |
02-003 |
Male, age 46 years; case of RTA with injuries over right upper and lower limb with active bleeding and swelling. Open grade IIIA fracture humerus right side with comminution. Open-grade IIIA fracture both bone leg right side with comminution. Compound knee with lateral patellar retinaculum right. Soft tissue injury right thigh. Fracture medial epicondyle humerus. Blunt trauma chest and abdomen Event occurrence (as per hospital records): 23 August 2017 at 4:30 p.m. Reporting at hospital: 23 August 2017 at 7:33 p.m. Received first dose of study drug: 23 August 2017 at 9:45 p.m. Surgery/procedure during hospitalization (if any): Application of external fixation right humerus, application of external fixation right leg., debridement and irrigation of arm, thigh and leg wound, lateral patellar retinaculum repair on 25 August 2017. ORIF with 2.4MM cortical screw. Right humerus medial epicondyle screw fixation. Debridement and irrigation of thigh and leg on 31 August 2017. Debridement and irrigation of wound with readjustment of external fixation right arm on 5 September 2017 |
| 02-007 |
Female, age 21 years; case of RTA with recurrent loss of consciousness, Degloving injury of right axilla extending dorsally to scapula with exposed brachial plexus and vessels. Degloving injury extending up to right elbow joint laterally with exposed muscles. Multiple right-side rib fractures with haemothorax. Head Injury: Small epidural haemorrhage with subarachnoid haemorrhage Event occurrence (as per hospital records): 5 March 2018 at 9:00 a.m. Reporting at hospital: 5 March 2018 at 9:50 a.m. Received first dose of study drug: 5 March 2018 at 1:35 p.m. Surgery/procedure during hospitalization (if any): Skin grafting with rib fixation performed on 7 March 2018 |
02-006 |
Male, age 60 years; case of RTA with pelvic fracture, pre-vesicle haematoma, extra-peritoneal rupture of urinary bladder, B/L nasal bone fracture and fracture roof of left orbit Event occurrence (as per hospital records): 26 February 2018 at 7:00 p.m. Reporting at hospital: 26 February 2018 at 9:20 p.m. Received first dose of study drug: 26 February 2018 at 11:30 p.m. Surgery/procedure during hospitalization (if any): Shoulder repair left side was performed on 1 March 2018. Anterior bladder was repaired om 23 March 2018 |
| 02-009 |
Male, age 26 years; case of RTA with blunt trauma abdomen with ortho injury Abrasion on Left (5 × 1 cm) and right leg, laceration (4 × 2 cm) right thigh, abrasion dorsum of B/L hand, laceration (3 × 1 cm) below chin Open grade II fracture shaft femur. On MRI knee: Intercondylar fracture with avulsion of posterior cruciate ligament from its tibial attachment. Marrow edema of bilateral femoral condyles, lateral tibial condyles, patellae CT abdomen: Moderate haemoperitoneum. Small mesenteric haematoma Event occurrence (as per hospital records): 22 March 2018 at 10:00 a.m. Reporting at hospital: 22 March 2018 at 11:30 a.m. Received first dose of study drug: 22 March 2018 at 1:35 p.m. Surgery/procedure during hospitalization (if any): None |
02-008 |
Male, age 35 years; case of RTA, transient loss of consciousness, pain in chest and abdomen, pain in back with shock. Abrasion left side of chest, large abrasion (25 × 25 cm) on back, abrasion right ear, laceration wound right temporo-parietal injury. USG abdomen-moderate fluid seen in peritoneal cavity. CT abdomen-American Association for Surgery of Trauma (AAST) grade III pancreatic injury with peripancreatic haematoma and active blood, AAST grade II splenic injury, AAST grade V left renal injury, lung contusion Event occurrence (as per hospital records): 6 March 2018 at 5:00 p.m. Reporting at hospital: 6 March 2018 at 6:15 p.m. Received first dose of study drug: 6 March 2018 at 8:00 p.m. Surgery/procedure during hospitalization (if any): Laparotomy with splenectomy performed on 7 March 2018 |
| 02-012 |
Male, age 37 years; case of RTA, loss of consciousness with bleeding from right ear and nasal cavity. Left lower limb fracture tibia, fracture left shaft femur with comminuted fracture left patella and Fracture parasymphyseal mandible with B/L condyle. Abnormal bony mobility left thigh with bony crepitus with tenderness, right-side facial swelling and abnormally mobile mandible and malocclusion present Event occurrence (as per hospital records): 3 July 2018 at 10:30 a.m. Reporting at hospital: 3 July 2018 at 2:25 p.m. Received first dose of study drug: 3 July 2018 at 3:05 p.m. Surgery/procedure during hospitalization (if any): Tibia nail (21 × 20), ORIF of fracture shaft femur with IMLN (11 × 40 cm) and fixation of fracture lateral condyle, ORIF-plating of fracture mandible performed on 4 July 2018 |
02-010 |
Male, age 24 years; case of fall from height. Pelvic fracture, haemoperitoneum, liver laceration, rhabdomyolysis, multiple rib fracture right side with pneumothorax. CT abdomen: Liver injury ASST grade II, moderate haemoperitoneum, pelvic fracture, mild bilateral pleural effusion with multiple rib fracture and right pneumothorax Event occurrence (as per hospital records): 7 June 2018 Reporting at hospital: 8 June 2018 at 11:56 a.m. Received first dose of study drug: 8 June 2018 at 2:30 p.m. Surgery/procedure during hospitalization (if any): Right acetabular plating with olecranon fracture repaired on 16 June 2018. Left fracture acetabulum repaired on 19 June 2018 |
| 02-013 |
Male, age 67 years; case of RTA with blunt trauma abdomen and chest. CT scan reveals subdural haematoma with subarachnoid haematoma and intraventricular haemorrhage. Fracture of left parietal bone extending into squamous and mastoid part of left temporal bone with opacification of mastoid air cells. Multi-detector row computed tomography whole abdomen reveals mesenteric haematoma, mild ascites, and multiple fractures Event occurrence (as per hospital records): 25 July 2018 at 7:30 a.m. Reporting at hospital: 25 July 2018 at 11:15 a.m. Received first dose of study drug: 25 July 2018 at 2:15 p.m. Surgery/Procedure during hospitalization (if any): None |
02-011 |
Male, age 61 years; case of RTA with wound over posterior aspect of right knee, pain in right knee, right thigh and right leg with pain in pelvic region. Lacerated wound over right proximal leg anterior aspect (4 × 5 cm). Lacerated wound over popliteal area (35 × 15 cm). Open grade IIIB fracture distal femur and proximal tibia right side with compound knee. Pubic diastasis with right sacroiliac joint disruption Event occurrence (as per hospital records): 22 June 2018 at 5:30 a.m. Reporting at hospital: 22 June 2018 at 12:30 p.m. Received first dose of study drug: 22 June 2018 at 2:20 p.m. Surgery/procedure during hospitalization (if any): Open reduction and stabilization of fracture distal femur and proximal tibia with external fixator across right knee performed on 23 June 2018. Closed reduction and stabilization of pubic diastasis with pelvic external fixator. Removal of pelvic external fixator. ORIF of pubic diastasis with anterior pelvic plate, split skin grafting on wound over right popliteal region with graft harvested from contra-lateral thigh was performed on 3 July 2018 |
| 02-016 |
Male, age 28 years; case of RTA, followed by vomiting, chest and abdomen pain Mesenteric injury with right tibia fracture. CT scan reveals splenic injury grade II, mesenteric haematoma with active extravasation of contrast, significant haemoperitoneum USG chest reveals bilateral pleural effusion Event occurrence (as per hospital records): 17 August 2018 Reporting at hospital: 17 August 2018 at 11:55 a.m. Received first dose of study drug: 17 August 2018 at 4:40 p.m. Surgery/procedure during hospitalization (if any): Exploratory laparotomy for blunt trauma abdomen and resection and anastomosis of jejunal segment with ligation of mesenteric bleeders was performed on 17 August 2018 Closed reduction and internal fixation of fracture tibia with tibial nail performed on 23 August 2018 |
02-014 |
Female, age 43 years; case of RTA, loss of consciousness, head injury, ENT bleed with chest and abdomen pain. Brain CT scan reveals head injury with non-haemorrhagic contusions, infarct in right thalamus with right parietal and left frontal subarachnoid haemorrhage with pneumocephalus. Evidence of comminuted depressed fracture all the walls of bilateral frontal and maxillary sinus and bilateral ethmoid septa with evidence of haemosinus, fracture bilateral nasal bones, bony nasal septum, left lateral pterygoid plate, all the walls of left orbit, medial and posterolateral walls and floors of right orbit. MRI spine reveals large haematoma in subcutaneous tissue in dorsal cervical region, prevertebral collection opposite C1-4 level, fracture of 1st rib on right side with right eye exposure keratopathy with conjunctival chemosis Event occurrence (as per hospital records): 8 August 2018 Reporting at hospital: 8 August 2018 at 5:10 a.m. Received first dose of study drug: 8 August 2018 at 1:20 p.m. Surgery/procedure during hospitalization (if any): Bilateral maxilla and left zygoma plating with soft tissue repair on face and scalp on 11 August 2018 |
| 05-005 |
Female, age 24 years; post-operative case of exploratory laparotomy followed by hysterectomy. Perforating molar pregnancy. Haemorrhagic collection in peritoneal cavity, B/L theca-lutein cysts, perforation at right border of cervix. Severe acidosis, hypotension due to haemorrhagic shock. Patient was admitted on 27 May 2018 with bleeding per vaginal and abdominal pain. The ultrasonography shows corrosive mole (molar pregnancy). The general condition of the patient was very poor, and patient was suffering from severe respiratory distress Event occurrence (as per hospital records): 1 June 2018 at 5:56 p.m. Reporting at hospital: 1 June 2018 at 5:56 p.m. Received first dose of study drug: 1 June 2018 at 7:00 p.m. Surgery/procedure during hospitalization (if any): Exploratory laparotomy followed by hysterectomy performed on 1 June 2018 |
02-015 |
Male, age 59 years; machine cut injury on right limbs and fell to ground and suffered laceration followed by bleeding from cut. Traumatic amputation right lower limb, sepsis, pneumonitis, and acute kidney injury (recovering phase) Event occurrence (as per hospital records): 17 August 2018 at 8:00 a.m. Reporting at hospital: 17 August 2018 at 10:25 a.m. Received first dose of study drug: 17 August 2018 at 12:15 p.m. Surgery/procedure during hospitalization (if any): Patient underwent haemodialysis |
| 05-007 |
Male, age 24 years; post-operative case of re-exploration of abdomen after open adrenalectomy. On 26 July 2018 resection of right-side supra-renal mass adrenalectomy done. On same day re-surgery was done to stop bleeding Event occurrence (as per hospital records): 26 July 2018 at 11:00 p.m. Reporting at hospital: 26 July 2018 at 11:00 p.m. Received first dose of study drug: 27 July 2018 at 2:00 a.m. Surgery/procedure during hospitalization (if any): On 26/07/2018 at 10:30 a.m. Resection of right-side supra-renal mass (Pheochromocytoma) adrenalectomy performed on 26 July 2018. Re-exploration of abdomen after open adrenalectomy on 26 July 2018. Re-surgery done to stop bleeding on 26 July 2018 |
05-003 |
Female, age 26 years; post-operative case of haemobilia, right hepatic artery pseudoaneurysm, status open cholecystectomy. Blood loss in the OT room. Cholestatic liver, no free fluid inside the abdomen. A 3 × 4-cm pseudoaneurysm arising from RHA palpated in lateral aspect of HDL. Pseudoaneurysm erodes the CBD near hilum. Pseudoaneurysm wall opened and around 100 cc clot removed Event occurrence (as per hospital records): 17 March 2018 at 8:55 p.m. Reporting at hospital: 17 March 2018 at 8:55 p.m. Received first dose of study drug: 18 March 2018 at 12:00 a.m. Surgery/procedure during hospitalization (if any): Excision of hepatic artery aneurysm and T-tube placement on 17 March 2018 |
| 08-003 |
