Abstract
Objectives
This study aimed to explore the diagnostic significance of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT for predicting the presence of epidermal growth factor receptor (EGFR) mutations in patients with non-small cell lung cancer (NSCLC).
Design
A systematic review and meta-analysis.
Data sources
The PubMed, EMBASE and Cochrane library databases were searched from the earliest available date to December 2020.
Eligibility criteria for selecting studies
The review included primary studies that compared the mean maximum of standard uptake value (SUVmax) between wild-type and mutant EGFR, and evaluated the diagnostic value of 18F-FDG PET/CT using SUVmax for prediction of EGFR status in patients with NSCLC.
Data extraction and synthesis
The main analysis was to assess the sensitivity and specificity, the positive diagnostic likelihood ratio (DLR+) and DLR−, as well as the diagnostic OR (DOR) of SUVmax in prediction of EGFR mutations. Each data point of the summary receiver operator characteristic (SROC) graph was derived from a separate study. A random effects model was used for statistical analysis of the data, and then diagnostic performance for prediction was further assessed.
Results
Across 15 studies (3574 patients), the pooled sensitivity for 18F-FDG PET/CT was 0.70 (95% CI 0.60 to 0.79) with a pooled specificity of 0.59 (95% CI 0.52 to 0.66). The overall DLR+ was 1.74 (95% CI 1.49 to 2.03) and DLR− was 0.50 (95% CI 0.38 to 0.65). The pooled DOR was 3.50 (95% CI 2.37 to 5.17). The area under the SROC curve was 0.68 (95% CI 0.64 to 0.72). The likelihood ratio scatter plot based on average sensitivity and specificity was in the lower right quadrant.
Conclusion
Meta-analysis results showed 18F-FDG PET/CT had low pooled sensitivity and specificity. The low DOR and the likelihood ratio scatter plot indicated that 18F-FDG PET/CT should be used with caution when predicting EGFR mutations in patients with NSCLC.
Keywords: nuclear radiology, respiratory tract tumours, genetics
Strengths and limitations of this study.
To our knowledge, this is the first review that systematically analyses the diagnostic accuracy of 18F-fluorodeoxyglucose/CT for predicting epidermal growth factor receptor status.
Weight mean difference analysis was performed prior to inclusion of studies in the diagnostic accuracy meta-analysis.
High heterogeneous effect should be mentioned in the results interpretation.
Introduction
Lung cancer is a common malignant tumour that is associated with considerable social and economic burden. Global statistics show that among malignant tumours, morbidity and mortality from lung cancer ranks first in males, while in females lung cancer is second only to breast cancer.1 Non-small cell lung cancer (NSCLC) accounts for 85%–90% of lung cancers, with lung adenocarcinomas (LUAD) being the most diagnosed histological subtype of NSCLC.2 In Asia, up to 50% of patients with LUAD have activating mutations of the tyrosine kinase domain of epidermal growth factor receptor (EGFR).3 Tyrosine-kinase inhibitor (TKI), which targets EGFR kinase domain mutations, seems to trigger a form of oncogenic shock, resulting in a favourable response in NSCLC.4 The clinical outcome of the patients with NSCLC harbouring EGFR alteration was significantly improved by three different generations of EGFR TKIs. Therefore, EGFR mutations are considered to have a predictive role in the success of TKI treatment in NSCLC. The standard approach to detecting EGFR status is genetic testing, which is based on tumour specimens captured by resection, fine needle aspiration or biopsy. However, this method does not reflect the status of the entire tumour, and usually results in failure or poor reproducibility due to insufficient materials. Liquid biopsy can identify mutant target gene in circulating cell-free tumour DNA, which is sometimes inconsistent with specimens biopsy,5 limiting it clinical application. Moreover, neither biopsies nor plasma samples can provide accurate anatomical information such as position, size, boundary and relationship with adjacent structures of the tumours, which is critical for clinical treatment planning and response assessment.
Image-based phenotyping, which provides a non-invasive method to visualise tumour phenotypic characteristics, is a promising tool for precision medicine.6 X-ray CT imaging have been systematically analysed to discover anatomical risk factors for EGFR mutations prediction in NSCLC.7 Molecular imaging is an attractive option for evaluating patients with NSCLC receiving targeted treatment because it can non-invasively capture the molecular and genomic characteristics of the tumour. The use of positron emission tomography (PET)/CT as a molecular imaging modality for precision medicine is unique. 18F-fluorodeoxyglucose (18F-FDG) PET/CT can provide information on glucose metabolism and is widely used for cancer diagnosis and image-guided therapy. Semi-quantitative parameters can be used for PET image analysis, with the mean maximum of standard uptake value (SUVmax) being the most effective and commonly used parameter. It has been reported that 18F-FDG PET/CT can predict EGFR status in patients with NSCLC, but this remains controversial. Some studies have confirmed that higher uptake of 18F-FDG is predictive of mutant EGFR in patients with NSCLC,8–10 while several other studies have shown the opposite result.11–13 A systematic review is needed to clarify this point.
Although 18F-FDG PET/CT was used to predict many biological features or other genetic mutations of certain malignancies through meta-analysis,14–16 as far as we know, no meta-analysis has summarised the association between 18F-FDG PET/CT and EGFR mutation status in NSCLC. The purpose of our study was to conduct a meta-analysis of the diagnostic performance of 18F-FDG PET/CT in predicting EGFR mutations, thereby providing more evidence for precise treatment of patients with NSCLC.
