Abstract
To generate new mechanistic hypotheses on the pathogenesis and disease progression of neuroHIV and identify novel therapeutic targets to improve neuropsychological function in people with HIV, we investigated host genes and pathway dysregulations associated with brain HIV RNA load in gene expression profiles of the frontal cortex, basal ganglia, and white matter of HIV+ patients. Pathway analyses showed that host genes correlated with HIV expression in all three brain regions were predominantly related to inflammation, neurodegeneration, and bioenergetics. HIV RNA load directly correlated particularly with inflammation genesets representative of cytokine signaling, and this was more prominent in white matter and the basal ganglia. Increases in interferon signaling were correlated with high brain HIV RNA load in the basal ganglia and the white matter although not in the frontal cortex. Brain HIV RNA load was inversely correlated with genesets that are indicative of neuronal and synaptic genes, particularly in the cortex, indicative of synaptic injury and neurodegeneration. Brain HIV RNA load was inversely correlated with genesets that are representative of oxidative phosphorylation, electron transfer, and the tricarboxylic acid cycle in all three brain regions. Mitochondrial dysfunction has been implicated in the toxicity of some antiretrovirals, and these results indicate that mitochondrial dysfunction is also associated with productive HIV infection. Genes and pathways correlated with brain HIV RNA load suggest potential therapeutic targets to ameliorate neuropsychological functioning in people living with HIV.
Subject terms: Diseases of the nervous system, Neural circuits
Introduction
The prevalence of severe human immunodeficiency virus (HIV)-associated dementia (HAD) has decreased since the introduction of antiretrovirals, but the incidence of milder and chronic forms of HIV-associated neurocognitive disorder (HAND) and HIV-associated major depressive disorder have increased1–10.
HIV persists in the brain despite combination antiretroviral therapy (cART)10–15. HIV RNA load in the central nervous system (CNS) tends to be associated with a range of neurological manifestations10,16–18. Individuals with neurological symptoms but sustained serum HIV suppression often have significant cerebrospinal fluid (CSF) HIV RNA loads16,17. Asymptomatic CSF virus escape has also been documented by lumbar punctures19. Combination antiretroviral therapy decreases brain viral load, improves immunohistochemical markers of neuronal injury in primates20, and is associated with a reduction of neurodegeneration in humans21. The low CNS bioavailability of antiretrovirals (e.g., protease inhibitors, such as atazanavir) has been implicated in higher CSF HIV-1 RNA load, together with the accumulation of resistance mutations in the CNS22. Neurologically symptomatic CSF escape has been linked to drug resistance mutations17,23,24.
Despite the advent of genome-wide Omics strategies, including transcriptomic, proteomic, and metabolomic investigations, their implementation in studies of the pathogenesis of neuroHIV and the identification of druggable targets remain somewhat limited25. Relatively few transcriptomic studies of HIV have been conducted in human samples, although they have provided considerable insights into pathogenesis, disease progression, latency, and reactivation26–30.
The present study sought to identify correlations between host gene expression patterns and brain HIV RNA loads in gene expression profiles of cases in the National NeuroAIDS Tissue Consortium (NNTC), consisting of samples from three brain regions: white matter, the basal ganglia, and the frontal cortex26,28. We used gene set enrichment analysis (GSEA) for pathway analysis31 in conjunction with genesets from the Molecular Signatures Database (MSigDb), including canonical pathways in the C2 collection, including Kyoto Encyclopedia of Genes and Genomes (KEGG)32,33. This approach aids interpretations of genome-wide expression profiles by revealing common biological processes that are dysregulated in pathological conditions. Transcriptional analyses showed that multiple molecular systems correlated with brain HIV RNA load. Genesets that correlated with brain HIV RNA load and were concordantly dysregulated in the three brain regions studied could mostly be grouped into three broad biological processes: greater inflammation, increased transcriptional evidence of neurodegeneration, and bioenergetics dysfunction. Transcriptional evidence of neurodegeneration and bioenergetics dysfunction were also correlated with HIV RNA levels in gene expression profiles of the prefrontal cortex in a rodent model of HIV. Results also indicate that HIV expression is itself associated with mitochondrial dysfunction and that impairments in brain energetics are a central aspect of the progression and severity of neuroHIV.
Methods
RNA brain gene expression profiles
The gene expression dataset consisted of Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays of the frontal cortex (Brodmann area 9), basal ganglia (head of the caudate nucleus), and white matter (deep frontal lobe) and was conducted by NNTC (GEO Accession No. GSE35864). A summary of the patients' clinical and demographic aspects is in Supplemental Table S1. The gene expression data were filtered and normalized as described previously26. Briefly, CEL files that were retrieved from GEO (Accession No. GSE35864) were analyzed using the GC Robust Multi-array Average (GCRMA) package available from Bioconductor (http://www.bioconductor.org). Normalization was implemented with the GCRMA procedure with the option fast set to FALSE in order to correctly retrieve gene–gene correlation34. RNA load was determined by PCR of HIV gag/pol by NNTC in each sample.
Gene expression and pathway analysis
For pathway analysis, we used Gene Set Enrichment Analysis (GSEA), a computational method to assess whether a priori defined sets of genes show statistically significant differences between biological states31. GSEA was used in conjunction with genesets from the Molecular Signatures Database (MSigDb), including canonical pathways in the C2 collection32,35. GSEA uses the Kolmogorov–Smirnov statistical test to assess whether a predefined geneset, here a pathway from the C2 collection, is statistically enriched in differentially expressed genes by testing their distribution in the full list of genes ranked by their differential expression between two biological states31. The MSigDB is a comprehensive database of genesets for performing geneset enrichment analysis that represents a wide range of biological processes and diseases32,35. A total of 1322 canonical Pearson pathways from the MSigDB C2 collection were scored and ranked using the GSEA algorithm as described also in12 using R. Multiple testing adjustment was performed and significance was assessed using the False Discovery Rate (FDR) in GSEA algorithm.
Correlation between HIV transcription and host gene and pathway expression in humans
We first computed Pearson correlations and associated p values between the expression levels of host genes and HIV RNA load. HIV transcription was used as a single value to estimate the overall virus expression level. Adjustments of p values were performed using the Benjamini–Hochberg FDR and significantly correlated host genes with HIV transcripts were selected by applying an appropriate threshold (e.g., 5% threshold by the FDR). Next, we separated the samples into two groups based on the mean expression of HIV transcripts to create groups with graded levels of HIV transcription (e.g., high and low HIV, or higher levels of expression as warranted by the results). Differential expression between groups was computed with Limma and used as an input for GSEA to identify pathways that were differentially regulated between samples with high and low HIV expression (i.e., load). Pathways that were tested were extracted from the MSigDb C2 canonical pathway collection. We selected pathways that were significantly associated with brain HIV load using the GSEA FDR < 0.05.
