Abstract
To compare pregnancy rate and implantation rate in poor responder women, aged over 40 years, who underwent natural cycle versus conventional ovarian stimulation. This is a retrospective single-center cohort study conducted at the GENERA IVF program, Rome, Italy, between September 2012 and December 2018, including only poor responder patients, according to Bologna criteria, of advanced age, who underwent IVF treatment through Natural Cycle or conventional ovarian stimulation. Between September 2012 and December 2018, 585 patients were included within the study. Two hundred thirty patients underwent natural cycle and 355 underwent conventional ovarian stimulation. In natural cycle group, both pregnancy rate per cycle (6.25 vs 12.89%, respectively, p = 0.0001) and pregnancy rate per patient101 with at least one embryo-transfer (18.85 vs 28.11% respectively, p = 0.025) resulted significant reduced. Pregnancy rate per patient managed with conventional ovarian stimulation resulted not significantly different compared with natural cycle (19.72 vs 15.65% respectively, p = 0.228), but embryo implantation rate was significantly higher in patients who underwent natural cycle rather than patient subjected to conventional ovarian stimulation (13 vs 8.28% respectively, p = 0.0468). No significant difference could be detected among the two groups in terms of abortion rate (p = 0.2915) or live birth pregnancy (p = 0.2281). Natural cycle seems to be a valid treatment in patients over 40 years and with a low ovarian reserve, as an alternative to conventional ovarian stimulation.
Keywords: Natural cycle, Poor responder, Ovarian stimulation, Bologna criteria, IVF
Introduction
Poor ovarian response (POR) remains a challenge of scientific and clinical relevance for in vitro fertilization (IVF) treatment because of the increased failure rate and the negative influence on the patients. Although many treatments have been suggested also with a high quantity of gonadotropins, poor responder patients are refractory to stimulation protocols with very low chance of pregnancy, resulting in less than 10% for oocyte retrieval [1]. For infertile women, there is a well-known association between advanced age and IVF treatment failure [2]. Nowadays, societal changes have imposed women to achieve educational goals and established careers before programming a pregnancy. In this context the age of women seeking pregnancy has increased, and this is progressively pointing out a worldwide social problem impacting future sustainability [3].
Up to 2011, there was the lack of a universal definition of “poor responder women”: patients were identified retrospectively after the ovarian stimulation considering the number of developed follicles and/or oocytes retrieved [4–7]. The European Society of Human Reproduction and Embryology (ESHRE) in 2011 published the so-called Bologna criteria for the definition of poor ovarian responders (POR) [8]. Many studies proposed different treatment protocols to attempt for these patients, with discordant results [9]. In this scenario, the best treatment option for poor responders is still needed to be clarified. Some evidences suggested that natural cycle (NC) works at least similar to the conventional ovarian stimulation (COS), with a pregnancy rate per cycle of 6.1 and 14.9% per transfer [10]. Natural cycle is defined as IVF treatment without any gonadotrophin administration: the biological rationale for this approach, indeed, is to obtain the natural follicle recruitment and selection and subsequent in vitro fertilization and embryo culture [11].
The objective of our study was to evaluate the fertility outcomes of NC IVF compared with conventional IVF (cIVF) carried out with high-dose ovarian stimulation in a large-size cohort of advanced age Bologna criteria poor responder women.
Materials and Methods
This was a retrospective single-center cohort study conducted at the GENERA IVF program, in Rome, Italy. Clinical and demographic data were collected from Italian Caucasian patients’ clinical chart who underwent intracytoplasmic sperm injection (ICSI) cycles between September 2012 and December 2018.
The institutional review board evaluated and approved the study.
All included patients had previously signed written informed consent regarding collection of data for research purposes. All patients had undergone a standard evaluation for infertility, including hormone test on the second day of the menstrual cycle (mainly FSH, LH, estradiol), hysterosalpingogram, hysteroscopy, complete blood tests, and semen analysis, AMH measure irrespective of the cycle day, Antral Follicle Count (AFC) on day 2–4, in a previous cycle.