Methods
Screening of publications
A systematic review of publications relevant to PET and EGFR mutations in NSCLC was undertaken using the electronic databases of PubMed, Embase and the Cochrane library from the earliest available date of indexing up to 31 December 2020. A search algorithm based on combined terms was used: (1) “FDG” OR “Fluorodeoxyglucose” OR “2-Fluoro-2-deoxyglucose” OR “2-Fluoro-2-deoxy-D-glucose” and (2) “PET” OR “positron emission tomography” and (3) “Epidermal Growth Factor Receptor” OR “EGFR” OR “c-erbB-1” OR “erbB-1” OR “v-erbB” and (4) “pulmonary cancer” OR “pulmonary cancer” OR “lung neoplasm” OR “lung cancer” and (5) “mutation” (see online supplemental file for further details on search strategy). In order to expand the scope of our search, we also screened the references of the included studies for other studies to include.
bmjopen-2020-044313supp001.pdf (263.3KB, pdf)
Inclusion of studies and data extraction
Only original articles focusing on 18F-FDG PET/CT and EGFR status in patients with NSCLC were eligible for inclusion. To compare the differences in 18F-FDG uptake between EGFR mutant and wild-type patients, the publications that reported SUVmax and SD of EGFR mutant and wild-type groups were first selected. Next, articles using 18F-FDG PET/CT to predict EGFR status in patients with NSCLC were included based on whether they provided sufficient data to re-evaluate the sensitivity and specificity, or provided absolute data including true-positive, true-negative, false-positive and false-negative without data overlap. Duplicate publications and publications that do not contain original data, such as case reports, conference papers, review articles and letters, were excluded. Non-relevant studies and basic research were also excluded. Only English articles were evaluated. Two researchers independently reviewed the abstracts of the selected articles using the above inclusion criteria. When there were disagreements between authors, a consensus was reached through a third author who was consulted. The same researchers independently evaluated the full text to determine whether they were eligible for final inclusion.
Quality assessment and publication bias
For pooled weighted mean difference (WMD) analysis, risk of bias, including random sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting were assessed. Publication bias was assessed using a funnel plot, and plot asymmetry was considered to be suggestive of publication bias. For diagnostic performance analysis, the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was employed to assess the risk of bias in diagnostic accuracy studies. The tool consisted of four domains of risk of bias, including patient selection, index test, reference standard and flow and timing. Publication bias was evaluated using a funnel plot and Egger’s regression test.
Data synthesis and analysis
A WMD was calculated through SUVmax extracted from the retrieved articles. A random effects model was used for statistical analysis of the data. Pooled data were displayed using forest plots and presented with 95% CIs. An I2 test was performed to analysis the heterogeneity between studies (I2 value >50% was considered significant). Diagnostic performance for prediction was further assessed. The main purpose was to assess the sensitivity and specificity, the positive and negative diagnostic likelihood ratios (DLR+ and DLR−, respectively), as well as the diagnostic OR (DOR). Publication bias was evaluated using a Deeks’ funnel plot of the effective sample size. The bivariate model allowed us to incorporate the correlation that might exist between the logit-transformed values of paired sensitivity and specificity across studies. Each data point of the summary receiver operator characteristic (SROC) graph was derived from a separate study. Based on these points, the smooth SROC curve was formed to reveal the accuracy of the pooled measures. The likelihood ratio scatter plots graphically showed summary spots of likelihood ratios obtained from the average sensitivity and specificity. Statistical analyses were performed using STATA V.15.1 (StataCorp LP) and RevMan V.5.3 (Cochrane Collaboration, Copenhagen, Denmark). P≤0.05 was considered statistically significant.
Patient and public involvement statement
Neither patients nor the public were involved in the design and planning of the study.
Results
Literature search and selection of studies
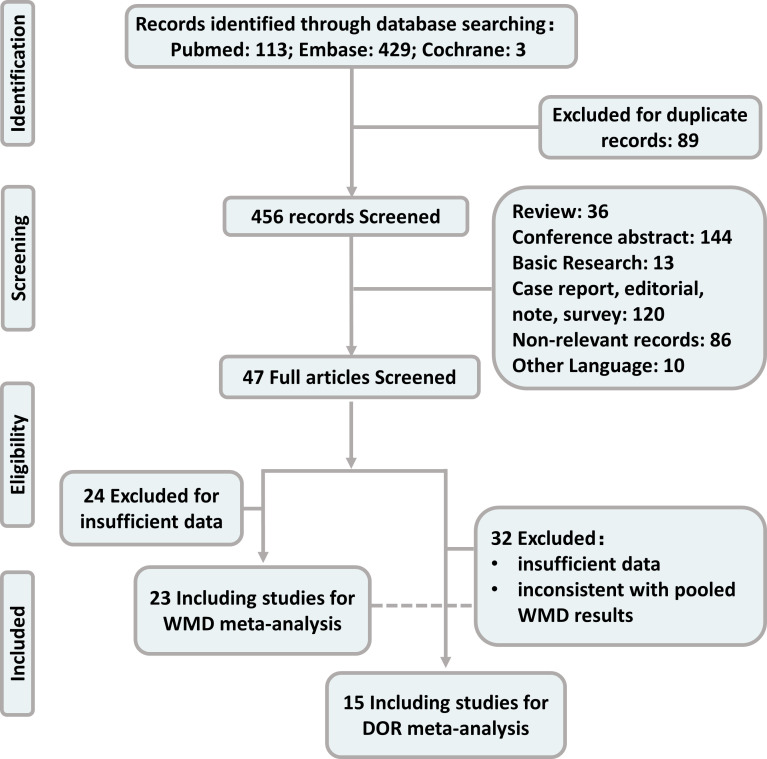
The comprehensive search yielded 545 records for analysis. Records with duplicate titles and abstracts (89) were excluded. Additionally, 36 review articles, 144 conference abstracts, 13 basic research articles, 120 case reports, editorials, notes and surveys, 86 non-relevant records and 10 other language studies were excluded. The remaining 47 full-text articles were further assessed for eligibility. For calculating pooled WMD, 24 articles were excluded due to insufficient data and 23 studies were included. For the pooled DOR analysis, 29 articles were excluded due to insufficient data and 3 articles were excluded due to inconsistent results according to pooled WMD results (18F-FDG uptake was significantly lower in EGFR mutant group; the pooled sensitivity, specificity and DOR were also calculated without excluding the three studies). The remaining 15 studies were included in the meta-analysis. The detailed procedure of study selection is shown in figure 1.