Male, age 45 years; case of RTA followed by loss of consciousness, two episodes of vomiting, nasal bleed and wound over forehead. Right knee septic arthritis with open head injury with pneumocephalus with wound over frontal region right side secondary to accident with nasal bone fracture, supra- and infra-orbital rim fracture Event occurrence (as per hospital records): 18 July 2018 at 8:00 p.m. Reporting at hospital: 19 July 2018 at 12:00 a.m. Received first dose of study drug: 19 July 2018 at 12:30 a.m. Surgery/procedure during hospitalization (if any): Exploration and debridement of wound was performed on 19/07/2018 |
05-004 |
Male, age 43 years; post-traumatic post-infective fracture clavicle with suspected pseudoaneurysm at right shoulder. Excision of right clavicle bone exploration of right subclavian area done. Right subclavian artery seen ruptured with pseudoaneurysm and rupture of subclavian vein cuts repaired. Blood loss approximate 3 l Event occurrence (as per hospital records): 7 April 2018 at 5:36 p.m. Reporting at hospital: 7 April 2018 at 5:36 p.m. Received first dose of study drug: 7 April 2018 at 8:10 p.m. Surgery/procedure during hospitalization (if any): Right clavicular fracture and right clavicular fixation on 7 April 2018. Right subclavian artery pseudoaneurysm-vessel repaired |
| 08-005 |
Male, age 20 years; case of RTA, pubic (Pelnic) diastasis with sacrum fracture. MRI and CT scan revealed fracture of right transverse process of L5 vertebral body, comminuted fracture of S-3 vertebral body and posterior element posterior subluxation of proximal fracture fragments causing compression over corresponding traversing sacral nerve roots, comminuted fracture of transverse process of all sacral vertebrae involving neural foramina retro-peritoneal haematoma Event occurrence (as per hospital records): 16 September 2018 at 2:30 p.m. Reporting at hospital: 16 September 2018 at 09:35 p.m. Received first dose of study drug: 17 September 2018 at 11:30 a.m. Surgery/procedure during hospitalization (if any): ORIF with CC screw for scram fracture. ORIF with plating for pubic diastasis performed on 23 September 2018 |
05-006 |
Female, age 31 years; post-operative case of laparotomy followed by hysterectomy, placental abruption, and uterine rupture. Around 2 l blood found in peritoneal cavity. Tear found in anterior and posterior wall of uterus. Patient 19 weeks of gestation with lower abdominal pain USG suggesting amniotic membrane separation, retro-amniotic and retro-peritoneal collection, tear in right lateral uterine wall through which collection extended to peri-uterine region. Interloop fluid is also noted Event occurrence (as per hospital records): 29 June 2018 at 4:06 p.m. Reporting at hospital: 29 June 2018 at 3:30 a.m. Received first dose of study drug: 29 June 2018 at 6:30 p.m. Surgery/procedure during hospitalization (if any): Laparotomy followed by hysterectomy done due to placental abruption and uterine rupture with B/L internal Iliac artery ligation on 29 June 2018 |
| 10-001 |
Male, age 32 years; RTA with fracture left I/T neck of femur, fracture left tibia condyle with fibular head fracture with right-side pneumothorax. Undisplaced fracture in the anterior portion of right 7th rib. Liver shows subcapsular haematoma correspond to grade III–IV liver injury Event occurrence (as per hospital records): 29 May 2018 at 11:30 a.m. Reporting at hospital: 30 May 2018 at 9:45 a.m. Received first dose of study drug: 30 May 2018 at 1:15 p.m. Surgery/procedure during hospitalization (if any): ORIF with PFN and ORIF with tibia condyle plating performed for left I/T fracture with tibia condyle fracture on 1 June 2018 |
07-001 |
Male, age 52 years; patient complaint of altered sensorium, haematuria, stool melena, fever, vomiting, and breathlessness. History of fever, on and off with vomiting from 2 months. Diagnosis: multiple liver abscess, septicaemia, hyponatraemia, and generalized tonic clonic convulsion Event occurrence (as per hospital records): 13 January 2018 at 2:50 p.m. Reporting at hospital: 13 January 2018 at 2:45 p.m. Received first dose of study drug: 13 January 2018 at 6:22 p.m. Surgery/procedure during hospitalization (if any): None |
| 10-004 |
Male, age 51 years; patient underwent endoscopic retrograde cholangiopancreatography (ERCP) stenting for obstructive jaundice on 26 May 2018. Post-ERCP patient was in shock with distension of abdomen. CT abdomen showed duodenal perforation Event occurrence (as per hospital records): 6 June 2018 at 6:45 p.m. Reporting at hospital: 28 May 2018 at 6:45 p.m. Received first dose of study drug: 6 June 2018 at 11:10 p.m. Surgery/procedure during hospitalization (if any): Exploratory laparotomy, duodenum repair, cholecystectomy + CBD exploration, T tube insertion done, stone removed with right DJ stenting, feeding jejunostomy done on 6 June 2018 |
08-001 |
Male, age 27 years; case of RTA, head and neck injury, pain in neck, loss of sensation, seizures, vomiting. MRI spine-wedge compression fracture of the C5 vertebral body with retropulsion of the fracture fragment causing significant compression on the subarachnoid space and spinal cord resulting. CT brain-epidural haematoma noted in occipital region on left side, 6 mm on bone window, fracture of the parietal bone along the sagittal suture, subgaleal haematoma, noted in the parieto-temporal and occipital regions Event occurrence (as per hospital records): 29 March 2018 at 2:00 p.m. Reporting at hospital: 29 March 2018 at 4:15 p.m. Received first dose of study drug: 29 March 2018 at 9:33 p.m. Surgery/procedure during hospitalization (if any): C5 corpectomy with bone grafting with C4–C6 plating performed on 11 April 2018 |
| 10-005 |
Female, age 41 years; known case of obstructive uropathy intravenous pyelogram with moderate hydronephrosis, calculus in renal pelvis. Operated on 9 June 2018 for right percutaneous nephrolithotomy and left stenting done. This was an intra-operative case of hypovolemic shock Event occurrence (as per hospital records): 9 June 2018 at 8:00 p.m. Reporting at hospital: 9 June 2018 at 8:00 p.m. Received first dose of study drug: 9 June 2018 at 11:45 p.m. Surgery/procedure during hospitalization (if any): percutaneous nephrolithotomy and left DJ stenting performed on 9 June 2018 |
08-002 |
Male, age 30 years; case of RTA, sustained injury to right lower limb, knee, and thigh. Right-side type III B open distal 1/3rd comminuted tibia fibula fracture with bone loss and soft tissue loss. Open wound of (20 × 10 cm) over right lower leg and ankle. Right lower limb: Open comminuted fracture of right tibia and fibula bone exposed with bleeding. Soft tissue defect over anterior aspect of ankle. Anterior tendon and bone-exposed, abrasion wound over knee and thigh Event occurrence (as per hospital records): 31 March 2018 at 11:30 p.m. Reporting at hospital: 1 April 2018 at 6:10 a.m. Received first dose of study drug: 1 April 2018 at 12:03 p.m. Surgery/procedure during hospitalization (if any): External fixation for right tibia + TENS nail for fibula bone under spinal anaesthesia on 1 April 2018. Debridement of wound bone. IMIL nailing for right tibia fracture bone and debridement + split thickness skin grafts done on 10 April 2018 |
| 10-007 |
Female, age 28 years; patient admitted with the complaint of pain in lower abdomen, minimal PV bleeding, vomiting (10–12 times), fainting episode once. Retention of urine (catheterized outside) initially treated and referred to site for further management. Diagnosis: Left-side ruptured ectopic pregnancy Event occurrence (as per hospital records): 20 July 2018 at 5:32 p.m. Reporting at hospital: 20 July 2018 at 5:32 p.m. Received first dose of study drug: 20 July 2018 at 8:00 p.m. Surgery/procedure during hospitalization (if any): Laparoscopic salpingectomy performed on 20 July 2018 for ectopic pregnancy |
10-002 |
Male, age 24 years; patient admitted with complaint of active P/R bleeding, abdominal pain. Caecal telangiectasia with bleeding from caecum. USG abdomen and pelvis reveals mild ascites (bilateral iliac fossae and hepato-renal pouch) Event occurrence (as per hospital records): 30 May 2018 at 5:00 PM Reporting at hospital: 30 May 2018 at 5:00 p.m. Received first dose of study drug: 30 May 2018 at 9:00 p.m. Surgery/procedure during hospitalization (if any): Telangiectasia at IC junction with limited hemicolectomy performed on 30 May 2018 |
| 11-001 |
Female, age 19 years; case of haematemesis, black-coloured stool for 3 days (3–4 episodes per day). History of intermittent haematemesis and melaena for 6–7 years. Pallor + + UGG: Mild splenomegaly. CT abdomen: Ostial narrowing of celiac artery hepato-splenomegaly Event occurrence (as per hospital records): 17 June 2018 at 10:02 p.m. Reporting at hospital: 17 June 2018 at 10:02 p.m. Received first dose of study drug: 18 June 2018 at 11:53 a.m. Surgery/procedure during hospitalization (if any): None |
10-003 |
Female, age 30 years; admitted with complaint of bilateral ovarian masses. CT abdomen and pelvis reveals bilateral ovarian masses with multiple enlarged iliac and retroperitoneal lymph nodes Event occurrence (as per hospital records): 26 May 2018 at 5:00 p.m. Reporting at hospital: 26 May 2018 at 5:00 p.m. Received first dose of study drug: 2 June 2018 at 12:30 a.m. Surgery/procedure during hospitalization (if any): Laparotomy for ovarian masses with hysterectomy with rectosigmoid endometriotic resection with bladder endometriotic resection done on 1 June 2018 |
| 11-004 |
Male, age 26 years; case of haematemesis for 2 days. Upper GI endoscopy: D1-clean based 1.5 × 0.5 cm ulcer present at D1/D2 junction with surrounding oedematous margins Event occurrence (as per hospital records): 22 July 2018 at 22:23 p.m. Reporting at hospital: 22 July 2018 at 22:23 p.m. Received first dose of study drug: 23 July 2018 at 11:15 a.m. Surgery/procedure during hospitalization (if any): None |
10-006 |
Male, age 58 years; case of fall, fracture of lower end left radius, and fracture of intertrochanteric left femur Event occurrence (as per hospital records): 14 June 2018 at 8:30 a.m. Reporting at hospital: 16 June 2018 at 10:50 a.m. Received first dose of study drug: 16 June 2018 at 3:00 p.m. Surgery/procedure during hospitalization (if any): ORIF with long PFN and SS wire performed on 16 June 2018. K-wire lower end radius left done on 18 June 2018 |
| 11-005 |
Male, age 60 years; case of peptic ulcer, haematemesis, pain in abdomen since 5 months, epigastric pain, diffuse, non-colicky, generalized weakness, melena since 15 days, severely malnourished, pallor++, hypovolemic shock Event occurrence (as per hospital records): 23 July 2018 at 10:11 a.m. Reporting at hospital: 23 July 2018 at 10:11 a.m. Received first dose of study drug: 23 July 2018 at 13:40 a.m. Surgery/procedure during hospitalization (if any): None |
11-002 |
Male, age 63 years; case of peptic ulcer, chief complaint of haematemesis (3–4 episodes) since one day. Pallor++ history of retching, nausea, abdominal pain, and abdominal distension. UGI endoscopy-circumferential ulcer in distal anterior, duodenum-ulcer seen in distal body. D1-multiple superficial ulcer Event occurrence (as per hospital records): 26 June 2018 at 9:27 a.m. Reporting at hospital: 26 June 2018 at 9:27 a.m. Received first dose of study drug: 26 June 2018 at 12:06 p.m. Surgery/procedure during hospitalization (if any): Pyloric catheterization |
| 11-003 |
Female, age 50 years; complaint of abdominal pain, haematemesis (4–5 episodes), malena + syncope, altered sensorium, decrease urine output. Pallor + and hypotension, tender hepatomegaly, spleen palpable, upper GI endoscopy showed large, ulcerated area with sloughed out base Event occurrence (as per hospital records): 19 July 2018 at 5:12 a.m. Reporting at hospital: 19 July 2018 at 5:12 a.m. Received first dose of study drug: 19 July 2018 at 11:05 a.m. Surgery/procedure during hospitalization (if any): None |
||
Primary Outcome
All patients received centhaquine or saline when they were in hypovolemic shock with SBP ≤ 90 mmHg. The standard of care (SOC) for shock was provided to all the patients in both groups. SOC in both groups was practically similar (Table 3).
Table 3.