Patients’ Selection and Eligibility Criteria
Patients were selected according to the definition of poor ovarian responders by the Bologna criteria [8] and exclusively aged ≥ 40 years.
Inclusion criteria were:
Advanced maternal age: patients over 40 years old
and at least one of the following:
Abnormal ovarian reserve biomarker: AMH < 0.5–1.1 ng/mL; AFC < 5–7
Previous POR: ≤ 3 oocytes with conventional stimulation
Two episodes of POR after maximal stimulation
Exclusion criteria were:
Body mass index (BMI) greater than 35 kg/m2
Irregular menstrual cycles
Previous monolateral oophorectomy
The presence of untreated endocrine abnormalities
The presence of comorbidities
Patients who underwent pre-implantation genetic testing (PGT)
Follicles Recruitment and IVF Procedure
In the cIVF group, recombinant follitropin alfa and lutropin alfa were initiated on Day 2 of cycle. The starting dose of follitropin alfa was 225 IU/day and the dose of lutropin alfa was 75 UI/daily for all patients. Endogenous LH surge was avoided by GnRh antagonist administration when the leading follicles reached 16 mm of diameters. In both groups, from the 6th day of the cycle, the patients underwent transvaginal sonography to monitor follicle size and structures within the ovary, endometrial thickness, and estradiol and progesterone dosage.
In cIVF group, when follicle size reached 18–20 mm in mean diameter and plasma estradiol value reached at least 200 pg/ml per follicle, triggering ovulation with 10,000 IU of human chorionic gonadotropin (hCG, Gonasi HP 5000; AMSA, Rome, Italy) was performed.
In NC group, no medical treatment was administrated for selection and recruitment of follicles. From the 6th day of the cycle, patients underwent transvaginal sonography to monitor follicle size and structures within the ovary, endometrial thickness, and estradiol and progesterone dosage. When follicle size reached 16 mm in mean diameter, triggering ovulation with 10,000 IU of human chorionic gonadotropin (hCG, Gonasi HP 5000; AMSA, Rome, Italy) was performed.
Oocyte Retrieval, Laboratory Procedures, Embryo Transfer
Thirty-six hours after the injection of hCG, oocyte retrieval was performed under ultrasound control in local or general anesthesia. We performed ICSI in all cases according to previously published procedures [12], to obtain a higher fecundation rate, and to maximize the success of embryo transfer, due to the very low number of oocytes harvested in these women, and to avoid differences in the fertilization rate among patients treated with different techniques. Patients gave their informed consent accepting the possible risks occurring to offspring from ICSI. Oocytes were observed 18 h after ICSI for their pronuclei and 44 h after insemination for embryo development.
Embryos were transferred 72 h after insemination using the Sydney embryo transfer catheter (Cook Ltd., Brisbane, Queensland, Australia). All transfer procedures were performed by the same physician to avoid inter-operator variability.
All pregnancies were confirmed by a rising titer of serum b-hCG from 12 days after embryo transfer and by ultrasound demonstration of the gestation sac 4 weeks after the transfer.
The same luteal phase support was used in all cycles: 75 mg daily of intramuscular progesterone from the day of embryo transfer.
Main Outcomes
The main outcomes were assessment of pregnancy rate per patient, per cycle, per transfer, and the embryo implantation rate.
Pregnancy was defined as the visualization of intrauterine sac with embryo with cardiac activity at the ultrasound exam.
Statistical Analysis
Descriptive statistics were used to characterize patients’ population. The quantitative variables are expressed as the mean and categorical variables are expressed as the median or as a number (percentage). To determine whether there was a statistically significant difference between the categories of qualitative and quantitative variables, student’s t test was used. The Chi-square test was used to analyze the relationship between the 2 categorical data.
Parameters analyzed were the number of cycles with oocytes, number of cycles with embryos, number of embryo transfers, pregnancy rate (per patients, per cycle started and per transfer), implantation rate (number of embryos observed by ultrasound per number of embryos transferred), abortion rate, and live birth rate. All statistical analyses were performed using the Statistical Package for the Social Sciences Statistical Package (SPSS, Inc., Chicago, IL) version 25. A p value ≤ 0.05 was considered to be statistically significant.