Figure 1.
Publication screening flowchart. DOR, diagnostic OR; WMD, weighted mean difference.
Study description and publication bias
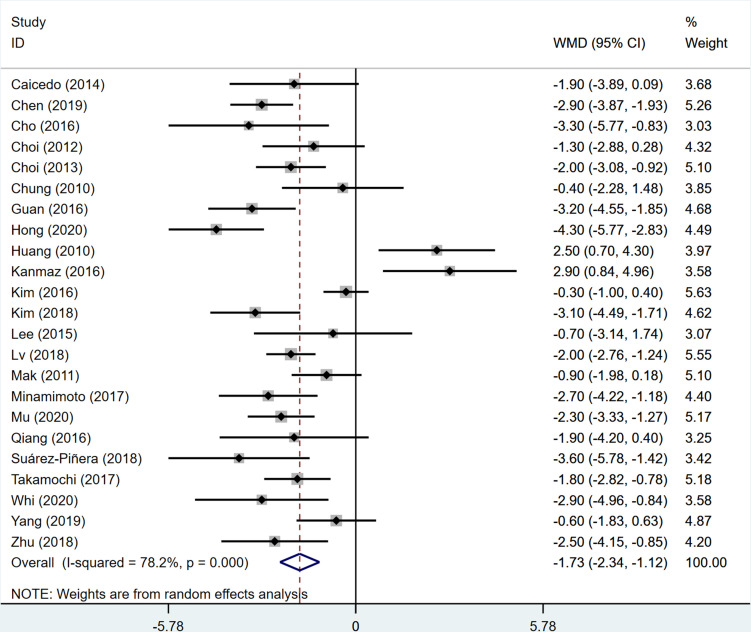
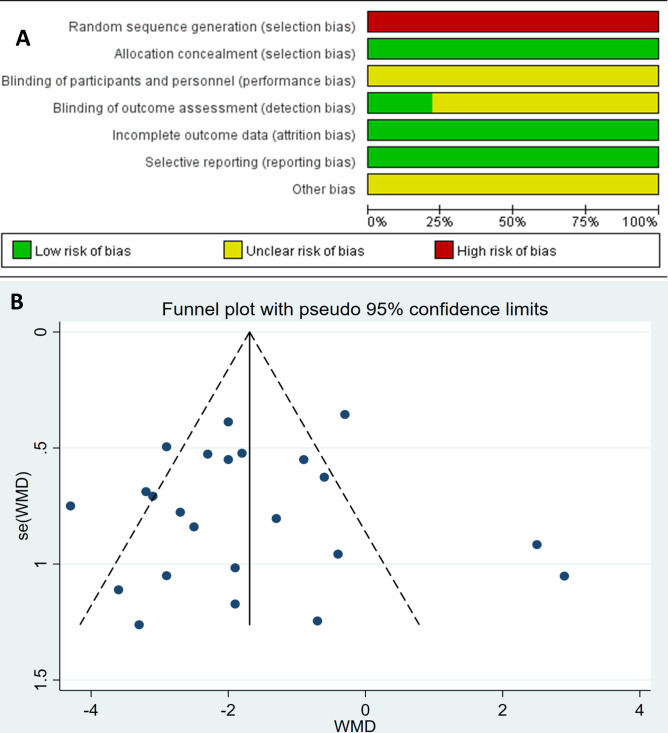
All included patients underwent a 18F-FDG PET/CT examination and EGFR gene test. EGFR mutations analysis was carried out on tissue specimens obtained from resection, aspiration or biopsy. A total of 5220 patients were included in the WMD analysis, and SUVmax between the EGFR mutant and wild-type groups were compared. The patients were enrolled retrospectively in all 23 of the included studies. The pooled comparison of the studies demonstrated that 18F-FDG uptake was significantly lower in the EGFR mutant group (WMD −1.73; 95% CI −2.34 to −1.12; p<0.05; I2=78.2%, figure 2). The most common domains with reporting deficiencies related to the patient selection, as there was no random sequence generation for retrospective studies (figure 3A). Visual analysis of the funnel plot was not suggestive of publication bias using Egger’s test (p=0.786; figure 3B). The principal characteristics of the included 23 studies are shown in table 1.
Figure 2.
Forest plot for analysis of 18F-fluorodeoxyglucose uptake in epidermal growth factor receptor mutant versus wild-type in patients with non-small cell lung cancer. WMD, weighted mean difference.
Figure 3.
(A) Risk of bias of included studies.(B) Funnel plot of maximum of standard uptake value in epidermal growth factor receptor mutant versus wild-type in patients with non-small cell lung cancer. WMD, weighted mean difference.
Table 1.