Correlation between HIV transcription and host gene and pathway expression in a rodent model of HIV
HIV transgenic (Tg) rats on a mixed Wistar-Fischer background, which ensures variability of host genes and HIV expression levels, were used for the study36. Male HIV Tg rats, weighing between 350 and 400 g, were housed two per cage on a reverse 12 h/12 h light/dark cycle (lights off at 8:00 AM) in a temperature (20–22°C) and humidity (45–55%) controlled vivarium with ad libitum access to tap water and food pellets (PJ Noyes, Lancaster, NH, USA). All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute and ARRIVE guidelines (http://www.nc3rs.org.uk/page.asp?id=1357). A total of 18 gene expression profiles from prefrontal cortices of HIV Tg rats profiled by RNA-Seq as previously described36 were separated into two groups based on the mean expression of brain HIV transcripts to create groups with graded levels of HIV transcription (n = 7 for high brain HIV RNA load and n = 10 for low brain HIV RNA load). Differential expression between groups was computed with DESeq2 and used as an input for the GSEA to identify pathways that were differentially regulated between samples with high and low HIV expression. A normalized enrichment score and p-value were calculated for each pathway.
Real-time PCR (RT-PCR)
Validation of gene expression was carried out by quantitative real-time PCR (RT-PCR) analysis in an independent set of human frontal cortex samples with either high or low HIV brain RNA loads as determined by RT-PCR (n = 5) also obtained from the NNTC. RT-PCR experiments were performed on the CFX Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Total RNA was reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad) with 100 ng of total RNA. Amplification was performed on a cDNA amount equivalent to 1.25 ng total RNA. Oligonucleotide primers were designed using the PrimerQuest online tool (IDT, San Diego, CA, USA). Calculations of the relative abundance of the genes under study were done by the comparative cycle threshold (Ct) method and expressed as 2-exp(ΔCt) using an empirically determined Ct of 40 as the detection threshold.
Results
Host transcriptional correlates of brain HIV RNA loads in human brain samples
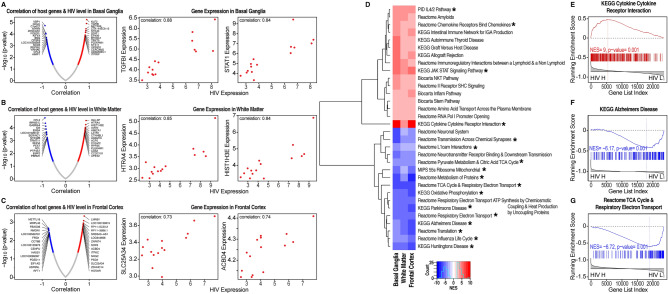
Gene expression profiles from three brain regions of HIV-infected patients, including the basal ganglia, white matter, and the frontal cortex, were used for the study. The gene expression data were filtered and normalized as described previously26. Pearson correlations and associated p values were computed between the expression levels of host genes and HIV transcripts for each brain region as outlined in the Methods section. Genes with a high degree of correlation with brain HIV RNA loads in each of the three brain regions studied are shown in Fig. 1A–C and Supplemental Table S2.
Figure 1.
Correlation of gene expression and pathway dysregulation with brain HIV RNA load in brain regions of patients with HIV. (A–C) Host genes that correlated with brain HIV RNA load in three brain regions of patients with HIV. Volcano plots depict significant correlation with brain HIV RNA loads in the three brain regions under study (left side; also see Supplemental Table S2) and representative examples of genes (plots on the right side). (A) Among the top genes that correlated with HIV expression in the basal ganglia were transforming growth factor-β-induced protein (TGFBI) and signal transducer and activator of transcription 1 (STAT1). (B) Among the top genes that correlated with HIV expression in white matter were HtrA serine peptidase 4 (HTRA4) and histone H3.1 (HIST1H3E). (C) Among the top genes that correlated with HIV expression in the frontal cortex were the mitochondrial carrier protein SLC25A34 and acyl-coenzyme A binding domain containing 4 protein (ACBD4). (D) Gene Set Enrichment Analysis of host genes that were concordantly correlated with HIV expression in all three regions (basal ganglia [BG], white matter [WM], and frontal cortex [FC]). Asterisk indicates pathways significantly concordantly dysregulated in all three brain regions (FDR < 0.05). (E–G) Representative host pathways that were concordantly correlated with HIV expression in all three brain regions that were indicative of (E) increase in cytokine signaling, (F) neurodegeneration, and (G) dysregulation of bioenergetics, between samples from patients with high brain HIV RNA load (HIV-H) and samples from patients with low brain HIV RNA load (HIV-L). The example in panel (E) is from the basal ganglia, the one in panel F is from the frontal cortex, and the one in panel (G) is from white matter. Similar results were obtained for these pathways in the three brain regions as shown in (D). Changes in the expression of the pathway in the GSEA plots, such as the ones in (E–G), are indicated by the asymmetric distribution of genes in the geneset (vertical bars) and of the line plot of the running normalized enrichment score31.
We applied GSEA, a computational method that assesses whether a priori-defined genesets show statistically significant differences between biological states31, to compare gene expression profiles associated with high brain HIV RNA loads. GSEA was used in conjunction with genesets from the MSigDb, including canonical pathways in the C2 collection32. In total, we identified 254 pathways in the basal ganglia, 104 pathways in white matter, and 393 pathways in the frontal cortex that were significantly enriched/decreased in samples with high brain HIV load (FDR < 0.05).
The top differentially regulated pathways in the three brain regions (concordantly changed in all regions) ranked by the normalized enrichment score (NES) are shown in Fig. 1D. Overall, the pathway analysis indicated that host genesets correlated with brain HIV RNA load and were concordantly dysregulated in the three brain regions studied, which could mostly be grouped into three broad biological processes: inflammation, neurodegeneration, and bioenergetics. Pathways related to inflammation, neurodegeneration, and bioenergetics were found to be correlated with HIV levels in all three brain regions (Fig. 1D–G). Pathways that are representative of inflammation and were concordantly dysregulated across brain regions were primarily representative of increased cytokine signaling and directly correlated with brain HIV RNA load. Pathways that are representative of neurodegeneration included pathways that are representative of gene expression changes in Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) were found to be dysregulated across all brain regions.