Results
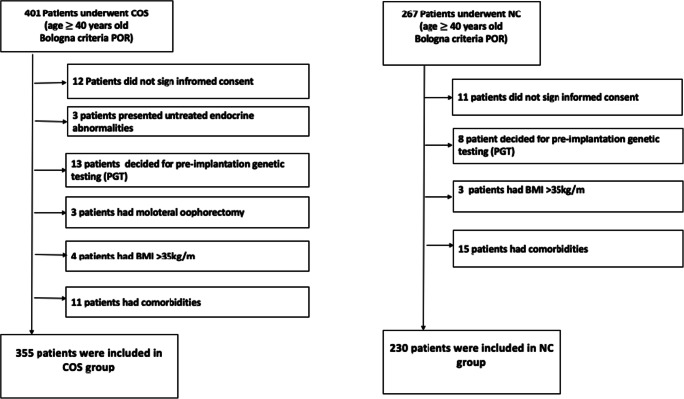
The patient selection flowchart is shown in Fig. 1.
Fig. 1.
Patients selection flowchart
Among 401 patients who underwent IVF COS protocol, 355 patients met the inclusion criteria. Among 367 patients who underwent IVF NC protocol, 230 patients met the inclusion criteria.
So 585 poor responder patients were included: 230 patients underwent NC and 355 underwent COS.
Al patients’ characteristics were listed in Table 1. Globally, the two study groups were homogeneous with regard to median age, BMI, FSH, menarche, previous pregnancies, AFC, and smoke status. AMH levels were significantly higher in stimulated patients compared with patients who underwent NC.
Table 1.
Study group characteristics
| Parameters | COS group | NC group | P value |
|---|---|---|---|
| Age (mean) | 41 (40–42) | 41 (40–42) | 0.956 |
| BMI | 24 (22–27) | 24 (22–28) | 0.785 |
| FSH | 9.55 | 11.59 | 0.059 |
| AMH | 1.46 | 0.58 | 0.02 |
| Smoke | %31,8 (113) | % 26,5 (61) | 0.17 |
| Menarche | 12 (10–14) | 12 (10–15) | 0.654 |
| Previous Pregnancies | 4,5% (16) | 2,2% (5) | 0.138 |
| AFC | 4 (2–7) | 3(1–5) | 0.846 |
The total number of ovarian cycles was 1119:576 in NC patient group and 543 in COS patients group.
Reproductive outcomes are summarized in Table 2. No significant differences resulted in the number of pregnancies per patient in COS and NC groups (19.72 vs 15.65% respectively, p = 0.228). The abortion rate and the live birth rate were no significantly different among the two study groups. Both pregnancy rate per cycle (6.25 vs 12.89% respectively, p = 0.0001) and pregnancy rate per patients who underwent at least one embryo-transfer (18.85% vs 28.11% respectively, p = 0.025) were significantly reduced in NC group compared with COS group.
Table 2.