Characteristics of the included studies
| Authors | Year | Country | Study design | Patient number | Age (mean) | Gender (M/F) | Smoker | LUAD | Genetic test | EGFR mutant/wild-type | 18F-FDG injection dose | Cut-off value | Meta-analysis |
| Caicedo et al33 | 2014 | Spain | R | 102 | 62 | 62/40 | 73 | 90 | PCR | 22/80 | NA | NA | WMD |
| Chen et al10 | 2019 | China | R | 157 | 66 | 84/73 | 68 | 144 | PCR | 54/103 | 481 MBq | 9.92 | WMD/DOR |
| Cho et al19 | 2016 | Korea | R | 61 | 61 | 33/28 | 29 | 58 | PCR | 30/31 | 5.5 MBq/kg | 9.6 | WMD/DOR |
| Choi et al34 | 2012 | Korea | R | 163 | 60 | 99/64 | 73 | 130 | PCR | 57/106 | 5.18 MBq/kg | NA | WMD |
| Choi et al35 | 2013 | Korea | R | 331 | 62 | 158/173 | 145 | 331 | PCR | 156/175 | 5.18 MBq/kg | NA | WMD |
| Chung et al25 | 2010 | Korea | R | 106 | 64 | 63/43 | 60 | 97 | PCR | 42/64 | 4.8 MBq/kg | NA | WMD |
| Gao et al36 | 2020 | China | R | 167 | 58 | 87/80 | 67 | 162 | PCR | 72/94 | 370 MBq | 11.5 | DOR |
| Gu et al21 | 2017 | China | R | 210 | 59 | 132/78 | 90 | 161 | PCR | 70/140 | 5.18 MBq/kg | 9 | DOR |
| Guan et al20 | 2016 | China | R | 316 | 60 | 216/100 | 162 | 242 | PCR | 126/190 | NA | 8.1 | WMD/DOR |
| Hong et al37 | 2020 | Korea | R | 134 | 69 | 89/45 | 76 | 134 | PCR | 62/72 | 52/7 MBq/kg | 9.6 | WMD/DOR |
| Huang et al11 | 2010 | China | R | 77 | 62 | 44/33 | 16 | 77 | PCR | 49/28 | 370 MBq | NA | WMD |
| Kanmaz et al12 | 2016 | Turkey | R | 218 | 62 | 151/67 | 155 | 218 | PCR | 63/155 | 3.7–5.2 MBq/kg | NA | WMD |
| Kim et al38 | 2016 | Korea | R | 198 | 62 | 113/85 | 68 | 183 | PCR | 101/97 | 5.18 MBq/kg | NA | WMD |
| Kim et al39 | 2018 | Korea | R | 232 | 64 | 104/128 | 93 | 232 | PCR | 132/100 | 5.18 MBq/kg | NA | WMD |
| Lee et al18 | 2015 | Korea | R | 206 | 68 | 148/58 | 71 | 135 | PCR | 47/159 | 481 MBq | 11.7 | DOR |
| Lee et al40 | 2015 | China | R | 71 | 65 | 33/38 | 19 | 71 | PCR | 48/23 | 370 MBq | NA | WMD |
| Liao et al26 | 2020 | China | R | 191 | 63 | 101/90 | 65 | 191 | PCR | 63/128 | 3.7 MBq/kg | 7.78 | DOR |
| Lv et al22 | 2018 | China | R | 808 | 59 | 468/340 | 310 | 731 | PCR | 371/437 | 5.5 MBq/kg | 7 | WMD/DOR |
| Liu et al41 | 2017 | China | R | 87 | 60 | 49/38 | 32 | 78 | PCR | 41/46 | NA | 10.4 | DOR |
| Mak et al24 | 2011 | USA | R | 100 | 65 | 39/61 | 73 | 90 | PCR | 24/76 | 5.55–7.4 MBq | NA | WMD |
| Minamimoto et al42 | 2017 | USA | R | 127 | 67 | NA | NA | 127 | PCR | 32/95 | 12~17 mCi | NA | WMD |
| Mu et al30 | 2020 | China, USA | R | 681 | 63 | 378/303 | 315 | 567 | PCR | 312/369 | NA | NA | WMD |
| Na et al17 | 2010 | Korea | R | 100 | 64 | 68/32 | 57 | 53 | PCR | 21/79 | 370 MBq | 9.2 | DOR |
| Qiang et al43 | 2016 | China | R | 97 | 65 | 50/47 | 51 | 97 | PCR | 44/53 | 7.4 MBq/kg | NA | WMD |
| Suárez-Piñera et al44 | 2018 | Spain | R | 106 | 71 | NA | NA | 106 | PCR | 24/82 | 5.29 MBq/kg | NA | WMD |
| Takamochi et al23 | 2017 | Japan | R | 734 | 68 | 367/367 | 363 | 734 | PCR | 334/400 | 3.5 MBq/kg | 2.69 | WMD/DOR |
| Whi et al45 | 2020 | Korea | R | 64 | 66 | 34/30 | 25 | 64 | PCR | 29/35 | 5.18 MBq/kg | 9.5 | WMD/DOR |
| Yang et al8 | 2019 | China | R | 200 | 61 | 108/92 | 68 | 200 | PCR | 115/85 | 3.7–6.66 MBq/kg | 6.15 | WMD/DOR |
| Zhu et al9 | 2018 | China | R | 139 | 62 | 62/77 | 46 | 139 | PCR | 74/65 | 4.2 MBq/kg | 11.19 | WMD/DOR |
DOR, diagnostic OR; EGFR, epidermal growth factor receptor; 18F-FDG, 18F-fluorodeoxyglucose; LUAD, lung adenocarcinoma; WMD, weighted mean difference.