Details of the treatment provided to the patients in the control and centhaquine group
| Patient no. | Control (N = 22) | Patient no. | Centhaquine (N = 23) |
|---|---|---|---|
| 01-004 | Inj. Streptokinase, Inj. Piperacillin + Tazobactam, Inj. Tranexamic Acid, Inj. Levetiracetam, Inj. Mannitol, Inj. Pantoprazole, Inj. Ondansetron, Inj. Cerebroprotein hydroxylate, Inj. Citicoline, Inj. Ondansetron, Inj. Vitamin K, Inj. Ceftriaxone + Sulbactam, Inj. Vitamin B Complex, Inj. Tetanus Toxoid, Inj. Diclofenac, Inj. Metronidazole, Tab. Trypsin + Chymotrypsin, Syrup Lactulose, Tab. Rutoside + Aceclofenac + Trypsin + Bromide, Tab. Betahistine, Inj. Enoxaparin, Tab. Pantoprazole + Domperidone, Tab. Aspirin + Clopidogrel, Tab. Rosuvastatin, Tab. Vitamin C, Tab. Lactobacillus, Tab. Vitamin D3 Calcium | 01-001 | Inj. Ceftriaxone + Sulbactam, Inj. Metronidazole, Inj. Amikacin, Inj. Pantoprazole, Inj. Ondansetron, Inj. Diclofenac, Inj. Tetanus toxoid, Inj. Vitamin K, Inj. Piperacillin + Tazobactam, Powder Chymotrypsin, Respule Levosalbutamol + Ipratropium bromide, Tab. Vitamin C, Cap. Vitamin B complex, Inj. Piperacillin + Tazobactam, Tab. Cefuroxime, Cap. Pantoprazole + Domperidone, Tab. Etoricoxib |
| 01-005 | Inj. Pantoprazole, Inj. Ondansetron, Inj. Tramadol, Inj. Tranexamic Acid, Inj. Diclofenac, Inj. Vitamin K, Inj. Ceftriaxone + Sulbactam, Inj. Levetiracetam, Inj. Metronidazole, Inj. Amikacin, Tab. Trypsin + Chymotrypsin, Tab. Lactulose, Inj. Cefuroxime, Tab. Vitamin C, Tab. Vitamin B Complex, Tab. Pantoprazole + Domperidone, Tab. Trypsin + Bromelain + Rutoside + Diclofenac | 01-002 | Inj. Ceftriaxone + Sulbactam, Inj. Diclofenac, Inj. Amikacin, Inj. Pantoprazole, Inj. Ondansetron, Inj. Tetanus Toxoid, Inj. Paracetamol, Inj. Tramadol, Tab. Vitamin C, Tab. Trypsin + Chymotrypsin, Tab. Lactobacillus, Inj. Methylprednisolone, Tab. Vitamin B Complex, Tab. Enoxaparin, Inj. Piperacillin + Tazobactam, Tab. Cefuroxime, Amlodipine, Protein Powder, Tab. Baclofen, Tab. Pantoprazole + Domperidone, Tab. Calcium, Tab. Vitamin D3 |
| 01-007 | Inj. Ceftriaxone + Sulbactam, Inj. Pantoprazole, Inj. Ondansetron, Inj. Metronidazole, Inj. Mannitol, Inj. Levetiracetam, Inj. Cerebroprotein, Inj. Citicoline, Inj. Lacosamide, Inj. Cefuroxime, Tab. Piracetam, Tab. Trypsin, Tab. Bromelain, Tab. Rutoside, Tab. Diclofenac, Tab. Coenzyme Q10 + Omega 3 Fatty Acid, Syp. Lactulose | 01-006 | Inj. Ceftriaxone + Sulbactam, Inj. Pantoprazole, Inj. Ondansetron, Inj. Diclofenac, Inj. Metronidazole, Inj. Tramadol, Inj. Tetanus Toxoid, Inj. Amikacin, Inj. Piperacillin + Tazobactam, Respule Levosalbutamol + Ipratropium, Respule Budesonide, Syp. Lactulose, Protein Powder, Tab. Enoxaparin, Tab. Vitamin C, Cap. Vitamin B Complex, Tab. Amlodipine, Inj. Ceftriaxone + Sulbactam, Linezolid, Tab. Atorvastatin + Aspirin |
| 02-001 | Inj. Cefuroxime, Inj. Pantoprazole, Inj. Ondansetron, Inj. Tramadol, Inj. Phenytoin Sodium, Inj. Tranexamic Acid, Inj. Etamsylate, Inj. Vitamin K, Tab. Ursodeoxycholic Acid, Tab. Becozyme-c forte, Tab. Tramadol Hydrochloride, Tab. Paracetamol, Tab. Vitamin C, Tab. Silymarin | 01-008 | Inj. Pantoprazole, Inj. Ondansetron, Inj. Tetanus Toxoid, Tab. Diclofenac, Inj. Hydrocortisone, Inj. Piperacillin + Tazobactam, Inj. Metronidazole, Inj. Amikacin, Inj. Ceftriaxone, Inj. Enoxaparin, Inj. Paracetamol, Inj. Levetiracetam, Tab. Vitamin C, Inj. Tetanus Immunoglobin, Tab. Multivitamin, Hydroxyl Protein Powder, Tab. Aspirin, Tab. Cefuroxime + Clavulanic Acid, Tab. Linezolid, Paracetamol, Tab. Domperidone + Pantoprazole, Lactobacillus, Tab. Coenzyme Q10 + Magnesium Oxide + Vitamin C + Zinc Sulphate + Selenic Acid, Tab. Levocetirizine, Tab. Rivaroxaban, Tab. Paracetamol + Tramadol, Tab. Aceclofenac + Thiocolchicoside, Tab. Calcium + Vitamin D3 |
| 02-004 | Inj. Piperacillin, Inj. Tazobactam, Inj. Metronidazole, Inj. Pantoprazole, Inj. Paracetamol, Inj. Diclofenac Sodium, Inj. Tranexamic Acid, Inj. Ondansetron, Inj. Phytomenadione, Inj. Ranitidine, Inj. Metoclopramide, Syp. Dextromethorphan, Tab. Serratiopeptidase, Tab. Ferrous fumarate, Tab. Folic Acid, Tab. Zink Sulphate, Tab. Pantoprazole, Tab. Nicotinamide, Tab. Vitamin C, Tab. Calcium carbonate, Tab. Vitamin D3 | 02-002 | Inj. Metronidazole, Inj. Cefuroxime, Inj. Pantoprazole, Inj. Ondansetron, Inj. Diclofenac Sodium, Inj. Tramadol, Inj. Gentamicin, Inj. Phenytoin Sodium, Inj. Piperacillin, Inj. Tazobactam, Inj. Calcium Gluconate, Inj. Paracetamol, Inj. Insulin, Inj. Ofloxacin, Inj. Dalteparin Sodium, Inj. Insulin Isophane, Tab. Alprazolam, Tab. Lactobacillus, Susp. Racecadotril, Tab. Tramadol hydrochloride, Tab. Cefuroxime Axetil, Tab. Ofloxacin, Tab. Aspirin |
| 02-005 | Inj. Cefuroxime Sodium, Inj. Pantoprazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Diclofenac Sodium, Inj. Metronidazole, Inj. Enoxaparin sodium, Inj. Levetiracetam, Inj. Tramadol, Inj. Lorazepam, Inj. Meropenem, Inj. Midazolam, Inj. Hydrocortisone Sodium Succinate, Inj. Atracurium Besylate, Inj. Fentanyl, Inj. Levetiracetam, Syrup Potassium Chloride, Tab. Quetiapine Magnesium, Tab. Magnesium Trisilicate, Tab. Aluminium hydroxide, Tab. Teicoplanin, Inh. Mesna, Tab. Ferrous Fumarate, Tab. Folic Acid | 02-003 | Inj. Phenytoin Sodium, Inj. Pantoprazole, Inj. Cefuroxime, Inj. Paracetamol, Inj. Tramadol, Inj. Diclofenac, Inj. Gentamycin, Inj. Metronidazole, Respule Ipratropium Bromide, MDI- Levosalbutamol, MDI- Budesonide, Inj. Phenytoin Sodium, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Vitamin K, Inj. Cefoperazone, Inj. Sulbactam, Inj. Fentanyl, Inj. Piperacillin, Inj. Tazobactam, Inj. Teicoplanin, Inj. Ciprofloxacin |
| 02-007 | Inj. Levofloxacin, Inj. Pantoprazole, Inj. Ondansetron, Inj. Tramadol, Inj. Tranexamic Acid, Inj. Etamsylate, Inj. Vitamin K, Inj. Fentanyl Citrate, Inj. Phytonadione, Inj. Meropenem, Inj. Levetiracetam, Inj. Metronidazole, Inj. Teicoplanin, Inj. Calcium Gluconate, Inj. Magnesium Sulphate, Inj. Paracetamol, Inj. Levosulpiride, Tab. Lactulose, Inj. Bethadoxin, Tab. Folic Acid, Inj. Colistin, Inj. Tigecycline, Inj. Deltapine Sodium, Inj. Midazolam, Inj. Cynocal, Inj. Zink Sulphate, Inj. Metoclopramide Hydrochloride, Inj. Domperidone, Tab. Ranitidine, Tab. Naproxen | 02-006 | Inj. Cefuroxime Sodium, Inj. Pantoprazole), Inj. Ondansetron, Inj. Paracetamol, Inj. Diclofenac Sodium, Inj. Metronidazole, Inj. Hydrocortisone Sodium, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Phytonadione, Inj. Amiodarone Hydrochloride, Inj. Insulin, MDI- Levosalbutamol, Inj. Ofloxacin, Inj. Piperacillin Sodium |
| 02-009 | Inj. Cefuroxime Sodium, Inj. Pantoprazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Metronidazole, Inj. Tranexamic Acid, Inj. Tramadol, Inj. Gentamycin, Inj. Tranexamic Acid, Inj. Etamsylate, Inj. Levosalbutamol | 02-008 | Inj. Cefuroxime Sodium, Inj. Pantoprazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Metronidazole, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Phytonadione, Inj. Fentanyl, Inj. Sulbactam, Inj. Cefoperazone, Inj. Piperacillin, Inj. Tazobactam, Inj. Ipratropium Bromide, Inj. Levosalbutamol, Inj. Calcium Gluconate |
| 02-012 | Inj. Cefuroxime, Inj. Pantoprazole, Inj. Ondansetron, Inj. Levetiracetam, Inj. Tranexamic Acid, Inj. Etamsylate, Inj. Piperacillin, Inj. Teicoplanin, Inj. Diclofenac, Inj. Dalteparin, Inj. Linezolid, Inj. Paracetamol + Tramadol, Tab. Esomeprazole, Tab. Multivitamin, Tab. Vitamin C, Tab. Calcium + Zinc + Magnesium + Vitamin D3, Tab. Ferrous Fumarate + Zinc | 02-010 | Inj. Cefuroxime Sodium + Sulbactam, Inj. Pantoprazole, Inj. Paracetamol, Inj. Lorazepam, Inj. Haloperidol, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Levetiracetam, Inj. Phytonadione, Inj. Furosemide, Inj. Calcium Gluconate, Inj. Dexmedetomidine, Inj. Diclofenac, Inj. Methylprednisolone, MDI- Levosalbutamol, MDI- Budesonide, Inj. Fentanyl Citrate, Tab. Charcoal, Tab. Unienzyme (Fungal Diastase, Charcoal, Papain), Cap. Livogen, Inj. Dexmedetomidine Hydrochloride, Tab. Amlodipine, Tab. Potassium Citrate, Tab. Calcium Citrate + Vitamin D3, Inj. Dalteparin, Inj. Ofloxacin, Inj. Enoxaparin, Tab. Alprazolam, Inj. Dexamethasone, Tab. Vitamin C, Syp. Neocardio, Inj. Haloperidol, Cap. Methylcobalamin + Thiamine + Vitamin B6 + Nicotinamide + D- Panthenol |
| 02-013 | Inj. Cefuroxime, Inj. Pantoprazole, Inj. Ondansetron, Inj. Paracetamol + Tramadol, Inj. Levetiracetam, Inj. Ethamsylate, Inj. Tranexamic Acid, Inj. Metoclopramide, Inj. Calcium Gluconate, Inj. Phytonadione, Tab. Baclofen, Tab. Spironolactone, Tab. Chlorpromazine, Protein Powder | 02-011 | Inj. Cefuroxime, Inj. Pantoprazole, Inj. Ondansetron, Inj. Tramadol, Inj. Tranexamic Acid, Inj. Phytonadione (Vitamin K1), Inj. Tazobactam, Inj. Ofloxacin, Inj. Metronidazole, Inj. Calcium Gluconate, Inj. Amlodipine, Tab. Amlodipine + Atenolol, Tab. Calcium + Vitamin D3, Tab. Vitamin B complex, Inj. Enoxaparin, Tab. Trypsin Chymotrypsin, Inj. Paracetamol, Syp. Potassium Chloride, Inj. Dalteparin, Tab. Alprazolam, Inj. Metoclopramide |