Study results
| Parameters | All cases | Natural cycle | Controlled ovarian stimulation | p |
|---|---|---|---|---|
| N. of patients | 585 | 230 | 355 | – |
| N. of cycles | 1119 | 576 | 543 | – |
| N. transfer | 1042 | 277 | 765 | – |
| Transfer/cycle | (1042/1119) 93% | (277/576) 48% | (765/543) 140% | < 0.0001 |
| Cycles without transfer | (467/1119) 41.7% | (329/576) 57% | (138/543) 25.4% | 0.00001 |
| Pregnancy/cycle | (106/1119) 9.47% | (36/576) 6.25% | (70/543) 12.89% | 0.0001 |
| Pregnancy/ transfer | (106/1042) 10% | (36/277) 13% | (70/765) 9.1% | 0.0814 |
| Implantation rate (Pregnancy/embryos) | (106/1122) 9.45% | (36/277) 13% | (70/845) 8.28% | 0.0468 |
| Pregnancy/patients transfer | (106/440) 24% | (36/191) 18.85% | (70/249) 28.11% | 0.025 |
| Pregnancy/patients | (106/585) 18.12% | (36/230) 15.65% | (70/355) 19.72% | 0.2281 |
| live birth pregnancy | (73/106) 68.87% | (22/36) 61% | (51/70) 72% | 0.2693 |
| Abortion rate | (37/106) 34.91% | (10/36) 27.78% | (27/70) 38.57% | 0.2915 |
The number of transfers was higher in patients who were conventionally stimulated (765 compared with 277 transfers of NC patients, p < 0.0001), but there was a trend towards significance for pregnancy rate per single transfer that was 13% in the NC group compared with 9.1% in COS group (p = 0.0814). Furthermore, embryo implantation rate resulted significantly higher in patients who underwent NC than COS (13 vs 8.28% respectively, p = 0.0468).
Discussion
To our knowledge, at the present with 585 patients studied, this is the largest study in the literature with a comparison of NC, intended as a natural follicle selection and recruitment without medical treatment, with cIVF in POR patients older than 40 years. Studies on NC outcomes compared with COS in advanced age Bologna POR women are rare. Other previously published studies evaluating NC pregnancy rate examined inhomogeneous populations with no precise and standardized pre-selection of patients, with a wide diversity in the criteria used to specify POR women, thus resulting in a wide variability pregnancy rate, which ranged from 10.2 to 50% [10, 12–17]. Contrarily, our study focused specifically on the most difficult subgroup of women requiring IVF treatment, selected in accordance with the strict requirements of Bologna criteria.
Results of the present study firstly highlighted no difference in pregnancy rate per patient among women subjected to NC compared with COS.
Recently, a smaller retrospective series assessed the outcomes of the same subgroup of patients by comparing conventional stimulation with high doses of gonadotropins versus the spontaneous cycle but modified, with a minimal stimulation. It showed that the type of treatment strategy was not significantly associated with differences in ongoing pregnancy rate (OR 2.56, 95% CI: 0.9–7.6) [18].
In the present study, 15.65% of women treated with NC achieved pregnancy. These findings are more encouraging than outcomes reported in our previously published cohort, 10 years ago, when the pregnancy rate was 9.7% in poor responder patients older than 40 years managed with NC [12].
Analyzing pregnancy rate per cycle was significantly higher in cIVF than in NC. These data are in accordance with the studies that reported a higher number of NC treatments is required to achieve the same success rate as cIVF with a longer time to pregnancy [19–21].
In the present study, pregnancy rate per number of patients who get at least one transfer in COS group was higher being 28% compared with 18.85% of NC group.
Three hundred fifty-five conventionally stimulated patients achieved 845 embryos, and they needed 765 embryo transfers, while the 230 patients in the NC underwent 277 transfer with 277 embryos.
Nevertheless, analyzing the number of pregnancies per total of transfers and per total of embryos, the percentages were reversed.
In NC group, with less number of embryos, women get higher number of pregnancies, being the second most interesting finding the higher embryo implantation rate among patients who underwent NC rather than COS.
The transfer number in patients stimulated, indeed, was significantly higher but resulted in a lower number of pregnancies per transfer. We found 9.1% of pregnancies per transfer in stimulated women compared with 13% of pregnancies per transfer when embryos developed from natural follicles. This result reached statistical significance if the implantation rate was evaluated: the number of pregnancies for embryos transferred passed from 13% of the spontaneous cycle to 8.28% of cIVF.
Our results are particularly meaningful considering that embryo transfer procedure means for women to support an economic expenditure [21] of clinical and laboratory procedures and also an emotional expense related to the stress of undergoing an invasive procedure and to the hope of waiting for a positive pregnancy test.