In order to predict the presence of EGFR mutations in patients with NSCLC, a total of 3574 patients were included in the analysis, including 2046 male and 1528 female cases. The average age was 62.9 years old, 90.3% had LUAD and 42.8% were smokers. All 15 studies enrolled patients retrospectively. The EGFR mutation incidence rate was 41.2% with a range of 21.0%–57.5%. SUVmax was used for the interpretation of 18F-FDG PET/CT to predict the EGFR mutation status. The principal characteristics of the 15 included studies are also shown in table 1. Most of the observational studies demonstrated a low risk of bias as assessed by the QUADAS-2 tool (figure 4A). Deek’s funnel plot asymmetry tests were performed to assess a possible publication bias. No significant bias was found (p=0.089; figure 4B).
Figure 4.
(A) Assessment of risk of bias of the included studies using QUADAS-2 tool. (B) Deeks’s funnel plot of asymmetry test for publication bias showed no significant bias was found. ESS, effective sample size; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies-2; WMD, weighted mean difference.
Diagnostic effectiveness of 18F-FDG PET/CT
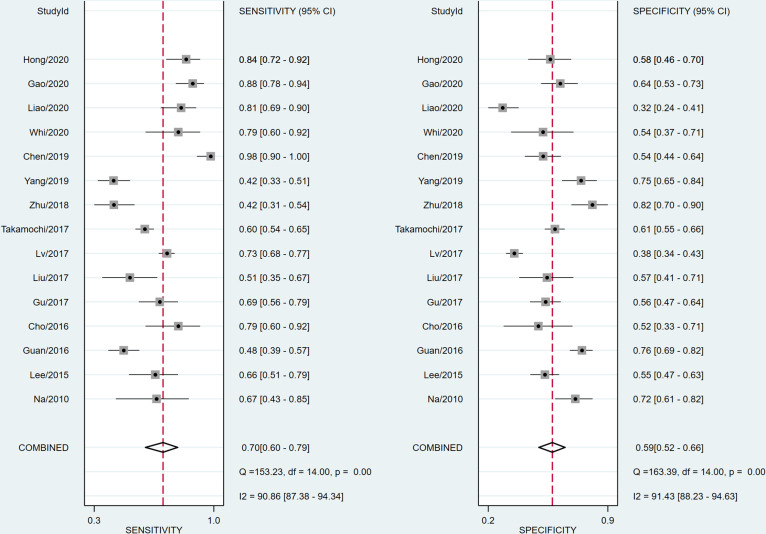
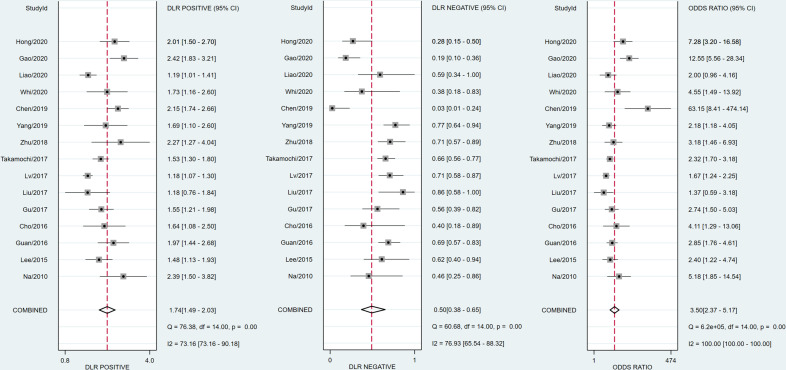
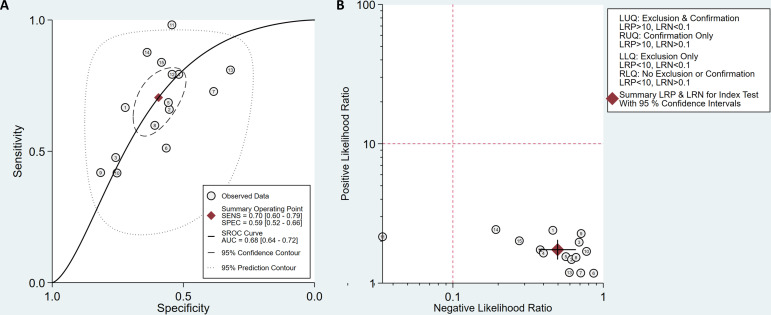
The diagnostic effectiveness of 18F-FDG PET/CT in predicting EGFR mutation in patients with NSCLC was meta-analysed across 15 studies. The pooled sensitivity was 0.70 (95% CI 0.60 to 0.79) with heterogeneity (I2=90.86, 95% CI 87.38 to 94.34, p<0.05). The pooled specificity was 0.59 (95% CI 0.52 to 0.66) with heterogeneity (I2=91.43, 95% CI 88.23 to 94.63, p<0.05; figure 5). DLR syntheses gave an overall DLR+ of 1.74 (95% CI 1.49 to 2.03) and DLR− of 0.50 (95% CI 0.38 to 0.65; figure 6). The pooled DOR was 3.50 (95% CI 2.37 to 5.17; figure 6). The area under curve (AUC) obtained from SROC was 0.68 (95% CI 0.64 to 0.72; figure 7A). Lower pooled sensitivity, specificity and DOR were shown with the three studies included in the prediction of EGFR mutations in patients with NSCLC (see online supplementary figure S1).
Figure 5.
Forest plot of pooled sensitivity and specificity of 18F-fluorodeoxyglucose positron emission tomography/CT for predicting epidermal growth factor receptor mutations in patients with non-small cell lung cancer.
Figure 6.
Forest plot of pooled positive, negative diagnostic likelihood ratio (DLR) and diagnostic OR of 18F-fluorodeoxyglucose emission tomography/CT for predicting epidermal growth factor receptor mutations in patients with non-small cell lung cancer.
Figure 7.