| 02-016 | Inj. Paracetamol + Tramadol, Inj. Ondansetron, Inj. Diclofenac, Inj. Pantoprazole, Inj. Cefuroxime, Inj. Tranexamic Acid, Inj. Tranexamic Ethamsylate, Inj. Phytomenadione, Inj. Cefoperazone Sulbactam, Inj. Metronidazole, Inj. Paracetamol, Inj. Calcium Gluconate, Inj. Fentanyl Citrate, Inj. Ceftriaxone, Inj. Magnesium Sulphate, Inj. Teicoplanin, Tab. Chymotrypsin, Inj. Paracetamol, Inj. Piperacillin Tazobactam, Tab. Montelukast, Tab. Calcitriol Citrate, Inj. Fluconazole, Inj. Furosemide, Lactulose | 02-014 | Inj. Piperacillin + Tazobactam, Inj. Pantoprazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Tetanus Toxoid, Inj. Diclofenac, Inj. Tramadol, Inj. Levetiracetam, Inj. Haloperidol, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Phytonadione, Inj. Teicoplanin, Inj. Fentanyl Citrate, Inj. Calcium Gluconate, Inj. Magnesium Sulphate, Inj. Dalteparin (Heparin), Inj. Teicoplanin, Inj. furosemide, Respule Levosalbutamol, Respule Budesonide, Drop. Chloramphenicol, Tab. Quetiapine, Tab. Lorazepam |
| 05-005 | Inj. Tranexamic Acid, Inj. Ceftriaxone Sodium, Inj. Metoclopramide Hydrochloride, Inj. Ranitidine, Inh. Ipratropium Bromide, Inh. Levosalbutamol, Inh. Budesonide, Inj. Piperacillin, Inj. Tazobactam, Inj. Metronidazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Sodium bicarbonate, Inj. Phytonadione | 02-015 | Inj. Piperacillin + Tazobactam, Inj. Pantoprazole, Inj. Ondansetron, Inj. Metronidazole, Inj. Levetiracetam, Inj. Tranexamic Acid, Inj. Ethamsylate, Inj. Vitamin K, Inj. Diclofenac Sodium, Inj. Tramadol, Inj. Cefuroxime Sodium, Inj. Gentamycin, Tab. Chymotrypsin, Tab. Ferrous Fumarate + Zinc, Inj. Furosemide, Inj. Cynocal (Vitamin B1 + Vitamin B6 + D-Panthenol, Tab. Folic Acid, Inj. Paracetamol, Tab. Clonazepam, Tab. Pregabalin + Methylcobalamin, Tab. Amlodipine, Tab. Cilnidipine, Respule Levosalbutamol, Inj. Ofloxacin, Inj. Insulin, Inj. Insulin Isophane, Inj. Dalteparin, Tab. Domperidone, Inj. Meropenem, Tab. Diltiazem |
| 05-007 | Tab. Elemental Iron, Tab. Folic Acid, Tab. Multivitamin, Tab. Paracetamol, Inj. Tetanus Toxoid, Tab. Pantoprazole, Inj. Ceftriaxone Sodium, Inj. Ondansetron, Inj. Sodium Bicarbonate, Inj. Atropine Sulphate | 05-003 | Inj. Meropenem, Inj. Metronidazole, Inj. Pantoprazole, Inj. Paracetamol, Inj. Paracetamol, Inj. Meropenem, Inj. Metoclopramide, Inj. Levosulpiride, Respule Levosalbutamol, Respule Ipratropium Bromide, MDI-Budesonide, Tab. Levofloxacin, Tab. Pantoprazole, Tab. Domperidone, Syp. Lactulose, Sol. Potassium Chloride, Tab. Metoclopramide, Inj. Amoxicillin, Inj. Clavulanic Acid, Tab. Pantoprazole, Tab. Domperidone, Tab. Paracetamol, Inj. Diclofenac Sodium, Inj. Ranitidine, Respule Levosalbutamol, Respule Ipratropium Bromide, Respule Budesonide, Sol. Acetylcysteine, Inj. Metoclopramide, Inj. Paracetamol, Tab. Amitriptyline, Inj. Amikacin, Tab. Levosulpiride, Syp. Lactulose, Inj. Ceftriaxone, Inj. Tetanus Toxoid |
| 08-003 | Inj. Ondansetron, Tab. Levetiracetam, Inj. Mannitol, Inj. Paracetamol, Inj. Rabeprazole, Inj. Moxifloxacin + Vancomycin, Inj. Meropenem, Tab. Trypsin + Bromelain + Rutoside Trihydrate, Tab. Ascorbic Acid, Tab. Lactulose, Tab. Acetazolamide, B-Protein-po, Tab. Levopride, Inj. Amikacin, Eye Drop Mezol, Inj. Ciprofloxacin, Tab. Glycerine, Tab. Amitriptyline, Inj. Ceftriaxone, Tab. Silymarin + Ursodeoxycholic Acid + Ursodiol | 05-004 | Inj. Paracetamol, Inj. Calcium Gluconate |
| 08-005 | Inh. Respule Levosalbutamol, Inj. Vitamin K, Piperacillin + Tazobactam, Inj. Tramadol, Inj. Pantoprazole, Inj. Paracetamol, Tab. Charcoal, Inj. Dexamethasone, Inj. Amikacin, Inj. Ondansetron. Inj. Amoxicillin Clavulanate, Tab. Trypsin Chymotrypsin, Tab. Paracetamol + Tramadol, Tab. Ranitidine, Tab. Calcitriol + Elemental Calcium, Methylcobalamin, Syp. Lactulose, Tab. Azithromycin, Tab. Clavulanic Acid | 05-006 | Inj. Paracetamol, Inj. Meropenem, Inj. Sodium Bicarbonate, Inj. Pantoprazole, Inj. Hydrocortisone Succinate, Inj. Promethazine, Inj. Tranexamic Acid, Inj. Ondansetron, Inj. Metoclopramide, Tab. Eltroxin, Tab. Propranolol, Syp. Lactose, Tab. Linezolid, Tab. Dextromethorphan, Tab. Metronidazole |
| 10-001 | Inj. Augmentin, Inj. Clindamycin, Inj. Pantoprazole, Inj. Piperacillin + Tazobactam, Inj. Paracetamol, Inj. Amikacin, Inj. Metronidazole, Tab. Lactulose + Galactose + Epilactose + Fructose, Inj. Potassium Chloride, Tab. Bifilac | 07-001 | Inj. Meropenem, Inj. Metronidazole, Inj. Pantoprazole, Inj. Phenytoin, Inj. Calcium, Inj. Menaphthone Sodium Bisulphate, Inj. Levetiracetam, Inj. Furosemide, Inj. Torsemide, Inj. Paracetamol, Inj. Linezolid, Inj. Tramadol |
| 10-004 | Inj. Polymyxin B, Inj. Vitamin B Complex, Inj. Mineral Supplement, Inj. Fluconazole, Inj. Imipenem, Inj. Octreotide, Inj. Vitamin K, Inj. Vitamin C, Inj. Vitamin A), Inj. Magnesium Sulphate, Inj. Paracetamol, Sucralfate, Inj. Pantoprazole, Inj. Terlipressin | 08-001 | Inj. Cefoperazone Sodium, Inj. Sulbactam, Inj. Rabeprazole, Inj. Ondansetron, Inj. Paracetamol, Inj. Methylprednisolone, Respule Levosalbutamol, Respule Ipratropium Bromide, Respule Budesonide, Powder Macrogel, Inj. Paracetamol, Inj. Amikacin, Inj. Dexamethasone, Tab Nutrinz. Nutrinz, Tab. Acetylcysteine, B-Protein Powder, Lotion Nadifloxacin, Tab. Calcium, Tab. Cefpodoxime |
| 10-005 | Inj. Ceftriaxone, Inj. Amikacin, Inj. Pantoprazole, Inj. Paracetamol, Tab. Ivabradine, Tab. Diltiazem Hydrochloride, Tab. Hyoscine Butylbromide, Tab. Mefenamic Acid, Tab. Tamsulosin, Tab. Ethamsylate, Tab. Magnesium Citrate, Tab. Potassium Citrate, Tab. Faropenem, Tab. Prulifloxacin | 08-002 | Inj. Tramadol, Inj. Piperacillin, Inj. Tazobactam, Inj. Omeprazole, Inj. Cefoperazone Sodium, Inj. Sulbactam, Inj. Amikacin, Inj. Pantoprazole, Inj. Diclofenac Sodium, Tab. Bromelain + Trypsin + Rutoside, Tab. Calcium Carbonate, Powder Cholecalciferol, Gel Mucaine, Tab. Ilaprazole, Tab. Linezolid |
| 10-007 | Inj. Paracetamol, Inj. Ceftriaxone, Inj. Pantoprazole, Bisacodyl, Tab. Amoxicillin, Tab. Potassium Chloride, Tab. Diclofenac Sodium, Tab. Aceclofenac + Paracetamol + Serratiopeptidase, Tab. Biotin, Tab. Copper Sulphate, Tab. Folic Acid, Tab. Inositol, Tab. Iodine, Tab. Taurine, Tab. Vanadium | 10-002 | Inj. Ceftriaxone, Inj. Metronidazole, Inj. Ethamsylate, Tab. Diosmin, Inj. Vitamin K, Inj. Pantoprazole, Inj. Paracetamol, Inj. Furosemide, Inj. Calcium Carbonate, Inj. Piperacillin + Tazobactam, Tab. Faropenem, Tab. Multivitamin |
| 11-001 | Tab. Albendazole, Inj. Pantoprazole, Inj. Optineuron, Inj. Ceftriaxone, Inj. Phenylephrine, Inj. Hydrocortisone | 10-003 | Inj. Piperacillin + Tazobactam, Inj. Metronidazole, Inj. Pantoprazole, Inj. Paracetamol, Inj. Ondansetron, Powder Lactobacillus + Streptococcus, Powder Clostridium Bacillus, Tab. Faropenem, Tab. Tolterodine, Tab. Biotin, Tab. Copper Sulphate, Tab. Inositol, Syp. Iodine, Tab. Taurine, Tab. Vanadium, Cap. Vitamin B 12, B2, B6, C, D3, E |
| 11-004 | Inj. Omeprazole, Inj. Pantoprazole, Inj. Vitamin K, Inj. Ceftriaxone | 10-006 | Inj. Ceftriaxone, Inj. Pantoprazole, Inj. Ondansetron, Inj. Diclofenac Sodium, Inj. Amikacin, Tab. Linezolid, Tab. Bisoprolol, Lactulose Solution |
| 11-005 | Peglec Powder, Bisacodyl, Inj. Pantoprazole, Inj. Optineuron, Inj. Ceftriaxone | 11-002 | Tab. Sucralfate, Inj. Magnesium Sulphate, Inj. Tramadol, Inj. Pantoprazole, Inj. Optineuron (Vitamin B1 + Vitamin B6 + Cyanocobalamin + Vitamin B2 + Nicotinamide + D-Panthenol), Inj. Ondansetron, Inj. Ceftriaxone, Inj. Diclofenac Sodium, Inj. Calcium Gluconate |
| 11-003 | Inj. Pantoprazole, Inj. Optineuron (Vitamin B1 + Vitamin B6 + Cyanocobalamin + Vitamin B2 + Nicotinamide + D-Panthenol), Inj. Ceftriaxone, Inj. Metronidazole, Powder Sporlac Lactobacillus, Tab. Thyroxin, Cap. Doxycycline, Syp. Sucralfate, Cap. Racecadotril |
Vital Signs
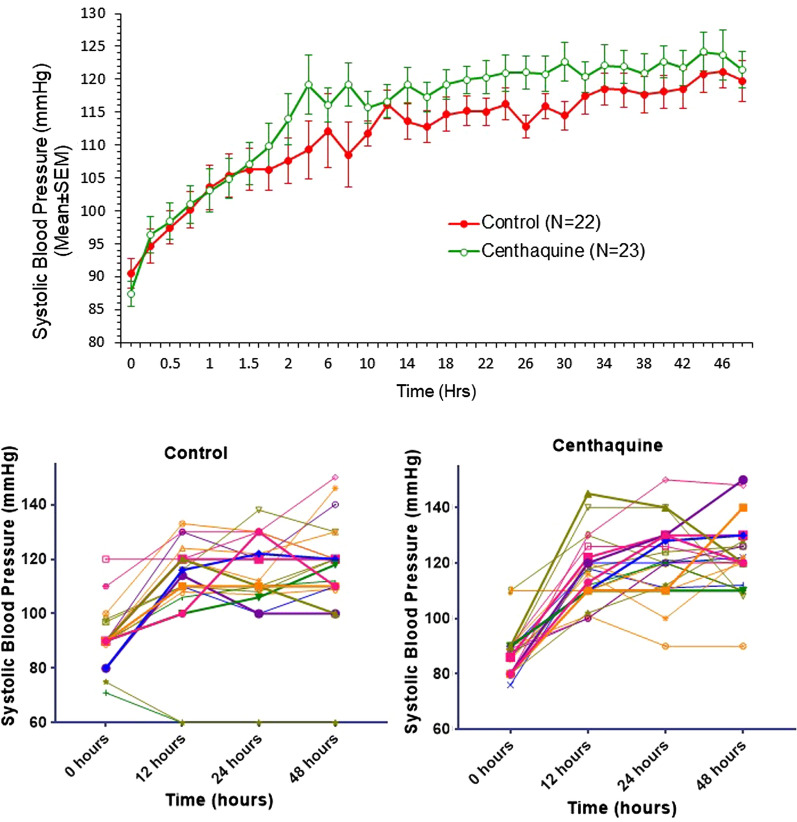
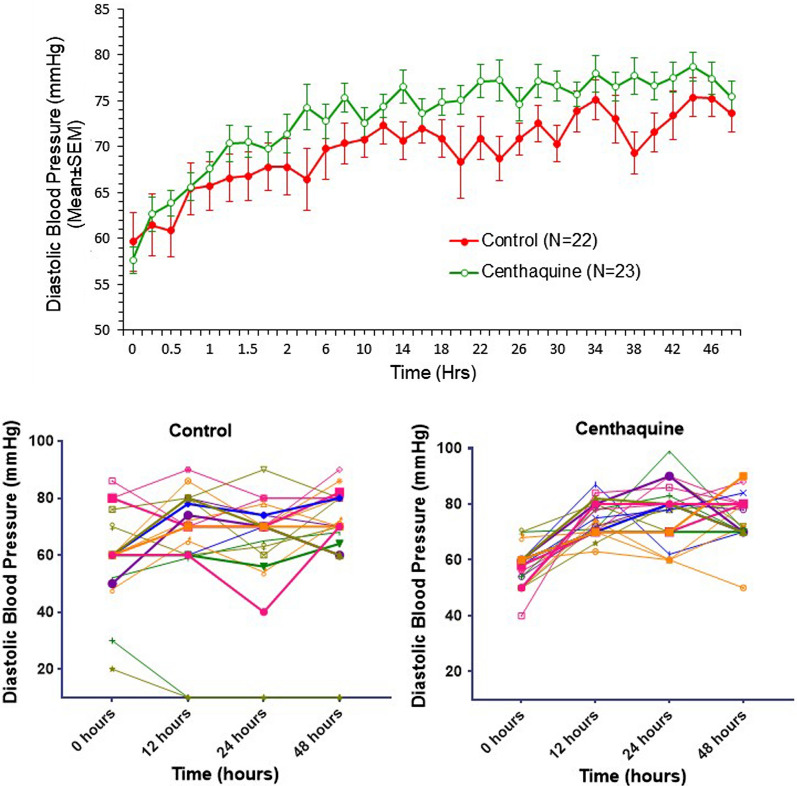
The vital signs of control and centhaquine groups are presented in Table 4. There was an improvement in vital signs following resuscitation in both the control and centhaquine groups; however, improvement in systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure were more significant in the centhaquine group. Once recovered from hypovolemic shock, vital signs were similar in both groups of patients.