Some studies hypothesized that implantation rate was lower in cIVF than in NC because of the dysregulation of endometrium for high estradiol concentration during conventional stimulation [19, 22], while other supported embryo quality as responsible [23–25]. The success of pregnancy rate of cIVF is directly correlated with the number of oocytes collected, so the chances are decreased in poor responders, while the success rate is higher when undergoing NC-IVF. A meta-analysis on conventional stimulation identified as negative prognostic factors for pregnancy: female age (OR 0.95, 95% CI: 0.94–0.96), duration of subfertility (OR 0.99, 95% CI: 0.98–1.0), basal FSH (OR 0.94, 95% CI: 0.88–1.0), and a positive correlation with oocytes number (OR 1.04, 95%CI:1.02–1.07) and embryo quality [26].
It must be also considered that emotional stress due to cIVF can cause premature treatment ending, thus reducing IVF success [27, 28]. Some contributing factors are daily injections, side effects, and costs but also embryo selection and cryopreservation [29].
This study has limitations of being retrospective and not homogeneous for the AMH levels, those were significant higher in stimulated patients compared with patients who underwent NC. Since AMH is a prognostic index of response to conventional ovarian stimulation [30], in the present study, patients with too low AMH did not receive pharmacologic stimulation as therapeutic solution but underwent natural cycle treatment. Therefore, two groups had different AMH values. However, although AMH of patients in natural cycle group was lower, they had a higher implantation rate. Furthermore, pregnancy rate per patients did not change between the two groups as a demonstration that AMH value did not correlate with pregnancy success, as recently described by von Wolff M et al. [31].
On the other hand, the strength points of this research were the large sample size and the homogeneity of the selected population in term of median age, BMI, FSH, menarche, previous pregnancies, AFC, and smoke status.
In conclusion our finding clearly showed that NC may be an option to consider in patients of advanced age identified as poor responder according to Bologna criteria, being outcomes comparable to cIVF but with a lower implantation failure.
Authors’ Contribution
M Schimberni and M Schimberni conceived of the presented idea.
MP De Marco, G Montanari, and Flavia Costanzi collected and processed the experimental data and performed the analysis.
M Schimberni, M Schimberni, MP De Marco, and I Ruscito contributed to the interpretation of the results.
MP De Marco wrote the manuscript with support from I Ruscito, M Schimberni, and M Schimberni.
A Giallonardo, FM Ubaldi, L Rienzi, and D Caserta were involved in planning and supervised the work.
Funding
Open Access funding provided by Università degli Studi di Roma La Sapienza. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Compliance with Ethical Standards
Conflict of Interest
The author Maria Paola De Marco declares that she has no conflict of interest. Author Giulia Montanari declares that she has no conflict of interest. The author Ilary Ruscito declares that she has no conflict of interest. The author Annalise Giallonardo declares that she has no conflict of interest. The author Filippo Maria Ubaldi declares that he has no conflict of interest. The author Laura Rienzi declares that she has no conflict of interest. The author Flavia Costanzi declares that she has no conflict of interest. The author Donatella Caserta declares that she has no conflict of interest. The author Mauro Schimberni declares that he has no conflict of interest. The author Matteo Schimberni declares that he has no conflict of interest.
Ethical Statement
Procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
This descriptive and retrospective study was conducted in the GENERA IVF program, in Rome, Italy, upon approval of the Institution Ethical Committee. All included patients had previously signed written informed consent regarding collection of data for research purposes. For research confidentiality, data analysis was performed anonymously; for that purpose, an identification number was assigned for each patient, and the patients’ names were not mentioned during analysis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Paola De Marco, Email: demarco.mariapaola@gmail.com.
Giulia Montanari, Email: giulia.montanari26@gmail.com.
Ilary Ruscito, Email: ilary.ruscito@uniroma1.it.
Annalise Giallonardo, Email: giallonardoannalise@gmail.com.
Filippo Maria Ubaldi, Email: ubaldi.fm@gmail.com.
Laura Rienzi, Email: rienzi@generaroma.it.
Flavia Costanzi, Email: costanzi.flavia@gmail.com.
Donatella Caserta, Email: donatella.caserta@uniroma1.it.