Pathways that are representative of bioenergetics, including pathways that are related to oxidative phosphorylation, electron transfer, and the tricarboxylic acid (TCA) cycle, were inversely correlated in all three brain regions studied (Fig. 2). Transcripts of several genes involved in energy metabolism were inversely correlated with HIV RNA load in all three brain regions, including α-enolase (ENO1), a glycolytic enzyme that catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which catalyzes the sixth step in glycolysis, converting glyceraldehyde 3-phosphate to d-glycerate 1,3-bisphosphate, and the mitochondrial membrane ATP synthase subunit C locus 3 (ATP5G3), Supplemental Table S2.
Figure 2.
Correlation between pathway dysregulation and brain HIV RNA load in three brain regions of patients with HIV. (A–C) Scatter (volcano) plots that depict differentially regulated pathways by GSEA between high brain HIV RNA load (HIV H) and low brain HIV RNA load (HIV L) in (A) the basal ganglia, (B) white matter, and (C) the frontal cortex. NES, running normalized enrichment score. Tables for each brain region show representative downregulated and upregulated pathways. Pathways with substantial overlap are omitted from these lists. Complete lists are shown in Supplementary Tables S3-6.
The differential expression of genes involved in energy metabolism and the TCA cycle was confirmed in an independent set of frontal cortices from individuals with high and low brain HIV RNA loads by quantitative real time PCR (RT-PCR), Fig. 3.
Figure 3.
Dysregulation of the expression of genes related to energy metabolism and the tricarboxylic acid (TCA) cycle in patients with high brain HIV RNA load. The mRNA levels of genes related to energy metabolism and the TCA cycle were compared in an independent sample of frontal cortices of patients with low brain HIV RNA load (HIV L) and high brain HIV RNA load (HIV H) by RT-PCR (n = 5). Reduced levels were observed for the mRNAs of mitochondrial TCA cycle enzymes, including (A) aconitase 2 (ACO2), (B) isocitrate dehydrogenase subunits α (IDH3A) and (C) γ (IDH3G), (D) α-ketoglutarate dehydrogenase (2-oxoglutarate dehydrogenase E1 component, OGDH), (E) succinyl-CoA ligase subunit β (SUCLA2), (F) succinate dehydrogenase (SDHB) subunit β, (G) fumarate hydratase (FH), and (H) malate dehydrogenase 1 (MDH1) and 2 (MDH2) (I) as well as for the glycolytic enzymes (J) glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and (K) α-enolase (enolase 1 [ENO1]) and mitochondrial respiratory chain proteins (L) succinate dehydrogenase complex, subunit A (SDHA) and (M) mitochondrial membrane ATP synthase subunit C locus 3 (ATP5G3) and for the mRNA of (N) mitochondrial glutaminase (GLS). *p < 0.05, **p < 0.01, ***p < 0.001.
Pathway dysregulations in each of the three brain regions studied are reviewed next.
Basal ganglia
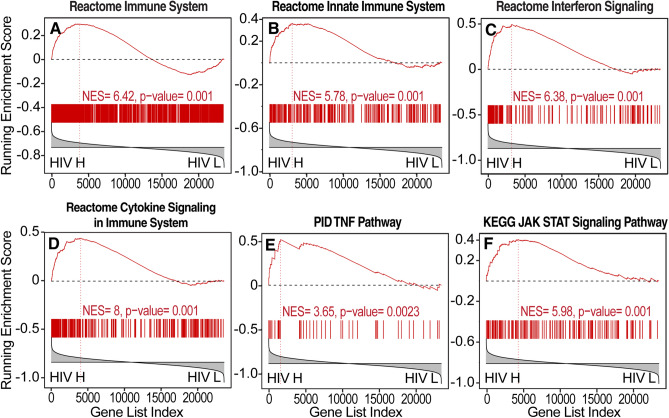
The pathways that showed the highest correlation with brain HIV load in the basal ganglia included pathways indicative of inflammation and immune system activation, including interferon type I and II and cytokine signaling, Toll-like receptor signaling, tumor necrosis factor-α signaling, JAK-STAT signaling, and interferon (Figs. 2 and 4). Inversely correlated with brain HIV RNA load in the basal ganglia were several pathways that are indicative of neural degeneration, including pathways that are representative of gene expression changes in AD, PD, and HD (Figs. 2 and 5), indicative of a reduction of neuronal signaling and trophism. Also inversely correlated with brain HIV RNA load were pathways related to synaptic plasticity, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking, and axonal function (Figs. 2 and 5, Supplemental Table S3). Pathways that are related to energy metabolism, including the TCA cycle, oxidative phosphorylation, and glycolysis, were concomitantly reduced with higher virus brain RNA load (Figs. 2 and 5). Transcriptional evidence of a catabolic milieu and evidence of impairments in proteostasis and mitochondrial protein synthesis were also associated with higher brain HIV RNA load (Figs. 2 and 5). Among the genes that showed higher correlations with virus titers were transforming growth factor, β-induced (TGFBI), signal transducer and activator of transcription 1 (STAT1), meteorin-like (METRNL), interferon-induced with helicase C domain 1 (IFIH1), interferon-inducible protein 16 (IFI16), IFI30, interferon-inducible transmembrane 1 (IFITM1), and γ-interferon-inducible lysosomal thiol reductase (GILT; Fig. 1A, Supplemental Table S4).
Figure 4.
Increase in neuroinflammation correlated with brain HIV RNA load in the basal ganglia. (A, B) Pathway analysis by GSEA provided extensive evidence of immune activation and inflammation, including (C) increases in interferon signaling, (D) cytokine signaling, (E) tumor necrosis factor α (TNF-α) signaling, and (F) JAK STAT pathway activation. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
Figure 5.