(A) Summary receiver operating characteristic (SROC) curves of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT for predicting epidermal growth factor receptor (EGFR) mutations in patients with non-small cell lung cancer (NSCLC). (B) Likelihood ratio scatter plot of 18F-FDG PET/CT predicting EGFR mutations in patients with NSCLC. AUC, area under curve.
Likelihood ratio scatter plot
The summary value of likelihood ratios obtained from the average sensitivity and specificity shown in the likelihood ratio scatter plot (figure 7B) was located in the lower right quadrant, which indicated that 18F-FDG PET/CT may not be useful for predicting whether there is an EGFR mutation (when positive) or not (when negative).
Discussion
In light of the advances in the precise treatment of lung cancer, identifying targetable mutations at the time of diagnosis has become the key to determining the best treatment strategies. The identification of the EGFR mutation led to an important paradigm shift in the treatment and survival of patients with NSCLC. A typical molecular imaging technique, 18F-FDG PET/CT has been used in prediction of EGFR status in patients with NSCLC. However, various studies have published contradictory results. This is the first systematic review and meta-analysis to summarise current evidence for the use of 18F-FDG PET/CT to predict EGFR status in patients with NSCLC. The principal findings of this meta-analysis showed low sensitivity and specificity of 18F-FDG PET/CT in the prediction of EGFR mutations.
Previous studies on the value of 18F-FDG PET in predicting EGFR status have been conflicting. Accumulation of 18F-FDG was reported to be lower in patients with NSCLC, which can be used to predict EGFR status. Na et al first reported that patients with low SUVmax were more likely to have EGFR mutations than those with high SUVmax. When using 9.2 as the cut-off value, the specificity and sensitivity reached 72% and 67%, respectively.17 Lee et al concluded that 18F-FDG avidity had no significant clinical value in predicting EGFR status, while the univariate analysis showed that SUVmax was significantly correlated with EGFR mutation using 11.7 as the cut-off value.18 Cho et al also found that mutant EGFR had relatively lower glycolysis compared with wild-type EGFR. A cut-off SUVmax value of 9.6 had the highest sensitivity (79.3 %) in predicting EGFR mutations.19 Research by Guan et al showed that 18F-FDG uptake values could effectively predict the EGFR mutation status of patients with NSCLC. ROC curve analysis revealed the AUC was 0.65, with an SUVmax value of 8.1 as the cut-off point.20 Next, other studies further demonstrated that low SUVmax was a significant predictor of EGFR mutations using different cut-off values.8 9 21–23 Chen et al demonstrated that using 9.92 as the SUVmax cut-off point can best discriminate the EGFR mutation status with an AUC of 0.75, and they identified that the mechanism responsible for the decreased FDG uptake associated with mutant EGFR was through the NOX4/ROS/GLUT1 axis.10 However, multiple groups have reported no association between SUVmax and EGFR status. Mak et al reported that high normalised SUVmax only correlated with the EFGR wild-type genotype.24 Moreover, several studies have reported conflicting results. Huang et al found that a higher 18F-FDG uptake with a SUVmax cut-off value of 9.5 correlates with the presence of EGFR mutations.11 While Ko et al showed a trend of higher SUVmax in patients with an EGFR mutation, with an optimal cut-off was 6.13 Kanmaz et al made a similar conclusion, with an SUVmax cut-off value of 13.65 as the predictor.12
Our results indicated the 18F-FDG PET/CT has low sensitivity and specificity in predicting EGFR mutations. Comparison of mean SUVmax between EGFR mutant and wild-type was first pooled with WMD to determine the relationship between EGFR status and FDG uptake. According to result of WMD meta-analysis,18F-FDG uptake was significantly lower in the EGFR mutant group. Thus, studies that reported higher 18F-FDG uptake for prediction of EGFR mutation in patients with NSCLC were excluded in the DOR analysis. The meta-analysis showed low pooled sensitivity of 70% and specificity of 59% for prediction. The low DOR of 0.68 as well as the likelihood ratio scatter plot indicated that 18F-FDG PET/CT might not be useful—or, at least, should be used with caution—for predicting EGFR mutations in patients with NSCLC. In addition, the obvious heterogeneity, especially for the main parameters, indicated that the differences between studies cannot be ignored and conclusion should be drawn carefully.
Many efforts have been made to improve prediction efficacy, which may be the direction of future research. More 18F-FDG PET/CT semi-quantitative parameters including metabolic tumour volume and total glucose glycolysis were investigated to potentially predict EGFR mutations.25 26 Recent studies also focused on 18F-FDG PET/CT radiomics.27 28 Radiomics refers to the extraction of quantitative characteristics from medical images.29 The PET/CT-based radiomic characteristics showed good performance in the prediction of EGFR mutations in patients with NSCLC.30 31 Although the predication efficacy improved, its clinical application requires additional studies to confirm and optimise. Beyond 18F-FDG, novel radiotracers have also been investigated. 18F-MPG PET/CT was demonstrated to be a valid strategy for stratifying patients with NSCLC with EGFR-activating mutations for EGFR-TKI treatment,32 but this radiotracer is not routinely available. Other promising studies are under way to translate these novel approaches into the clinic to guide effective precision therapy for patients with NSCLC.
Strengths and limitations
The strength of this study is that the conflicting results were first analysed using WMD analysis, so that a more reasonable meta-analysis can be performed on the accuracy of the diagnosis. The high level of heterogeneity is the main limitation. However, this can be addressed using a random effects model. The first area of heterogeneity is related to NSCLC subtypes. LUAD is the main pathological type of NSCLC, but even within LUAD, there are different subtypes. For example, alveolar carcinoma demonstrates relatively low 18F-FDG uptake. Second, SUVmax is the most stable and commonly used index, but there are many factors that affect SUVmax, including tumour size, glucose level, and image acquisition and reconstruction, especially for different PET/CT equipment with different acquisition parameters. Third, the number of studies included in this study was small, especially for subgroup analysis. To further study these issues, an increased number of high-quality studies need to be carried out in the future.