Table 4.
Patients' vitals recorded through day 1 (baseline) to day 28
| Vitals | Group | Baseline | After administration of study drug | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 28 | ||
| Systolic blood pressure (mmHg) | Control | 90.45 ± 2.27 | 115.00 ± 1.99 | 120.70 ± 2.48 | 121.00 ± 3.36 | 124.92 ± 3.97 | 123.38 ± 4.40 | 120.31 ± 3.59 | 120.10 ± 2.23 |
| Centhaquine | 87.36 ± 1.85 | 119.08 ± 2.63 | 121.65 ± 2.36 | 119.90 ± 2.78 | 124.30 ± 3.45 | 122.20 ± 2.95 | 122.73 ± 3.74 | 118.60 ± 1.92 | |
| Diastolic blood pressure (mmHg) | Control | 59.63 ± 3.16 | 72.35 ± 1.98 | 71.50 ± 1.73 | 75.62 ± 2.44 | 79.07 ± 3.03 | 77.53 ± 2.29 | 74.69 ± 3.32 | 78.63 ± 1.44 |
| Centhaquine | 57.63 ± 1.49 | 73.65 ± 1.84 | 74.21 ± 2.35 | 72.95 ± 1.87 | 76.20 ± 2.05 | 75.40 ± 2.17 | 76.89 ± 1.93 | 76.95 ± 1.64 | |
| Pulse pressure (mmHg) | Control | 30.81 ± 1.93 | 42.65 ± 1.38 | 49.20 ± 2.29 | 45.37 ± 2.49 | 45.85 ± 2.73 | 45.84 ± 3.33 | 45.61 ± 3.54 | 41.47 ± 1.61 |
| Centhaquine | 29.72 ± 1.76 | 45.43 ± 2.40 | 47.43 ± 1.94 | 46.95 ± 2.08 | 48.52 ± 2.48 | 46.63 ± 2.91 | 46.16 ± 2.98 | 41.65 ± 1.17 | |
| Heart rate (beats/min) | Control | 94.36 ± 5.09 | 87.80 ± 3.84 | 83.36 ± 4.16 | 86.87 ± 4.43 | 88.28 ± 4.36 | 86.00 ± 5.22 | 85.15 ± 5.10 | 87.89 ± 2.18 |
| Centhaquine | 98.91 ± 5.07 | 95.13 ± 3.29 | 93.47 ± 3.51 | 88.52 ± 3.10 | 91.45 ± 4.35 | 88.25 ± 3.56 | 88.63 ± 4.34 | 85.39 ± 2.66 | |
| Respiratory rate (breaths/min) | Control | 20.72 ± 0.77 | 20.45 ± 0.78 | 20.30 ± 0.68 | 19.62 ± 0.69 | 20.57 ± 0.70 | 20.00 ± 1.18 | 19.76 ± 0.89 | 19.89 ± 0.47 |
| Centhaquine | 21.22 ± 0.93 | 19.72 ± 0.79 | 20.40 ± 0.65 | 19.71 ± 0.52 | 20.61 ± 0.83 | 19.50 ± 0.68 | 20.84 ± 0.95 | 19.73 ± 0.40 | |
| Body temperature (°C) | Control | 36.72 ± 0.07 | 36.88 ± 0.10 | 36.78 ± 0.10 | 38.83 ± 1.92 | 36.83 ± 0.07 | 36.96 ± 0.15 | 36.94 ± 0.18 | 36.79 ± 0.05 |
| Centhaquine | 36.61 ± 0.06 | 36.90 ± 0.09 | 36.91 ± 0.11 | 37.01 ± 0.14 | 36.85 ± 0.09 | 36.90 ± 0.13 | 36.60 ± 0.09 | 36.71 ± 0.07 | |
Data are presented as mean ± standard error of the mean
Haematology, Coagulation Parameters, and Lipid Profile
The haematological parameters were similar in control and centhaquine groups at the time of inclusion in the study (day 1, baseline). The haematological parameters improved from day 1 (baseline) to day 28, and this improvement was similar in both groups. Centhaquine does not alter patients' haematological parameters in hypovolemic shock any differently from in the control group. (Table 5). The baseline (at the time the patient was included in the study) coagulation parameters (platelet count, prothrombin time, fibrinogen value, and international normalized ratio) in the control and centhaquine groups were similar. The improvement in coagulation parameters from day 1 (baseline) to day 3 and day 28 was similar in both groups. There was no significant difference observed between the groups (Table 5). The baseline lipid profile of the control and centhaquine groups was similar. The change in lipid profile from day 1 (baseline) to day 28 was similar in both groups. Centhaquine does not significantly affect the lipid profile of patients in hypovolemic shock (Table 5).
Table 5.
Haematological, biochemical, and serum electrolyte levels of patients
| Control (N = 22) | Centhaquine (N = 23) | |||||
|---|---|---|---|---|---|---|
| Day 1 (baseline) | Day 3 | Day 28 | Day 1 (baseline) | Day 3 | Day 28 | |
| Haematology | ||||||
| Haemoglobin (g/dl) | 9.38 ± 0.71 | 9.84 ± 0.33 | 12.26 ± 0.44 | 8.73 ± 0.55 | 8.71 ± 0.30 | 11.00 ± 0.38 |
| Haematocrit (%) | 28.79 ± 2.11 | 30.26 ± 1.00 | 38.01 ± 1.36 | 26.71 ± 1.81 | 26.43 ± 0.96 | 34.31 ± 1.16 |
| Red blood cells (mill/cumm) | 3.35 ± 0.23 | – | 4.44 ± 0.19 | 3.05 ± 0.20 | – | 4.04 ± 0.13 |
| White blood cells | 15.17 ± 1.22 | – | 8.65 ± 0.49 | 16.33 ± 1.40 | – | 8.37 ± 0.64 |
| Neutrophils (%) | 81.50 ± 2.21 | – | 65.18 ± 2.48 | 82.97 ± 2.27 | – | 68.46 ± 2.08 |
| Lymphocytes (%) | 12.96 ± 2.13 | – | 24.42 ± 1.97 | 12.33 ± 2.01 | – | 22.86 ± 2.02 |
| Monocytes (%) | 4.53 ± 0.63 | – | 5.84 ± 0.62 | 3.43 ± 0.60 | – | 5.55 ± 0.62 |
| Eosinophils (%) | 0.91 ± 0.24 | – | 4.35 ± 0.91 | 1.24 ± 0.29 | – | 2.93 ± 0.38 |
| Basophils (%) | 0.18 ± 0.10 | – | 0.22 ± 0.08 | 0.27 ± 0.13 | – | 0.33 ± 0.14 |
| Reticulocytes (%) | 2.39 ± 0.31 | – | 1.93 ± 0.25 | 2.26 ± 0.73 | – | 1.83 ± 0.23 |
| Mean corpuscular volume (fl) | 84.40 ± 2.68 | – | 86.13 ± 1.54 | 87.63 ± 1.47 | – | 85.74 ± 1.59 |
| Mean corpuscular haemoglobin (Pg) | 27.29 ± 1.11 | – | 27.86 ± 0.62 | 28.74 ± 0.56 | – | 27.48 ± 0.46 |
| Lipid profile | ||||||
| Triglyceride (mg/dl) | 109.42 ± 12.66 | – | 198.46 ± 25.92 | 151.88 ± 21.47 | – | 160.51 ± 10.04 |
| Total cholesterol (mg/dl) | 109.58 ± 10.71 | – | 156.96 ± 8.53 | 132.51 ± 14.92 | – | 152.25 ± 10.07 |
| High-density lipoprotein (mg/dl) | 30.68 ± 2.50 | – | 39.53 ± 2.64 | 34.13 ± 2.68 | – | 40.17 ± 6.79 |
| Low-density lipoprotein (mg/dl) | 63.26 ± 8.97 | – | 91.16 ± 7.56 | 73.30 ± 11.60 | – | 93.04 ± 8.44 |
| Very-low-density lipoprotein (mg/dl) | 22.70 ± 2.24 | – | 40.09 ± 5.16 | 31.03 ± 4.36 | – | 34.59 ± 3.38 |
| Kidney function | ||||||
| Serum creatinine (mg/dl) | 0.94 ± 0.10 | – | 0.57 ± 0.03 | 1.13 ± 0.10 | – | 0.75 ± 0.06 |
| Blood urea nitrogen (mg/dl) | 12.46 ± 1.06 | – | 8.85 ± 0.63 | 15.24 ± 1.61 | – | 12.64 ± 1.58 |
| Glomerular filtration rate (ml/min/1.73 m2) | 114.08 ± 10.47 | – | 171.48 ± 11.41 | 90.86 ± 9.72 | 139.97 ± 15.36 | |
| Liver function | ||||||
| Alanine aminotransferase (U/l) | 172.52 ± 67.80 | – | 37.37 ± 5.13 | 91.58 ± 34.12 | – | 39.77 ± 7.31 |
| Aspartate aminotransferase (U/l) | 211.55 ± 64.76 | – | 29.68 ± 2.51 | 111.02 ± 28.69 | – | 35.46 ± 7.51 |
| Serum bilirubin (mg/dl) | 0.82 ± 0.13 | – | 0.84 ± 0.17 | 1.22 ± 0.42 | – | 0.64 ± 0.10 |
| Alkaline phosphatase (IU/l) | 88.36 ± 10.57 | – | 179.22 ± 24.00 | 83.56 ± 10.51 | – | 195.45 ± 38.77 |
| Serum albumin (g/dl) | 3.04 ± 0.16 | – | 3.89 ± 0.18 | 3.04 ± 0.24 | – | 3.53 ± 0.23 |
| Coagulation parameters | ||||||
| Platelet count (/cumm) | 201.59 ± 16.78 | 162.80 ± 20.80 | 329.75 ± 31.49 | 262.74 ± 42.81 | 191.81 ± 33.10 | 308.70 ± 22.66 |
| Prothrombin time (S) | 18.55 ± 2.53 | 14.82 ± 0.52 | 14.15 ± 0.45 | 17.51 ± 2.06 | 15.71 ± 0.88 | 15.43 ± 0.73 |
| Fibrinogen value | 259.26 ± 20.64 | 392.92 ± 23.12 | 388.34 ± 34.87 | 306.47 ± 44.74 | 339.49 ± 30.89 | 361.48 ± 28.07 |
| International normalized ratio | 1.93 ± 0.50 | 1.25 ± 0.03 | 1.20 ± 0.03 | 1.40 ± 0.09 | 1.34 ± 0.08 | 1.23 ± 0.03 |
| Blood glucose (mg/dl) | 158.23 ± 19.73 | – | 114.38 ± 7.10 | 185.14 ± 24.95 | –- | 111.13 ± 7.92 |
| Serum electrolyte | ||||||
| Sodium (mmol/l) | 140.02 ± 0.80 | – | 136.87 ± 1.10 | 138.32 ± 1.02 | – | 137.26 ± 1.01 |
| Potassium (mmol/l) | 3.98 ± 0.15 | – | 4.29 ± 0.15 | 4.03 ± 0.14 | – | 4.19 ± 0.09 |
| Calcium (mmol/l) | 1.48 ± 0.14 | – | 1.99 ± 0.13 | 1.57 ± 0.14 | – | 1.86 ± 0.11 |
Data are presented as mean ± standard error of the mean
Kidney and Liver Function Markers
The baseline (day 1, time of patient inclusion) serum creatinine, blood urea nitrogen, and glomerular filtration rate of the control and centhaquine groups were similar. The improvement in kidney function parameters from day 1 (baseline) to day 28 was similar in both groups. No significant difference was observed between the groups (Table 5). The baseline levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum bilirubin, alkaline phosphatase, and serum albumin of the control and centhaquine groups was similar. The improvement in liver function parameters from day 1 (baseline) to day 28 was similar in both groups (Table 5). Centhaquine does not affect patients' kidney and liver function markers in hypovolemic shock.
Serum Electrolytes and Random Blood Glucose
The baseline serum electrolytes (sodium, potassium, and calcium) and blood glucose of control and centhaquine groups were similar. The improvement in electrolytes and blood glucose from day 1 (baseline) to day 28 was similar in both groups. There was no significant difference observed between the groups (Table 5). Centhaquine does not affect serum electrolyte and blood glucose levels in hypovolemic shock patients.
Safety and Tolerability
All patients who received treatment were included in the safety analysis. Two out of 22 patients died in the control group and none in the centhaquine group. Three adverse events were reported in three patients of the control group (N = 22). Of these three, two events were serious (death), and one was moderate (viral hepatitis), which was resolved with medical intervention. Two moderate adverse events, diarrhoea in one and acute kidney injury (due to severe traumatic injury resulting in the amputation of a right lower limb combined with sepsis) in the other patient of the centhaquine group (N = 23), were reported, both resolved with medical intervention. None of the adverse events were related to the study drug.