Evidence of increase in neurodegeneration and impairments in bioenergetics correlated with brain HIV RNA load in the basal ganglia. (A–C) Transcriptional changes that are indicative of neurodegeneration, including pathways that are representative of gene expression changes in (A) Alzheimer’s disease (AD), (B) Parkinson’s disease (PD), and (C) Huntington’s disease (HD), were associated with high brain HIV RNA load. (D) Gene expression changes indicated that reductions of neuronal signaling that are indicative of a reduction of trophism and synaptodendritic injury were associated with high brain HIV RNA load. (E) Transcriptional evidence of impairments in mitochondrial function and energy metabolism were also correlated with brain HIV RNA load in the basal ganglia as well as evidence of impairments in (F) RNA metabolism, (G, H) proteostasis, and (I, J) lower mitochondrial protein synthesis. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
White matter
Transcriptional evidence of immune activation and inflammation in white matter that were positively correlated with brain HIV RNA load included pathways indicative of increases in cytokines, interferons, and chemokines, including increases in JAK STAT and nuclear factor κB (NFκB) signaling, interferon signaling, and inflammasome activation (Figs. 2 and 6, Supplemental Table S5). Transcriptional evidence of increased neurodegeneration and impaired bioenergetics was also correlated with brain HIV RNA load in white matter, including pathways representative of AD, PD, and HD, reductions of TCA cycle and electron transport, and glycolytic genes (Figs. 2 and 7). Concomitant evidence of a catabolic milieu was also associated with higher brain HIV RNA load (Figs. 2 and 7).
Figure 6.
Evidence of increase in neuroinflammation correlated with brain HIV RNA load in white matter. (A–C) The pathway analysis by GSEA demonstrated broad immune activation and inflammation, characterized by increases in (D) cytokine, (E) interferon, and (F) chemokine signaling, (G) a decrease in transforming growth factor β (TGF-β) pathway signaling, (H) an increase in JAK STAT pathway signaling, and (I) an increase in nuclear factor κB (NFκB) pathway signaling. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
Figure 7.
Evidence of increase in neurodegeneration and impairments in bioenergetics correlated with brain HIV RNA load in white matter. (A–C) The pathway analysis by GSEA demonstrated transcriptional changes that are indicative of neurodegeneration and included pathways representative of gene expression changes in AD, PD, and HD. (D) Gene expression changes indicative of a reduction of energy metabolism, including reduced gene expression of the TCA cycle, electron transport, (E) reduced expression of glycolytic genes; (F) reduced expression of RNA processing genes, and (G–J) reduced expression of amino acid metabolic pathways. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
Frontal cortex
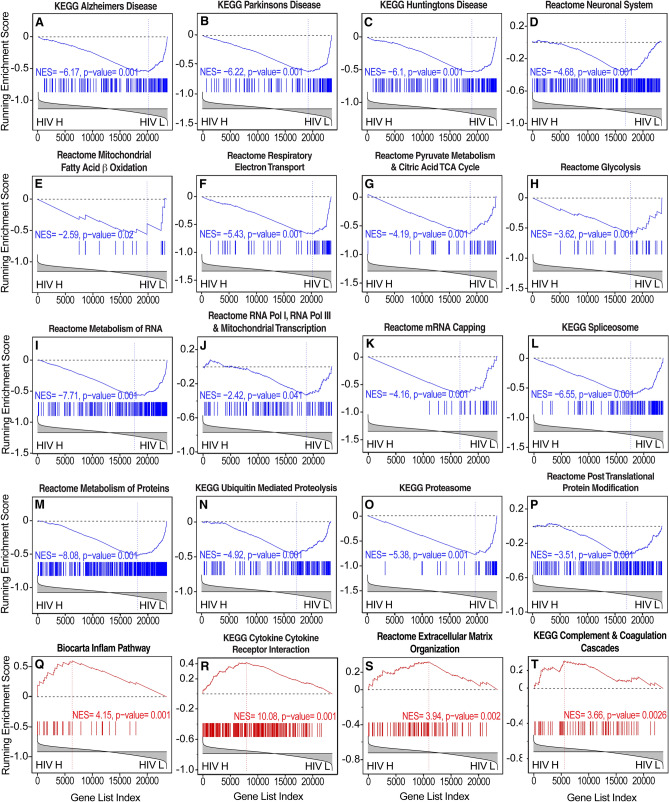
Pathways reflecting changes in the expression of neuronal genes, including axonal and synaptic transcripts, trophic signaling, and mitochondrial and other key genes that are associated with neurodegenerative conditions were dysregulated in the frontal cortex (Figs. 2 and 8). Like the basal ganglia and white matter, these included genesets involved in AD, PD, and HD and evidence of reductions of synaptodendritic injury, synaptic signaling and plasticity, neurotrophin signaling, and energy metabolism (Fig. 7). The latter included reductions of genesets that are representative of TCA cycle, electron transport, and glycolytic genes (Figs. 2, 3 and 8). A catabolic milieu was also indicated by impairments in RNA synthesis and processing and impairments in proteostasis and protein synthesis that are associated with brain HIV RNA load (Fig. 8). Immune activation and inflammation were also correlated with brain HIV RNA load (Fig. 8). Pathways representative of increased inflammation and cytokine signaling were also correlated with brain HIV RNA load in the frontal cortex (Fig. 8).
Figure 8.
Representative pathways indicative of neurodegeneration, impairments in bioenergetics, and neuroinflammation correlated with brain HIV RNA load in the frontal cortex. (A–C) The pathway analysis by GSEA demonstrated transcriptional changes that are indicative of neurodegeneration, including pathways representative of gene expression changes in AD, PD, and HD. (D) Gene expression changes indicated a reduction of neuronal signaling that is consistent with synaptodendritic injury, (E–H) impairments in mitochondrial function and energy metabolism, including reductions of TCA cycle, electron transport, and glycolytic genes, (I–L) impairments in RNA synthesis and processing, (M–P) proteostasis and protein synthesis, (Q, R) increased inflammation and cytokine signaling, (S) extracellular matrix, and (T) complement genes. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
Transcriptional correlates of brain HIV RNA load in a rodent model
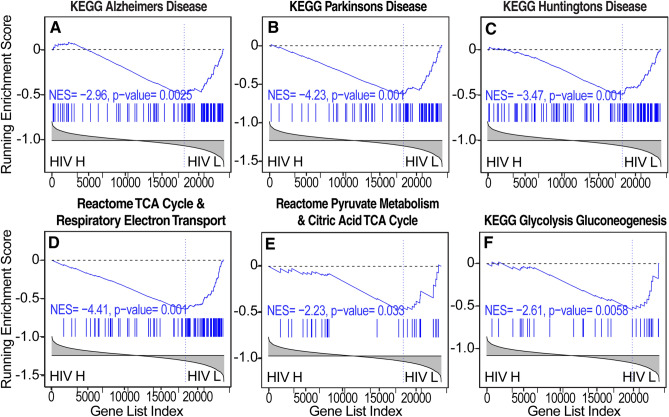
HIV transgenic rats express multiple HIV products in disease-relevant cells in the brain, such as microglia and astrocytes, under control of the viral LTR promoter37–39. These rats also have similar brain gene expression changes as humans with HIV26,36,38. The repertoire of pathway dysregulations in humans with HIV in the present study was generally consistent with observations in HIV transgenic rats36,38. As depicted in Fig. 9, using previously reported gene expression profiles of the frontal cortex in HIV transgenic rats on a mixed genetic background36, the expression of genesets representative of neurodegeneration and bioenergetics, although not inflammation-related genesets, was significantly correlated with brain HIV RNA loads in the frontal cortex in HIV transgenic rats, despite a lower HIV RNA dynamic range (Fig. 9).