Conclusion
Our meta-analysis results showed that 18F-FDG PET/CT had low pooled sensitivity and specificity for EGFR mutation prediction. The low DOR and the likelihood ratio scatter plot indicated that 18F-FDG PET/CT might not be useful—or, at least, that it should be used with caution—for predicting EGFR mutations in patients with NSCLC.
Supplementary Material
Footnotes
Contributors: BD is the first author. BD and YL obtained funding. BD, XL and YL designed the study. BD, YC, GL and SW collected and analysed the data. BD drafted the manuscript. BD and YL contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content, and approved the final version of the manuscript. All authors have read and approved the final manuscript. BD and YL are the study guarantors.
Funding: This work was supported by the National Natural Science Foundation of China (81971652) and Young Scholars Programme of China Medical University (QGZ-2018036).
Disclaimer: This study was a systematic review and meta-analysis. Ethics committee approval was not necessary because all data were carefully extracted from existing literature.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No additional data are available.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669–92. 10.1016/j.ccm.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 3.McLoughlin EM, Gentzler RD. Epidermal growth factor receptor mutations. Thorac Surg Clin 2020;30:127–36. 10.1016/j.thorsurg.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 4.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169–81. 10.1038/nrc2088 [DOI] [PubMed] [Google Scholar]
- 5.Del Re M, Crucitta S, Gianfilippo G, et al. Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int J Mol Sci 2019;20. 10.3390/ijms20163951. [Epub ahead of print: 14 Aug 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aerts HJWL. The potential of Radiomic-Based phenotyping in precision medicine: a review. JAMA Oncol 2016;2:1636–42. 10.1001/jamaoncol.2016.2631 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Cai W, Wang Y, et al. Ct and clinical characteristics that predict risk of EGFR mutation in non-small cell lung cancer: a systematic review and meta-analysis. Int J Clin Oncol 2019;24:649–59. 10.1007/s10147-019-01403-3 [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Wang QG, Lu M, et al. Correlations Study Between 18F-FDG PET/CT Metabolic Parameters Predicting Epidermal Growth Factor Receptor Mutation Status and Prognosis in Lung Adenocarcinoma. Front Oncol 2019;9:589. 10.3389/fonc.2019.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu L, Yin G, Chen W, et al. Correlation between EGFR mutation status and F18 -fluorodeoxyglucose positron emission tomography-computed tomography image features in lung adenocarcinoma. Thorac Cancer 2019;10:659–64. 10.1111/1759-7714.12981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Zhou Y, Tang X, et al. Egfr mutation decreases FDG uptake in non‑small cell lung cancer via the NOX4/ROS/GLUT1 axis. Int J Oncol 2019;54:370–80. 10.3892/ijo.2018.4626 [DOI] [PubMed] [Google Scholar]
- 11.Huang C-T, Yen R-F, Cheng M-F, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol 2010;27:9–15. 10.1007/s12032-008-9160-1 [DOI] [PubMed] [Google Scholar]
- 12.Kanmaz ZD, Aras G, Tuncay E, et al. Contribution of ¹⁸fluorodeoxyglucose positron emission tomography uptake and TTF-1 expression in the evaluation of the EGFR mutation in patients with lung adenocarcinoma. Cancer Biomark 2016;16:489–98. 10.3233/CBM-160588 [DOI] [PubMed] [Google Scholar]
- 13.Ko K-H, Hsu H-H, Huang T-W, et al. Value of ¹⁸F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging 2014;41:1889–97. 10.1007/s00259-014-2802-y [DOI] [PubMed] [Google Scholar]
- 14.Machado Medeiros T, Altmayer S, Watte G, et al. 18F-Fdg PET/CT and whole-body MRI diagnostic performance in M staging for non-small cell lung cancer: a systematic review and meta-analysis. Eur Radiol 2020;30:3641–9. 10.1007/s00330-020-06703-1 [DOI] [PubMed] [Google Scholar]
- 15.Kim S-J, Pak K, Kim K. Diagnostic performance of F-18 FDG PET/CT for prediction of KRAS mutation in colorectal cancer patients: a systematic review and meta-analysis. Abdom Radiol 2019;44:1703–11. 10.1007/s00261-018-01891-3 [DOI] [PubMed] [Google Scholar]
- 16.Ayati N, Sadeghi R, Kiamanesh Z, et al. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021;48:428–48. 10.1007/s00259-020-04967-9 [DOI] [PubMed] [Google Scholar]
- 17.Na II, Byun BH, Kim KM, et al. 18F-Fdg uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer 2010;67:76–80. 10.1016/j.lungcan.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Bae SK, Jung SJ, et al. Fdg uptake in non-small cell lung cancer is not an independent predictor of EGFR or KRAS mutation status: a retrospective analysis of 206 patients. Clin Nucl Med 2015;40:950–8. 10.1097/RLU.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 19.Cho A, Hur J, Moon YW, et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer 2016;16:224. 10.1186/s12885-016-2251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan J, Xiao NJ, Chen M, et al. 18F-Fdg uptake for prediction EGFR mutation status in non-small cell lung cancer. Medicine 2016;95:e4421. 10.1097/MD.0000000000004421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Xu S, Huang L, et al. Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer. J Thorac Dis 2018;10:723–31. 10.21037/jtd.2017.12.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv Z, Fan J, Xu J, et al. Value of 18F-FDG PET/CT for predicting EGFR mutations and positive ALK expression in patients with non-small cell lung cancer: a retrospective analysis of 849 Chinese patients. Eur J Nucl Med Mol Imaging 2018;45:735–50. 