Secondary Outcomes
All-Cause Mortality
Twenty-eight-day all-cause mortality was 9.1% in the control group, whereas it was 0% in the centhaquine group. Two out of 22 patients died within the first 48 h of resuscitation in the control group, while all 23 patients in the centhaquine group survived.
Time in Hospital, in ICU, and on a Ventilator
Centhaquine group patients were in the hospital for a longer duration (14.87 ± 1.89 days) than control (10.75 ± 2.01 days). Major surgery was performed in 91.30% (21 out of 23) patients in the centhaquine group and 68.18% (15 out 22) in the control group (p = 0.0526). However, the ICU stay was only a little longer for centhaquine group patients (6.23 ± 1.31 days) than control group patients (5.26 ± 1.27 days). Percent stay in ICU was higher for control group patients (48.93%) compared to those taking centhaquine (41.89%). The difference between the means using a two-tailed, unpaired t-test with Welch's correction was − 10.10 ± 10.16, 95% CI − 30.63 to 10.44, p = 0.3262. Centhaquine group patients were on ventilator support for a shorter duration (0.89 ± 0.45 days) than control group patients who stayed on ventilator support for 1.96 ± 1.10 days (Table 6). The difference between the means was − 1.063 ± 1.186, 95% CI − 3.493 to 1.367, p = 0.3778.
Table 6.
Time spent in hospital, in ICU, and on a ventilator by control and centhaquine group patients
| Group | Duration of hospital stay (days) | Time spent in ICU (days) | Time spent on ventilator support (days) | Time spent in ward (days) |
|---|---|---|---|---|
| Control (N = 22) | 10.75 ± 2.01 | 5.26 ± 1.27 | 1.96 ± 1.10 | 5.87 ± 1.59 |
| Centhaquine (N = 23) | 14.87 ± 1.89 | 6.23 ± 1.31 | 0.89 ± 0.45 | 8.61 ± 1.44 |
Data are presented as mean ± standard error of the mean
Total Fluids and Blood Products During First 48 Hours
When treated with centhaquine, patients with hypovolemic shock required a lesser (statistically insignificant) volume of fluids (4.26 ± 0.23 l) in the first 48 h compared to control (4.59 ± 0.41 l) group patients (two-tailed, unpaired t-test, p = 0.4919). Patients with hypovolemic shock enrolled in the control and centhaquine groups required an almost similar amount of blood products in the first 48 h (control 0.88 ± 0.13 l and centhaquine 0.92 ± 0.15 l; two-tailed, unpaired t-test, p = 0.8933). Around 86.36% of patients in the control group and 86.96% of patients in the centhaquine group required blood products (Fig. 2).
Fig. 2.
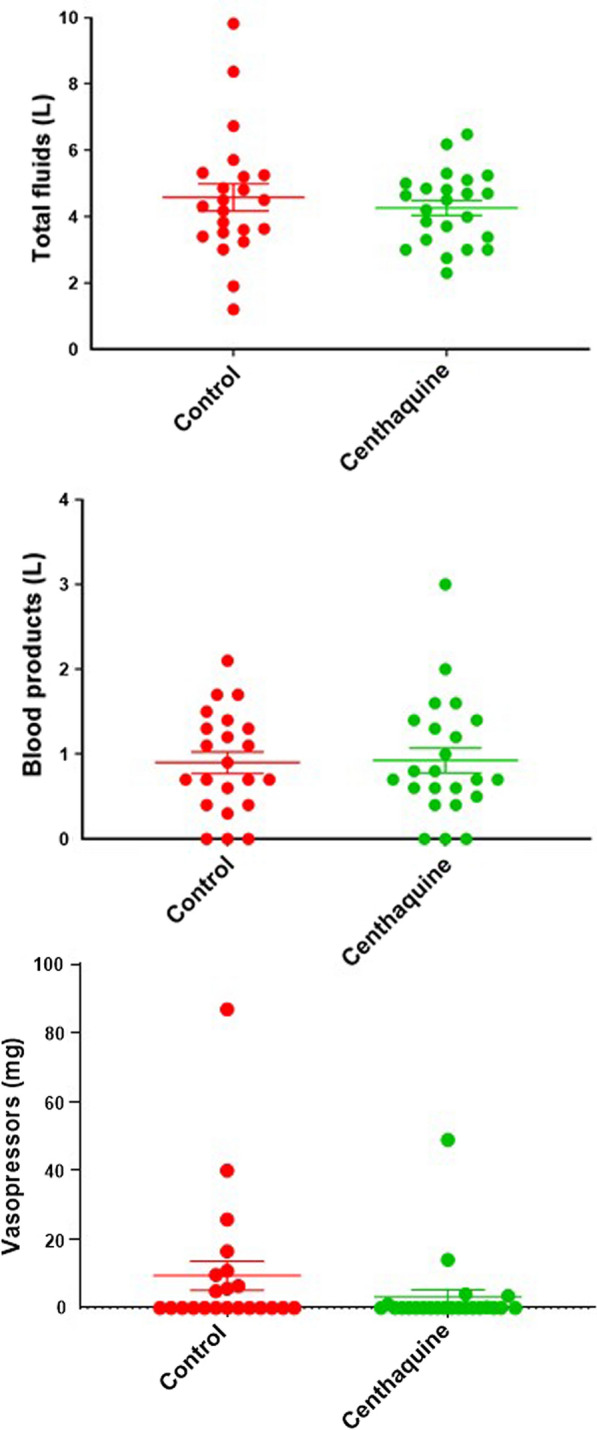
Total volume of fluid, blood products, and vasopressors administered during the first 48 h in the control and centhaquine group of patients. Data presented as the mean ± standard error. Each dot represents the amount administered to each patient
Amount of Vasopressors Infused in First 48 Hours
The total amount of vasopressors needed in the first 48 h of resuscitation was 9.39 ± 4.28 mg for patients in the control group, while only 3.12 ± 2.18 mg (p = 0.2013) in the centhaquine group (Fig. 2). A two-tailed, unpaired t-test with Welch's correction indicated that the difference between the means was − 6.272 ± 4.805, 95% CI − 16.07 to 3.524, p = 0.2013. A total of 40.91% of patients from the control group required vasopressors, while only 26.09% needed them in the centhaquine group.
Number of Study Drug Doses in First 48 Hours
A total of 30 doses of placebo (normal saline) were required in 22 patients of the control group (1.36 ± 0.17 doses per patient) in the first 48 h of randomization, while in the centhaquine group, a total of 28 doses were required in 23 patients (1.22 ± 0.11 doses per patient). The number of doses required in the centhaquine group was about 10.29% less than those required in the control group.
Haemodynamic Changes in the First 48 Hours
Both the control and centhaquine groups of patients showed a significant increase in SBP in the first 48 h of randomization. The increase in SBP from baseline to 12, 24, and 48 h of resuscitation with centhaquine was highly significant (p < 0.0001). In the control group of patients, the increase in SBP from baseline was comparatively less significant (Fig. 3). At 12 h of resuscitation, the mean difference from baseline was 14.86 mmHg (95% CI 1.313–28.41, p = 0.0261) in the control group and 29.39 mmHg (95% CI 20.94–37.85, p < 0.0001) in the centhaquine group. Similarly, at 24 h of resuscitation, the mean difference from baseline was 15.23 mmHg (95% CI 1.677–28.78, p = 0.0216) in the control group and 33.70 mmHg (95% CI 25.24–42.15, p < 0.0001) in the centhaquine group (Fig. 3). The mean difference between baseline and 48 h of resuscitation was 34.13 mmHg (95% CI 25.68–42.59) in centhaquine compared to 18.41 mmHg (95% CI 4.859–31.96) in the control group.
Fig. 3.
Systolic blood pressure during the first 48 h in the control and centhaquine groups of patients. The upper panel shows data as the mean ± standard error. The lower panel shows a change in each patient's systolic blood pressure with time. Two-way ANOVA showed a significant change in systolic blood pressure in the centhaquine (p < 0.0001) but less in the control (p = 0.0261) group during the first 12 h of resuscitation
An increase in DBP at 12, 24, and 48 h from baseline occurred following resuscitation in both the control and centhaquine groups. An increase in DBP was more marked in the centhaquine group than in the controls (Fig. 4). The mean difference from baseline was 5.545 mmHg (95% CI − 1.595 to 12.69, p = 0.1812) at 12 h of resuscitation in the control group and 17.13 mmHg (95% CI 10.81–23.45, p < 0.0001) in the centhaquine group. Similarly, at 24 h of resuscitation, the mean difference from baseline was 2.818 mmHg (95% CI − 4.323 to 9.959, p = 0.7258) in the control group and 19.91 mmHg (95% CI 13.59–26.23, p < 0.0001) in the centhaquine group (Fig. 4). The mean difference between baseline and 48 h of resuscitation was 18.13 mmHg (95% CI 11.81–24.45) in the centhaquine compared to 7.273 mmHg (95% CI 0.1318–14.41) in the control group.
Fig. 4.
Diastolic blood pressure during the first 48 h in the control and centhaquine group of patients. The upper panel shows data as the mean ± standard error. The lower panel shows a change in each patient's diastolic blood pressure with time. Two-way ANOVA showed a significant change in diastolic blood pressure in the centhaquine (p < 0.0001) but not in the control (p = 0.1812) group during the first 12 h of resuscitation
Change in Blood Lactate
Blood lactate levels (mmol/l) were 4.30 ± 0.96 and 4.34 ± 0.78 in the control and centhaquine groups, respectively, at baseline. It decreased to 3.19 ± 1.07 and 1.44 ± 0.13 in the control and centhaquine groups, respectively, at day 3. Centhaquine significantly (two-tailed, unpaired t-test with Welch's correction, the difference between the means was − 2.902 ± 0.7865, 95% CI − 4.529 to − 1.276, p = 0.0012; 66.8%) decreased blood lactate levels with a high level of statistical significance. The decrease in blood lactate was not significant in control group patients (the difference between the means was − 1.110 ± 1.437, 95% CI − 4.013 to 1.792, p = 0.4441; 25.8%). (Fig. 5). Analysis of change in lactate levels in individual patients using two-way ANOVA had a p-value of 0.0682 in the control and 0.0007 in the centhaquine group of patients (Fig. 5). On comparison of blood lactate levels on day 3 between the control and centhaquine groups using a two-tailed, unpaired t-test with Welch's correction, the difference between the means was − 1.752 ± 1.077, 95% CI − 3.988 to 0.4839, p = 0.1183.
Fig. 5.
Blood lactate levels on days 1 and 3 of resuscitation in the control and centhaquine group of patients. The upper panel shows data as the mean ± standard error in the control and centhaquine groups. The lower panel shows a change in each patient's blood lactate levels on days 1 and 3 of the control (p = 0.0682) and centhaquine (p = 0.0007) groups
Change in Base Deficit
Base deficit levels (mmol/l) improved from − 7.40 ± 1.42 at baseline to − 2.58 ± 1.49 at day 3 in the control group (difference between the means 4.823 ± 2.054, 95% CI 0.6768–8.969, p = 0.0237) and from − 5.78 ± 1.22 at baseline to 1.33 ± 0.76 at day 3 in the centhaquine group (difference between the means 7.114 ± 1.439, 95% CI 4.913–10.03, p < 0.0001).
Change in Glasgow Coma Scale (GCS), Multiple Organ Dysfunction Score (MODS), and Adult Respiratory Distress Syndrome (ARDS)
The baseline GCS score was similar in control and centhaquine group patients, and it improved through day 28 in both groups. On day 3, centhaquine-treated patients had a lower MODS (1.17 ± 0.27) than controls (3.68 ± 1.45). The difference between the means of the control and centhaquine groups was 2.508 ± 1.486, 95% CI − 0.5713 to 5.587, p = 0.1054). MODS improved through day 28 in both groups, but it was a little higher in the control group (0.26 ± 0.17) compared to centhaquine (0.17 ± 0.10). Centhaquine-treated patients had lower ARDS scores (0.08 ± 0.03) on day 2 than controls (0.57 ± 0.25). The difference between the means of the control and centhaquine groups was 0.4921 ± 0.2622, 95% CI − 0.0519 to 1.036, p = 0.074). The ARDS score was higher in the control group during the 7-day hospitalization period. ARDS improved through day 28 in both groups (Table 7).
Table 7.