Figure 9.
Representative pathways indicative of neurodegeneration and impairments in bioenergetics correlated with brain HIV RNA load in the prefrontal cortex in a rodent model of HIV. (A–C) The pathway analysis by GSEA showed transcriptional changes that are indicative of neurodegeneration-like changes, including pathways that are representative of gene expression changes in AD, PD, and HD, and (D–F) gene expression changes that are indicative of a reduction of energy metabolism, including reductions of TCA cycle, electron transport, and glycolytic genes. HIV H, high brain HIV RNA load; HIV L, low brain HIV RNA load.
Discussion
The molecular mechanisms of HIV-associated neurotoxicity have been only partially elucidated. cART has itself been implicated in neurotoxicity40–42. Brain HIV viral load and indirect indicators, such as CSF HIV RNA load, are generally, albeit variably, inversely correlated with neuropsychological scores10,43–45. To better highlight genes and pathway dysregulations that have greater potential relevance to neuroHIV severity and progression, we correlated transcriptional alterations of functional pathways and genes in three brain regions in human cases of HIV with brain HIV RNA load. We used GSEA, a knowledge-based computational strategy that interrogates a genome-wide expression profile dataset using a priori-defined genesets of functionally related genes46. GSEA was used in conjunction with the MSigDb, including canonical pathways in the C2 collection32.
Here, we found that transcriptional evidence of inflammation was more pronounced in white matter and the basal ganglia. Pathways that are representative of cytokine signaling were correlated with brain HIV RNA load in all three brain regions, consistent with the contribution of inflammatory processes to cortical, subcortical, and white matter injury47. Increases in interferon signaling were associated with high brain HIV RNA load in the basal ganglia and white matter. However, despite considerable activation of interferon-related pathways in the frontal cortex of HIV cases versus uninfected control individuals in the present human neuroHIV dataset26, their overall expression was not significantly correlated with brain HIV RNA load in the frontal cortex. In fact, significant activation of interferon signaling is seen in the frontal cortex even in the absence of neurocognitive impairment26. Conversely, the present results highlight that in subcortical regions such as the basal ganglia and white matter, the activation of interferon signaling was directly correlated with brain HIV RNA load.
Effective cART is associated with lower intrathecal inflammation48–50. A multilevel analysis of the neuropathogenesis of neurocognitive impairment in HIV showed that brain HIV RNA load was significantly correlated with inflammation and markers of neurodegeneration45. Significant correlations were also found between inflammatory markers, particularly type I interferon, and brain or CSF HIV RNA load has been observed in both recent cohorts10,51–53 and earlier ones54–56.
In the present study, brain HIV RNA load was inversely correlated with genesets that are indicative of neuronal and synaptic genes, particularly in the cortex, indicative of synaptic injury and neurodegeneration. Genesets that are related to neurodegeneration and were found to be dysregulated in cases with high brain HIV RNA load included pathways that are representative of gene expression changes in AD, PD, and HD. Established immunohistochemical markers of neuropathological changes that underlie the progression of neuroHIV include pre- and postsynaptic markers, such as synaptophysin (SYP) and microtubule-associated protein-2 (MAP2)45,57, and abnormal protein aggregates, such as β-amyloid58–62. MAP2 and SYP are indicators of synaptic integrity, and their expression in the frontal cortex is inversely correlated with plasma and brain HIV RNA load45. β-amyloid plaque burden in the frontal cortex also correlated with brain HIV RNA load, and SYP and β-amyloid were negatively correlated with HIV RNA load45. Transcriptional evidence of alterations of proteostasis that were associated with brain HIV RNA load was also found, particularly in the basal ganglia and frontal cortex. Altogether, these results indicate that brain HIV RNA load is correlated with synaptodendritic injury and dysfunctional protein clearance. Evidence of neurodegeneration was also found in the basal ganglia and white matter, in addition to the frontal cortex. This is consistent with mounting evidence of the involvement of the basal ganglia and white matter in aging and neurodegenerative diseases, such as AD and PD63–71.
Emerging neuropathology concepts in neuroHIV also suggest a degree of pathogenic similarity and possibly overlap between neuroHIV and AD. Several studies reported increases in amyloid deposition in older HIV-infected individuals, suggesting that long-term HIV and antiretroviral therapy might interfere with the clearance of proteins, such as amyloid-β peptide, and worsen neuronal damage and cognitive impairment in this population58,60,61,72, 73. In one study, amyloid-β plaques in HIV+ brains, although immunohistologically different from those in symptomatic AD brains, were associated with HAND among apolipoprotein E (APOE) ε4 carriers, suggesting the possibility of convergent mechanisms of pathogenesis61. In vitro studies indicate that HIV proteins can disrupt different steps of amyloid pathways59,74–76. The HIV protein Tat, which is produced by the HIV reservoir in the brain even in the setting of the suppression of viral replication77, has been shown to bind amyloid-β peptide, promoting amyloid aggregation and neurotoxicity78. When Tat was injected in the brains of amyloid precursor protein and presenilin-1 transgenic mice (APP-PS1), it colocalized with APP78. Furthermore, crossing Tat transgenic mice with APP-PS1 transgenic mice resulted in an increase in amyloid-β deposition, neurodegeneration, neuronal apoptotic signaling, and phosphorylated Tau compared with PSAPP mice79, indicating that Tat likely contributes to AD-like pathology in HIV. As the HIV-positive population ages, individuals with both HIV and canonical AD pathology are beginning to be diagnosed. A recent case report of AD in an HIV-infected patient who was positive for amyloid on positron emission tomography80 was followed by approximately 20 more cases81. Thus, current issues of debate include the possibility of HAND/AD mixed dementia and the hypothesis that HIV may represent a predisposing factor for AD80–83. Supporting the latter possibility, combined CSF biomarker risk for AD in an Australian HIV-positive cohort was found to be more than 10-times greater than in age-matched controls82.