Mauro Schimberni, Email: mauro.schimberni@uniroma1.it.
Matteo Schimberni, Email: matteo.schimberni@gmail.com.
References
- 1.Jenkins JM, Davies DW, Devonport H, Anthony FW, Gadd SC, Watson RH, Masson GM. Comparison of ‘poor’ responders with ‘good’ responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum Reprod. 1991;6:918–921. doi: 10.1093/oxfordjournals.humrep.a137459. [DOI] [PubMed] [Google Scholar]
- 2.Pellicer A, Ballester MJ, Serrano MD, Mir A, Serra-Serra V, Remohi J, Bonilla-Musoles FM. Aetiological factors involved in the low response to gonadotrophins in infertile women with normal basal serum follicle stimulating hormone levels. Hum Reprod. 1994;9:806–811. doi: 10.1093/oxfordjournals.humrep.a138600. [DOI] [PubMed] [Google Scholar]
- 3.Mark V. Sauer MD reproduction at an advanced maternal age and maternal health. Fert Ster. 2015;103(5):1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Ben Rafael Z, Orvieto R, Feldberg D. The poor responder patient in an in vitro fertilization–embryo transfer (IVF–ET) program. Gynecol Endocrinol. 1994;8:277–286. doi: 10.3109/09513599409023632. [DOI] [PubMed] [Google Scholar]
- 5.Surrey ER, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fert Ster 2000; 73, 667–. [DOI] [PubMed]
- 6.Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–1190. doi: 10.1016/S0015-0282(01)02893-X. [DOI] [PubMed] [Google Scholar]
- 7.Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9:61–76. doi: 10.1093/humupd/dmg007. [DOI] [PubMed] [Google Scholar]
- 8.Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G. Gianaroli L on behalf of the ESHRE working group on poor ovarian response definition ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 9.Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi. FM What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol. 2018; 10.1016/j.beem.2018.10.005 [DOI] [PubMed]
- 10.Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, Aragona C. A controlled trial of natural cycle versus microdose gonadotropin releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil Steril 2004; 81,1542–. [DOI] [PubMed]
- 11.Nargund G, Fauser BC, Macklon NS, et al. Rotterdam ISMAAR consensus group on terminology for ovarian stimulation for IVF. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum Reprod. 2007;22:2801e4. doi: 10.1093/humrep/dem285. [DOI] [PubMed] [Google Scholar]
- 12.Schimberni M, Morgia F, Colabianchi J, Giallonardo A, Piscitelli C, Giannini P, Montigiani M, Sbracia M. Natural-cycle in vitro fertilization in poor responder patients: a survey of 500 consecutive cycles. Fertil Steril. 2009;4:1297–1301. doi: 10.1016/j.fertnstert.2008.07.1765. [DOI] [PubMed] [Google Scholar]
- 13.Bassil S, Godin PA, Donnez J. Outcome of in-vitro fertilization through natural cycles in poor responders. Hum Reprod. 1999;14:1262–1265. doi: 10.1093/humrep/14.5.1262. [DOI] [PubMed] [Google Scholar]
- 14.Ubaldi FM, Rienzi L, Ferrero S, Baroni E, Sapienza F, Cobellis L, Greco E. Management of poor ovarian responders in IVF. Reprod BioMed Online. 2005;10:235–246. doi: 10.1016/S1472-6483(10)60946-7. [DOI] [PubMed] [Google Scholar]
- 15.Elizur SE, Aslan D, Shulman A, Weisz B, Bider D, Dor J. Modified natural cycle using GnRH antagonist can be an optional treatment in poor responders undergoing IVF. J Assist Reprod Genet. 2005;22:75–79. doi: 10.1007/s10815-005-1496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ata B, Yakin K, Balaban B, Urman B. Embryo implantation rates in natural and stimulated assisted reproduction treatment cycles in poor responders. Reprod BioMed Online. 2008;17:207–212. doi: 10.1016/S1472-6483(10)60196-4. [DOI] [PubMed] [Google Scholar]