Patients' GCS, MODS, and ARDS recorded through day 28
| Score | Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 28 |
|---|---|---|---|---|---|---|---|---|---|
| GCS | Control | 13.95 ± 0.44 | 13.91 ± 0.75 | 14.85 ± 0.11 | 14.75 ± 0.25 | 14.66 ± 0.27 | 14.27 ± 0.73 | 14.26 ± 0.73 | 15.00 ± 0.00 |
| Centhaquine | 13.78 ± 0.45 | 14.43 ± 0.31 | 14.69 ± 0.26 | 14.67 ± 0.29 | 14.71 ± 0.28 | 14.70 ± 0.30 | 14.68 ± 0.32 | 15.00 ± 0.00 | |
| MODS | Control | 3.68 ± 1.45 | 1.31 ± 0.36 | 0.93 ± 0.35 | 1.07 ± 0.38 | 1.00 ± 0.32 | 0.26 ± 0.17 | ||
| Centhaquine | 1.17 ± 0.27 | 1.28 ± 0.42 | 0.95 ± 0.31 | 1.00 ± 0.30 | 0.94 ± 0.34 | 0.17 ± 0.10 | |||
| ARDS | Control | 0.25 ± 0.11 | 0.57 ± 0.25 | 0.26 ± 0.12 | 0.34 ± 0.15 | 0.25 ± 0.14 | 0.23 ± 0.14 | 0.20 ± 0.13 | 0.00 ± 0.00 |
| Centhaquine | 0.12 ± 0.05 | 0.08 ± 0.03 | 0.13 ± 0.05 | 0.09 ± 0.05 | 0.05 ± 0.03 | 0.05 ± 0.03 | 0.05 ± 0.03 | 0.00 ± 0.00 |
Data are presented as mean ± standard error of the mean
Discussion
This multicentre trial provides evidence that centhaquine administration to hypovolemic shock patients had no evidence of adverse effects or complications and improved clinical outcome. The trial used the randomization method to ensure that participating clinicians did not know the treatment allocation and that the intervention did not influence the outcome assessment. The factors that could affect the prognosis were well balanced at the baseline, and all patients' follow-up was carried out randomly till the end of the study with minuscule potential for bias.
Treatment of blood loss resulting in haemorrhagic shock has been guided by traditional practices rather than randomized clinical trials. In the past 10 years, a decrease in crystalloids and an increase in blood product use in ratios that depict blood transfusion have improved clinical outcome [6, 31]. Administering a sufficient fluid volume is essential during the early phases to stabilize haemodynamics, and fluid restriction may be helpful in later phases [32]. Fluids and vasopressors are still recognized as an essential part of resuscitation and are associated with undesired effects such as fluid responsiveness, extravasation of fluids, and cardiac complications [10, 11, 33]. There is a need to develop novel resuscitative agents that either work as a single agent or improve existing therapeutics.
Centhaquine is a resuscitative agent acting as an α-adrenergic receptor agonist. It acts on venous α2B adrenergic receptors to produce constriction and increase venous return to the heart, increasing cardiac output and tissue perfusion. It also acts on central α2A adrenergic receptors to reduce sympathetic drive and decrease systemic vascular resistance leading to improved tissue blood perfusion [22, 34]. The resuscitative effect of centhaquine is significantly blocked by α2 adrenergic receptor antagonists, yohimbine, or atipamezole [34]. Centhaquine does not act on beta-adrenergic receptors; therefore, the risk of cardiac arrhythmia is mitigated.
There is a drop in cardiac pre-load in hypovolemic conditions to a critical level, resulting in a dramatic drop in cardiac output, leading to a decrease in tissue and organ perfusion, ultimately leading to multiple organ dysfunction and death. Patients' clinical outcome is predominantly monitored using biomarkers, blood pressure, and blood lactate levels. Vasopressors tend to increase blood pressure by causing arterial vasoconstriction and increasing heart rate. An increase in heart rate augments cardiac output. However, the force or rate of contraction cannot explain a significant cardiac output increase [35]. About two-thirds of blood volume is pooled in the venous system serving as an adjustable reservoir [36]. An increase in venous return from systemic veins into the right atrium significantly increases cardiac output by the Frank-Starling mechanism, resulting in increased arterial blood pressure and tissue perfusion. Based on the mechanism of action, centhaquine increases venous return to the heart and increases cardiac output and tissue perfusion, making it an ideal candidate for use as a resuscitative agent in treating patients with hypovolemic patients. The venous system is critical following haemorrhage in mobilizing unstressed blood volume to preserve or increase venous blood return to the heart and increase cardiac output [37, 38]. Centhaquine helps the venous system convert unstressed blood volume to stressed blood volume and optimize cardiac output to maintain blood circulation in a state of shock.
The safety of centhaquine in hypovolemic shock patients was assessed based on adverse events, vital signs, and clinical laboratory parameters. Centhaquine did not show any clinically significant effect on vital signs, haematology, lipid profile, kidney functions, liver functions, and serum electrolytes (Table 4). Patients with hypovolemic shock showed a fall in SBP, DBP, and pulse pressure. Centhaquine improved these vital parameters. Once patients recovered from hypovolemic shock, no clinically significant effect of centhaquine was observed on vital signs (Tables 4, 5). This study excluded patients with GCS < 8 to avoid the added complication of damage to the CNS in patients with a severe head injury. Severe injury to the head can damage the brain and alter system haemodynamics and modify cardiovascular responses and influence the investigational drug outcome.
Two out of 22 patients died in the control group and none in the centhaquine group. Three adverse events were reported in three patients of the control group (N = 22). Out of these three, two events were serious (death), and one was moderate (viral hepatitis), which was resolved with medical intervention. Two adverse events, diarrhoea in one and acute kidney injury (due to severe traumatic injury resulting in the amputation of a right lower limb combined with sepsis) in the other, were reported in the centhaquine group (N = 23); both were moderate in severity and resolved with medical intervention. All these adverse events in the control and centhaquine groups were not related to the study drug and were associated entirely with the patient's disease progression. Centhaquine was found to be safe and well tolerated in hypovolemic shock patients.
Efficacy of centhaquine was assessed based on survival at 28 days; days in ICU; days on ventilator support; total fluids and blood product requirement during first 48 h; amount of total vasopressor infused in the first 48 h; haemodynamic variables; blood lactate; base deficit; MOD and ARDS scores. A significant correlation exists between blood loss and change in haemoglobin [39], and a drop in haemoglobin reflects the blood loss [40]. Since the haemoglobin level was lower by 0.65 g/dl in the centhaquine group than in the controls, the blood loss was to some extent more in the centhaquine group at the time of enrolment.
Two out of 22 patients died within the first 48 h of enrolment in the control group, while all 23 patients in the centhaquine group survived. The severity of injuries was significantly more in the centhaquine group, where 21 out of 23 (91.30%) patients needed major surgery following trauma, while only 15 out of 22 (68.18%) in the control group underwent surgery (p = 0.0526). However, centhaquine group patients stayed in the hospital for a longer duration than controls. However, the percent stay in ICU was higher for control group patients (48.93%) than for the centhaquine group (41.89%). The more extended hospital stays of centhaquine group patients were because of their health conditions, which were a little more severe than those of the control group patients as seen from their ISS score (control 20.63 ± 2.45 vs. centhaquine 23.14 ± 3.30), baseline haemoglobin (control 9.38 ± 0.71 g/dl vs. centhaquine 8.73 ± 0.55 g/dl) and haematocrit (control 28.79 ± 2.11% vs. centhaquine 26.71 ± 1.81%) levels. Centhaquine group patients were on ventilator support for a shorter duration (0.89 ± 0.45 days) than the control group patients who stayed on ventilator support for 1.96 ± 1.10 days. Although it did not reach the statistical significance level, the results indicate a better recovery of patients with centhaquine treatment (Table 6).
Centhaquine treated patients required a similar volume of fluids (4.26 ± 0.23 l) in the first 48 h of randomization compared to control (4.59 ± 0.41 L) group patients. Almost a similar volume of blood products was required by control and centhaquine group patients in the first 48 h of randomization. These data of fluids and blood products and other treatments shown in Table 4 indicate that patients from both groups received almost the same standard of care, avoiding biases.
Most patients show improved blood pressure in response to the fluids shortly after their administration; however, this response is transient and is lost rapidly over time [41]. Using fluids is an attempt to improve tissue perfusion by improving cardiac output. Adding centhaquine to fluid administration augments cardiac output improvement through a different mechanism of action that complements each other. Vasopressors are used when patients in hypovolemic shock are poorly or not responding to fluids. Norepinephrine, phenylephrine, epinephrine, and dopamine are the commonly used vasopressors. These catecholamines enhance cardiac contractility and vascular tone and influence overall arterial, venous, and capillary pressures and blood flow. The dose of vasopressors needs to be carefully titrated and is associated with many adverse effects like arrhythmias, fluid extravasation, and ischaemia [10, 11]. Vasopressin is used both as adjunctive and first-line therapy, but studies have equivocal results [42–44]. Angiotensin II has recently been introduced for patients with vasodilatory shock [42, 45]. These vasopressors act on different receptors and through different pathways. Agents affecting the sympathetic system through α- and β-adrenergic receptors are available, but additional stimulation of these receptors is not apparent and could be helpful or even harmful [46]. The present study is critical, given that centhaquine acts through α2-adrenergic receptors to increase cardiac output (via α2B) and decreased arterial resistance (via α2A) has shown promising efficacy in hypovolemic shock patients.
A comparatively lesser amount of vasopressors was needed by the centhaquine group patients in the first 48 h of resuscitation than by the control group patients. Only 26.09% of patients from the centhaquine group required vasopressors, while 40.91% of patients from the control group needed them. Though the difference in mean values of total vasopressors between the groups was not statistically significant, the trend indicates a reduction in vasopressors' requirement in centhaquine treatment. Our findings are consistent with our preclinical studies conducted in an animal model of hypovolemic shock where rats treated with centhaquine required less norepinephrine to maintain their blood pressure [47]. A significant reduction in SBP and DBP occurs in hypovolemic shock, leading to a reduction in peripheral blood perfusion. Treatment with centhaquine showed a highly significant increase (p < 0.0001) in SBP from baseline in the first 48 h of resuscitation, while in the control group, the increase in SBP was comparatively less significant than with centhaquine. A similar increase (p < 0.0001) was seen in DBP in centhaquine treated patients, while in the control group of patients, minimal improvement in DBP occurred (Figs. 3, 4).
Changes in lactate levels provide an early and objective evaluation of a patient's response to therapy, and repeated lactate determinations have proved to be a reliable prognostic index for patients with haemorrhagic shock [48]. Early return of lactate levels to normal levels (< 2.0 mmol/l) within 24 h is associated with improved mortality. A significant correlation was established between the admission base deficit and transfusion requirements within the first 24 h and the risk of post-traumatic organ failure or death [49]. In the present study, all the enrolled patients showed a significant increase in blood lactate and base deficit levels. Centhaquine treatment showed a significant reduction in blood lactate (p = 0.0012; 66.8%), while in the control group the reduction was not significant (0.441; 25.8%). Similarly, the centhaquine group showed a highly significant improvement in base deficit (p < 0.0001), while in the control group, the reduction was less significant (p = 0.0237).
Mortality in hypovolemic shock patients is mainly associated with multiple organ dysfunction because of hypoperfusion and severe acidosis. On day 3 of resuscitation, centhaquine-treated patients showed a lower MODS (1.17 ± 0.27) than controls (3.68 ± 1.45). Studies in a swine model of haemorrhagic shock showed that centhaquine significantly improved the Horowitz index (327 ± 10 and 392 ± 16 in the control and centhaquine group, respectively) and reduced pulmonary oedema [22, 25]. In the present study, centhaquine-treated patients showed comparatively lower ARDS scores than the control group patients with p = 0.074 indicating significance at 90% CI but not at 95% CI (Table 7).
In this phase II study, centhaquine was highly efficacious, with statistically significant improvements in blood lactate levels, base deficit, and blood pressure. An improvement in all the above clinical and biological markers appears to contribute towards improved outcomes and reduced deaths when centhaquine was added to the SOC. Results of this phase II study have confirmed previously observed efficacy in preclinical studies [21–27].
This study's limitation is that it was conducted in a small number of patients and was conducted in one country. Since this study involves a first-in-class drug product, we took this approach to have an appropriate comparison with a limited number of patients with similar baseline characteristics. Another limitation of the study was the assessment of tissue perfusion, including cardiac output, which was not done because of lack of accessibility. Information about a patient's cardiac functions is clinically desirable to manage hypovolemic shock better. However, this study's promising results have led to an efficacy study in a larger group of hypovolemic shock patients, investigating centhaquine in a multicentric, randomized, blinded, controlled efficacy clinical trial phase III (NCT04045327). In the future, other investigators and we may conduct further studies to test whether centhaquine and vasopressors complement each other in improving clinical outcomes of patients with hypovolemic shock. We would also like to investigate this hypothesis in septic shock patients. Another question is how chronic co-morbidities, such as hypertension and congestive heart failure, impact the efficacy of centhaquine. We recognize the demographics and SOC for the treatment of hypovolemic shock across the world may vary and that the efficacy of centhaquine needs to be established in populations across the world.
Conclusion
Centhaquine (Lyfaquin®) was safe and well tolerated in hypovolemic shock patients. This study is the first to assess the effect of centhaquine on clinical outcomes in patients in hypovolemic shock due to blood loss. Centhaquine is a highly effective resuscitative agent and appears to improve hypovolemic shock patients' clinical outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the study participants, patients, and their families.
Funding
Pharmazz, Inc., Willowbrook, IL, USA, provided funding for this study and paid the journal's Rapid Service and Open Access fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Study concept and design: Anil Gulati and Manish S. Lavhale; Investigation: Dinesh Jain, Nilesh Radheshyam Agrawal, Prashant Rahate, Rajat Choudhuri, Soumen Das, Deba Prasad Dhibar, Madhav Prabhu, Sameer Haveri, and Rohit Agarwal; Acquisition of data: Dinesh Jain, Nilesh Radheshyam Agrawal, Prashant Rahate, Rajat Choudhuri, Soumen Das, Deba Prasad Dhibar, Madhav Prabhu, Sameer Haveri, and Rohit Agarwal; Analysis and interpretation of data: Anil Gulati and Manish S. Lavhale; Drafting of the manuscript: Manish S. Lavhale and Anil Gulati; Review of the manuscript: Dinesh Jain, Nilesh Radheshyam Agrawal, Prashant Rahate, Rajat Choudhuri, Soumen Das, Deba Prasad Dhibar, Madhav Prabhu, Sameer Haveri, Rohit Agarwal, Manish S. Lavhale and Anil Gulati; Funding acquisition: Anil Gulati.