The present data also show that the gene expression evidence of impairments in bioenergetics are associated with brain HIV RNA load and are correlated with transcriptional evidence of inflammation and neurodegeneration. Mitochondrial dysfunction has been implicated in neurodegenerative disease (e.g., AD84–86 and PD86,87), aging88, and neuroHIV, particularly in adverse ageing in older people who live with HIV89. Frontal cortices from patients with HAND showed mitochondrial abnormalities, including a reduction of mitochondrial biogenesis90. The accumulation of mutations and deletions of mitochondrial DNA (mtDNA)91,92 altered mitochondrial fission and fusion93. Experimental evidence shows that HIV products, such as Tat and gp120, can cause mitochondrial damage94–100. Mitochondrial dysfunction is also implicated in the toxicity of some antiretrovirals101. The present data indicate that mitochondrial dysfunction is a key component of the neuropathogenesis of HIV infection itself.
In the present study, we found that a rodent model of HIV, in which several HIV products are expressed in disease-relevant glial cells (e.g., microglia and astrocytes) but not in neurons37,38, exhibited dysregulations of bioenergetics- and neurodegeneration-related genesets that were associated with brain HIV RNA paralleled findings in gene expression profiles of patients with HIV. In agreement with the present results, mitochondria from this HIV rodent model were found to have significantly lower oxygen consumption rates, basal respiration, ATP production, maximal respiratory capacity, spare capacity, proton leakage, and non-mitochondrial respiration96.
Emphasis has been rightfully placed on impairments in oxidative phosphorylation in neuroHIV and degenerative diseases, such as AD84,102–105. The present pathway analysis underscores the potential contribution of disruptions of the TCA cycle and β-oxidation in the mitochondrial matrix, in which oxidative phosphorylation depends on the supply of reducing equivalents from the end-oxidation of nutrients106. The TCA cycle and oxidative phosphorylation are tightly coupled. The TCA cycle produces the reducing equivalents NADH and FADH2, which are required for electron transfer through the mitochondrial respiratory chain or electron transport chain (ETC). The oxidation of NADH and FADH2 in complexes I and II of the ETC is required to maintain the function of the TCA cycle106. As electrons are transported through the complexes, the ETC produces a mitochondrial membrane potential that generates ATP. Mitochondrial complexes I and II in the ETC replenish NAD+ and FAD, respectively, allowing the oxidative TCA cycle to function106. Thus, lower expression of TCA cycle-related genes may play a crucial role in impairments in mitochondrial oxidative phosphorylation in neuroHIV. Tricarboxylic acid cycle metabolites are also important for the biosynthesis of nucleotides, lipids, and proteins106. Mounting evidence indicates that metabolites in the TCA cycle are also involved in controlling chromatin modifications, DNA methylation, and post-translational modifications of proteins106. Acetyl-coenzyme A levels regulate chromatin dynamics by providing acetyl groups for the acetylation of histones by histone acetyltransferases107–110. The TCA cycle predominates in neurons, whereas glycolysis predominates in astrocytes111. Thus, decreases in TCA cycle-related gene expression in neuroHIV may be a correlate of lower neuronal energy metabolism. Evidence of alterations of TCA cycle capacity and expression that are induced by HIV infection in the scientific literature is limited. Interestingly, CSF metabolomics showed that worsening cognitive status in HIV-infected patients is associated with the accumulation of citrate and acetate, which are suggestive of disruptions of TCA cycle function112. A separate CSF metabolomic analysis of young individuals with HIV who were on ART found a dysregulation of TCA cycle intermediates, such as succinate and malate, among other metabolites, differed when compared with HIV-negative controls113. Relevant to the present results, we recently found that escalated (dependent) methamphetamine self-administration in HIV transgenic rats was associated with the lower expression of TCA cycle-related genes in conjunction with transcriptional evidence of increases in inflammation and neurodegeneration36.
Selected genes that encode enzymes that are involved in glycolysis, such as ENO1 and GAPDH, were concordantly dysregulated in all three brain regions that were studied herein, and the expression of genesets that are representative of glycolysis was reduced particularly in the basal ganglia and frontal cortex, thus indicating that alterations of glycolysis and reductions of the TCA cycle and electron transport are key bioenergetic alterations that are correlated with brain HIV RNA load.
In conclusion, we explored functional pathways that are associated with brain HIV load as a correlate of neuroHIV disease severity and progression by the pathway analyses of transcriptional profiles from brain regions of humans with HIV. We found that brain HIV RNA load correlated with transcriptional evidence of an increase in inflammation. Evidence of increased cytokine signaling was associated with high HIV RNA across the three brain regions studied. Increases in interferon signaling were correlated with high brain HIV RNA load in the basal ganglia and white matter although not in the frontal cortex. We also found transcriptional evidence of neurodegeneration that involved pathways that reflect changes in axonal and synaptic genes and trophic signaling, among others. Lastly, we found transcriptional evidence of impairments in bioenergetics that involved oxidative phosphorylation and the TCA cycle across all three brain regions. Transcriptional evidence of mitochondrial dysfunction was also associated with brain HIV RNA load and correlated with transcriptional evidence of inflammation and neurodegeneration, indicating that mitochondrial dysfunction is a key component of the neuropathogenesis of HIV infection itself.
Supplementary Information
Acknowledgements
Supported by National Institutes of Health grants DA041750, DA043268, DA046170, DA046204, and DA048882. This publication uses NNTC resources and thus was made possible by NIH funding from the NIMH and NINDS (Texas NeuroAIDS Research Center: U24MH100930, California NeuroAIDS Tissue Network: U24MH100928, National Neurological AIDS Bank: U24MH100929, Manhattan HIV Brain Bank: U24MH100931, and NNTC Data Coordinating Center: U24MH100925); its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH. The authors thank Michael Arends for assistance with manuscript preparation.
Author contributions
P.P.S. and V.R.C. conceived the study. Y.F. and C.L. performed the analyses. P.P.S., Y.F., E.M., C.L., V.R.C. interpreted the data. P.P.S., Y.F., C.L., V.R.C. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pietro Paolo Sanna, Email: psanna@scripps.edu.
Vez Repunte-Canonigo, Email: canonigo@scripps.edu.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88052-7.