- 17.Roesner S, Pflaumer U, Germeyer A, et al. Natural cycle IVF: evaluation of 463 cycles and summary of the current literature. Arch Gynecol Obstet. 2014;289:1347e54. doi: 10.1007/s00404-013-3123-2. [DOI] [PubMed] [Google Scholar]
- 18.Drakopoulos P, Romito A, Errazuriz J et al. Modified natural cycle IVF versus conventional stimulation in advanced-age Bologna poor responders. Reprod Biomed Online 2019; 39(4), 698–703. 39(4):698–703. [DOI] [PubMed]
- 19.von Wolff M, Rohner S, Santi A, et al. Modified natural cycle in-vitro fertilization e an alternative IVF treatment with lower costs per achieved pregnancy but longer treatment time. J Reprod Med. 2014;59:553e9. [PubMed] [Google Scholar]
- 20.Sunkara SK, LaMarca A. Polyzos NP et al live birth and perinatal outcomes following stimulated and unstimulated IVF: analysis of over two decades of a nationwide data. Hum Reprod. 2016;31:2261e7. doi: 10.1093/humrep/dew184. [DOI] [PubMed] [Google Scholar]
- 21.Groen H, Tonch N, Simons AHM, van der Veen F, Hoek A, Land JA. Modified natural cycle versus controlled ovarian hyperstimulation IVF: a cost-effectiveness evaluation of three simulated treatment scenarios. Hum Reprod. 2013:3236–46. [DOI] [PubMed]
- 22.Horcajadas JA, Mínguez P, Dopazo J, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500e10. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 23.Lainas TG, Sfontouris IA, Venetis CA, et al. Live birth rates after modified natural cycle compared with high-dose FSH stimulation using GnRH antagonists in poor responders. Hum Reprod. 2015;30:2321e30. doi: 10.1093/humrep/dev198. [DOI] [PubMed] [Google Scholar]
- 24.Kollmann Z, Bersinger NA, McKinnon BD, et al. Anti-Müllerian hormone and progesterone levels produced by granulosa cells are higher when derived from natural cycle IVF than from conventional gonadotropin-stimulated IVF. Reprod Biol Endocrinol. 2015;13:21. doi: 10.1186/s12958-015-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollmann Z, Schneider S, Fux M, et al. Gonadotrophin stimulation in IVF alters the immune cell profile in follicular fluid and the cytokine concentrations in follicular fluid and serum. Hum Reprod. 2017;15:1e12. doi: 10.1093/humrep/dex005. [DOI] [PubMed] [Google Scholar]
- 26.van Loendersloot LL, van Wely M, Limpens J, et al. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577e89. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 27.Haemmerli Keller K, Alder G, Faeh M, et al. Three natural cycle IVF treatment imposes less psychological stress than one conventional IVF treatment cycle. Acta Obstet Gynecol Scand. 2018;97:269e76. doi: 10.1111/aogs.13281. [DOI] [PubMed] [Google Scholar]
- 28.von Wolff M. The role of natural cycle IVF in assisted reproduction. Best Pract Res Clin Endocrinol Metab. 2019;33(1):35–45. doi: 10.1016/j.beem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Svanberg AS, Boivin J, Bergh T. Factors influencing the decision to use or discard cryopreserved embryos. Acta Obstet Gynecol Scand. 2001;80:849e55. doi: 10.1034/j.1600-0412.2001.080009849.x. [DOI] [PubMed] [Google Scholar]
- 30.Arce J-C, Nyboe Andersen A, Fernández-Sánchez M, Visnova H, Bosch E, García-Velasco JA, et al. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimüllerian hormone–stratified, dose–response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102:1633–1640.e5. doi: 10.1016/j.fertnstert.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 31.von Wolff M, Schwartz AK, Bitterlich N, Stute P, Fäh M. Only women's age and the duration of infertility are the prognostic factors for the success rate of natural cycle IVF. Arch Gynecol Obstet. 2019;299(3):883–889. doi: 10.1007/s00404-018-5034-8. [DOI] [PubMed] [Google Scholar]