Disclosures
Anil Gulati (AG) and Manish S. Lavhale (MSL) are employees of Pharmazz, Inc., and have issued and pending patents relating to this study. Dinesh Jain, Nilesh Radheshyam Agrawal, Prashant Rahate, Rajat Choudhuri, Soumen Das, Deba Prasad Dhibar, Madhav Prabhu, Sameer Haveri, and Rohit Agarwal have no conflicts of interest that are directly relevant to the content of this article. Present address of Soumen Das: Netaji Subhas Chandra Bose Cancer Hospital, Kolkata, India.
Compliance with Ethics Guidelines
The study was performed according to the Declaration of Helsinki principles, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for Good Clinical Practice (ICH-GCP), and local regulatory requirements. The study protocol (PMZ-02, version 2.0/dated 10 March 2016) was approved by the Drugs Controller General of India, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India (DCGI CT NOC. no. CT/ND/37/2016). Each institutional ethics committee also reviewed and approved the study protocol before initiating patient enrolment. The trial was registered at the Clinical Trials Registry, India (CTRI/2017/03/008184), and the US National Library of Medicine, ClinicalTrials.gov (NCT04056065). Supplementary Table 1 shows that the list of sites where institutional ethics committees approved the study.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Anil Gulati, Email: agulati@midestern.edu.
Dinesh Jain, Email: drjaindinesh@yahoo.co.in.
Nilesh Radheshyam Agrawal, Email: anileshr@gmail.com.
Prashant Rahate, Email: prashantrahate84@yahoo.com.
Rajat Choudhuri, Email: rajat.choudhuri@gmail.com.
Soumen Das, Email: soumendoc.das@gmail.com.
Deba Prasad Dhibar, Email: drdeba_prasad@yahoo.co.in.
Madhav Prabhu, Email: maddy2380@gmail.com.
Sameer Haveri, Email: drsameerhaveri@gmail.com.
Rohit Agarwal, Email: drrohit1977@gmail.com.
Manish S. Lavhale, Email: manish.lavhale@pharmazz.com
References
- 1.Taghavi S, Jones G, Duchesne J, McGrew P, Guidry C, Schroll R, et al. Impact of trauma center volume on major vascular injury: an analysis of the National Trauma Data Bank (NTDB) Am J Surg. 2020;220(3):787–792. doi: 10.1016/j.amjsurg.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378(4):370–379. doi: 10.1056/NEJMra1705649. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haut ER, Kalish BT, Cotton BA, Efron DT, Haider AH, Stevens KA, et al. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Ann Surg. 2011;253(2):371–377. doi: 10.1097/SLA.0b013e318207c24f. [DOI] [PubMed] [Google Scholar]
- 5.Haut ER, Kalish BT, Cotton BA, Efron DT, Haider AH, Stevens KA, et al. Reply to letter: "Prehospital intravenous fluid administration is associated with higher mortality in trauma patients". Ann Surg. 2014;259(2):e20–e21. doi: 10.1097/SLA.0b013e3182456b7c. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 8.Nederpelt CJ, El Hechi MW, Kongkaewpaisan N, Kokoroskos N, Mendoza AE, Saillant NN, et al. Fresh frozen plasma-to-packed red blood cell ratio and mortality in traumatic hemorrhage: nationwide analysis of 4,427 patients. J Am Coll Surg. 2020;230(6):893–901. doi: 10.1016/j.jamcollsurg.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Havel C, Arrich J, Losert H, Gamper G, Mullner M, Herkner H. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2011;11(5):003709. doi: 10.1002/14651858.CD003709.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Abid O, Akca S, Haji-Michael P, Vincent JL. Strong vasopressor support may be futile in the intensive care unit patient with multiple organ failure. Crit Care Med. 2000;28(4):947–949. doi: 10.1097/00003246-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Aoki M, Abe T, Saitoh D, Hagiwara S, Oshima K. Use of vasopressor increases the risk of mortality in traumatic hemorrhagic shock: a nationwide cohort study in Japan. Crit Care Med. 2018;46(12):e1145–e1151. doi: 10.1097/CCM.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 12.Georgoff PE, Nikolian VC, Higgins G, Chtraklin K, Eidy H, Ghandour MH, et al. Valproic acid induces prosurvival transcriptomic changes in swine subjected to traumatic injury and hemorrhagic shock. J Trauma Acute Care Surg. 2018;84(4):642–649. doi: 10.1097/TA.0000000000001763. [DOI] [PubMed] [Google Scholar]
- 13.Ganster F, Burban M, de la Bourdonnaye M, Fizanne L, Douay O, Loufrani L, et al. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care. 2010;14(5):R165. doi: 10.1186/cc9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wepler M, Merz T, Wachter U, Vogt J, Calzia E, Scheuerle A, et al. The mitochondria-targeted H2S-Donor AP39 in a murine model of combined hemorrhagic shock and blunt chest trauma. Shock. 2019;52(2):230–239. doi: 10.1097/SHK.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 15.Thakral S, Wolf A, Beilman GJ, Suryanarayanan R. Development and in vivo evaluation of a novel lyophilized formulation for the treatment of hemorrhagic shock. Int J Pharm. 2018;537(1–2):162–171. doi: 10.1016/j.ijpharm.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Gulati A, Sen AP. Dose-dependent effect of diaspirin cross-linked hemoglobin on regional blood circulation of severely hemorrhaged rats. Shock. 1998;9(1):65–73. doi: 10.1097/00024382-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Gulati A, Sen AP, Sharma AC, Singh G. Role of ET and NO in resuscitative effect of diaspirin cross-linked hemoglobin after hemorrhage in rat. Am J Physiol. 1997;273(2 Pt 2):H827–H836. doi: 10.1152/ajpheart.1997.273.2.H827. [DOI] [PubMed] [Google Scholar]
- 18.Sloan EP, Koenigsberg M, Gens D, Cipolle M, Runge J, Mallory MN, et al. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999;282(19):1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 19.Sloan EP, Koenigsberg MD, Philbin NB, Gao W, Group DCTHSS, European HI. Diaspirin cross-linked hemoglobin infusion did not influence base deficit and lactic acid levels in two clinical trials of traumatic hemorrhagic shock patient resuscitation. J Trauma. 2010;68(5):1158–71. [DOI] [PubMed]
- 20.Vincent JL, Privalle CT, Singer M, Lorente JA, Boehm E, Meier-Hellmann A, et al. Multicenter, randomized, placebo-controlled phase III study of pyridoxalated hemoglobin polyoxyethylene in distributive shock (PHOENIX) Crit Care Med. 2015;43(1):57–64. doi: 10.1097/CCM.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 21.Gulati A. Vascular endothelium and hypovolemic shock. Curr Vasc Pharmacol. 2016;14(2):187–195. doi: 10.2174/1570161114666151202210221. [DOI] [PubMed] [Google Scholar]
- 22.Gulati A, Lavhale M, Giri R, Andurkar S, Xanthos T. Centhaquine citrate. Alpha2B-Adrenoceptor ligand, resuscitative agent for hypovolemic shock. Drugs Fut. 2020;45(3):153–163. [Google Scholar]
- 23.Gulati A, Lavhale MS, Garcia DJ, Havalad S. Centhaquin improves resuscitative effect of hypertonic saline in hemorrhaged rats. J Surg Res. 2012;178(1):415–423. doi: 10.1016/j.jss.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Gulati A, Zhang Z, Murphy A, Lavhale MS. Efficacy of centhaquin as a small volume resuscitative agent in severely hemorrhaged rats. Am J Emerg Med. 2013;31(9):1315–1321. doi: 10.1016/j.ajem.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Kontouli Z, Staikou C, Iacovidou N, Mamais I, Kouskouni E, Papalois A, et al. Resuscitation with centhaquin and 6% hydroxyethyl starch 130/04 improves survival in a swine model of hemorrhagic shock: a randomized experimental study. Eur J Trauma Emerg Surg. 2019;45(6):1077–1085. doi: 10.1007/s00068-018-0980-1. [DOI] [PubMed] [Google Scholar]
- 26.Lavhale MS, Havalad S, Gulati A. Resuscitative effect of centhaquin after hemorrhagic shock in rats. J Surg Res. 2013;179(1):115–124. doi: 10.1016/j.jss.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 27.Papapanagiotou P, Xanthos T, Gulati A, Chalkias A, Papalois A, Kontouli Z, et al. Centhaquin improves survival in a swine model of hemorrhagic shock. J Surg Res. 2016;200(1):227–235. doi: 10.1016/j.jss.2015.06.056. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds PS, Michael MJ, Cochran ED, Wegelin JA, Spiess BD. Prehospital use of plasma in traumatic hemorrhage (The PUPTH Trial): study protocol for a randomised controlled trial. Trials. 2015;30(16):321. doi: 10.1186/s13063-015-0844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulger EM, May S, Kerby JD, Emerson S, Stiell IG, Schreiber MA, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9(12):1447–1454. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
- 31.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. doi: 10.1001/jamasurg.2014.940. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa K. Aggressive fluid management in the critically ill: Pro. J Intensive Care. 2019;7:9. doi: 10.1186/s40560-019-0361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi L, Costantini TW, Coimbra R. Hypovolemic shock resuscitation. Surg Clin N Am. 2012;92(6):1403–1423. doi: 10.1016/j.suc.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Gulati A, Voshtina E, Zhang Z, Murphy A. Alpha adrenergic receptors mediate resuscitative effect of centhaquin in hemorrhaged rats. Crit Care Med. 2012;40(12):U162–U163. [Google Scholar]
- 35.Berlin DA, Bakker J. Understanding venous return. Intensive Care Med. 2014;40(10):1564–1566. doi: 10.1007/s00134-014-3379-4. [DOI] [PubMed] [Google Scholar]
- 36.Jansen JR, Maas JJ, Pinsky MR. Bedside assessment of mean systemic filling pressure. Curr Opin Crit Care. 2010;16(3):231–236. doi: 10.1097/MCC.0b013e3283378185. [DOI] [PubMed] [Google Scholar]
- 37.Chalkias A, Koutsovasilis A, Laou E, Papalois A, Xanthos T. Measurement of mean systemic filling pressure after severe hemorrhagic shock in swine anesthetized with propofol-based total intravenous anesthesia: implications for vasopressor-free resuscitation. Acute Crit Care. 2020;35(2):93–101. doi: 10.4266/acc.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen T, Baker K. Venous return and clinical hemodynamics: how the body works during acute hemorrhage. Adv Physiol Educ. 2015;39(4):267–271. doi: 10.1152/advan.00050.2015. [DOI] [PubMed] [Google Scholar]
- 39.Jansen AJ, leNoble PJ, Steegers EA, van Rhenen DJ, Duvekot JJ. Relationship between haemoglobin change and estimated blood loss after delivery. BJOG. 2007;114(5):657. doi: 10.1111/j.1471-0528.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 40.Yefet E, Yossef A, Suleiman A, Hatokay A, Nachum Z. Hemoglobin drop following postpartum hemorrhage. Sci Rep. 2020;10(1):21546. doi: 10.1038/s41598-020-77799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez G, Ospina-Tascon GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla LS, Ostermann M, Forni L, Tidmarsh GF. Broad spectrum vasopressors: a new approach to the initial management of septic shock? Crit Care. 2019;23(1):124. doi: 10.1186/s13054-019-2420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gazmuri RJ, Whitehouse K, Whittinghill K, Baetiong A, Shah K, Radhakrishnan J. Early and sustained vasopressin infusion augments the hemodynamic efficacy of restrictive fluid resuscitation and improves survival in a liver laceration model of hemorrhagic shock. J Trauma Acute Care Surg. 2017;82(2):317–327. doi: 10.1097/TA.0000000000001318. [DOI] [PubMed] [Google Scholar]
- 44.Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316(5):509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 45.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 46.Lat I, Coopersmith CM, De Backer D, Research Committee of the Surviving Sepsis C, Members of the Surviving Sepsis Campaign Research Committee contributing to this article are as f, Co-chair AGA, et al. The surviving sepsis campaign: fluid resuscitation and vasopressor therapy research priorities in adult patients. Crit Care Med. 2021;49(4):623–35. [DOI] [PMC free article] [PubMed]
- 47.Gulati A, Zhang Z, Arshad K. Centhaquin decreases the requirement of norepinephrine, maintains blood pressure and improves survival following resuscitation of hemorrhaged rats. Crit Care Med. 2011;39(12):114. [Google Scholar]
- 48.Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI, et al. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg. 2013;74(4):999–1004. doi: 10.1097/TA.0b013e3182858a3e. [DOI] [PubMed] [Google Scholar]
- 49.Doran CM, Doran CA, Woolley T, Carter A, Male K, Midwinter MJ, et al. Targeted resuscitation improves coagulation and outcome. J Trauma Acute Care Surg. 2012;72(4):835–843. doi: 10.1097/TA.0b013e318248347b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.