References
- 1.Heaton RK, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J. Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi V, et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res. Hum. Retroviruses. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- 4.Neuenburg JK, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2002;31:171–177. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 5.McArthur JC. HIV dementia: an evolving disease. J. Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18(Suppl 1):S75–78. doi: 10.1097/00002030-200418001-00011. [DOI] [PubMed] [Google Scholar]
- 7.Everall I, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J. Neurovirol. 2009;15:360–370. doi: 10.1080/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr. Opin. HIV AIDS. 2014;9:517–520. doi: 10.1097/COH.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelman BB, et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J. Acquir. Immune Defic. Syndr. 2013;62:487–495. doi: 10.1097/QAI.0b013e31827f1bdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamers SL, et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J. Virol. 2016;90:8968–8983. doi: 10.1128/JVI.00674-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman BB, Endsley J, Kolson D. When do models of NeuroAIDS faithfully imitate "the real thing"? J. Neurovirol. 2018;24:146–155. doi: 10.1007/s13365-017-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray LR, et al. Is the central nervous system a reservoir of HIV-1? Curr. Opin. HIV AIDS. 2014;9:552–558. doi: 10.1097/COH.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko A, et al. Macrophages but not astrocytes harbor HIV DNA in the brains of HIV-1-infected aviremic individuals on suppressive antiretroviral therapy. J. Neuroimmune Pharmacol. 2019;14:110–119. doi: 10.1007/s11481-018-9809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tso FY, et al. Brain is a potential sanctuary for subtype C HIV-1 irrespective of ART treatment outcome. PLoS ONE. 2018;13:e0201325. doi: 10.1371/journal.pone.0201325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canestri A, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 17.Peluso MJ, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bingham R, et al. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int. J. STD AIDS. 2011;22:608–609. doi: 10.1258/ijsa.2011.010507. [DOI] [PubMed] [Google Scholar]
- 19.Eden A, et al. asymptomatic cerebrospinal fluid HIV-1 viral blips and viral escape during antiretroviral therapy: a longitudinal study. J. Infect. Dis. 2016;214:1822–1825. doi: 10.1093/infdis/jiw454. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez RG, et al. Temporal/compartmental changes in viral RNA and neuronal injury in a primate model of NeuroAIDS. PLoS ONE. 2018;13:e0196949. doi: 10.1371/journal.pone.0196949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant AK, et al. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS. 2015;29:323–330. doi: 10.1097/QAD.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukerji SS, et al. Impact of antiretroviral regimens on cerebrospinal fluid viral escape in a prospective multicohort study of antiretroviral therapy-experienced human immunodeficiency virus-1-infected adults in the United States. Clin. Infect. Dis.. 2018;67:1182–1190. doi: 10.1093/cid/ciy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nightingale S, et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J. Neurovirol. 2016;22:852–860. doi: 10.1007/s13365-016-0448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukerji SS, et al. Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J. Acquir. Immune Defic. Syndr. 2017;75:246–255. doi: 10.1097/QAI.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciuffi A, Mohammadi P, Golumbeanu M, di Iulio J, Telenti A. Bioinformatics and HIV latency. Curr. HIV/AIDS Rep. 2015;12:97–106. doi: 10.1007/s11904-014-0240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanna PP, Repunte-Canonigo V, Masliah E, Lefebvre C. Gene expression patterns associated with neurological disease in human HIV infection. PLoS ONE. 2017;12:e0175316. doi: 10.1371/journal.pone.0175316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi P, et al. Dynamics of HIV latency and reactivation in a primary CD4+ T cell model. PLoS Pathog. 2014;10:e1004156. doi: 10.1371/journal.ppat.1004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelman BB, et al. The national neuro AIDS tissue consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS ONE. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siangphoe U, Archer KJ. Gene expression in HIV-associated neurocognitive disorders: a meta-analysis. J. Acquir. Immune Defic. Syndr. 2015;70:479–488. doi: 10.1097/QAI.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 30.Borjabad A, Volsky DJ. Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer's disease, and multiple sclerosis. J. Neuroimmune Pharmacol. 2012;7:914–926. doi: 10.1007/s11481-012-9409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberzon A, et al. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim WK, Wang K, Lefebvre C, Califano A. Comparative analysis of microarray normalization procedures: effects on reverse engineering gene networks. Bioinformatics. 2007;23:i282–288. doi: 10.1093/bioinformatics/btm201. [DOI] [PubMed] [Google Scholar]
- 35.Liberzon A, et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Guglielmo G, et al. Increases in compulsivity, inflammation, and neural injury in HIV transgenic rats with escalated methamphetamine self-administration under extended-access conditions. Brain Res. 2020;1726:146502. doi: 10.1016/j.brainres.2019.146502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reid W, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Repunte-Canonigo V, et al. Gene expression changes consistent with neuroAIDS and impaired working memory in HIV-1 transgenic rats. Mol. Neurodegener. 2014;9:26. doi: 10.1186/1750-1326-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royal W, 3rd, et al. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J. Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen BK, Roth LM, Grinspan JB, Jordan-Sciutto KL. White matter loss and oligodendrocyte dysfunction in HIV: A consequence of the infection, the antiretroviral therapy or both? Brain Res. 2019;1724:146397. doi: 10.1016/j.brainres.2019.146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Benedetto I, Trunfio M, Guastamacchia G, Bonora S, Calcagno A. A review of the potential mechanisms of neuronal toxicity associated with antiretroviral drugs. J. Neurovirol. 2020;26:642–651. doi: 10.1007/s13365-020-00874-9. [DOI] [PubMed] [Google Scholar]
- 42.Treisman GJ, Soudry O. Neuropsychiatric effects of HIV antiviral medications. Drug Saf. 2016;39:945–957. doi: 10.1007/s40264-016-0440-y. [DOI] [PubMed] [Google Scholar]
- 43.Ellis RJ, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann. Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 44.Everall I, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J. Neurovirol. 2009;15:360–370. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine AJ, et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol. 2016;22:431–441. doi: 10.1007/s13365-015-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 47.Anderson AM, et al. Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2015;69:29–35. doi: 10.1097/QAI.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair E, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J. Infect. Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 50.Yilmaz A, et al. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand. J. Infect. Dis. 2004;36:823–828. doi: 10.1080/00365540410025320. [DOI] [PubMed] [Google Scholar]
- 51.Eden A, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J. Infect. Dis. 2010;202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yilmaz A, et al. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl V, et al. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rho MB, et al. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav. Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 55.Perrella O, et al. Transforming growth factor beta-1 and interferon-alpha in the AIDS dementia complex (ADC): possible relationship with cerebral viral load? Eur. Cytokine Netw. 2001;12:51–55. [PubMed] [Google Scholar]