10.1007/s00259-017-3885-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamochi K, Mogushi K, Kawaji H, et al. Correlation of EGFR or KRAS mutation status with 18F-FDG uptake on PET-CT scan in lung adenocarcinoma. PLoS One 2017;12:e0175622. 10.1371/journal.pone.0175622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak RH, Digumarthy SR, Muzikansky A, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist 2011;16:319–26. 10.1634/theoncologist.2010-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung HW, Lee KY, Kim HJ, et al. Fdg PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol 2014;140:89–98. 10.1007/s00432-013-1545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao X, Cui Y, Chen X, et al. Primary metabolic tumor volume from 18F-FDG PET/CT associated with epidermal growth factor receptor mutation in lung adenocarcinoma patients. Nucl Med Commun 2020;41:1210–7. 10.1097/MNM.0000000000001274 [DOI] [PubMed] [Google Scholar]
- 27.Li X, Yin G, Zhang Y, et al. Predictive Power of a Radiomic Signature Based on 18F-FDG PET/CT Images for EGFR Mutational Status in NSCLC. Front Oncol 2019;9:1062. 10.3389/fonc.2019.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair JKR, Saeed UA, McDougall CC, et al. Radiogenomic models using machine learning techniques to predict EGFR mutations in non-small cell lung cancer. Can Assoc Radiol J 2021;72:109–19. 10.1177/0846537119899526 [DOI] [PubMed] [Google Scholar]
- 29.Park H, Sholl LM, Hatabu H, et al. Imaging of precision therapy for lung cancer: current state of the art. Radiology 2019;293:15–29. 10.1148/radiol.2019190173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu W, Jiang L, Zhang J, et al. Non-Invasive decision support for NSCLC treatment using PET/CT radiomics. Nat Commun 2020;11:5228. 10.1038/s41467-020-19116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Zhao X, Zhao Y, et al. Value of pre-therapy 18F-FDG PET/CT radiomics in predicting EGFR mutation status in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2020;47:1137–46. 10.1007/s00259-019-04592-1 [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Xiao Z, Chen G, et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci Transl Med 2018;10. 10.1126/scitranslmed.aan8840. [Epub ahead of print: 07 03 2018]. [DOI] [PubMed] [Google Scholar]
- 33.Caicedo C, Garcia-Velloso MJ, Lozano MD, et al. Role of [¹⁸F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:2058–65. 10.1007/s00259-014-2833-4 [DOI] [PubMed] [Google Scholar]
- 34.Choi Y-J, Cho BC, Jeong YH, et al. Correlation between (18)f-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in advanced lung cancer. Nucl Med Mol Imaging 2012;46:169–75. 10.1007/s13139-012-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi H, Paeng JC, Kim D-W, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242–7. 10.1016/j.lungcan.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 36.Gao X-C, Wei C-H, Zhang R-G. 18F-Fdg PET/CT SUVmax and serum CEA levels as predictors for EGFR mutation state in Chinese patients with non-small cell lung cancer. Oncol Lett 2020;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong IK, Lee JM, Hwang IK, et al. Diagnostic and Predictive Values of 18F-FDG PET/CT Metabolic Parameters in EGFR-Mutated Advanced Lung Adenocarcinoma. Cancer Manag Res 2020;12:6453–65. 10.2147/CMAR.S259055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TJ, Lee C-T, Jheon SH, et al. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann Thorac Surg 2016;101:473–80. 10.1016/j.athoracsur.2015.07.062 [DOI] [PubMed] [Google Scholar]
- 39.Kim Y-I, Paeng JC, Park YS, et al. Relation of EGFR mutation status to metabolic activity in localized lung adenocarcinoma and its influence on the use of FDG PET/CT parameters in prognosis. AJR Am J Roentgenol 2018;210:1346–51. 10.2214/AJR.17.18916 [DOI] [PubMed] [Google Scholar]
- 40.Lee EYP, Khong P-L, Lee VHF, EYP L, VHF L, et al. Metabolic phenotype of stage IV lung adenocarcinoma: relationship with epidermal growth factor receptor mutation. Clin Nucl Med 2015;40:e190–5. 10.1097/RLU.0000000000000684 [DOI] [PubMed] [Google Scholar]
- 41.Liu A, Han A, Zhu H, et al. The role of metabolic tumor volume (MTV) measured by [18F] FDG PET/CT in predicting EGFR gene mutation status in non-small cell lung cancer. Oncotarget 2017;8:33736–44. 10.18632/oncotarget.16806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamimoto R, Jamali M, Gevaert O, et al. Prediction of EGFR and KRAS mutation in non-small cell lung cancer using quantitative 18F FDG-PET/CT metrics. Oncotarget 2017;8:52792–801. 10.18632/oncotarget.17782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiang G, Huang W, Liang C, et al. Association between histopathological subtype, 18F-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in lung adenocarcinoma. Oncol Lett 2016;11:1769–77. 10.3892/ol.2016.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suárez-Piñera M, Belda-Sanchis J, Taus A, et al. Fdg PET-CT SUVmax and IASLC/ATS/ERS histologic classification: a new profile of lung adenocarcinoma with prognostic value. Am J Nucl Med Mol Imaging 2018;8:100–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Whi W, Ha S, Bae S, et al. Relationship of EGFR mutation to glucose metabolic activity and Asphericity of metabolic tumor volume in lung adenocarcinoma. Nucl Med Mol Imaging 2020;54:175–82. 10.1007/s13139-020-00646-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044313supp001.pdf (263.3KB, pdf)
Data Availability Statement
No additional data are available.