- 56.Krivine A, et al. Measuring HIV-1 RNA and interferon-alpha in the cerebrospinal fluid of AIDS patients: insights into the pathogenesis of AIDS Dementia complex. J. Neurovirol. 1999;5:500–506. doi: 10.3109/13550289909045379. [DOI] [PubMed] [Google Scholar]
- 57.Moore DJ, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- 58.Achim CL, et al. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J. Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 60.Green DA, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 61.Soontornniyomkij V, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26:2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J. Neurol. Neurosurg. Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan PL, et al. Gray matter atrophy in Parkinson's disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol. Sci. 2013;34:613–619. doi: 10.1007/s10072-012-1250-3. [DOI] [PubMed] [Google Scholar]
- 64.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiol. Dis. 2012;46:590–596. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 65.Jesse S, et al. Neurochemical approaches in the laboratory diagnosis of Parkinson and Parkinson dementia syndromes: a review. CNS Neurosci. Ther. 2009;15:157–182. doi: 10.1111/j.1755-5949.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tromp D, Dufour A, Lithfous S, Pebayle T, Despres O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2015;24:232–262. doi: 10.1016/j.arr.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Alves GS, et al. Neuroimaging findings related to behavioral disturbances in Alzheimer's disease: a systematic review. Curr. Alzheimer Res. 2017;14:61–75. doi: 10.2174/1567205013666160603010203. [DOI] [PubMed] [Google Scholar]
- 68.Brickman AM, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch. Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kandiah N, et al. Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat. Disord. 2013;19:680–683. doi: 10.1016/j.parkreldis.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Provenzano FA, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J. Neuroimmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clifford DB, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci. Lett. 2010;475:174–178. doi: 10.1016/j.neulet.2010.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pulliam L. HIV regulation of amyloid beta production. J. Neuroimmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- 76.Daily A, Nath A, Hersh LB. Tat peptides inhibit neprilysin. J. Neurovirol. 2006;12:153–160. doi: 10.1080/13550280600760677. [DOI] [PubMed] [Google Scholar]
- 77.Johnson TP, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. U.S.A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hategan A, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat. Struct. Mol. Biol. 2017;24:379–386. doi: 10.1038/nsmb.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giunta, B. et al. HIV-1 Tat contributes to Alzheimer's disease-like pathology in PSAPP mice. Int J Clin Exp Pathol.2, 433–443. (2009). PMID: 19294002 [PMC free article] [PubMed]
- 80.Turner RS, et al. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst) 2016;4:1–5. doi: 10.1016/j.dadm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakradhar S. A tale of two diseases: aging HIV patients inspire a closer look at Alzheimer's disease. Nat. Med. 2018;24:376–377. doi: 10.1038/nm0418-376. [DOI] [PubMed] [Google Scholar]
- 82.Cysique LA, et al. APOE epsilon4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals—a cross-sectional observational study. BMC Neurol. 2015;15:51. doi: 10.1186/s12883-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer's disease: an emerging issue in geriatric neuroHIV. Curr. HIV/AIDS Rep. 2017;14:123–132. doi: 10.1007/s11904-017-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swerdlow RH. Mitochondria in Alzheimer brains: a PET project shows complex changes. Neurology. 2020;94:646–647. doi: 10.1212/WNL.0000000000009236. [DOI] [PubMed] [Google Scholar]
- 85.Cardoso S, Seica RM, Moreira PI. Mitochondria as a target for neuroprotection: implications for Alzheimer s disease. Expert Rev. Neurother. 2017;17:77–91. doi: 10.1080/14737175.2016.1205488. [DOI] [PubMed] [Google Scholar]
- 86.Monzio-Compagnoni G, et al. The role of mitochondria in neurodegenerative diseases: the lesson from Alzheimer's disease and Parkinson's disease. Mol. Neurobiol. 2020;57:2959–2980. doi: 10.1007/s12035-020-01926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 88.Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM. Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann. Neurol. 1998;43:217–223. doi: 10.1002/ana.410430212. [DOI] [PubMed] [Google Scholar]
- 89.Hunt M, Payne BAI. Mitochondria and ageing with HIV. Curr. Opin. HIV AIDS. 2020;15:101–109. doi: 10.1097/COH.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 90.Swinton MK, et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: implications for therapeutic targeting of astroglia. Neurobiol. Dis. 2019;130:104502. doi: 10.1016/j.nbd.2019.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, et al. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 92.Miro O, et al. Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clin. Infect. Dis. 2004;39:710–716. doi: 10.1086/423176. [DOI] [PubMed] [Google Scholar]
- 93.Fields JA, et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol. Dis. 2016;86:154–169. doi: 10.1016/j.nbd.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teodorof-Diedrich C, Spector SA. Human immunodeficiency virus type 1 gp120 and tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J. Virol. 2018;92:1. doi: 10.1128/JVI.00993-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rozzi SJ, et al. Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox Res. 2017;32:723–733. doi: 10.1007/s12640-017-9776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thangaraj A, et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14:1596–1619. doi: 10.1080/15548627.2018.1476810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J. Immunol. 2007;178:869–876. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 98.Lecoeur H, et al. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis. 2012;3:e282. doi: 10.1038/cddis.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 2018;4:8. doi: 10.1038/s41420-017-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avdoshina V, et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox Res. 2016;29:583–593. doi: 10.1007/s12640-016-9608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stauch KL, Emanuel K, Lamberty BG, Morsey B, Fox HS. Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J. Neurovirol. 2017;23:795–807. doi: 10.1007/s13365-017-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butterfield TR, Landay AL, Anzinger JJ. Dysfunctional immunometabolism in HIV Infection: Contributing Factors and Implications for Age-Related Comorbid Diseases. Curr HIV/AIDS Rep. 2020;17:125–137. doi: 10.1007/s11904-020-00484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J. Bioenergy Biomembr. 2009;41:441–446. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cenini G, Voos W. Mitochondria as potential targets in Alzheimer disease therapy: an update. Front. Pharmacol. 2019;10:902. doi: 10.3389/fphar.2019.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kallianpur KJ, et al. Systemic mitochondrial oxidative phosphorylation protein levels correlate with neuroimaging measures in chronically HIV-infected individuals. AIDS Res. Hum. Retroviruses. 2020;36:83–91. doi: 10.1089/AID.2019.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moussaieff A, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Lee JV, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mews P, et al. Alcohol metabolism contributes to brain histone acetylation. Nature. 2019;574:717–721. doi: 10.1038/s41586-019-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 112.Dickens AM, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29:559–569. doi: 10.1097/QAD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.